Abstract
AIMS
To investigate the relationships between pretreatment folate concentrations, MTX pharmacokinetics and acute toxicities following high dose methotrexate (HD MTX) therapy.
METHODS
MTX and its major extracellular metabolite 7-OH-MTX were measured in eight serum samples per HD MTX cycle in 65 consecutive osteosarcoma patients (288 cycles) and AUC (area under the blood concentration–time curve) was calculated. Pretreatment concentrations of folate in serum (S) and erythrocytes (ER) were determined. Hepatic, renal and haematological toxicities, assessed by routine laboratory parameters, as well as mucositis were graded according to National Cancer Institute Common Terminology Criteria for adverse events (CTCAE v.3.0). Dermatitis and pleuritis were reported as occurred or not.
RESULTS
S- and ER-folate pretreatment concentrations increased significantly with increasing number of HD MTX cycles (P < 0.001). ER-folate pretreatment concentrations were higher among males (median 610 nmol l−1, 95% CI 550, 680) compared with females (median 465 nmol l−1, 95% CI 430, 520, P < 0.001), but showed no correlation with MTX or 7-OH-MTX pharmacokinetics. We found correlations between alanine aminotransferase peak concentration (ALATmax) and clearance of MTX (P < 0.001), gender (P < 0.001), age (P < 0.001) and 7-OH-MTX concentrations (P < 0.001), the latter being the main factor influencing ALATmax.
CONCLUSION
Our results suggest that 7-OH-MTX is involved in the development of HD MTX hepatic toxicity and that young female patients are most affected.
Keywords: 7-hydroxy-methotrexate, folate, gender, methotrexate, osteosarcoma, toxicity
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
High dose methotrexate (HD MTX) is generally well tolerated, although unpredictable acute toxicities occur frequently.
Low overall toxicity in paediatric patients and increased liver toxicity in adults has been reported, but there are no reports addressing the relationship between acute liver toxicity and gender in patients treated with HD MTX.
Previous studies in animals suggested involvement of the 7-hydroxy-methotrexate metabolite in acute liver toxicity, but this has not been investigated in humans and the aetiology of acute liver toxicity remains unclear.
WHAT THIS STUDY ADDS
Survival has increased dramatically over the past decades for patients with malignancies such as osteosarcoma and since these patients are frequently children or adolescents at the time of high dose methotrexate, identification of risk factors for toxicity increases the possibility for tailoring treatment.
Our study presents a detailed analysis of acute toxicity, folate concentrations and pharmacokinetics of both methotrexate and its major extracellular metabolite 7-hydroxy-methotrexate and identifies several factors that are highly correlated with acute liver toxicity.
Introduction
High dose methotrexate (HD MTX), defined as >1 g m−2, is given in combination with doxorubicin and a platinum agent in most osteosarcoma protocols [1]. The use of leucovorin (LV; 5-formyl-tetrahydrofolic acid) rescue [2] has facilitated the escalation of MTX doses to high plasma concentrations with enhanced anticancer as well as cytotoxic effects. Dihydrofolate reductase is the primary drug target for MTX, although polyglutamated forms of antifolates inhibit other enzymes involved in intracellular folate metabolism [3], thus interrupting normal cellular metabolism and inducing cell death. It is believed that the mechanism of action is responsible both for drug efficacy and toxicity. MTX is hydroxylated to 7-OH-MTX mainly in hepatocytes where concentrations of 7-OH-MTX exceed those of the parent compound shortly after infusion of HD MTX [4]. MTX and 7-OH-MTX may precipitate in renal tubules [5–8] and contribute to renal toxicity [9], but their solubility in urine is enhanced by adequately hydrating and alkalinizing the patient. Routine monitoring of plasma MTX concentrations with early detection of abnormal clearance has permitted countermeasures, such as adjustments of LV doses and intensified hydration regimes, to prevent excessive host toxicity [10, 11]. Despite LV rescue, hydration and urinary alkalinization, HD MTX is associated with toxicities affecting the liver, kidneys, skin, lungs, bone marrow and gastrointestinal system, particularly the oral mucosa. In osteosarcoma patients with normal kidney function, HD MTX-induced renal toxicity occurs in approximately 2% of patients in clinical trials [12, 13]. As MTX is primarily cleared renally, MTX-induced renal toxicity can be life-threatening, because it delays MTX excretion, resulting in sustained, elevated plasma MTX concentration and enhancement of other adverse events.
Since MTX targets several critical events in folate metabolism and competes with endogenous folate both for cellular uptake and for the formation of polyglutamates, it might be expected that pretreatment serum (S-) and/or erythrocyte (ER-) folate could predict subsequent toxicity of the drug. Serum or plasma folate describes extracellular concentrations and current changes in folate intake, whereas ER-folate reflects body storage levels at the time of erythropoiesis when folate enters the erythroblasts and is trapped intracellularly [14]. In the present study, both S- and ER-folate concentrations were determined prior to HD MTX cycles.
Previous studies of HD MTX pharmacokinetics have generally relied on limited sampling strategies, with three to five serum samples per cycle, while measuring only the parent compound by immunoassay techniques. In the present study a total of eight serum samples were collected per patient for each cycle of chemotherapy, one prior to the HD MTX infusion and additional samples at 2, 4, 6, 12, 24, 48 and 72 h after the start of the infusion. Herein, we present a detailed analysis of MTX and 7-OH-MTX concentrations from 288 HD MTX cycles, with corresponding toxicity data.
Methods
Patients
Sixty-five consecutive patients with high grade osteosarcoma treated with HD MTX at the Norwegian Radium Hospital between September 1994 and April 2003 were included. Patients were defined as paediatric (<15 years) or adults (≥15 years), and the patient characteristics are summarized in Table 1. In two patients, osteosarcoma occurred in previously irradiated areas (bilateral retinoblastoma and Hodgkin's lymphoma). Concomitant drugs with known interference with MTX were not administered during the HD MTX treatment cycles. The patients did not have a history of renal, hepatic or cardiac disease and none of the patients had underlying Epstein Barr, herpes or cytomegalovirus infection. One patient was hepatitis C virus antibody positive. Informed consent was obtained and the study was approved by the Regional Ethics Committee (REK, Oslo, Norway) and the local Institutional Review Board.
