Abstract
Alterations in androgen levels lead to reproductive defects in both males and females, including hypogonadotropic hypogonadism, anovulation, and infertility. Androgens have been shown to down-regulate GnRH mRNA levels through an androgen receptor (AR)-dependent mechanism. Here, we investigate how androgen regulates expression from the GnRH regulatory region in the GT1-7 cell line, a model of GnRH neurons. A synthetic androgen, R1881, repressed transcription from the GnRH promoter (GnRH-P) in an AR-dependent manner, and liganded AR associated with the chromatin at the GnRH-P in live GT1-7 cells. The three known octamer-binding transcription factor-1 (Oct-1) binding sites in GnRH-P were required for AR-mediated repression, although other sequences were also involved. Although a multimer of the consensus Oct-1 binding site was not repressed, a multimer of the cluster of Oct-1, Pre-B cell leukemia transcription factor (Pbx)/Prep, and NK2 homeobox 1 (Nkx2.1) binding sites, found at −106/−91 in GnRH-P, was sufficient for repression. In fact, overexpression of any of these factors disrupted the androgen response, indicating that a balance of factors in this tripartite complex is required for AR repression. AR bound to this region in EMSA, indicating a direct interaction of AR with DNA or with other transcription factors bound to GnRH-P at this sequence. Collectively, our data demonstrate that GnRH transcription is repressed by AR via multiple sequences in GnRH-P, including three Oct-1 binding sites, and that this repression requires the complex interaction of several transcription factors.
A fundamental concept in hypothalamic-pituitary-gonadal (HPG) axis regulation is the steroid hormone feedback loop, whereby gonadal steroids feedback to regulate hypothalamic control of reproduction. Due to the lack of sensitivity of classical methods used to colocalize steroid receptors with GnRH-expressing neurons, it was believed that this feedback occurred indirectly through interneurons expressing the steroid hormone nuclear receptors. However, it has become clear that GnRH neurons do express steroid hormone receptors that can serve as direct targets of the feedback loop. Hypothalamic GnRH neurons have been shown to express estrogen receptor (ER)β in vivo (1–3), and a GnRH-expressing cell line, GT1, expresses androgen receptor (AR), ERα, ERβ, and progesterone receptor (PR)A (4–6).
The AR is a ligand-activated transcription factor, a member of the nuclear receptor superfamily, and is closely related to PR and glucocorticoid receptor (GR). AR mediates various biological effects of androgens in a wide array of reproductive processes, including sexual differentiation and maturation, spermatogenesis, and gonadotropin regulation. Proper regulation of androgen signaling in the HPG pathway is important to maintain mammalian fertility. Despite the importance of androgens in overall reproductive health, the mechanism by which they act upon the hypothalamus remains unclear.
It is difficult to colocalize GnRH neurons and nuclear receptors due to the low numbers, heterogeneity, and dispersion of GnRH neurons in vivo, as well as the low abundance of nuclear receptors. The difficulty of studying the small, dispersed population of GnRH neurons in vivo led to the creation of a GnRH-expressing neuronal cell line, GT1-7. The GT1-7 cell line was created using GnRH-simian virus 40 T-antigen transgenic mice (7). GT1-7 cells express GnRH mRNA and exhibit pulsatile secretion of GnRH peptide with the 30-min interpulse interval appropriate for the mouse (8–10). They also express neuronal markers, including presynaptic vesicle proteins (11) and extend neurites ending in growth cones or contacts with other cells (7). Thus, the GT1-7 cell line represents an excellent model system for the GnRH neuron that allows the study of GnRH synthesis and secretion in response to steroid hormone treatments.
Androgens decrease GnRH expression and secretion from the hypothalamus in vivo (12, 13). Androgens have a repressive effect on GnRH gene expression in vitro (14, 15), although the mechanism of this effect has not been determined. In addition, due to the complex feedback loops of the HPG axis, the necessity for AR expression in the GnRH neuron in vivo and its effects on fertility are yet to be elucidated. Previous reports demonstrated that androgens regulate synthesis and secretion of gonadotropins at the level of both the hypothalamus and the pituitary. Androgens have been reported to decrease GnRH expression and secretion from the hypothalamus, which leads to repression of both FSH and LH secretion from the pituitary (12, 13, 16–20). Belsham et al. (14) showed decreased levels of GnRH mRNA in GT1-7 cells after 24 h of treatment with 5α-dihydrotestosterone (DHT). Interestingly, GnRH secretion from GT1-7 cells was stimulated by DHT through a membrane-initiated event, and this was distinct from the mechanism of transcriptional repression (15).
In the present study, we assessed whether androgen-mediated repression of GnRH transcription occurs via AR interaction with the GnRH 5′ regulatory region. We determined that AR interacts with the proximal promoter region to repress GnRH transcriptional activity and that multiple sites, including a cluster of octamer-binding transcription factor-1 (Oct-1), NK2 homeobox 1 (Nkx1.2), and Pre-B cell leukemia transcription factor (Pbx)/Prep binding elements at −106/−91, are required for androgen-mediated repression.
Results
GnRH transcriptional activity is repressed by androgen in GT1-7 cells via the proximal promoter
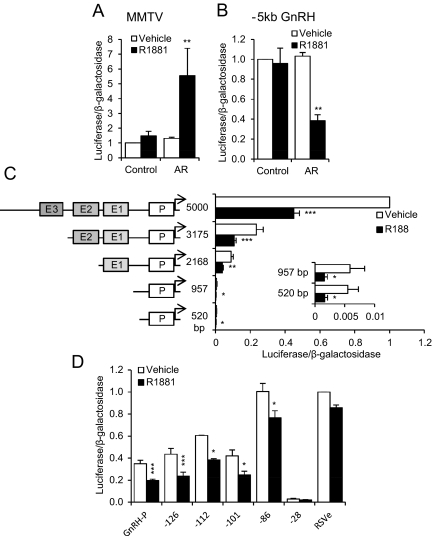
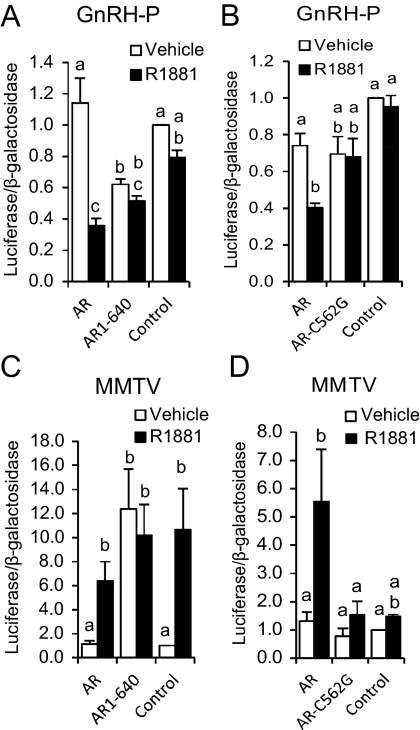
Although GT1 cells have been shown to express AR and respond to androgens (5, 14, 15, 21), receptor expression can be lost in immortalized cell lines, depending on culture conditions (22, 23). To determine whether the GT1-7 cells we were utilizing expressed functional AR, a mouse mammary tumor virus (MMTV)-luciferase reporter plasmid, a positive control for activation by liganded AR, was transfected into GT1-7 cells, with or without an AR expression plasmid. Cells then were treated for 24 h with 100 nm methyltrienolone (R1881), a synthetic AR agonist. MMTV promoter activity was activated by R1881 only when AR was exogenously expressed (Fig. 1A). AR overexpression was also confirmed by Western blotting of GT1-7 cells transfected with empty vector (control) or AR expression plasmid (AR) (Supplemental Fig. 1A, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). We continued to overexpress AR for the remainder of this study.
Fig. 1.
Liganded AR represses GnRH expression via the proximal promoter. GT1-7 cells were transiently transfected with expression plasmid containing AR cDNA or empty vector (control) and a luciferase reporter plasmid containing: the MMTV promoter (A), −5 kb of the GnRH 5′ regulatory region (B), 5000 bp of the GnRH 5′ regulatory region or indicated truncations (C), or 5′ truncations of GnRH-P with RSVe (or RSVe alone) (D). Cells were treated for 24 h with 100 nm R1881 (closed bars) or vehicle (ethanol; open bars) and subjected to luciferase assay. Data were normalized to vehicle-treated control or 5000-bp GnRH. *, P < 0.05; **, P < 0.01; and ***, P < 0.001 vs. vehicle by one-way ANOVA followed by Student's t test.
