Abstract
The hormone glucagon has long been dismissed as a minor contributor to metabolic disease. Here we propose that glucagon excess, rather than insulin deficiency, is the sine qua non of diabetes. We base this on the following evidence: (a) glucagon increases hepatic glucose and ketone production, catabolic features present in insulin deficiency; (b) hyperglucagonemia is present in every form of poorly controlled diabetes; (c) the glucagon suppressors leptin and somatostatin suppress all catabolic manifestations of diabetes during total insulin deficiency; (d) total β cell destruction in glucagon receptor–null mice does not cause diabetes; and (e) perfusion of normal pancreas with anti-insulin serum causes marked hyperglucagonemia. From this and other evidence, we conclude that glucose-responsive β cells normally regulate juxtaposed α cells and that without intraislet insulin, unregulated α cells hypersecrete glucagon, which directly causes the symptoms of diabetes. This indicates that glucagon suppression or inactivation may provide therapeutic advantages over insulin monotherapy.
Introduction
The opposing hormonal actions of insulin and glucagon first became evident as long ago as 1921, when Banting and Best administered a crude extract of canine pancreas to a diabetic dog (1). The subsequent destinies of the two components of the extract, however, could not have been more different. The discovery of insulin was acclaimed as the greatest achievement in medical history and won a Nobel Prize within one year of its first injection into a human. Since then, insulin has been considered the single most important metabolic regulator, and the catabolic derangements of type 1 diabetes (T1DM) have been directly attributed to insulin lack; this insulinocentric view of diabetes has persisted for 90 years (Sidebar 1).

In contrast, the hyperglycemic factor was consigned to the category of unwelcome distraction. In 1971, Charles Best wrote to Pierro Foa that he had “a very clear recollection of the immediate rise in blood sugar lasting about one-half hour. We thought that this might have been due to epinephrine and for this reason we failed to investigate it thoroughly” (personal communication).
In 1923, the hyperglycemic factor was separated from insulin by Kimball and Murlin and named glucagon (2). However, the contaminant stigma persisted among rank-and-file physicians long after it became patently untenable. Glucagon did, however, attract the interest of biochemists and physiologists (3–7), who identified its glycogenolytic, gluconeogenic, and ketogenic activities. It was purified and sequenced at Eli Lilly Co. (8), and shortly thereafter was made commercially available for the treatment of severe hypoglycemic reactions to insulin.
Five decades later, glucagon finally gained recognition as a hormone (9). In 1959, the development of a RIA for glucagon (10, 11) made possible specific confirmation of glucagon responses to changes in fuel needs and abundance (12). The evidence suggesting that elevated glucagon is the glucoregulatory partner of insulin was reviewed in the 1975 Banting Lecture of the American Diabetes Association (9). However, the importance of glucagon in normal glucose homeostasis and in the diabetic phenotype remained controversial. Clearly, the vast majority of clinicians and scientists continued to believe that insulin did it all and that glucagon had, at most, a relatively minor modulatory role. Even today, few scientists or clinicians accept the glucagonocentric premise that α cell dysfunction is the sine qua non of the diabetic phenotype and that its correction — independent of insulin treatment — would provide important therapeutic benefit (Sidebar 1).
Here, we review evidence that the insulinocentric view of metabolic homeostasis is incomplete and that glucagon is indeed a key regulator of normal fuel metabolism, albeit under insulin’s paracrine guidance and control. Most importantly, we emphasize that, whenever paracrine control by insulin is lacking, as in T1DM, the resulting unbridled hyperglucagonemia is the proximal cause of the deadly consequences of uncontrolled diabetes and the glycemic volatility of even “well-controlled” patients.
The practical goal of this review is to highlight the targeting α cells as part of the therapeutic strategy of T1DM to eliminate the glycemic volatility that characterizes current insulin monotherapy. It should be noted that inhibition of glucagon receptor action has been associated with α cell hyperplasia (13) as well as abnormal lipid metabolism (14, 15), making inhibition of α cell hypersecretion the more appealing strategy for diabetes treatment.
Metabolic credentials
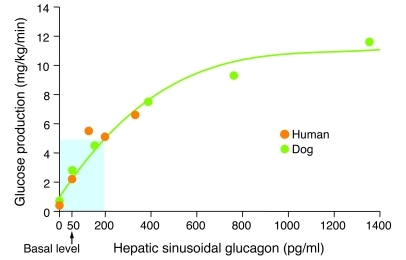
It soon became obvious that the effects of insulin and glucagon on the liver were in diametric opposition (3), which suggested that the two hormones share responsibility for regulating hepatic glucose metabolism. The ability of glucagon to stimulate glucose production in vivo was demonstrated in studies in which somatostatin was used to disable the endocrine pancreas, so that plasma insulin could be clamped at basal levels while plasma glucagon was varied. In the dog, it was possible to replace insulin intraportally, thus maintaining basal insulin levels in both liver and nonhepatic tissues. Under such conditions, a selective decrease in glucagon resulted in a rapid fall in glucose production (16), whereas a selective increase in the hormone caused a rapid rise in hepatic glucose output (17, 18). In fact, after an overnight fast, the basal glucagon level accounted for up to 70% of glucose production (16). In addition, a rise in plasma glucagon of only 100 pg/ml in the liver sinusoids tripled glucose production (19, 20). Thus, the control strength of glucagon is profound, with a dynamic range of approximately 5 mg/kg/min over the physiologic range of plasma glucagon concentrations (Figure 1 and refs. 21–26). Not only is the liver very sensitive to changes in plasma glucagon, it also responds rapidly, with a half-maximal activation time of only 8 minutes (27). Human studies, although less well controlled, confirmed that the observations made in the dog extend to man (22–25, 28–30). Thus, it is evident that after an overnight fast, basal levels of glucagon drive resting glucose production, thereby allowing insulin to link hepatic glucose output to the body’s need for glucose.
Figure 1. Relationship between hepatic sinusoidal glucagon and glucose production in vivo.
A pancreatic clamp was used to keep plasma insulin basal and constant. The glucose production rate reflects the maximal effect of glucagon and was observed approximately 15 minutes after the change in the hormone level. In this way, the accompanying hyperglycemia was limited such that its inhibitory effect on glucose production was minimal. When glucagon was made deficient (i.e., 0 pg/ml), euglycemia was maintained by glucose infusion. The region shaded blue denotes the physiologic range of plasma glucagon. Figure adapted with permission from Handbook of Physiology (96).
Whenever there is an increased demand for glucose (i.e., starvation, hypoglycemia, and exercise), insulin secretion falls, stimulating glucagon secretion. This removes insulin’s inhibitory action on the liver while augmenting glucagon’s stimulatory effect on fuel production. As a result, glucose production is increased to meet the needs of the organism. When glucose is abundant, as with an oral glucose load, the reverse occurs.
Glucagon also modulates hepatic glucose uptake (HGU) (28, 31, 32) and hepatic glycogen synthesis (33). A decrease in plasma glucagon has little effect on HGU in the presence of elevated insulin (31), but the effect can be quite marked when insulin is deficient (32), which has obvious implications for diabetes. Insulin is a key determinant of hepatic glucokinase (GK) expression, which is required for HGU. It is unclear whether, in the presence of complete insulin deficiency, glucagon suppression would increase liver glucose uptake, a possibility that still needs to be directly examined. On the other hand, it is clear that an increase in glucagon can interfere with the ability of a rise in plasma insulin to enhance glucose uptake by the liver (31). This suggests that glucagon and insulin jointly control hepatic production (in times of deficit) and storage (in times of plenty) of glucose. When glucose is scarce, as in starvation, lipolysis increases, as does the delivery of nonesterified fatty acids to the liver. It is also now clear that insulin and glucagon interact to govern hepatic fatty acid synthesis (34) and hepatic ketogenesis (4). Likewise, the two hormones oppose each other with regard to liver protein metabolism (35).