Table 1.
Patient characteristics
| Number of patients | |
|---|---|
| Characteristic | (n= 65) |
| Gender | |
| Male | 39 |
| Female | 26 |
| Age | |
| <15 years | 18 |
| ≥15 years | 47 |
| Median | 18 |
| Range | (9–51) |
| Tumour | |
| Extremity localized | 46 |
| Non-extremity localized | 19 |
| Newly diagnosed | 59 |
| Recurrent disease | 6 |
| Without overt metastases at diagnosis | 43 |
| With metastases at diagnosis | 22 |
| Number of MTX cycles received | |
| 1–4 | 38 |
| 5–9 | 15 |
| 10 | 12 |
Chemotherapy
Patients <40 years were treated according to current institutionally approved protocols, including SSG (Scandinavian Sarcoma Group) VIII [15], ISG (Italian Sarcoma Group)/SSG I [16, 17], ISG/SSG II [18] and ISG/SSG XIV [19]. All protocols were multi-drug regimens consisting of HD MTX, cisplatin, doxorubicin, ifosfamide and, in some patients, etoposide. The number of HD MTX cycles planned to be given in each treatment protocol varied from 2 to 10 cycles. For patients who could not follow the ongoing protocol due to toxicity, disease progression or age ≥ 40 years, protocols were modified. Actual body surface area at each cycle was determined from height and weight by standard nomograms for adults and for children and the methotrexate dose was adjusted accordingly. Patients were treated with MTX as a 4 h infusion with LV rescue started 24 h after initiation of MTX and continued until blood samples showed that MTX concentrations were below 0.2 µmol l−1[20]. Eighteen paediatric and 47 adult patients received a median of five and three HD MTX cycles, respectively. Ablative surgery was performed 9–12 weeks after initiation of treatment. Postoperatively, patients received adjuvant chemotherapy for up to 30 weeks.
Blood samples and analyses
Eight blood samples (one before the MTX infusion and at 2, 4, 6, 12, 24, 48 and 72 h after start of infusion) per patient per cycle were collected in serum tubes (Vacutainer, Brand, UK) and centrifuged at 1300 g for 12 min. Samples were stored at −20°C. MTX and 7-OH-MTX concentrations were measured in each serum sample using the HPLC method developed by Lawson et al. [21] and modified as described by Seidel et al. [22]. Briefly: serum samples (200 µl) were deproteinized by addition of 40 µl perchloric acid (2 mol l−1) and centrifuged prior to injection of 25–50 µl of the clear supernatant onto the chromatographic system. Patient samples collected up to 24 h after the start of infusion were manually diluted 1:10 in serum from healthy volunteers before preparation. The within and between run coefficients of variation (CVs) were 4% and 8% at the relevant concentrations of both compounds (40 µm of MTX and 2 µm of 7-OH-MTX). At low concentrations the within run CV increased 8% at 0.1 µm and 15% at 0.05 µm. The lower limit of quantification (signal to noise ratio 5:1) was 0.02 µmol l−1 for both MTX and 7-OH-MTX. Lack of interference from endogenous substances or drugs was verified by visual inspection of all chromatograms, including chromatograms from samples taken just before the start of MTX infusion. No interference was observed from relevant folates or other study drugs. S-folate and ER-folate were measured in pre-treatment samples using time-delayed immunofluorescence and a semiautomated analyzer (AutoDelfia, Wallac, Turku, Finland). The detection limit was 1.9 nmol l−1 (calculated from the mean value – 2SD of the null response). Reproducibility (total CV) for both analytes was <10%. Crossreactivities (at the 50% displacement level) with folinic acid and methotrexate were 0.04% and 2.4% respectively. In the same pretreatment samples methotrexate concentrations were not detectable by HPLC. In blood samples before infusion and 4, 12, 24, 48 and 72 h subsequent to start of MTX infusion, alanine aminotransferase (ALAT), alkaline phosphatase (ALP), gamma-glutamyl transferase (γGT), total bilirubin, creatinine, platelet and white blood cell (WBC) counts were analyzed by routine methods and data were extracted from patient files.
Toxicity
The National Cancer Institute Common Terminology Criteria for Adverse Events v.3.0 (CTCAE) score system was applied for grading hepatic, renal, haematologic and mucosal toxicity, while occurrence of dermatitis and pleuritis was categorized as absent or present [23].
Calculations
Individual pharmacokinetic parameters were calculated using PK Solutions 2.0 (Summit Research Services, CO, USA). Drug exposure during HD MTX cycles was expressed as AUC(0,72 h) (area under the blood concentration–time curve) calculated by the summation of integrals defined by the drug concentrations and the respective time spans over which they had been obtained (trapezoidal rule). MTX clearance (l m−2 h−1) was calculated as CLMTX= MTX dose/AUCMTX. For comparison of 7-OH-MTX concentrations between cycles the ratio AUC7-OH-MTX : AUCtotal (where AUCtotal= AUCMTX+ AUC7-OH-MTX) was calculated. The first treatment cycle was used as baseline (cycle1) for calculating the intra-individual cycle-to-cycle relative differences (Δcycle = cyclen− cycle1) of pharmacokinetic parameters and of folate pretreatment concentrations (Δ S-folate and Δ ER-folate). A two-way analysis of variance (anova) was used to identify cycle-to-cycle and interindividual variation in pharmacokinetic and toxicity-related parameters. Interindividual variation was expressed as 95% confidence intervals for each cycle. While CLMTX was normally distributed, all other parameters (MTX and 7-OH-MTX peak values, ratio AUC7-OH-MTX : AUCtotal, S-folate, ER-folate, WBC and platelet nadirs, creatinine, ALAT, γGT, ALP and total bilirubin peak values) had to be log transformed to exhibit a normal distribution. Patients were grouped by gender and age (paediatric or adults) and differences between groups were assessed by Student's t-test for normally distributed data and Mann-Whitney test (M-W) elsewhere. P values were corrected for multiple comparisons by means of Bonferroni's rule. The level of significance was set to 0.05. Bivariate associations between markers of toxicity and folate concentrations, age, gender, cycle number and pharmacokinetic parameters were evauated by two-sided Spearman's rank correlation coefficients (ρSp). The first treatment cycle was used as baseline and relationships between toxicity markers (dependent variables) and folate concentrations, age, gender, cycle number and pharmacokinetic parameters (independent variables) were investigated by backward multiple linear regression analysis. Table tests were performed as Chi squared test with Yates' correction. Data were analyzed with the Excel AnalyzeIT XP and SPSS version 13 (SPSS, Inc., Chicago, IL, USA).
