Abstract
In the CNS, the hypothalamic arcuate nucleus (ARN) energy-balance circuit plays a key role in regulating body weight. Recent studies have shown that neurogenesis occurs in the adult hypothalamus, revealing that the ARN energy-balance circuit is more plastic than originally believed. Changes in diet result in altered gene expression and neuronal activity in the ARN, some of which may reflect hypothalamic plasticity. To explore this possibility, we examined the turnover of hypothalamic neurons in mice with obesity secondary to either high-fat diet (HFD) consumption or leptin deficiency. We found substantial turnover of neurons in the ARN that resulted in ongoing cellular remodeling. Feeding mice HFD suppressed neurogenesis, as demonstrated by the observation that these mice both generated fewer new neurons and retained more old neurons. This suppression of neuronal turnover was associated with increased apoptosis of newborn neurons. Leptin-deficient mice also generated fewer new neurons, an observation that was explained in part by a loss of hypothalamic neural stem cells. These data demonstrate that there is substantial postnatal turnover of the arcuate neuronal circuitry in the mouse and reveal the unexpected capacity of diet and leptin deficiency to inhibit this neuronal remodeling. This insight has important implications for our understanding of nutritional regulation of energy balance and brain function.
Introduction
The hypothalamus is a critical regulator of energy balance. Lesions of the ventromedial nucleus (1) or arcuate nucleus (ARN) (2) result in obesity, while lesions of the lateral hypothalamic area (3) result in weight loss. Energy-related signals such as leptin (4, 5) are integrated by responsive neurons in the ARN and ventromedial hypothalamus. In particular, leptin stimulates the anorexigenic proopiomelanocortin (POMC) neurons and inhibits orexigenic neuropeptide Y (NPY) neurons. In situations of negative energy balance, reduced leptin levels inhibit the activity of POMC neurons and activate NPY neurons. While this feedback circuit is essential for normal energy homeostasis (see review refs. 6, 7), most obesity is associated with dysregulation of the circuit and leptin resistance (8).
Two commonly studied mouse models of obesity, the high-fat diet–induced (HFD-induced) obesity (DIO) model (9) and the more severe obesity arising from the lack of leptin (4, 5), share features such as increased fat mass, hyperglycemia, hyperinsulinemia, and activation of the adrenal axis but are distinct in that DIO mice have elevated peripheral leptin levels and are resistant to both endogenous and exogenous leptin (8). DIO mice also become resistant to centrally administered leptin (10). This phenomenon is reversible, with leptin responsiveness returning several months after cessation of HFD consumption (11).
The molecular and cellular mechanisms underlying hypothalamic dysfunction in the context of obesity over long-time frames are still not fully understood. Possible mechanisms include impaired leptin transport across the blood brain barrier (10, 12–14), endoplasmic reticulum stress (15), and defects in the leptin receptor signaling pathway (16–18). In addition, we and others have shown that neurogenesis, the generation of neurons, occurs in adult hypothalamus (19, 20).
Our recent work showed that i.c.v. infusion of the growth factor CNTF in DIO mice results in both enhanced hypothalamic neurogenesis and long-term body weight loss (19). Hence, it might be the case that neurons that compose the energy-balance circuit are themselves differentially remodeled during the course of obesity. Here we investigate hypothalamic neuronal turnover in mice, comparing those fed a standard diet with DIO mice. We found that ARN neurons involved in energy-balance regulation exhibited substantial turnover in the adult mouse, with more than half of those neurons being replaced between 4 and 12 weeks of age. Surprisingly, this remodeling of hypothalamic neurons is suppressed in DIO, in part due to increased apoptosis of the newly divided cells. Furthermore, this effect of HFD can be reversed by a short period of calorie restriction. Like DIO mice, genetically obese mice lacking leptin (ob/ob mice) also have reduced hypothalamic neurogenesis. However, unlike DIO mice, leptin-deficient mice lack hypothalamic neural stem cells. This is consistent with our observation that leptin stimulates neural stem cell expansion in vivo. These observations provide evidence for an unexpected degree of dynamic remodeling of the hypothalamic neuronal circuitry, its regulation by diet, its failure in DIO, and the role of the adipokine leptin.
Results
The hypothalamic energy-balance circuit is dynamically remodeled in the adult mouse.
We have recently shown that neurogenesis occurs in the adult mouse hypothalamus (19, 21). Since the generation of new neurons is not accompanied by an expansion of the hypothalamus with age, neuronal turnover is likely to occur to maintain the integrity of the tissue. To evaluate the postnatal turnover of neurons in the hypothalamic ARN, we developed a strategy to label neurons born during embryogenesis and then to quantify those remaining in adulthood.
During development, most ARN neurons are generated between E10.5 and E12.5 (22, 23). Energy-balance neurons were the earliest born, with substantial numbers present at E10.5 that express the neuropeptide POMC (Figure 1A) shortly after cell-cycle exit (22, 23). These immature POMC+ neurons do not solely become mature POMC+ neurons but also give rise to mature NPY+ neurons (24). We confirmed our previous findings that embryonic generation of these energy-balance neurons occurred around E10.5 by counting the number of POMC+ immature ARN neurons in developing embryos within the E10.5 gestational window (n = 6). The mean number of POMC neurons present was 1,032 ± 202, ranging from 544 POMC+ neurons in the most immature embryo to 1,780 neurons in the most mature embryo (Figure 1A and data not shown). By E12.5, the number of immature POMC neurons had risen to 3,039 ± 154 POMC+ neurons (n = 6), similar to that present in the adult (22, 25). These data indicate that the E10.5 gestational window coincides with the main phase of energy-balance neuron generation.
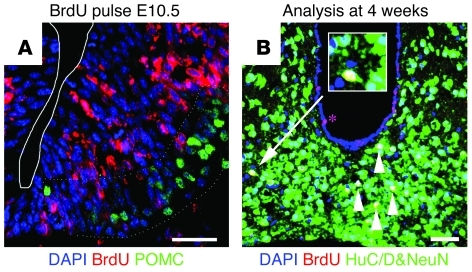
Figure 1. BrdU administration at E10.5 labels embryo-born neurons in the adult hypothalamus.
(A) Coronal sections of ARN at E10.5, showing proliferative neural stem cells (labeled with BrdU [red]) within the hypothalamic neuroepithelium giving rise to energy-balance neurons (POMC+ [green] counterstained with DAPI [blue]). (B) By 4 weeks of age, all strongly BrdU-labeled (red) cells within the parenchyma (white arrowheads) had differentiated into neurons (NeuN+HuC/D+ [green] counterstained with DAPI [blue]). The inset shows a high-magnification view of a labeled ARN neuron. Rare ependymal glia retain strong BrdU label (asterisk) and may represent adult neural stem cells. Scale bar: 50 μm. Original magnification, ×2 (inset).
To label a sample of energy-balance neurons across this window, FVB female mice were time mated with male mice expressing humanized Renilla green fluorescent protein from the Npy promoter (NPY-hrGFP mice) (FVB background), and the resulting embryos were treated with BrdU at E10.5 to label the neural progenitors giving rise to the ARN neurons (22). At 4 weeks of age, the resulting mice were sacrificed, and BrdU+ cells were revealed by immunofluorescence.
Using WT mice, BrdU+ ARN cells were exclusively neurons, as revealed by their immunoreactivity for the neuronal markers HuC/D+ and NeuN+ (Figure 1B). Next, to quantify adult ARN neuronal turnover, we assayed the replacement of these BrdU+ neurons by new unlabeled neurons between 4 weeks and 12 to 26 weeks of age (Figure 2). Between 4 and 12 weeks, there was no change in the total number of cells within the ARN (DAPI staining in Figure 2, A–D; P = 0.25). However, the number of embryo-born BrdU+ neurons was reduced by 63% (P = 0.0013). There was no further depletion of BrdU+ neurons between 12 and 26 weeks (P = 0.9), although the total number of ARN cells decreased by 23% (P = 0.0003). Similar data was obtained when the number of BrdU+ neurons was calculated as a percentage of ARN cells at each stage (data not shown).
