Abstract
AIM
We compared three times daily dual therapy with standard triple therapy for effectiveness and safety in H. pylori infection.
METHODS
Two hundred and four H. pylori positive patients with peptic ulcer were randomly assigned to one of two regimens: (i) triple therapy with amoxicillin, clarithromycin and lansoprazole twice daily for 2 weeks or (ii) dual therapy with amoxicillin and lansoprazole three times daily for 2 weeks. The success of eradication was evaluated 4 to 5 weeks after completing treatment.
RESULTS
The eradication rate was 82.8% in the triple therapy group and 78.4% in the dual therapy group by per protocol analysis. This difference was not significant (P= 0.573). Adverse events were more frequent in the triple therapy group than in the dual therapy group (P= 0.002).
CONCLUSIONS
Because dual therapy had fewer side effects than triple therapy and a similar eradication rate, dual therapy may provide an acceptable alternative first line therapy for H. pylori eradication in Korea.
Keywords: amoxicillin/administration and dosage, combination, drug therapy, Helicobacter pylori/drug therapy, proton pump inhibitors/administration and dosage
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Triple therapy using a proton pump inhibitor with two antibiotics is the standard treatment for H. pylori infection. However, the increasing prevalence of antibiotic resistance has eroded the high success rates initially reported for the standard triple therapy.
WHAT THIS STUDY ADDS
Compared with standard triple therapy, amoxicillin/lansoprazole dual therapy, given three times daily for H. pylori had a similar eradication rate with fewer side effects.
Introduction
To eradicate Helicobacter pylori infection, the globally accepted standard regimen is a triple therapy that combines a proton pump inhibitor (PPI) and two antibiotics, such as clarithromycin and amoxicillin or metronidazole [1]. However, with increasing prevalence of clarithromycin resistant H. pylori strains, eradication rates with triple therapy have fallen below 80% [2]. Given this development, a dual therapy with amoxicillin and a PPI, without clarithromycin, could provide an alternative regimen if the eradication rate is acceptable.
A key component of this approach would be the optimal reduction in gastric acidity during treatment, in addition to effective antimicrobial action [3]. A sustained increase in intragastric pH level may predict successful outcome for H. pylori eradication using dual therapy with omeprazole and amoxicillin [4]. Increasing the dose and the dosing frequency of the PPI may also increase the success rate [5–7]. The purpose of this study was to compare a three times daily dual therapy using lansoprazole and amoxicillin, with the standard triple therapy using lansoprazole, amoxicillin and clarithromycin for effectiveness and safety in H. pylori eradication in Korea.
Methods
Patient selection
From May 2009 through April 2010, outpatients at Korea University Ansan Hospital who were at least 18 years of age and had endoscopically proven peptic ulcer disease were asked to participate in the study. The exclusion criteria were the absence of H. pylori infection, a history of H. pylori eradication, concurrent critical illness, pregnancy, recent and high frequency use of non-steroidal anti-inflammatory drugs, an allergy to the study medications, history of gastric surgery and the presence of gastric cancer. Written informed consent for participation was obtained from each patient before the study. The Institutional Review Board of our institution reviewed and approved this study (ED09049).
Diagnosis of H. pylori infection
During endoscopy, a rapid urease test (CLO test; Kimberly-Clark Ballard Medical Products, Roswell, GA, USA) and histologic examination were performed using biopsy specimens taken from the antrum and body of the stomach. Biopsies were fixed and stained with cresyl-violet for histologic examination. Patients were diagnosed as infected if one of these two tests were positive.
Treatment and eradication
Patients were randomly assigned to one of two regimens: (i) 1000 mg amoxicillin with 500 mg clarithromycin and 30 mg lansoprazole twice daily for 2 weeks (triple therapy group) or (ii) 750 mg amoxicillin with 30 mg lansoprazole three times daily for 2 weeks (dual therapy group). Subjects and physicians were not blinded to the treatments patients received. To evaluate the success of eradication, follow-up endoscopy with a rapid urease test and histologic examination or a 13C-urea breath test were performed 4–5 weeks after completing therapy. The cut-off value for the 13C-urea breath test was 2.5%. Eradication failure was defined as a positive result for any of these three tests. Patients answered the questionnaire about the side effects of medication 1 week after completion of treatment. Compliance was evaluated by remaining pill count and poor compliance was defined as taking less than 80% of the total medication prescribed. Side effects were described as symptoms occurring after ingestion of medication, and the side effects that caused patients to discontinue the medication were considered severe.
Statistical analysis
Assuming a difference of 10% between eradication rates achieved by the two regimens and a statistical power of at least 80% with a value of α < 0.05, the minimum number of patients to be included in each group was calculated to be 83. Assuming a withdrawal rate of 20%, at least 104 patients were required in each group. The cure rate was estimated to be 0.80 based on previous studies [8, 9].