Treatment of GT1-7 cells with DHT resulted in a 55% reduction in GnRH endogenous mouse mRNA levels, and repression was blocked by hydroxyflutamide, an AR antagonist (15). Thus, androgen acts directly through AR to repress GnRH mRNA levels. To establish whether AR-mediated repression is at the transcriptional level as opposed to decreased mRNA half-life and/or processing, GT1-7 cells were transiently transfected with a luciferase reporter driven by −5 kb of the rat GnRH regulatory region (−5kb GnRH), with or without AR expression plasmid. Activity was reduced by 60% after treatment with 100 nm R1881 when AR was cotransfected (Fig. 1B), indicating that androgen represses transcription of the GnRH gene by exerting effects within the 5-kb regulatory region. No repression was seen with empty vector (control), indicating that this repression is AR dependent.
We have described four evolutionarily conserved regions of the GnRH regulatory region: GnRH enhancer (GnRH-E)3, GnRH-E2, GnRH-E1, and the 173-bp proximal promoter (GnRH-P) (24). Truncation analysis was performed on the −5-kb GnRH regulatory region to determine which of these regions are involved in repression. As expected (24, 25), basal activity decreased with subsequent truncations as the evolutionarily conserved enhancers were removed (Fig. 1C). However, AR-mediated repression was retained within 520 bp upstream of the transcriptional start site (Fig. 1C, inset).
The −520-bp truncation includes GnRH-P (−173 to +1), which has been shown to be critical for proper cell-specific regulation of GnRH (26, 27). Truncation to GnRH-P alone [with addition of the Rous sarcoma virus enhancer (RSVe) to increase luciferase expression to detectable levels] was sufficient for AR-mediated repression (Fig. 1D). To characterize the regions of GnRH-P necessary for repression by AR, truncation analysis was performed by cloning reporter plasmids with serial deletions from the 5′ end of the proximal promoter, beginning with −126 bp (relative to the start site of transcription). Truncation to −101 bp retained approximately 40% repression, compared with 44% for −126 bp and GnRH-P. Truncation of GnRH-P to −86 bp resulted in a loss of most of the androgen-mediated repression (24% repressed), and further truncation to −28 bp resulted in a loss of basal activity.
Multiple elements in GnRH-P contribute to androgen repression
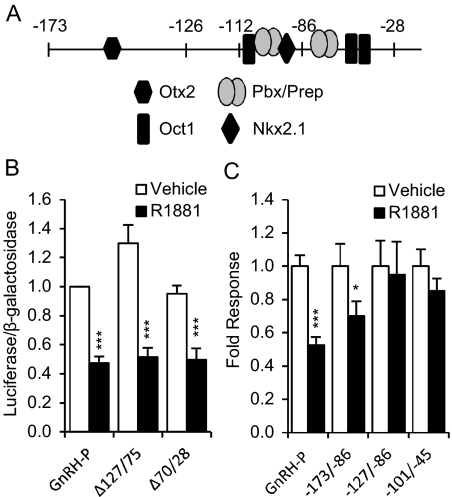
GnRH-P is known to bind to specific transcription factors that are important for GnRH expression and/or GnRH neuron development, including Oct-1 (28), Pbx/Prep1 (29), Nkx2.1 (30), Otx2 (31, 32), and Dlx and Msx (Fig. 2A) (33). The −101/−86-bp sequence identified by truncation analysis includes two known transcription factor binding sites, Pbx (29) and Nkx2.1 (30). It is also within the negative progesterone-response element that has been shown to bind PR and mediate its repression of GnRH (34), suggesting a shared mechanism may exist between AR and PR regulation of the GnRH-P.
Fig. 2.
Multiple elements in GnRH-P are involved in AR-mediated repression. A, Known transcription factor binding sites in GnRH-P. GT1-7 cells were transiently transfected with the AR expression plasmid and reporter plasmids containing RSVe and GnRH-P with block deletions of the bases between −127 and −75 (Δ127/75) or between −70 and −28 (Δ70/28) (B) or GnRH-P or heterologous reporters containing the indicated promoter region between RSVe and RSVp (C). Cells were treated for 24 h with 100 nm R1881 (closed bars) or ethanol vehicle (open bars) and subjected to luciferase assay. Data are expressed relative to vehicle-treated GnRH-P. *, P < 0.05; and ***, P < 0.001 vs. vehicle by one-way ANOVA followed by Student's t test.
Interestingly, deletion of the −127- to −75-bp region from the full-length GnRH-P did not relieve androgen repression (Δ127/75) (Fig. 2B), and neither did deletion of the region between −70 and −28 bp (Δ70/28). Thus, although cis elements between −101 and −86 are involved in androgen-mediated repression, there are redundant AR-responsive elements throughout GnRH-P.
Because repression was lost when the proximal promoter was truncated to −86 bp, we tested whether regions upstream were sufficient for AR-mediated repression. Subregions of GnRH-P were cloned upstream of RSV promoter (RSVp) and tested with or without R1881 treatment in transient transfection assays. The heterologous promoter containing the sequence from −173 to −86 was significantly repressed by androgen, but the sequences between −127 and −86, and between −101 and −45, were not (Fig. 2C). Therefore, although the −101/−86 region is required for androgen repression in the absence of upstream elements, it is not sufficient and requires elements within either the −173/−128 or −45/+112 regions. These data further confirm the presence of multiple androgen-responsive sites in the promoter region and indicate that some minimum number of sites is required for repression.
Requirement of transcription factor binding sites for transcriptional repression of GnRH-P by androgen
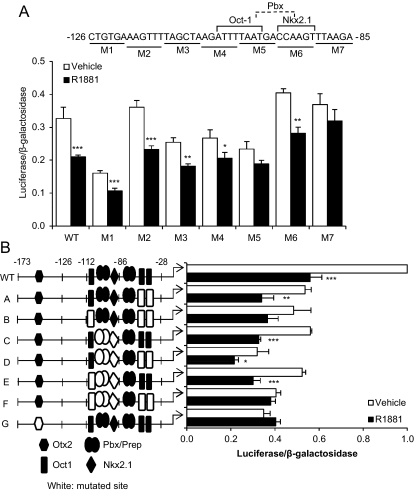
Steroid receptors have been shown to activate genes by binding directly to a hormone-response element and recruiting coactivators. However, transcriptional repression is often due to indirect actions, e.g. by interfering with DNA binding of other transcription factors or by interacting with another protein in a DNA-independent manner to form a transcriptionally inactive protein complex (35). Analysis of the sequence between −173 and −86 using the Transcription Element Search System (36) did not reveal any potential hormone-response elements (data not shown). Thus, it is likely that repression of GnRH-P by AR is via an indirect mechanism. The −126/−86 region is the most well-studied region and contains several known transcription factor binding sites, and several of these transcription factors have been shown to interact with AR. To determine specific sequences in the −126/−86 region that were required for repression, reporters containing 6-bp mutations were created. The −126-bp truncated promoter was used to avoid compensation by elements in the −173/−126-bp region. Mutations at either −102/−97 (M5) or −90/−85 (M7) resulted in a statistically significant loss of androgen repression (Fig. 3A).
Fig. 3.
Multiple elements, including three Oct-1 binding sites, are required for androgen repression of GnRH-P. GT1-7 cells were transiently transfected with an AR expression vector and the −126-bp truncated GnRH-P containing the indicated scanning mutations shown below the sequence (A) or GnRH-P containing mutations in transcription factor binding sites (B), as indicated in the schematic at the left. Cells were treated for 24 h with 100 nm R1881 (closed bars) or ethanol vehicle (open bars) and subjected to luciferase assay. Data were expressed relative to RSVe (data not shown) or GnRH-P. *, P < 0.05; **, P < 0.01; and ***, P < 0.001 vs. vehicle by one-way ANOVA followed by Student's t test. WT, Wild type.
Several of the transcription factors known to bind GnRH-P also interact with steroid hormone receptors. For example, AR interacts directly with Oct-1 (37), and this interaction is important in the transcriptional repression of the muscle atrophy factor muscle atrophy F-box (49) and dehydroepiandrosterone sulfotransferase genes (38). Also, GR represses GnRH transcription by tethering to Oct-1 at the promoter (40). To determine whether the transcriptional repression by androgen is due to the interaction of AR with Oct-1 bound to GnRH-P, we focused on three previously known Oct-1 binding sites (at −106/−99, −61/−56, and −54/−48) that have been shown to be important for basal GnRH expression. Each site was mutated both singly and in combination, in the context of the −173-bp GnRH-P. Mutation of any Oct-1 binding site singly or doubly had no effect on the repression of GnRH-P by R1881 (data not shown and Fig. 3B, mutant A). However, a reporter containing mutations of all three Oct-1 sites in combination was no longer significantly repressed by R1881 (Fig. 3B, mutant B). As expected, mutation of any or all of the Oct-1 sites resulted in reduced basal expression. Mutation of the Pbx/Prep and Nkx2.1 binding sites at −99/−94 and −96/−91, respectively, did not affect repression (due to extensive overlap of the two sites, they could not be mutated singly; see Fig. 3A). Mutation of these Pbx/Prep and Nkx2.1 sites, in addition to mutation of the three proximal Oct-1 sites, completely blocked repression (Fig. 3B, mutant F). Thus, androgen repression of GnRH-P requires participation of multiple cis elements, including the Oct-1 binding sites.