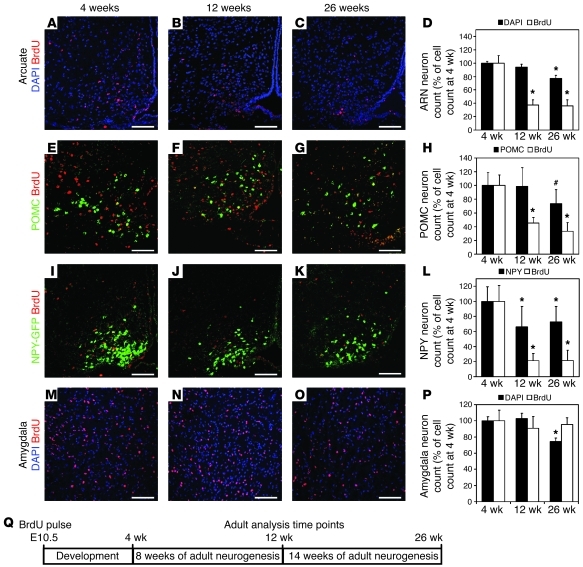
Figure 2. ARN energy-balance neurons are turned over by ongoing neurogenesis.
(A–C) Nuclei labeled with BrdU (red) at E10.5 remaining in the ARN at 4, 12, and 26 weeks, counterstained with DAPI (blue). (D) Quantification of ARN neuron survival, showing that labeled ARN neurons were lost between 4 and 12 weeks of age but not between 12 and 26 weeks of age. (E–G) POMC neurons (green) labeled with BrdU (red) at E10.5 remaining in the ARN at 4, 12, and 26 weeks. (H) Quantification of POMC neuron survival, showing that labeled POMC neurons were lost between 4 and 12 weeks of age but not between 12 and 26 weeks of age. (I–K) NPY neurons (green) labeled with BrdU (red) at E10.5 remaining in the ARN at 4, 12, and 26 weeks. We made use of NPY-hrGFP mice to reveal NPY neurons. (L) Quantification of NPY neuron survival, showing that labeled NPY neurons were lost between 4 and 12 weeks of age but not between 12 and 26 weeks of age. (M–O) Nuclei labeled with BrdU (red) at E10.5 remaining in the amygdala at 4, 12, and 26 weeks, counterstained with DAPI (blue). (P) Quantification of amygdala neuron survival, showing that labeled amygdala neurons were not lost between 4 and 12 weeks of age or between 12 and 26 weeks of age. (Columns indicate total cell counts as a percentage of cell count at 4 weeks.) *P < 0.05 compared with 4 weeks; #P < 0.05 compared with 12 weeks. (Q) Schematic of analysis. Mean ± SEM; n = 10–19 at 4 weeks, 7–11 at 12 weeks, 4–9 at 26 weeks. Scale bar: 100 μm.
POMC neurons were turned over at a similar rate as those in the ARN as a whole (compare black bars in Figure 2, D and H). The number of BrdU+ POMC neurons was reduced by 55% between 4 and 12 weeks (P = 0.0196), with no further reduction between 12 and 26 weeks (P = 0.2; Figure 2, E–H). Likewise, the number of BrdU+ POMC neurons as a proportion of the total POMC population decreased from 9.9% at 4 weeks to 4.5% at 12 weeks (P = 0.0051) with no further change by 26 weeks (P = 0.4, data not shown).
Using NPY-hrGFP, the turnover of NPY neurons was assessed. Compared with that of POMC neurons, the total population of NPY neurons decreased to a lesser extent, by 34% (P = 0.0059) between 4 weeks and 12 weeks, with no further reduction by 26 weeks (P = 0.6; Figure 2, I–L). Similar to POMC neurons, however, the number of BrdU+ NPY neurons initially decreased by 79% between 4 and 12 weeks (P = 0.0094), with no further depletion between 12 and 26 weeks (P = 0.9). At 4 weeks, BrdU+ NPY neurons represented 4.6% of the total NPY population, while this number decreased to 1.4% by 12 weeks (P = 0.0147), with no further depletion by 26 weeks (P = 0.9; data not shown).
The turnover of ARN neurons between 4 and 12 weeks of age was confirmed in a second cohort of mice using WT C57BL/6 mice, which received 3 BrdU pulses, one each at E10.5, E11.5, and E12.5 (n = 4 each stage, data not shown). Like E10.5 labeled FVB mice, E10.5–E12.5 labeled C57BL/6 mice also showed a loss of labeled ARN neurons, with 51% of labeled neurons remaining at 12 weeks compared with those at 4 weeks (P = 0.0190; data not shown). At 4 weeks, BrdU+ neurons represented 11.9% of the total ARN population decreasing to 6.9% by 12 weeks (P = 0.0184; data not shown). This confirms that remodeling is not limited to the FVB strain or the E10.5 labeling window. Together, these data unexpectedly reveal that more than half of the POMC and NPY neurons present at 4 weeks of age are replaced by ongoing neurogenesis during the following 8 weeks. This represents a substantial and unanticipated remodeling of the energy-balance circuit in adulthood.
Although few forebrain neurons are generated at E10.5 outside the hypothalamus, a distinct population of neural progenitor cells within the amygdala was found to have entered the cell cycle by BrdU staining at E10.5 (data not shown). However, in contrast to that in the ARN, there was no significant variation in the number of BrdU+ amygdala neurons between 4 and 12 weeks (P = 0.3) or 12 and 26 weeks (P = 0.9; Figure 2, M–P), suggesting that postnatal neuronal turnover, as seen in the ARN, is not found uniformly in the brain but only in discrete regions.
DIO leads to decreased hypothalamic neurogenesis in the adult.
Our previous results indicate that increasing hypothalamic neurogenesis via CNTF treatment leads to a long-term reduction in body weight (19). We, therefore, then decided to investigate whether DIO has an effect on hypothalamic neurogenesis in the adult mouse.
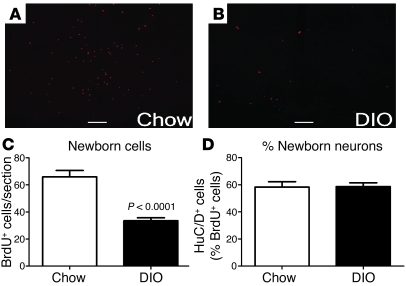
The generation of new cells in the adult hypothalamus was evaluated by i.c.v. infusion of BrdU in 16-week-old DIO mice compared with that in chow-fed controls (Supplemental Table 1; supplemental material available online with this article; doi: 10.1172/JCI43134DS1). Mice were sacrificed 4 weeks after infusion, and BrdU+ cells were then detected by immunofluorescence. The number of newborn BrdU+ cells in adult ARN was substantial in mice fed chow: an average of 66 ± 5 BrdU+ cells were revealed in each 12-μm section. Strikingly, in DIO mice, the number of BrdU+ cells was half of that seen in chow-fed mice (P = 0.0001; Figure 3, A–C). The proportion of BrdU+ cells that differentiated into neurons (revealed by the neuronal marker HuC/D+) was similar between DIO and control mice (Figure 3D). Together, these data indicate that DIO mice have a decrease in the number of newly generated cells in the ARN, including neurons.
Figure 3. DIO inhibits adult hypothalamic neurogenesis.
Sixteen-week-old mice were i.c.v. infused with BrdU for 7 days and harvested 4 weeks later. (A–C) The number of newborn (BrdU-labeled) cells, including neurons, was significantly reduced in the hypothalamus of DIO mice compared with that in lean controls. (D) However, there was no difference in the percentage of cells adopting a neuronal fate between the 2 groups, as indicated by an equal proportion of BrdU-labeled cells adopting a HuC/D+ fate. Scale bar: 100 μm. Data are mean ± SEM. n = 5 chow; n = 6 DIO.
DIO leads to the depletion of actively proliferating progenitor-like cells in the hypothalamus.
In the adult CNS, neurons are generated from precursor glial cells. A simplified view of the multistep process leading to generation of neurons is that slowly proliferating multipotent stem cells give rise to highly proliferative progenitor cells that have limited capacity for self-renewal (neuroblasts) and ultimately give rise to neurons (for reviews, see refs. 26, 27). Stem-like cells capable of generating multipotent neurospheres ex vivo are present in the hypothalamus (28). Likewise, we have previously shown that highly proliferative progenitor cells are present in the hypothalamus and give rise to neurons (19, 21). Therefore, the decreased neurogenesis observed in the hypothalamus of DIO mice may result from decreased availability of neural stem-like cells and/or of highly proliferative progenitor-like cells.