Calculations were performed using SPSS version 15.0 (SPSS Inc., Chicago, IL, USA). To compare the two groups, t-tests and chi-square tests were used. The 95% confidence intervals (CIs) for eradication rates were calculated using standard methods. P values of less than 0.05 were considered statistically significant.
Results
During the study period, 219 patients were diagnosed with peptic ulcer and H. pylori infection. Eight patients refused to participate the study and three patients with gastric cancer were excluded (Figure 1). The 208 patients in the study were randomly assigned to either the triple therapy group (n= 104) or the dual therapy group (n= 104). During the follow-up period, 11 patients in the triple therapy group and 12 patients in the dual therapy group were lost. Demographic characteristics of the two groups did not differ significantly (Table 1). Four patients in the dual therapy group demonstrated poor compliance (less than 80%) and all patients in the triple therapy group showed good compliance.
Figure 1.
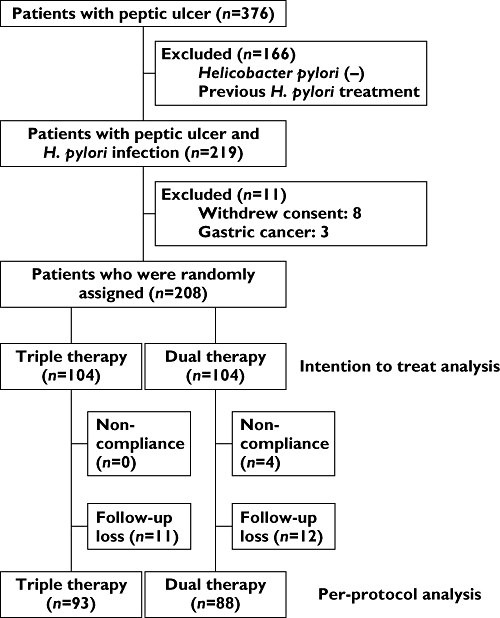
Flow chart of the study
Table 1.
Demographic data, eradication rates and adverse events for the triple and dual therapy groups
| Triple (n= 104) | Dual (n= 104) | P value | |
|---|---|---|---|
| Age (years, mean ± SD) | 49.6 ± 12.3 | 50.9 ± 11.8 | 0.458 |
| Gender (M/F) | 63/41 | 70/34 | 0.360 |
| Hypertension | 19 (18.3%) | 18 (17.3%) | 1.000 |
| Diabetes mellitus | 12 (11.5%) | 9 (8.7%) | 0.629 |
| Alcohol | 46 (44.2%) | 53 (50.9%) | 0.379 |
| Smoking | 32 (30.8%) | 35 (33.7%) | 0.755 |
| Endoscopic findings | 0.457 | ||
| Gastric ulcer | 26 (25.0%) | 34 (32.7%) | |
| Duodenal ulcer | 64 (61.5%) | 55 (52.9%) | |
| Both gastric and duodenal ulcer | 14 (13.5%) | 15 (14.4%) | |
| Eradication rate (%) | |||
| Intention to treat analysis | 77/104 (74.0%) | 70/104 (67.3%) | 0.361 |
| Difference (95% CI, %) | −6.7 (−19.0, 5.6) | ||
| Per-protocol analysis | 77/93 (82.8%) | 69/88 (78.4%) | 0.573 |
| Difference (95% CI, %) | −4.4 (−15.9, 7.1) | ||
| Adverse events | 37 (35.6%) | 19 (18.3%) | 0.002 |
| Bitter taste | 28 (26.9%) | 5 (4.8%) | <0.001 |
| Diarrhoea | 17 (16.3%) | 2 (1.9%) | <0.001 |
| Loose stool | 2 (1.9%) | 3 (2.9%) | 1.000 |
| Abdominal discomfort | 4 (3.8%) | 4 (3.8%) | 1.000 |
| Nausea | 3 (2.9%) | 1 (1.0%) | 0.621 |
| Dry mouth | 2 (1.9%) | 0 | 0.498 |
For intention to treat (ITT) analyses, the eradication rate was 74% (95% CI 65.5, 82.4%, 77/104) in the triple therapy group and 67.3% (95% CI 58.3, 76.3%, 70/104) in the dual therapy group. By per protocol analysis, the eradication rate was 82.8% (95% CI 75.1, 90.5%, 77/93) in the triple therapy group and 78.4% (95% CI, 69.8, 86.9%, 69/88) in the dual therapy group. These differences between the two groups were not significant (P= 0.361 and P= 0.573, respectively) (Table 1).
Side effects were generally mild, and none of the patients stopped taking medication because of side effects. Mild adverse events were more frequently reported in the triple therapy group (35.6%) than in dual therapy group (18.3%) (P= 0.002; Table 1).
