Abstract
Recombinant viruses hold promise as vectors for vaccines to prevent infectious diseases with significant global health impacts. One of their major limitations is that preexisting anti-vector neutralizing antibodies can reduce T cell responses to the insert antigens; however, the impact of vector-specific cellular immunity on subsequent insert-specific T cell responses has not been assessed in humans. Here, we have identified and compared adenovirus-specific and HIV-specific T cell responses in subjects participating in two HIV-1 vaccine trials using a vaccine vectored by adenovirus serotype 5 (Ad5). Higher frequencies of pre-immunization adenovirus-specific CD4+ T cells were associated with substantially decreased magnitude of HIV-specific CD4+ T cell responses and decreased breadth of HIV-specific CD8+ T cell responses in vaccine recipients, independent of type-specific preexisting Ad5-specific neutralizing antibody titers. Further, epitopes recognized by adenovirus-specific T cells were commonly conserved across many adenovirus serotypes, suggesting that cross-reactivity of preexisting adenovirus-specific T cells can extend to adenovirus vectors derived from rare serotypes. These findings provide what we believe to be a new understanding of how preexisting viral immunity may impact the efficacy of vaccines under current evaluation for prevention of HIV, tuberculosis, and malaria.
Introduction
Vaccines offer the most effective and durable interventions for the prevention of infectious diseases. Classic approaches to developing highly efficacious vaccines for major global health threats, including HIV, malaria, and tuberculosis, have been unsuccessful. Recombinant vectors that encode pathogen proteins hold promise, and adenovirus and pox vectors have been commonly employed for this purpose. For example, adenovirus serotype 5 (Ad5) vectors containing HIV-1 gene inserts are notable for their ability to induce strong HIV-1–specific cellular immune responses (1, 2) and have advanced to efficacy trials. One obstacle in the use of vectors based on human viruses is that naturally acquired immunity to these viruses has the potential to modulate ensuing insert-specific immune responses. The dampening effect of preexisting Ad5-specific humoral immunity, defined by type-specific neutralizing antibodies (nAbs), on the induction of HIV-specific cellular immune responses has previously been recognized (1, 2). This has led to the development of alternative human adenovirus vectors based on Ad35 and Ad26, which are under evaluation for HIV-1, tuberculosis, and malaria vaccines and may offer advantages over Ad5-based vectors because of their lower seroprevalence (3).
Concerns about reduced immunogenicity due to preexisting Ad5 immunity have focused largely on vector-specific antibodies, but little is known about Ad5-specific cellular immune responses after natural Ad5 exposure or vaccination using an Ad5 vector. The unexpected results from the Step study, showing an increased number of HIV infections in vaccinees and an early association of this risk with higher baseline Ad5 nAb titers, highlighted the need for improved understanding of vector-specific T cells, to assess their role in serving as susceptible targets for HIV infection and their interaction with their humoral counterparts (1). Identifying the epitope specificities of these responses is critical, particularly since multiple adenovirus serotypes are under evaluation as vaccine vectors, and the extent of T cell cross-reactivity between serotypes has not been defined at the epitope level. Previous studies suggested that any adenovirus could induce T cells that partially protect against many circulating serotypes (4–6) and that primarily target the hexon capsid protein (7–9). The recognition of MHC class I– and II–restricted Ad epitopes within other proteins has rarely been shown (9, 10), although most published studies are based on small cohorts and more comprehensive analyses of T cell memory responses among Ad5 proteins have not been reported.
To further our understanding of cellular immune responses against Ad5 and their impact on HIV-specific T cell responses, we conducted a comprehensive analysis in samples from more than 400 vaccine and placebo recipients enrolled in the Step study evaluating the MRKAd5/HIV-1 vaccine, as well as 35 recipients of the same vaccine from another trial (HVTN 071) conducted in parallel. We observed that a higher magnitude of baseline Ad-specific CD4+ T cell responses was inversely associated with the magnitude of HIV-specific CD4+ T cell responses and the breadth of HIV-specific CD8+ T cell responses, independent of Ad5 nAb titers. Using polychromatic flow cytometry, we determined preferred T cell targets in participants with or without preexisting Ad5 nAbs and mapped the adenovirus responses to distinct epitopic regions. We found preferential T cell targeting of epitopes within regions conserved across many serotypes, including Ad26 and Ad35. Our results emphasize that preexisting serotype-specific nAb titers do not predict cellular immunity to adenoviruses and that Ad-specific T cell responses play a major role in determining the outcome of vaccination using Ad vectors. This is an important consideration in the choice of alternate Ad vectors to circumvent preexisting adenovirus immunity in regions with high Ad5 seroprevalence.
Results
Adenovirus-specific T cell responses are readily detectable in unvaccinated Ad5-seronegative subjects.
To determine the influence of Ad5 serostatus as well as vaccination on cellular immunity to the MRKAd5 vector, we assessed Ad-specific T cell responses in 410 Step study participants: 94 Ad5-seronegative (Ad5 nAb titers ≤18) and 71 Ad5-seropositive (Ad5 nAb titers >18) placebo recipients; and 113 baseline Ad5-seronegative and 132 baseline Ad5-seropositive vaccinees. Responses were measured 4 weeks after the final (third) immunization by expression of intracellular IFN-γ and/or IL-2 after 6-hour ex vivo stimulation with empty Ad5 vector (Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI60202DS1). All magnitudes are reported as percentage of cells expressing IFN-γ and/or IL-2. Of note, baseline PBMCs were not collected in the Step study, precluding longitudinal analyses, but since participants were randomized to treatment, Ad-specific T cell responses in placebo recipients receiving saline alone should reflect those at baseline in vaccinees considering this sample size.