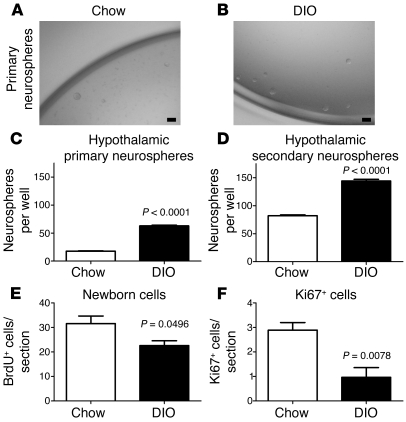
To investigate the cause of reduced adult neurogenesis in the context of DIO, we first examined the presence of hypothalamic neural stem-like cells in 16-week-old DIO mice using a neurosphere assay (28). The ventricular lining of the hypothalamus was dissociated and grown in serum-free conditions containing EGF and bFGF. Neurospheres derived from DIO mice were slightly larger than those from lean controls (diameter: lean, 0.47 ± 0.02 mm [n = 100] vs. DIO, 0.55 ± 0.02 mm [n = 100]; P = 0.0137; Figure 4, A and B). Unexpectedly, the hypothalamus of DIO mice generated 3.5 times more neurospheres per 2,000 cells plated than that of lean controls (P = 0.0002; Figure 4C). In addition, the number of DIO-derived neurospheres after 1 passage remained higher than that in lean controls (P = 0.0008; Figure 4D). These data indicate that DIO does not result in the loss of hypothalamic neural stem-like cells.
Figure 4. Obese mice do not lack hypothalamic stem cells but have a reduced number of actively proliferating cells.
The number of hypothalamic neural stem/progenitor cells in 16-week-old mice was examined using the neurosphere assay. (A and B) Neurospheres derived from DIO mice were of a similar size and appearance as those derived from lean controls. (C) However, DIO mice contain a higher number of hypothalamic neurosphere-forming cells than lean controls (n = 4 mice each; 2,000 cells plated per well). (D) Neurospheres from both DIO mice and lean controls expanded after passage. However, a greater number of secondary neurospheres were formed in DIO mice than in lean controls. (E) Sixteen-week-old mice were i.p. injected with BrdU and harvested 48 hours later. The number of newborn BrdU+ cells was significantly decreased in DIO mice compared with that in lean controls, although this decrease appeared mild compared with the loss observed 4 weeks after BrdU administration (see Figure 1). (F) This decrease in BrdU-labeled cells was mirrored by a reduction in Ki67-expressing glia in DIO mice compared with that in lean controls. Data are mean ± SEM. n = 4–7 chow; n = 4–5 DIO. Scale bar: 1 mm.
To then evaluate the number of highly proliferative progenitor-like cells, mice on chow or HFD were repeatedly injected with BrdU (i.p.) over a day to cumulatively label cells in S-phase. Mice were sacrificed 48 hours after the first injection. The number of hypothalamic BrdU+ cells was significantly reduced in DIO mice compared with that in lean controls (P = 0.0496; Figure 4E), indicating a lower number of highly proliferative progenitor-like cells in DIO mice. This result was confirmed by immunohistochemistry against the proliferating cell markers Ki67 and proliferating cell nuclear antigen (PCNA) (P = 0.0078 for Ki67, Figure 4F; P = 0.0089 for PCNA, data not shown).
All together, these results indicate that DIO leads to an expansion of the pool of hypothalamic neural stem-like cells while leading to a depletion of the pool of highly proliferative progenitor-like cells. This suggests that DIO inhibits the differentiation of stem-like cells into more proliferative progenitor-like cells and/or impairs the survival of these progenitor-like cells.
DIO selectively increases apoptosis of newly divided cells.
Interestingly, the number of hypothalamic BrdU+ cells in DIO mice was only reduced by 28% compared with that in control mice at 48 hours after labeling (Figure 4E), while it was reduced by 50% at 4 weeks after labeling (Figure 3C). This discrepancy suggests that many newborn cells are lost after S-phase in the hypothalamus of DIO mice.
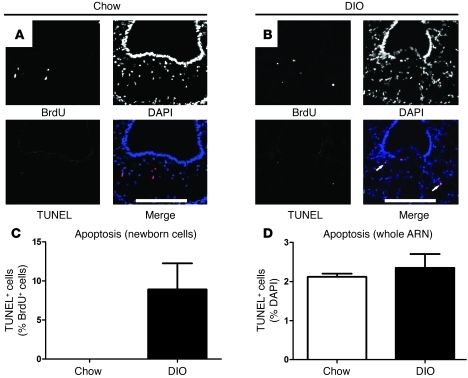
To investigate the loss of these newborn cells, the rate of apoptosis was determined using the TUNEL method at 48 hours after BrdU labeling. In lean controls, no TUNEL+BrdU+ cells could be detected, whereas 8.9% ± 3.3% of BrdU+ cells were TUNEL+ in DIO mice (Figure 5, A–C). Although, the apoptosis of newborn cells was increased, there was no overall difference in the rate of apoptosis in the hypothalamus of DIO mice (lean, 2.1% ± 0.1% vs. DIO, 2.4% ± 0.4%; P = 0.54; Figure 5D). This result indicates that the loss of newborn neurons in DIO mice is due in part to the selectively increased apoptosis of newly divided cells.
Figure 5. DIO results in the apoptosis of newly dividing cells.
Proliferating cells in 16-week-old DIO mice and lean controls were cumulatively labeled with 5 i.p. injections of BrdU and assayed for apoptosis 48 hours later using the TUNEL method. (A–C) Unlike lean mice in which no newly divided cells were apoptotic, several newly divided cells were apoptotic in DIO mice, indicating that the failure of hypothalamic neurogenesis is partially due to the apoptosis of newborn cells. (D) There was no difference in the overall rate of apoptosis in the ARN between DIO mice and lean controls. Data are mean ± SEM. n = 5 chow; n = 5 DIO. Scale bar: 100 μm.
A short-term period of calorie restriction rescues DIO-impaired hypothalamic neurogenesis.
In order to investigate the permanence of the neurogenic defects seen in DIO mice, 16-week-old DIO mice were calorie restricted to 70% of the normal intake of chow-fed mice. After 4 weeks of restriction, body weight (32.5 ± 0.8 g) had reduced to similar levels as those of age-matched chow-fed mice (34.2 ± 0.6 g; P = 0.1). A second cohort of DIO mice was maintained on ad libitum HFD as a control group (45.3 ± 1.5 g). The availability of hypothalamic neural stem-like cells was examined using the neurosphere assay, while the number of actively proliferating progenitor-like cells was evaluated in vivo with repeated BrdU injections followed by sacrifice 48 hours later, as described above.
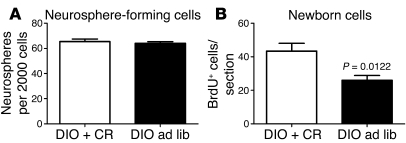
After calorie restriction, the number of hypothalamic neurosphere-forming cells (neural stem-like cells) was similar to that obtained with DIO control mice (P = 0.84; Figure 6A). In contrast, the number of actively proliferating progenitor-like cells was increased by 69% after calorie restriction, as measured by in vivo BrdU incorporation (P = 0.0122; Figure 6B).
Figure 6. Calorie restriction partially restores neurogenesis in DIO mice.
Sixteen-week-old DIO mice were either maintained on HFD or calorie restricted. (A) Four weeks of calorie restriction did not affect the number of hypothalamic neurosphere-forming cells observed with DIO mice. (B) However, calorie restriction restored the proliferation of neuronal progenitor cells in DIO mice. Data are mean ± SEM. n = 3–5 chow; n = 4–5 DIO. ad lib, ad libitum; CR, calorie restriction.
All together, these results suggest that the loss of highly proliferative progenitor-like cells caused by DIO is a dynamic phenotype that can be rescued by calorie restriction, while the DIO-induced expansion of the hypothalamic stem-like cell pool may be a more long-lasting phenotype. This supports the view that the loss of the newborn progenitor cells occurs via a process that is distinct from that causing an increase in the number of hypothalamic stem-like cells.
DIO alters the dynamic remodeling of the hypothalamic energy-balance circuit and leads to a relative ageing of the neuronal population.
