Abstract
Background
Adipose-derived stromal cells (ASCs) are a multipotent cell type with the ability to undergo osteogenic differentiation. A small number of studies have examined the ability of systemically administered ASCs to participate in various biologic repair processes. In the following study, we sought to examine whether systemically administered ASCs would migrate to and heal surgically created defects of the mouse cranial skeleton.
Methods
Mouse ASCs were harvested from Luciferase+ transgenic mice; human ASCs were harvested from human lipoaspirate and labeled with Luciferase and GFP. A 4mm calvarial defect (critical sized) was performed in the mouse parietal bone; skin incisions alone were used as a control (n=5 per group). ASCs were injected intravenously (IV; 200,000 cells / animal) and compared to saline injection only. Methods of analyses included micro computed tomography (microCT), In Vivo Imaging System (IVIS) detection of Luciferase activity, and standard histology.
Results
Migration of ASCs to calvarial defect sites was confirmed by accumulation of Luciferase activity and GFP stain, as early as 4 days and persisting up to 4 weeks. In comparison, little activity was observed among control groups. Intravenous administration of either mouse or human ASCs resulted in histologic evidence of bone formation within the defect site, in comparison to an absence of bone among control defects. By microCT analysis, human but not mouse ASCs stimulated significant osseous healing.
Conclusions
Intravenously administered ASCs migrate to sites of calvarial injury. Thereafter, IV hASCs contribute to bony calvarial repair. The intravenous administration of ASCs may be an effective delivery method for future efforts in skeletal regeneration.
Keywords: Adipose derived stromal cells, Skeletal tissue engineering, Tissue regeneration, Multipotent stromal cells, Critical size calvarial defect, Systemic Migration
INTRODUCTION
Multiple lines of evidence suggest that human adipose-derived stromal cells (hASCs) hold promise for future use in skeletal regeneration 1. Human ASCs are easily harvested by simple lipoaspiration procedures and are readily expandable as compared to bone marrow mesenchymal cells 2. Moreover, hASCs undergo rapid osteogenic differentiation.3, 4 In our laboratory, we have observed convincingly that ASCs, whether derived from mouse or human origin, contribute to osseous healing of mouse calvarial defects 5, 6. Perhaps most exciting are those applications of hASCs to the human patient. In small pilot studies, defects of the cranium 7, maxilla 8, and mandible 9 have been either healed or enabled to heal faster with the use of hASCs 10.
Despite accumulating animal research and intriguing case reports, much remains unknown regarding the optimum mode of ASC delivery. A significant and growing number of studies have examined the intravenous (IV) administration of ASCs. Such studies have examined the natural distribution of ASCs following IV injection. Interestingly, most organs show uptake of ASCs after administration, including bone and bone marrow tissues 11, 12, and at least some studies show long-term persistence within the host without oncogenic transformation 13. These studies were geared toward disparate interests, but all rooted in the regenerative capabilities of ASCs. For example studies have examined intravenous injection of ASCs for repair of the liver 12, heart 14, endothelium 15, and even olfactory epithelium 16. An emerging interest as well is in the intravenous administration of ASCs for autoimmune and inflammatory disorders, such as experimental colitis and abdominal sepsis 17, 18, muscular dystrophy 19, experimental arthritis 20, and multiple sclerosis 21. Two provocative case studies have also been published in humans showing beneficial outcomes of intravenous ASCs: the first in rheumatoid arthritis, and the second in colitis 17, 22. Thus, the use of intravenous ASCs may be a safe and beneficial future therapeutic modality.
In the following study, we utilized a well established mouse calvarial defect model to address the possible application of intravenous ASCs to skeletal regenerative medicine. After defining a safety window in which ASCs can be injected without systemic toxicity, we observed that IV ASCs traffic to sites of calvarial skeletal injury. Next, by histological analysis we found that animals injected with either mouse or human ASCs formed bone within a defect site. Finally, IV human, but not mouse ASCs were observed to lead to significant osseous healing by microCT analysis. These data broaden the field of ASC-mediated skeletal tissue regeneration to suggest that systemic, as well as locoregional, application of ASCs may be a viable future therapy.
MATERIALS AND METHODS
Chemicals, supplies and animals
Dulbecco’s Modified Eagles Medium (DMEM), fetal bovine serum (FBS), and penicillin/streptomycin were purchased from GIBCO Life Technologies, (Carlsbad, CA). Cell culture wares were purchased from Corning Inc, (San Mateo, CA). All chemicals were purchased from Sigma-Aldrich unless otherwise specified. CD-1 wild-type mice, CD-1 nude mice (Crl:CD-1 Foxn1nu), and CD-1 mice expressing Luciferase transgene were obtained from Charles Rivers, (Wilmington, MA).
Cell Harvest
Human ASCs were harvested from lipoaspirate derived from three women under 50 years of age, under BMI of 28 kg/m2, and without significant co-morbidities from the flank and thigh regions by suction assisted liposuction as previously described 6. Digestion was carried out with a collagenase solution and cultures were established at 37°C, 21% O2, 5% CO2 in standard growth medium (DMEM, 10% FBS). Cells were expanded in primary culture for a period of 72 hours, at which time they were pooled yielding a single population of hASCs. Mouse ASCs were harvested from the inguinl fat pads of CD-1 mice expressing Luciferase transgene and processed in a similar fashion. For all assays, first passage cells only were used as later passage cells have been associated with decreased osteogenesis 23, 24.
Creation of calvarial defects
Nude and wild type animals were divided into two surgical groups: 1) those without a defect but with a sham skin incision only, and 2) those with calvarial defect. A non-healing (4mm) calvarial defect was created in the right parietal bone of 60 day-old mice (n=5) as previously described25.