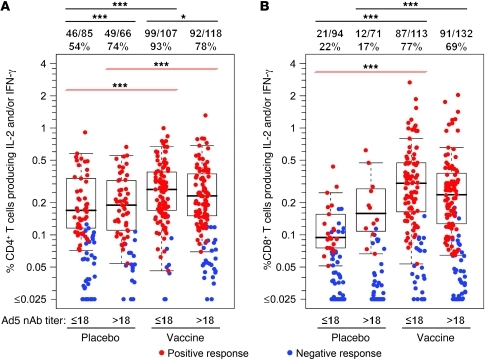
Circulating Ad-specific CD4+ T cells were readily detectable in 54% of Ad5-seronegative placebo recipients (Figure 1A) and in 74% of Ad5-seropositive placebo recipients (P = 0.0004). The magnitude of responses was similar in both groups (median 0.17% vs. 0.19% of CD4+ T cells in Ad5-seronegative vs. Ad5-seropositive placebo recipients, respectively). Following vaccination, Ad-specific CD4+ T cell responses were observed in 93% of Ad5-seronegative participants (P < 0.0001 compared with Ad5-seronegative placebo recipients) but in only 78% of Ad5-seropositive vaccinees (P = 0.05 compared with Ad5-seronegative vaccinees). Vaccination led to a significant increase in the magnitude of Ad-specific CD4+ T cell responses that was not affected by baseline Ad5 serostatus (median 0.27% in Ad5-seronegative and 0.23% in Ad5-seropositive vaccinees; P < 0.0001 each compared with placebo recipients).
Figure 1. Rates and magnitudes of Ad5 vector-specific T cell responses in 410 Step study participants 4 weeks after final immunization (week 30).
Percentages of T cells producing IFN-γ and/or IL-2 after stimulation with empty Ad5 vector are shown for CD4+ (A) and CD8+ (B) T cells. Red symbols represent positive responses; blue symbols represent responses below the positivity threshold. Box plots represent positive responses only and show medians, interquartile ranges (IQRs), and whiskers extending to the furthest point within 1.5 times the IQR from the upper or lower quartile. Subjects are grouped by treatment (placebo or vaccine) and baseline Ad5 nAb titers (≤18 or >18) as indicated. Numbers above the panels show response rates. Significant differences in response rates are shown by black lines above the figure; significant differences in response magnitudes are shown by red lines (*P = 0.05; ***P < 0.001). Participants with high background cytokine production (>0.1%) by CD4+ T cells were excluded from analysis, resulting in reduced numbers compared with CD8+ T cell responses.
Ad-specific CD8+ T cell response rates were lower than for CD4+ T cells in all groups (Figure 1B), and with the exception of one individual, CD8+ T cell responses in placebo recipients were restricted to individuals with positive CD4+ T cell responses, while vaccination led to the induction of CD8+ T cell responses in the absence of Ad-specific CD4+ T cells in 14 vaccine recipients. Ad-specific CD8+ T cell responses were detected in 22% of Ad5-seronegative placebo recipients, with a median magnitude of 0.09%. Similarly, 17% of Ad5-seropositive placebo recipients had Ad-specific CD8+ T cell responses, with a median magnitude of 0.16%. Vaccination significantly boosted response rates in both groups, to 77% in Ad5-seronegative and 69% in Ad5-seropositive vaccinees (P < 0.0001 each compared with placebo recipients), with increases in magnitude to 0.30% (P < 0.0001) and 0.24% (P = 0.06), respectively.
Baseline Ad-specific CD4+ T cell responses, in addition to Ad5 nAb titers, influence MRKAd5-induced HIV-specific responses.
While adenovirus nAbs are type specific and therefore potentially affect only a single serotype vector platform, cross-reactivity of Ad-specific T cell responses could influence vaccine regimens based on virtually any Ad vector. Therefore, we assessed whether baseline Ad-specific T cell responses influenced the breadth or magnitude of ensuing HIV-specific T cell responses after administration of Ad5-based vaccines, similar to the dampening effect of Ad5 nAb titers observed in the Step study (1). We analyzed baseline vector-specific responses in 35 recipients of the MRKAd5/HIV-1 vaccine in the HVTN 071 study. Response rates and magnitude of HIV-specific responses were measured at peak (4 weeks after the first vaccination) and at memory (1 year after the first vaccination). The influence of baseline Ad-specific responses on vaccine-induced HIV-specific immunity was assessed using models incorporating sex, number of vaccinations, Ad5 nAb titer, and Ad-specific CD4+ T cell responses (see Methods). Univariate analyses suggested that age and Ad-specific CD8+ T cell responses were not predictive of HIV-specific responses; therefore, these two parameters were not included in the final models. Of note, as shown in the Step study (1), higher Ad5 nAb titers significantly dampened both CD4+ and CD8+ insert-specific T cell responses in HVTN 071 participants.
