Abstract
Acute lung injury and its more severe form, acute respiratory distress syndrome, are characterized by an acute inflammatory response in the airspaces and lung parenchyma. The nuclear receptor farnesoid X receptor (FXR) is expressed in pulmonary artery endothelial cells. Here, we report a protective role of FXR in a lipopolysaccharide-induced mouse model of acute lung injury. Upon intratracheal injection of lipopolysaccharide, FXR−/− mice showed higher lung endothelial permeability, released more bronchoalveolar lavage cells to the alveoli, and developed acute pneumonia. Cell adhesion molecules were expressed at higher levels in FXR−/− mice as compared with control mice. Furthermore, lung regeneration was much slower in FXR−/− mice. In vitro experiments showed that FXR activation blocked TNFα-induced expression of P-selectin but stimulated proliferation of lung microvascular endothelial cells through up-regulation of Foxm1b. In addition, expression of a constitutively active FXR repressed the expression of proinflammatory genes and improved lung permeability and lung regeneration in FXR−/− mice. This study demonstrates a critical role of FXR in suppressing the inflammatory response in lung and promoting lung repair after injury.
Acute lung injury (ALI) and its more severe form, acute respiratory distress syndrome (ARDS), are characterized by an acute inflammatory response in the airspaces and lung parenchyma (1). The clinical course of ALI is a complex, variable process associated with severe lung dysfunction. The first stage, the exudative phase, is an acute inflammatory response accompanied by a marked influx of neutrophils that injure epithelial and endothelial cells (EC). Proinflammatory cytokines are produced locally in the lung by alveolar macrophages, epithelial cells, and fibroblasts. This enhances neutrophil-dependent injury to epithelial cells and EC, thereby leading to the loss of barrier function of the alveolar epithelial and pulmonary capillary EC (basement membrane disruption).
Insufficient repair of lung injury results in deposition of excessive collagen and extracellular matrix, which may lead to alveolar wall fibrosis (1, 2). Increasing Foxm1b levels is linked to enhanced cellular proliferation during aging and in lung diseases, such as emphysema (3). Zhao et al. (4) showed that EC-restricted deletion of Foxm1b markedly impaired pulmonary EC regeneration and increased vascular permeability, thus reducing survival after ALI due to severe pulmonary edema. We recently demonstrated that the nuclear receptor farnesoid X receptor (FXR) directly regulates Foxm1b gene expression by binding to an FXR-responsive element to regulate liver regeneration (5–8). FXR also plays an important role in the pathogenesis of cardiovascular diseases by regulating the metabolism and transport of cholesterol (9). Furthermore, FXR is expressed in human and rat pulmonary EC (10). More recently, roles for FXR in atherosclerosis and inflammation have also been identified. For example, FXR can antagonize nuclear factor κB in hepatic inflammatory response (11) and inhibits vascular smooth muscle cell inflammation (12). Additional reports suggest that FXR is a modulator of intestinal and hepatic innate immunity (13–15).
The endothelium plays a key regulatory role during inflammatory responses, controlling leukocyte adhesion and emigration through selective expression of cytokines, chemokines, and adhesion molecules. Recruitment of leukocytes into extravascular tissues occurs through a concerted multistep process that involves leukocyte rolling, adhesion, and emigration across the endothelium. The cell adhesion molecule P-selectin plays an essential role in leukocyte rolling on EC. Several studies have shown that nuclear receptors are also involved in regulating leukocyte adhesion and rolling on the endothelium (16, 17). Whether FXR is involved in mediating this process during inflammation is not known. Therefore, in this work, we aim to determine whether FXR plays a role in protecting lung from lipopolysaccharide (LPS)-induced injury. We found that FXR activation suppressed the expression of P-selectin and induced Foxm1b expression, thereby decreasing the permeability of the lung and suppressing the movement of leukocytes out of the circulation into inflamed tissues to promote lung repair. These results highlight FXR as a novel target for treating inflammation-induced lung injury.
Materials and Methods
Reagents
Chenodeoxycholic acid (CDCA) and LPS (from Escherichia coli 0111:B4) were purchased from Sigma Chemical (St. Louis, MO). 6-ethylchenodeoxycholic acid (6eCDCA) was synthesized through a modified procedure (our unpublished data).