Human ASC labeling
Human ASCs were stably transduced with the lentivirus carrying a triple fusion reporter genes, including firefly luciferase (Fluc), red fluorescence protein (RFP) and herpes simplex virus truncated thymidine kinase (HSV-ttk) genes. Stably expressed hASCs were purified by fluorescence activated cell sorting based on the RFP expression.
ASC in vivo administration
For in vivo applications, passage one Luciferase+ mASCs or hASCs were trypsinized and resuspended in normal saline. First, various methods of administration of mASCs were compared: In the postoperative period, mASCs were either injected intravenously, injected intraperitoneally, or directly applied to the defect site on a hydroxyappetite coated PLGA scaffold. For intravenous or intraperitoneal injection, 200,000 Luc+ mASCs were resuspended in 200 uL Normal Saline (NS) were injection (n=5 per group). Finally, further experiments were performed to assess defect healing upon IV administration of either human or mouse ASCs. Human ASCs or Luc+ mASCs were injected intravenously in the postoperative period (200,000 ASCs in 200 uL based on a dose curve NS; n=5 animals per group). As a control, Luc+ mASCs were applied directly to a calvarial defect by operative means, seeded onto a hydroxy-apatite coated PLGA scaffold as previously described (n=5 animals in total) 6. Briefly, 200,000 ASCs were placed on a hydroxyappatite coated PLGA scaffold for 12 hours. Subsequently the scaffold was rinsed copiously in normal saline and placed into the defect. For all experiments, equal numbers of animals (n=5) were used with sham skin incisions only. In the case of Luc+ mASC administration, host CD-1 wild-type mice were utilized. In the case of hASC administration, host CD-1 nude mice were utilized.
In vivo imaging
Micro-computed tomography was performed on live animals in a serial manner postoperatively (up to 4 weeks healing), using a high-resolution MicroCAT II™ (ImTek Inc., Knoxville, TN) small animal imaging system as previously described 25. Fraction bony healing was quantified using Adobe Photoshop, comparing scans to those on the same animal taken immediately postoperatively 6.
IVIS imaging was performed at stratified timepoints postoperatively to assess migration of systemically injected Luc+ mASCs. Mice were anesthetized with Aerrane; luciferin was injected intraperitoneally 10 minutes prior to imaging (150mg/kg in 200 uL). Animals were imaged using the IVIS 200b imaging system and imaged under anesthesia with a 1–3 minute capture, medium binning. Total photon emission was quantifed in a standardized area encompassing 1) the calvarial defect sites, 2) pulmonary sites using LivingImage software.
Histologic analyses
Up to 4 weeks postoperatively, animals were sacrificed by CO2 asphyxiation and cervical dislocation to confirm imaging findings by histology. Calvaria were harvested, formalin-fixed, decalcified in 19% EDTA, paraffin-embedded and sectioned at 8 um thickness. Approximately 100 slides were generated per calvarial defect. Aniline blue staining was performed on every 10th section throughout the sample to provide detailed histology of the regenerate. Histomorphometry of each aniline blue section was performed as previously described 26. Next, select slides were stained with pentachrome, alkaline phosphatase and picrosirius red as previously described 27. GFP immunohistochemistry was performed on select slides as previously described 28, per manufacturers instructions. Photographs were obtained at 10× and 40× for presentation.
Statistical analysis
Means and standard deviations were calculated from numerical data, as presented in the text, figures and figure legends. In figures, bar graphs represent means, whereas error bars represent one standard deviation. Statistical analysis was performed using a Student’s t-test at each time to directly compare two groups with a post-op Bonferroni correction. Inequality of standard deviations was excluded by employing the Levene’s test. *P<0.05 was considered to be significant.
RESULTS
Route of ASC administration
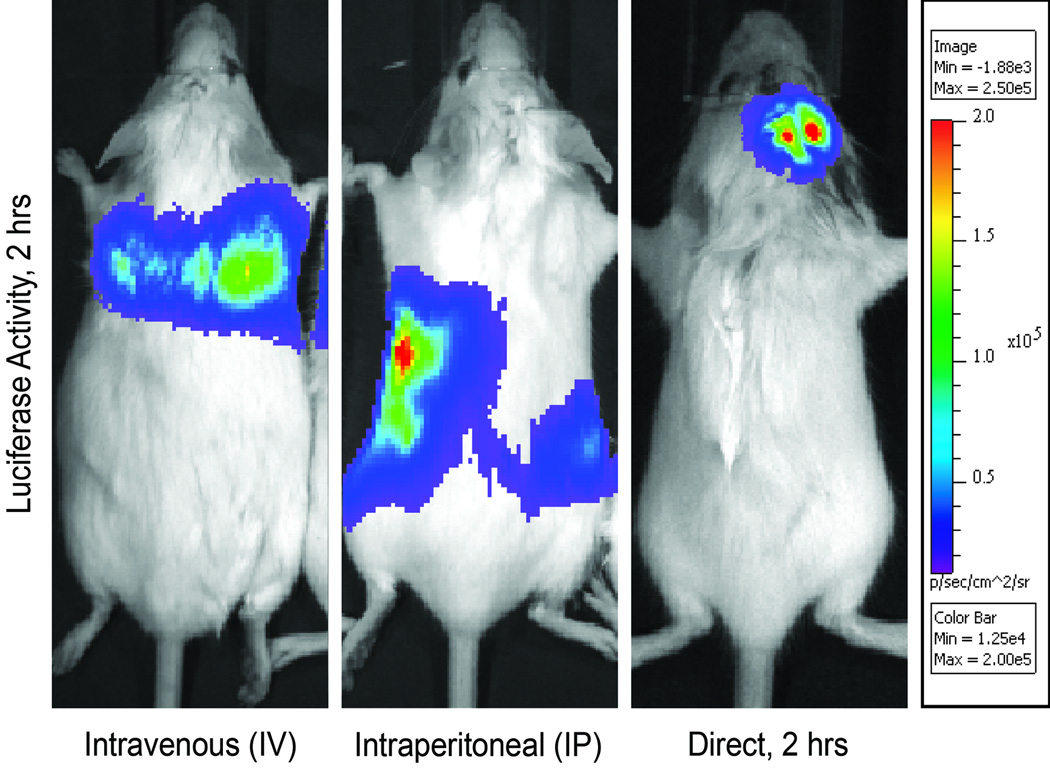