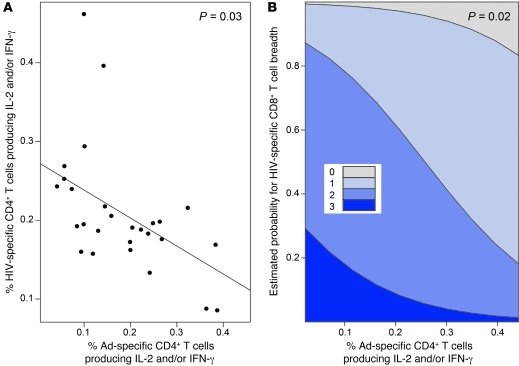
Regardless of Ad5 nAb titer, higher-magnitude baseline Ad-specific CD4+ T cell responses were associated with a reduced magnitude of memory HIV-specific CD4+ T cell responses measured 1 year after first vaccination: a 0.1% increase in the magnitude of Ad-specific CD4+ T cell frequencies was associated with a 0.04% decrease in the magnitude of HIV-specific CD4+ T cell responses (95% CI, –0.07% to –0.004%, P = 0.03; Figure 2A). Moreover, a higher-magnitude baseline Ad-specific CD4+ T cell response was also associated with decreased breadth of HIV-specific CD8+ T cell response during the peak responses at week 4. For every 0.1% increase in baseline Ad-specific CD4+ T cell magnitude, a 56% decrease in odds of a broader response was observed at week 4 (odds ratio [OR] 0.44, 95% CI 0.22–0.87, P = 0.02, Figure 2B). Thus, preexisting Ad-specific CD4+ T cell responses, in addition to Ad5 nAbs, dampen HIV-specific CD4+ and CD8+ T cell responses induced by Ad5-vectored vaccination.
Figure 2. Baseline Ad-specific CD4+ T cell responses are inversely associated with insert-specific T cell responses.
The magnitude of the baseline Ad-specific CD4+ T cell response (defined as %CD4+ T cells producing IFN-γ and/or IL-2 following stimulation with empty Ad5 vector) on the x axis is plotted against (A) the magnitude of the HIV-specific CD4+ T cell response at the memory time point or (B) the estimated probability of having HIV-specific CD8+ T cells recognizing 0, 1, 2, or 3 proteins at the peak time point on the y axes. (A) The solid line is the regression line of the predicted HIV-specific CD4+ T cell response at the memory time point given the magnitude of the baseline Ad-specific CD4+ T cell response. (B) Estimated probabilities are based on a proportional odds model (see Methods). The regression line in A and probability estimates in B have been adjusted for sex, number of vaccinations, and log10 Ad5 nAb titer distributions.
Adenovirus-specific T cells recognize epitopes that are conserved across multiple adenovirus serotypes.
To understand why T cell responses to Ad5 proteins can be detected in Ad5-seronegative individuals, we examined adenovirus epitope specificities and compared the targeted sequences across a range of adenovirus serotypes. We designed a peptide panel covering about 40% of the Ad5 genome, including core, capsid, and non-structural proteins; the peptides chosen represent early and late viral proteins contained in the Ad5 vaccine vector as well as those indicated by previous work to induce T cell responses (11). Samples were tested from 102 of the 410 participants described above, distributed equally by treatment and Ad5 nAb titer; seropositive participants were distributed equally within Ad5 nAb titer strata of 19–200, 201–1000, and greater than 1,000. To determine whether responses to the peptide pools adequately recapitulated the Ad-specific T cell responses measured by stimulation ex vivo with the Ad5 vector, we compared the response rates and magnitudes in the same donors. We observed a highly significant correlation in the responses to peptide pool versus empty Ad5 vector stimulation for both CD4+ and CD8+ T cells (Supplemental Figure 2) and confirmed the predominance of CD4+ T cell responses with use of the peptide pools.
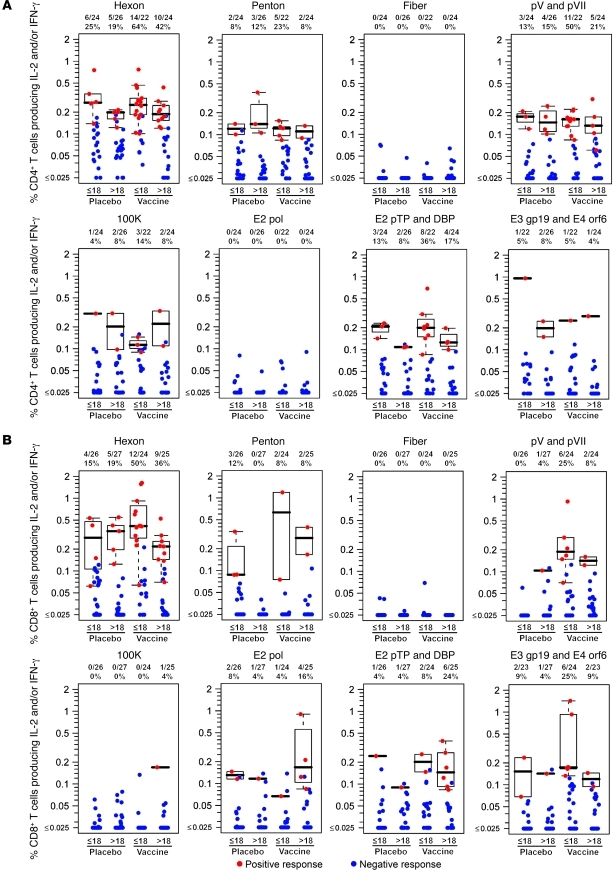
Ad-specific CD4+ T cells targeted the majority of tested proteins (Figure 3A); only fiber and polymerase failed to induce detectable responses. Hexon was most frequently targeted by CD4+ T cells, followed by pV and pVII and E2 pre-terminal protein (E2 pTP). Likewise, hexon was the preferred target of Ad5-specific CD8+ T cells (Figure 3B), followed by E3 gp19 and E4 orf6 and pV and pVII. Only fiber failed to induce a detectable CD8+ T cell response. No major differences were observed in CD4+ or CD8+ T cell specificity when stratifying by treatment or Ad5 nAb titer.