Otx2 has been shown to bind GnRH-P at −152/−143 to activate transcription (31, 32). This site also has been shown to be involved in repression of GnRH-P by PR (34). Mutation of the Otx2 binding site in GnRH-P resulted in decreased basal activity and a loss of repression by androgen (Fig. 3B, mutant G).
The Oct-1, Pbx/Prep, and Nkx2.1 binding site cluster in GnRH-P is sufficient for repression by AR
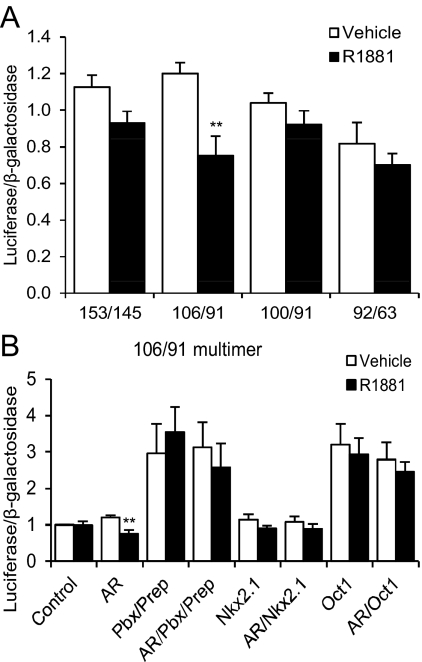
To determine whether the sites identified by mutational analyses were sufficient for AR-mediated repression, reporter constructs containing multimers of the −106/−91, −101/−91, −92/−63, and −153/−145 regions, upstream of a thymidine kinase (TK) promoter, were used. A multimer of the Oct-1, Pbx/Prep, and Nkx2.1 binding site region (106/91 multimer) was repressed by androgen treatment when AR was cotransfected (Fig. 4A). However, when the Oct-1 site was removed (101/91 multimer), repression was lost. We conclude that the Oct-1 binding site is required for AR repression of this region. Although mutation of −90/−85 (M7) resulted in a loss of androgen repression, a multimer of the −92/−63 region was not sufficient for repression. Similarly, although a mutation of the Otx2 site resulted in a loss of androgen repression, a multimer of this site (153/145 multimer) was not repressed. Thus, the −90/−85 and Otx2 sites are not sufficient for repression, whereas the Oct-1, Pbx/Prep, and Nkx2.1 binding site cluster is.
Fig. 4.
A multimer of the region between −106 and −91 of GnRH-P is sufficient for androgen repression. GT1-7 cells were transiently transfected with the AR expression plasmid and the indicated reporter plasmid, containing multiple copies of the indicated region, upstream of the minimal TK promoter (A) or the 106/−91 multimer and the indicated expression plasmid(s) (B). Cells were treated for 24 h with 100 nm R1881 (closed bars) or ethanol vehicle (open bars) and subjected to luciferase assay. Data were expressed relative to vehicle-treated control. **, P < 0.01 vs. vehicle by one-way ANOVA followed by Student's t test.
AR repression of GnRH-P may be via indirect interaction with DNA through other transcription factors. We tested whether overexpression of Pbx2 and Prep1, Nkx2.1, or Oct-1 affected AR-mediated repression of 4x106/91. Overexpression of Pbx2 and Prep1, or of Oct-1, increased basal transcription from this reporter; Nkx2.1 had no effect (Fig. 4B). However, overexpression of any of these transcription factors completely disrupted androgen repression of the multimer, indicating that the balance of transcription factor binding at this sequence is very important.
The AR DNA-binding domain (DBD) is required for repression
AR contains three distinct regions that are involved in its activity: the amino-terminal activation domain, the DBD, and the carboxy-terminal ligand-binding domain (LBD). Deletion of the LBD (AR1-640) resulted in constitutive, ligand-independent repression of GnRH-P (compare AR1-640 with vehicle-treated control) (Fig. 5A). However, mutation of 1 bp within a zinc finger in the DBD of full-length AR (AR-C562G) resulted in a loss of GnRH-P repression (Fig. 5B), indicating that the AR DBD is required for repression of GnRH-P activity. AR1-640 also constitutively activated MMTV activity (Fig. 3C), whereas AR-C562G was inactive (Fig. 5D). The activation of MMTV activity by endogenous AR (Fig. 5C, control) has been observed inconsistently in some lower-passage GT1-7 cells (Brayman, M. J., and P. L. Mellon, unpublished observations). These data indicated that the AR DBD, but not the LBD, is required for repression of GnRH transcription.
Fig. 5.
The AR DBD is required for repression. GT1-7 cells were transiently transfected with GnRH-P and human AR lacking the LBD (AR1-640) (A), GnRH-P and rat AR containing a single bp mutation in the DBD (AR-C562G) (B), MMTV and AR1-640 (C), or MMTV and AR-C562G (D). Control: empty vector, no AR. Cells were treated for 24 h with 100 nm R1881 (closed bars) or ethanol vehicle (open bars) and subjected to luciferase assay. Data are expressed relative to vehicle-treated control. Bars with different letters are significantly different from each other by one-way ANOVA followed by Tukey HSD post hoc test.
AR interacts with GnRH-P in the GT1-7 cell line
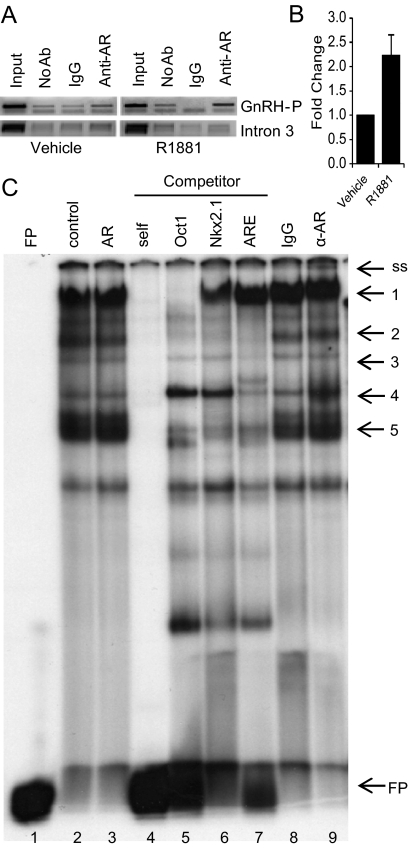
Chromatin immunoprecipitation (ChIP) assays were performed to determine whether AR interacts with endogenous GnRH-P in GT1-7 cells. GT1-7 cells were transfected with AR expression plasmid, treated with R1881 for 2 h, and nuclei were isolated. An antibody specific for AR was used to immunoprecipitate chromatin associated with AR. The resulting isolated DNA was used in a PCR with primers specific for GnRH-P (24), and PCR products were visualized via gel electrophoresis. AR interaction with GnRH-P, but not GnRH intron 3, increased upon treatment with R1881 (Fig. 6A). This increased interaction was confirmed by real-time PCR (Fig. 6B). Thus, liganded AR preferentially interacts, directly or indirectly, with the androgen-regulated GnRH regulatory regions in this GnRH neuronal cell line.
Fig. 6.
AR binds the −110/−86 region of GnRH-P. A, GT1-7 cells were transiently transfected with AR and treated for 2 h with 100 nm R1881 or vehicle. Nuclei were extracted and subjected to ChIP with an antibody specific for AR or normal IgG control. The resulting chromatin was analyzed by PCR with primers to GnRH-P or GnRH intron 3, and the resulting DNA was visualized by gel electrophoresis. The gels shown were representative of three independent experiments. B, Quantitative real-time PCR was performed on the chromatin samples from A using primers to GnRH-P. C, Nuclear extracts from GT1-7 cells, transfected with pSG5 (lane 2) or AR (lanes 3–9), were incubated with a radiolabeled probe containing the −110/−86 region of GnRH-P. Extracts were further incubated with unlabeled competitors or antibody: lane 4, −110/−86; lane 5, consensus Oct-1 binding site; lane 6, known Nkx2.1 binding site; lane 7, androgen-response element (ARE); lane 8, rabbit IgG; lane 9, anti-AR antibody. FP, Free probe; ss, supershift. Ab, Antibody.
