Abstract
Many of the transcriptional and growth regulating activities of 1α,25-dihydroxyvitamin D3 [1,25-(OH)2D3] in the intestine and colon are recapitulated in the human colorectal cancer cell LS180. We therefore used this line together with chromatin immunoprecipitation-seq and gene expression analyses to identify the vitamin D receptor (VDR)/retinoid X receptor (RXR) and transcription factor 7-like 2 (TCF7L2/TCF4)/β-catenin cistromes and the genes that they regulate. VDR and RXR colocalized to predominantly promoter distal, vitamin D response element-containing sites in a largely ligand-dependent manner. These regulatory sites control the expression of both known as well as novel 1,25-(OH)2D3 target genes. TCF4 and β-catenin cistromes partially overlapped, contained TCF/lymphoid enhancer-binding factor consensus elements, and were only modestly influenced by 1,25-(OH)2D3. However, the two heterodimer complexes colocalized at sites near a limited set of genes that included c-FOS and c-MYC; the expression of both genes was modulated by 1,25-(OH)2D3. At the c-FOS gene, both VDR/RXR and TCF4/β-catenin bound to a single distal enhancer located 24 kb upstream of the transcriptional start site. At the c-MYC locus, however, binding was noted at a cluster of sites between −139 and −165 kb and at a site located −335 kb upstream. Examined as isolated enhancer fragments, these regions exhibited basal and 1,25-(OH)2D3-inducible activities that were interlinked to both VDR and β-catenin activation. These data reveal additional complexity in the regulation of target genes by 1,25-(OH)2D3 and support a direct action of both VDR and the TCF4/β-catenin regulatory complex at c-FOS and c-MYC.
1α,25-Dihydroxyvitamin D3 [1,25-(OH)2D3], the active metabolite of vitamin D3, is widely known for its role in the regulation of calcium and phosphorus homeostasis (1). Accordingly, 1,25-(OH)2D3 modulates uptake of dietary mineral by the intestine, retention of calcium via the kidney, and remodeling of the skeleton through control of both bone-forming osteoblasts and activation and replenishment of bone-resorbing osteoclasts. 1,25-(OH)2D3 is also known to control the proliferation and differentiation of a variety of cell types including those of mesenchymal, hematopoietic, and epidermal origin, and to promote the differentiation of tumor cells as well (2, 3). This latter activity is manifested in both cancer cells in culture and in a variety of in vivo models of tumor formation (3). The anticancer effects of vitamin D were suggested early on through epidemiological studies and supported more recently through studies in vitamin D receptor (VDR)-null mouse models wherein loss of the receptor leads, among other effects, to enhanced tumor formation in breast (4), increased incidence of squamous cell carcinomas in skin (5), and accelerated development of colon tumors (6).
The mechanisms through which vitamin D mediates its anticancer effects are tumor type specific and extremely diverse, and include VDR-mediated suppression of cyclins, induction of cyclin-dependent kinases such as p21 and p27 (7, 8), and modulation of a wide variety of genes associated with differentiated cell phenotypes. In the case of colorectal cancer (CRC) cells, it is hypothesized that the antitumor effects of the VDR are mediated by antagonist actions of ligand-activated VDR on pathways that include canonical WNT signal transduction (6, 9, 10). The former pathway represents a regulatory master switch that controls epithelial cell proliferation and is instrumental in stimulating activation and translocation of the coregulator β-catenin from the cytoplasmic compartment to the nucleus (11). There, this factor interacts directly with DNA-bound T-cell specific factor (TCF)/lymphoid enhancer-binding factor (LEF) transcription factors, reverses the suppressive effects of these latter factors on specific gene activity, and facilitates their transcriptional up-regulation. Gene targets of the TCF/β-catenin complex are known to include among others c-FOS, c-JUN, and c-MYC (12). In that regard, the VDR has been shown to suppress the activity of β-catenin, divert its activity away from TCF-regulated target genes, and regulate the export of this transcription factor from the nucleus (10). Muñoz and colleagues (13) have pioneered the study of many these actions of 1,25-(OH)2D3 extensively over the past decade, finding that the vitamin D ligand also modulates the expression of genes that regulate the activity of the WNT pathway including the antagonists DKK1, DKK4, and membrane-associated E-cadherin, all of which function to reduce the nuclear activity of β-catenin. Importantly, these investigators have also discovered that the VDR gene itself is down-regulated via the transcription factor SNAIL (14, 15). This reduction in VDR expression increasingly limits the inhibitory activity imposed by 1,25-(OH)2D3 at the level of the WNT/β-catenin pathway during cancer progression, and this activity may contribute to eventual resistance to 1,25-(OH)2D3 (16). Given the underlying complexity of unregulated growth in cancer cells, however, it seems likely that additional mechanisms of inhibition by the vitamin D hormone may also contribute.
In the current studies, we defined the cistromes for both VDR/retinoid X receptor (RXR) and TCF7L2(TCF4)/β-catenin in the LS180 CRC cell line and identified a number of genes that were regulated by each of these two pathways independently in the colon in vivo. We then used these data sets to identify an overlapping cohort of genes that were regulated by both signaling mechanisms. This small subset of genes was strongly associated with growth control and included both c-FOS and c-MYC. Additional studies of these two genes defined the locations and activities of the enhancers that mediate the responses of these genes to both WNT activators and 1,25-(OH)2D3. Our results suggest that, in addition to the indirect mechanisms that appear to mediate the antiproliferative properties of 1,25-(OH)2D3 in CRC cells, the hormone also exerts direct effects on both c-FOS and c-MYC.
Results
The LS180 CRC cell line
The LS180 cell line represents an excellent model for exploring underlying transcriptional mechanisms that are responsible for the control of gene expression by 1,25-(OH)2D3 in the intestinal tract (17). Our previous studies described the induction by 1,25-(OH)2D3 of several genes including CYP24A1 and TRPV6 and others (18, 19). To evaluate transcriptional response to 1,25-(OH)2D3 in a more comprehensive manner, we treated cells with either vehicle or 1,25-(OH)2D3 and conducted a DNA microarray analysis of the isolated RNA at 24 h. As documented in Supplemental Fig. 1 and Supplemental Table 1 published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org, the results of this analysis indicate that many of the genes known to be induced in the intestinal tract and responsible for vitamin D catabolism, calcium and phosphate uptake, secondary bile acid metabolism, and xenobiotic degradation were all modulated by 1,25-(OH)2D3, including CYP24A1, TRPV6, CLDN2, CALB1, ABCB1, CYP3A4, and CYP2B6. For a selected subset of genes, these transcriptional responses to 1,25-(OH)2D3 were further characterized by direct RT-PCR analysis as a function of both time- and dose dependency (Supplemental Fig. 1); the results together with many previous studies indicate that these responses likely represent direct responses to the vitamin D hormone. Genes such as c-FOS, c-JUN, CCND1, CDH1, and AXIN2 that control cell proliferation were also regulated, highlighting this facet of 1,25-(OH)2D3 activity. Given this transcriptional responsiveness, we also examined the antiproliferative effect of the ligand on the LS180 cell line. As seen in Fig. 1A, 1,25-(OH)2D3 suppressed LS180 cell growth as well and, although modest, did so in a fashion similar to that observed for previously investigated colorectal cancer cell lines such as Caco-2, HCT116, and adherent SW480 (10, 20). The biological effects of 1,25-(OH)2D3 observed on gene expression in the LS180 cell line as well as its inhibitory effect on proliferation prompted us to employ this line for further analysis of 1,25-(OH)2D3 action at the genome-wide level.
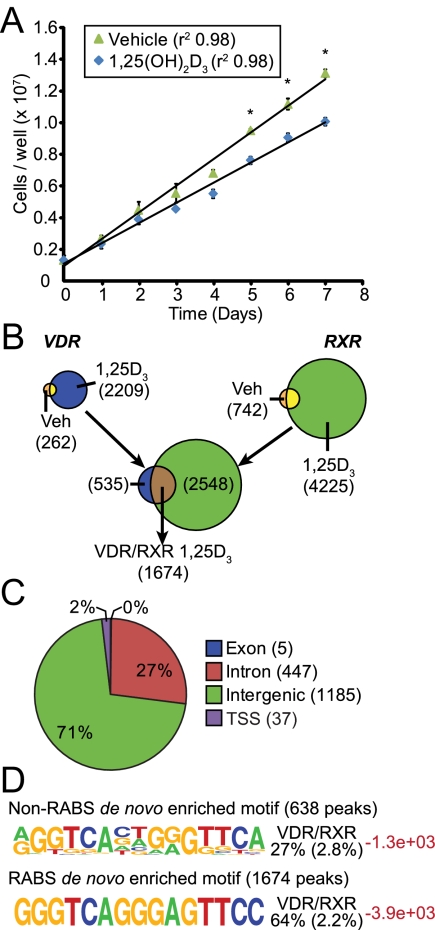
Fig. 1.
1,25-(OH)2D3 activation of VDR and RXR defines the transcriptional cistrome. A, LS180 cells are growth inhibited after treatment with 1,25-(OH)2D3. Cells were grown in culture with ethanol vehicle (green triangles) or 10−7 m 1,25-(OH)2D3 (blue diamonds) for 7 d in culture. Data are displayed from a triplicate analysis ± sem (*, P < 0.05). B, The VDR and RXR binding sites are depicted schematically as Venn diagrams with the diagram size proportional to the number of sites discovered in parentheses. The VDR+1,25-(OH)2D3 (1,25D3) (2209) and RXR+1,25-(OH)2D3 (1,25D3) (4225) peaks yielded an overlap of 1674 peaks in direct contact with each other and are listed as VDR/RXR 1,25D3. C, Peaks were annotated to the hg18 genome to the nearest gene promoter and defined as intragenic (exon, intron), promoter (within −500/+500 bp), or intergenic (>−500 bp or any distance downstream of 3′-untranslated region). D, HOMER analysis revealed the top de novo enriched motifs (frequency logos) in the VDR/RXR peaks associated with RABS (bottom) or NRABS. Each logo is accompanied by the percent enrichment within the peak population compared with sequence enrichment in 50,000 random sequences (in parentheses). Log P values are provided in red. Veh, Vehicle.
