Abstract
AIM
Metformin is the most commonly prescribed oral anti-diabetic drug in young people. It is also prescribed for polycystic ovarian syndrome (PCOS) and obesity treatment in adults in an unlicensed fashion. Little is known as to the extent metformin has been used in young people. We investigated the use of metformin in children and adolescents aged 0–18 years in the UK.
METHODS
Population-based prescribing data were obtained from the UK IMS Disease Analyzer between January 2000 and December 2010.
RESULTS
A total of 2674 metformin prescriptions were issued to 337 patients (80% female) between 2000 and 2010. The prevalence of metformin prescribing increased from 0.03 per 1000 person-years [95% confidence interval (CI) 0.02, 0.05] to 0.16 per 1000 person-years (95% CI 0.12, 0.20) (P= 0.001). There was a steady increase in metformin prescribing in girls aged 16–18 years. There were 290 metformin treated patients (81% female; n= 235) who had at least one diagnosis of diabetes, PCOS or obesity. Among these patients, PCOS was the most common indication for metformin prescribing in girls (n= 120) followed by diabetes. There were 22 patients (7.6%) who received metformin for obesity treatment only.
CONCLUSIONS
Prescribing of metformin increased between 2000 and 2010, in particular amongst girls aged 16–18 years. The main indication for metformin prescribing was PCOS. At present, metformin is not licensed for PCOS and obesity treatment in adults or children. As there is a steady increase in the prescribing of metformin in young people, further studies are required to investigate the efficacy and safety of these prescriptions.
Keywords: adolescents, children, diabetes, metformin, obesity, polycystic ovary syndrome
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Metformin is licensed for type 2 diabetes mellitus (DM) treatment in the UK.
Evidence has shown the moderate efficacy of metformin treatment for polycystic ovarian syndrome (PCOS) and obesity.
WHAT THIS STUDY ADDS
Metformin prescribing increased in children and adolescents between 2000 and 2010, in particular in girls aged 16–18 years.
PCOS and obesity were the main, but unlicensed, indications for metformin prescribing amongst female adolescents.
Introduction
Metformin is the most commonly prescribed oral anti-diabetic drug for diabetes mellitus (DM) in children and adolescents in the UK [1]. As metformin has been shown to be effective in reducing testosterone concentrations and improving irregular menstrual cycles [2], it has also been prescribed for the treatment of polycystic ovarian syndrome (PCOS) in women of reproductive age [3, 4]. However, there is still controversy regarding metformin use in PCOS. Two large randomized controlled trials (RCTs) did not show metformin to be more efficacious than placebo in adult women with PCOS [5, 6]. In adolescents, RCTs have shown the effectiveness of metformin treatment in girls with PCOS [7–9]. In contrast to these findings, a recent RCT did not show benefit from metformin treatment along with lifestyle modification in adolescents with PCOS [10]. Despite the controversy, metformin is still recommended as one of the therapeutic options for PCOS in teenage girls [2]. In addition to PCOS, metformin is also effective as an anti-obesity drug due to its effect on insulin resistance [11]. Metformin may also have other effects on weight loss, as it reduces hepatic glucose production, inhibits fat cell lipogenesis, increases peripheral insulin sensitivity and may reduce food intake [12]. Studies have shown metformin is associated with moderate BMI reduction in obese non-diabetic adolescents [13, 14]. In the UK, metformin is licensed for children over the age of 10 years with type 2 diabetes who have failed strict dieting [15]. At present, metformin is not licensed for the treatment of PCOS and obesity in adults or children in the UK [15, 16]. Little is known about the extent to which this drug has been used in young people in UK primary care. Therefore, this study was conducted to examine metformin prescribing patterns in children and adolescents in the UK primary care setting.
Methods
A retrospective cohort study was conducted using a primary care database, the IMS Disease Analyzer (IMS DA) database. This database contains approximately 2 million anonymous patient records and over 95 million prescriptions from about 125 general practices and more than 500 general practitioners (GPs) [17]. In the UK, virtually all patient care is managed by GPs in primary care. When patients are seen in secondary care (e.g. hospital), consultants or specialists will make the diagnosis and initiate treatment, and GPs will usually continue to monitor patients and issue prescriptions. Electronic medical records are routinely used in UK general practice. GPs usually enter diagnosis and prescription information into the electronic medical records to assist them in patient management [18].