Endocrine and paracrine credentials
The demonstration that glucagon has powerful glycogenolytic activity exerted via the second messenger cAMP (7) provided strong biochemical evidence for it being a true hormone. In vivo evidence of its physiologic activity was provided by Foa’s elegant pancreatic-femoral cross-circulation studies in dogs, which demonstrated that the pancreas was indeed the source of the hyperglycemic factor (36). Histochemical evidence reinforced the conclusion that glucagon came from pancreatic α cells (37).
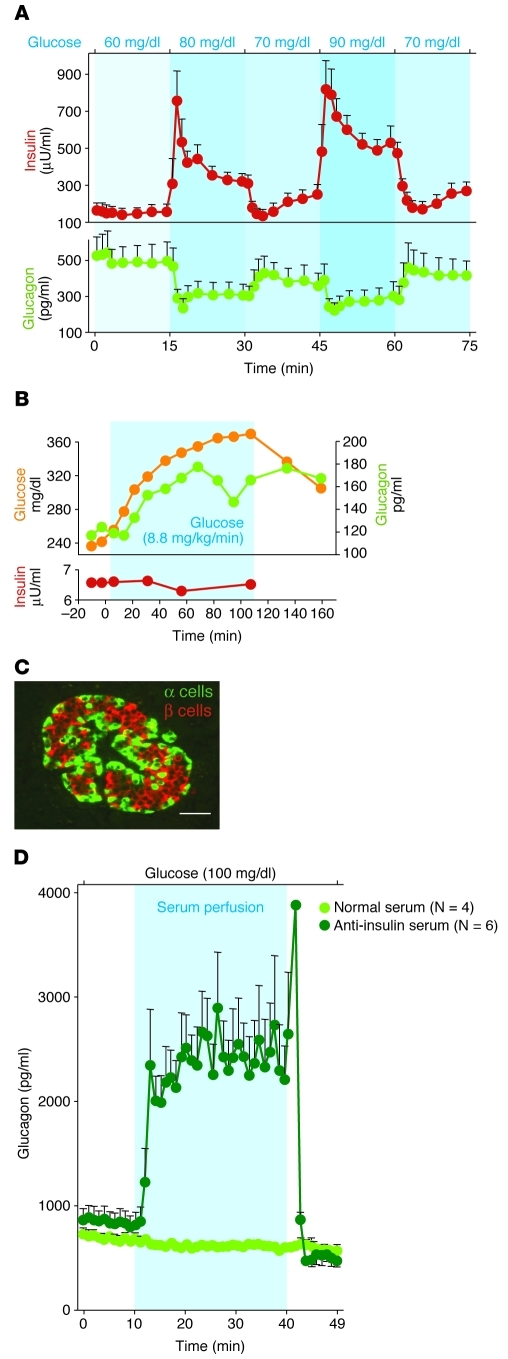
The development of highly specific RIAs for insulin (38) and glucagon (10, 11) demonstrated reciprocal behavior of the 2 hormones. Insulin levels fell during glucopenia and rose during glucose administration (Figure 2A and ref. 38), and glucagon levels rose during glucopenia and fell during glucose administration, fully consistent with its glycogenolytic and gluconeogenic actions (5–7). Glucagon was localized immunocytochemically to α cells of the pancreas (39), confirming the histochemical findings of Ferner (37). Nevertheless, the importance of its role continued to be debated, despite metabolic, physiologic, and anatomical clues suggesting a bihormonal homeostatic relationship between insulin and glucagon (12, 40, 41).
Figure 2. Relationship between insulin and glucagon secretion.
(A) Responses of insulin and glucagon to minor changes in glucose perfused into isolated pancreata of normal dogs. The perfusate glucose concentration varied from 60 to 90 mg/dl. Modest changes in the perfusing glucose concentration led to major reciprocal responses of both insulin and glucagon. Figure adapted with permission from Diabetologia (38). (B) Demonstration that a rise in glucose “paradoxically” stimulates glucagon secretion when it is not accompanied by the rise in insulin that normally accompanies elevations in glucose concentration. Figure adapted from Journal of Clinical Investigation (43). (C) Topographic scheme of a normal human islet showing the extensive juxtaposition of β cells (red) to α cells (green) that facilitates instantaneous insulin control of glucagon secretion via the interstitial space separating the two cells. Scale bar: 50 μm. Figure reproduced with permission from Diabetes (48). (D) Direct physiologic evidence of the paracrine role of insulin on α cell function in rodents. The isolated pancreata of normal rats are perfused with either nonimmune serum, as control, or a potent anti-insulin serum. The sudden rise in glucagon upon infusion of the anti-insulin serum indicates an ongoing paracrine inhibition of glucagon secretion by the insulin in the islets. Figure adapted from Journal of Clinical Investigation (53).
Another clue to the critical nature of this bihormonal relationship was the demonstration that when insulin rises after glucose feeding, the accompanying suppression of glucagon secretion is caused not by hyperglycemia, but by increased insulin levels (42), Indeed, if a rise in blood glucose is unaccompanied by insulin release, hyperglycemia stimulates glucagon secretion (Figure 2B and refs. 43, 44). This established insulin as a glucagon-suppressing hormone and, as detailed below, made it increasingly clear that the glucagon-suppressing action of insulin was largely a paracrine function (45), providing further support for the concept of bihormonal control of glucose homeostasis (Sidebar 1 and refs. 5, 6).
The reciprocal changes in insulin and glucagon secretion that occur in response to relatively minor perturbations in plasma glucose (Figure 2A and ref. 38) give further credence to the concept of bihormonal control at the level of the islets, as well as of the liver (46).
Anatomical credentials
Finally, anatomical clues suggested that paracrine insulin reaches the α cells before insulin reaches any other targets in the body in concentrations far above the endocrine levels delivered to peripheral insulin targets. In rodents, the first clue (47) was the “portal” microcirculation that carries insulin from the β cell core to the α cell mantle of the islet (48). In addition, the demonstration of gap junctions between α and β cells (49) raised the possibility that their activities are also coordinated via intracellular signals. In human islets, there is extensive juxtaposition of β cells and α cells that should permit insulin to reach α cells across their shared interstitium in a paracrine relationship. (Figure 2C and refs. 48, 50, 51). Interestingly, although the topographic arrangements of α and β cells differ in different species, they all appear to enable insulin to control glucagon secretion via some type of intraislet action. The tightly coupled reciprocal nature of changes in the secretion of the two hormones (Figure 2A) was suggestive of coordinated relationships analogous to the reciprocal innervations of skeletal muscle contraction described in the Second Law of Sherrington, which states that whenever the biceps contracts, the triceps relaxes (52).
Powerful evidence that insulin controls the secretion of glucagon via a paracrine mechanism was obtained by perfusing the isolated pancreas of normal rats with a potent neutralizing anti-insulin serum. Whereas perfusion of nonimmune serum had no effect, perfusion of the anti-insulin serum caused a prompt and dramatic increase in glucagon secretion (Figure 2D and ref. 53). This demonstrates that insulin acts inside the islets to inhibit glucagon secretion.