Results
With a median follow-up of 88 months, the overall survival rate at 5 years for the population of 65 patients was 62%. There was no significant difference in survival between genders.
Folate concentrations
In pretreatment samples from 227 HD MTX cycles, ER-folate concentrations ranged from 150 to 2050 nmol l−1 with a median of 565 nmol l−1 and 95% CI 210, 1390 (reference range 315–835 nmol l−1). ER-folate was higher in males (median 610 nmol l−1, 95% CI 550, 680) compared with females (median 465 nmol l−1, 95% CI 430, 520, M-W, P < 0.001).
S-folate, ranged from 3 to 55 nmol l−1 with a median of 11.3 nmol l−1 and 95% CI 4, 45 (reference range was 5.8–23 nmol l−1) and was slightly higher in males (median 11.9 nmol l−1, 95% CI 10.4, 14.0) compared with females (median 9.7 nmol l−1, 95% CI 7.9, 14.0, M-W, P= 0.02).
Figure 1 depicts intra- and interindividual relative differences in S-folate and ER-folate concentrations. Variations in ER- and S-folate concentrations between cycles within patients (CV 22.6% and 7.1% respectively) were not significantly greater than variation between patients (CV 15.7% and 3.3% respectively). Both S- and ER-folate concentrations increased significantly with increasing number of HD MTX cycles (ρSp= 0.26 and 0.13; P < 0.001 and 0.05, respectively).
Figure 1.
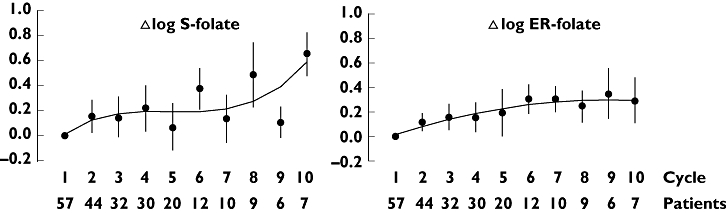
Serum (S-) and erythrocyte (ER-) folate concentrations were measured prior to therapy and before each cycle of high dose methotrexate. Relative cycle-to-cycle differences (Δ) are shown using first cycle pretreatment concentrations as baseline. Interindividual variation in 65 patients at each cycle is illustrated as 95% confidence intervals of mean relative differences. Polynomial regression curves are fitted to the experimental data to indicate dependency of cycle number
Pharmacokinetics
There were sufficient samples available in 288 HD MTX cycles to enable calculation of pharmacokinetic parameters. Following a 4 h continuous HD MTX infusion, MTX was eliminated from the plasma in a biphasic manner with α and β half-lives (t1/2,α and t1/2,β) while 7-OH-MTX elimination fitted with a monophasic course (Figure 2). The biphasic elimination curves for MTX and the monophasic curves described for 7-OH-MTX were apparent from the chosen sampling strategy with a total of eight serum samples over 72 h. However, a calculation based on the two latest time points for both compounds would increase the estimated terminal half-life for 7-OH-MTX from 10 h to 15 h, and increase the estimated terminal half-life for MTX by a factor of almost two, which may suggest that the elimination pharmacokinetics in serum for MTX and 7-OH-MTX are most adequately described by tri- and bi-phasic processes, respectively. On the other hand, this is entirely based on single measurements at the 72 h time point, when analytical variability is at its most disadvantageous, and we have not pursued this. Thus, elimination kinetics are described as bi- and monophasic for MTX and its 7-hydroxylated metabolite. The estimated values for CLMTX, the ratio AUC7-OH-MTX : AUCtotal, MTX t1/2,α, MTX t1/2,β and 7-OH-MTX t1/2,β are listed in Table 2. Analyses were based on observed, and not extrapolated, AUCs. This may underestimate the AUCs by a magnitude of less than 0.1% (median) for MTX and less than 2% (median) for 7-OH-MTX, which was considered negligible. There was up to nine-fold difference in the 7-OH-MTX concentrations between patients, and although 7-OH-MTX peak concentrations were significantly correlated with MTX dose, neither MTX dose nor MTX peak value could predict the ratio AUC7-OH-MTX : AUCtotal.
Figure 2.
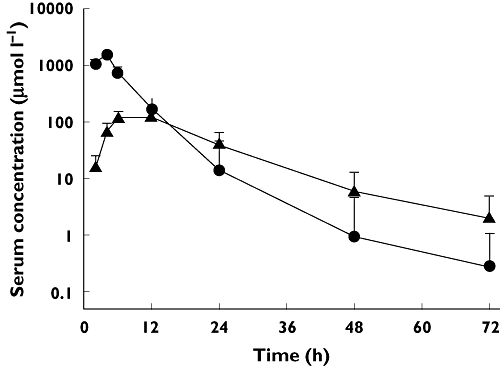
Methotrexate (•) and 7-hydroxy-methotrexate (▴) concentration–time data from 288 high dose methotrexate cycles in 65 patients. Data are presented as mean + 1SD
Table 2.