The VDR/RXR cistrome
We next quantitated the number of DNA-binding sites for the VDR and its heterodimer partner RXR across the LS180 genome using ChIP-seq analysis, characterizing the properties of these binding sites using the bioinformatic tools described in Materials and Methods. Cells were treated with either vehicle or 1,25-(OH)2D3 for 3 h [a time point at which VDR DNA binding was determined previously to be maximal (19)] and then subjected to the above analyses using validated antibodies to either VDR or RXR. The RXR antibody interacts preferentially with RXRα but maintains some cross-reactivity for both RXRβ and RXRγ. Both RXRα and RXRβ transcripts represent the most abundant of RXR isoforms found in LS180 cells (data not shown). As documented in Fig. 1B, 262 binding sites were present for the VDR across the LS180 genome in vehicle-treated cells [false discovery rate (FDR) < 0.001]. This number increased to 2209 after activation by 1,25-(OH)2D3, indicating that VDR DNA binding was largely dependent upon the presence of ligand. Under basal conditions, a substantially higher number of binding sites for RXR (742) was observed, a number which was similarly increased approximately 6-fold upon 1,25-(OH)2D3 treatment. Although this number for RXR is higher than that for VDR, many of these RXR peaks were associated with modest VDR binding levels that did not meet the statistical threshold we had established initially for these analyses, suggesting the possibility that the antibody used to detect RXR binding activity was more efficient. Perhaps most important, however, was the finding that RXR co-occupied 1674 of the 2209 binding sites identified statistically for VDR, an observation that supports the prevailing hypothesis that activation of gene expression by 1,25-(OH)2D3 frequently involves both VDR and RXR. Interestingly, as seen in Fig. 1C, genomic annotation coupled to nearest gene neighbor analysis revealed that the majority of these sites were positioned distal rather than proximal to gene promoters (within 1 kb of the TSS). Thus, 98% of VDR/RXR binding sites were located in either distal intergenic regions or within introns. This observation is consistent with findings for many transcription factors the genomic binding sites of which have been determined recently using unbiased, genome-scale techniques (21–23).
Vitamin D response element (VDRE) de novo motif discovery
We also performed de novo motif finding analysis across the 1674 VDR/RXR peaks to identify a DNA sequence(s) that could function as a direct binding site for the VDR heterodimer. As observed in Fig. 1D, the most common DNA sequence element found in this analysis was a known VDRE-like motif comprised of two directly repeated hexanucleotide half-sites separated by 3 bp. Interestingly, however, whereas 638 of these were located within genomic segments that were unique [termed “non-repeat-associated sites” or NRABS (24)], 1073 were found within Long Interspersed Nuclear Element (LINE) repeat segments of DNA (termed “repeat associated sites” or RABS), as seen in Supplemental Fig. 2. Indeed, more than 80% of the sequences found within this latter class of sites contained nearly exact sequence identity. The significance of VDR/RXR binding within these repeated regions, which has also been identified for other transcriptions factors including the estrogen receptor (ER), MYC, SOX2, and CTCF (24, 25), is unclear, although we note that at least one of these sites is positioned near a gene (TNFA1P8LS) that appears to be modestly regulated by 1,25-(OH)2D3 (see Supplemental Fig. 2A). Despite this surprising complexity, our analyses have revealed the quantitative nature of VDR/RXR binding activity across the LS180 genome and highlight both anticipated, as well as unanticipated, features of this binding activity. In view of the uncertainty of VDR/RXR function at the RABS in the repeated regions, we focused our subsequent analyses on the collection of 638 sites of VDR action identified in unique regions of the LS180 genome.
De novo motif finding reveals additional participating transcription factors
The presence of a recognizable DNA motif(s) located near authenticated transcription factor binding sites has been used to predict the involvement of additional regulatory proteins at specific gene loci. In a genome-wide context, these factors may represent chromatin pioneering, cell lineage-determining or tissue-specific factors the actions of which predispose unique gene subsets for subsequent secondary regulation (26). Given our identification of the VDR/RXR cistrome in LS180 cells, we probed regions adjacent to the heterodimer cistrome for motif enrichment that could be representative of such binding sites. As seen in Supplemental Fig. 3A, this analysis revealed an increased frequency of potential binding sites for CCAAT enhancer binding protein (C/EBP)β, ETS1, activator protein 1, and caudal type homeobox 2 (CDX2), many located within 100 bp of the peak centers for the 638 non-RABS VDR/RXR binding sites. To confirm several of these relationships, we conducted a genome-wide ChIP-seq analysis for both C/EBPβ and CDX2. As documented in Supplemental Fig. 3, B and C, the cistromes for C/EBPβ and CDX2 at the LS180 cell genome were comprised under basal conditions of 16236 and 6288 high-confidence binding sites, respectively, and were only modestly affected by 1,25-(OH)2D3 treatment. Additional features of these collections of binding sites are enumerated in Supplemental Fig. 3D and Supplemental Fig. 4. For example, as seen in Supplemental Fig. 3D, direct analysis of the tag densities for these two factors, at sites of VDR/RXR interaction, suggest that 25% of the 638 VDR/RXR binding sites contained C/EBPβ or CDX2 sites nearby. Identification of the roles of these two factors in the regulation of genes by 1,25-(OH)2D3 is the subject of ongoing work.
VDR/RXR and the regulation of representative target genes
The results of the above experiments confirm and extend mechanistic studies conducted previously on several genes known to be regulated by 1,25-(OH)2D3 in the intestine or colon including CYP24A1, TRPV6, CYP2B6, and CYP3A4 (see representative ChIP-seq profiles in Supplemental Fig. 5). Importantly, the studies not only confirmed the presence and location of most previously characterized enhancers but, in several cases, also identified additional novel regions with regulatory potential as well. The gene expression studies, together with ChIP-seq analyses, also provide new mechanistic insight into how previously unidentified gene targets are modulated by 1,25-(OH)2D3. One interesting example is the activation by 1,25-(OH)2D3 of members of the peptidylarginine deiminase (PADI) gene family (27, 28). Several members of this family have been shown to be responsive to 1,25-(OH)2D3 in human keratinocytes (29), although the mechanism of activation was not defined. As can be seen in Fig. 2A, a single VDR/RXR regulatory site was located within an intron of PADI1 (see an expanded analysis of this locus in Supplemental Fig. 5) and could facilitate the strong induction by 1,25-(OH)2D3 of PADI3 and perhaps other members as well, as seen in Fig. 2B. To determine whether this enhancer was capable of mediating response to 1,25-(OH)2D3, we cloned both a DNA segment of 600 bp (A/B) that encompassed the entire regulatory site and two smaller subfragments (A and B) into the thymidine kinase-luciferase reporter plasmid and assessed the activity of these segments after transfection into the LS180 cell line. The results, as seen in Fig. 2C, clearly show that this isolated fragment retains the capacity to mediate activity by 1,25-(OH)2D3. We also noted a potential VDR/RXR binding site (VDRE) within this intronic segment as well (data not shown). Although neither basal nor inducible enhancer activity was examined in the context of the gene cluster itself, an effort that will require the recombinant preparation and stable integration of large DNA segments into the LS180 cell line, it is clear that this isolated DNA fragment derived from the PADI1 intron is able to mediate a robust response to 1,25-(OH)2D3.
Fig. 2.
The PADI locus is regulated by novel VDR/RXR binding. A, The PADI locus is comprised of all PADI genes within a 350-kb region (chr1: 17,257,762–17,609,924). ChIP-seq tag density profiles for VDR-1,25D3, RXR-1,25D3, and input in the presence of 1,25-(OH)2D3 are displayed centered around the PADI1 and PADI3 genomic locus (chr1: 17,400,675–17,469,931) with gene transcriptional direction indicated by the arrow. The ChIP-seq tag densities have been normalized to 1 × 107 tags with the tag maximum for the data depicted on the top left of each track. FDR threshold of 0.001 is represented as a dashed line for each tag density track. The major peak of VDR/RXR binding occurs within an intron of PADI1. B, LS180 cells were treated with increasing concentrations of 1,25-(OH)2D3 (10−9 to 10−7) or ethanol vehicle (V) for 24 h. RNA was reverse transcribed and analyzed by qPCR for PADI1, PADI2, PADI3, PADI4, and PADI6 and displayed as fold change over vehicle. Each point was averaged and normalized to β-actin ± sem for a triplicate set of assays. *, P < 0.05 as compared with vehicle treatment. C, DNA fragments (400–600 bp) containing either the TRPV6 −2.1 kb and −4.3 kb regions, control regions around the PADI1 promoter (1-PRO), PADI3 promoter (3-PRO) or the full peak region (A/B) as well as partial A or B regions were cloned into the pTK-luciferase (tkluc) reporter vector and were evaluated for transcriptional activity in LS180 cells after treatment for 16 h with either ethanol vehicle (V) or 1,25-(OH)2D3 (10−9 to 10−7 m). Each point represents the relative light unit average normalized to β-gal ± sem for a triplicate set of assays. *, P < 0.05 as compared with vehicle treatment within construct. a, P < 0.05 to determine differences in basal levels as compared with vehicle treatment of 1-PRO and 3-PRO constructs. These results are representative of at least three similar experiments. chr, Chromosome; Norm, normalized.