Expression from the Oct-1, Pbx/Prep, and Nkx2.1 binding site cluster multimer (106/91 multimer) was repressed by AR (Fig. 4A). To determine whether AR interacts with this region in vitro, EMSA was performed with a radiolabeled probe containing the binding site cluster (−110/−86). No apparent differences were seen between nuclear extracts from GT1-7 cells transfected with pSG5 empty vector (Fig. 6C, lane 2) vs. transfected with AR (Fig. 6C, lane 3). Incubation with unlabeled oligonucleotide containing the Oct-1 consensus binding site resulted in the loss of band 1 (Fig. 6C, lane 5), suggesting that band 1 corresponds to Oct-1 binding. This high molecular weight band is consistent with our previous reports of Oct-1 binding in EMSA (26, 37). Incubation with unlabeled oligonucleotide containing the androgen-response element resulted in a loss of band 3 and a partial loss of band 4 (Fig. 6C, lane 7). Incubation with either of these oligonucleotides, or unlabeled Nkx2.1 binding site (Fig. 6C, lane 6), resulted in a loss of band 2 and the triplet of bands, 5, suggesting that these represent either a complex of proteins or that they are competed nonspecifically. Addition of an antibody specific for AR resulted in a supershifted band (Fig. 6C, lane 9, band ss). These data indicate that AR interacts with the −110/−86 region of GnRH-P.
Nkx2.1 enhances AR repression of a multimer of the Nkx2.1 consensus binding site and represses GnRH-P
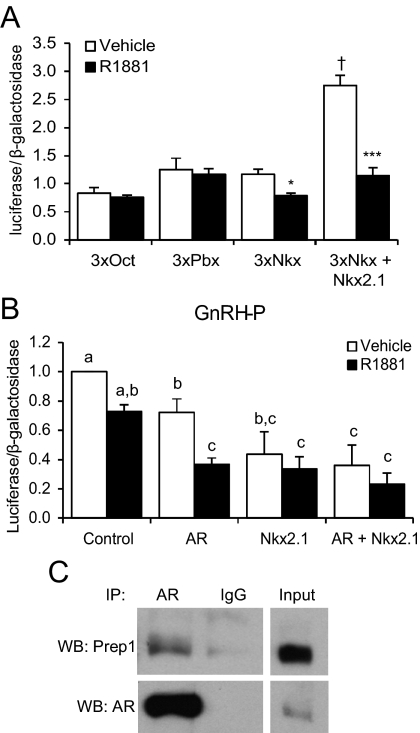
To test whether AR can modulate transcription with Oct-1, Pbx/Prep, or Nkx2.1, reporter constructs were created that contained three copies of a known binding site for each transcription factor, placed between RSVe and RSVp. Liganded AR was unable to repress the Oct-1 or Pbx/Prep consensus binding sites (Fig. 7A). In contrast, the Nkx2.1 binding site multimer was significantly repressed by R1881 when AR expression plasmid was cotransfected, suggesting an interaction between AR and Nkx2.1. Further overexpression of Nkx2.1 enhanced basal activity and resulted in approximately 62% repression by R1881. Overexpression of Oct-1 or Prep with AR did not affect either basal or R1881-treated activity from their respective binding sites (data not shown).
Fig. 7.
AR represses expression from a multimer of a known Nkx2.1 binding site, and overexpression of Nkx2.1 represses GnRH-P. A, GT1-7 cells were transiently transfected with AR and/or Nkx2.1 expression plasmids and reporter plasmids containing multimers of known Oct, Pbx, or Nkx2.1 binding sites. Cells were treated for 24 h with 100 nm R1881 (closed bars) or ethanol vehicle (open bars) and subjected to luciferase assay. Data were expressed relative to empty vector without AR (data not shown). *, P < 0.05; ***, P < 0.001 vs. vehicle; †, P < 0.001 vs. vehicle-treated 3xNkx2.1 (without Nkx2.1 overexpression) by one-way ANOVA followed by Student's t test. B, GT1-7 cells were transiently transfected with AR and/or Nkx2.1 expression plasmids and GnRH-P. Cells were treated for 24 h with 100 nm R1881 (closed bars) or ethanol vehicle (open bars) and subjected to luciferase assay. Data were expressed relative to vehicle-treated control. Bars with different letters are significantly different from each other by one-way ANOVA followed by Tukey HSD post hoc test. C, Cell lysates from GT1-7 cells, transfected with AR, were immunoprecipitated with an antibody specific for AR (or IgG) followed by immunoblotting with an antibody specific for Prep1 (top) or AR (bottom). IP, Immunoprecipitation; WB, Western blot.
Nkx2.1 is expressed in GT1-7 cells, as determined by RT-PCR (Supplemental Fig. 1C) and transcriptome deep sequencing (RNA-seq; Brayman, M. J., and P. L. Mellon, unpublished results). Because the −90/−85 mutation that eliminated AR repression (Fig. 3A, M7) overlapped the Nkx2.1 binding site, we hypothesized that Nkx2.1 may be involved in GnRH repression by androgen. Overexpression of Nkx2.1 repressed basal GnRH-P activity and abrogated repression by androgen (Fig. 7B). However, the observed loss of AR-mediated repression could be due to the low level of activity of the reporter as a result of Nkx2.1 repression, rather than an interaction between AR and Nkx2.1, per se.
Although the ability of AR to interact with Oct-1 has been established (38, 39), there are no reports of an interaction between AR and Prep1. To determine whether these two proteins interact in our system, coimmunoprecipitation was performed using an antibody specific for AR. A band corresponding to Prep1 was observed after immunoblotting with an anti-Prep1 antibody (Fig. 7C), indicating that AR and Prep1 interact in GT1-7 cells. Interaction between AR and a flag-tagged Nkx2.1 was not observed by coimmunoprecipitation (data not shown).
Discussion
Progestins, glucocorticoids, estrogens, and androgens have all been shown to down-regulate GnRH transcription via their classical nuclear receptors. GR interacts with the mouse GnRH-P in two regions, −237/−201 and −184/−150 (40). These regions correspond to −132/−96 and −79/−45 of the rat GnRH-P, respectively, and are approximately 95% homologous to the rat sequence (40, 41). GR tethers to Oct-1 at the distal site via its DBD (40, 42), although similar interaction between GR and Oct-1 at the proximal site was not observed (40). Nonetheless, Oct-1 does bind the rat GnRH-P at the proximal GR site (26, 37). Liganded ERβ also repressed transcription from the same regions of the mouse GnRH-P (−225/−201 and −184/−150) (43), although the possibility of an interaction with Oct-1 was not examined. Similarly, PR was shown to interact with the −126/−73 region of the rat promoter, and the TTAAT sequence of the Otx2 site at −152 was also implicated (32, 34). PR repression also required the DBD. Taken together, these studies suggest that GR, ERβ, and PR act through similar sequences of GnRH-P to repress transcription, although the precise mechanisms of repression have not been fully resolved. Similarly, although liganded AR has been shown to repress GnRH mRNA levels (14), the mechanism had not been determined.
We have shown that AR repression of GnRH transcription occurs through AR interaction with GnRH-P and requires AR DBD. GnRH-P contains multiple transcription factor binding sites, including Oct-1 (28), Pbx/Prep1 (29), Nkx2.1 (30), Dlx and Msx (33), CCAAT/enhancer binding protein-β (44), and SIX homeobox 6 (45). Because negative regulation by steroid hormone receptors is often indirect (e.g. via tethering), rather than by direct binding to DNA, and no AR consensus binding site is present, we hypothesized that AR interacts with one or more of these transcription factors to repress GnRH expression.
Our data suggest that multiple sites in GnRH-P are required for AR-mediated repression. Truncation to −101 bp (relative to the transcriptional start site) retained repression, which was largely, but not completely, lost by further truncation to −86. Yet deletion of the region between −127 and −75 did not affect repression, indicating that the −173 to −127 and/or −75 to +112 regions are also involved. Additionally, although the −127/−86 and −101/−45 regions were not sufficient for repression, a multimer of the −106/−91 region was repressed by R1881. This suggests either that AR-mediated repression requires some threshold number of elements, or that the sequences surrounding −106/−91 contain cis elements that interfere with repression.