Interestingly, recent reports suggest that insulin may also regulate glucagon secretion through an action in the ventromedial hypothalamus, as well as by an effect on the α cell directly (54, 55), a dual control system.
Glucagon, sine qua non of hyperglycemia in all forms of insulin deficiency
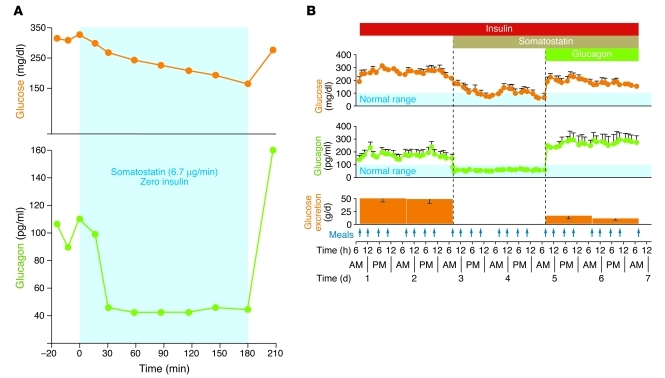
The similarity between the glycogenolytic, gluconeogenic, and ketogenic actions of glucagon (Sidebar 1) and the metabolic abnormalities of insulin deficiency suggested that the α cell hormone played a central pathogenic role in diabetes. Using the glucagon RIA, it was demonstrated that hyperglucagonemia is present in untreated T1DM in humans and animal models (40). Absolute proof that endogenous glucagon plays an essential role in the pathogenesis of diabetes requires that suppression of glucagon secretion or action reduces the metabolic manifestations of insulin deficiency. In 1974, Koerker et al. (56) reported that somatostatin (57) could suppress glucagon. Several groups quickly exploited this to test the effects of glucagon suppression on the metabolic manifestations of insulin deficiency. When somatostatin was infused into alloxan-diabetic dogs (Figure 3A and ref. 58) or in insulin-deprived humans with T1DM, as first shown by Gerich et al. (Figure 3B and refs. 59, 60), hyperglucagonemia was suppressed and hyperglycemia was markedly decreased, even though insulin had been reduced or discontinued. Notably, infusion of exogenous glucagon restored the hyperglycemia. Physiologic studies by Stevenson et al. (20), using the depancreatized dog, demonstrated that when insulin was replaced intraportally at a basal rate, the plasma glucagon level (3,500 MW glucagon produced by α cells in the gut) fell markedly. It was the fall in glucagon that was responsible for most of the insulin-driven improvement in glycemia, since it ceased when glucagon was replaced. These experiments provided the first concrete evidence that glucagon might be playing an essential pathogenic role in the hyperglycemia of insulin deficiency. They also called into question for the first time the dogma of insulinocentrism, suggesting that glucagon excess, rather than insulin deficiency, causes the catabolism of insulin deficiency.
Figure 3. Glucagon is essential in diabetic hyperglycemia.
(A) Perfusion of a severely diabetic, insulin-deprived dog with somatostatin. The hyperglycemia and hyperglucagonemia are promptly suppressed by the somastotatin infusion, and both reappear when it is stopped. Figure adapted with permission from Science (58). (B) A similar experiment in type 1 diabetic humans receiving a suboptimal insulin dose administered by intravenous infusion (60). Their hyperglucagonemia, hyperglycemia, and glycosuria are suppressed soon after beginning an infusion of somastotatin, confirming earlier work by Gerich et al. (59). When hyperglucagonemia was restored by infusion of recombinant glucagon, hyperglycemia and glycosuria reappeared. Figure adapted with permission from New England Journal of Medicine (60).
The main opposition to this idea was based on the fact that total pancreatectomy causes diabetes. This argument was based on the false assumption that α cells are located only in the pancreatic islets (61). However, in the 1970s, several groups reported measurable glucagon levels in insulin-deprived, totally pancreatectomized humans and animals (62–65). The stomach was found to be an important source of the nonpancreatic hyperglucagonemia, and classical α cells were found in the gastric fundus and duodenum of animals and humans (66, 67). Gastric α cells were shown to oversecrete glucagon during insulin deficiency and to be more sensitive than pancreatic α cells to small amounts of insulin. Interestingly, immunoassayable glucagon was present in a totally depancreatized, totally gastrectomized human (68), which suggests that α cells are present in the digestive tract below the pylorus. The recent demonstration by Thorel et al. that ablation of 98% pancreatic α cells does not lower glucagon levels sufficiently to suppress streptozotocin-induced diabetes (69) may have a similar explanation.
These insights invalidated the only argument against an essential diabetogenic role for glucagon (67). Glucagonocentrism had become plausible.
Glucagon and the glycemic volatility of T1DM?
Glycemic volatility, a hallmark of insulin-treated T1DM, is its most challenging day-to-day clinical problem. T1DM patients must constantly monitor glucose levels in order to respond to and correct major glycemic deflections with supplemental insulin or glucose (70), profoundly reducing quality of life. Given that T1DM is the only condition in which such glucose volatility occurs and that T1DM is the only condition in which the islets are devoid of β cells, the possibility of a causal relationship between the volatility and the loss of paracrine control of glucagon secretion by insulin seems quite plausible.
For example, it is not widely appreciated that, when hyperglycemia is unaccompanied by an increase in insulin, it stimulates rather than suppresses glucagon secretion. This paradoxical increase in glucagon could be an important factor in the exaggerated postprandial hyperglycemia of T1DM. If β cells are not juxtaposed to α cells to provide a glucose-stimulated paracrine “squirt” of insulin, postprandial hyperglycemia will stimulate a paradoxical rise of glucagon secretion, rather than trigger suppression of its release (Figure 2B and refs. 43, 44). This adds an endogenous source of glucose to the exogenous glucose from the meal.
Glucagon and the hypoglycemia of T1DM
Another burden of T1DM is that hypoglycemia (precipitable by physical exertion or by delays in feeding) is unalleviated in the absence of the normal glucagon response. In this case, the circulating insulin derived from the injection does not decline when blood glucose levels fall, thus preventing the glucagon rise that would otherwise defend against hypoglycemia. In addition, the observation that high levels of insulin in the brain can inhibit glucagon secretion through a neural mechanism (54, 55) suggests that central insulin action may also contribute to high hypoglycemia incidence in patients with TIDM.
Glucagonocentrism: insulin actions are mediated by glucagons
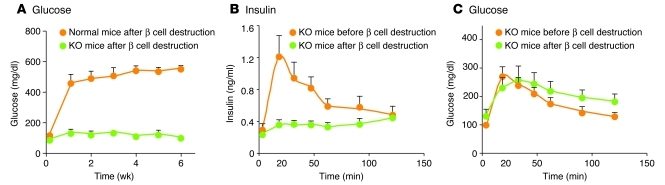
Studies in glucagon receptor–null (Gcgr–/–) mice indicate that glucagon mediates the catabolic consequences of insulin lack (71). In these Gcgr–/– mice, which exhibit no response to glucagon at any concentration, total β cell destruction did not result in any of the diabetic abnormalities thought to be caused by insulin deficiency. Destruction of β cells in wild-type controls resulted in the familiar catabolic consequences of insulin deficiency, with death due to ketoacidosis within 6 weeks, whereas in the Gcgr–/– mice, none of the clinical or laboratory manifestations of insulin deficiency was detected (Figure 4). The insulin-deficient Gcgr–/– mice did not become hyperglycemic or hyperketonemic, and their livers exhibited no increase either in phospho–cAMP response element–binding protein (p-CREB; a mediator of glucagon action) (72) or in the gluconeogenic enzyme phosphoenolpyruvate carboxykinase, both of which are elevated in uncontrolled diabetes.