First, we sought to compare various routes of non-invasive mASC delivery to the more commonly employed direct application of ASCs to a surgical defect site.6 To permit in vivo tracking of ASCs, cells were harvested from Luc+ transgenic mice. Luc+ mASCs were either injected intravenously (IV), intraperitonealy (IP), or directly placed into a calvarial defect (direct) (Fig. 1). In all cases 200,000 mASCs were applied, either resuspended in normal saline for IV or IP injection, or directly into a calvarial defect (n=5 per group) with the structural support of a modified PLGA scaffold per standard protocol 5. Localization of Luciferase activity was performed 2 hrs thereafter by IVIS imaging, to confirm that all methods of application maintained immediate cell viability. Across all groups, cells remained viable within the host mouse (Fig. 1A–C). After IV administration, accumulation was apparent in the lungs at 2hrs post-injection, (Fig. 1A). After IP administration, cells remained clearly delimited by the intraperitoneal cavity at 2 hrs (Fig. 1B). In comparison, when mASCs were directly applied to a calvarial injury site, they remained in place and well circumscribed by the defect site (Fig. 1C). Administration of mASCs by either intraperitoneal or intravenous methods appeared to be a feasible alternative to direct application, however would cells traffic outside the lungs or peritoneal cavity, respectively? To answer this, IVIS imaging was performed at 2, 24 and 96 hrs post mASC injection. With intravenous injection, Luciferase activity was again observed to accumulate in the lungs after 2 hrs (See figure, Supplemental Digital Content 1A which displays Intravenous ASC administration: IVIS imaging after intravenously injected Luc+ mASCs, imaged at 2, 24 and 96hrs (n=5)). At 24 hrs, the distribution of Luciferase activitiy remained similar, but with decreased absolute activity (See Figure, Supplemental Digital Content 1B). At 96 hrs, this dissipation of pulmonary Luciferase activity was followed accompanied by a wide distribution of Luciferase activity throughout the mouse, including the head (See Figure, Supplemental Digital Content 1C). Luciferase activity in the mouse thorax was quantified (n=5 mice), and confirmed a rapid dissipation from 2 to 24 hours post-injection, and a nearly undetectable presence of luciferase activity in the lungs after 96 hours (See figure, Supplemental Digital Content 2, which displays Quantification of luciferase activity in the lung fields at 2, 24 and 96 hours post-injection, presented as total photon emission in a standardized square area encompassing the thorax (n=5, *P<0.05). A Student’s t-test with a Bonferroni correction was used to assess significance). After intraperitoneal injection, Luciferase activity again remained within the peritoneal cavity at 2 hrs post injection and dissipated thereafter (See figure, supplemental Digital Content 3, which displays Intraperitoneal ASC administration: IVIS imaging after intraperitoneal injected Luc+ mASCs, imaged at 2, 24 and 96 hrs (n=5)). Thus, intravenous but not intraperitoneal injection appeared to be a suitable method for non-invasive systematic ASC application.
Figure 1. Route of ASC administration.
(A) Luc+ mASCs were injected by tail vein and imaged 2 hrs thereafter showing accumulation in the lungs by IVIS imaging. (B) Luc+ mASCs delivered by intraperitoneal injection remained in the peritoneal cavity after 2 hours. (C) For comparison, Luc+ mASCs directly applied to a calvarial defect and imaged after 2 hours. The strongest luciferase activity appears red, followed by yellow, green, blue, purple in decreasing intensity (see color scale), n=5 mice for all groups.
Safety of mASC administration
Next, to define the safety window for IV injection, mouse ASCs were resuspended in an increasing gradient of cell number (from 200,000–1,000,000 cells per 200 ul). Increasing turbidity was noted at the higher concentrations. No deleterious effects on mouse survival occurred when 200,000 mASCs were administered (5/5 survival at up to 4 wks). Starting at a cell burden of 400,000 mASCs, an increasing number of mice died within minutes after injection (3/5 survival). With administration of 1,000,000 mASCs, total mortality occurred within minutes (0/5 survival). No deaths occurred outside the immediate post-injection period. Necropsy was performed on all animals. Gross examination failed to reveal any cause of death. Microscopic examination was performed on all organs, showing evidence of disseminated intravascular coagulation, effecting the lungs, liver and kidneys. All other organs including the brain, thyroid, thymus, heart, gastrointestinal tract, spleen, adrenals and genitourinary system were without gross or microscopic pathology (data not shown).
