Abstract
Epoxyeicosatrienoic acids (EETs) generated from arachidonic acid by cytochrome P450 (CYP) epoxygenases have beneficial effects in certain cardiovascular and kidney diseases. Hence, great efforts have been made to develop drugs targeting the EET pathway. Some of these agents are currently under evaluation in clinical trials for treatment of hypertension and diabetes. In this issue of the JCI, Panigrahy et al. evaluate in a systematic way the role of CYP epoxygenases and the metabolites they generate in cancer progression. Their findings, along with previous studies, raise concerns about using these drugs in humans.
High dietary fat intake is reported to be associated with several human diseases, including diabetes and heart disease. Moreover, epidemiologic and experimental observations support the hypothesis that high dietary fat intake is also a risk factor for cancers. However, the mechanisms underlying the link between high dietary fat intake and cancer progression are poorly understood. One factor thought to be involved is arachidonic acid (AA), a major component of animal fats that is primarily found in red meats, egg yolks, and organ meats. The bioactive lipids derived from AA play critical roles in cancer progression (1).
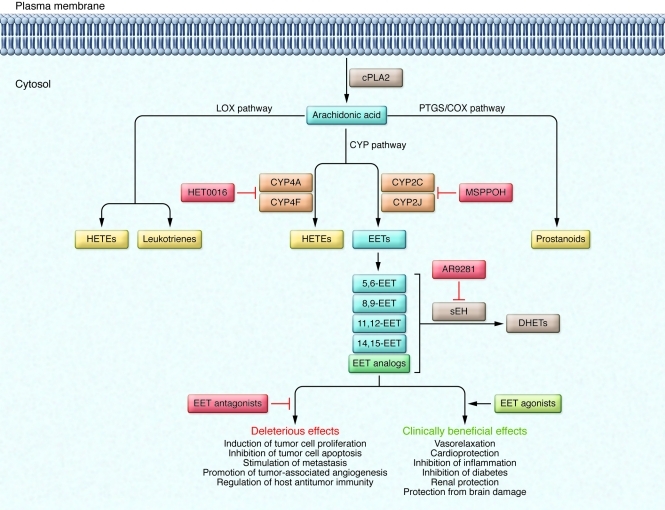
Eicosanoid synthesis pathways
AA is a polyunsaturated omega-6 fatty acid that constitutes the phospholipid domain of most cell membranes. AA is liberated by cytoplasmic phospholipase A2 (cPLA2) (Figure 1). Free AA can be metabolized to eicosanoids through three major pathways: the prostaglandin-endoperoxide synthase/cyclooxygenase (PTGS/COX) pathway, the lipoxygenase (LOX) pathway, and the cytochrome P450 (CYP) pathway. Prostanoids are the eicosanoids generated by the PTGS/COX pathway, while the LOX pathway generates leukotrienes and hydroxyeicosatetraenoic acids (HETEs). The CYP enzymes that convert AA into eicosanoids include CYP epoxygenase and CYP ω-hydroxylase enzymes. CYP epoxygenases, such as members of the CYP2C and CYP2J families, metabolize AA to four biologically active epoxyeicosatrienoic acids (EETs) (5,6-EET, 8,9-EET, 11,12-EET, and 14,15-EET). CYP ω-hydroxylases, such as members of the CYP4A and CYP4F families, convert AA to HETEs. Among the members of the CYP2C and CYP2J families of CYP enzymes, CYP2J2, CYP2C8, and CYP2C9 are the predominant epoxygenase isoforms that convert AA into EETs. All EETs are then further metabolized to less active dihydroxyeicosatrienoic acids (DHETs) by soluble epoxide hydrolase (sEH).
Figure 1. An overview of CYP epoxygenase pathways and how they can be modulated.
AA is a polyunsaturated fatty acid that constitutes the phospholipid domain of most cell membranes. It is liberated from cellular membranes by cPLA2. Free AA can be metabolized through three major pathways: the PTGS/COX pathway, the LOX pathway, and the CYP pathway. In the CYP pathway, AA is converted to EETs and HETEs by CYP epoxygenases and CYP ω-hydroxylases, respectively. The EETs are then further metabolized to less active DHETs by sEH. MSPPOH is a selective inhibitor of CYP epoxygenases, and HET0016 is a selective inhibitor of CYP ω-hydroxylases. AR9281 is a selective inhibitor of sEH and is currently under evaluation in phase II clinical trials as a treatment for patients with hypertension and type 2 diabetes.