Figure 4. Glucagon is the sine qua non of diabetes in mice.
(A) Glucose levels in normal wild-type mice and in Gcgr–/– mice after destruction of β cells by double-dose streptozotocin treatment. Gcgr–/– mice remain normoglycemic and exhibit no detectable metabolic consequence of total insulin deficiency. (B) Insulin response to oral glucose in Gcgr–/– mice before and after β cell destruction. (C) Oral glucose tolerance curve of Gcgr–/– mice before and after β cell destruction. Remarkably, although streptozotocin-treated Gcgr–/– mice were incapable of secreting insulin in response to an oral glucose tolerance test, their glucose tolerance curves did not differ significantly from Gcgr–/– mice with intact β cells and a robust insulin response. In other words, in this model of congenital absence of glucagon activity, insulin has become irrelevant. (A–C) Figure adapted with permission from Diabetes (71).
These findings agree with other work in which glucagon receptors were blocked with antibodies (73, 74) or with glucagon receptor antagonists (75). Such maneuvers also improved the metabolic state in insulin deficiency (76–80). These results strongly suggest that the catabolic actions heretofore considered the direct consequences of insulin lack are actually mediated by a relative or absolute excess of glucagon to insulin.
By far the most surprising observation in the Gcgr–/– mice was the fact that oral or intraperitoneal glucose tolerance tests remained normal (Figure 4B), despite destruction of virtually all β cells and lack of an insulin response to glucose (Figure 4C). Since a normal glucose tolerance test excludes the diagnosis of diabetes, one must conclude that the diabetic state cannot be manifest without glucagon action — at least in the mouse. Therefore, the abnormalities of glucose and ketone metabolism associated with T1DM in the mouse are mediated by dysregulated glucagon secretion, rather than by insulin lack per se (Sidebar 1 and refs. 51, 71).
Gcgr–/– mice reportedly have very high plasma levels of the incretin hormone glucagon-like peptide 1, but this is not thought to account for their improved oral glucose tolerance, although the plasticity of the incretin system in this model is striking (81). If these rodent findings extend to humans, as suggested by the somatostatin studies of Gerich et al. (59) and Raskin and Unger (60), the excess of unsuppressed and unopposed glucagon, rather than the lack of insulin by itself, would be the direct cause of the catabolic cascade in insulin deficiency states (Sidebar 1). It should be stressed that, at present, there is no basis for questioning a direct role for insulin lack alone in the enhanced lipolysis seen in adipose tissue or in the increased proteolysis seen in muscle in individuals with uncontrolled T1DM (Sidebar 1). In fact, there are no known glucagon receptors in muscle (82). Therefore, why insulin deficiency in Gcgr–/– mice does not appear to alter fat or muscle metabolism is unclear. It is worth noting that glucose tolerance is not altered in muscle-specific insulin receptor KO mice (83) or in whole-body Glut4-null mice (84). In the normal dog and human, on the other hand, when insulin and glucagon secretion were simultaneously made deficient using somatostatin (30, 85), insulin lack resulted in a significant decrease in glucose clearance and a consequent doubling of the plasma glucose level. Thus, in large mammals, the effect of insulin deficiency on muscle glucose uptake — at least acutely — is apparent even in the face of glucagon lack.
Glucagon suppression as therapeutic strategy
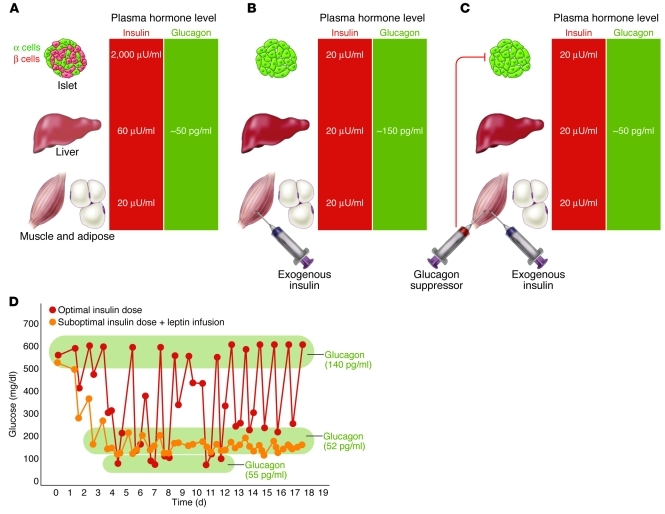
If glucagon hypersecretion is in fact the direct cause of major metabolic aberrations in human diabetes, including the glycemic volatility of T1DM, glucagon suppression becomes an attractive therapeutic strategy for managing the disease. The glycemic volatility of T1DM observed with insulin monotherapy could easily result from the sharp differences in the insulin concentrations required by various targets of the hormone. By virtue of their proximity to β cells, nondiabetic pancreatic α cells are exposed to insulin in concentrations at least 100 times those reaching skeletal muscle (Figure 5A). In contrast, injected insulin provides a similar insulin concentration for all tissues (Figure 5B), which results either in underinsulinization of α cells or in overinsulinization of peripheral tissues. The obvious solution is to use insulin in doses that meet the requirements of peripheral tissues but are not high enough to suppress hyperglucagonemia and to reassign the duty of α cell suppression to a noninsulin agent, such as leptin (Figure 5B).
Figure 5. Why insulin monotherapy in T1DM cannot restore normal glycemic stability.
(A) Concentration disparity of secreted insulin normally delivered to target organs. Normal α cells receive 100 times more insulin than do peripheral tissues. (B) In T1DM, all targets receive the same concentration of injected insulin. Levels high enough to suppress α cells are too high for the liver and the peripheral tissues. (C) By lowering the insulin dose and suppressing hyperglucagonemia with a noninsulin glucagon suppressor, glycemic stability is achieved. (D) Suppression of glycemic volatility in T1DM. NOD mice were treated with optimal insulin dose (0.2 U twice daily); other mice were treated with a suboptimal insulin dose (0.02 U twice daily) and a subcutaneous infusion of leptin. Mean glucose values were determined at 10 a.m. and 5 p.m. Leptin suppressed glucose volatility in these mice by preventing hyperglucagonemia, and hypoglycemia was prevented by reducing the insulin. Figure adapted with permission from Proceedings of the National Academy of Sciences of the United States of America (51).
Noninsulin glucagon suppressors
In 1978, the first clinical trial of glucagon suppression in T1DM was reported (60). Patients were treated with somatostatin infusion after reduction of their insulin (Figure 3B and refs. 59, 60). When hyperglucagonemia was suppressed, hyperglycemia and glycosuria were markedly reduced. Unfortunately, side effects of somatostatin precluded its long-term use in T1DM, and more than 20 years passed before another glucagon suppressor was identified.