Since hypothalamic neurogenesis is altered by DIO with a selective loss of newborn cells, but the overall rate of cell death remains unchanged compared with that of lean controls, we investigated whether the survival of old neurons is increased in DIO to compensate for the reduced generation of new neurons, in order to maintain hypothalamic integrity. C57BL/6 mouse embryos were labeled with BrdU at E10.5 as described above, weaned on chow, and were either given a HFD at 6 weeks of age or kept on chow until sacrifice at 16 weeks of age (Figure 7). The retention of the neurons born during embryogenesis was then examined by BrdU immunofluorescence detection.
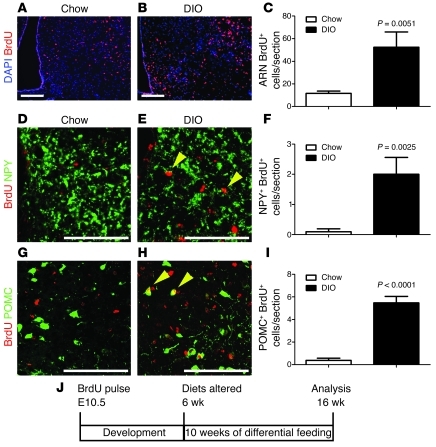
Figure 7. HFD feeding inhibits remodeling of the ARN energy-balance circuit.
ARN neurons were labeled with BrdU during embryogenesis. Mice were divided into a HFD-fed and a chow-fed cohort at 6 weeks, and BrdU-labeled embryonic neurons were assayed after 10 weeks of differential feeding. (A–C) In mice continually fed a chow diet, few labeled ARN neurons (BrdU [red] and DAPI [blue]) remained at 16 weeks, but a significantly greater number of labeled ARN neurons remained in littermates placed on HFD at 6 weeks of age. (D–F) A similar retention of labeled NPY neurons (yellow arrowheads) was found in HFD-fed mice (BrdU [red] and NPY [green]). (G–I) A similar retention of labeled POMC neurons (yellow arrowheads) was found in HFD-fed mice (BrdU [red] and POMC [green]). (J) Schematic of analysis. Mean ± SEM. Scale bar: 100 μm.
At 16 weeks, the number of BrdU+ ARN cells was greater in DIO mice than that in chow-fed controls (Figure 7, A–C). This indicates that DIO reduces the normal postnatal depletion of old ARN neurons. Although the number of BrdU+ neurons was increased, there was no difference in the total number of ARN cells between DIO and control mice (P = 0.13; data not shown), suggesting that the increased retention of old neurons in DIO is balanced by the decreased generation of new neurons. Likewise, the survival of embryo-born, specific energy-balance neurons was enhanced by DIO, with an increase in both the number of BrdU+NPY+ neurons (Figure 7, D–F) and BrdU+POMC+ neurons (Figure 7, G–I) remaining in DIO mice compared with those in lean controls. Together, these results suggest that neurons forming the energy-balance circuit in the hypothalamus of DIO mice are older than those of control mice.
Leptin is required for maintenance of hypothalamic stem cells.
To investigate whether the loss of hypothalamic neurogenesis occurs in another model of obesity with distinct molecular etiology, we examined neurogenesis and neural stem cell biology in a genetic model of obesity lacking leptin, the ob/ob mouse.
The generation of new cells in the adult hypothalamus was evaluated by i.c.v. infusion of BrdU in 16-week-old ob/ob mice compared with that in lean controls. Mice were sacrificed 4 weeks after infusion, and BrdU+ cells were then detected by immunofluorescence. The number of newborn BrdU+ cells in adult ARN was severely reduced in ob/ob mice compared with that in WT controls (n = 5 vs. n = 5; P = 0.0005; Figure 8, A–C). The proportion of BrdU+ cells that differentiated into neurons (revealed by the neuronal marker HuC/D+) was similar between ob/ob and lean controls (Figure 8D). This indicates that leptin-deficient mice have a severely reduced neurogenesis, more extensive than that seen in DIO mice.
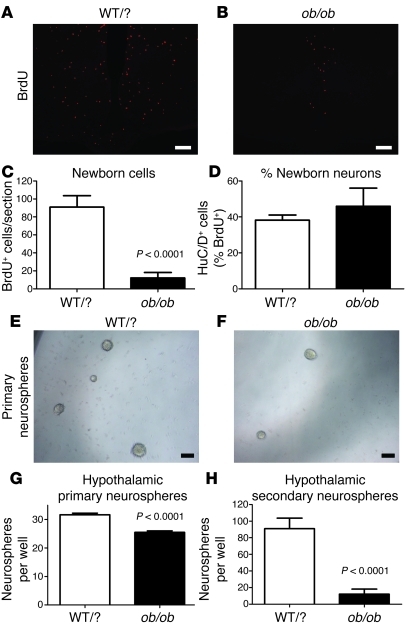
Figure 8. Loss of neural stem cells and neurogenesis in mice lacking leptin.
(A–C) Hypothalamic neurogenesis is almost completely abolished in 16-week-old obese ob/ob mice lacking leptin compared with that in lean littermates (WT/WT or WT/ob [WT/?]). (D) However, there was no difference in the percentage of cells adopting a neuronal fate (HuC/D+) between the 2 groups. (E–G) The number of neurosphere-generating cells is reduced in the hypothalamus of ob/ob mice compared with that in lean littermates (2,000 cells plated per well). (H) The majority of primary neurosphere-forming cells present in ob/ob mice are not stem cells, as they fail to generate new neurospheres after passage. Mean ± SEM. Scale bar: 1 mm.
To investigate the cause of this reduced adult neurogenesis in ob/ob mice, the ventricular lining of the hypothalamus was dissociated and grown in serum-free conditions containing EGF and bFGF. In contrast to findings in the DIO mouse, the hypothalamus of ob/ob mice generated fewer neurosphere-forming cells compared with that of lean controls (P < 0.0001; Figure 8, E–G). Although ob/ob-derived neurospheres were of a similar size compared with WT neurospheres, few of the neurosphere-forming cells derived from ob/ob mice were neural stem cells, as they failed to expand after passage (P < 0.0001; Figure 8H). This was confirmed using immunohistochemistry, which showed a widespread loss of the neural stem cell markers GFAP, Vimentin, Nestin, and Sox2 in the hypothalamus of ob/ob mice (data not shown). The loss of hypothalamic stem cells in ob/ob mice could be detected as early as 4 weeks of age (data not shown). These data indicate that leptin is required for postnatal hypothalamic neurogenesis at the level of neural stem cells.
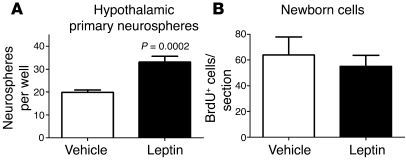
To further explore the ability of leptin to regulate hypothalamic neurogenesis, 8-week-old male C57BL/6 mice were infused with leptin using a subcutaneously implanted osmotic minipump. After 14 days of leptin infusion, mice lost 2.1 ± 0.2 g of body weight (n = 5, P = 0.0016; data not shown). Then, the ventricular lining of the hypothalamus was dissociated and grown in serum-free conditions containing EGF and bFGF. The hypothalamus of leptin-infused mice contained 68% more neurosphere-forming cells per 2,000 cells plated compared with that of vehicle-infused controls (P = 0.0002; Figure 9A). This indicates that leptin is not only required for the maintenance of hypothalamic neural stem cells but is also able to induce their expansion in vivo.
Figure 9. Leptin infusion increases the number of neurosphere-forming neural stem/progenitor cells in vivo but does not directly increase neurogenesis acutely.
(A) Sixteen-week-old mice were infused with leptin peripherally for 14 days. Leptin-treated mice contained higher numbers of neurosphere-forming cells than vehicle-infused control mice. (B) Acute central infusion of leptin and BrdU did not alter the rate of hypothalamic neurogenesis compared with that in mice infused with BrdU alone. Mean ± SEM.
A direct role for leptin in the generation of new neurons in the adult hypothalamus was evaluated by i.c.v. infusion of leptin and BrdU for 7 days in 8-week-old C57BL/6 mice. Mice were sacrificed 4 weeks after infusion, and BrdU+ cells were then detected by immunofluorescence. After 7 days of leptin infusion, mice lost 3.6 ± 0.3 g of body weight (n = 5, P = 0.0013; data not shown). However, the number of newborn BrdU+ cells in adult ARN was unchanged in leptin-infused mice compared with that in mice infused with BrdU alone (n = 5 vehicle vs. n = 4 leptin, P = 0.3204; Figure 9B). Likewise, the proportion of BrdU+ cells that differentiated into neurons (revealed by the neuronal marker HuC/D+) was similar between leptin- and mock-infused mice (P = 0.1199; data not shown). Together, these data suggests that leptin acts primarily at the stem cell level and does not regulate progenitor cell proliferation or specification.