Figure 3. Rates and magnitudes of Ad5 peptide pool–specific T cell responses in Step study participants at 4 weeks (week 30) after final immunization.
(A) Percentage of CD4+ T cells producing IFN-γ and/or IL-2 after stimulation with Ad5 peptide pools in 96 participants. (B) Percentage of CD8+ T cells producing IFN-γ and/or IL-2 after stimulation with Ad5 peptide pools in 102 participants. Red symbols represent positive responses; blue symbols represent responses below the positivity threshold. Box plots represent positive responses only and show medians, IQR, and whiskers extending to the furthest point within 1.5 times the IQR from the upper or lower quartile. Subjects are grouped by treatment (placebo or vaccine) and baseline Ad5 nAb titers (≤18 or >18) as indicated. Numbers above each panel show response rates. Participants with high background cytokine production (>0.1%) by CD4+ T cells were excluded from analysis, resulting in reduced numbers compared with CD8+ T cell responses. The peptide pools are described in Supplemental Table 1. DBP, ssDNA binding protein; pol, polymerase; pTP, preterminal protein.
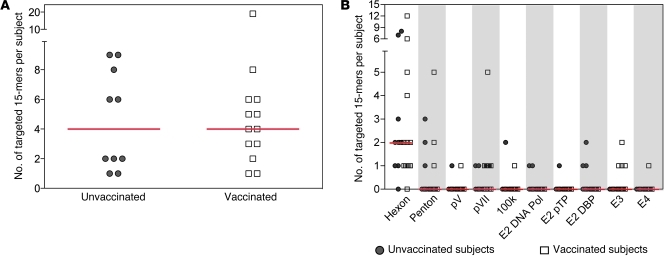
We next mapped Ad-specific T cells to single 15-mer peptides in 18 vaccinees (11 of 18 Ad5-seronegative) and 14 unvaccinated control individuals (7 of 14 Ad5-seronegative) using leukapheresis samples obtained from HVTN 071 and Seattle Area Control (SAC; see Methods) participants. Responses to at least one 15-mer were mapped in 72% (13 of 18) of vaccinees and 71% (10 of 14) unvaccinated controls. Overall, a median of 4 Ad5 epitopic regions was recognized in both groups (Figure 4A), with medians of 2 and 1.5 proteins targeted by responding vaccinees and controls, respectively (data not shown).
Figure 4. Breadth and distribution of T cell responses to Ad5 15-mers.
Responses to single Ad5 15-mer peptides were mapped by IFN-γ ELISPOT in 18 HVTN 071 vaccine recipients and 14 unvaccinated HIV-uninfected SACs. Breadth (A) and distribution (B) for each targeted 15-mer are shown for 13 HVTN 071 vaccinees (open squares) and 10 SACs (gray circles) with at least 1 positive response. Red bars represent medians. (A) Total breadth as measured by the number of targeted 15-mers across all tested proteins; two overlapping 15-mers were counted as a single response. (B) Number of targeted 15-mers per Ad5 protein (fiber generated no responses).
Responses were broadly distributed across all tested proteins (Figure 4B) except fiber (data not shown); in addition, similar to the peptide pool responses in Figure 3, hexon was the predominant target in both groups. A single hexon peptide was targeted by 10 individuals (43% of responders, PQKFFAIKNLLLL), while all other 15-mers were targeted by 5 subjects or fewer (Supplemental Table 2). Slight differences were observed in the proteins targeted by vaccinated and unvaccinated subjects, with E3 gp19 and E4 orf6 targeted only by vaccinees and E2 proteins targeted only by unvaccinated subjects, but due to the small number of individuals tested, these differences were not significant.
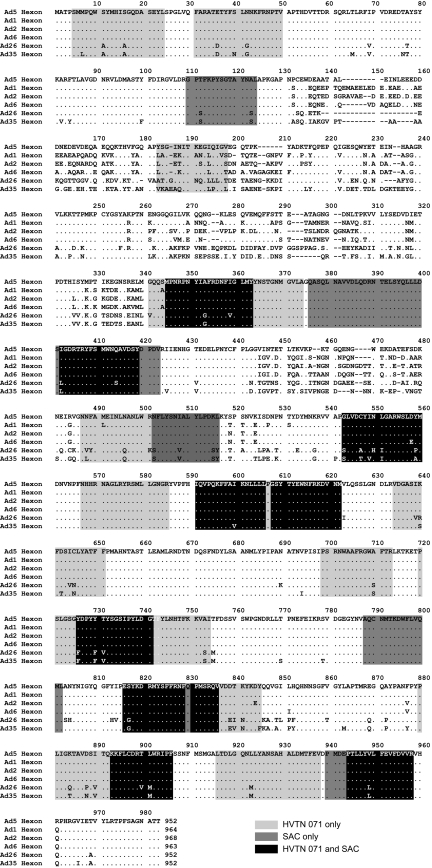
Hexon-specific T cell clones obtained from unvaccinated individuals were previously shown to be cross-reactive between adenovirus serotypes (12). To determine whether Ad5 15-mer sequences targeted by T cells from our study subjects were conserved among other serotypes, we analyzed an amino acid alignment of all group C adenoviruses (Ad1, Ad2, Ad5, and Ad6) as well as two additional serotypes currently under evaluation as vaccine vectors: Ad26 (group D) and Ad35 (group B). We focused on hexon, since it is the immunodominant target, but other proteins show similar conservation across serotypes. The majority of targeted hexon sequences fell into regions of extensive sequence homology between all analyzed serotypes (Figure 5). The overall degree of identity within hexon (the proportion of amino acids identical between Ad5 and the other serotypes) was 84% (Ad1), 85% (Ad2), and 86% (Ad6), but the degree of identity between Ad5 and other group C adenoviruses within targeted regions was significantly higher, ranging from 97% (Ad1) to 98% (Ad2, Ad6) (P < 0.0001 for all comparisons, Fisher’s exact test).