Pharmacokinetic parameters of methotrexate (MTX) and 7-hydroxy-methotrexate (7-OH-MTX)
| High dose methotrexate cycles | Quartiles | |||
|---|---|---|---|---|
| (n= 288) | Mean | Median | (Q−, Q+) | Range |
| MTX dose (g m−2) | 12 | (12, 12) | 8–16 | |
| Peak MTX (µm) | 1509 | (1325, 1683) | 722–2512 | |
| Peak 7-OH-MTX (µm) | 130 | (101, 160) | 27–253 | |
| MTX clearance (l m−2 h−1)* | 2.9 | (2.49, 3.19) | 1.10–4.95 | |
| Ratio AUC7-OH-MTX : (AUCtotal) | 0.20 | (0.16, 0.24) | 0.03–0.43 | |
| MTX t1/2,α† | 2.49 | (2.23, 2.85) | 1.67–6.49 | |
| MTX t1/2,β‡ | 8.38 | (7.55, 9.30) | 5.63–21.74 | |
| 7-OH-MTX t1/2,β‡ | 10.16 | (8.44, 12.14) | 6.40–27.11 |
MTX dose/AUCMTX.
at 4–6–12 h.
at 24–48–72 h.
Variations in 7-OH-MTX peak concentrations and ratio AUC7-OH-MTX : AUCtotal between cycles in individual patients (anova; CV 26.3% and 26.5% respectively) were of the similar magnitude as the variations in levels between patients (anova; CV 20.9% and 21.0% respectively). Variations in CLMTX and MTX peak concentrations between cycles within the same patient (anova; CV = 16.0% and 15.6% respectively) were not significantly different compared with concentrations between patients (anova; CV = 10.6% and 10.1% respectively). Figure 3 shows relative differences in CLMTX and MTX peak concentrations, the ratio AUC7-OH-MTX : AUCtotal and 7-OH-MTX peak concentrations. Interindividual variation was increased after cycle number 4 for all pharmacokinetic parameters, most likely due to the declining number of patients receiving additional therapy cycles. In a multiple backward regression model there was a significant trend of increasing MTX peak concentrations (ρSp=−0.26, P < 0.001), decreasing 7-OH-MTX peak concentrations (ρSp=−0.23, P < 0.001) and CLMTX slightly decreasing with increasing cycle number (ρSp=−0.22, P < 0.001). The ratio AUC7-OH-MTX : AUCtotal increased during cycles 1–4 and then decreased. There was no correlation between CLMTX, ratio AUC7-OH-MTX : AUCtotal, MTX or 7-OH-MTX peak concentrations and age, gender or pretreatment folate concentrations, respectively.
Figure 3.
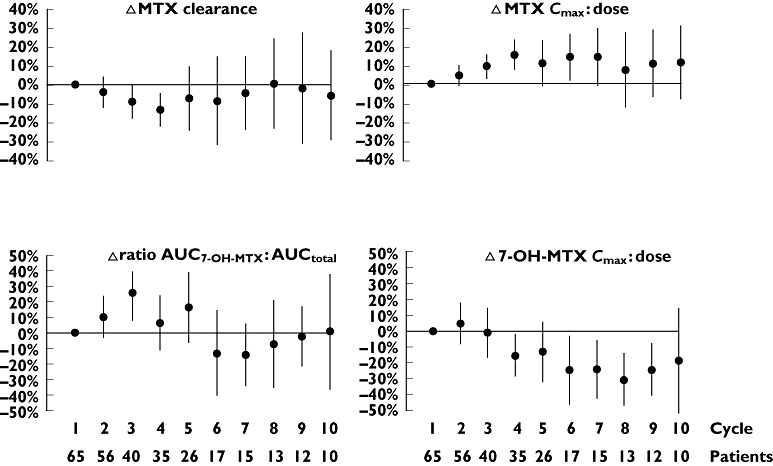
Cycle-to-cycle relative differences (Δ) in methotrexate clearance and peak concentrations (MTXcmax : MTX dose) and in 7-hydroxy-methotrexate ratio (AUC7-OH-MTX : AUCtotal) and peak concentrations (7-OH-MTX cmax : dose) obtained in 288 infusions of high dose methotrexate using concentrations at first cycle as baseline. The points represent mean relative differences and the bars indicate the 95% confidence intervals illustrating interindividual variation at each cycle
Toxicity
Table 3 summarizes the hepatic, renal, haematological and oral mucosal toxicities encountered. There were no life-threatening adverse events or therapy related deaths during the HD MTX treatment cycles. Most patients had transient elevations in ALAT concentrations. Peak values (at 4 h) were significantly higher among female patients (median 10.0 × ULN, 95% CI 7.6, 13.9) compared with male patients (median 5.5 × ULN, 95% CI 4.6, 7.1, M-W, P < 0.001) and were significantly higher among paediatric patients (median 10.5 × ULN, 95% CI 8.0, 11.5) compared with adults (median 4.8 × ULN, 95% CI 4.2, 6.6, M-W, P= 0.02). The relationship between ALAT peak values and pharmacokinetic parameters was studied in a backward linear multiple regression analysis with log ALAT as an independent variable and log CLMTX, log MTX peak concentration, log ratio AUC7-OH-MTX : AUCtotal, log 7-OH-MTX peak concentration, age and gender as dependent variables. We found significant positive correlations between log ALAT and log ratio AUC7-OH-MTX : AUCtotal (P < 0.001), age (P < 0.001), gender (P < 0.001) and log CLMTX (P < 0.001). Age contributed with a factor of 2.5 for the youngest patients relative to the oldest and gender contributed with a factor of 1.7 for female (1.0 for male). Comparing cycles with high ratio AUC7-OH-MTX : AUCtotal (≥90thpercentile) with low ratio (≤10thpercentile), this variable contributed with a 3.3 fold increase in peak ALAT concentrations (Figure 4). Comparing cycles with low CLMTX (≤10thpercentile) with high CLMTX (≥90thpercentile), this variable contributed with a 1.3 fold increase in peak ALAT concentrations. These analyses indicated that high relative concentrations of 7-OH-MTX, young age, female gender and low CLMTX were among the contributing factors for hepatocellular damage, assessed as increases in ALAT concentrations after MTX infusion. γGT peak values were significantly higher among female patients (median 3.1 × ULN, 95% CI 2.7, 4.0) than among male patients (median 1.4 × ULN, 95% CI 1.3, 1.7, M-W, P < 0.001), but was not correlated with age.