Migration of intravenously administered ASCs to a critical size mouse calvarial defect
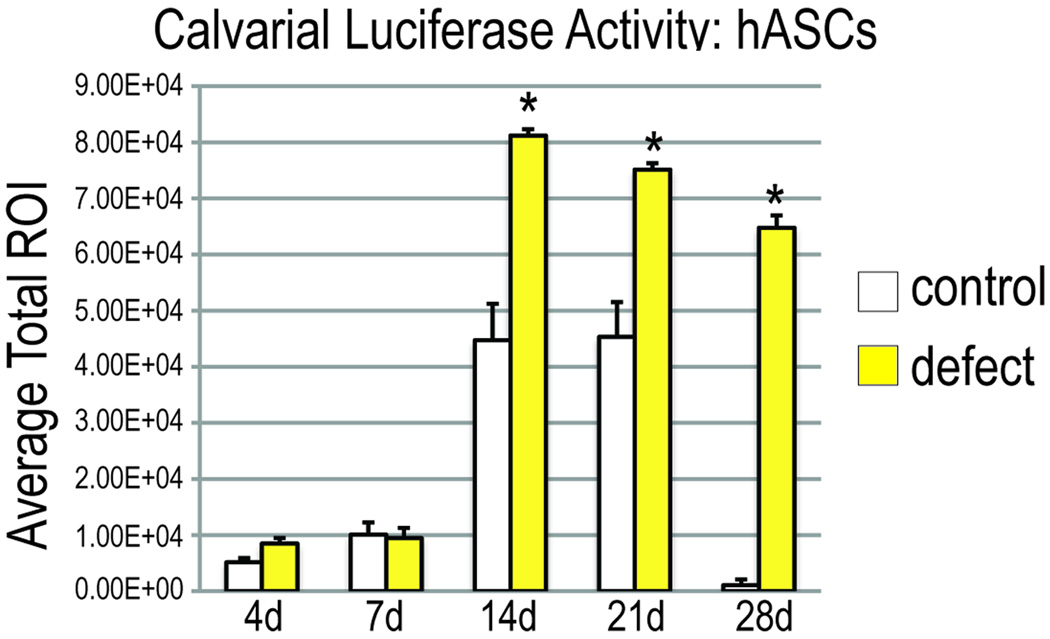
Having demonstrated intravenous administration as the ideal method of administration, we next sought to evaluate the trafficking of mASCs to a calvarial injury site. A well studied critical sized (4mm) full thickness defect model was utilized, created in the right parietal bone 6, followed immediately by IV injection of 200,000 Luc+ mASCs (Fig. 2). As a control, sham skin incisions over the calvaria were performed; Luciferase activity was measured at stratified timepoints postoperatively by IVIS imaging. IVIS or luciferase imaging provides a heat map as to the location of viable cells expressing luciferase but since the camera is external, one cannot tell if the signal is coming from the skin or deeper structures. Thus, having a control of a skin incision only is crucial to further demonstrate that the cells were trafficking in response to the calvarial defect and not in response to the skin injury. For injection of mASCs, a significant accumulation of Luciferase activity was apparent in the calvaria as early as 2 weeks postoperatively, remaining localized at up to 4 wks postoperatively (Fig. 2A, bottom row). This was in comparison to animals with sham incisions only, which showed some sparse accumulation at the incision site (Fig. 2A, top row). Luciferase activity was quantified using a standardized circular area encompassing the skull (Fig. 2C). A significant increase in Luciferase activity was observed in defects in comparison to skin incision only control at all timepoint (Fig. 2C). With hASCS, it took two weeks for the hASCs to migrate to the calvarial defect and the cells remained in the site of the defect up to four weeks in comparison to control (Fig 2B). Luciferase activity also was significantly higher than controls from day 14 to day 28 (Fig 2D). The final question, however, is if those cells actually participate in the osteogenesis at the defect site. GFP stain of the bony island of a nude mouse 4 weeks after injection of hASCs indeed demonstrates GFP staining (Fig. 2E, left). This is in comparison to a bony island from a direct application of non-GFP transfected ASCs which demonstrates no staining (Fig 2E, right). Thus, hASCs traffic to the defect, and more importantly, participate in osteogenesis of the defect at every time point assessed (p<0.05) using a Student’s t test with a Bonferroni correction.
Figure 2. ASC migration to mouse critical size cranial defects.
(A) IVIS imaging of Luciferase activity of mASC injected mice at stratified timepoints postoperatively (2 and 4 wks). Preferential migration to the calvaria was noted among animals with calvarial defects (defect), compared to those with sham skin incisions only (control). (B) IVIS imaging of Luciferase activity of hASC injected mice at stratified timepoints postoperatively (1 to 4 wks). Preferential migration to the calvaria was noted among animals with calvarial defects (defect), compared to those with sham skin incisions only (control). (C) Quantification of Luciferase activity in mASC injected mice in a standardized circular field encompassing the calvaria among sham skin incisions (control) or with calvarial defects (defect), n=5 per group (see color scale for intensity quantification). (D) Quantification of Luciferase activity in hASC injected mice in a standardized circular field encompassing the calvaria among sham skin incisions (control) or with calvarial defects (defect), n=5 per group (see color scale for intensity quantification). A Student’s t-test with a Bonferroni correction was used to assess significance, *p<0.05.