Discussion
We have recently shown that neurogenesis occurs in the adult mouse hypothalamus and, in particular, in the ARN energy-balance circuit. We have also demonstrated that increasing hypothalamic neurogenesis with CNTF administration leads to long-term body weight reduction (19, 21). We speculated that the opposite might also be true, that long-term weight gain might be associated with reduced neurogenesis within the ARN energy-balance circuit. Using BrdU labeling, we first investigated the extent of neurogenesis in the adult ARN in lean mice and found it to be more substantial than initially thought. We then examined neurogenesis in obese mice and found that obese animals have reduced adult hypothalamic neurogenesis, confirming our speculations. This results in reduced neuronal turnover of the ARN energy-balance circuit in DIO.
The extent of ARN neurogenesis.
The fact that neurogenesis occurs in the adult hypothalamus implies that the hypothalamic neuronal network is dynamically remodeled in the adult mouse. However, the extent of such remodeling was unclear. Similar data based upon BrdU incorporation in adult mice could be generated either by a small, rapidly turning over population or by a large, slowly turning over population. This uncertainty is due to the narrow time window of BrdU incorporation and is particularly relevant to small neuronal populations, such as the ARN energy-balance circuit, in which only an occasional newborn energy-balance neuron can be detected with this technique.
To circumvent these issues and investigate neuronal cell turnover in the ARN, we labeled adult neurons during their embryonic neurogenesis with a pulse of BrdU at E10.5. As embryos present at E10.5 are at a range of developmental stages (around 2 days of developmental time), this technique has the advantage of not labeling a single discrete population of neurons but labeling overlapping populations, ranging from immature embryos at the beginning of energy-balance neurogenesis to mature embryos near the end of this process. We then charted the loss of these labeled neurons at different postnatal time points. We found that the majority of ARN neurons present at weaning (4 weeks of age) are lost by adulthood (12 weeks of age), indicating that most of the ARN neuronal network is remodeled within 8 weeks. As this neuronal loss occurs without a significant reduction in the size of the ARN, adult neurogenesis must balance the loss of embryonic labeled neurons.
Although the main phase of embryonic energy-balance neurogenesis occurs at E10.5, the population of neurons that was labeled at that time may not be representative of all ARN neurons, as this embryonic wave of neurogenesis continues until E12.5 (22). This possibility was excluded by labeling progenitor cells across the entire period of ARN neurogenesis, using 3 successive pulses of BrdU at E10.5, E11.5, and E12.5. This approach confirmed the widespread remodeling of the ARN between 4 and 12 weeks of age.
Between 12 weeks of age and 26 weeks of age we did not detect any further loss of the few labeled neurons that remained. This may indicate that the ARN consists of a main population of neurons that remodels and a smaller relatively static population. However, given the considerable levels of apoptosis and neurogenesis at 16 weeks of age, this apparent lack of ongoing neurogenesis beyond 12 weeks may simply reflect a lack of sensitivity, due to the small number of BrdU-labeled neurons remaining. Distinguishing between these two possibilities and accurately measuring total ARN turnover in older animals will require the development of novel methodologies that are not reliant on the continuing presence of embryo-born ARN neurons.
Suppression of neurogenesis in DIO.
We examined adult neurogenesis in DIO mice compared with that in lean mice. First, we labeled newborn cells within the hypothalamus of adult DIO mice and lean controls with BrdU and counted the number of adult-born cells 4 weeks after BrdU infusion to allow time for proliferating cells to adopt mature fates. We found that the number of such newborn neurons was severely reduced in DIO mice compared with that in lean mice, showing reduced adult hypothalamic neurogenesis in this state. However, while the total number of newborn neurons was reduced, the number of newborn hypothalamic neurons as a proportion of all newborn hypothalamic cells was normal in DIO mice. This indicates that there is no defect in the specification of neuronal cell fate under these conditions.
Next, we investigated the causes of decreased neurogenesis in DIO. The prevailing view of adult neurogenesis in the mammalian CNS is that neural stem cells, a subtype of macroglia located in or near the ventricular lining, are relatively quiescent but capable of both self-renewal and the generation of highly proliferative progenitors that in turn adopt neuronal fates (26, 27). Reduced neurogenesis may result from a loss of stem-like cells and/or from decreased survival of newborn neurons.
We measured the number of hypothalamic stem-like cells using a neurosphere assay. Strikingly, a significant increase in the number of such stem-like cells was observed in DIO mice. This excludes a simple direct cause, i.e., reduced stem-like cells resulting in reduced neuronal generation. Conversely, the results of acute BrdU incorporation, confirmed by Ki67 and PCNA staining, indicated a reduction in the pool of highly proliferative progenitor-like cells within the hypothalamus of DIO mice compared with that in lean controls. The reduction in the number of BrdU-labeled cells after 48 hours was mild compared with that evident 4 weeks later, suggesting that in addition to reduced replication of progenitor-like cells, many newborn cells fail to survive between the 2 time points in the context of DIO. This was confirmed by examining apoptosis in newly divided cells using the TUNEL method. Whereas we were unable to detect any newborn cells undergoing apoptosis in lean controls, 8.9% of newborn cells underwent apoptosis in DIO mice. This increased apoptosis may not be limited to cells fated to become neurons and may also include cells fated to remain proliferating progenitors.
Within the ARN as a whole, we detected a considerable degree of apoptosis in chow-fed mice at 16 weeks of age, supporting the view of neuronal turnover. However, we did not observe any difference in the overall rate of apoptosis in DIO mice compared with that in chow-fed controls. This suggests that the rate of cell death within older neurons may be decreased to compensate for the increased apoptosis in newborn cells, leading to maintenance of ARN energy-balance circuit integrity. Indeed we found no difference in the size of the ARN between obese and lean mice (data not shown), supporting this view.
To confirm that the ARN energy-balance circuit remains largely intact in DIO mice due to increased survival of ARN neurons, we labeled these neurons in the embryo at E10.5 and charted their survival during exposure to differential postnatal diets. After 10 weeks of HFD feeding, labeled neurons, including POMC and NPY neurons, were extensively retained within the ARN energy-balance circuit compared with that observed in mice fed chow. This suggests a reduced turnover of the ARN energy-balance circuit during HFD exposure. However, we were unable to count all NPY neurons with confidence, and the apparent retention of NPY neurons could be due to increased detection of NPY neurons in DIO mice. However, as both ARN neurons overall and POMC neurons specifically are retained, the NPY result is unlikely to be an isolated artifact.
We also found that calorie restriction was able to recover neurogenesis in DIO without rescue of the expanded neural stem cell pool phenotype, supporting the view that the loss of neurogenesis in DIO mice and the expansion of neural stem-like cells are independently regulated processes.
Suppression of neurogenesis in leptin-deficient obesity.
To determine whether reduced hypothalamic neurogenesis may be seen in other states of obesity apart from DIO, we examined the leptin-deficient ob/ob model of genetic obesity. Genetically obese ob/ob mice have a greater loss of hypothalamic neurogenesis compared with that in the environmentally induced DIO model of obesity, supporting the view that reduced neurogenesis may have general relevance to hypothalamic dysfunction in obesity. However, ob/ob mice show a distinct neural stem cell phenotype compared with DIO mice; adult ob/ob mice have a severe deficiency of hypothalamic neural stem cells. This may be a direct result of the lack of leptin as long-term leptin infusion increases the number of hypothalamic neural stem-like cells in vivo.
This long-term role of leptin in hypothalamic neurogenesis may be similar to the role of leptin in hippocampal neurogenesis (29). In agreement with the role of leptin in the hippocampus, we were unable to detect an acute effect of leptin in cell generation or specification within the adult hypothalamus. However, leptin does not appear to act directly on hypothalamic stem cells as it does in the hippocampus (29). In the adult, the ventricular lining of the hypothalamus (the location of hypothalamic neural stem cells) shows little, if any, response to leptin in vivo (ref. 30 and data not shown). Likewise, we were unable to reliably and robustly detect an effect of leptin on hypothalamic neurospheres in vitro (data not shown). This supports the view that leptin regulates hypothalamic stem cells indirectly via other leptin-responsive cell types in line with the recent finding that NPY neurons are able to regulate hypothalamic neurogenesis (20).