Defining the TCF4/β-catenin cistrome
Constitutive activation of the WNT pathway through mutation of the adenomatous polyposis coli complex provides proliferative drive for many CRC cell lines (30, 31). Although the precise WNT pathway defect in LS180 cells in not entirely clear, it is certain that the transcriptional activity of the coactivator β-catenin is exerted in an uncontrolled constitutive fashion in part by an adenomatous polyposis coli mutation. Previous studies have suggested that 1,25-(OH)2D3 suppresses the activity of the WNT-signaling pathway through a direct interaction with β-catenin, through up-regulation of e-cadherin or, possibly, through induction of antagonists of the WNT pathway (6, 10, 16). One or more of these or perhaps a novel mechanism may mediate the suppression we observed with 1,25-(OH)2D3, as seen in Fig. 1A. To explore this suppression further, we used ChIP-seq analysis to identify the β-catenin and TCF4 cistromes in LS180 cells and to evaluate the influence of 1,25-(OH)2D3 on these cistromes. We then identified genes regulated by both the VDR/RXR and the TCF4/β-catenin heterodimers. As seen in Fig. 3, A and B, ChIP-seq analysis revealed approximately 828 β-catenin and 3161 TCF4 binding sites in LS180 cells. After treatment with 1,25-(OH)2D3, these numbers decreased modestly for the former and increased significantly for the latter. Not surprisingly, given that genome binding of the coactivator β-catenin is mediated directly by TCF4 and other members of the TCF/LEF family, the most common de novo motif enriched at these collections of TCF4 and β-catenin binding sites is a TCF/LEF consensus site. Several additional characteristics of these cistromes are provided in Supplemental Fig. 6. We then examined the overlap between the binding peaks for TCF4 and β-catenin. Interestingly, as can be seen in Fig. 3C, only about 20% of β-catenin binding sites colocalized with those for TCF4, supporting the idea that the β-catenin antibody may be less efficient than that for TCF4, but also because β-catenin functions as a coregulator for other transcription factors such as the FOXO family as well (32). A direct analysis of the tag density of β-catenin across the 3612 TCF4 sites, however, indicated that in those instances where overlap was present, β-catenin binding was centered at sites that bound TCF4. Interestingly, as also observed in Supplemental Fig. 6, the binding sites of C/EBPβ and particularly CDX2 were also strongly colocalized to the TCF4 sites as well. These results suggest that TCF4 is prebound to numerous sites across the LS180 genome and that a significant percentage of these sites also contain activated β-catenin as well. Finally, treatment with 1,25-(OH)2D3 appears to reduce the total number of β-catenin binding sites on the genome, suggesting a possible impact of the vitamin D ligand on redistribution of this cofactor to other sites within the cell.
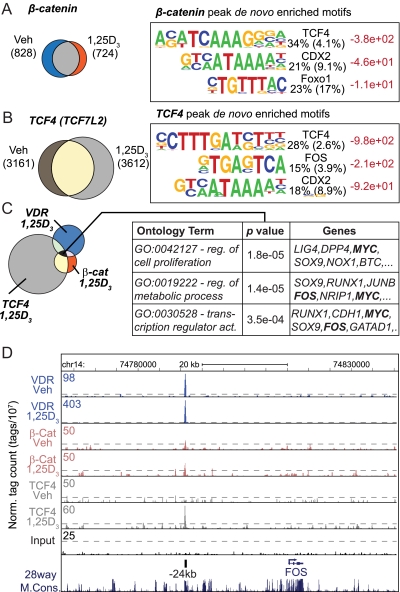
Fig. 3.
β-Catenin and TCF4 cistrome analysis. A, Cistrome analysis of β-catenin reveals 828 peaks in the untreated and 724 in the 1,25-(OH)2D3-treated state; 431 peaks overlap as displayed by Venn diagram (left). The β-catenin peaks were interrogated with the HOMER analysis for de novo motif discovery. Each logo is displayed as in Fig. 1D. B, Cistrome analysis of TCF4 reveals 3161 peaks in the untreated and 3612 in the 1,25-(OH)2D3-treated state; 2026 peaks overlap as displayed by Venn diagram (left). The TCF4 peaks were interrogated by HOMER analysis for de novo motif discovery. Each logo is displayed as in Fig. 1D. C, Cistrome analysis of TCF4, β-catenin, and VDR was performed and the peak overlaps were displayed by Venn diagram (left). Seventy four peaks were common to VDR, TCF4, and β-catenin (black) and annotated to the nearest gene promoters. Gene Ontology (GO) analysis was performed on the annotated set of genes from the 74 peaks. A few of the top enriched GO terms are displayed (left). The Gene Ontology (GO) terms (reg.. regulation; act., activation) (left), P values (center), and associated genes (right) are listed. D, ChIP-seq tag density profiles for VDR (Veh, 1,25D3), β-catenin (β-cat) (Veh, 1,25D3), TCF4 (Veh, 1,25D3) and input centered on the c-FOS genomic locus (chr14: 74,762,115–74,840,552) are displayed with the direction of transcription indicated by the arrow. The ChIP-seq tag densities are normalized to 1 × 107 tags with the tag maximum for the data depicted on the top left of each track. FDR threshold of 0.001 is represented as a dashed line for each tag density track. The major peak of VDR/RXR binding is annotated at −24 kb upstream of the c-FOS TSS. Chr, Chromosome; Veh, vehicle.
VDR/RXR and TCF4/β-catenin co-occupy a small subset of growth-regulating targets
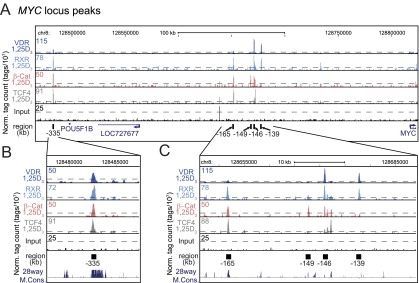
Examination of the overlap between cistromes for TCF4, β-catenin, and VDR, as seen in Fig. 3C, indicates that VDR binds to a small but significant number of nonoverlapping β-catenin and TCF4 sites and to an equally restricted set of 74 sites that are bound by both β-catenin and TCF4. To explore this set of targets, we conducted an ontology analysis of the genes that were located nearby. As can be seen in Fig. 3C, these genes were found to represent regulators of metabolic processes, transcriptional activity, and cell proliferation. Of the genes highlighted, perhaps the most interesting were those for SOX9, c-FOS, and c-MYC. We therefore examined the ChIP-seq profiles for two of these factors directly. As can be seen in Fig. 3D, VDR/RXR heterodimer binding was strongly induced by 1,25-(OH)2D3 at the c-FOS gene locus to a single novel site located −24 kb upstream of the c-FOS promoter. TCF4 and β-catenin, as well as CDX2, were also associated with this region in the presence of 1,25-(OH)2D3 (Supplemental Fig. 5). Little activity was seen near the c-FOS promoter or at other sites surrounding the c-FOS transcription unit itself. With regard to the c-MYC locus, multiple activities were observed upstream of the TSS at −139, −146, −149, −165, and −335 kb, as seen in Fig. 4A. The results in Fig. 4, B and C, also show that 1,25-(OH)2D3-inducible VDR and RXR binding was found primarily at the −139 and −146 regions and that inducible TCF4 and β-catenin binding was found at −146 kb. Basal levels of β-catenin were similarly observed at −149 kb (Supplemental Fig. 5). Both of these sets of transcription factors were either present or induced by 1,25-(OH)2D3 at the −165 kb site. Finally, the site at −335 kb, shown previously to be associated with elevated risk for colorectal, breast, and prostate cancer and to mediate activation by the TCF4/β-catenin complex (33, 34) was occupied by not only that complex, but by inducible VDR and RXR as well (Fig. 4B). The transcription factor binding activities at each of these sites were all validated within the c-MYC locus through traditional ChIP-qPCR analyses, as documented in Supplemental Fig. 7. These binding profiles were also replicated on the c-MYC gene in Caco-2 (Supplemental Fig. 8A) but not HCT116 (Supplemental Fig. 8B) cells, the latter from a tumor (35) different from the Duke's type B tumor type from which LS180 cells were derived (36). In the HCT116 cell line, 1,25-(OH)2D3 did not induce the VDR at the −139 through −165 kb regions despite the presence under basal conditions of other transcription factors including RXR (Supplemental Fig. 8B). Binding activities at these intermediate sites (−139 through −165 kb) may be CRC cell-specific because they were not observed in MCF7 breast cancer cells despite detection of residual activities at the −335 kb enhancer (Supplemental Fig. 8C and Ref. 37). We conclude from these analyses that our results have both confirmed and extended transcription factor activities at the c-MYC enhancer discovered previously at −335 kb (33, 34) but have also identified an additional set of enhancers between −139 kb and −165 kb that bind differential sets of potentially regulatory transcription factors as well. Surprisingly, little activity was seen elsewhere across the locus including areas immediately surrounding the c-MYC gene itself or near its promoter (38). These findings suggest that a more complex set of novel regulatory regions may be responsible for the regulation of c-MYC expression in CRC cells.
Fig. 4.
VDR/RXR, β-catenin, and TCF4 colocalize to the −335 kb region as well as to a novel region approximately −145 kb from the c-MYC gene. A, ChIP-seq tag density profiles for VDR-1,25D3, RXR-1,25D3, β-catenin-1,25D3, TCF4–1,25D3, and input are centered on the c-MYC genomic locus (chr8: 128,451,376–128,834,335) and displayed with the direction of gene transcription indicated by the arrow. The ChIP-seq tag densities have been normalized to 1 × 107 tags with the tag maximum for the data depicted on the top left of each track. FDR threshold of 0.001 is represented as a dashed line for each tag density track. B, Focused view of the −335 kb c-MYC binding region (chr8: 128,478,500–128,487,500). The −335 kb region is annotated as a black box. C, Focused view of the −145 kb c-MYC binding region (chr8: 128,646, 218–128,689,044). The binding regions −165 kb, −149 kb, −146 kb, and −139 kb are annotated by black boxes. Chr, Chromosome; Norm, normalized; β-Cat, β-catenin.