Discussion
In the mid 1990s to early 2000s, dual therapy using amoxicillin and a PPI showed eradication rates ranging from 57–90%) [10–12]. When given three or four times daily, however, PPI plus amoxicillin dual therapy produced high eradication rates (77–100%) [10, 11, 13, 14]. A regimen of lansoprazole 30 mg three times daily, produced significantly greater acid suppression than lansoprazole 30 mg twice daily [15]. In addition, amoxicillin showed time-dependent killing, and its effectiveness increased with dosing frequency [16]. Based on this record, we expected the three times daily dual therapy to result in better acid control and more efficient eradication than triple therapy; but results did not meet our expectation. Graham et al[17]. reported that the intention to treat cure rate as 72.2% (95% CI 56, 84%, 26/36) and as 74.2% by per protocol (95% CI 56, 87%, 26/35) using esomeprazole 40 mg plus amoxicillin 750 mg every 8 h for 14 days. These findings may indicate that the maximum eradication rate using PPI plus amoxicillin three times daily has been achieved. However, the eradication rates for the two groups in our study were similar, and the dual therapy group experienced a lower incidence of adverse events, which were mild. Side effects of clarithromycin, such as bitter taste and headache, limit its usefulness in some patients and that is why the dual therapy group in this study experienced fewer adverse events.
Clarithromycin is contraindicated in patients with hypersensitivity to the macrolide antibiotics, and should be used with caution in patients with liver disease or certain heart problem such as QT prolongation, or who are taking HIV drugs [18]. For these patients, dual therapy may be a useful therapeutic option for H. pylori infection, especially in regions, including Korea, where metronidazole resistance is high.
One limitation of our study was the loss to follow-up of a relatively high proportion (11.5%) of patients. This may weaken the power of the study. In addition, we did not investigate clarithromycin resistance, genetic status of cytochrome P450 2C19 or the eradication rate in correlation with directly measured intragastric pH.
In conclusion, dual therapy with 750 mg amoxicillin and 30 mg lansoprazole three times daily for 2 weeks was similar in effectiveness to the standard triple therapy and had fewer side effects, when given as first line treatment for H. pylori. This dual therapy may therefore serve as an alternative first line therapy for H. pylori in Korea.
Acknowledgments
This study was supported by Korea University Grant.
Competing Interests
There are no competing interests to declare.
REFERENCES
- 1.Malfertheiner P, Megraud F, O'Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772–81. doi: 10.1136/gut.2006.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graham DY, Shiotani A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat Clin Pract Gastroenterol Hepatol. 2008;5:321–31. doi: 10.1038/ncpgasthep1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunt RH. pH and Hp-gastric acid secretion and Helicobacter pylori: implications for ulcer healing and eradication of the organism. Am J Gastroenterol. 1993;88:481–3. [PubMed] [Google Scholar]
- 4.Sugimoto M, Furuta T, Shirai N, Kodaira C, Nishino M, Ikuma M, Ishizaki T, Hishida A. Evidence that the degree and duration of acid suppression are related to Helicobacter pylori eradication by triple therapy. Helicobacter. 2007;12:317–23. doi: 10.1111/j.1523-5378.2007.00508.x. [DOI] [PubMed] [Google Scholar]
- 5.Furuta T, Shirai N, Takashima M, Xiao F, Hanai H, Sugimura H, Ohashi K, Ishizaki T, Kaneko E. Effect of genotypic differences in CYP2C19 on cure rates for Helicobacter pylori infection by triple therapy with a proton pump inhibitor, amoxicillin, and clarithromycin. Clin Pharmacol Ther. 2001;69:158–68. doi: 10.1067/mcp.2001.113959. [DOI] [PubMed] [Google Scholar]
- 6.Furuta T, Shirai N, Xiao F, Takashita M, Sugimoto M, Kajimura M, Ohashi K, Ishizaki T. High-dose rabeprazole/amoxicillin therapy as the second-line regimen after failure to eradicate H. pylori by triple therapy with the usual doses of a proton pump inhibitor, clarithromycin and amoxicillin. Hepatogastroenterology. 2003;50:2274–8. [PubMed] [Google Scholar]