EET signaling in cardiovascular and kidney diseases
EETs are produced primarily by endothelial cells, although they are also produced by other cell types, such as astrocytes and cardiomyocytes. Because EETs induce vasodilation and exert antiinflammatory effects in blood vessels in an autocrine manner (2), they can lower blood pressure, protect the myocardium and brain from ischemia, attenuate hypertension-induced renal damage, and reduce cigarette smoke–induced lung inflammation (3). Increasing evidence reveals that EETs govern these various biological functions by inducing endothelial cell proliferation, survival, and tube formation and stimulating renal epithelial cell proliferation and survival through multiple signaling pathways (4). Hence, great efforts have been made to develop drugs targeting these pathways. For example, 11,12-EET has been shown to improve coronary artery endothelial function when it is added to transplant preservation solutions (5). Moreover, an sEH inhibitor (AR9281) is currently under evaluation in phase II clinical trials as a treatment for patients with hypertension and type 2 diabetes on the basis of evidence that sEH inhibitors have beneficial effects in animal models of hypertension and cardiovascular diseases (3, 6). However, emerging evidence shows that EETs can promote cancer progression by directly promoting cancer cell proliferation, survival, migration, and invasion. In this issue of the JCI, Panigrahy et al. report breakthroughs in our understanding of how EET signaling in the tumor microenvironment contributes to tumor growth and metastasis (7). These results raise concerns about using EET analogs and agonists as well as sEH inhibitors to treat cardiovascular diseases.
EET signaling and cancer
Although little is known about the role of EET signaling in cancer progression, emerging evidence indicates that CYP epoxygenases and the metabolites they generate are involved in tumor biology. CYP2J2 expression is elevated in human malignant solid tumors in esophageal, liver, breast, lung, and colorectal organs, and high levels of EETs have been detected in urine and blood samples obtained from patients with these cancers (8, 9). Interestingly, CYP2C9 is specifically expressed in the tumor-associated vasculature of human renal carcinomas (10). Moreover, hypoxia induces the expression of CYP2C8 and CYP2C9 (11). These results indicate that CYP epoxygenases may be involved in tumor-associated angiogenesis.
In vitro studies have shown that both overexpression of CYP2J2 in cancer cells and treatment of cancer cells with EETs stimulate cell proliferation, survival, migration, and invasion (8, 12). Further, in mouse xenograft models, overexpression of CYP2J2 in breast cancer cells promotes lung metastasis (12), whereas treatment with a selective inhibitor of CYP2J2 suppresses breast tumor growth and lung metastasis (13). Moreover, disruption of the Cyp2c44 gene of the xenograft recipient inhibits tumor growth and tumor-associated angiogenesis (10). Yet another study showed that preventive treatment of mice with CYP2J peptide inhibited tumor growth by activating host antitumor immunity at an initial stage of an implanted murine bladder tumor, whereas continuous treatment of mice with this peptide accelerated tumor growth by suppressing host antitumor immunity at an advanced stage (14). These findings indicate that CYP epoxygenases and EETs may play an important role in the tumor microenvironment.
Although EETs are well known to stimulate angiogenesis by promoting endothelial cell proliferation, survival, migration, and tube formation, surprisingly little research has directly addressed the question of how modification of EET signaling in endothelial cells affects neoplastic growth and metastasis. In this issue of the JCI, Panigrahy and colleagues present the first direct evidence showing that elevation of EET levels in endothelial cells leads to the promotion of tumor-associated angiogenesis and metastasis (7). They found that treatment of endothelial cells with 14,15-EET promoted primary tumor growth and metastasis. Similarly, elevation of EET levels by overexpression of either CYP2C8 or CYP2J2 in endothelial cells and by cell type–nonspecific deletion of sEH also stimulated tumor growth and metastasis. By contrast, reduction of EET levels by overexpression of sEH in endothelial cells inhibited tumor growth and metastasis. Importantly, these investigators used a mouse model of prostate cancer in which the tumor develops spontaneously to confirm the results from their xenograft (subcutaneous and orthotopic) studies. Similarly, they found that treatment of mice with inhibitors of sEH accelerated tumor growth and metastasis. These data generated by Panigrahy and colleagues significantly extend our understanding of how EET signaling in the tumor microenvironment contributes to tumor growth and metastasis.