Possibility of artifact.
Our conclusions regarding postnatal turnover of neurons rely in part on the postulate that the loss of BrdU labeling postnatally is due to the replacement of BrdU-labeled neurons by adult-born unlabeled neurons. Two other possible causes for the loss of BrdU labeling would be DNA repair or the loss of neuronal gene expression but not the actual neurons.
Several factors argue against a potential role for DNA repair as the cause of loss of the BrdU labeling in our system. If it were to be the case, similar BrdU loss should also be observed with amygdala neurons that are generated concomitantly with ARN neurons, and loss of label should remain constant between all postnatal time points. Neither of these features was observed (Figure 2). In addition, obesity is a highly oxidative state (31) that would predict greater nucleotide exchange via DNA repair and hence increased BrdU incorporation in neurons of obese mice (32), not less, as observed (Figure 3). Finally, DNA repair in human cortical neurons does not eliminate or reduce DNA labeled at terminal S-phase over the course of many decades (33). For all of these reasons, DNA repair is an extremely unlikely cause for the changes in BrdU labeling reported here.
The second possibility is that neuronal turnover is an artifact of dramatically altered expression of neuronal markers. This can be rejected for ARN neurons generally, as we first showed that BrdU-labeled parenchymal ARN cells are neurons and then counted BrdU-labeled ARN cells without further identification as neurons. In the case of POMC neurons specifically, if there is a general loss of POMC detection between 4 and 12 weeks then a reduction in the number of detected POMC neurons overall should also occur. This is not apparent (Figure 2), and likewise we do not see a significant change in the total number of POMC+ neurons between DIO mice and chow-fed controls. Therefore, this possibility can be rejected for POMC neurons.
In the case of NPY neurons, although we found that the total number of NPY+ neurons was reduced between 4 and 12 weeks of age, the loss of BrdU-labeled NPY+ neurons was greater than the loss of NPY+ neurons overall, supporting the conclusion of that NPY neurons are replaced postnatally. We are unable to reliably count all NPY+ neurons in nontransgenic mice and relied on identifying only double BrdU+NPY+ neurons. It is possible that the increase of double BrdU+NPY+ neurons in DIO mice could be an artifact due to increased detection of NPY neurons. However, given that ARN neurons overall (and POMC neurons specifically) are retained in DIO mice, the retention of NPY neurons is unlikely to be an artifact.
It is theoretically possible that the BrdU incorporation we detect in adult mice does not reflect uptake during S-phase in proliferating neural precursors but rather reflects DNA synthesis after an abortive S-phase in postmitotic neurons undergoing programmed cell death. This can be excluded in the case of adult labeling, as we tested for the presence of apoptosis in BrdU-incorporating cells using TUNEL and found no BrdU+TUNEL+ cells in chow-fed mice.
Finally, due to teratogenic effects, embryos exposed to large or repeated doses of BrdU may display developmental defects. To reduce this possibility to minimum, the E10.5 BrdU incorporation pulse was carried out using a single relatively low dose of BrdU (100 mg/kg). Postnatally, BrdU-labeled animals were physiologically normal and responded to HFD feeding as expected (Supplemental Table 1 and data not shown).
Cellular mechanism of reduced neurogenesis in obesity.
Both leptin-deficient and HFD obese mice have reduced neurogenesis, but the underlying cellular mechanism appears to be distinct. In ob/ob mice, the loss of neurogenesis stands at the level of neural stem cells. DIO mice do not lack neural stem cells; rather, the loss of neurogenesis in this model appears to occur primarily at the level of progenitor cell generation/survival. The molecular mechanism by which HFD inhibits the turnover of energy-balance neurons at this level is unresolved. Consumption of free fatty acids increases neuronal hypothalamic apoptosis in rats potentially via a TLR4-regulated process (34). Diabetes also increases hypothalamic apoptosis, resulting in the loss of proliferating glia (35, 36), perhaps involving activation of the adrenal axis, as it does in the hippocampus (37). Whether inflammation-dependent mechanisms, adrenal axis activation, and/or other factors are involved in the alteration of hypothalamic turnover in DIO needs further investigation. However, the recent discovery that reduced mitochondrial function in NPY/AgRP neurons but not POMC neurons results in compensatory neurogenesis (20), suggests that energy-balance neurons may regulate their own turnover in a cell-autonomous manner. In addition, it is intriguing to note that the genetic induction of neurodegeneration in the ARN results in neurogenic and metabolic changes similar to those induced by nutrition. That is, degeneration of orexigenic NPY/AgRP neurons increases neurogenesis much as calorie restriction does in the current study, while degeneration of POMC neurons does not and results in obesity (38).
Our results are consistent with the recent findings that HFD-induced obesity inhibits adult neurogenesis in the mouse hippocampus without resulting in neuronal loss (39). In this case, a role for reduced BDNF expression in DIO mice was suggested. Given the central role of BDNF in energy balance regulation (40), BDNF may play a similar role in the hypothalamus as in the hippocampus. However, DIO mice are also diabetic, and diabetes has also been shown to inhibit adult neurogenesis in the hippocampus (37). In this study, the role of glucocorticoid-mediated effects was emphasized. Taken together, these studies suggest that both nutrition and the endocrine system can regulate adult neurogenesis, but the molecular mechanism remains elusive.
The consequences of reduced neurogenesis in obesity.
As adult hypothalamic neurogenesis is reduced in both an environmental (HFD) and a genetic (ob/ob) model of obesity by apparently distinct mechanisms, loss of hypothalamic neurogenesis may play a more general role in the hypothalamic dysfunction seen in obesity. However, the mechanism by which this occurs is unclear, and a number of possible scenarios are apparent.
First, in both leptin-deficient and DIO mice, few newborn neurons are generated and/or survive. These newborn/immature neurons may play a critical role in regulation of energy balance and be specifically required for long-term weight management. Second, in lean mice, mature neurons integrated into the local network circuitry are lost, and new neurons are generated that require integration into local circuitry. In obese animals, this mechanism of network plasticity is reduced, and network adaptability may be critical to long-term energy balance. Third, the metabolic milieu may be damaging to energy-balance neurons in all mice, and it may be the failure to replace damaged neurons in obese mice that directly leads to hypothalamic dysfunction rather than the presence of a specific damaging “metabolic milieu of obesity.” Finally, the failure to regenerate ARN energy-balance neurons will lead to a premature “ageing” of the energy-balance circuit. An aged energy-balance circuit may be less able to achieve energy homeostasis.
Much remains to be learned about the regulation of hypothalamic neuronal turnover, the mechanism by which HFD suppresses it, and the potential implications of these changes for the pathogenesis of obesity. We believe that answers to these questions may have major implications for our understanding of nutritional regulation of energy balance and brain function more generally. In addition, this discovery raises new questions as to the interpretation of transgenic models in which the energy-balance circuit is permanently altered during embryogenesis or early life. Furthermore, DIO induced by 8 to 10 weeks of differential feeding, beginning at 6 to 8 weeks of age, is a standard model in obesity research. Since neurons of the ARN in an obese mouse may be of an older neuronal population than those in their lean counterparts, we may need new interpretations of the changes observed in neuronal gene expression within the ARN of DIO mice.
Overall, our data establish the existence of a substantial postnatal turnover of ARN neuronal circuitry, verifying and substantially extending previous observations. Even more remarkably, our studies reveal an unexpected and profound capacity of postnatal exposure to HFD to suppress this neuronal remodeling, through enhanced survival of preexisting neurons and reduced generation of new neurons, the latter in part due to accelerated apoptosis of postnatally born cells. Likewise, we have discovered that leptin regulates hypothalamic neurogenesis, with leptin-deficient mice having reduced postnatal neurogenesis in part due to leptin being required for maintenance of the hypothalamic stem cell pool. Although much remains to be learned about the mechanisms for these findings and their implications for energy balance regulation, we believe these observations force a change in the operative paradigm for the field, suggesting that it goes beyond changes in the activity of existing neurons, to include regulated plasticity of the underlying neural circuitry.
Methods
Mice.