(E) GFP stain of bony island in the calvarial defect 4 weeks after hASCs injection. (Left) The bone in a GFP+ hASC injected mouse demonstrates staining whereas the negative control, non-GFP labeled hASCs does not stain for GFP (Right)
Effect of intravenously administered ASCs on mouse calvarial defect healing (micro Computed Tomography)
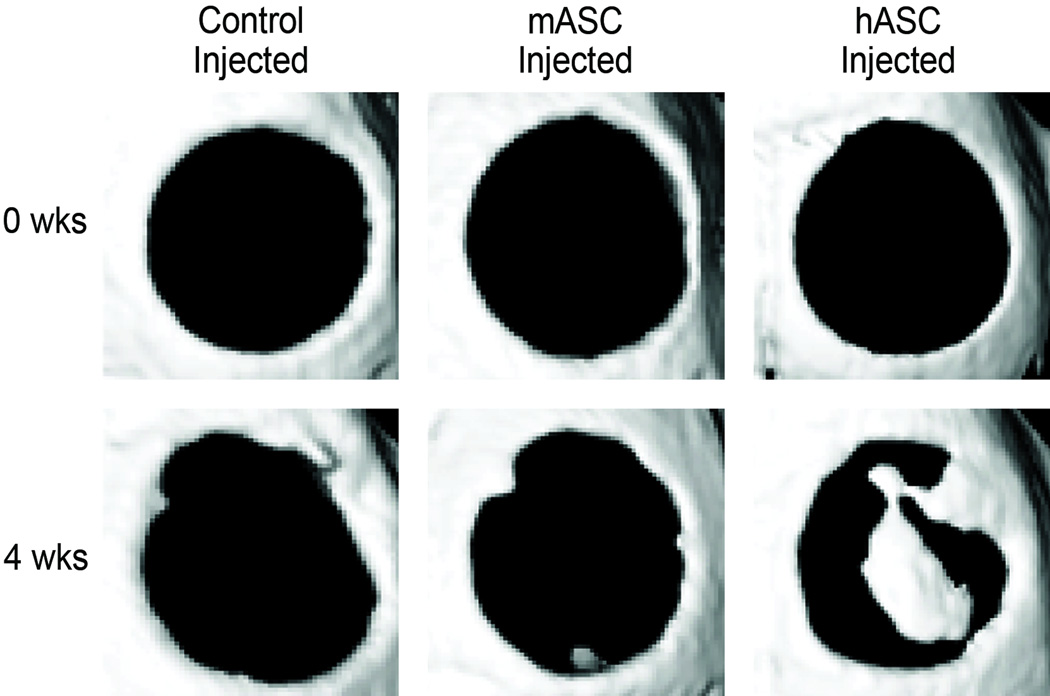
Next, calvarial healing was assessed by micro CT (Fig. 3). MicroCT images were obtained immediately postoperatively, verifying equal initial defect size among each group (Fig. 3A, top row). Consistent with a critical sized defect, little healing was observed among the control treated group (normal saline injected); only an approximate 7% healing was observed after four weeks (n=5, Fig. 3A,B). No difference in calvarial healing was observed among mASC injected animals (n=5, Fig. 3A,B). In marked contrast, a near 40% healing was observed among hASC injected animals (n=5, Fig. 3,A,B *P<0.05). Thus in summary, ASCs migrate to a defect site after calvarial injury. Their effect, however, on osseous healing was dependent on species of derivation: injected human ASCs significantly increased defect ossification whereas mouse ASCs did not.
Figure 3. ASC healing of mouse critical size cranial defects by microCT.
(A) Micro-computed tomography of defect sites at 0 and 4 wks postoperatively in representative calvaria (n=5 mice per group). Animals were injected with normal saline as a control, or an NS cell suspension of either 200,000 mASCs or 200,000 hASCs. (B) Quantification of healing by microCT utilizing Adobe Photoshop, as an average fraction bone formation of the average original defect size. A Student’s t-test with a Bonferroni correction was used to assess significance, *p<0.05.
Effect of intravenously administered ASCs on mouse calvarial defect healing (histology)
Detailed histologic analysis was next undertaken. Each calvarial defect was sectioned in entirety, generating approximately 100 slides. Every 10th section was stained with Aniline blue, to detect bone formation within the defect site (Fig. 4 A–C, top row). Images are taken at 10× magnification of the mid-defect. As expected of a critical size defect, a complete absence of bone was observed among defects treated with vehicle only (normal saline injected, Fig. 4 A). Bone nodules within the defect site were observed among IV mASC treated animals, although infrequently (Fig. 4B). Larger bone islands were present throughout most histologic sections of IV hASC treated animals (Fig. 4C). Photometric quantification of Aniline Blue positive bone within the defect site was performed using Adobe Photoshop, utilizing approximately 10 sections per defect (n=50 slides in total per group) (Fig 4D). Consistent with microCT evaluation, the intravenous injection of mASCs showed no difference in comparison to control treated mice (Fig. 4D). In contrast, an over 700% increase in Aniline blue staining was observed among hASC treated animals within the defect site (*P<0.05).
Figure 4. Histological analysis of defects after intravenous ASC administration.
(A) Histological appearance of defects treated after 4 wks postoperatively under control conditions, (Control Injected), (B) or treated with IV mASCs or (C) IV hASCs. Photomicrographs are at 10×. (Above) Aniline blue in which osteoid appears dark blue. (Below) Pentachrome in which bone appears bright yellow. (D) Quantification of Aniline Blue staining within the defect site after 4 wks healing.
Stains specific for osteoid and bone formation were performed to verify indeed that IV ASC treated animals, in contrast to controls, formed bone within a critical size cranial defect. Pentachrome staining was performed, demonstrating yellow bony tissue in those injected with mASC and hASC (Fig. 4 A–C, bottom row). Alkaline phosphatase staining was performed, in which the phosphatase activity turns histologic sections purple which was present only in those groups treated with ASCs (data not shown). Finally the anionic dye picrosirius red staining was performed, demonstrating that mature lamellar bone appears (green under polarized light) in the defect site of those treated with ASCs (data not shown). Thus in summary and utilizing multiple staining techniques specific for bone, we confirmed that control injected mice showed an essential absence of bone islands within the defect site. Among mouse ASC injected animals, infrequent bone nodule formation was detected, although this did not lead to significant bone formation. Among hASC injected, numerous bone islands were observed within the defect site, leading to marked bony healing.