Table 3.
High dose methotrexate (HD MTX) toxicity in 65 osteosarcoma patients
| CTCAE* | CTCAE | CTCAE | CTCAE | ||
|---|---|---|---|---|---|
| No toxicity | grade 1 | grade 2 | grade 3 | grade 4 | |
| Alanine aminotransferase | ≤ULN† | >ULN −2.5 × ULN | >2.5–5.0 × ULN | >5.0 −20 × ULN | >20 × ULN |
| Cycles (%) | 2 | 14 | 25 | 42 | 17 |
| Patients | 0 | 7 | 6 | 26 | 26 |
| Alkaline phosphatase | ≤ULN | >ULN −2.5 × ULN | >2.5–5.0 × ULN | >5.0 −20 × ULN | >20 × ULN |
| Cycles (%) | 97 | 3 | 0 | 0 | 0 |
| Patients | 58 | 7 | 0 | 0 | 0 |
| Gamma-glutamyl transferase | ≤ULN | >ULN −2.5 × ULN | >2.5–5 × ULN | >5.0 −20 × ULN | >20 × ULN |
| Cycles (%) | 27 | 43 | 23 | 7 | 0 |
| Patients | 12 | 18 | 23 | 12 | 0 |
| Bilirubin | ≤ULN | >ULN −1.5 × ULN | >1.5–3.0 × ULN | >3.0–10 × ULN | >10 × ULN |
| Cycles (%) | 41 | 35 | 24 | 0 | 0 |
| Patients | 13 | 21 | 31 | 0 | 0 |
| Creatinine | ≤ULN | >ULN −1.5 × ULN | >1.5–3.0 × ULN | >3.0–6.0 × ULN | >6.0 × ULN |
| Cycles (%) | 95 | 3 | 2 | 0 | 0 |
| Patients | 54 | 8 | 3 | 0 | 0 |
| Leucocyte nadir | ≥3.0 × 109 l−1 | <LLN‡−3.0 × 109 l−1 | <3.0–2.0 × 109l−1 | <2.0–1.0 × 109l−1 | <1.0 × 109l−1 |
| Cycles (%) | 32 | 5 | 30 | 27 | 6 |
| Patients | 6 | 1 | 14 | 30 | 14 |
| Thrombocyte nadir | ≥75 × 109l−1 | <LLN (75 × 109l−1 | <75–50 × 109l−1 | <50–25 × 109l−1 | <1.0 × 109l−1 |
| Cycles (%) | 53 | 20 | 7 | 10 | 10 |
| Patients | 9 | 14 | 5 | 15 | 22 |
| Mucositis | |||||
| Cycles (%) | 64 | 13 | 8 | 15 | 0 |
| Patients | 18 | 9 | 8 | 30 | 0 |
Common Terminology Criteria Adverse Events v 3.0.
Upper level of normal.
Lower level of normal.
Figure 4.
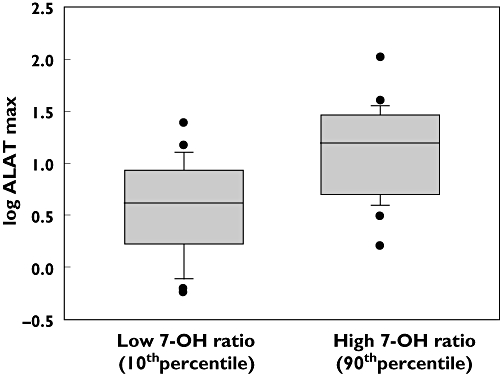
Box plot showing significant (P < 0.001) difference in ALAT peak value between cycles having a high (≥90thpercentile) 7-hydroxy-methotrexate ratio (AUC7-OH-MTX : AUCtotal) and cycles having a low (≤10thpercentile) 7-hydroxy-methotrexate ratio
Renal toxicity grade 1–2 developed in 13 patients with creatinine elevations after the first cycle in 11 patients and after the fourth cycle in two patients. Five of these patients continued therapy according to protocol (four patients <40 years), while five patients were excluded from further HD MTX cycles due to renal toxicity (four patients >40 years), and the remaining three patients (all < 40 years) did not receive further HD MTX due to other causes.
Platelet nadirs did not correlate with gender, but were slightly higher among paediatric patients (median 172 × 109 l−1, 95% CI 128, 169) compared with adults (median 125 × 109 l−1, 95% CI 106, 136, M-W, P < 0.001).
WBC nadirs were slightly lower (median 2.2 × 109l−1, 95% CI 1.8, 2.7) in patients with low baseline S-folate concentrations, compared with patients with high concentrations (median 2.8 × 109 l−1, 95% CI 2.5, 3.2, M-W, P= 0.005), using the same cut-off value for S-folate as described by Sterba et al. [24], thus dividing patients by low (≤10 nmol l−1) and high folate concentrations (>10 nmol l−1).