Figure 5. Targeted hexon 15-mers are conserved across adenovirus serogroups.
Amino acid alignment is shown for group C adenoviruses (Ad1, Ad2, Ad5, and Ad6), Ad26 (group D), and Ad35 (group B). Light gray represents 15-mer sequences targeted in HVTN 071 vaccine recipients only; dark gray represents 15-mer sequences targeted in unvaccinated SAC subjects only; black represents 15-mer sequences recognized by participants from both cohorts.
Interestingly, while the degree of homology between the hexon proteins of Ad5 and Ad26 as well as Ad35 was lower overall (75% and 74% identity, respectively), the degree of identity was still significantly higher within targeted regions (89% each for Ad26 and Ad35, P < 0.0001 for each comparison). Therefore, cross-reactivity of Ad-specific T cell responses with serotypes within and outside group C can be explained by targeting of conserved regions in hexon.
The N-terminal variable region of hexon (aa 130–340, Figure 5) was targeted by only one of 32 tested individuals. While the variable region could be inherently poorly immunogenic, the paucity of Ad-specific T cell responses in this region could also be due to infection by more seroprevalent adenoviruses, such as Ad1 or Ad2 (13), which would have induced T cells that only recognize regions in Ad5 that are homologous to Ad1 or Ad2. To test this hypothesis, we generated overlapping 15-mer peptides based on the sequences of Ad1 and Ad2 covering amino acids 124–353 and tested them in 12 (7 Ad5-seronegative) of the subjects described above based on sample availability. Nine of these individuals had detectable T cell responses: 5 targeted both Ad1 and Ad2, while 2 subjects each recognized either Ad1 or Ad2 (Supplemental Table 3). Ad1 and Ad2 nAb titers were determined in 8 of the 9 subjects with detectable Ad1 or Ad2 T cell responses; all were seropositive for at least 1 of the 2 viruses, with 6 seropositive for both. There was no correlation between Ad1, Ad2, or Ad5 nAb titers and the number of responses to Ad1 and Ad2 peptides (data not shown). These findings suggest that cellular immune responses could indeed have been induced by alternate adenoviral serotypes, and explain the detection of adenovirus-specific T cell responses in Ad5-seronegative individuals (Figure 1).
Discussion
Our comprehensive investigations of Ad-specific T cell responses are, to our knowledge, the first to define the impact of vector-specific cellular immunity on HIV-specific, vaccine-induced T cell responses in the context of a clinical trial. Our analyses show that higher baseline cellular immune responses to the vector attenuate vaccine-induced T cell responses to the HIV insert. Based on our epitope mapping studies and sequence analysis across serotypes, we demonstrate that adenovirus regions targeted by Ad-specific T cells are conserved not only between group C adenoviruses such as Ad5, but also adenoviruses outside group C that are currently under development as vaccine vectors based on their lower seroprevalence in target populations. Together, these findings have potentially profound implications for the use of other adenoviruses as vectors based solely on their reduced seroprevalence, without considering T cell reactivity. The cross-reactivity of Ad-specific T cell responses could potentially lead to reduced vaccine-induced immune responses in all populations with prevalent adenovirus infections of any serotype.
Unfortunately, while some of the data presented in this study were obtained using samples from the Step study, the impact of preexisting cellular Ad-specific immunity cannot be measured directly in this trial, because baseline cryopreserved PBMC samples were not stored. Since HIV-specific immune responses were extensively measured in Step and found not to correlate with risk for HIV-1 infection, it is possible but unlikely that circulating preexisting cellular immune responses to Ad5 would have had an effect on HIV acquisition in Step study vaccinees.
Interestingly, although vaccination with Ad5-vectored immunogens leads to preferred induction of insert-specific CD8+ T cell responses (1), Ad-specific responses are dominated by CD4+ T cells both in natural infection and after vaccination, even though CD8+ T cell responses increase significantly in all vaccinees regardless of Ad5 serostatus. In the current study, CD4+ T cell response rates were only boosted in Ad5-seronegative vaccinees, while no increase in CD4+ T cell response rates in Ad5-seropositive vaccinees versus placebo recipients was observed 1 month after the final vaccination. This could be explained by the requirement of both naive (14) and memory (15) CD4+ T cells for prolonged antigenic stimulus for expansion, while short antigen pulses are apparently sufficient to induce CD8+ T cells (16). The presentation of Ad5 antigens in the context of immune complexes may have limited the duration of antigen exposure, leading to the observed increase in CD8+ T cell response rates in the absence of a concurrent increase in CD4+ T cell responses. However, the possibility remains that the MRKAd5 vaccine does boost Ad-specific CD4+ T cells, which then rapidly migrate to the mucosa, precluding their detection in peripheral blood at the time points available after vaccination.
Identification of peptide-specific responses confirmed the previously reported predominance of hexon-specific CD4+ and CD8+ T cell responses (7–9), but also showed broad recognition of additional adenoviral proteins. Overall, CD4+ and/or CD8+ T cell responses were detected in 10 of the 11 tested proteins, with fiber being the only protein not targeted. The preferred targeting of hexon could be explained by its abundance in the virus particle: the 720 copies of hexon assembling the capsid account for 58% of Ad5 protein content (17). Similarly, the frequently targeted core protein pVII also accounts for greater than 10% of Ad5 protein content (17), suggesting preferred induction of immune responses to proteins present at high levels in the virion.