C57BL/6, FVB, and ob/ob mice were obtained from The Jackson Laboratory. NPY-hrGFP mice were a gift of Brad Lowell (Beth Israel Deaconess Medical Center) (41). The NPY-hrGFP mice used were on a FVB background (this strain has been subsequently backcrossed to C57BL/6, generating The Jackson Laboratory strain 006417). All other experiments were carried out using the WT C57BL/6 strain. Animals were housed under a 12-hour-light/12-hour-dark cycle, and noon of the morning of vaginal plug discovery was assumed to be E0.5. This study was carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals. The animal protocol was approved by the institutional animal care and use committee of Beth Israel Deaconess Medical Center.
DIO.
Male C57BL/6 mice were divided into 2 cohorts at 8 weeks of age. One cohort was maintained on a standard lab chow (3.1 kcal/g, 19% calories from fat, 31% calories from protein, 50% calories from carbohydrates; no. 8664, Harlan Teklad). The second cohort was maintained on a HFD (5.6 kcal/g, 58% kcal from fat, 16% calories from protein, 26% calories from carbohydrate; D-12331, Research Diets), as used previously (19). Mice were used at 16 weeks, after 8 weeks on the respective diets. Obesity, hyperglycemia, and hyperinsulinemia were confirmed in 16-week-old mice HFD-fed mice as previously described (18).
Calorie restriction.
Sixteen-week-old HFD-fed mice were divided into 2 cohorts. One cohort was maintained on HFD for a further 4 weeks, and the second cohort was fed 70% of the daily intake of age-matched chow-fed controls for 4 weeks (see Supplemental Table 2).
BrdU labeling of newborn neurons in the adult by i.c.v. infusion.
Adult mice were infused with BrdU using i.c.v. minipumps, as previously described (18, 20). In brief, mice under ketamine and xylazine anesthesia (45 mg/kg and 5 mg/kg, respectively) were implanted with a steel guide cannula (Plastics One) into the right lateral cerebroventricle (anteroposterior –0.3 mm; lateral +1.0 mm to bregma; and dorsoventral –2.5 mm below skull). The cannula was connected to an osmotic minipump (flow rate 0.5 μl/h, 7 d; 1007D, Alzet). Each minipump was filled with artificial cerebrospinal fluid (aCSF) (19) containing 1 μg/μl mouse serum albumin (Sigma-Aldrich) and 1 μg/μl 5-bromodeoxyuridine (BrdU, Sigma-Aldrich). In the case of leptin infusion, leptin minipumps contained 6 nmol/μl leptin in addition to aCSF and BrdU. Mice were then housed singly and harvested 4 weeks after minipump implantation.
BrdU labeling of newborn neurons by acute i.p. injection.
Mice received 6 i.p. injections with BrdU (100 mg/kg body weight) 2.5 hours apart, during the light cycle, and were euthanized 48 hours after the first injection.
Chronic subcutaneous leptin infusion.
Infusion was delivered by osmotic minipump (flow rate 0.25 μl/h, 14 days; 1002, Alzet) placed subcutaneously and filled with either PBS alone or PBS containing 2 μg/μl leptin.
Embryonic neuronal survival.
WT FVB female mice were mated with transgenic NPY-hrGFP males on the same genetic background, and pregnant dams were injected with a single pulse of BrdU at E10.5 (100 mg/kg body weight) to label a discrete population of newborn neurons in the anterior portion of the ARN (22, 42). Mice were separated into 2 cohorts, a NPY-hrGFP+ and a WT cohort, which were analyzed for NPY and POMC neuronal turnover, respectively. The number of labeled cells was quantified at 4, 12, and 26 weeks of age.
Embryonic neuronal survival in adult HFD-fed mice.
Two i.p. injections of BrdU were given 2.5 hours apart at E10.5. Male mice were separated into 2 cohorts. One cohort was maintained on a standard lab chow. The second cohort was maintained on a standard lab chow from weaning to 6 weeks of age before being fed a HFD. Mice were sacrificed at 16 weeks.
Immunohistochemistry.
Brain sections were treated with 2 N HCl for 1 hour prior to incubation in primary antibody diluted in PBS plus 0.1% Triton X-100 overnight at 4°C. A solution of secondary antibody was then applied for 60 minutes at room temperature, with DAPI added to visualize the cell nuclei. In the cases of POMC and HuC/D, samples were subjected to heat-mediated antigen retrieval in citrate buffer prior to HCl treatment. Primary antibodies used were as follows: β-endorphin (1:200, Novus Biologicals), NPY (1:200, Novus Biologicals), HuC/D (1:70, Molecular Probes), NeuN (1:100, Chemicon), and BrdU (1:200, Accurate). Secondary antibodies were conjugated to FITC or Cy3 (1:200, Jackson ImmunoResearch Inc.), as previously described (19).
Imaging.
The brain sections were examined using an Axio Imager.A1 microscope (Zeiss) or LSM 510 META upright confocal microscope (Zeiss). Images were processed using Photoshop 7.0 (Adobe).
Neurosphere culture.
Neurospheres were derived from the ventricular lining of the hypothalamus. Briefly, the brain was removed and placed in a dish with the median eminence up. Fine forceps were then used to cut around the median eminence to a depth such that the entire dorsal-ventral lining of the third ventricle and adjacent parenchymal cells immediately dorsal to the median eminence was removed from each mouse as a single cone of tissue. This sample was used as donor tissue for neurosphere generation. Neurospheres were cultured as previously described (28), except that additional insulin present in the N2 supplement (Invitrogen) was replaced with 6 nM IGF-1 (43), while insulin remained present in the B27 supplement, and the glucose was reduced to 100 mg/dl (Invitrogen). Neurospheres from a single mouse were grown in 24 wells of an uncoated 96-well culture plate. The number of primary neurospheres per well was assessed 7 days after plating. The results of all 24 wells were averaged for each mouse. To passage, all neurospheres derived from a single mouse were collected, dissociated into single cells, and replated in the original volume of fresh media. Neurospheres were imaged using a Micromaster Digital Inverted Microscope (Fisher Scientific).
TUNEL.
A TUNEL Detection Kit was used following the manufacturer’s instructions (Roche).
Histology, cell counting, and statistics.
Mice were perfused with 10% formalin. Brains were immersed in 30% sucrose (w/v), embedded in OCT compound (VWR), and cut into 12-μM coronal sections using a cryostat (10 series). The entire hypothalamus containing the ARN was sectioned and processed for BrdU and other stains, as described below. The slides were coded, and an observer blinded to the experiments counted DAPI+, GFP+, POMC+, TUNEL+, and BrdU+ cells in 3 consecutive sections, and then averaged the number of cells as a single data point for each mouse. BrdU+ cells were also quantified automatically using ImageJ ( http://rsbweb.nih.gov/ij/), which produced equivalent results. The Student t test was used to determine the significance of differences between means in all cases. P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Brad Lowell (Beth Israel Deaconess Medical Center) for the gift of the NPY-hrGFP mouse line and Alexander Real for technical support. This work was supported by NIH grant DK R37 28082 (to J.S. Flier and E. Maratos-Flier), a grant from the Picower Foundation (to J.S. Flier and E. Maratos-Flier), and NIH grant 2P01DK56116 to the Neuroanatomy core of the Beth Israel Deaconess Medical Center, Division of Endocrinology.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2012;122(1):142–152. doi:10.1172/JCI43134.
David E.G. McNay’s present address is: Integrative Physiology, University of Aberdeen, Aberdeen, United Kingdom.
Nadege Briançon’s present address is: Harvard Medical School, Department of Cell Biology, Boston, Massachusetts, USA.
Maia V. Kokoeva’s present address is: Department of Medicine, Division of Endocrinology and Metabolism, McGill University, Montreal, Quebec, Canada.
See the related Commentary beginning on page 22.