The data from human specimens and the in vitro and in vivo studies discussed here support the hypothesis that EETs may promote cancer progression by directly inducing cancer cell proliferation, survival, migration, and invasion and/or by changing the tumor microenvironment by influencing angiogenesis and immunosuppression in an autocrine and/or paracrine manner. This hypothesis has been tested in preclinical studies in which inhibitors of epoxygenase and EET antagonists were evaluated for their ability to inhibit tumor formation and growth (Figure 1). For example, treatment of glioblastoma-bearing rats with CYP epoxygenase inhibitors was found to attenuate tumor growth and tumor-associated angiogenesis (15). Similarly, the work reported in this issue of the JCI by Panigrahy et al. (7) showed for the first time that an EET antagonist could inhibit tumor growth and metastasis as well as prolong survival in several animal models. These in vivo data are consistent with a previous study in which an EET antagonist inhibited EET-induced prostate carcinoma cell migration and invasion in vitro (16). Collectively, the results discussed here not only raise concerns about developing sEH inhibitors as well as EET analogs and agonists for human use to treat cardiovascular diseases, but also support the rationale for developing EET antagonists and inhibitors of CYP epoxygenase enzymes as antitumor agents (Figure 1).
EET downstream signaling pathways in cancer
Although no EET receptor(s) have yet been clearly identified, EETs have been shown to bind to GPCRs (17, 18) and to facilitate binding activity of the PPAR/RXR heterodimer to a peroxisome proliferator response element (19, 20). Moreover, 14,15-EET induces EGFR transactivation in cancer cells in vitro (21). Indeed, EETs induce cancer cell proliferation via the EGFR/PI3K/Akt and EGFR/MAPK pathways and promote cancer cell survival through multiple pathways, including the TNF-α pathway and antioxidant enzyme–mediated pathways (8, 22). Moreover, pro-metastatic MMPs may mediate the effects of EETs on metastasis (12). The report by Panigrahy et al. (7) reveals that a VEGF signaling pathway is affected by EETs in endothelial cells. Furthermore, they found that VEGF signaling was required for EET-induced tumor-associated angiogenesis, which accelerated tumor growth and metastasis. However, it remains unclear whether EETs promote cancer progression by binding to cell-surface receptors and/or intracellular receptors such as nuclear receptors, with subsequent enhancement of cell proliferation, promotion of angiogenesis, inhibition of apoptosis, and stimulation of invasion/motility. Identification of specific EET receptors will be critical not only to further understanding of the molecular, cellular, and biological mechanisms underlying the involvement of EETs in malignant diseases, but also to enable the development of EET receptor–specific antagonists as antitumor agents.
Summary
CYP epoxygenases and the metabolites they generate, EETs, clearly have cardiovascular protective effects. However, the findings by Panigrahy et al. in this issue of the JCI (7) and other published results (8–16) indicate that EETs also promote tumor growth and metastasis in some contexts. This warrants further investigation before sEH inhibitors as well as EETs and their analogs and agonists can be considered as therapies for cardiovascular disease. Clarifying this issue is of critical importance in order to avoid harmful effects in patients who may be considered for treatment with this class of drugs.
Acknowledgments
This work is supported, in part, by NIH MERIT award R37 DK47297, R01 DK 62112, NCI P01 CA77839, and CPRIT RP100960. We also thank the National Colorectal Cancer Research Alliance (NCCRA) for generous support (to R.N. DuBois).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2012;122(1):19–22. doi:10.1172/JCI61453
See the related article beginning on page 178.