Regulation of c-FOS and c-MYC by ligand-activated VDR and TCF4/β-catenin
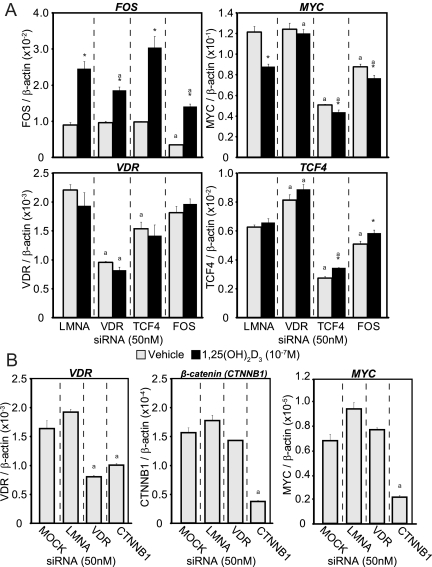
Gene expression analysis (see Supplemental Fig. 1 and Supplemental Table 1) revealed that levels of c-FOS were strongly induced and that levels of c-MYC were slightly decreased by 1,25-(OH)2D3. To assess these effects in more detail, we transfected LS180 cells with small interfering RNA (siRNA) for LMNA, VDR, c-FOS, and TCF4 and 48 h later were treated with either vehicle or 1,25-(OH)2D3 for an additional 24 h. As observed in Fig. 5A, each of the siRNA effectively and selectively reduced the expression of each of their mRNA targets by at least 50% or more (including LMNA control, data not shown). 1,25-(OH)2D3 treatment induced c-FOS expression while suppressing c-MYC expression; no effect was observed on transcripts for either VDR or TCF4. Reduction in VDR expression by siRNA, however, reduced 1,25-(OH)2D3 induction of c-FOS and eliminated the suppression observed on c-MYC. These results support the anticipated effects of the hormone on these two regulatory genes. Reduction of TCF4 expression had no effect on c-FOS expression, and only a modest negative effect on basal VDR expression, but strikingly down-regulated c-MYC mRNA, suggesting that basal c-MYC expression was indeed supported strongly through activation of the TCF4/β-catenin pathway. Finally, down-regulation of c-FOS had no effect of VDR expression but resulted in a significant suppression of both c-MYC and TCF4 transcripts. In a separate experiment, we also examined the effects of siRNA for β-catenin on VDR and c-MYC expression. As can be seen in Fig. 5B, down-regulation of β-catenin transcripts resulted in a suppression of both VDR and c-MYC RNA as well. These results support the idea that 1,25-(OH)2D3 does indeed regulate both c-FOS and c-MYC and that both c-FOS and the TCF4/β-catenin heterodimer contribute to the basal expression of c-MYC.
Fig. 5.
Knockdown via siRNAs decreases the 1,25-(OH)2D3-mediated effect on c-MYC transcription. A, siRNA for Lamin A (LMNA, control), VDR, TCF4, or c-FOS (50 nm) were transfected into LS180 cells and 48 h later treated for an additional 24 h with ethanol vehicle (gray) or 1,25-(OH)2D3 (10−7m, black) in triplicate. RNA was reverse transcribed and analyzed by qPCR for c-FOS, c-MYC, VDR, and TCF4. *, P < 0.05 as compared with vehicle treatment within same siRNA. a, P < 0.05 as compared with LMNA control levels within the same treatment. These results are representative of at least three similar experiments. B, An independent experiment was performed to determine the basal contributions of β-catenin to each gene. siRNA for mock (no RNA control), Lamin A (LMNA, control), VDR, or β-catenin (50 nm) were transfected into LS180 cells and 48 h later treated for an additional 24 h with ethanol vehicle (gray) in triplicate. RNA was reverse transcribed and analyzed by qPCR for VDR, c-MYC, and β-catenin expression. a, P < 0.05 as compared with LMNA control levels within the same treatment. These results are representative of at least three similar experiments.
Isolated c-FOS and c-MYC enhancer segments exhibit complex transcriptional responses to 1,25-(OH)2D3
As a result of the above observations, we examined in a final set of experiments the relative activities of the numerous regulatory regions in both the c-FOS and c-MYC gene loci identified by ChIP-seq analysis, assessed their ability to mediate 1,25-(OH)2D3 response, and mapped through mutagenesis several of the regulatory elements present that bound VDR/RXR or TCF4/β-catenin dimers. Accordingly, we cloned the promoter regions for both genes, the −24 kb segment of the c-FOS gene and the −139, −146, −148, −165, and −335 kb segments of the c-MYC gene upstream of the thymidine kinase reporter gene and assessed the individual basal and 1,25-(OH)2D3-inducible activities of these constructs after transfection into LS180 cells. As can be seen in Fig. 6A, although the c-FOS promoter was unresponsive to 1,25-(OH)2D3, the enhancer identified at −24 kb mediated a strong response to the hormone. The isolated enhancer regions from the c-MYC gene, on the other hand, exhibited a range of activities. Thus, segments from −139 and −146 kb that bound the VDR mediated a dose-dependent induction of transcriptional activity by 1,25-(OH)2D3 whereas the region from −165 kb that also bound VDR mediated a down-regulation. The region at −149 kb that did not bind the VDR was unresponsive to the ligand as was the c-MYC promoter itself. The basal activities of these constructs were also illuminating. Thus, the regions at −149 kb and particularly at −165 kb that were shown to bind β-catenin and TCF4 under basal conditions exhibited much higher basal activities. Finally, the previously identified enhancer located at −335 kb exhibited very high basal activity but no response to 1,25-(OH)2D3 despite the presence of dimers for both VDR/RXR and TCF4/β-catenin.
Fig. 6.
Novel c-MYC regions contain transcriptional activity that is selectively abrogated after mutation at potential TCF4 and VDR response elements. A, DNA fragments (400–600 bp) containing either the TRPV6 −2.1 kb and −4.3 kb regions, control regions around the c-MYC promoter (Pro), c-MYC upstream regions (−139, −146, −149, −165, −335), c-FOS promoter (PRO) and c-FOS −24 kb upstream region were cloned into the pTK-luciferase (tkluc) reporter vector and were evaluated for transcriptional activity in LS180 cells after treatment for 16 h with either ethanol vehicle (V) or 1,25-(OH)2D3 (10−9 to 10−7 m). Each point represents the relative light unit average normalized to β-gal ± sem for a triplicate set of assays. *, P < 0.05 compared with vehicle treatment within each construct. a, P < 0.05 to determine differences in basal levels compared with vehicle treatment of c-MYC Pro or c-FOS PRO constructs, respectively. These results are representative of at least three similar experiments. B, Mutations were introduced in the c-MYC region constructs at −146 kb (chr8: 128,670,964–128,671,012), −165 kb (chr8: 128,651,782–128,651,900), and −335 kb (chr8: 128,482,449–128,482,497). All genomic locations are based on the hg18 genomic build. The sequences listed are the wild-type gene. Underlined portions of the sequence were mutated to TTT nucleotides in all cases except c-MYC-146-VDRE2 (mutated to CCC). C, Point mutations were created in the corresponding wild-type (wt) pTK-luciferase (tkluc) reporter vector and were evaluated for transcriptional activity in LS180 cells after treatment for 16 h with either ethanol vehicle (V) or 1,25-(OH)2D3 (10−7 m). Each point represents the relative light unit average normalized to β-gal ± sem for a triplicate set of assays. *, P < 0.05 compared with vehicle treatment within each construct. a, P < 0.05 to determine differences in basal levels as compared with vehicle treatment of c-MYC Pro. #, P < 0.05 compared with wild-type (wt) condition. These results are representative of at least three similar experiments. chr, Chromosome.
To identify and characterize several of the regulatory elements located within these transcriptionally active regions, we performed mutagenesis on candidate target VDRE and TCF/LEF binding sites localized at the peak centers for the enhancers at −146, −165, and −335 kb. Potential sites that were mutated and tested are summarized in Fig. 6B and assessed in Fig. 6C. Surprisingly, although mutation of the putative TCF/LEF site at −146 kb had no effect on basal activity (neither TCF4 nor β-catenin were present at this site under basal conditions), its alteration was as effective as the mutation in VDRE1 in blocking induction by 1,25-(OH)2D3. Likewise, mutation in both the TCF/LEF1 and VDRE1 motifs reduced basal activity of the −165 kb region but generally did not eliminate the suppressive response to 1,25-(OH)2D3. Finally, mutation of both the VDRE and the TCF4 sites 1 and 2 in the −335 kb region strikingly reduced basal activity but did not reverse the lack of response to 1,25-(OH)2D3. Creation of the single nucleotide polymorphism (SNP) previously found in the TCF4 site 2 (34) only modestly reduced the basal activity of the isolated enhancer at this site. Interestingly, this SNP (either G/T, shown, or G/C, not shown) was located in a LEF site that contributed least to basal activity. These experiments suggest that whereas basal activity of several of these constructs is due to constitutive TCF4/β-catenin activity, unusual interactions between this complex and the VDR/RXR heterodimer at several regions where VDRE and TCF/LEF motifs are proximal to each other may influence both basal and inducible activity of the constructs. It is also clear, however, that unraveling the independent activities of these enhancers will require much additional work, likely involving the development of a large DNA construct containing the entire c-MYC locus into which selective enhancer mutations can be introduced. Regardless, the data suggest a highly complex role of both VDR/RXR and TCF4/β-catenin at these multiple regulatory sites identified initially through ChIP-seq analysis.