There was a significant correlation between mucositis grade and age. Mucositis in paediatric patients occurred in 32 of 124 HD MTX cycles: grade 0 in 92 cycles, grade 1 in nine cycles, grade 2 in 13 cycles and grade 3 in 10 cycles. Among adults mucositis occurred in 85 of 171 HD MTX cycles: grade 0 in 86 cycles, grade 1 in 31 cycles, grade 2 in 13 cycles and grade 3 in 31 cycles (chi-squared test with Yates' correlation = 16.4; P < 0.001). There was no correlation between mucositis and gender, pretreatment folate concentrations, MTX dose, pharmacokinetic parameters, number of MTX cycles or renal toxicity. Dermatitis developed after 7% of cycles (18 patients) and pleuritis after 1% (three patients, all female).
Discussion
Acute toxicities in HD MTX therapy are often unexpected and not typically dose dependent. Being a folate analogue, MTX competes with natural folates both for cellular uptake and metabolism, and it could be expected that patients with poor pretreatment folate status would be more likely to develop toxicity. In our study S- and ER-folate concentrations increased significantly with increasing number of HD MTX cycles. These findings are in agreement with those reported by Sterba et al. [24], who suggested that increasing folate baseline concentrations could originate from repetitive LV administration. We also found lower initial folate concentrations in females compared with males, but except for slightly lower WBC nadirs in patients with low baseline folate concentrations, there was no correlation between folate concentrations and the markers of acute toxicity that we studied. Publications concerning the impact of pretreatment folate status on HD MTX related acute toxicity are limited. In a case report, severe encephalopathy was described in association with the first but not the second cycle of HD MTX, where pretreatment folate plasma concentrations were low before the first cycle and then 10-fold higher before the second cycle [25].
MTX targets several critical events in folate metabolism and baseline folate concentrations could be possible predictors of in vivo MTX pharmacobiological action. In our study approximately 2200 serum samples were analyzed allowing a detailed analysis of pharmacokinetics, but there were no significant correlations between pretreatment folate concentrations (227 HD MTX cycles) and pharmacokinetic variables.
Acute hepatic toxicity related to HD MTX occurs frequently and we observed elevated liver transaminases, bilirubin and γGT in 98%, 59% and 73% of the cycles with peak values up to 105, 2.9 and 7.9 times normal, respectively. The pathophysiology has remained unclear, but in our study we found that ALAT peak values were correlated with 7-OH-MTX exposure and were 3.3 fold increased at the 90thpercentile compared with the 10thpercentile of AUC7-OH-MTX : AUCtotal.
In contrast to earlier reports of lower overall toxicity in paediatric patients [26] and reports of increased liver toxicity in adults [27], we found significant positive correlations between acute liver toxicity and young age. The highest ALAT concentrations were apparent in paediatric patients (paediatric : adult ratio for ALATmax was 2.5:1.0) and although the paediatric patients received a higher number of HD MTX infusions, there was no correlation between number of cycles and toxicity.
We also found significant correlations between the maximum ALAT concentration and gender (female : male ratio for ALATmax was 1.7:1.0). There are, however, no previous reports addressing the relationship between acute liver toxicity and gender in patients treated with HD MTX. It is notable, though, that Becker et al. [28] found significantly greater risk of liver toxicity in females after low dose MTX, and suggested that hormonal interactions or differences in MTX pharmacodynamics between genders might be involved.
Polymorphisms in folate-metabolizing enzymes and transport proteins may modify the toxicity and drug elimination of folate antagonists [29–31]. Baggott et al. [32] described a bimodal distribution of 7-OH-MTX production in a group of 29 patients with rheumatoid arthritis receiving low dose MTX (one cycle per patient) and suggested the existence of two phenotypes in the metabolism of MTX to 7-OH-MTX. Although we found large differences in 7-OH-MTX concentrations between patients, a unimodal distribution of AUC7-OH-MTX, with intermediate concentrations of the metabolite was displayed in the majority of patients.
While Erttmann et al. [4] reported that 7-OH-MTX concentrations decreased with repeated cycles in osteosarcoma patients, Borsi et al. [33] described that increasing number of cycles did not affect 7-OH-MTX concentrations in children with ALL. In our study, however, a biphasic trend was apparent. From the first to the fourth cycle 7-OH-MTX concentrations increased significantly, but after the fifth cycle 7-OH-MTX concentrations decreased. Our suggestion is that this could possibly be caused by increased activity of renal and hepatic ATP-binding cassette transporter proteins, thus increasing elimination of 7-OH-MTX.
Since both MTX and cisplatin are included in the osteosarcoma treatment protocols, it is likely that repetitive exposure to these nephrotoxic drugs would alter MTX clearance. The effect of repeated HD MTX administration on the pharmacokinetics of MTX in our study revealed the same trend as described by Crews et al. [34]. As the number of HD MTX infusions increased, CLMTX decreased slightly, but we found no association between HD MTX cycle number and creatinine elevation. Renal toxicity (creatinine CTCAE grade ≥ 1) was seen in 5% of the cycles, and of the 65 patients in our study, five were excluded from further cycles due to MTX toxicity. It should be noted, however, that serum creatinine is not a sensitive marker of nephrotoxicity, and osteosarcoma patients today are monitored by measurement of glomerular filtration rate.
To our knowledge, this is the first report of an association between 7-OH-MTX concentrations, age, gender and acute hepatocellular damage in humans. Putative mechanisms remain obscure, but it is noteworthy that the 7-hydroxylated MTX metabolite formed in the liver has regained some cytotoxic activity and possesses lesser solubility at relevant pH compared with the parent compound. Thus, the association may reflect a direct toxic effect of 7-OH-MTX on hepatic parenchyma cells.
Acknowledgments
We thank Clinical Chemistry Departments at Oslo University Hospital for processing blood samples. This study was supported by the Norwegian Cancer Society.
Competing Interests
There are no competing interests to declare.