Animal maintenance and treatments
FXR−/− mice were described previously (5, 31). Wild-type (WT) C57BL/6 mice were bred and maintained in the City of Hope Animal Resources Center. All procedures followed the National Institutes of Health guidelines for the care and use of laboratory animals. Mice were housed in a pathogen-free animal facility under standard 12-h light, 12-h dark cycle and fed standard rodent chow and water ad libitum. Male mice between 8 and 10 wk old were used in experiments.
LPS-induced lung injury
For LPS treatment, 8- to 10-wk-old mice were anesthetized by ip injection with ketamine (150 mg/kg) and xylazine [10 mg/kg body weight (BW)] and subjected to intranasal instillation (i.t.) of LPS (4 μg/10 g of BW) or vehicle (saline) in 50 μl. The mice were then either subjected to bronchoalveolar lavage (BAL), injection with Evans blue, or killed 24 or 48 h after the i.t.
BAL fluid collection and cell counting
Twenty-four or 48 h after LPS instillation, mice were anesthetized and BAL fluids collected by performing BAL three times with sterile saline (1.0 ml). The recovered BAL fluids were centrifuged (300 rpm, 10 min), and the total number of BAL cells was counted using a hemocytometer (16).
Analysis of lung permeability
The microvascular permeability index was measured in all groups using a modification of the Evans blue dye extravasation technique as described previously (18). Briefly, mice received 20 mg/kg of Evans blue dye iv 2 h before being euthanized. At the time of death, a heparinized sample of blood was taken from the cannulated femoral vein, and plasma was removed by centrifugation. The lungs were then perfused free of blood with 0.9% saline (20 ml), after which they were removed from the thoracic cavity and surrounding mediastinal structures and weighed. Pulmonary tissue was then homogenized in 0.9% saline (3 ml), added to two volumes of deionized formamide, and incubated (60 C, 12 h). Supernatant was separated from the lung tissue by centrifugation (2000 × g, 30 min). Evans blue dye in plasma and lung tissue was quantified by dual wavelength spectrophotometric analysis. This method corrects specimen absorbance at 620 nm for the absorbance of contaminating heme pigments. Correction was calculated using the following formula: corrected absorbance at 620 nm = actual absorbance at 620 nm − [1.426 (absorbance at 740 nm) −0.03]. The permeability index, which reflects the degree of extravasation of Evans blue dye into the extravascular pulmonary tissue compartment, was calculated by dividing the corrected pulmonary tissue Evans blue dye absorbance (620 nm) by the corrected plasma Evans blue dye absorbance (620 nm).
Lung histology
After mice were euthanized, their lungs were removed, fixed in 4% formaldehyde-PBS solution, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin staining. For WT lung FXR and Pecam staining, frozen tissue was embedded in Tissue Tek O.C.T. compound (Sarura Finetek, CA) and sectioned at 10 μm using a Leica CM3050S Cryostat (Leica, Heerbrugg, Switzerland). Sections were fixed in Acetone for 20 min at − 20 C. Then washed three times in PBS and permeabilized with 0.5% Triton X-100 for 5 min and blocked with 5% normal donkey serum in PBS for 1 h at room temperature. The sections were then incubated with primary antibodies, including FXR antibody (Abcam) and Pecam polyclonal goat antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Sections were washed three times in PBS including 0.5% Tween 20 and incubated with secondary antibodies diluted 1:200 in PBS including 0.5% Tween 20 with 5% donkey serum for 1 h at room temperature. For secondary antibodies, Alexa Fluor 488 (donkey antirabbit) and Alexa Fluor 647 (donkey antigoat IgG), from Molecular Probes (Eugene, OR), were used. After being washed three times with PBS, sections were mounted with Prolong Gold antifade reagent (Molecular Probes) to delay fluorescence quenching. After being covered with coverslips and sealed with nail polisher, sections were visualized using Zeiss laser-scanning confocal microscope 510 (Carl Zeiss Microscopy, Oberkochen, Germany). 4′,6-diamidino-2-phenylindole (DAPI) staining was used to show the nuclei. For bromodeoxyuridine (BrdU) immunofluorescence, mice were ip injected with bromodeoxyuridine (BrdU) (75 mg/kg BW) 4 h before tissue collection (4). Mouse lung sections were stained with fluorescein isothiocyanate (FITC)-conjugated anti-BrdU following the manufacturer's instructions (In Situ Cell Proliferation kit; Roche Diagnostics, Indianapolis, IN), and the nuclei were counterstained with propidium iodide. We used the In Situ Cell Death Detection kit (Roche Diagnostics) for in situ apoptosis detection.