- 7.Miehlke S, Kirsch C, Schneider-Brachert W, Haferland C, Neumeyer M, Bastlein E, Papke J, Jacobs E, Vieth M, Stolte M, Lehn N, Bayerdorffer E. A prospective, randomized study of quadruple therapy and high-dose dual therapy for treatment of Helicobacter pylori resistant to both metronidazole and clarithromycin. Helicobacter. 2003;8:310–9. doi: 10.1046/j.1523-5378.2003.00158.x. [DOI] [PubMed] [Google Scholar]
- 8.Fischbach L, Evans EL. Meta-analysis: the effect of antibiotic resistance status on the efficacy of triple and quadruple first-line therapies for Helicobacter pylori. Aliment Pharmacol Ther. 2007;26:343–57. doi: 10.1111/j.1365-2036.2007.03386.x. [DOI] [PubMed] [Google Scholar]
- 9.Kim N, Park SH, Seo GS, Lee SW, Kim JW, Lee KJ, Shin WC, Kim TN, Park MI, Park JJ, Hong SJ, Shim KN, Kim SW, Shin YW, Chang YW, Chun HJ, Lee OJ, Jeon WJ, Park CG, Cho CM, Park CH, Won SY, Lee GH, Park KS, Shin JE, Kim HU, Park JY, Chae HS, Song GA, Kim JG, Yoon BC, Seol S, Jung HC, Chung IS. Lafutidine versus lansoprazole in combination with clarithromycin and amoxicillin for one versus two weeks for Helicobacter pylori eradication in Korea. Helicobacter. 2008;13:542–9. doi: 10.1111/j.1523-5378.2008.00648.x. [DOI] [PubMed] [Google Scholar]
- 10.Bayerdorffer E, Miehlke S, Mannes GA, Sommer A, Hochter W, Weingart J, Heldwein W, Klann H, Simon T, Schmitt W, Bastlein E, Eimiller A, Hatz R, Lehn N, Dirschedl P, Stolte M. Double-blind trial of omeprazole and amoxicillin to cure Helicobacter pylori infection in patients with duodenal ulcers. Gastroenterology. 1995;108:1412–7. doi: 10.1016/0016-5085(95)90689-4. [DOI] [PubMed] [Google Scholar]
- 11.Furuta T, Shirai N, Takashima M, Xiao F, Hanai H, Nakagawa K, Sugimura H, Ohashi K, Ishizaki T. Effects of genotypic differences in CYP2C19 status on cure rates for Helicobacter pylori infection by dual therapy with rabeprazole plus amoxicillin. Pharmacogenetics. 2001;11:341–8. doi: 10.1097/00008571-200106000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Wong BC, Xiao SD, Hu FL, Qian SC, Huang NX, Li YY, Hu PJ, Daldiyono, Manan C, Lesmana L, Carpio RE, Perez JY, Jr, Fock KM, Kachintorn U, Phornphutkul K, Kullavanijaya P, Ho J, Lam SK. Comparison of lansoprazole-based triple and dual therapy for treatment of Helicobacter pylori-related duodenal ulcer: an Asian multicentre double-blind randomized placebo controlled study. Aliment Pharmacol Ther. 2000;14:217–24. doi: 10.1046/j.1365-2036.2000.00689.x. [DOI] [PubMed] [Google Scholar]
- 13.Shirai N, Sugimoto M, Kodaira C, Nishino M, Ikuma M, Kajimura M, Ohashi K, Ishizaki T, Hishida A, Furuta T. Dual therapy with high doses of rabeprazole and amoxicillin versus triple therapy with rabeprazole, amoxicillin, and metronidazole as a rescue regimen for Helicobacter pylori infection after the standard triple therapy. Eur J Clin Pharmacol. 2007;63:743–9. doi: 10.1007/s00228-007-0302-8. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz H, Krause R, Sahba B, Haber M, Weissfeld A, Rose P, Siepman N, Freston J. Triple versus dual therapy for eradicating Helicobacter pylori and preventing ulcer recurrence: a randomized, double-blind, multicenter study of lansoprazole, clarithromycin, and/or amoxicillin in different dosing regimens. Am J Gastroenterol. 1998;93:584–90. doi: 10.1111/j.1572-0241.1998.169_b.x. [DOI] [PubMed] [Google Scholar]
- 15.Blum RA, Hunt RH, Kidd SL, Shi H, Jennings DE, Greski-Rose PA. Dose-response relationship of lansoprazole to gastric acid antisecretory effects. Aliment Pharmacol Ther. 1998;12:321–7. doi: 10.1046/j.1365-2036.1998.00306.x. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman A, Danenberg HD, Katzhendler I, Shuval R, Gilhar D, Friedman M. Pharmacodynamic and pharmacokinetic rationales for the development of an oral controlled-release amoxicillin dosage form. J Control Release. 1998;54:29–37. doi: 10.1016/s0168-3659(97)00165-x. [DOI] [PubMed] [Google Scholar]
- 17.Graham DY, Javed SU, Keihanian S, Abudayyeh S, Opekun AR. Dual proton pump inhibitor plus amoxicillin as an empiric anti-H. pylori therapy: studies from the United States. J Gastroenterol. 2010;45:816–20. doi: 10.1007/s00535-010-0220-x. [DOI] [PubMed] [Google Scholar]
- 18.Williams JD. Evaluation of the safety of macrolides. Int J Antimicrob Agents. 2001;18(Suppl 1):S77–81. doi: 10.1016/s0924-8579(01)00399-5. [DOI] [PubMed] [Google Scholar]