Information held on the database includes patient demographics, indications for treatment and prescription details. Prescribed drugs are coded based on the Anatomical Therapeutic Chemical (ATC) classification issued by the European Pharmaceutical Market Research Association [19], and medical diagnoses are coded according to the International Classification of Disease (ICD) version 10 codes [20]. The database has been shown to be of high quality and is widely used in paediatric pharmacoepidemiological studies [21–23]. This study consisted of children and adolescents aged 0–18 years registered with a GP who contributed data to the IMS DA between January 2000 and December 2010. All subjects needed to have a minimum of 6 months valid data in the database. Age bands were based on the modification of the International Conference of Harmonization (ICH) as follows: <2, 2–11, 12–15 and 16–18 years [24]. Prevalence was calculated as the total number of subjects with at least one prescription of metformin during each year of investigation divided by the total number of study subjects registered on the database in the same year, stratified by age and gender. Annual prevalence of metformin prescribing was calculated using Poisson distribution with a 95% confidence interval (CI). A χ2 test (Cochran-Armitage test for trend) was used to examine the yearly trend of metformin prescribing. As IMS DA directly links prescriptions to medical indications, the following indications were examined for metformin prescriptions: diabetes (ICD10 E10-E14), PCOS (E282) and obesity (E66). Analyses were carried out using Stata version 11.0 (Stata Corp., College Station, TX, USA).
This study protocol was approved by the IMS Independent Scientific and Ethical Advisory Committee.
Results
A total number of 2674 metformin prescriptions were issued to 337 children and adolescents between 2000 and 2010, 80% were female (n= 270). There were no metformin prescriptions issued to children aged under 2 years. The majority of children were taking metformin for diabetes treatment aged 2–11 years. The metformin prescribing started at age 5 years old for diabetes treatment. The number of female adolescents aged 12–18 years who received metformin treatment steadily increased over time (Table 1).The annual prevalence of metformin prescribing increased from 0.03 per 1000 person-years (95% CI 0.02, 0.05) to 0.16 per 1000 person-years (95% CI 0.12, 0.20) (P= 0.001) (Figure 1). There were a total of 290 patients with at least one diagnosis of DM, PCOS or obesity in their medical records, of whom 235 patients were female (81%) (Table 2). Of 290 patients, 120 female patients were prescribed metformin for the treatment of PCOS and obesity. There were 23 female patients with DM, PCOS and obesity diagnoses who received metformin treatment during the study period. There were 22 patients (7.6%, 22/290) who were prescribed metformin for obesity treatment alone. A total number of 47 patients were prescribed metformin without specific relevant diagnosis. After scrutinizing their medical records, the most common diagnosis for prescribing were ‘unknown and unspecified causes of morbidity’. As IMS DA only contains data from GPs, there is no hospital record in the database to verify diagnoses for these prescriptions.
Table 1.
Characteristics of study subjects between 2000 and 2010 by age and calendar year
| Aged <2 years | Aged 2–11 years | Aged 12–15 years | Aged 16–18 years | Person-time | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | Boys | Girls | Boys | Girls | Boys | Girls | Boys | Girls | Total | Boys | Girls | Total |
| 2000 | 0 | 0 | 2 | 4 | 0 | 3 | 1 | 10 | 20 | 266 537.2 | 257 100.8 | 523 638.0 |
| 2001 | 0 | 0 | 1 | 9 | 1 | 3 | 5 | 18 | 38 | 265 419.0 | 256 182.8 | 521 601.7 |
| 2002 | 0 | 0 | 4 | 5 | 2 | 7 | 1 | 18 | 37 | 263 778.0 | 254 243.0 | 518 020.9 |
| 2003 | 0 | 0 | 7 | 5 | 7 | 10 | 3 | 29 | 61 | 258 312.5 | 248 746.9 | 507 059.4 |
| 2004 | 0 | 0 | 5 | 7 | 6 | 15 | 2 | 38 | 73 | 244 468.2 | 235 088.5 | 479 556.7 |
| 2005 | 0 | 0 | 5 | 6 | 5 | 9 | 5 | 34 | 64 | 233 251.9 | 223 886.7 | 457 138.6 |
| 2006 | 0 | 0 | 7 | 8 | 5 | 9 | 5 | 41 | 75 | 226 924.2 | 218 008.5 | 444 932.7 |
| 2007 | 0 | 0 | 1 | 6 | 4 | 11 | 3 | 41 | 66 | 219 137.0 | 210 379.6 | 429 516.6 |
| 2008 | 0 | 0 | 4 | 6 | 1 | 15 | 10 | 29 | 65 | 211 939.1 | 203 246.9 | 415 186.0 |
| 2009 | 0 | 0 | 2 | 4 | 3 | 14 | 7 | 28 | 58 | 201 208.5 | 192 740.3 | 393 948.8 |
| 2010 | 0 | 0 | 3 | 3 | 3 | 13 | 5 | 34 | 61 | 191 773.4 | 183 644.6 | 375 418.0 |
Figure 1.