REFERENCES
- 1.Ferguson WS, Goorin AM. Current treatment of osteosarcoma. Cancer Invest. 2001;19:292–315. doi: 10.1081/cnv-100102557. [DOI] [PubMed] [Google Scholar]
- 2.Jaffe N. Recent advances in the chemotherapy of metastatic osteogenic sarcoma. Cancer. 1972;30:1627–31. doi: 10.1002/1097-0142(197212)30:6<1627::aid-cncr2820300631>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 3.Allegra CJ, Chabner BA, Drake JC, Lutz R, Rodbard D, Jolivet J. Enhanced inhibition of thymidylate synthase by methotrexate polyglutamates. J Biol Chem. 1985;260:9720–6. [PubMed] [Google Scholar]
- 4.Erttmann R, Bielack S, Landbeck G. Kinetics of 7-hydroxy-methotrexate after high-dose methotrexate therapy. Cancer Chemother Pharmacol. 1985;15:101–4. doi: 10.1007/BF00257517. [DOI] [PubMed] [Google Scholar]
- 5.Ekstrom PO, Andersen A, Saeter G, Giercksky KE, Slordal L. Continuous intratumoral microdialysis during high-dose methotrexate therapy in a patient with malignant fibrous histiocytoma of the femur: a case report. Cancer Chemother Pharmacol. 1997;39:267–72. doi: 10.1007/s002800050571. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs SA, Stoller RG, Chabner BA, Johns DG. 7-Hydroxymethotrexate as a urinary metabolite in human subjects and rhesus monkeys receiving high dose methotrexate. J Clin Invest. 1976;57:534–8. doi: 10.1172/JCI108308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smeland E, Fuskevag OM, Nymann K, Svendesn JS, Olsen R, Lindal S, Bremnes RM, Aarbakke J. High-dose 7-hydromethotrexate: acute toxicity and lethality in a rat model. Cancer Chemother Pharmacol. 1996;37:415–22. doi: 10.1007/s002800050406. [DOI] [PubMed] [Google Scholar]
- 8.Lankelma J, van der Klein E, Ramaekers F. The role of 7-hydroxymethotrexate during methotrexate anti-cancer therapy. Cancer Lett. 1980;9:133–42. doi: 10.1016/0304-3835(80)90117-2. [DOI] [PubMed] [Google Scholar]
- 9.Slordal L, Sager G, Aarbakke J. Pharmacokinetic interactions with methotrexate: is 7-hydroxy-methotrexate the culprit? Lancet. 1988;1:591–2. doi: 10.1016/s0140-6736(88)91387-6. [DOI] [PubMed] [Google Scholar]
- 10.Isacoff WH, Townsend CM, Eiber FR, Forster T, Morton DL, Block JB. High dose methotrexate therapy of solid tumors: observations relating to clinical toxicity. Med Pediatr Oncol. 1976;2:319–25. doi: 10.1002/mpo.2950020313. [DOI] [PubMed] [Google Scholar]
- 11.Stoller RG, Hande KR, Jacobs SA, Rosenberg SA, Chabner BA. Use of plasma pharmacokinetics to predict and prevent methotrexate toxicity. N Engl J Med. 1977;297:630–4. doi: 10.1056/NEJM197709222971203. [DOI] [PubMed] [Google Scholar]
- 12.Widemann BC, Adamson PC. Understanding and managing methotrexate nephrotoxicity. Oncologist. 2006;11:694–703. doi: 10.1634/theoncologist.11-6-694. [DOI] [PubMed] [Google Scholar]
- 13.Zelcer S, Kellick M, Wexler LH, Gorlick R, Meyers PA. The Memorial Sloan Kettering Cancer Center experience with outpatient administration of high dose methotrexate with leucovorin rescue. Pediatr Blood Cancer. 2008;50:1176–80. doi: 10.1002/pbc.21419. [DOI] [PubMed] [Google Scholar]
- 14.Bailey LB. Folate status assessment. J Nutr. 1990;120(Suppl. 11):1508–11. doi: 10.1093/jn/120.suppl_11.1508. [DOI] [PubMed] [Google Scholar]
- 15.Smeland S, Muller C, Alvegard TA, Wiklund T, Wiebe T, Bjork O, Stenwig AE, Willen H, Holmstrom T, Folleras G, Brosjo O, Kivioja A, Jonsson K, Monge O, Saeter G. Scandinavian Sarcoma Group Osteosarcoma Study SSG VIII: prognostic factors for outcome and the role of replacement salvage chemotherapy for poor histological responders. Eur J Cancer. 2003;39:488–94. doi: 10.1016/s0959-8049(02)00747-5. [DOI] [PubMed] [Google Scholar]
- 16.Bacci G, Ferrari S, Longhi A, Picci P, Mercuri M, Alvegard TA, Saeter G, Donati D, Manfrini M, Lari S, Briccoli A, Forni C. High dose ifosfamide in combination with high dose methotrexate, adriamycin and cisplatin in the neoadjuvant treatment of extremity osteosarcoma: preliminary results of an Italian Sarcoma Group/Scandinavian Sarcoma Group pilot study. J Chemother. 2002;14:198–206. doi: 10.1179/joc.2002.14.2.198. [DOI] [PubMed] [Google Scholar]
- 17.Ferrari S, Smeland S, Mercuri M, Bertoni F, Longhi A, Ruggieri P, Alvegard TA, Picci P, Capanna R, Bernini G, Muller C, Tienghi A, Wiebe T, Comandone A, Bohling T, Del Prever AB, Brosjo O, Bacci G, Saeter G. Neoadjuvant chemotherapy with high-dose ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity: a joint study by the Italian and Scandinavian Sarcoma Groups. J Clin Oncol. 2005;23:8845–52. doi: 10.1200/JCO.2004.00.5785. [DOI] [PubMed] [Google Scholar]
- 18.Del Prever AB, Smeland S, Tienghi A, Hall KS, Aglietta G, Bernini G, Bohling T, Berta M, Brosjo O, Ferrari S, Alvegaard TA. High-risk osteosarcoma (OS): preliminary results of the ISG-SSG II protocol. J Clin Oncol. 2005;23:8845–52. No. 16S, Part I of II. [Google Scholar]