Discussion
In this report, we have described an analysis of the regulatory cistromes for the VDR and its partner RXR as well as for the DNA-binding protein TCF4 and its coactivator β-catenin in the CRC cell line LS180. Previously, two groups examined VDR binding throughout the genome in lymphoblastoid (39) and monocytic leukemic cells (40), two nonclassical vitamin D-responsive tissues. These binding data varied greatly from the LS180 CRC line and neither group were able to confirm VDR binding near the CYP24A1 gene. Our studies in LS180 cells have led to the identification of some 1674 binding sites for the ligand-activated VDR/RXR heterodimer, a portion of which (1073 sites) was associated with repeat regions from the LINE family of RABS (24, 25, 41). Although the biological relevance of these latter sites is unclear, we have observed that several of the genes with which these repeats are associated are indeed regulated by 1,25-(OH)2D3. 638 NRAB sites were analyzed further and found to be associated with the majority of known and newly discovered genes modulated by 1,25-(OH)2D3 in LS180 cells, including CYP24A1, TRPV6, CYP2B6, CYP3A4, and PADI. The most prevalent binding motif discovered in this NRABS set of binding sites was a classic VDRE defined by us and others previously (42, 43) comprised of two hexameric half-sites A/G GGTCA separated by 3 bp. The remaining 36% of these sites could represent a differential organization of the two half-sites that have been identified less frequently in a variety of gene targets. Although some of these novel motifs appear active in the context of genes themselves, most have been evaluated in purely heterologous contexts and currently require validation (44). Interestingly, the motif associated with the RABS represents a nearly classic VDRE, differentiated from this traditional motif by only one base. Whereas 3612 and 828 sites were found to contain residual prebound TCF4 and β-catenin, respectively, only about 20% of these sites were discovered to overlap in this CRC cell line, suggesting significant but limited colocalization of both TCF4 and β-catenin at the TCF/LEF binding site motif. Finally, although TCF4 and β-catenin binding was not strongly influenced by 1,25-(OH)2D3, a small cohort of sites for this heterodimer was found to overlap with those of VDR and RXR. These overlapping sites were adjacent to a set of genes involved in transcriptional regulation and cell proliferation, and included c-FOS, c-MYC and SOX9. Regulatory sites for both TCF4/β-catenin and VDR/RXR were located far upstream of the transcriptional units for c-FOS and c-MYC and were characterized further. We conclude that both of these genes, as well as others in the CRC cell line, represent direct targets of both VDR and β-catenin modulation.
An analysis of both the 1,25-(OH)2D3-induced transcriptome and the VDR/RXR cistrome has revealed a number of gene targets that are consistent with the ion transport and xenobiotic degradation functions of vitamin D in the intestine and colon. Accordingly, we noted the recruitment of the VDR/RXR heterodimer to genes such as the calcium channel TRPV6, the calcium binding protein CALB1, the paracellular calcium channel CLDN2, the phosphate transporter SLC34A3, CA2, and CA9 as well as to the KLK locus near KLK9 (data available through GEO at GSE31939). Although the presence of enhancers has been identified for some of these genes, our studies not only confirmed previously identified regulatory sites (TRPV6 and SLC34A3), but in several cases also identified additional regions of regulation as well. In addition, novel regulatory sites for other vitamin D targets were also observed (CALB1, CA2, CLDN2, and KLK9). Similar observations were made for genes involved in both vitamin D metabolism as well as xenobiotic handling and catabolism including CYP24A1, CYP3A4, CYP2B6, and ABCB1 (MDR1) (18, 45–47). Interestingly, several genes in the PADI (peptidylarginine deiminases) gene cluster were also shown to be targets of 1,25-(OH)2D3 action in the intestine. These proteins convert arginine residues of protein targets into citrulline, were first shown to be regulated by 1,25-(OH)2D3 in human keratinocytes, and are essential for epidermal barrier function (27, 28). Their regulation by nuclear factor-κB, JUNB, and activator protein 1 has been documented recently through chromatin-looping interactions in keratinocytes (48–50). PADI genes have been also discovered to target histone deacetylases and DNA methyltransferases, directly affecting transcriptional regulation (51, 52). Interestingly, the regulatory element that controls robust PADI3 expression may be located in an intron of PADI1; this enhancer may also regulate the expression of other PADI members within this gene cluster, although additional studies will be required to prove this concept. PADIs are not the only epigenetic modifiers regulated by 1,25-(OH)2D3; recent studies by Muñoz (53) have also demonstrated that a histone demethylase, KDM6B/JMJD3, is also regulated by this hormone. These and other direct targets identified here both confirm and extend the normal biological actions of 1,25-(OH)2D3 in intestinal/colonic cells.
Perhaps the most interesting observation made in this study is the finding that several genes known to be involved in intestinal cell proliferation and differentiation are direct targets of both WNT-TCF4/β-catenin and 1,25-(OH)2D3/VDR/RXR-signaling pathways. The WNT pathway can stimulate proliferation whereas 1,25-(OH)2D3 functions to suppress proliferation and promote differentiation (10, 43). Of the genes identified, perhaps the most significant is c-MYC. TCF4/β-catenin is known to induce c-MYC expression directly in intestinal lineage cells (54). 1,25-(OH)2D3 and its receptor, on the other hand, function to suppress c-MYC expression, thus countering β-catenin-induced growth; the mechanism underlying this suppression is unknown, although it is believed to occur via a DNA-independent interaction between the VDR and β-catenin, an interaction that occurs with other nuclear receptors as well (10, 43). Muñoz and co-workers (6, 9, 55) have also shown that inhibition of proliferation by 1,25-(OH)2D3 is also mediated through indirect mechanisms and involves up-regulation of antagonists such as DKK1 and DKK4, induction of E-cadherin, and perhaps other mechanisms. We show here an additional mechanism; 1,25-(OH)2D3 can exert a direct inhibitory effect on c-MYC expression while simultaneously inducing c-FOS. Interestingly, our ChIP-seq analyses not only confirm that c-MYC is a direct target of TCF4/β-catenin activation at an enhancer identified previously −335 kb upstream of the c-MYC transcription unit, but also identify several new enhancers located −146 kb and −165 kb upstream as well. The specific TCF4 DNA binding site previously described at −335 kb together with the putative TCF4 binding motifs at −146 kb and −165 kb were explored further. Surprisingly, no activity was observed either immediately upstream (<10 kb) or downstream of the c-MYC locus, as has been documented in other cell types. It should be noted that the regions at −146 kb and −149 kb partially overlap a LINE repeat from the L2 family of transposons; however, their sequence diverges from the RABS observed above. Surprisingly, we also found that 1,25-(OH)2D3 induced VDR and RXR binding at not only the well-established −335 kb site but also at the sites located at −165 kb, −146 kb, and −139 kb. Interestingly, although similar binding activities were observed in Caco-2 cells, 1,25-(OH)2D3 did not induce binding of the VDR at these sites in HCT116 cells. These findings highlight the apparent complex nature of CRC cells of different origins. Direct assessment of the functional activity of these isolated segments of DNA after transfection into LS180 cells revealed that 1,25-(OH)2D3 induced activity at −146 kb and −139 kb, suppressed activity at −165 kb, and was inactive at −335 kb. This unexpected finding of diverse 1,25-(OH)2D3 activities with regard to these individual fragments is likely due to the absence of appropriate chromatin structure after transient transfection and the lack of context relative to the c-MYC locus in vivo. Despite this, these findings strongly support the ability of 1,25-(OH)2D3 to modulate c-MYC expression. Future studies utilizing recombinant bacterial artificial chromosome clones, as we have shown recently for genes such as CYP24A1 (18) and the VDR (56), or targeted deletion of specific c-MYC enhancers in vivo may be necessary to define fully the relative contributions and interrelationships between these regulatory regions.
The extended upstream region of the c-MYC gene is located within the interval 8q24 that is associated with increased risk of colorectal, prostate, and breast cancer (33, 37, 57). Although a number of variants linked to these cancers have been identified, it was the variant rs6983267 located within a TCF/LEF binding sites at −335 kb that has been most revealing (33). Our studies both confirmed the interaction of the TCF4/β-catenin heterodimer at this enhancer and identified additional heterodimer interactions as well. The functionality of these regions may also be supported by deoxyribonuclease I (DNAse I) hypersensitivity data developed in the Caco-2 CRC cell line as documented through the ENCODE project (58, 59) and, more recently, as a result of ChIP-seq analysis of an extensive set of transcription factors in other cell lines as well (54). Although risk-relevant SNP have not been observed at the c-MYC locus within the more proximal regulatory regions we have identified, recent studies using Chromosome Conformation Capture analysis have shown that, in addition to the long-range interactions shown for the −335 kb region with the c-MYC promoter in LS174T cells (an LS180 subclone), LNCaP, and MCF-7 cancer cells, similar interactions are also evident with the −139 kb to −165 kb regions (33, 37). This may be important because at least two annotated transcripts (LOC727677 and POU5F1B) are located between the −165 kb enhancer and the −335 kb enhancer upstream of the c-MYC gene. Our studies therefore provide important insight into the complexity of c-MYC regulation and the roles of TCF4/β-catenin, VDR/RXR, and other transcription factors in this regulation.
The studies described herein define complex cistromes for both VDR/RXR and TCF4/β-catenin in the LS180 cell line. We demonstrate that although complex, 1,25-(OH)2D3 manifests a direct action on a substantial cohort of intestinal-specific genes and more ubiquitously on growth-regulating genes such as c-FOS and c-MYC. Although these studies have relevance to CRC, it remains to be determined how both VDR/RXR and TCF4/β-catenin cistromes correspond to those that define the normal transcriptome in the intestine and in other tissue types.
Materials and Methods
Reagents
1,25-(OH)2D3 was obtained from Tetrionics, Inc. (Madison, WI). Antibodies to VDR (C-20, sc-1008), RXR (ΔN-197, sc-774), C/EBPβ (C-19, sc-150), and β-catenin (H-102, sc-7199; C-18, sc-1496; E-5, sc-7963) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). TCF-4 (clone 6H5–3) antibody was purchased from Millipore Corp. (Billerica, MA). CDX-2 (CDX2–88) antibody was purchased from Biogenex (San Ramon, CA). Anti-tetra-acetyl H4 antibody (06-866) was acquired from Upstate (Charlottesville, VA). All quantitative real-time PCR (qPCR) reagents (Power SYBR green) were obtained from ABI (Foster City, CA) or Fast Start SYBR Green Master Mix (w/ rox) from Roche (Indianapolis, IN). All qPCR were conducted on the StepOnePlus from ABI. Primers were obtained from Integrated DNA Technologies, Inc. (Coralville, IA), and all sequences are freely available upon request. Sequencing reagents for ChIP-seq (no. 11257047 RevA) and GAIIx sequencing were obtained from Illumina (San Diego, CA).