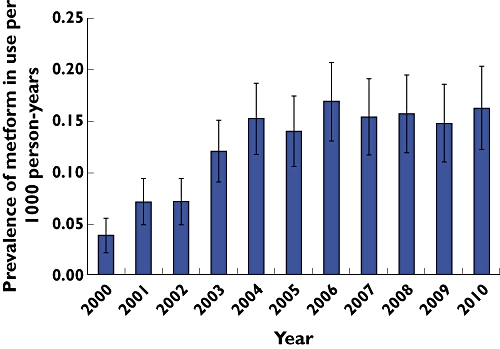
Overall prevalence of metformin prescribing in children and adolescents aged 0–18 years between 2000 and 2010
Table 2.
Metformin prescriptions for diabetes, polycystic ovarian syndrome (PCOS) and obesity between 2000 and 2010
| Number of patients | |||
|---|---|---|---|
| Diagnosis | Boys | Girls | Total |
| Diabetes only | 48 | 66 | 114 |
| Obesity only | 4 | 18 | 22 |
| PCOS and obesity | NA | 120 | 120 |
| Diabetes and obesity | 3 | 8 | 11 |
| Diabetes, PCOS and obesity | NA | 23 | 23 |
NA, not applicable.
Discussion
Our study showed that the use of metformin in the paediatric population has increased markedly between 2000 and 2010 in primary care, with prescribing prevalence increasing from 0.03 to 0.16 per 1000 person-years. This increase was particularly marked amongst girls aged 12–18 years.
There are limited data on paediatric metformin prescribing patterns in the UK. As some prescribing databases do not have links with indications for prescribing, an added strength of this study was that we were able to identify the disease indication for metformin therapy. However, our findings are subject to some limitations. First, the IMS DA only records prescriptions issued in primary care, excluding prescriptions dispensed from hospitals. It is possible that our data did not include a small number of initial hospital prescriptions. While the great majority of these would have been continued in primary care, unfortunately there are no data to investigate the extent of metformin prescribing in hospitals. Second, we were unable to identify whether subjects were treated with lifestyle modification along with metformin, as healthy diet and exercise are mainstays of the treatment for obesity, PCOS and type 2 diabetes [2, 4]. Third, we have no information on diagnostic criteria used for any of the conditions under study as diagnoses are often made in secondary care and as the dataset does not record criteria for those made in primary care. While diagnostic criteria for type 2 diabetes are internationally accepted, a number of different definitions exist for obesity and PCOS. Fourth, the IMS DA does not contain data on ethnicity and socioeconomic status and thus their impact on prescribing patterns remains unstudied.
Our finding that PCOS was the main indication for metformin prescribing in female adolescents was unexpected. This indicates that the use of metformin for PCOS in teenage girls is increasing in general practice. It has been well documented that adolescent obesity is increasing in population-based studies in the US [25] and UK [26]. In addition, a previous study has shown an increased prevalence of type 2 diabetes in adolescents aged 12–18 years in the UK [1]. Therefore, it is possible that the prevalence of PCOS in adolescents may also have increased. Although metformin has been shown to be of benefit in teenage girls with PCOS in a number of studies [7–9], the current evidence is limited and inconsistent. The efficacy of metformin treatment in adolescents with PCOS remains to be confirmed by well designed randomized controlled studies. In addition, to extrapolate treatment from adults to adolescents with PCOS is questionable since the risks and benefits are unclear.