Mouse lung EC isolation
WT and FXR knockout lung EC were isolated as previously reported (18) with slight modification. Lung tissues were removed aseptically, rinsed in Hanks' balanced salt solution (Invitrogen, Carlsbad, CA), minced into approximately 1 × 2-mm pieces, and digested in 20 ml of collagenase A (1 mg/ml; Boehringer, Mannheim, Germany) at 37 C for 45 min with occasional agitation. The cellular digest was filtered through a sterile 100-μm nylon mesh, centrifuged (100 × g for 10 min), and washed twice in 10% fetal bovine serum (FBS)-DMEM. The cell pellet was resuspended in 10% FBS-DMEM.
Dynabeads (Invitrogen) coated with sheep antirat IgG (30-μl aliquot per 5 ml of tube) were mixed with rat-antimouse CD31 overnight and washed, then the CD31-cojugated beads were incubated in mouse EC supernatant (1 ml, 37 C, 2 h) with occasional agitation and washed three times with 10% FBS-DMEM. The bead-bound cells were recovered, washed five times with 10% FBS-DMEM and once with FBS-free DMEM, and then digested at 37 C in trypsin/EDTA (Invitrogen) to release the beads. The bead-free cells were centrifuged in 10% FBS-DMEM and resuspended in growth medium (20% FBS-M199, 2 mmol/liter l-glutamine, 2 mmol/liter sodium pyruvate, 20 mmol/liter HEPES, 1% nonessential amino acids, 100 μg/ml streptomycin, 100 UI/ml penicillin, freshly added heparin, and 5 ng/ml vascular endothelial growth factor) for culture. The isolates were cultured in six-well plates precoated overnight with 1% gelatin (type B from bovine skin; Sigma Chemical) in PBS. Cells were either passed routinely or treated as indicated.
EC proliferation assay
Isolated lung EC were cultured for 5–7 d, and then the media were changed to M199 plus resin and charcoal stripped 5% serum for 2 d. Vehicle control or FXR ligands [CDCA (50 μm) or 6eCDCA (2 μm)] were added to the media, and 24 h later, RNA extraction or BrdU staining (In Situ Cell Proliferation kit; Roche Diagnostics) was performed.
RNA analysis
Total lung RNA was extracted using TRIzol Reagent (Invitrogen) according to the manufacturer's instructions. Quantitative real-time PCR was performed using SYBR Green PCR Master Mix and an ABI prim 7300 Sequence Detection System (Applied Biosystems, Foster City, CA). Murine 36B4 primers were used as internal controls. PCR primers specific for each gene are listed below (5′-3′): 36B4-forward (F), GCC CTG CAC TCT CGC TTT CT and 36B4-reverse (R), CAA CTG GGC ACC GAG GCA ACA GTT G; Foxm1b-F, GGA TTC CAA GAG AGC AGA GGT G and Foxm1b-R, CCG ATT CTG CTC CAG GTG ACA A; CyclinD1-F, GCG TAC CCT GAC ACC AAT CTC and CyclinD1-R, CTC CTC TTC GCA CTT CTG CTC; TNFα-F, TGT CTA CTG AAC TTC GGG GTG ATC and TNFα- R, GGT TGT CTT TGA GAT CCA TGC CGT; IL-6-F, GTC TTC TGG AGT ACC ATA GC and IL-6-R, CAC TCC TTC TGT GAC TCC AG; interferon (IFN)γ-F, CAG CAA CAG CAA GGC GAA A and IFNγ-R, CTG GAC CTG TGG GTT GTT GAC; monocyte chemoattractant protein (MCP)1-F, ATT CCT TCA CGG GGA CTA GG and MCP1-R, GGG AGC CAT CTA TCC CAA A; inducible nitric oxide synthase (iNOS)-F, GAC GAG ACG GAT AGG CAG AG and iNOS-R, CTT CAA GCA CCT CCA GGA AC; and P-selectin-F, CAT CTG GTT CAG TGC TTT GAT CT and P-selectin-R, ACC CGT GAG TTA TTC CAT GA.