Cell culture
Human LS180 CRC cells were obtained from American Type Culture Collection (Manassas, VA). LS180 cells were cultured in minimum Eagle's medium supplemented with 10% non-heat-inactivated fetal bovine serum from Hyclone Laboratories (Logan, UT), 1% nonessential amino acids, 1% sodium pyruvate, and 1% penicillin-streptomycin from Invitrogen (Carlsbad, CA) as previously reported (19).
Proliferation analysis
LS180 cells were plated at low density (100,000 cells per well) in six-well tissue culture plates in triplicate. The cells were treated with ethanol vehicle or 10−7 m 1,25-(OH)2D3 every other day (d 0, d 2, d 4, and d 6). Each day for 7 consecutive days, cells were trypsinized, counted, and averaged.
Gene expression analysis
LS180 cells were grown to confluency and treated with vehicle or 10−7 m 1,25-(OH)2D3 for 0, and 24 h before RNA isolation. RNA was isolated using the TRI-Reagent protocol (MRC, Cincinnati, OH) and amplification methods according Roche NimbleGen protocols. Briefly, 10 μg of total RNA was DNase treated and reverse transcribed with the Superscript double-stranded cDNA synthesis system (Invitrogen). Double stranded cDNA was verified for integrity by an Agilent 2100 Bioanalyzer and qPCR analysis. Double stranded cDNA was then labeled with Cy3 as described by Roche NimbleGen. Labeled samples were hybridized to human (HG18) 385k microarrays (Roche NimbleGen) in triplicate. Extracted data were processed with the Arraystar 4.0.0 package from DNASTAR (Madison, WI) using a moderated t test and multiple testing correction; FDR (Benjimini-Hochberg). All data passing 95% confidence limits greater than 2-fold were included in up- or down-regulated data analysis. Data are displayed as log2 values (log2) as well as fold change values [1,25-(OH)2D3/vehicle] in Supplemental Tables 1–3. For validation studies, LS180 cells were treated for 24 h in triplicate with either vehicle or 10−9, 10−8, 10−7 m 1,25-(OH)2D3 or cells were treated over a 24-h time course (0, 1, 3, 6, 12, 24 h) with 10−7 m 1,25-(OH)2D3. Isolated total RNA (1 μg) was DNAse treated, reverse transcribed using the High Capacity cDNA Kit (ABI), and then treated with ribonuclease H. qPCR was performed using primers specific to a select set of differentially expressed genes by SYBR green or Taqman analyses and are included as Supplemental Fig. 1.
Molecular cloning
The pCH110-β-galactosidase reporter plasmid was previously described (60). pTK-TRPV6–2.1 and pTK-TRPV6–4.3 were previously described (19). All c-MYC, PADI, and c-FOS pTK plasmids were constructed by cloning the appropriate human DNA fragments obtained through DNA amplification of bacterial artificial chromosome DNA into the pTK-luc vector using BamHI and HindIII restriction sites. Mutations were introduced using the QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) as per the manufacturer's protocol and recommendations.
Analysis of enhancer activity by using reporter genes
LS180 cells were seeded into 24-well plates in α-MEM containing 10% fetal bovine serum at a concentration of 5.0 × 104 cells per well and transfected 24 h later with Lipofectamine PLUS (Invitrogen) in serum- and antibiotic-free medium. Individual wells were cotransfected with 250 ng of a luciferase reporter vector and 50 ng of pCH110-βgal. After transfection, the cells were cultured in medium supplemented with 20% fetal bovine serum with or without 1,25-(OH)2D3. Cells were harvested 18 h after treatment, and the lysates assayed for luciferase and β-galactosidase activities as previously described (61). Luciferase activity was normalized to β-galactosidase activity in all cases.
Chromatin immunoprecipitation (ChIP)
Chromatin immunoprecipitation was performed as described previously (60, 62–64). Briefly, LS180 cells were treated for 3 h with vehicle or 10−7 m 1,25-(OH)2D3 as was optimized as previously reported (19). The treated cells were washed several times with PBS and then subjected to a 15 min cross-linking reaction with 1.5% formaldehyde. After the cross-linking reaction, isolated cell extracts were sonicated to prepare chromatin fragments (average DNA size of 500–600 bp DNA, as assessed by agarose gel electrophoresis) using a Fisher Model 100 Sonic Dismembranator (Fisher Scientific, Pittsburgh, PA) at a power setting of 2. Precleared samples were subjected to immunoprecipitation using either a control IgG antibody or the indicated experimental antibody [VDR, RXR, C/EBPβ, CDX2, TCF4 (TCF7L2), β-catenin]. The precipitated DNA was then washed, the cross-links were reversed, and the DNA fragments were purified using QIAquick PCR Purification Kits (QIAGEN, Valencia, CA). The isolated DNA [or DNA acquired before precipitation (input)] was then subjected to quantitative real-time PCR (qPCR) and further preparation for ChIP-chip or ChIP-seq analysis. ChIP-chip analysis of histone H4 modification was performed as recently described (18).
Chromatin immunoprecipitation coupled to deep sequencing (ChIP-seq) analysis
ChIP-DNA was prepared and amplified using the Illumina ChIP-seq DNA preparation kit (1003473, no. 11257047 RevA), and clusters were formed and sequenced on the Illumina GAIIx sequencers by the University of Wisconsin-Madison DNA Sequencing Facility in the University of Wisconsin-Madison Biotechnology Center. DNA clusters were generated using either a Standard Cluster Generation kit (version 4) on an Illumina cluster station (Illumina, San Diego, CA), [for all samples sequenced before April, 2010) or using a cBot Single Read Cluster Generation kit on an Illumina cBot (Illumina) after April, 2010] according to the manufacturer's instructions, to obtain an average of 2.0 × 107 clusters for each lane on a flow cell. All sequencing runs for 36mers were performed using the Illumina Sequencing kit (version 4). Fluorescent images were analyzed using the Illumina base-calling pipeline 1.6.0 to obtain FASTQ-formatted sequence data. Sequences were mapped to the human genome (March 2006 assembly NCBI36/hg18) using BOWTIE (Bowtie 0.12.5 – mismatch = 3; solexa1.3-quals on) (65). All sequencing tag statistics (total reads, mapped reads, uniquely placed reads) are listed in Supplemental Table 4.
Bioinformatic and statistical analyses
Samples were further processed by QuEST and HOMER. Peaks were accepted if they passed criteria for both methods. QuEST 2.4 (66) was run using the recommend settings for transcription factor-like binding with the following exceptions: kde_bandwith = 30; region_size = 600; ChIP threshold =35; enrichment fold = 3; rescue fold = 3. HOMER (26) analysis was run using the default settings for peak finding with the following exception: clonal filter = 5. FDR cut off was 0.001 (0.1%) for all peaks. All peaks mapping to coordinates corresponding to centromeric attachment sites were eliminated from the final peak count. The tag density for each factor was normalized to 1 × 107 tags and displayed using the UCSC genome browser. The FDR threshold is indicated on each graph containing ChIP-seq tag density profiles with a dashed line. Positive peak regions were interrogated further using the Genomatix Software Suite (67) as well as HOMER. Motif analysis (de novo and known) was performed using the HOMER software and Genomatix. Peak overlaps were processed with Galaxy (68). All data are deposited in the Gene Expression Omnibus (GSE31939).
Acknowledgments
We thank members of the Pike laboratory for their helpful discussions and contributions to this manuscript. We also thank Drs. Jamie Thomson, Ron Stewart, and Jeff Nie for their contributions to our bioinformatic program (Morgridge Instititute, University of Wisconsin-Madison). We also acknowledge the University of Wisconsin DNA Sequencing Facility supported by Marie Adams, Josh Hyman, and Eric Cabot (University of Wisconsin-Madison Biotechnology Center). We also thank Chris Benner (University of California-San Diego) for his helpful discussion and HOMER data analysis software. Linux server maintenance was performed by Mindy Preston and Rebecca Hudson (University of Wisconsin-Madison Department of Biochemistry).
This work was supported by National Institutes of Health Grant DK-073995 (to J.W.P.).
Disclosure Summary: The authors have nothing to declare.
NURSA Molecule Pages†:
Nuclear Receptors: VDR | RXR-α;
Ligands: Calcitriol.
Annotations provided by Nuclear Receptor Signaling Atlas (NURSA) Bioinformatics Resource. Molecule Pages can be accessed on the NURSA website at www.nursa.org.
- C/EBP
- CCAAT enhancer binding protein
- CDX2
- caudal type homeobox 2
- CRC
- colorectal cancer
- DNAse I
- deoxyribonuclease I
- FDR
- false discovery rate
- LEF
- lymphoid enhancer-binding factor
- NRABS
- non-repeat-associated sites
- 1,25-(OH)2D3
- 1α,25-dihydroxyvitamin D3
- qPCR
- quantitative real-time PCR
- RABS
- repeat-associated sites
- RXR
- retinoid X receptor
- siRNA
- small interfering RNA
- SNP
- single nucleotide polymorphism
- TCF
- T-cell specific factor
- TSS
- transcriptional start site
- VDR
- vitamin D receptor
- VDRE
- vitamin D response element.