There were a small number of patients who received metformin for obesity treatment in our study. This prescribing trend may increase in the next few years. In January 2010, the European Medicines Agency (EMA) recommended removing sibutramine from all markets in the European Union (EU) due to the risk of developing cardiovascular events in adults [27]. Consequently orlistat is the only licensed anti-obesity drug in the EU. As there is limited drug choice for obesity treatment, metformin is likely to gain its popularity for obesity treatment. In addition, a systematic review on economic consequences of obesity treatments has shown that metformin appeared to be a cost saving treatment for obese patients with type 2 diabetes [28]. At present, metformin has not yet received a paediatric licence for PCOS or obesity treatment. As it is mainly prescribed for these unlicensed indications in the paediatric population, more studies are needed to demonstrate its efficacy and safety in this population.
In conclusion, metformin prescribing in children and adolescents has increased substantially in the past decade. There is an increased number of teenage girls receiving metformin for PCOS treatment in general practice. As metformin is not licensed for PCOS and obesity treatment in the paediatric population, further studies are required to investigate its long-term efficacy and safety for these conditions.
Acknowledgments
The authors thank the general practitioners who contributed data to the IMS DA database.
This paper presents independent research funded by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research programme (Grant Reference Number RP-PG-0608-10035) – the Paediatric Research in Obesity Multi-model Intervention and Service Evaluation (PROMISE) programme. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Competing Interests
There are no competing interests to declare.
REFERENCES
- 1.Hsia Y, Neubert AC, Rani F, Viner RM, Hindmarsh PC, Wong IC. An increase in the prevalence of type 1 and 2 diabetes in children and adolescents: results from prescription data from a UK general practice database. Br J Clin Pharmacol. 2009;67:242–9. doi: 10.1111/j.1365-2125.2008.03347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harwood K, Vuguin P, DiMartino-Nardi J. Current approaches to the diagnosis and treatment of polycystic ovarian syndrome in youth. Horm Res. 2007;68:209–17. doi: 10.1159/000101538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352:1223–36. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]
- 4.Mastorakos G, Lambrinoudaki I, Creatsas G. Polycystic ovary syndrome in adolescents: current and future treatment options. Paediatr Drugs. 2006;8:311–8. doi: 10.2165/00148581-200608050-00004. [DOI] [PubMed] [Google Scholar]
- 5.Moll E, Bossuyt PM, Korevaar JC, Lambalk CB, van der Veen F. Effect of clomifene citrate plus metformin and clomifene citrate plus placebo on induction of ovulation in women with newly diagnosed polycystic ovary syndrome: randomised double blind clinical trial. BMJ. 2006;332:1485. doi: 10.1136/bmj.38867.631551.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Legro RS, Barnhart HX, Schlaff WD, Carr BR, Diamond MP, Carson SA, Steinkampf MP, Coutifaris C, McGovern PG, Cataldo NA, Gosman GG, Nestler JE, Giudice LC, Leppert PC, Myers ER. Cooperative Multicenter Reproductive Medicine Network. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med. 2007;356:551–66. doi: 10.1056/NEJMoa063971. [DOI] [PubMed] [Google Scholar]
- 7.Ibáñez L, Ferrer A, Ong K, Amin R, Dunger D, de Zegher F. Insulin sensitization early after menarche prevents progression from precocious pubarche to polycystic ovary syndrome. J Pediatr. 2004;144:23–9. doi: 10.1016/j.jpeds.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 8.Bridger T, MacDonald S, Baltzer F, Rodd C. Randomized placebo-controlled trial of metformin for adolescents with polycystic ovary syndrome. Arch Pediatr Adolesc Med. 2006;160:241–6. doi: 10.1001/archpedi.160.3.241. [DOI] [PubMed] [Google Scholar]
- 9.De Leo V, Musacchio MC, Morgante G, Piomboni P, Petraglia F. Metformin treatment is effective in obese teenage girls with PCOS. Hum Reprod. 2006;21:2252–6. doi: 10.1093/humrep/del185. [DOI] [PubMed] [Google Scholar]