Mutation of the Otx2 binding site at −152 (within the 173-bp GnRH-P) completely blocked repression by androgen. Interestingly, this site was implicated in PR repression of GnRH gene transcription (34). Because the −152 mutation resulted in a dramatic reduction in basal transcription, it is possible that this reporter could not be further repressed by AR, a possibility that is supported by the lack of repression observed on the −152 multimer. Interestingly, AR is known to functionally interact with Groucho-related gene (Grg)5 (46), and Otx2 interaction with Grg4 represses transcription from GnRH-P (32). Thus, it is possible that AR interaction with GnRH-P, at the Otx2 site or upstream, recruits Grg proteins (i.e. Grg4) that interact with Otx2 to repress transcription. Loss of the Otx2 binding site would remove this mechanism, but if AR is not interacting directly with Otx2 or the −152 site, a multimer of the site would not be repressed by AR. Still, this could not be the sole mechanism of androgen repression of GnRH-P, because removal of the Otx2 site in truncation analysis did not ablate repression.
Other mutational analyses further confirmed the hypothesis that multiple elements are involved in GnRH-P repression by androgen. In the absence of the −173/−127 region, mutation of −102/−97 or −90/−85 resulted in a loss of repression. However, mutation of either or both sites in the full −173-bp promoter did not affect repression (data not shown). Multiple mutations in GnRH-P, specifically in all three known Oct-1 binding sites, were required to abrogate AR-mediated repression. The −102/−97 mutation overlapped the distal Oct-1 site. A multimer containing the distal Oct-1 binding site, along with the nearby Pbx/Prep and Nkx2.1 binding sites, was repressed by androgen. Like repression by GR and PR, AR-mediated repression requires receptor DBD. Moreover, these steroid hormone receptors interact with Oct-1 and other transcription factors via their DBD (47). Collectively, these data suggest that AR, like GR, may exert its regulation via tethering to DNA through protein-protein interactions with Oct-1.
Although AR interaction with Oct-1 is one mechanism of androgen action on GnRH-P, it is likely that other transcription factors are also involved, because multiple elements with GnRH-P were necessary. Prep1 and Pbx1 can form complexes with Oct-1 to increase GnRH transcription (29), and we showed that AR and Prep1 interact in GT1-7 cells. Thus, AR may disrupt Oct-1 interaction with Prep1, thereby reducing transcription levels. Alternatively, AR interaction with GnRH-P may be stabilized by its interaction with both Oct-1 and Prep1, allowing the recruitment of corepressors to the region. In addition, a consensus Oct-1 binding site was not repressed by androgen. Rather, the GnRH Oct-1 site, which matches the consensus at 6–8 bp (26), along with the Pbx/Prep and Nkx2.1 sites, was required, further suggesting the importance of this cluster of binding sites in steroid hormone responsiveness. Remarkably, glucocorticoid repression of GnRH-P was reduced when the distal Oct-1 site was mutated to a consensus site (42), further supporting this hypothesis. Nkx2.1 also appears to be involved in the repression by androgen, because a known Nkx2.1 binding site was repressed by liganded AR, and overexpression of Nkx2.1 enhanced this repression. Nkx2.1 overexpression decreased basal transcription from GnRH-P, possibly due to disruption of Pbx/Prep binding to the overlapping site, indicating that AR repression could involve Nkx2.1 recruitment to the region. Finally, overexpression of Prep1, Nkx2.1, or Oct-1 resulted in a loss of androgen repression from the 4x−106/−91 multimer. We conclude that the balance of binding by these transcription factors is essential for AR action.
The steroid hormone receptors have been shown to repress gene transcription through several mechanisms, including direct DNA binding, antagonism of DNA binding by other transcription factors, and tethering to DNA by protein-protein interactions with transcription factors (48). AR interaction with Oct-1 has been shown to be required for androgen-mediated repression of muscle atrophy factor muscle atrophy F-box (49) and sulfotransferase (50). Interestingly, like GnRH-P, the prolactin gene is activated by the interaction between Oct-1 and Pbx, and binding of both transcription factors was required for GR-mediated repression (51). We showed that AR interacts with the binding site cluster, suggesting a role for AR tethering to Oct-1, Pbx, and/or Prep in repression of GnRH-P. Moreover, GR has been shown to inhibit surfactant protein-A transcription via interaction with Nkx2.1 and nuclear factor κB and recruitment of histone deacetylases (52). AR interaction with Oct-1, Pbx, and/or Prep may recruit corepressors and histone deacetylases and/or result in steric hindrance of basal transcription factor binding. Further work is required to determine whether AR repression involves chromatin remodeling in this region.
Steroid hormone regulation of GnRH transcription and secretion is crucial for the proper function of the HPG axis. This report identifies a complex interaction between multiple transcription factors at GnRH-P that is involved in AR repression, with Oct-1 playing a central role. This mechanism is similar to that previously shown for GR, the only other nuclear receptor whose interaction with Oct-1 on GnRH-P has been studied. The similarity of GnRH transcriptional regulation by AR and GR suggests that direct repression of GnRH gene expression by classical nuclear receptors is an important aspect of the hormonal regulation of fertility.
Materials and Methods
Plasmids and cloning
The expression plasmids used were: rat AR, pSG5-rAR (53); mutant DBD AR, AR-C562G (54); human AR and AR1-640 (55); cytomegalovirus-Nkx2.1; and human Oct-1 in the pcDNA1.1 vector (29). The pGL3 MMTV plasmid was provided by Jeff Miner of Ligand Pharmaceuticals (La Jolla, CA) (56). The truncated rat GnRH-P constructs were created from the −5-kb GnRH-luc plasmid using the QuikChange Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA) (24). The Otx2 mutation in GnRH-P, Otx2 expression vector, and −152 Otx2 multimer (32); GnRH-PΔ127/75 and Δ70/28 (27); and reporter constructs containing GnRH-P, as well as RSVe, (26, 27) have been described. The GnRH-P truncations, scanning mutations, and mutated promoter Oct-1 binding site reporter constructs were created with the QuikChange Site-Directed Mutagenesis kit, using the primers given in Supplemental Table 1 and their reverse complements. For the scanning mutations in Fig. 3A, EclXI sites were added. No unintentional binding sites were formed, based on Transcription Element Search System analysis. Heterologous promoters were created by PCR using GnRH-P as the template, followed by cloning the products into pGL3 containing RSVe/p. The primer sets used were: −173/−86, 5′-TTTCCCGGGGGAATTCAACATGTCTGGCTTTT (sense) and 5′-TTTACCTGGTCTTAAACTTGGTCATTAAAATCTTAGC (antisense); −127/−86, 5′-CCGGGCTGTGAAAGTTTTAGCTAAGATTTTAATGACCAAGTTTAAGA (sense) and 5′-CCAGGTCTTAAACTTGGTCATTAAAATCTTAGCTAAAACTTTCACAGC (antisense); and −101/−45, 5′-TTTCCCGGGTAATGACCAAGTTTAAGAAAATGCAAC (sense) and 5′-TTTACCTGGTAATGTAATTGGAACACCTGCTG (antisense). Multimers were made by designing complimentary single-stranded oligonucleotides (Supplemental Table 1) that were ligated into the pGL3 vector containing either RSVe/RSVp or the TK promoter.
Cell culture and transfections
GT1-7 cell were maintained in DMEM (Cellgro, Mediatech, Inc., Herndon, VA), supplemented with 10% (vol/vol) fetal bovine serum (FBS) (Gemini Bio-Products, West Sacramento, CA), penicillin (100 U/ml), and streptomycin (0.1 mg/ml) (Invitrogen, Carlsbad, CA) at 37 C with 5% CO2. One day before transfection, cells were plated in 24-well plates with phenol red-free DMEM, supplemented with 10% (vol/vol) charcoal/dextran-treated (cs) FBS (Gemini Bio-Products) and 2 mm Glutamax (Invitrogen). Cells were transfected with FuGENE 6 reagent (Roche Applied Science, Indianapolis, IN) using 400 ng of AR expression plasmid (unless otherwise indicated), 200 ng of other expression plasmid(s) (where indicated), 400 ng of the indicated reporter plasmid, and 100 ng of the internal control (herpes simplex virus TK −109 promoter upstream of the β-galactosidase gene). Transfection efficiency of GT1-7 cells under similar conditions ranges from 25 to 60% with cytomegalovirus-green fluorescent protein expression plasmid (10). Twenty-four hours after transfection, cells were treated with 100 nm R1881 (Sigma, St. Louis, MO) or ethanol vehicle in phenol red-free DMEM, supplemented with 10% (vol/vol) cs-FBS and 2 mm Glutamax. Cells were lysed 24 h after treatment and assayed for luciferase and β-galactosidase expression as previously described (57). In all transfection experiments, luciferase values were normalized to the values from a cotransfected TK-β-galactosidase reporter to control for transfection efficiency, and the data represent the mean ± sem of at least three experiments done in quadruplicate.