DISCUSSION
This study extends prior observations regarding the intravenous administration of ASCs for regeneration and repair. We observed that ASCs, whether derived from mouse or human origin, migrated to sites of cranial injury. We then focused specifically on calvarial healing, utilizing a critical sized (or non-healing) 4mm defect. We found that ASCs traffic to a site of cranial surgical injury. Next, we found that IV administered human as compared to mouse ASCs led to significantly more bone within the calvarial defect site. These findings offer a distinct alternative to the more traditional and invasive, direct placement of ASCs in a skeletal injury site. Intravenous administration of cells alleviates the need to make an incision to perform a dissection to directly apply ASCs into a skeletal defect.
Our technique of transfecting hASCs with a luciferase GFP virus has significant scientific implications as it allows us to continuously monitor the viability of hASCs in vivo real time rather than having to sacrifice these animals at specific timepoints and relying on non-specific staining protocols. We believe these transfected cells will allow future studies to further delineate if the effect of hASCs is caused by them simply secreting cytokines or specifically playing a role in osteogenesis.
A significant gap exists between the current knowledge regarding ASC biology and their future translation to clinical use. First, the safety of hASCs must be determined. Pertinent to the use of intravenously injected cells, the potential for thrombus formation must be thoroughly investigated. In the current study, IVIS imaging suggested that even low numbers of IV ASCs produced subclinical and transient pulmonary migration, while larger doses led to numerous micro pulmonary emboli and death. Related studies have observed similar phenomenon in bone marrow mesenchymal cells (MSCs), coining the term ‘pulmonary passage’ as of major impedence in the use of intravenous stem cells 29–31.
Once the cells pass through the lungs, the question exists of whether they will traffic to an osseous defect. Along these lines, a related study previously demonstrated that systemically administered ASCs incorporate into the bones of developing mice 11. The authors hypothesized that the infused cells were exposed to a “bone microenvironment” and subsequently acquired “osteoblast-like properties.” Regardless the etiology, this finding brings to the fore exciting possibilities specific to our study.
An intuitive result in the present study was that IV injected human ASCs were observed to contribute to calvarial healing to a greater degree than mouse ASCs.
Numerous differences have been observed between mouse and human ASCs 32, 33. For example, mouse ASCs require a significant pro-osteogenic stimulus for significant in vitro osteogenic differentiation whereas human ASCs do not, whether this be retinoic acid, BMP, or Sonic Hedgehog 32, 34–36. In fact, human ASCs stain sporadically for bone nodules at one day of differentiation and robustly after one week in standard ODM 4. Given these in vitro differences in cellular behavior between mouse and human ASCs, the fact that hASCs demonstrated more healing by CT scan than mASCs is not surprising and most likely represents their differential capacities to undergo osteogenic differentiation. Of note, anytime when administering human cells into mice, an immunocompromised mouse must be used to avoid an inflammatory reaction. The mice used for human cell administration were Crl:CD-1-Foxn1nu, which are athymic and thus deficient in T cells, developed by nude gene transfer onto a CD-1 background. For mouse cell experiments, CD-1 wild-type mice were used. Importantly for our current experimental design, calvarial defect healing has been confirmed to be similar between athymic and wild-type mice 37. Moreover, relative cell migration was approximately equal between mouse and human experiments, suggesting that differences in immune mediated sequestration of mouse versus human cells did not introduce an artifactual difference in calvarial healing between mouse and human sources. Thus, though we expect different responses in a CD-1 nude and a CD-1 wild type mouse, we would not anticipate it would cause large differences in the osteogenic capabilies of injected ASCs. Furthermore, since we would forsee hASCs in the future to be harvested and reinjected into the same patient, we would not expect a large T-cell response. Future studies in large animals including non-human primates will be required to fully understand and translate our benchtop findings to the bedside. The findings in this study, recapitulate the potential clinical utility of human-derived ASCs in skeletal regeneration, and lie in contrast to previous studies using murine-derived ASCs 38.
This study has several potential clinical implications. First, clinically we can often reconstruct a calvarial defect with bone grafts, or alloplastic material and surgeons have begun to apply scaffolds implanted with cells to defect sites that are easily accessible. If, however, a surgeon is treating a defect that is difficult to expose such as that of the skull base, a scaffold and cell based treatment may not be an option. In such cases, surgeons would benefit from perfecting a mechanism to traffic cells to the defect such as intravenous administration. In a previous study we demonstrated that an hASCs seeded scaffold can heal a critical sized defect within four weeks.25 From a surgical standpoint, however, if a surgeon needs to take a patient back to the operating room to reconstruct a defect with a scaffold, the surgeon may be faced with a complicated and bloody dissection. We would hope that this study would translate into having the ability to forgo this dissection and simply injecting hASCs into the patient.
There may also exist a reconstruction that would benefit from both direct application of a scaffold seeded with hASCs along with adjuvant treatments with a stem cell type administered intravenously before or after the reconstruction to further augment osteogenesis of the defect site or scaffold placement site.
CONCLUSIONS
Intravenously administered ASCs migrate to sites of calvarial injury. Thereafter, IV hASCs contribute to bony calvarial repair. The intravenous administration of ASCs may be a safe and effective delivery method for future efforts in calvarial regeneration.
Supplementary Material
See figure, Supplemental Digital Content 1 which displays Intravenous ASC administration: (A–C)IVIS imaging after intravenously injected Luc+ mASCs, imaged at 2, 24 and 96hrs (n=5).