- 19.Smeland S, Bruland OS, Hjorth L, Brosjo O, Bjerkehagen B, Osterlundh G, Jakobson A, Hall KS, Monge OR, Bjork O, Alvegaard TA. Scandinavian experience in classical osteosarcoma Results of the SSG XIV protocol. Acta Orthop Suppl. 2010;80:60–6. doi: 10.3109/17453674.2011.566141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saeter G, Alvegard TA, Elomaa I, Stenwig AE, Holmstrom T, Solheim OP. Treatment of osteosarcoma of the extremities with the T-10 protocol, with emphasis on the effects of preoperative chemotherapy with single-agent high-dose methotrexate: a Scandinavian Sarcoma Group study. J Clin Oncol. 1991;9:1766–75. doi: 10.1200/JCO.1991.9.10.1766. [DOI] [PubMed] [Google Scholar]
- 21.Lawson GJ, Dixon PF, Aherne GW. Rapid and simple method for the measurement of methotrexate and 7-hydroxymethotrexate in serum by high-performance liquid chromatography. J Chromatogr. 1981;223:225–31. doi: 10.1016/s0378-4347(00)80091-2. [DOI] [PubMed] [Google Scholar]
- 22.Seidel H, Andersen AM, Nygaard R, Moe PJ, Jacobsen G, Slørdal L. Variability in methotrexate serum and cerebrospinal fluid pharmacokinetics in children with acute lymphocytic leukemia: relation to assay methodology and physiological variables. Leuk Res. 2000;24:193–9. doi: 10.1016/s0145-2126(99)00181-2. [DOI] [PubMed] [Google Scholar]
- 23.U.S. National Institutes of Health. National Cancer Institute CTEP CTCAE v3.0. 2011. Available at http://ctep.cancer.gov/reporting/ctc.html (last accessed June 2011.
- 24.Sterba J, Dusek L, Demlova R, Valik D. Pretreatment plasma folate modulates the pharmacodynamic effect of high-dose methotrexate in children with acute lymphoblastic leukemia and non-Hodgkin lymphoma: ‘folate overrescue’ concept revisited. Clin Chem. 2006;52:692–700. doi: 10.1373/clinchem.2005.061150. [DOI] [PubMed] [Google Scholar]
- 25.Valik D, Sterba J, Bajciova V, Demlova R. Severe encephalopathy induced by the first but not the second course of high-dose methotrexate mirrored by plasma homocysteine elevations and preceded by extreme differences in pretreatment plasma folate. Oncology. 2005;69:269–72. doi: 10.1159/000088334. [DOI] [PubMed] [Google Scholar]
- 26.Wang YM, Sutow WW, Romsdahl MM, Perez C. Age-related pharmacokinetics of high-dose methotrexate in patients with osteosarcoma. Cancer Treat Rep. 1979;63:405–10. [PubMed] [Google Scholar]
- 27.Rask C, Albertioni F, Bentzen SM, Schroeder H, Peterson C. Clinical and pharmacokinetic risk factors for high-dose methotrexate-induced toxicity in children with acute lymphoblastic leukemia–a logistic regression analysis. Acta Oncol. 1998;37:277–84. doi: 10.1080/028418698429586. [DOI] [PubMed] [Google Scholar]
- 28.Becker ML, Rosé CD, Cron RQ, Sherry DD, Bilker WB. Effectiveness and toxicity of methotrexate in juvenile idiopathic arthritis: comparison of 2 initial dosing regimens. J Rheumatol. 2010;37:870–5. doi: 10.3899/jrheum.090826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robien K, Boynton A, Ulrich CM. Pharmacogenetics of folate-related drug targets in cancer treatment. Pharmacogenomics. 2005;6:673–89. doi: 10.2217/14622416.6.7.673. [DOI] [PubMed] [Google Scholar]
- 30.Rau T, Erney B, Gores R, Eschenhagen T, Beck J, Langer T. High-dose methotrexate in pediatric acute lymphoblastic leukemia: impact of ABCC2 polymorphisms on plasma concentrations. Clin Pharmacol Ther. 2006;80:468–76. doi: 10.1016/j.clpt.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 31.Hulot JS, Villard E, Maguy A, Morel V, Mir L, Tostivint I, William-Faltaos D, Fernandez C, Hatem S, Deray G, Komajda M, Leblond V, Lechat P. A mutation in the drug transporter gene ABCC2 associated with impaired methotrexate elimination. Pharmacogenet Genomics. 2005;15:277–85. doi: 10.1097/01213011-200505000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Baggott JE, Bridges SL, Jr, Morgan SL. Evidence for two phenotypes in the metabolism of methotrexate to 7-hydroxymethotrexate in patients with rheumatoid arthritis. Arthritis Rheum. 2005;52:356–8. doi: 10.1002/art.20742. [DOI] [PubMed] [Google Scholar]
- 33.Borsi JD, Sagen E, Romslo I, Moe PJ. Comparative study on the pharmacokinetics of 7-hydroxy-methotrexate after administration of methotrexate in the dose range of 0.5–33.6 g/m2 to children with acute lymphoblastic leukemia. Med Pediatr Oncol. 1990;18:217–24. doi: 10.1002/mpo.2950180310. [DOI] [PubMed] [Google Scholar]
- 34.Crews KR, Liu T, Rodriguez-Galindo C, Tan M, Meyer WH, Panetta JC, Link MP, Daw NC. High-dose methotrexate pharmacokinetics and outcome of children and young adults with osteosarcoma. Cancer. 2004;100:1724–33. doi: 10.1002/cncr.20152. [DOI] [PubMed] [Google Scholar]