The preferential detection of Ad-specific T cells targeting conserved regions suggests that such responses may be boosted by repeated exposure to different adenoviruses. The recognition of Ad1- and Ad2-specific sequences from the variable region of hexon in subjects who were seropositive for Ad1 and/or Ad2 indicates that the variable region is immunogenic, and previous infections with either of these high-prevalence adenoviruses may result in preferential expansion of cross-reactive memory T cells targeting conserved regions rather than induction of new, serotype-specific T cells upon Ad5 exposure. The conservation of the targeted regions described here explains the presence of Ad-specific T cell responses in the majority of Ad5-seronegative subjects in our cohort, confirming findings by other groups (18–20). Our analyses further suggest that Ad-directed cellular responses observed in Ad5-seronegative subjects probably result from exposure to other adenoviruses. Of note, baseline T cell responses detected by O’Brien et al. using the rare Ad26, Ad35, and Ad48 viruses as antigens were of magnitude equivalent to that measured using Ad5 as stimulus in both Ad5-seropositive and Ad5-seronegative participants (18), supporting our finding that targeted epitopes are cross-reactive between rare and common adenovirus serotypes.
The mechanism for the dampening effect of preexisting Ad-specific CD4+ T cell responses on ensuing insert-specific responses is not entirely clear. The predominant function of CD4+ T cells is helping CD8+ T cells or B cells, but Ad-specific CD8+ T cell responses were not associated with HIV-specific immunity in our analysis, and nAb titers — while clearly having an effect — were included as a covariate, suggesting that the observed reduction in insert-specific immunity is not mainly explained by CD4+ T cell help for higher nAb responses. Consequently, direct interaction of Ad-specific CD4+ T cells with antigen-presenting cells that take up the replication-defective vector may be responsible for the effect. Alternatively, a CD4+ T cell cytotoxic or an immunomodulatory pathway mediated by T regulatory cells may be responsible for attenuated insert-specific immune responses. Such possibilities await further study. Regardless of the mechanism, our work may have broad implications for the use of Ad vectors in HIV vaccines. We have shown that determining the impact of preexisting immunity for adenoviruses will not only need to involve study of serotype-specific nAb responses, but should also include analyses of cellular immune responses to other cross-reactive adenoviruses both within and outside the vector’s subgroup.
Methods
Study populations.
Samples were obtained from participants enrolled in the Step (21) and HVTN 071 (a phase Ib open-label clinical trial using the same vaccine product) studies and from unvaccinated individuals enrolled in the study “Establishing Immunologic Assays for Determining HIV-1 Prevention and Control,” also referred to as SACs. Vaccine recipients received MRKAd5 HIV-1 gag/pol/nef at weeks 0, 4, and 26 in the Step study, while HVTN 071 was halted based on the Step results before participants received the third vaccination; all participants received at least one vaccination, but none received all three. Ad5 nAb titers were measured at baseline as described previously (22); titers of 18 or less defined seronegativity. Ad1 and Ad2 nAb titers were measured as described in Supplemental Methods.
Ad5 empty vector–specific immune responses.
Ad5-specific immune responses were assessed by intracellular cytokine staining using an empty Ad5 vector (ΔE1, ΔE3, ΔE4) provided by Gary Nabel at the Dale and Betty Bumper Vaccine Research Center, Bethesda, Maryland, USA (23), as previously described (1) (see Supplemental Methods). A study was performed in 17 individuals to compare the VRC vector to the empty Ad5 vector (ΔE1, WT E3, WT E4) provided by Merck (2). Responses to both vectors were highly correlated (ρ = 0.9, P < 0.0001 for both CD4+ and CD8+ T cells; data not shown), validating the use of the VRC vector for this study. Responses are reported as percentage of CD4+ or CD8+ T cells producing IFN-γ and/or IL-2. Positivity was established according to a previously published method (24).
Ad5 and HIV peptides.
We synthesized 1,773 overlapping peptides for 11 Ad5 proteins/ORFs, including core, capsid, and non-structural proteins, covering about 40% of the Ad5 genome (5,134 amino acids; Supplemental Table 1). Ad5 peptides were synthesized as 15-mers overlapping by 11 amino acids (BioSynthesis). Lyophilized peptides were resuspended in DMSO and pooled as described in Supplemental Table 1.
HIV peptides covering the inserts in the studied vaccines were global potential T cell epitope (PTE-g) peptides representing Gag, Nef, and Pol that have been previously described (25) (see Supplemental Methods).
Ad5 epitope mapping.
Epitope mapping, performed using IFN-γ ELISPOT, consisted of 4 peptide testing stages: master pool, sub-pool, matrix, and 15-mers. First, 12 Ad5 peptide master pools, consisting of 121–168 15-mer peptides each at a concentration of 1 μg/ml, were tested. Positive master pools were separated into 4 equal sub-pools and tested at 2 μg/ml. Positive sub-pools were tested in a 6 × 7 or 5 × 7 matrix at 2 μg/ml. Matrices were deconvoluted, and single 15-mers were tested at 2 μg/ml. In the first 3 rounds, the positive threshold was set at ≥2× background and ≥50 spot forming cells/million (SFC/M). At the final stage, the threshold was increased to ≥3× background. T cell responses to two consecutive 15-mer peptides were counted as a single response. Three consecutive 15-mers were counted as a single response if the overlapping sequence covered at least 8 amino acids; otherwise they were counted as two responses.
IFN-γ ELISPOT assays were performed using the Mabtech kit with 100,000 cells/well, as described in Supplemental Methods.