References
- 1. Plum LA, DeLuca HF. 2010. Vitamin D, disease and therapeutic opportunities. Nat Rev Drug Discov 9:941–955 [DOI] [PubMed] [Google Scholar]
- 2. Bikle DD. 2010. Vitamin D and the skin. J Bone Miner Metab 28:117–130 [DOI] [PubMed] [Google Scholar]
- 3. Deeb KK, Trump DL, Johnson CS. 2007. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer 7:684–700 [DOI] [PubMed] [Google Scholar]
- 4. Welsh J. 2004. Vitamin D and breast cancer: insights from animal models. Am J Clin Nutr 80:1721S–1724S [DOI] [PubMed] [Google Scholar]
- 5. Ellison TI, Smith MK, Gilliam AC, MacDonald PN. 2008. Inactivation of the vitamin D receptor enhances susceptibility of murine skin to UV-induced tumorigenesis. J Invest Dermatol 128:2508–2517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Larriba MJ, Ordóñez-Morán P, Chicote I, Martín-Fernández G, Puig I, Muñoz A, Pálmer HG. 2011. Vitamin D receptor deficiency enhances Wnt/β-catenin signaling and tumor burden in colon cancer. PLoS One 6:e23524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Flynn G, Chung I, Yu WD, Romano M, Modzelewski RA, Johnson CS, Trump DL. 2006. Calcitriol (1,25-dihydroxycholecalciferol) selectively inhibits proliferation of freshly isolated tumor-derived endothelial cells and induces apoptosis. Oncology 70:447–457 [DOI] [PubMed] [Google Scholar]
- 8. Liu M, Lee MH, Cohen M, Bommakanti M, Freedman LP. 1996. Transcriptional activation of the Cdk inhibitor p21 by vitamin D3 leads to the induced differentiation of the myelomonocytic cell line U937. Genes Dev 10:142–153 [DOI] [PubMed] [Google Scholar]
- 9. González-Sancho JM, Aguilera O, García JM, Pendás-Franco N, Peña C, Cal S, García de Herreros A, Bonilla F, Muñoz A. 2005. The Wnt antagonist DICKKOPF-1 gene is a downstream target of β-catenin/TCF and is downregulated in human colon cancer. Oncogene 24:1098–1103 [DOI] [PubMed] [Google Scholar]
- 10. Pálmer HG, González-Sancho JM, Espada J, Berciano MT, Puig I, Baulida J, Quintanilla M, Cano A, de Herreros AG, Lafarga M, Muñoz A. 2001. Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of β-catenin signaling. J Cell Biol 154:369–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ying Y, Tao Q. 2009. Epigenetic disruption of the WNT/β-catenin signaling pathway in human cancers. Epigenetics 4:307–312 [DOI] [PubMed] [Google Scholar]
- 12. Tice DA, Soloviev I, Polakis P. 2002. Activation of the Wnt pathway interferes with serum response element-driven transcription of immediate early genes. J Biol Chem 277:6118–6123 [DOI] [PubMed] [Google Scholar]
- 13. Pendás-Franco N, Aguilera O, Pereira F, González-Sancho JM, Muñoz A. 2008. Vitamin D and Wnt/β-catenin pathway in colon cancer: role and regulation of DICKKOPF genes. Anticancer Res 28:2613–2623 [PubMed] [Google Scholar]
- 14. Larriba MJ, Martín-Villar E, García JM, Pereira F, Peña C, de Herreros AG, Bonilla F, Muñoz A. 2009. Snail2 cooperates with Snail1 in the repression of vitamin D receptor in colon cancer. Carcinogenesis 30:1459–1468 [DOI] [PubMed] [Google Scholar]
- 15. Pálmer HG, Larriba MJ, García JM, Ordóñez-Morán P, Peña C, Peiró S, Puig I, Rodríguez R, de la Fuente R, Bernad A, Pollán M, Bonilla F, Gamallo C, de Herreros AG, Muñoz A. 2004. The transcription factor SNAIL represses vitamin D receptor expression and responsiveness in human colon cancer. Nat Med 10:917–919 [DOI] [PubMed] [Google Scholar]
- 16. Larriba MJ, Valle N, Pálmer HG, Ordóñez-Morán P, Alvarez-Díaz S, Becker KF, Gamallo C, de Herreros AG, González-Sancho JM, Muñoz A. 2007. The inhibition of Wnt/β-catenin signalling by 1α,25-dihydroxyvitamin D3 is abrogated by Snail1 in human colon cancer cells. Endocr Relat Cancer 14:141–151 [DOI] [PubMed] [Google Scholar]
- 17. Tom BH, Rutzky LP, Oyasu R, Tomita JT, Goldenberg DM, Kahan BD. 1977. Human colon adenocarcinoma cells. II. Tumorigenic and organoid expression in vivo and in vitro. J Natl Cancer Inst 58:1507–1512 [DOI] [PubMed] [Google Scholar]
- 18. Meyer MB, Goetsch PD, Pike JW. 2010. A downstream intergenic cluster of regulatory enhancers contributes to the induction of CYP24A1 expression by 1α,25-dihydroxyvitamin D3. J Biol Chem 285:15599–15610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meyer MB, Watanuki M, Kim S, Shevde NK, Pike JW. 2006. The human transient receptor potential vanilloid type 6 distal promoter contains multiple vitamin D receptor binding sites that mediate activation by 1,25-dihydroxyvitamin D3 in intestinal cells. Mol Endocrinol 20:1447–1461 [DOI] [PubMed] [Google Scholar]
- 20. Pálmer HG, Sánchez-Carbayo M, Ordóñez-Morán P, Larriba MJ, Cordón-Cardó C, Muñoz A. 2003. Genetic signatures of differentiation induced by 1α,25-dihydroxyvitamin D3 in human colon cancer cells. Cancer Res 63:7799–7806 [PubMed] [Google Scholar]
- 21. Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, Eeckhoute J, Shao W, Hestermann EV, Geistlinger TR, Fox EA, Silver PA, Brown M. 2005. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell 122:33–43 [DOI] [PubMed] [Google Scholar]
- 22. Hatzis P, van der Flier LG, van Driel MA, Guryev V, Nielsen F, Denissov S, Nijman IJ, Koster J, Santo EE, Welboren W, Versteeg R, Cuppen E, van de Wetering M, Clevers H, Stunnenberg HG. 2008. Genome-wide pattern of TCF7L2/TCF4 chromatin occupancy in colorectal cancer cells. Mol Cell Biol 28:2732–2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nielsen R, Pedersen TA, Hagenbeek D, Moulos P, Siersbaek R, Megens E, Denissov S, Borgesen M, Francoijs KJ, Mandrup S, Stunnenberg HG. 2008. Genome-wide profiling of PPARγ:RXR and RNA polymerase II occupancy reveals temporal activation of distinct metabolic pathways and changes in RXR dimer composition during adipogenesis. Genes Dev 22:2953–2967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bourque G, Leong B, Vega VB, Chen X, Lee YL, Srinivasan KG, Chew JL, Ruan Y, Wei CL, Ng HH, Liu ET. 2008. Evolution of the mammalian transcription factor binding repertoire via transposable elements. Genome Res 18:1752–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kunarso G, Chia NY, Jeyakani J, Hwang C, Lu X, Chan YS, Ng HH, Bourque G. 2010. Transposable elements have rewired the core regulatory network of human embryonic stem cells. Nat Genet 42:631–634 [DOI] [PubMed] [Google Scholar]
- 26. Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, Cheng JX, Murre C, Singh H, Glass CK. 2010. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell 38:576–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kamata Y, Taniguchi A, Yamamoto M, Nomura J, Ishihara K, Takahara H, Hibino T, Takeda A. 2009. Neutral cysteine protease bleomycin hydrolase is essential for the breakdown of deiminated filaggrin into amino acids. J Biol Chem 284:12829–12836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Méchin MC, Coudane F, Adoue V, Arnaud J, Duplan H, Charveron M, Schmitt AM, Takahara H, Serre G, Simon M. 2010. Deimination is regulated at multiple levels including auto-deimination of peptidylarginine deiminases. Cell Mol Life Sci 67:1491–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lu J, Goldstein KM, Chen P, Huang S, Gelbert LM, Nagpal S. 2005. Transcriptional profiling of keratinocytes reveals a vitamin D-regulated epidermal differentiation network. J Invest Dermatol 124:778–785 [DOI] [PubMed] [Google Scholar]
- 30. Kwong LN, Dove WF. 2009. APC and its modifiers in colon cancer. Adv Exp Med Biol 656:85–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Phelps RA, Broadbent TJ, Stafforini DM, Jones DA. 2009. New perspectives on APC control of cell fate and proliferation in colorectal cancer. Cell Cycle 8:2549–2556 [DOI] [PubMed] [Google Scholar]
- 32. Almeida M, Han L, Martin-Millan M, O'Brien CA, Manolagas SC. 2007. Oxidative stress antagonizes Wnt signaling in osteoblast precursors by diverting β-catenin from T cell factor- to forkhead box O-mediated transcription. J Biol Chem 282:27298–27305 [DOI] [PubMed] [Google Scholar]
- 33. Pomerantz MM, Ahmadiyeh N, Jia L, Herman P, Verzi MP, Doddapaneni H, Beckwith CA, Chan JA, Hills A, Davis M, Yao K, Kehoe SM, Lenz HJ, Haiman CA, Yan C, Henderson BE, Frenkel B, Barretina J, Bass A, Tabernero J, Baselga J, Regan MM, Manak JR, Shivdasani R, Coetzee GA, Freedman ML. 2009. The 8q24 cancer risk variant rs6983267 shows long-range interaction with MYC in colorectal cancer. Nat Genet 41:882–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tuupanen S, Turunen M, Lehtonen R, Hallikas O, Vanharanta S, Kivioja T, Björklund M, Wei G, Yan J, Niittymäki I, Mecklin JP, Järvinen H, Ristimäki A, Di-Bernardo M, East P, Carvajal-Carmona L, Houlston RS, Tomlinson I, Palin K, Ukkonen E, Karhu A, Taipale J, Aaltonen LA. 2009. The common colorectal cancer predisposition SNP rs6983267 at chromosome 8q24 confers potential to enhanced Wnt signaling. Nat Genet 41:885–890 [DOI] [PubMed] [Google Scholar]
- 35. Brattain MG, Fine WD, Khaled FM, Thompson J, Brattain DE. 1981. Heterogeneity of malignant cells from a human colonic carcinoma. Cancer Res 41:1751–1756 [PubMed] [Google Scholar]