Amylin is a second glucoregulatory β cell hormone that is normally co-secreted with insulin in response to meals and is deficient in patients with T1DM (86). Preclinical studies have shown that amylin slows nutrient absorption, acts as a satiety factor, and decreases glucagon secretion (86). In clinical studies in which pramlinitide (a commercially available amylin analog) was used as an adjunct to insulin therapy in patients with T1DM, there were decreases in plasma glucagon levels, glucose fluctuations, postprandial glucose levels, and plasma triglyceride concentrations (86–89). As one might expect, the patients’ insulin dose had to be decreased in order to prevent hypoglycemia. To the extent that these effects relate to the reduction in plasma glucagon, the data support the therapeutic concept described above.
In 2008, 14 years after its discovery (90), leptin was shown to suppress glucagon hypersecretion in T1DM rodents at least as effectively as somatostatin and without undesirable side effects (Figure 5C and refs. 91–93). Should similar results be demonstrated in humans, glucagon suppression with leptin could become a new treatment strategy for T1DM.
In rodents, continuous glucagon suppression is required to maintain glycemia within the normal range throughout the day (92). This can be achieved by continuous subcutaneous infusion of leptin to suppress the hyperglucagonemia caused by the 90% reduction of the insulin dose to eliminate hypoglycemia; the glycemic profile produced by low insulin plus leptin infusion is virtually normal. (Figure 5C). The low insulin plus leptin regimen reduces the expression of transcription factors and enzymes involved in lipogenesis and cholesterologenesis (34), presumably by eliminating the iatrogenic hyperinsulinemia required in the absence of glucagon suppression by paracrine insulin action. All in all, it would seem that conventional monotherapy with insulin is incomplete because it can provide paracrine suppression of glucagon secretion only by seriously overdosing the extrapancreatic tissues.
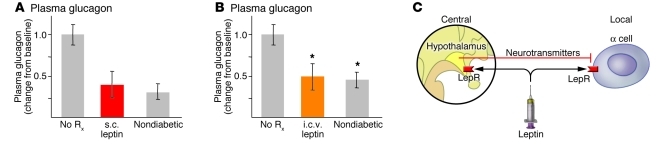
The antidiabetic glucagon-suppressing effects of peripherally induced hyperleptinemia (Figure 6A and ref. 92) have been duplicated by leptin infusion into the intracerebral ventricle (Figure 6B and ref. 91). This provides evidence for both a leptin-responsive hypothalamic pathway for glucagon expression (94) and direct leptin-mediated suppression of α cells. However, leptin could also act directly on the α cell, in a model of dual control similar to that proposed for insulin secretion (Figure 6C and ref. 55).
Figure 6. Pathways for the glucagon-suppressing action of leptin.
(A) Plasma glucagon in NOD mice treated with placebo (No Rx) or with leptin infused subcutaneously. Figure adapted with permission from Proceedings of the National Academy of Sciences of the United States of America (92). (B) Plasma glucagon in streptozotocin-diabetic mice treated with of placebo or leptin infused intracerebroventricularly. Figure adapted with permission from Proceedings of the National Academy of Sciences of the United States of America (91). (C) Proposed dual control model of α cell secretion. LepR, leptin receptor.
Summary
It is understandable, but nevertheless troubling, that the historic dimensions of the discovery of insulin in 1922 have distorted scientific and clinical perspectives of hormonal dysregulation in diabetes for so long. Even though nine decades of insulin monotherapy have taught us that insulin replacement alone cannot normalize glucose homeostasis in T1DM, while α cell research has repeatedly suggested the diabetogenic role of glucagon, no intensive effort to reduce or block glucagon actions in diabetes has yet been undertaken. Failure to translate decades of favorable preclinical evidence to the management of human diabetes must reflect insulinocentric skepticism concerning the pathophysiologic importance of diabetic hyperglucagonemia. Indeed, this is suggested in the title of the outstanding review by Gromada et al., “α-Cells of the endocrine pancreas: 35 years of research but the enigma remains” (95). It is hoped that this review will catalyze such efforts to determine whether this research can improve and extend life for diabetic patients.
Acknowledgments
This work was supported by Pfizer Inc., Takeda Inc., Amylin Pharmaceuticals, and private donations from the University of Texas Southwestern Medical School; by the High Impact Grant Fund of UT Southwestern Medical Center; and by a VA North Texas Health Care System Merit grant. Sara Kay McCorkle (Dallas VA North Texas Care System) contributed expert technical help and designed the figures. We thank Xinxin Yu (UT Southwestern Medical Center) for outstanding editorial work.
Footnotes
Conflict of interest: Alan D. Cherrington has received income from Biocon; has received income and research support from Novo Nordisk; holds equity in Amylin; and has received research support from Takeda, Pfizer, and Eli Lilly. Roger H. Unger holds equity in Merck, Amgen, and Johnson and Johnson and has received research support from Amylin, Takeda, and Pfizer.
Citation for this article: J Clin Invest. 2012;122(1):4–12. doi:10.1172/JCI60016.
References
- 1.Banting FG, Best CH, Collip JB, Campbell WR, Fletcher AA. Pancreatic extracts in the treatment of diabetes mellitus. Can Med Assoc J. 1922;12(3):141–146. [PMC free article] [PubMed] [Google Scholar]
- 2.Kimball CP, Murlin JR. Aqueous extracts of pancreas. III. Some precipitation reactions of insulin. J Biol Chem. 1923;58(1):337–346. [Google Scholar]
- 3.Cherrington A, Vranic M. Role of glucagon and insulin in control of glucose turnover. Metabolism. 1971;20(6):625–628. doi: 10.1016/0026-0495(71)90010-2. [DOI] [PubMed] [Google Scholar]
- 4.Keller U, Chiasson JL, Liljenquist JE, Cherrington AD, Jennings AS, Crofford OS. The roles of insulin, glucagon, and free fatty acids in the regulation of ketogenesis in dogs. Diabetes. 1977;26(11):1040–1051. doi: 10.2337/diab.26.11.1040. [DOI] [PubMed] [Google Scholar]
- 5.Cherrington AD, Chiasson JL, Liljenquist JE, Lacy WW, Park CR. Control of hepatic glucose output by glucagon and insulin in the intact dog. Biochem Soc Symp. 1978;43(43):31–45. [PubMed] [Google Scholar]
- 6.Exton JH, et al. The hormonal control of hepatic gluconeogenesis. Recent Prog Horm Res. 1970;26:411–461. doi: 10.1016/b978-0-12-571126-5.50014-5. [DOI] [PubMed] [Google Scholar]
- 7.Sutherland JP, McKinley B, Eckel RH. The metabolic syndrome and inflammation. Metab Syndr Relat Disord. 2004;2(2):82–104. doi: 10.1089/met.2004.2.82. [DOI] [PubMed] [Google Scholar]
- 8.Bromer WW, Sinn LG, Staub A, Behrens OK. The amino acid sequence of glucagon. Diabetes. 1957;6(3):234–238. doi: 10.2337/diab.6.3.234. [DOI] [PubMed] [Google Scholar]