Electrophoretic mobility shift assay
GT1-7 cells were transfected with FuGENE 6 reagent in 10-cm dishes with 8 μg of expression vector in phenol red-free DMEM containing 10% (vol/vol) cs-FBS and 2 mm Glutamax. Forty-eight hours after transfection, cells were treated for 2 h with 100 nm R1881 or vehicle (ethanol) in phenol red-free DMEM containing 2% (vol/vol) cs-FBS and 2 mm Glutamax.
Nuclear extracts were prepared as described previously (58), and protein concentration was determined by Bradford assay (59). Oligonucleotide probe was labeled and purified as previously described (60). Reactions were performed using 4 μg of nuclear extract in a solution containing gel shift binding buffer [10 mm N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) (pH 7.8), 50 mm KCl, 1 mm EDTA, 5 mm spermidine, and 2% (wt/vol) Ficoll], 0.125 μg/μl BSA, 1 mm dithiothreitol, 500 μm phenylmethylsulfonyl fluoride (PMSF), and 0.25 μg Poly(dI-dC). Unlabeled competitor, as indicated, was added to the reaction mixture and incubated on ice for 5 min before addition of 4 fmol labeled probe. The reaction mixture was further incubated for 20 min at room temperature, and DNA-protein complexes were resolved by electrophoresis on 8% acrylamide-N,N′-methylene bisacrylamide (30:1) gels at 10 V/cm. Dried gels were exposed to XAR-5 film (Kodak, New York, NY). Supershifts were performed by addition of anti-AR (PG-21; Upstate, Lake Placid, NY), or rabbit control IgG to the reaction mixture. The sense strand sequences of the double-stranded oligonucleotide probes and competitors are listed in Supplemental Table 2. Oligonucleotides were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA).
Chromatin immunoprecipitation
GT1-7 cells were transfected and treated as described for EMSA above. Two hours after addition of R1881, the nuclear fraction was obtained as described (24). Chromatin was sonicated to an average length of 200 bp, and immunoprecipitation was performed using the ChIP Assay kit (Millipore, Temecula, CA) and an anti-AR antibody (PG-21), as per the manufacturer's instructions. PCR was performed using primers as described (24) and as indicated in figure legends. Briefly, the GnRH-P primer amplified the −173/+53 region, and the intron 3 primer set amplified the +3623/+3820 region of the mouse GnRH gene. Real-time PCR was performed on the chromatin using the GnRH-P primers, as described (24).
Coimmunoprecipitation
GT1-7 cells were transfected and treated as described above. Whole-cell extracts were lysed in radioimmunoprecipitation assay buffer [50 mm Tris (pH 8.0), 150 mm NaCl, 1% (vol/vol) Nonidet P-40, 0.5% (wt/vol) sodium deoxycholate, 0.1% (wt/vol) sodium dodecyl sulfate, and 1 mm PMSF] containing protease inhibitors (Sigma). Samples were precleared by incubation with Protein A/G PLUS-Agarose (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) at 4 C for 30 min. Sample was incubated with 4 μg of rat anti-AR monoclonal antibody (C-20; Santa Cruz Biotechnology, Inc.) or rabbit control IgG at 4 C for 1 h before addition of Protein A/G beads. The mixture was incubated for 1 h at 4 C. Beads were washed three times with radioimmunoprecipitation assay buffer containing protease inhibitors. Protein-antibody complexes were eluted from the beads by incubation with sample buffer [78 mm Tris-Cl (pH 6.8), 30% (vol/vol) glycerol, 2.5% (wt/vol) sodium dodecyl sulfate, 0.01% (wt/vol) bromophenol blue, and 0.05% (vol/vol) β-mercaptoethanol] at 100 C for 2 min and electrophoresed as described above. Membranes were probed with rabbit anti-AR polyclonal antibody (PG-21) or anti-Prep1 (N-15; Santa Cruz Biotechnology, Inc.).
SDS-PAGE and Western blotting
GT1-7 cell extracts were made by incubation of the cells with lysis buffer [20 mm Tris (pH 7.4), 140 mm NaCl, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 25 μg/ml pepstatin, 1 mm PMSF, 1% (vol/vol) Nonidet P-40, 0.05 mm EDTA, and 1 mm EGTA]. Samples were diluted 1:1 in sample buffer (described above) before electrophoresis using an 8% SDS-PAGE gel. Proteins were transferred to a polyvinylidene diflouride membrane (Millipore). The membrane was blocked with 5% (wt/vol) nonfat dry milk in 1× PBS containing 2% (vol/vol) Tween 20 and probed with an anti-AR antibody (N-20, Santa Cruz Biotechnology, Inc.). A horseradish peroxidase-linked antirabbit IgG secondary (Amersham Biosciences, Piscataway, NJ) was then applied, and the signal was visualized using the SuperSignal West Pico chemiluminescent substrate (Pierce, Rockford, IL).
Reverse transcriptase PCR
RNA was extracted from GT1-7 cells using the RNeasy Plus Mini kit (QIAGEN, Valencia, CA). RNA was reverse transcribed using the SuperScript III First-Strand Synthesis System (Invitrogen). PCR was performed on the resulting cDNA, using primers specific for Nkx2.1. The primer sequences were: sense, 5′- CGAGCGGCATGAATATGAG; antisense, 5′- GACCTGCGTGGGTGTCAG (61). The resulting PCR products were run on a 2% (wt/vol) agarose gel containing ethidium bromide and visualized under UV light.
Statistical analysis
Statistical analysis was performed using JMP version 8 (SAS Institute, Cary, NC). All relative luciferase units were optimally transformed by the method of Box and Cox. Raw data were analyzed by ANOVA, followed by Student's t test or Tukey honestly significant difference (HSD), as indicated in figure legends. A P value of less than 0.05 was the requirement for declaring significance.
Acknowledgments
We thank Jorma Palvimo (University of Kuopio, Kuopio, Finland) for the pSG5-rAR and pSG5-rAR-C562G plasmids, Xiang-Dong Fu (University of California, San Diego) for the human AR and AR1-640 plasmids, Roberto Di Lauro (Università Federico II, Naples, Italy) for the Nkx2.1 expression plasmid, Sioko Kimura (National Institutes of Health, Bethesda, MD) for the flag-Nkx2.1 plasmid, and Xiuping Yu (Vanderbilt University, Nashville, TN) for technical advice regarding ChIP assays. We also thank Varykina G. Thackray, Anita K. Iyer, and Christine A. Glidewell-Kenney for manuscript editing and many helpful discussions.
This work was supported by National Institutes of Health (NIH) Grants R01 DK044838 and R01 HD020377 (to P.L.M.) and by National Institute of Child Health and Human Development/NIH through a cooperative agreement (U54 HD012303) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research (to P.L.M.). M.J.B. was partially supported by NIH grants F32 HD058460 and T32 HD007203. P.A.P. was partially supported by the Doris Howell Foundation. S.E.B. was partially supported by NIH grants P42 ES010337 and T32 DA007315.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AR
- Androgen receptor
- ChIP
- chromatin immunoprecipitation
- cs
- charcoal/dextran-treated
- DBD
- DNA-binding domain
- DHT
- 5α-dihydrotestosterone
- ER
- estrogen receptor
- FBS
- fetal bovine serum
- GnRH-E
- GnRH enhancer
- GnRH-P
- GnRH promoter
- Grg
- Groucho-related gene
- HPG
- hypothalamic-pituitary-gonadal
- HSD
- honestly significant difference
- LBD
- carboxy-terminal ligand-binding domain
- MMTV
- mouse mammary tumor virus
- Nkx2.1
- NK2 homeobox 1
- Oct-1
- octamer-binding transcription factor-1
- Otx
- orthodenticle homeobox 2
- Pbx
- Pre-B cell leukemia transcription factor
- PMSF
- phenylmethylsulfonyl fluoride
- PR
- progesterone receptor
- RSVe
- Rous sarcoma virus enhancer
- RSVp
- RSV promoter
- TK
- thymidine kinase.