- 10.Hoeger K, Davidson K, Kochman L, Cherry T, Kopin L, Guzick DS. The impact of metformin, oral contraceptives, and lifestyle modification on polycystic ovary syndrome in obese adolescent women in two randomized, placebo-controlled clinical trials. J Clin Endocrinol Metab. 2008;93:4299–306. doi: 10.1210/jc.2008-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wald AB, Uli NK. Pharmacotherapy in paediatric obesity: current agents and future directs. Rev Endocr Metab Disord. 2009;10:205–14. doi: 10.1007/s11154-009-9111-y. [DOI] [PubMed] [Google Scholar]
- 12.Freemark M. Pharmacotherapy of childhood obesity: an evidence-based, conceptual approach. Diabetes Care. 2007;30:395–402. doi: 10.2337/dc06-1569. [DOI] [PubMed] [Google Scholar]
- 13.Rogovik AL, Chanoine JP, Goldman RD. Pharmacotherapy and weight-loss supplements for treatment of paediatric obesity. Drugs. 2010;70:335–46. doi: 10.2165/11319210-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 14.Park MH, Kinra S, Ward KJ, White B, Viner RM. Metformin for obesity in children and adolescents: a systematic review. Diabetes Care. 2009;32:1743–5. doi: 10.2337/dc09-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joint Formulary Committee. British National Formulary For Children. 59. London: British Medical Association and Royal Pharmaceutical Society of Great Britain; 2009. [Google Scholar]
- 16.Joint Formulary Committee. British National Formulary. 59. London: British Medical Association and Royal Pharmaceutical Society of Great Britain; 2009. [Google Scholar]
- 17.Wong IC, Murray ML. The potential of UK clinical databases in enhancing paediatric medication research. Br J Clin Pharmacol. 2005;59:750–5. doi: 10.1111/j.1365-2125.2005.02450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stergachis A, Saunders KW, Davis RL. Examples of automated databases. In: Strom BL, editor. Textbook of Pharmacoepidemiology. 4. Chichester, West Sussex: John Wiley & Son Ltd; 2006. pp. 173–214. [Google Scholar]
- 19.European Pharmaceutical Market Research Association. ATC- Anatomical classification. Available at http://www.ephmra.org/PDF/ATC%20Guidelines%202010.pdf (last accessed June 2011.
- 20.WHO. International Statistical Classification of Diseases and Health Problem, 10th Revision. Geneva: World Health Organisation; 1992. Available at http://www.who.int/classifications/icd/en/ (last accessed June 2011. [Google Scholar]
- 21.Murray ML, Thompson M, Santosh PJ, Wong IC. Effects of the Committee on Safety of Medicines advice on antidepressant prescribing to children and adolescents in the UK. Drug Saf. 2005;28:1151–7. doi: 10.2165/00002018-200528120-00009. [DOI] [PubMed] [Google Scholar]
- 22.Hsia Y, Neubert A, Sturkenboom MC, Murray ML, Verhamme KM, Sen F, Giaquinto C, Ceci A, Wong IC, TEDDY Network of Excellence Antiepileptic drug prescribing comparison in three European countries. Epilepsia. 2010;51:789–96. doi: 10.1111/j.1528-1167.2009.02331.x. [DOI] [PubMed] [Google Scholar]
- 23.Neubert A, Verhamme K, Murray ML, Picelli G, Hsia Y, Sen FE, Giaquinto C, Ceci A, Sturkenboom M, Wong IC, TEDDY Network of Excellence The prescribing of analgesics and non-steroidal anti-inflammatory drugs in paediatric primary care in the UK, Italy and the Netherlands. Pharmacol Res. 2010;62:243–8. doi: 10.1016/j.phrs.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Rose K, Stotter H. ICH E11: clinical investigation of medicinal products in the paediatric population. In: Rose K, van den Anker JN, editors. Guide to Paediatric Clinical Research. Basel: Karger; 2007. pp. 33–7. [Google Scholar]
- 25.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999-2000. JAMA. 2002;288:1728–32. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 26.Viner RM, Hsia YF, Neubert AC, Wong IC. Rise in anti-obesity drug prescribing in children and adolescents in the UK: a population-based study. Br J Clin Pharmacol. 2009;68:844–51. doi: 10.1111/j.1365-2125.2009.03528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.European Medicines Agency (EMEA) Questions and answers on the suspension of medicines containing sibutramine. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Sibutramine_107/WC500094238.pdf (last accessed June 2011.
- 28.Avenell A, Broom J, Brown TJ, Poobalan A, Aucott L, Stearns SC, Smith WCS, Jung RT, Campbell MK, Grant AM. Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement. Health Technol Assess. 2004;8:iii–iv. 1–182. doi: 10.3310/hta8210. [DOI] [PubMed] [Google Scholar]