- 9.Unger RH. The Banting Memorial Lecture 1975. Diabetes and the alpha cell. Diabetes. 1976;25(2):136–151. doi: 10.2337/diab.25.2.136. [DOI] [PubMed] [Google Scholar]
- 10.Unger RH, Eisentraut AM, McCall MS, Keller S, Lanz HC, Madison LL. Glucagon antibodies and their use for immunoassay for glucagon. Proc Soc Exp Biol Med. 1959;102:621–623. doi: 10.3181/00379727-102-25338. [DOI] [PubMed] [Google Scholar]
- 11.Unger RH, Eisentraut AM, McCall MS, Madison LL. Glucagon antibodies and an immunoassay for glucagon. J Clin Invest. 1961;40:1280–1289. doi: 10.1172/JCI104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Unger RH. Glucagon physiology and pathophysiology. N Engl J Med. 1971;285(8):443–449. doi: 10.1056/NEJM197108192850806. [DOI] [PubMed] [Google Scholar]
- 13.Gelling RW, et al. Lower blood glucose, hyperglucagonemia, and pancreatic alpha cell hyperplasia in glucagon receptor knockout mice. Proc Natl Acad Sci U S A. 2003;100(3):1438–1443. doi: 10.1073/pnas.0237106100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Longuet C, et al. The glucagon receptor is required for the adaptive metabolic response to fasting. Cell Metab. 2008;8(5):359–371. doi: 10.1016/j.cmet.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang J, et al. Polyomic profiling reveals significant hepatic metabolic alterations in glucagon–receptor (GCGR) knockout mice: implications on anti–glucagon therapies for diabetes. BMC Genomics. 2011;12:281. doi: 10.1186/1471-2164-12-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cherrington AD, Liljenquist JE, Shulman GI, Williams PE, Lacy WW. Importance of hypoglycemia-induced glucose production during isolated glucagon deficiency. Am J Physiol. 1979;236(3):E263–E271. doi: 10.1152/ajpendo.1979.236.3.E263. [DOI] [PubMed] [Google Scholar]
- 17.Cherrington AD, Williams PE, Shulman GI, Lacy WW. Differential time course of glucagon’s effect on glycogenolysis and gluconeogenesis in the conscious dog. Diabetes. 1981;30(3):180–187. doi: 10.2337/diabetes.30.3.180. [DOI] [PubMed] [Google Scholar]
- 18.Stevenson RW, et al. Similar dose responsiveness of hepatic glycogenolysis and gluconeogenesis to glucagon in vivo. Diabetes. 1987;36(3):382–389. doi: 10.2337/diabetes.36.3.382. [DOI] [PubMed] [Google Scholar]
- 19. Cherrington AD. Control of glucose production in vivo by insulin and glucagon,. In: Jefferson LS, Cherrington AD, Goodman HM, eds.The Handbook of Physiology: The Endocrine Pancreas and Metabolic Regulation . Vol. 2. New York, New York, USA: Oxford Press; 2001:759–785. [Google Scholar]
- 20.Stevenson RW, Williams PE, Cherrington AD. Role of glucagon suppression on gluconeogenesis during insulin treatment of the conscious diabetic dog. Diabetologia. 1987;30(10):782–790. doi: 10.1007/BF00275744. [DOI] [PubMed] [Google Scholar]
- 21.Davis MA, Williams PE, Cherrington AD. Effect of glucagon on hepatic lactate metabolism in the conscious dog. Am J Physiol. 1985;248(4 pt 1):E463–E470. doi: 10.1152/ajpendo.1985.248.4.E463. [DOI] [PubMed] [Google Scholar]
- 22.Fradkin J, Shamoon H, Felig P, Sherwin RS. Evidence for an important role of changes in rather than absolute concentrations of glucagon in the regulation of glucose production in humans. J Clin Endocrinol Metab. 1980;50(4):698–703. doi: 10.1210/jcem-50-4-698. [DOI] [PubMed] [Google Scholar]
- 23.Magnusson I, Rothman DL, Gerard DP, Katz LD, Shulman GI. Contribution of hepatic glycogenolysis to glucose production in humans in response to a physiological increase in plasma glucagon concentration. Diabetes. 1995;44(2):185–189. doi: 10.2337/diabetes.44.2.185. [DOI] [PubMed] [Google Scholar]
- 24.Muller MJ, Moring J, Seitz HJ. Regulation of hepatic glucose output by glucose in vivo. Metabolism. 1988;37(1):55–60. doi: 10.1016/0026-0495(88)90029-7. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen MF, Wise S, Dinneen SF, Schwenk WF, Basu A, Rizza RA. Assessment of hepatic sensitivity to glucagon in NIDDM: use as a tool to estimate the contribution of the indirect pathway to nocturnal glycogen synthesis. Diabetes. 1997;46(12):2007–2016. doi: 10.2337/diabetes.46.12.2007. [DOI] [PubMed] [Google Scholar]
- 26.Wada M, Connolly CC, Tarumi C, Neal DW, Cherrington AD. Hepatic denervation does not significantly change the response of the liver to glucagon in conscious dogs. Am J Physiol. 1995;268(2 pt 1):E194–E203. doi: 10.1152/ajpendo.1995.268.2.E194. [DOI] [PubMed] [Google Scholar]
- 27.Dobbins RL, Davis SN, Neal DW, Cobelli C, Jaspan J, Cherrington AD. Compartmental modeling of glucagon kinetics in the conscious dog. Metabolism. 1995;44(4):452–459. doi: 10.1016/0026-0495(95)90051-9. [DOI] [PubMed] [Google Scholar]
- 28.Liljenquist JE, Bloomgarden ZT, Cherrington AD, Perry JM, Rabin D. Possible mechanism by which somatostatin-induced glucagon suppression improves glucose tolerance during insulinopaenia in man. Diabetologia. 1979;17(3):139–143. doi: 10.1007/BF01219739. [DOI] [PubMed] [Google Scholar]
- 29.Liljenquist JE, et al. An important role for glucagon in the regulation of glucose production in vivo. Metabolism. 1976;25(11 suppl 1):1371–1373. doi: 10.1016/S0026-0495(76)80144-8. [DOI] [PubMed] [Google Scholar]
- 30.Liljenquist JE, et al. Evidence for an important role of glucagon in the regulation of hepatic glucose production in normal man. J Clin Invest. 1977;59(2):369–374. doi: 10.1172/JCI108649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holste LC, Connolly CC, Moore MC, Neal DW, Cherrington AD. Physiological changes in circulating glucagon alter hepatic glucose disposition during portal glucose delivery. Am J Physiol. 1997;273(3 pt 1):E488–E496. doi: 10.1152/ajpendo.1997.273.3.E488. [DOI] [PubMed] [Google Scholar]
- 32.Shulman GI, Liljenquist JE, Williams PE, Lacy WW, Cherrington AD. Glucose disposal during insulinopenia in somatostatin-treated dogs. The roles of glucose and glucagon. J Clin Invest. 1978;62(2):487–491. doi: 10.1172/JCI109150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roden M, et al. The roles of insulin and glucagon in the regulation of hepatic glycogen synthesis and turnover in humans. J Clin Invest. 1996;97(3):642–648. doi: 10.1172/JCI118460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109(9):1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Charlton MR, Adey DB, Nair KS. Evidence for a catabolic role of glucagon during an amino acid load. J Clin Invest. 1996;98(1):90–99. doi: 10.1172/JCI118782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foa PP, Weinstein HR, Smith JA. Secretion of insulin and of a hyperglycemic substance studied by means of pancreatic-femoral cross-circulation experiments. Am J Physiol. 1949;157(2):197–204. doi: 10.1152/ajplegacy.1949.157.2.197. [DOI] [PubMed] [Google Scholar]