Statistics.
Group comparisons of treatment and serostatus were made for Ad5-specific CD4+ and CD8+ T cell response rates using multivariate logistic regression models. The covariates included to adjust for the potential imbalances and to increase the efficiency of the testing were region (North America or other), race (European descent or other), age (≤30 or >30), HSV-2 status, and circumcision status. A stepwise model selection procedure based on the Akaike information criterion (AIC) was used to reduce the number of predictors in the model (26). If a main effect (treatment or serostatus) was not included in the final model, the P value is reported as NS. For comparison of groups based on the magnitude of Ad5-specific CD4+ and CD8+ T cell responses, only samples with a positive response were included. The responses were log transformed to satisfy distributional assumptions of the subsequent linear regression modeling.
Associations between HIV-specific T cell responses and preexisting Ad5 immunity were tested using (a) multivariate linear regression for response magnitudes, (b) multivariate logistic regression for CD4+ T cell response rates, and (c) multivariate proportional odds logistic regression for CD8+ T cell response breadth. Since the CD8+ T cell response rate was high (32 of 34 vaccinees had detectable responses at peak), we assessed whether baseline Ad-specific responses influenced the breadth of the ensuing CD8+ T cell response (measured as the number of HIV proteins recognized, i.e., ranging from 0 to 3). First, univariate models were run on age (≤35, >35), sex, number of vaccinations, log10-transformed baseline Ad5 nAb titer, as well as baseline Ad-specific CD4+ and CD8+ T cell responses. These analyses suggested that number of vaccinations and sex could confound the relationship between the predictors of interest and the outcome, while age and Ad-specific CD8+ T cell responses were not predictive of HIV-specific cellular responses. Therefore, the final models included 4 baseline covariates: (a) sex, (b) number of vaccinations, (c) log10-transformed baseline Ad5 nAb titer, and (d) baseline Ad-specific CD4+ T cell response magnitude. A P value 0.05 or less was considered significant.
Study approval.
The institutional human subjects review committee at each clinical site approved all protocols prior to study initiation, and participants completed a thorough written informed consent process before study enrollment.
The following IRBs participated in the study: (a) HVTN sites: Fred Hutchinson Cancer Research Center, Seattle, Washington, USA; Emory University, Atlanta, Georgia, USA; University of Alabama at Birmingham, Birmingham, Alabama, USA; Brigham & Women’s Hospital, Boston, Massachusetts, USA; New York Blood Center, New York, New York, USA; Columbia University Medical Center, New York, New York, USA; University of Illinois at Chicago, Chicago, Illinois, USA; Fenway Community Health, Boston, Massachusetts, USA; AIDS Research Alliance, Los Angeles, California, USA; University of Pennsylvania, Philadelphia, Pennsylvania, USA; Saint Louis University New Hope Comprehensive Care Clinic, Saint Louis, Missouri, USA; Vanderbilt University Health Science Committee, Nashville, Tennessee, USA; San Francisco General Hospital (SFGH), San Francisco, California, USA; University of Rochester, Rochester, New York, USA; Asociación Civil Impacta Salud y Educación, Lima, Peru; Hospital Universitário Clementino Fraga Filho, Rio de Janeiro, Brazil; Centro de Referência e Treinamento, São Paulo, Brazil; University of Puerto Rico Medical Sciences, San Juan, Puerto Rico, USA; GHESKIO Centers, Port-au-Prince, Haiti; University of the West Indies, Kingston, Jamaica; Instituto Dermatológico y Cirugía de Piel, Santo Domingo, Dominican Republic; (b) Merck sites: North Jersey Community Research Initiative, Newark, New Jersey, USA; University of Colorado Health Sciences Center, Denver, Colorado, USA; New York University School of Medicine AIDS Clinical Trials Unit, New York, New York, USA; Center for Clinical Studies — Houston, Houston, Texas, USA; Center for Clinical Studies — Webster, Webster, Texas, USA; Care Resource, Miami, Florida, USA; AIDS Research Alliance, West Hollywood, California, USA; HIV Clinical Trials Unit IBAC, Sydney, New South Wales, Australia; Cohort St-Luc, Infectious Diseases, Montreal, Quebec, Canada; Canadian Immunodeficiency Research Collaborative Inc., Toronto, Ontario, Canada; Saint Paul’s Hospital — Immunodeficiency Clinic, Vancouver, British Columbia, Canada; Instituto Dominicano de Estudios Virológicos (IDEV), Santo Domingo, Dominican Republic; Asociación Vía Libre, Lima, Peru.
Supplementary Material
Acknowledgments
We thank the study participants for their time and effort; the HVTN Laboratory Program, SCHARP, and Core staff who contributed to study implementation and analysis; Patricia D’Souza at the NIH Division of AIDS for thoughtful discussions and support; and Stephen Voght for editorial assistance. Funding was provided by Public Health Service grant UM1 AI068618.
Footnotes
Conflict of interest: Danilo R. Casimiro and Michael N. Robertson are paid employees of Merck, own Merck stock, and have Merck stock options.
Citation for this article: J Clin Invest. 2012;122(1):359–367. doi:10.1172/JCI60202.
See the related Commentary beginning on page 25.