- 36. Tom BH, Rutzky LP, Jakstys MM, Oyasu R, Kaye CI, Kahan BD. 1976. Human colonic adenocarcinoma cells. I. Establishment and description of a new line. In Vitro 12:180–191 [DOI] [PubMed] [Google Scholar]
- 37. Ahmadiyeh N, Pomerantz MM, Grisanzio C, Herman P, Jia L, Almendro V, He HH, Brown M, Liu XS, Davis M, Caswell JL, Beckwith CA, Hills A, Macconaill L, Coetzee GA, Regan MM, Freedman ML. 2010. 8q24 prostate, breast, and colon cancer risk loci show tissue-specific long-range interaction with MYC. Proc Natl Acad Sci USA 107:9742–9746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yochum GS, Sherrick CM, Macpartlin M, Goodman RH. 2010. A β-catenin/TCF-coordinated chromatin loop at MYC integrates 5′ and 3′ Wnt responsive enhancers. Proc Natl Acad Sci USA 107:145–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ramagopalan S, Heger A, Berlanga A, Maugeri N, Lincoln M, Burrell A, Handunnetthi L, Handel A, Disanto G, Orton S, Watson C, Morahan J, Giovannoni G, Ponting C, Ebers G, Knight J. 2010. A ChIP-seq defined genome-wide map of vitamin D receptor binding: associations with disease and evolution. Genome Res 20:1352–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Heikkinen S, Väisänen S, Pehkonen P, Seuter S, Benes V, Carlberg C. 16 August 2011. Nuclear hormone 1α,25-dihydroxyvitamin D3 elicits a genome-wide shift in the locations of VDR chromatin occupancy. Nucleic Acids Res 10.1093/nar/gkr654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pan YF, Wansa KD, Liu MH, Zhao B, Hong SZ, Tan PY, Lim KS, Bourque G, Liu ET, Cheung E. 2008. Regulation of estrogen receptor-mediated long range transcription via evolutionarily conserved distal response elements. J Biol Chem 283:32977–32988 [DOI] [PubMed] [Google Scholar]
- 42. Pike JW. 2011. Genome-wide principles of gene regulation by the vitamin D receptor and its activating ligand. Mol Cell Endocrinol 347:3–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Haussler MR, Haussler CA, Whitfield GK, Hsieh JC, Thompson PD, Barthel TK, Bartik L, Egan JB, Wu Y, Kubicek JL, Lowmiller CL, Moffet EW, Forster RE, Jurutka PW. 2010. The nuclear vitamin D receptor controls the expression of genes encoding factors which feed the “Fountain of Youth” to mediate healthful aging. J Steroid Biochem Mol Biol 121:88–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Carlberg C. 2003. Molecular basis of the selective activity of vitamin D analogues. J Cell Biochem 88:274–281 [DOI] [PubMed] [Google Scholar]
- 45. Thummel KE, Brimer C, Yasuda K, Thottassery J, Senn T, Lin Y, Ishizuka H, Kharasch E, Schuetz J, Schuetz E. 2001. Transcriptional control of intestinal cytochrome P-4503A by 1a,25-dihydroxy vitamin D3. Mol Pharmacol 60:1399–1406 [DOI] [PubMed] [Google Scholar]
- 46. Drocourt L, Ourlin JC, Pascussi JM, Maurel P, Vilarem MJ. 2002. Expression of CYP3A4, CYP2B6, and CYP2C9 is regulated by the vitamin D receptor pathway in primary human hepatocytes. J Biol Chem 277:25125–25132 [DOI] [PubMed] [Google Scholar]
- 47. Saeki M, Kurose K, Tohkin M, Hasegawa R. 2008. Identification of the functional vitamin D response elements in the human MDR1 gene. Biochem Pharmacol 76:531–542 [DOI] [PubMed] [Google Scholar]
- 48. Adoue V, Chavanas S, Coudane F, Méchin MC, Caubet C, Ying S, Dong S, Duplan H, Charveron M, Takahara H, Serre G, Simon M. 2008. Long-range enhancer differentially regulated by c-Jun and JunD controls peptidylarginine deiminase-3 gene in keratinocytes. J Mol Biol 384:1048–1057 [DOI] [PubMed] [Google Scholar]
- 49. Chavanas S, Adoue V, Méchin MC, Ying S, Dong S, Duplan H, Charveron M, Takahara H, Serre G, Simon M. 2008. Long-range enhancer associated with chromatin looping allows AP-1 regulation of the peptidylarginine deiminase 3 gene in differentiated keratinocyte. PLoS One 3:e3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ying S, Kojima T, Kawada A, Nachat R, Serre G, Simon M, Takahara H. 2010. An intronic enhancer driven by NF-κB contributes to transcriptional regulation of peptidylarginine deiminase type I gene in human keratinocytes. J Invest Dermatol 130:2543–2552 [DOI] [PubMed] [Google Scholar]
- 51. Cherrington BD, Morency E, Struble AM, Coonrod SA, Wakshlag JJ. 2010. Potential role for peptidylarginine deiminase 2 (PAD2) in citrullination of canine mammary epithelial cell histones. PLoS One 5:e11768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Slack J, Causey C, Thompson P. 2011. Protein arginine deiminase 4: a target for an epigenetic cancer therapy. Cell Mol Life Sci 68:709–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pereira F, Barbáchano A, Silva J, Bonilla F, Campbell MJ, Muñoz A, Larriba MJ. 2 September 2011. KDM6B/JMJD3 histone demethylase is induced by vitamin D and modulates its effects in colon cancer cells. Hum Mol Genet 10.1093/hmg/ddr399 [DOI] [PubMed] [Google Scholar]
- 54. Verzi M, Hatzis P, Sulahian R, Philips J, Schuijers J, Shin H, Freed E, Lynch J, Dang D, Brown M, Clevers H, Liu X, Shivdasani R. 2010. TCF4 and CDX2, major transcription factors for intestinal function, converge on the same cis-regulatory regions. Proc Natl Acad Sci USA 107:15157–15162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pendás-Franco N, García JM, Peña C, Valle N, Pálmer HG, Heinäniemi M, Carlberg C, Jiménez B, Bonilla F, Muñoz A, González-Sancho JM. 2008. DICKKOPF-4 is induced by TCF/β-catenin and upregulated in human colon cancer, promotes tumour cell invasion and angiogenesis and is repressed by 1α,25-dihydroxyvitamin D3. Oncogene 27:4467–4477 [DOI] [PubMed] [Google Scholar]
- 56. Zella LA, Meyer MB, Nerenz RD, Lee SM, Martowicz ML, Pike JW. 2010. Multifunctional enhancers regulate mouse and human vitamin D receptor gene transcription. Mol Endocrinol 24:128–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wright JB, Brown SJ, Cole MD. 2010. Upregulation of c-MYC in cis through a large chromatin loop linked to a cancer risk-associated single-nucleotide polymorphism in colorectal cancer cells. Mol Cell Biol 30:1411–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sabo PJ, Humbert R, Hawrylycz M, Wallace JC, Dorschner MO, McArthur M, Stamatoyannopoulos JA. 2004. Genome-wide identification of DNaseI hypersensitive sites using active chromatin sequence libraries. Proc Natl Acad Sci USA 101:4537–4542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sabo PJ, Kuehn MS, Thurman R, Johnson BE, Johnson EM, Cao H, Yu M, Rosenzweig E, Goldy J, Haydock A, Weaver M, Shafer A, Lee K, Neri F, Humbert R, Singer MA, Richmond TA, Dorschner MO, McArthur M, Hawrylycz M, Green RD, Navas PA, Noble WS, Stamatoyannopoulos JA. 2006. Genome-scale mapping of DNase I sensitivity in vivo using tiling DNA microarrays. Nat Methods 3:511–518 [DOI] [PubMed] [Google Scholar]
- 60. Kim S, Yamazaki M, Zella LA, Shevde NK, Pike JW. 2006. Activation of receptor activator of NF-κB ligand gene expression by 1,25-dihydroxyvitamin D3 is mediated through multiple long-range enhancers. Mol Cell Biol 26:6469–6486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yamamoto H, Shevde NK, Warrier A, Plum LA, DeLuca HF, Pike JW. 2003. 2-Methylene-19-nor-(20S)-1,25-dihydroxyvitamin D3 potently stimulates gene-specific DNA binding of the vitamin D receptor in osteoblasts. J Biol Chem 278:31756–31765 [DOI] [PubMed] [Google Scholar]
- 62. Kim S, Shevde NK, Pike JW. 2005. 1,25-Dihydroxyvitamin D3 stimulates cyclic vitamin D receptor/retinoid X receptor DNA-binding, co-activator recruitment, and histone acetylation in intact osteoblasts. J Bone Miner Res 20:305–317 [DOI] [PubMed] [Google Scholar]
- 63. Fretz JA, Zella LA, Kim S, Shevde NK, Pike JW. 2006. 1,25-Dihydroxyvitamin D3 regulates the expression of low-density lipoprotein receptor-related protein 5 via deoxyribonucleic acid sequence elements located downstream of the start site of transcription. Mol Endocrinol 20:2215–2230 [DOI] [PubMed] [Google Scholar]
- 64. Zella LA, Kim S, Shevde NK, Pike JW. 2006. Enhancers located within two introns of the vitamin D receptor gene mediate transcriptional autoregulation by 1,25-dihydroxyvitamin D3. Mol Endocrinol 20:1231–1247 [DOI] [PubMed] [Google Scholar]
- 65. Langmead B, Trapnell C, Pop M, Salzberg SL. 2009. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Valouev A, Johnson DS, Sundquist A, Medina C, Anton E, Batzoglou S, Myers RM, Sidow A. 2008. Genome-wide analysis of transcription factor binding sites based on ChIP-Seq data. Nat Methods 5:829–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cartharius K, Frech K, Grote K, Klocke B, Haltmeier M, Klingenhoff A, Frisch M, Bayerlein M, Werner T. 2005. MatInspector and beyond: promoter analysis based on transcription factor binding sites. Bioinformatics 21:2933–2942 [DOI] [PubMed] [Google Scholar]
- 68. Giardine B, Riemer C, Hardison RC, Burhans R, Elnitski L, Shah P, Zhang Y, Blankenberg D, Albert I, Taylor J, Miller W, Kent WJ, Nekrutenko A. 2005. Galaxy: a platform for interactive large-scale genome analysis. Genome Res 15:1451–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]