- 37.Ferner H. The A- and B-cells of the pancreatic islets as sources of the antagonistic hormones glucagon and insulin; the shift of the AB-relation in diabetes mellitus. Am J Dig Dis. 1953;20(10):301–306. doi: 10.1007/BF02895538. [DOI] [PubMed] [Google Scholar]
- 38.Unger RH. Glucagon physiology and pathophysiology in the light of new advances. Diabetologia. 1985;28(8):574–578. doi: 10.1007/BF00281991. [DOI] [PubMed] [Google Scholar]
- 39.Baum J, Simons BE, Jr, Unger RH, Madison LL. Localization of glucagon in the alpha cells in the pancreatic islet by immunofluorescent technics. Diabetes. 1962;11:371–374. [PubMed] [Google Scholar]
- 40.Muller WA, Faloona GR, Unger RH. Hyperglucagonemia in diabetic ketoacidosis. Its prevalence and significance. Am J Med. 1973;54(1):52–57. doi: 10.1016/0002-9343(73)90083-1. [DOI] [PubMed] [Google Scholar]
- 41.Unger RH, Orci L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet. 1975;1(7897):14–16. doi: 10.1016/S0140-6736(01)18841-0. [DOI] [PubMed] [Google Scholar]
- 42. Samols E, Tyler J, Marks V. Glucagon insulin interrelationships. In: Lefebvre PJ, Unger RH, eds.Glucagon: Molecular Physiology, Clinical and Therapeutic Implications . Oxford, United Kindgom: Pergamon Press; 1972:157–174. [Google Scholar]
- 43.Braaten JT, Faloona GR, Unger RH. The effect of insulin on the alpha-cell response to hyperglycemia in long-standing alloxan diabetes. J Clin Invest. 1974;53(4):1017–1021. doi: 10.1172/JCI107638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le Marchand SJ, Piston DW. Glucose suppression of glucagon secretion: metabolic and calcium responses from alpha-cells in intact mouse pancreatic islets. J Biol Chem. 2010;285(19):14389–14398. doi: 10.1074/jbc.M109.069195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meier JJ, Ueberberg S, Korbas S, Schneider S. Diminished glucagon suppression after beta-cell reduction is due to impaired alpha-cell function rather than an expansion of alpha-cell mass. Am J Physiol Endocrinol Metab. 2011;300(4):E717–E723. doi: 10.1152/ajpendo.00315.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Flattem N, Igawa K, Shiota M, Emshwiller MG, Neal DW, Cherrington AD. Alpha- and beta-cell responses to small changes in plasma glucose in the conscious dog. Diabetes. 2001;50(2):367–375. doi: 10.2337/diabetes.50.2.367. [DOI] [PubMed] [Google Scholar]
- 47.Bonner-Weir S, Orci L. New perspectives on the microvasculature of the islets of Langerhans in the rat. Diabetes. 1982;31(10):883–889. doi: 10.2337/diabetes.31.10.883. [DOI] [PubMed] [Google Scholar]
- 48.Bosco D, et al. Unique arrangement of alpha- and beta-cells in human islets of Langerhans. Diabetes. 2010;59(5):1202–1210. doi: 10.2337/db09-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meda P, Kohen E, Kohen C, Orci L. [Heterocellular coupling in cultures of endocrine pancreatic cells]. C R Seances Acad Sci III. 1981;293(10):607–610. [PubMed] [Google Scholar]
- 50.Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A. 2006;103(7):2334–2339. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Unger RH, Orci L. Paracrinology of islets and the paracrinopathy of diabetes. Proc Natl Acad Sci U S A. 2010;107(37):16009–16012. doi: 10.1073/pnas.1006639107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sherrington CS. Further experimental note on the correlation of action of antagonistic muscles. Br Med J. 1893;1:1218. doi: 10.1136/bmj.1.1693.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maruyama H, Hisatomi A, Orci L, Grodsky GM, Unger RH. Insulin within islets is a physiologic glucagon release inhibitor. J Clin Invest. 1984;74(6):2296–2299. doi: 10.1172/JCI111658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paranjape SA, et al. Chronic reduction of insulin receptors in the ventromedial hypothalamus produces glucose intolerance and islet dysfunction in the absence of weight gain. Am J Physiol. Endocrinol Metab. 2011;301(5):E978–E983. doi: 10.1152/ajpendo.00304.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paranjape SA, et al. Influence of insulin in the ventromedial hypothalamus on pancreatic glucagon secretion in vivo. Diabetes. 2010;59(6):1521–1527. doi: 10.2337/db10-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koerker DJ, et al. Somatostatin: hypothalamic inhibitor of the endocrine pancreas. Science. 1974;184(135):482–484. doi: 10.1126/science.184.4135.482. [DOI] [PubMed] [Google Scholar]
- 57.Brazeau P, et al. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973;179(68):77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- 58.Dobbs R, et al. Glucagon: role in the hyperglycemia of diabetes mellitus. Science. 1975;187(4176):544–547. doi: 10.1126/science.1089999. [DOI] [PubMed] [Google Scholar]
- 59.Gerich JE, et al. Prevention of human diabetic ketoacidosis by somatostatin. Evidence for an essential role of glucagon. N Engl J Med. 1975;292(19):985–989. doi: 10.1056/NEJM197505082921901. [DOI] [PubMed] [Google Scholar]
- 60.Raskin P, Unger RH. Hyperglucagonemia and its suppression. Importance in the metabolic control of diabetes. N Engl J Med. 1978;299(9):433–436. doi: 10.1056/NEJM197808312990901. [DOI] [PubMed] [Google Scholar]
- 61.Barnes AJ, Bloom SR. Pancreatectomised man: A model for diabetes without glucagon. Lancet. 1976;1(7953):219–221. doi: 10.1016/s0140-6736(76)91339-8. [DOI] [PubMed] [Google Scholar]
- 62.Blazquez E, Munoz-Barragan L, Patton GS, Orci L, Dobbs RE, Unger RH. Gastric A-cell function in insulin-deprived depancreatized dogs. Endocrinology. 1976;99(5):1182–1188. doi: 10.1210/endo-99-5-1182. [DOI] [PubMed] [Google Scholar]
- 63.Mashiter K, et al. Persistent pancreatic glucagon but not insulin response to arginine in pancreatectomized dogs. Endocrinology. 1975;96(3):678–693. doi: 10.1210/endo-96-3-678. [DOI] [PubMed] [Google Scholar]
- 64.Muller WA, Girardier L, Seydoux J, Berger M, Renold AE, Vranic M. Extrapancreatic glucagon and glucagonlike immunoreactivity in depancreatized dogs. A quantitative assessment of secretion rates and anatomical delineation of sources. J Clin Invest. 1978;62(1):124–132. doi: 10.1172/JCI109096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ravazzola M, Baetens D, Engerman R, Kovacevic N, Vranic M, Orci L. Endocrine cells in oxyntic mucosa of a dog 5 years after pancreatectomy. Horm Metab Res. 1977;9(6):480–483. doi: 10.1055/s-0028-1093504. [DOI] [PubMed] [Google Scholar]
- 66.Munoz-Barragan L, Blazquez E, Patton GS, Dobbs RE, Unger RH. Gastric A-cell function in normal dogs. Am J Physiol. 1976;231(4):1057–1061. doi: 10.1152/ajplegacy.1976.231.4.1057. [DOI] [PubMed] [Google Scholar]