See figure, Supplemental Digital Content 2, which displays Quantification of luciferase activity in the lung fields at 2, 24 and 96 hours post-injection, presented as total photon emission in a standardized square area encompassing the thorax (n=5, *P<0.05). A Student’s t-test with a Bonferroni correction was used to assess significance.
See figure, supplemental Digital Content 3, which displays Intraperitoneal ASC administration: (A–C) IVIS imaging after intraperitoneal injected Luc+ mASCs, imaged at 2, 24 and 96 hrs (n=5).
ACKNOWLEDGEMENTS
We thank Mr. Greg Nelson in the Department of Veterinary Medicine, Stanford University for his excellent technical assistance and Mr. Deli Camara at the Plastic Surgery Center for his assistance in liposuction procedures.
Sources of Support:
This study was supported by National Institutes of Health, National Institute of Dental and Craniofacial Research grant 1 R21 DE019274-01 and RC2 DE020771-01, the Oak Foundation and Hagey Laboratory for Pediatric Regenerative Medicine and the National Endowment for Plastic Surgery to M.T.L. B.L was supported by the National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases grant 1F32AR057302-02.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement:
The authors above have no financial interest in any of the products, devices, procedures or anything else connected with the article. There was no internal or external funding received to complete this study.
University of Stanford IRB approval was obtained prior to commencement of the study (IRB # 2188, 9999, 8638).
REFERENCES
- 1.Kwan MD, Slater BJ, Wan DC, et al. Cell-based therapies for skeletal regenerative medicine. Hum Mol Genet. 2008;17:R93–R98. doi: 10.1093/hmg/ddn071. [DOI] [PubMed] [Google Scholar]
- 2.De Ugarte DA, Morizono K, Elbarbary A, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174:101–109. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- 3.Levi B, James AW, Wan DC, et al. Regulation of human adipose-derived stromal cell osteogenic differentiation by insulin-like growth factor-1 and platelet-derived growth factor-alpha. Plast Reconstr Surg. 126:41–52. doi: 10.1097/PRS.0b013e3181da8858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee J, Gupta D, Panetta NJ, et al. Elucidating Mechanisms of Osteogenesis in Human Adipose-Derived Stromal Cells via Microarray Analysis. J Craniofac Surg. doi: 10.1097/SCS.0b013e3181e488d6. [DOI] [PubMed] [Google Scholar]
- 5.Cowan CM, Shi YY, Aalami OO, et al. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004;22:560–567. doi: 10.1038/nbt958. [DOI] [PubMed] [Google Scholar]
- 6.Levi B, James AW, Nelson ER, et al. Human adipose derived stromal cells heal critical size mouse calvarial defects. PLoS ONE. 2010 doi: 10.1371/journal.pone.0011177. Accepted for Publication, May 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lendeckel S, Jodicke A, Christophis P, et al. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: case report. J Craniomaxillofac Surg. 2004;32:370–373. doi: 10.1016/j.jcms.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Mesimaki K, Lindroos B, Tornwall J, et al. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. International journal of oral and maxillofacial surgery. 2009;38:201–209. doi: 10.1016/j.ijom.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Kulakov AA, Goldshtein DV, Grigoryan AS, et al. Clinical study of the efficiency of combined cell transplant on the basis of multipotent mesenchymal stromal adipose tissue cells in patients with pronounced deficit of the maxillary and mandibulary bone tissue. Bulletin of experimental biology and medicine. 2008;146:522–525. doi: 10.1007/s10517-009-0322-8. [DOI] [PubMed] [Google Scholar]
- 10.Mao JJ, Giannobile WV, Helms JA, et al. Craniofacial tissue engineering by stem cells. Journal of dental research. 2006;85:966–979. doi: 10.1177/154405910608501101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liao X, Li F, Wang X, et al. Distribution of murine adipose-derived mesenchymal stem cells in vivo following transplantation in developing mice. Stem Cells Dev. 2008;17:303–314. doi: 10.1089/scd.2007.0086. [DOI] [PubMed] [Google Scholar]
- 12.Kim DH, Je CM, Sin JY, et al. Effect of partial hepatectomy on in vivo engraftment after intravenous administration of human adipose tissue stromal cells in mouse. Microsurgery. 2003;23:424–431. doi: 10.1002/micr.10178. [DOI] [PubMed] [Google Scholar]
- 13.Vilalta M, Degano IR, Bago J, et al. Biodistribution, long-term survival, and safety of human adipose tissue-derived mesenchymal stem cells transplanted in nude mice by high sensitivity non-invasive bioluminescence imaging. Stem Cells Dev. 2008;17:993–1003. doi: 10.1089/scd.2007.0201. [DOI] [PubMed] [Google Scholar]
- 14.Kim U, Shin DG, Park JS, et al. Homing of adipose-derived stem cells to radiofrequency catheter ablated canine atrium and differentiation into cardiomyocyte-like cells. International journal of cardiology. 2009 doi: 10.1016/j.ijcard.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 15.Miranville A, Heeschen C, Sengenes C, et al. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349–355. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- 16.Kim YM, Choi YS, Choi JW, et al. Effects of systemic transplantation of adipose tissue-derived stem cells on olfactory epithelium regeneration. The Laryngoscope. 2009;119:993–999. doi: 10.1002/lary.20187. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez-Rey E, Anderson P, Gonzalez MA, et al. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58:929–939. doi: 10.1136/gut.2008.168534. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez MA, Gonzalez-Rey E, Rico L, et al. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009;136:978–989. doi: 10.1053/j.gastro.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 19.Mizuno H. The Potential for Treatment of Skeletal Muscle Disorders with Adipose-Derived Stem Cells. Current stem cell research & therapy. 2009 doi: 10.2174/157488810791268573. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez MA, Gonzalez-Rey E, Rico L, et al. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum. 2009;60:1006–1019. doi: 10.1002/art.24405. [DOI] [PubMed] [Google Scholar]