References
- 1.McElrath MJ, et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372(9653):1894–1905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Priddy FH, et al. Safety and immunogenicity of a replication-incompetent adenovirus type 5 HIV-1 clade B gag/pol/nef vaccine in healthy adults. Clin Infect Dis. 2008;46(11):1769–1781. doi: 10.1086/587993. [DOI] [PubMed] [Google Scholar]
- 3.Barouch DH. Novel adenovirus vector-based vaccines for HIV-1. Curr Opin HIV AIDS. 2010;5(5):386–390. doi: 10.1097/COH.0b013e32833cfe4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith CA, Woodruff LS, Rooney C, Kitchingman GR. Extensive cross-reactivity of adenovirus-specific cytotoxic T cells. Hum Gene Ther. 1998;9(10):1419–1427. doi: 10.1089/hum.1998.9.10-1419. [DOI] [PubMed] [Google Scholar]
- 5.Leen AM, et al. Fiber-modified adenoviruses generate subgroup cross-reactive, adenovirus-specific cytotoxic T lymphocytes for therapeutic applications. Blood. 2004;103(3):1011–1019. doi: 10.1182/blood-2003-07-2449. [DOI] [PubMed] [Google Scholar]
- 6.Calcedo R, Vandenberghe LH, Roy S, Somanathan S, Wang L, Wilson JM. Host immune responses to chronic adenovirus infections in human and nonhuman primates. J Virol. 2009;83(6):2623–2631. doi: 10.1128/JVI.02160-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olive M, Eisenlohr L, Flomenberg N, Hsu S, Flomenberg P. The adenovirus capsid protein hexon contains a highly conserved human CD4+ T-cell epitope. Hum Gene Ther. 2002;13(10):1167–1178. doi: 10.1089/104303402320138952. [DOI] [PubMed] [Google Scholar]
- 8.Tang J, et al. Adenovirus hexon T-cell epitope is recognized by most adults and is restricted by HLA DP4, the most common class II allele. Gene Ther. 2004;11(18):1408–1415. doi: 10.1038/sj.gt.3302316. [DOI] [PubMed] [Google Scholar]
- 9.Tang J, et al. Human CD8+ cytotoxic T cell responses to adenovirus capsid proteins. Virology. 2006;350(2):312–322. doi: 10.1016/j.virol.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 10.Souberbielle BE, Russell WC. Human T cell proliferative response to polypeptides from adenovirus type 2. J Infect Dis. 1995;172(5):1421–1422. doi: 10.1093/infdis/172.5.1421-a. [DOI] [PubMed] [Google Scholar]
- 11.McKelvey T, Tang A, Bett AJ, Casimiro DR, Chastain M. T-cell response to adenovirus hexon and DNA-binding protein in mice. Gene Ther. 2004;11(9):791–796. doi: 10.1038/sj.gt.3302232. [DOI] [PubMed] [Google Scholar]
- 12.Leen AM, et al. Conserved CTL epitopes on the adenovirus hexon protein expand subgroup cross-reactive and subgroup-specific CD8+ T cells. Blood. 2004;104(8):2432–2440. doi: 10.1182/blood-2004-02-0646. [DOI] [PubMed] [Google Scholar]
- 13.Schmitz H, Wigand R, Heinrich W. Worldwide epidemiology of human adenovirus infections. Am J Epidemiol. 1983;117(4):455–466. doi: 10.1093/oxfordjournals.aje.a113563. [DOI] [PubMed] [Google Scholar]
- 14.Obst R, van Santen HM, Mathis D, Benoist C. Antigen persistence is required throughout the expansion phase of a CD4(+) T cell response. J Exp Med. 2005;201(10):1555–1565. doi: 10.1084/jem.20042521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ravkov EV, Williams MA. The magnitude of CD4+ T cell recall responses is controlled by the duration of the secondary stimulus. J Immunol. 2009;183(4):2382–2389. doi: 10.4049/jimmunol.0900319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat Immunol. 2001;2(5):415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Berk AJ. Adenoviridae: the viruses and their replication. In: Knipe DM, Howley PM, eds.Fields Virology . Vol. 1. Philadelphia, Pennsylvania, USA: Lippincott Williams and Wilkins; 2007:2355–2394. [Google Scholar]
- 18.O’Brien KL, et al. Adenovirus-specific immunity after immunization with an Ad5 HIV-1 vaccine candidate in humans. Nat Med. 2009;15(8):873–875. doi: 10.1038/nm.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koup RA, et al. Replication-defective adenovirus vectors with multiple deletions do not induce measurable vector-specific T cells in human trials. . J Virol. 2009;83(12):6318–6322. doi: 10.1128/JVI.00384-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hutnick NA, et al. Baseline Ad5 serostatus does not predict Ad5 HIV vaccine-induced expansion of adenovirus-specific CD4+ T cells. Nat Med. 2009;15(8):876–878. doi: 10.1038/nm.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buchbinder SP, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372(9653):1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aste-Amezaga M, et al. Quantitative adenovirus neutralization assays based on the secreted alkaline phosphatase reporter gene: application in epidemiologic studies and in the design of adenovector vaccines. Hum Gene Ther. 2004;15(3):293–304. doi: 10.1089/104303404322886147. [DOI] [PubMed] [Google Scholar]
- 23.Catanzaro AT, et al. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 candidate vaccine delivered by a replication-defective recombinant adenovirus vector. J Infect Dis. 2006;194(12):1638–1649. doi: 10.1086/509258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horton H, et al. Optimization and validation of an 8-color intracellular cytokine staining (ICS) assay to quantify antigen-specific T cells induced by vaccination. J Immunol Methods. 2007;323(1):39–54. doi: 10.1016/j.jim.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li F, et al. Peptide selection for human immunodeficiency virus type 1 CTL-based vaccine evaluation. Vaccine. 2006;24(47–48):6893–6904. doi: 10.1016/j.vaccine.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 26.Hirotugu A. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19(6):716–723. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.