- 67.Nakabayashi H, Dobbs RE, Unger RH. The role of glucagon deficiency in the Houssay phenomenon of dogs. J Clin Invest. 1978;61(5):1355–1362. doi: 10.1172/JCI109053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bringer J, et al. Glucagon immunoreactivity and antidiabetic action of somatostatin in the totally duodeno-pancreatectomized and gastrectomized human. Diabetes. 1981;30(10):851–856. doi: 10.2337/diabetes.30.10.851. [DOI] [PubMed] [Google Scholar]
- 69.Thorel F, et al. Normal glucagon signaling and {beta}-cell function after near-total {alpha}-cell ablation in adult mice. Diabetes. 2011;60(11):2872–2882. doi: 10.2337/db11-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Derr R, Garrett E, Stacy GA, Saudek CD. Is HbA(1c) affected by glycemic instability? Diabetes Care. 2003;26(10):2728–2733. doi: 10.2337/diacare.26.10.2728. [DOI] [PubMed] [Google Scholar]
- 71.Lee Y, Wang MY, Du XQ, Charron MJ, Unger RH. Glucagon receptor knockout prevents insulin-deficient type 1 diabetes in mice. Diabetes. 2011;60(2):391–397. doi: 10.2337/db10-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Altarejos JY, Montminy M. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nat Rev Mol Cell Biol. 2011;12(3):141–151. doi: 10.1038/nrm3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brand CL, Jorgensen PN, Svendsen I, Holst JJ. Evidence for a major role for glucagon in regulation of plasma glucose in conscious, nondiabetic, and alloxan-induced diabetic rabbits. Diabetes. 1996;45(8):1076–1083. doi: 10.2337/diabetes.45.8.1076. [DOI] [PubMed] [Google Scholar]
- 74.Brand CL, Rolin B, Jorgensen PN, Svendsen I, Kristensen JS, Holst JJ. Immunoneutralization of endogenous glucagon with monoclonal glucagon antibody normalizes hyperglycaemia in moderately streptozotocin-diabetic rats. Diabetologia. 1994;37(10):985–993. doi: 10.1007/BF00400461. [DOI] [PubMed] [Google Scholar]
- 75.Rivera N, et al. A novel glucagon receptor antagonist, NNC 25-0926, blunts hepatic glucose production in the conscious dog. J Pharmacol Exp Ther. 2007;321(2):743–752. doi: 10.1124/jpet.106.115717. [DOI] [PubMed] [Google Scholar]
- 76.Cascieri MA, et al. Characterization of a novel, non-peptidyl antagonist of the human glucagon receptor. J Biol Chem. 1999;274(13):8694–8697. doi: 10.1074/jbc.274.13.8694. [DOI] [PubMed] [Google Scholar]
- 77.Parker JC, et al. Effects of skyrin, a receptor-selective glucagon antagonist, in rat and human hepatocytes. Diabetes. 2000;49(12):2079–2086. doi: 10.2337/diabetes.49.12.2079. [DOI] [PubMed] [Google Scholar]
- 78.Petersen KF, Sullivan JT. Effects of a novel glucagon receptor antagonist (Bay 27-9955) on glucagon-stimulated glucose production in humans. Diabetologia. 2001;44(11):2018–2024. doi: 10.1007/s001250100006. [DOI] [PubMed] [Google Scholar]
- 79.Qureshi SA, et al. A novel glucagon receptor antagonist inhibits glucagon-mediated biological effects. Diabetes. 2004;53(12):3267–3273. doi: 10.2337/diabetes.53.12.3267. [DOI] [PubMed] [Google Scholar]
- 80.Unson CG, Gurzenda EM, Merrifield RB. Biological activities of des-His1[Glu9]glucagon amide, a glucagon antagonist. Peptides. 1989;10(6):1171–1177. doi: 10.1016/0196-9781(89)90010-7. [DOI] [PubMed] [Google Scholar]
- 81.Ali S, Lamont BJ, Charron MJ, Drucker DJ. Dual elimination of the glucagon and GLP-1 receptors in mice reveals plasticity in the incretin axis. J Clin Invest. 2011;121(5):1917–1929. doi: 10.1172/JCI43615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Edgerton DS, Cherrington AD. Glucagon as a critical factor in the pathology of diabetes. Diabetes. 2011;60(2):377–380. doi: 10.2337/db10-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bruning JC, et al. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell. 1998;2(5):559–569. doi: 10.1016/S1097-2765(00)80155-0. [DOI] [PubMed] [Google Scholar]
- 84.Charron MJ, Gorovits N, Laidlaw JS, Ranalletta M, Katz EB. Use of GLUT-4 null mice to study skeletal muscle glucose uptake. Clin Exp Pharmacol Physiol. 2005;32(4):308–313. doi: 10.1111/j.1440-1681.2005.04189.x. [DOI] [PubMed] [Google Scholar]
- 85.Cherrington AD, Lacy WW, Chiasson JL. Effect of glucagon on glucose production during insulin deficiency in the dog. J Clin Invest. 1978;62(3):664–677. doi: 10.1172/JCI109174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Levetan C, et al. Impact of pramlintide on glucose fluctuations and postprandial glucose, glucagon, and triglyceride excursions among patients with type 1 diabetes intensively treated with insulin pumps. Diabetes Care. 2003;26(1):1–8. doi: 10.2337/diacare.26.1.1. [DOI] [PubMed] [Google Scholar]
- 87.Buse JB, Weyer C, Maggs DG. Amylin replacement with pramlintide in type 1 1nd type 2 diabetes: a physiological approach to overcome barriers with insulin therapy. Clin Diabetes. 2002;20(3):137–144. doi: 10.2337/diaclin.20.3.137. [DOI] [Google Scholar]
- 88.Nyholm B, et al. The amylin analog pramlintide improves glycemic control and reduces postprandial glucagon concentrations in patients with type 1 diabetes mellitus. Metabolism. 1999;48(7):935–941. doi: 10.1016/S0026-0495(99)90232-9. [DOI] [PubMed] [Google Scholar]
- 89.Orskov L, Nyholm B, Yde Hove K, Gravholt CH, Moller N, Schmitz O. Effects of the amylin analogue pramlintide on hepatic glucagon responses and intermediary metabolism in Type 1 diabetic subjects. Diabet Med. 1999;16(10):867–874. doi: 10.1046/j.1464-5491.1999.00164.x. [DOI] [PubMed] [Google Scholar]
- 90.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 91.Fujikawa T, Chuang JC, Sakata I, Ramadori G, Coppari R. Leptin therapy improves insulin-deficient type 1 diabetes by CNS-dependent mechanisms in mice. Proc Natl Acad Sci U S A. 2010;107(40):17391–17396. doi: 10.1073/pnas.1008025107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang MY, et al. Leptin therapy in insulin-deficient type I diabetes. Proc Natl Acad Sci U S A. 2010;107(11):4813–4819. doi: 10.1073/pnas.0909422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yu X, Park BH, Wang MY, Wang ZV, Unger RH. Making insulin-deficient type 1 diabetic rodents thrive without insulin. Proc Natl Acad Sci U S A. 2008;105(37):14070–14075. doi: 10.1073/pnas.0806993105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kalra SP. Central leptin gene therapy ameliorates diabetes type 1 and 2 through two independent hypothalamic relays; a benefit beyond weight and appetite regulation. Peptides. 2009;30(10):1957–1963. doi: 10.1016/j.peptides.2009.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gromada J, Franklin I, Wollheim CB. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev. 2007;28(1):84–116. doi: 10.1210/er.2006-0007. [DOI] [PubMed] [Google Scholar]
- 96. Cherrinton AD. Control of glucose production in vivo by insulin and glucagon. In:Handbook of Physiology . Vol. 2. 2011:759–785. [Google Scholar]