References
- 1.Wang D, DuBois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10(3):181–193. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleming I. Vascular cytochrome p450 enzymes: physiology and pathophysiology. Trends Cardiovasc Med. 2008;18(1):20–25. doi: 10.1016/j.tcm.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Imig JD, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat Rev Drug Discov. 2009;8(10):794–805. doi: 10.1038/nrd2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu X, Zhang XA, Wang DW. The roles of CYP450 epoxygenases and metabolites, epoxyeicosatrienoic acids, in cardiovascular and malignant diseases. Adv Drug Deliv Rev. 2011;63(8):597–609. doi: 10.1016/j.addr.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Yang Q, Zhang RZ, Yim AP, He GW. Effect of 11,12-epoxyeicosatrienoic acid as an additive to St. Thomas’ cardioplegia and University of Wisconsin solutions on endothelium-derived hyperpolarizing factor-mediated function in coronary microarteries: influence of temperature and time. Ann Thorac Surg. 2003;76(5):1623–1630. doi: 10.1016/S0003-4975(03)00735-5. [DOI] [PubMed] [Google Scholar]
- 6.Shen HC. Soluble epoxide hydrolase inhibitors: a patent review. Expert Opin Ther Pat. 2010;20(7):941–956. doi: 10.1517/13543776.2010.484804. [DOI] [PubMed] [Google Scholar]
- 7.Panigrahy D, et al. Epoxyeicosanoids stimulate multiorgan metastasis and tumor dormancy escape in mice. J Clin Invest. 2012;122(1):178–191. doi: 10.1172/JCI58128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang JG, et al. Cytochrome P450 2J2 promotes the neoplastic phenotype of carcinoma cells and is up-regulated in human tumors. Cancer Res. 2005;65(11):4707–4715. doi: 10.1158/0008-5472.CAN-04-4173. [DOI] [PubMed] [Google Scholar]
- 9.Chen C, et al. Cytochrome P450 2J2 is highly expressed in hematologic malignant diseases and promotes tumor cell growth. J Pharmacol Exp Ther. 2010;336(2):344–355. doi: 10.1124/jpet.110.174805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pozzi A, et al. The anti-tumorigenic properties of peroxisomal proliferator-activated receptor alpha are arachidonic acid epoxygenase-mediated. J Biol Chem. 2010;285(17):12840–12850. doi: 10.1074/jbc.M109.081554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michaelis UR, Fisslthaler B, Barbosa-Sicard E, Falck JR, Fleming I, Busse R. Cytochrome P450 epoxygenases 2C8 and 2C9 are implicated in hypoxia-induced endothelial cell migration and angiogenesis. . J Cell Sci. 2005;118(pt 23):5489–5498. doi: 10.1242/jcs.02674. [DOI] [PubMed] [Google Scholar]
- 12.Jiang JG, et al. Cytochrome p450 epoxygenase promotes human cancer metastasis. Cancer Res. 2007;67(14):6665–6674. doi: 10.1158/0008-5472.CAN-06-3643. [DOI] [PubMed] [Google Scholar]
- 13.Chen C, et al. Selective inhibitors of CYP2J2 related to terfenadine exhibit strong activity against human cancers in vitro and in vivo. J Pharmacol Exp Ther. 2009;329(3):908–918. doi: 10.1124/jpet.109.152017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Homma S, et al. Antigenic stimulation with cytochrome P450 2J expressed in mouse hepatocellular carcinoma cells regulates host anti-tumour immunity. . Clin Exp Immunol. 2009;156(2):344–352. doi: 10.1111/j.1365-2249.2009.03900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zagorac D, Jakovcevic D, Gebremedhin D, Harder DR. Antiangiogenic effect of inhibitors of cytochrome P450 on rats with glioblastoma multiforme. J Cereb Blood Flow Metab. 2008;28(8):1431–1439. doi: 10.1038/jcbfm.2008.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nithipatikom K, et al. Inhibition of carcinoma cell motility by epoxyeicosatrienoic acid (EET) antagonists. . Cancer Sci. 2010;101(12):2629–2636. doi: 10.1111/j.1349-7006.2010.01713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spector AA. Arachidonic acid cytochrome P450 epoxygenase pathway. J Lipid Res. 2009;50(suppl):S52–S56. doi: 10.1194/jlr.R800038-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang W, et al. Characterization of 14,15-epoxyeicosatrienoyl-sulfonamides as 14,15-epoxyeicosatrienoic acid agonists: use for studies of metabolism and ligand binding. J Pharmacol Exp Ther. 2007;321(3):1023–1031. doi: 10.1124/jpet.107.119651. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, et al. The antiinflammatory effect of laminar flow: the role of PPARgamma, epoxyeicosatrienoic acids, and soluble epoxide hydrolase. Proc Natl Acad Sci U S A. 2005;102(46):16747–16752. doi: 10.1073/pnas.0508081102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang X, et al. 14,15-Dihydroxyeicosatrienoic acid activates peroxisome proliferator-activated receptor-alpha. Am J Physiol Heart Circ Physiol. 2006;290(1):H55–H63. doi: 10.1152/ajpheart.00427.2005. [DOI] [PubMed] [Google Scholar]
- 21.Cheng LM, et al. The epoxyeicosatrienoic acid-stimulated phosphorylation of EGF-R involves the activation of metalloproteinases and the release of HB-EGF in cancer cells. Acta Pharmacol Sin. 2010;31(2):211–218. doi: 10.1038/aps.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu L, et al. Epoxyeicosatrienoic acids attenuate reactive oxygen species level, mitochondrial dysfunction, caspase activation, and apoptosis in carcinoma cells treated with arsenic trioxide. J Pharmacol Exp Ther. 2011;339(2):451–463. doi: 10.1124/jpet.111.180505. [DOI] [PMC free article] [PubMed] [Google Scholar]