References
- 1. Hrabovszky E, Shughrue PJ, Merchenthaler I, Hajszán T, Carpenter CD, Liposits Z, Petersen SL. 2000. Detection of estrogen receptor-β messenger ribonucleic acid and 125I-estrogen binding sites in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology 141:3506–3509 [DOI] [PubMed] [Google Scholar]
- 2. Hrabovszky E, Steinhauser A, Barabás K, Shughrue PJ, Petersen SL, Merchenthaler I, Liposits Z. 2001. Estrogen receptor-β immunoreactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology 142:3261–3264 [DOI] [PubMed] [Google Scholar]
- 3. Herbison AE, Pape JR. 2001. New evidence for estrogen receptors in gonadotropin-releasing hormone neurons. Front Neuroendocrinol 22:292–308 [DOI] [PubMed] [Google Scholar]
- 4. Roy D, Angelini NL, Belsham DD. 1999. Estrogen directly represses gonadotropin-releasing hormone (GnRH) gene expression in estrogen receptor-α (ERα)- and ERβ-expressing GT1-7 GnRH neurons. Endocrinology 140:5045–5053 [DOI] [PubMed] [Google Scholar]
- 5. Poletti A, Rampoldi A, Piccioni F, Volpi S, Simeoni S, Zanisi M, Martini L. 2001. 5α-Reductase type 2 and androgen receptor expression in gonadotropin releasing hormone GT1-1 cells. J Neuroendocrinol 13:353–357 [DOI] [PubMed] [Google Scholar]
- 6. Navarro CE, Abdul Saeed S, Murdock C, Martinez-Fuentes AJ, Arora KK, Krsmanovic LZ, Catt KJ. 2003. Regulation of cyclic adenosine 3′,5′-monophosphate signaling and pulsatile neurosecretion by Gi-coupled plasma membrane estrogen receptors in immortalized gonadotropin-releasing hormone neurons. Mol Endocrinol 17:1792–1804 [DOI] [PubMed] [Google Scholar]
- 7. Mellon PL, Windle JJ, Goldsmith PC, Padula CA, Roberts JL, Weiner RI. 1990. Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis. Neuron 5:1–10 [DOI] [PubMed] [Google Scholar]
- 8. Martínez de la Escalera G, Choi AL, Weiner RI. 1992. b1-Adrenergic regulation of the GT1 gonadotropin-releasing hormone (GnRH) neuronal cell lines: stimulation of GnRH release via receptors positively coupled to adenylate cyclase. Endocrinology 131:1397–1402 [DOI] [PubMed] [Google Scholar]
- 9. Wetsel WC, Valença MM, Merchenthaler I, Liposits Z, López FJ, Weiner RI, Mellon PL, Negro-Vilar A. 1992. Intrinsic pulsatile secretory activity of immortalized LHRH secreting neurons. Proc Natl Acad Sci USA 89:4149–4153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chappell PE, White RS, Mellon PL. 2003. Circadian gene expression regulates pulsatile gonadotropin-releasing hormone (GnRH) secretory patterns in the hypothalamic GnRH-secreting GT1-7 cell line. J Neurosci 23:11202–11213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mellon PL, Wetsel WC, Windle JJ, Valença MM, Goldsmith PC, Whyte DB, Eraly SA, Negro-Vilar A, Weiner RI. 1992. Immortalized hypothalamic gonadotropin-releasing hormone neurons. In: Chadwick DJ, Marsh J, eds. Functional anatomy of the endocrine hypothalamus. Chichester, UK: John Wiley & Sons Ltd.; 104–126 [DOI] [PubMed] [Google Scholar]
- 12. Toranzo D, Dupont E, Simard J, Labrie C, Couet J, Labrie F, Pelletier G. 1989. Regulation of pro-gonadotropin-releasing hormone gene expression by sex steroids in the brain of male and female rats. Mol Endocrinol 3:1748–1756 [DOI] [PubMed] [Google Scholar]
- 13. Roselli CE, Kelly MJ, Ronnekleiv OK. 1990. Testosterone regulates progonadotropin-releasing hormone levels in the preoptic area and basal hypothalamus of the male rat. Endocrinology 126:1080–1086 [DOI] [PubMed] [Google Scholar]
- 14. Belsham DD, Evangelou A, Roy D, Duc VL, Brown TJ. 1998. Regulation of gonadotropin-releasing hormone (GnRH) gene expression by 5α-dihydrotestosterone in GnRH-secreting GT1-7 hypothalamic neurons. Endocrinology 139:1108–1114 [DOI] [PubMed] [Google Scholar]
- 15. Shakil T, Hoque AN, Husain M, Belsham DD. 2002. Differential regulation of gonadotropin-releasing hormone secretion and gene expression by androgen: membrane versus nuclear receptor activation. Mol Endocrinol 16:2592–2602 [DOI] [PubMed] [Google Scholar]
- 16. Gross DS. 1980. Effect of castration and steroid replacement on immunoreactive gonadotropin-releasing hormone in hypothalamus and preoptic area. Endocrinology 106:1442–1450 [DOI] [PubMed] [Google Scholar]
- 17. Kalra PS, Kalra SP. 1980. Modulation of hypothalamic luteinizing hormone-releasing hormone levels by intracranial and subcutaneous implants of gonadal steroids in castrated rats: effects of androgen and estrogen antagonists. Endocrinology 106:390–397 [DOI] [PubMed] [Google Scholar]
- 18. Selmanoff M, Shu C, Petersen SL, Barraclough CA, Zoeller RT. 1991. Single cell levels of hypothalamic messenger ribonucleic acid encoding luteinizing hormone-releasing hormone in intact, castrated, and hyperprolactinemic male rats. Endocrinology 128:459–466 [DOI] [PubMed] [Google Scholar]
- 19. Raabe EH, Yoshida K, Schwarting GA. 1997. Differential laminin isoform expression in the developing rat olfactory system. Brain Res Dev Brain Res 101:187–196 [DOI] [PubMed] [Google Scholar]
- 20. Tagami T, Madison LD, Nagaya T, Jameson JL. 1997. Nuclear receptor corepressors activate rather than suppress basal transcription of genes that are negatively regulated by thyroid hormone. Mol Cell Biol 17:2642–2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Poletti A, Melcangi RC, Negri-Cesi P, Maggi R, Martini L. 1994. Steroid binding and metabolism in the luteinizing hormone-releasing hormone-producing neuronal cell line GT1-1. Endocrinology 135:2623–2628 [DOI] [PubMed] [Google Scholar]
- 22. Strobl SJ, Lippman ME. 1978. Studies of steroid hormone effects on human breast cancer cells in long-term tissue culture. In: McGuire WL, ed. Hormones, receptors and breast cancer. New York: Raven Press; 85–106 [Google Scholar]
- 23. Page MJ, Field JK, Everett NP, Green CD. 1983. Serum regulation of the estrogen responsiveness of the human breast cancer cell line MCF-7. Cancer Res 43:1244–1250 [PubMed] [Google Scholar]
- 24. Iyer AK, Miller NL, Yip K, Tran BH, Mellon PL. 2010. Enhancers of GnRH transcription embedded in an upstream gene use homeodomain proteins to specify hypothalamic expression. Mol Endocrinol 24:1949–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Whyte DB, Lawson MA, Belsham DD, Eraly SA, Bond CT, Adelman JP, Mellon PL. 1995. A neuron-specific enhancer targets expression of the gonadotropin-releasing hormone gene to hypothalamic neurosecretory neurons. Mol Endocrinol 9:467–477 [DOI] [PubMed] [Google Scholar]
- 26. Givens ML, Kurotani R, Rave-Harel N, Miller NL, Mellon PL. 2004. Phylogenetic footprinting reveals functional upstream regions of the gonadotropin-releasing hormone gene that enhance cell-specific expression. Mol Endocrinol 18:2950–2966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nelson SB, Lawson MA, Kelley CG, Mellon PL. 2000. Neuron-specific expression of the rat gonadotropin-releasing hormone gene is conferred by interactions of a defined promoter element with the enhancer in GT1-7 cells. Mol Endocrinol 14:1509–1522 [DOI] [PubMed] [Google Scholar]
- 28. Clark ME, Mellon PL. 1995. The POU homeodomain transcription factor Oct-1 is essential for activity of the gonadotropin-releasing hormone neuron-specific enhancer. Mol Cell Biol 15:6169–6177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rave-Harel N, Givens ML, Nelson SB, Duong HA, Coss D, Clark ME, Hall SB, Kamps MP, Mellon PL. 2004. TALE homeodomain proteins regulate gonadotropin-releasing hormone gene expression independently and via interactions with Oct-1. J Biol Chem 279:30287–30297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kepa KJ, Wang C, Neeley CI, Raynolds MV, Gordon DF, Wood WM, Wierman ME. 1992. Structure of the rat gonadotropin releasing hormone (rGnGH) gene promoter and functional analysis in hypothalamic cells. Nuc Acids Res 20:1393–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kelley CG, Lavorgna G, Clark ME, Boncinelli E, Mellon PL. 2000. The Otx2 homeoprotein regulates expression from the gonadotropin-releasing hormone proximal promoter. Mol Endocrinol 14:1246–1256 [DOI] [PubMed] [Google Scholar]