References
- 1.Brobeck JR, Tepperma J, Long C. Experimental hypothalamic hyperphagia in the albino rat. Yale J Biol Med. 1943;15(6):831–853. [PMC free article] [PubMed] [Google Scholar]
- 2.Olney JW. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science. 1969;164(880):719–721. doi: 10.1126/science.164.3880.719. [DOI] [PubMed] [Google Scholar]
- 3.Anand BK, Brobeck JR. Localization of a feeding center in the hypothalamus of the rat. Proc Soc Exp Biol Med. 1951;77(2):323–324. doi: 10.3181/00379727-77-18766. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 5.Halaas JL, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269(5223):543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 6.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443(7109):289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz MW, Woods SC, Porte D, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404(6778):661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 8.Frederich RC, Hamann A, Anderson S, Lollmann B, Lowell BB, Flier JS. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med. 1995;1(12):1311–1314. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- 9.Schemmel R, Mickelsen O, Tolgay Z. Dietary obesity in rats: influence of diet, weight, age, and sex on body composition. Am J Physiol. 1969;216(2):373–379. doi: 10.1152/ajplegacy.1969.216.2.373. [DOI] [PubMed] [Google Scholar]
- 10.El-Haschimi K, Pierroz DD, Hileman SM, Bjørbaek C, Flier JS. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest. 2000;105(12):1827–1832. doi: 10.1172/JCI9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enriori PJ, et al. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab. 2007;5(3):181–194. doi: 10.1016/j.cmet.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Banks WA, et al. Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes. 2004;53(5):1253–1260. doi: 10.2337/diabetes.53.5.1253. [DOI] [PubMed] [Google Scholar]
- 13.Caro JF, et al. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet. 1996;348(9021):159–161. doi: 10.1016/S0140-6736(96)03173-X. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz MW, Peskind E, Raskind M, Boyko EJ, Porte D., Jr Cerebrospinal fluid leptin levels: relationship to plasma levels and to adiposity in humans. Nat Med. 1996;2(5):589–593. doi: 10.1038/nm0596-589. [DOI] [PubMed] [Google Scholar]
- 15.Ozcan L, et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9(1):35–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Mori H, et al. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat Med. 2004;10(7):739–743. doi: 10.1038/nm1071. [DOI] [PubMed] [Google Scholar]
- 17.Bence KK, et al. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat Med. 2006;12(8):917–924. doi: 10.1038/nm1435. [DOI] [PubMed] [Google Scholar]
- 18.Briancon N, McNay DE, Maratos-Flier E, Flier JS. Combined neural inactivation of suppressor of cytokine signaling-3 and protein-tyrosine phosphatase-1B reveals additive, synergistic, and factor-specific roles in the regulation of body energy balance. Diabetes. 2010;59(12):3074–3084. doi: 10.2337/db10-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kokoeva MV, Yin H, Flier JS. Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science. 2005;310(5748):679–683. doi: 10.1126/science.1115360. [DOI] [PubMed] [Google Scholar]
- 20.Pierce AA, Xu AW. De novo neurogenesis in adult hypothalamus as a compensatory mechanism to regulate energy balance. J Neurosci. 2010;30(2):723–730. doi: 10.1523/JNEUROSCI.2479-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kokoeva MV, Yin H, Flier JS. Evidence for constitutive neural cell proliferation in the adult murine hypothalamus. J Comp Neurol. 2007;505(2):209–220. doi: 10.1002/cne.21492. [DOI] [PubMed] [Google Scholar]
- 22.McNay DEG, Pelling M, Claxton S, Guillemot F, Ang S. Mash1 is required for generic and subtype differentiation of hypothalamic neuroendocrine cells. Mol Endocrinol. 2006;20(7):1623–1632. doi: 10.1210/me.2005-0518. [DOI] [PubMed] [Google Scholar]
- 23.Pelling M, et al. Differential requirements for neurogenin 3 in the development of POMC and NPY neurons in the hypothalamus. Dev Biol. 2011;349(2):406–416. doi: 10.1016/j.ydbio.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Padilla SL, Carmody JS, Zeltser LM. Pomc-expressing progenitors give rise to antagonistic neuronal populations in hypothalamic feeding circuits. Nat Med. 2010;16(4):403–405. doi: 10.1038/nm.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi S, Dallman MF. Hypothalamic obesity: multiple routes mediated by loss of function in medial cell groups. Endocrinology. 1999;140(9):4081–4088. doi: 10.1210/en.140.9.4081. [DOI] [PubMed] [Google Scholar]
- 26.Chojnacki AK, Mak GK, Weiss S. Identity crisis for adult periventricular neural stem cells: subventricular zone astrocytes, ependymal cells or both? Nat Rev Neurosci. 2009;10(2):153–163. doi: 10.1038/nrn2571. [DOI] [PubMed] [Google Scholar]
- 27.Duan X, Kang E, Liu CY, Ming GL, Song H. Development of neural stem cell in the adult brain. Curr Opin Neurobiol. 2008;18(1):108–115. doi: 10.1016/j.conb.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiss S, et al. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci. 1996;16(23):7599–7609. doi: 10.1523/JNEUROSCI.16-23-07599.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garza JC, Guo M, Zhang W, Lu X. Leptin increases adult hippocampal neurogenesis in vivo and in vitro. J Biol Chem. 2008;283(26):18238–18247. doi: 10.1074/jbc.M800053200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cottrell EC, et al. Developmental changes in hypothalamic leptin receptor: relationship with the postnatal leptin surge and energy balance neuropeptides in the postnatal rat. Am J Physiol Regul Integr Comp Physiol. 2009;296(3):R631–R639. doi: 10.1152/ajpregu.90690.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frisard M, Ravussin E. Energy metabolism and oxidative stress: impact on the metabolic syndrome and the aging process. Endocrine. 2006;29(1):27–32. doi: 10.1385/ENDO:29:1:27. [DOI] [PubMed] [Google Scholar]
- 32.Fishel ML, Vasko MR, Kelley MR. DNA repair in neurons: so if they don’t divide what’s to repair? Mutat Res. 2007;614(1–2):24–36. doi: 10.1016/j.mrfmmm.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 33.Bhardwaj RD, et al. Neocortical neurogenesis in humans is restricted to development. Proc Natl Acad Sci U S A. 2006;103(33):12564–12568. doi: 10.1073/pnas.0605177103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moraes JC, et al. High-fat diet induces apoptosis of hypothalamic neurons. PloS One. 2009;4(4):e5045. doi: 10.1371/journal.pone.0005045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lechuga-Sancho AM, et al. Reduction in the number of astrocytes and their projections is associated with increased synaptic protein density in the hypothalamus of poorly controlled diabetic rats. Endocrinology. 2006;147(11):5314–5324. doi: 10.1210/en.2006-0766. [DOI] [PubMed] [Google Scholar]
- 36.Garcia-Caceres C, Lechuga-Sancho A, Argente J, Frago LM, Chowen JA. Death of hypothalamic astrocytes in poorly controlled diabetic rats is associated with nuclear translocation of apoptosis inducing factor. J Neuroendocrinol. 2008;20(12):1348–1360. doi: 10.1111/j.1365-2826.2008.01795.x. [DOI] [PubMed] [Google Scholar]
- 37.Stranahan AM, Arumugam TV, Cutler RG, Lee K, Egan JM, Mattson MP. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat Neurosci. 2008;11(3):309–317. doi: 10.1038/nn2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu AW, et al. Effects of hypothalamic neurodegeneration on energy balance. PLoS Biol. 2005;3(12):e415. doi: 10.1371/journal.pbio.0030415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park HR, Park M, Choi J, Park KY, Chung HY, Lee J. A high-fat diet impairs neurogenesis: involvement of lipid peroxidation and brain-derived neurotrophic factor. Neurosci Lett. 2010;482(3):235–239. doi: 10.1016/j.neulet.2010.07.046. [DOI] [PubMed] [Google Scholar]
- 40.Xu B, et al. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci. 2003;6(7):736–742. doi: 10.1038/nn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van den Pol AN, et al. Neuromedin B and gastrin-releasing peptide excite arcuate nucleus neuropeptide Y neurons in a novel transgenic mouse expressing strong Renilla green fluorescent protein in NPY neurons. J Neurosci. 2009;29(14):4622–4639. doi: 10.1523/JNEUROSCI.3249-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Altman J, Bayer SA. Development of the diencephalon in the rat. II. Correlation of the embryonic development of the hypothalamus with the time of origin of its neurons. J Comp Neurol. 1978;182(4 pt 2):973–993. doi: 10.1002/cne.901820512. [DOI] [PubMed] [Google Scholar]
- 43.Arsenijevic Y, Weiss S, Schneider B, Aebischer P. Insulin-like growth factor-I is necessary for neural stem cell proliferation and demonstrates distinct actions of epidermal growth factor and fibroblast growth factor-2. J Neurosci. 2001;21(18):7194–7202. doi: 10.1523/JNEUROSCI.21-18-07194.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.