- 21.Constantin G, Marconi S, Rossi B, et al. Adipose-derived mesenchymal stem cells ameliorate chronic experimental autoimmune encephalomyelitis. Stem Cells. 2009;27:2624–2635. doi: 10.1002/stem.194. [DOI] [PubMed] [Google Scholar]
- 22.Fang B, Song YP, Li N, et al. Resolution of refractory chronic autoimmune thrombocytopenic purpura following mesenchymal stem cell transplantation: a case report. Transplant Proc. 2009;41:1827–1830. doi: 10.1016/j.transproceed.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 23.Wall ME, Bernacki SH, Loboa EG. Effects of serial passaging on the adipogenic and osteogenic differentiation potential of adipose-derived human mesenchymal stem cells. Tissue Eng. 2007;13:1291–1298. doi: 10.1089/ten.2006.0275. [DOI] [PubMed] [Google Scholar]
- 24.Quarto N, Longaker MT. FGF-2 inhibits osteogenesis in mouse adipose tissue-derived stromal cells and sustains their proliferative and osteogenic potential state. Tissue Eng. 2006;12:1405–1418. doi: 10.1089/ten.2006.12.1405. [DOI] [PubMed] [Google Scholar]
- 25.Levi B, James AW, Nelson ER, et al. Human adipose derived stromal cells heal critical size mouse calvarial defects. PLoS One. 5 doi: 10.1371/journal.pone.0011177. e11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.James AW, Theologis AA, Brugmann SA, et al. Estrogen/estrogen receptor alpha signaling in mouse posterofrontal cranial suture fusion. PLoS One. 2009;4 doi: 10.1371/journal.pone.0007120. e7120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu Y, Hammerick KE, James AW, et al. Inhibition of histone deacetylase activity in reduced oxygen environment enhances the osteogenesis of mouse adipose-derived stromal cells. Tissue Eng Part A. 2009;15:3697–3707. doi: 10.1089/ten.tea.2009.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.James AW, Leucht P, Levi B, et al. Sonic Hedgehog influences the balance of osteogenesis and adipogenesis in mouse adipose-derived stromal cells. Tissue Eng Part A. doi: 10.1089/ten.tea.2010.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fischer UM, Harting MT, Jimenez F, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009;18:683–692. doi: 10.1089/scd.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schrepfer S, Deuse T, Reichenspurner H, et al. Stem cell transplantation: the lung barrier. Transplant Proc. 2007;39:573–576. doi: 10.1016/j.transproceed.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 31.Furlani D, Ugurlucan M, Ong L, et al. Is the intravascular administration of mesenchymal stem cells safe? Mesenchymal stem cells and intravital microscopy. Microvasc Res. 2009;77:370–376. doi: 10.1016/j.mvr.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Quarto N, Longaker MT. Differential expression of specific FGF ligands and receptor isoforms during osteogenic differentiation of mouse Adipose-derived Stem Cells (mASCs) recapitulates the in vivo osteogenic pattern. Gene. 2008;424:130–140. doi: 10.1016/j.gene.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 33.Levi B, James AW, Xu Y, et al. Divergent modulation of adipose-derived stromal cell differentiation by TGF-beta1 based on species of derivation. Plast Reconstr Surg. 2010 doi: 10.1097/PRS.0b013e3181df64dc. Accepted for publication. [DOI] [PubMed] [Google Scholar]
- 34.Skillington J, Choy L, Derynck R. Bone morphogenetic protein and retinoic acid signaling cooperate to induce osteoblast differentiation of preadipocytes. J Cell Biol. 2002;159:135–146. doi: 10.1083/jcb.200204060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malladi P, Xu Y, Yang GP, et al. Functions of Vitamin D, Retinoic Acid, and Dexamethasone on Mouse Adipose-Derived Mesenchymal Cells (AMCs) Tissue Eng. 2006;12:2031–2040. doi: 10.1089/ten.2006.12.2031. [DOI] [PubMed] [Google Scholar]
- 36.Malladi P, Xu Y, Chiou M, et al. Effect of reduced oxygen tension on chondrogenesis and osteogenesis in adipose-derived mesenchymal cells. Am J Physiol Cell Physiol. 2006;290:C1139–C1146. doi: 10.1152/ajpcell.00415.2005. [DOI] [PubMed] [Google Scholar]
- 37.Gupta DM, Kwan MD, Slater BJ, et al. Applications of an athymic nude mouse model of nonhealing critical-sized calvarial defects. J Craniofac Surg. 2008;19:192–197. doi: 10.1097/scs.0b013e31815c93b7. [DOI] [PubMed] [Google Scholar]
- 38.Yoshimura H, Muneta T, Nimura A, et al. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007;327:449–462. doi: 10.1007/s00441-006-0308-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See figure, Supplemental Digital Content 1 which displays Intravenous ASC administration: (A–C)IVIS imaging after intravenously injected Luc+ mASCs, imaged at 2, 24 and 96hrs (n=5).
See figure, Supplemental Digital Content 2, which displays Quantification of luciferase activity in the lung fields at 2, 24 and 96 hours post-injection, presented as total photon emission in a standardized square area encompassing the thorax (n=5, *P<0.05). A Student’s t-test with a Bonferroni correction was used to assess significance.
See figure, supplemental Digital Content 3, which displays Intraperitoneal ASC administration: (A–C) IVIS imaging after intraperitoneal injected Luc+ mASCs, imaged at 2, 24 and 96 hrs (n=5).