- 32. Larder R, Mellon PL. 2009. Otx2 induction of the gonadotropin-releasing hormone promoter is modulated by direct interactions with Grg co-repressors. J Biol Chem 284:16966–16978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Givens ML, Rave-Harel N, Goonewardena VD, Kurotani R, Berdy SE, Swan CH, Rubenstein JL, Robert B, Mellon PL. 2005. Developmental regulation of gonadotropin-releasing hormone gene expression by the MSX and DLX homeodomain protein families. J Biol Chem 280:19156–19165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kepa JK, Jacobsen BM, Boen EA, Prendergast P, Edwards DP, Takimoto G, Wierman ME. 1996. Direct binding of progesterone receptor to nonconsensus DNA sequences represses rat GnRH. Mol Cell Endocrinol 117:27–39 [DOI] [PubMed] [Google Scholar]
- 35. Meyer T, Starr DB, Carlstedt-Duke J. 1997. The rat glucocorticoid receptor mutant K461A differentiates between two different mechanisms of transrepression. J Biol Chem 272:21090–21095 [DOI] [PubMed] [Google Scholar]
- 36. Schug J, Overton GC. 1997. TESS: transcription element search software on the www. In: Technical report CBIL-TR-1997-1001-v00 computational biology and informatics laboratory school of medicine. Philadelphia: University of Pennsylvania [Google Scholar]
- 37. Eraly SA, Nelson SB, Huang KM, Mellon PL. 1998. Oct-1 binds promoter elements required for transcription of the gonadotropin-releasing hormone gene. Mol Endocrinol 12:469–481 [DOI] [PubMed] [Google Scholar]
- 38. Gonzalez MI, Robins DM. 2001. Oct-1 preferentially interacts with androgen receptor in a DNA-dependent manner that facilitates recruitment of SRC-1. J Biol Chem 276:6420–6428 [DOI] [PubMed] [Google Scholar]
- 39. González MI, Tovaglieri A, Robins DM. 2002. Androgen receptor interactions with Oct-1 and Brn-1 are physically and functionally distinct. Mol Cell Endocrinol 190:39–49 [DOI] [PubMed] [Google Scholar]
- 40. Chandran UR, Attardi B, Friedman R, Zheng Z, Roberts JL, DeFranco DB. 1996. Glucocorticoid repression of the mouse gonadotropin-releasing hormone gene is mediated by promoter elements that are recognized by heteromeric complexes containing glucocorticoid receptor. J Biol Chem 271:20412–20420 [DOI] [PubMed] [Google Scholar]
- 41. Eraly SA, Mellon PL. 1995. Regulation of GnRH transcription by protein kinase C is mediated by evolutionarily conserved, promoter-proximal elements. Mol Endocrinol 9:848–859 [DOI] [PubMed] [Google Scholar]
- 42. Chandran UR, Warren BS, Baumann CT, Hager GL, DeFranco DB. 1999. The glucocorticoid receptor is tethered to DNA-bound Oct-1 at the mouse gonadotropin-releasing hormone distal negative glucocorticoid response element. J Biol Chem 274:2372–2378 [DOI] [PubMed] [Google Scholar]
- 43. Pak TR, Chung WC, Roberts JL, Handa RJ. 2006. Ligand-independent effects of estrogen receptor β on mouse gonadotropin releasing hormone (GnRH) promoter activity. Endocrinology 147:1924–1931 [DOI] [PubMed] [Google Scholar]
- 44. Belsham DD, Mellon PL. 2000. Transcription factors Oct-1 and C/EBP β (CCAAT/enhancer binding protein-β) are involved in the glutamate/nitric oxide/cyclic guanosine 5′-monophosphate-mediated repression of gonadotropin-releasing hormone gene expression. Mol Endocrinol 14:212–228 [DOI] [PubMed] [Google Scholar]
- 45. Larder R, Clark DD, Miller NL, Mellon PL. 2011. Hypothalamic dysregulation and infertility in mice lacking the homeodomain protein Six6. J Neurosci 31:426–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yu X, Li P, Roeder RG, Wang Z. 2001. Inhibition of androgen receptor-mediated transcription by amino-terminal enhancer of split. Mol Cell Biol 21:4614–4625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Préfontaine GG, Walther R, Giffin W, Lemieux ME, Pope L, Haché RJ. 1999. Selective binding of steroid hormone receptors to octamer transcription factors determines transcriptional synergism at the mouse mammary tumor virus promoter. J Biol Chem 274:26713–26719 [DOI] [PubMed] [Google Scholar]
- 48. De Bosscher K, Vanden Berghe W, Haegeman G. 2003. The interplay between the glucocorticoid receptor and nuclear factor-κB or activator protein-1: molecular mechanisms for gene repression. Endocr Rev 24:488–522 [DOI] [PubMed] [Google Scholar]
- 49. Zhao W, Pan J, Wang X, Wu Y, Bauman WA, Cardozo CP. 2008. Expression of the muscle atrophy factor muscle atrophy F-box is suppressed by testosterone. Endocrinology 149:5449–5460 [DOI] [PubMed] [Google Scholar]
- 50. Song CS, Jung MH, Kim SC, Hassan T, Roy AK, Chatterjee B. 1998. Tissue-specific and androgen-repressible regulation of the rat dehydroepiandrosterone sulfotransferase gene promoter. J Biol Chem 273:21856–21866 [DOI] [PubMed] [Google Scholar]
- 51. Subramaniam N, Cairns W, Okret S. 1998. Glucocorticoids repress transcription from a negative glucocorticoid response element recognized by two homeodomain-containing proteins, Pbx and Oct-1. J Biol Chem 273:23567–23574 [DOI] [PubMed] [Google Scholar]
- 52. Islam KN, Mendelson CR. 2008. Glucocorticoid/glucocorticoid receptor inhibition of surfactant protein-A (SP-A) gene expression in lung type II cells is mediated by repressive changes in histone modification at the SP-A promoter. Mol Endocrinol 22:585–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ikonen T, Palvimo JJ, Jänne OA. 1998. Heterodimerization is mainly responsible for the dominant negative activity of amino-terminally truncated rat androgen receptor forms. FEBS Lett 430:393–396 [DOI] [PubMed] [Google Scholar]
- 54. Ikonen T, Palvimo JJ, Kallio PJ, Reinikainen P, Jänne OA. 1994. Stimulation of androgen-regulated transactivation by modulators of protein phosphorylation. Endocrinology 135:1359–1366 [DOI] [PubMed] [Google Scholar]
- 55. Céraline J, Cruchant MD, Erdmann E, Erbs P, Kurtz JE, Duclos B, Jacqmin D, Chopin D, Bergerat JP. 2004. Constitutive activation of the androgen receptor by a point mutation in the hinge region: a new mechanism for androgen-independent growth in prostate cancer. Int J Cancer 108:152–157 [DOI] [PubMed] [Google Scholar]
- 56. Thackray VG, Mellon PL. 2008. Synergistic induction of follicle-stimulating hormone β-subunit gene expression by gonadal steroid hormone receptors and smad proteins. Endocrinology 149:1091–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lawson MA, Whyte DB, Mellon PL. 1996. GATA factors are essential for activity of the neuron-specific enhancer of the gonadotropin-releasing hormone gene. Mol Cell Biol 16:3596–3605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schreiber E, Matthias P, Müller MM, Schaffner W. 1989. Rapid detection of octamer binding proteins with mini-extracts prepared from a small number of cells. Nucl Acids Res 17:6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principles of protein-dye binding. Anal Biochem 72:248–254 [DOI] [PubMed] [Google Scholar]
- 60. Tang Q, Mazur M, Mellon PL. 2005. The protein kinase C pathway acts through multiple transcription factors to repress gonadotropin-releasing hormone gene expression in hypothalamic GT1-7 neuronal cells. Mol Endocrinol 19:2769–2779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nóbrega-Pereira S, Kessaris N, Du T, Kimura S, Anderson SA, Marín O. 2008. Postmitotic Nkx2–1 controls the migration of telencephalic interneurons by direct repression of guidance receptors. Neuron 59:733–745 [DOI] [PMC free article] [PubMed] [Google Scholar]