Adenovirus injection
Adenovirus that expressed VP16 (the transactivation domain of herpes simplex virus) alone (Ad-VP16) and murine FXRα2 fused to VP16 (FXRα2-VP16; constitutively active FXR) were provided by Peter A. Edwards (University of California at Los Angeles, CA). Adenovirus was amplified in HEK293 cells and purified with an Adeno-X Virus Purification kit (CLONTECH Laboratories, Inc., Mountain View, CA). FXR−/− mice were injected in the tail vein with 109 plaque-forming units per mouse of either Ad-VP16 or FXRα2-VP16. After 5 d, mice received i.t. of a single dose of LPS or saline alone. After 24 or 48 h, mice were subjected to lung permeability analysis, BAL analysis, or killed for further analysis.
Statistical analyses
Data are expressed as means ± sd. Two-tailed Student's t test was used to determine differences between two groups. All analyses were performed using one-way ANOVA. P < 0.05 (*) was considered statistically significant, and P < 0.01 (**) was considered highly significant.
Results
FXR−/− mice are more susceptible to LPS-induced acute injury
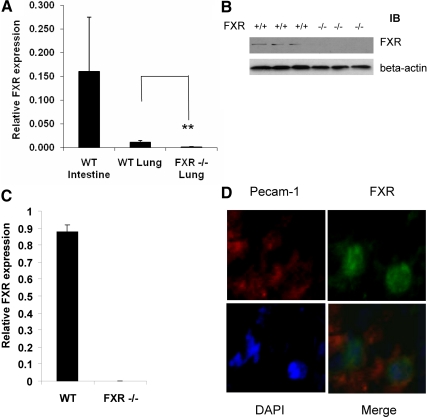
We first used quantitative RT-PCR (QRT-PCR) to measure FXR mRNA expression in lung tissues. WT lung showed expression of FXR transcript though the transcription level is lower than that of the ileum (Fig. 1A). The results were confirmed at protein levels by Western blotting, which showed FXR protein expressing in WT lung tissues but not in FXR−/− lung tissues (Fig. 1B). The results were further confirmed by immunostaining using an FXR-specific antibody (data not shown). Next, we isolated the lung EC and QRT-PCR results showed expression of FXR transcript in those cells only from WT but not from FXR−/− mice (Fig. 1C). Finally, we did immunofluorescence of FXR and Pecam-1 in WT lung sections, and the results showed that FXR was expressed in Pecam-1 positive cells, further confirming the expression of FXR in lung EC (Fig. 1D).
Fig. 1.
FXR is expressed in the mouse lung. A, RT-QPCR analysis of expression levels of FXR transcript in WT mouse ileum, WT lung, and FXR−/− lung. B, Immunoblot analysis of FXR protein levels in WT and FXR−/− lung tissues. C, QRT-PCR analysis of expression levels of FXR transcript from isolated lung EC. D, Immunofluorescence staining of FXR and Pecam-1 on frozen lung tissues sections (green, FXR; red, Pecam-1; blue, DAPI). **, P < 0.01.
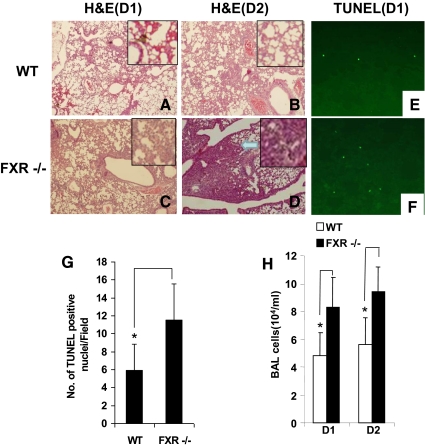
We next investigated the roles of FXR in a LPS-induce lung injury model. Eight- to 10-wk-old mice were subjected to i.t. of 50 μl of LPS (4 μg/10 g of BW) or vehicle (saline). Mortality of FXR−/− mice was greater than that observed in WT mice (data not shown). Histologic analysis revealed elevated neutrophilic alveolar and interstitial infiltration by d 1 and 2 after LPS inhalation in FXR−/− mice, with increased interstitial thickening and mixed cellular infiltration on d 2 in the lungs of FXR−/− mice compared with the WT mice (Fig. 2, A–D). In addition, measurement of lung injury, including number of apoptotic lung cells (Fig. 2, E–G) and BAL cells (Fig. 2H), indicated that more severe damage was present in FXR−/− mice than in WT mice after LPS treatment.
Fig. 2.
FXR−/− mice are more susceptible to LPS-induced ALI. A–D, Histologic analysis revealed elevated neutrophilic alveolar and interstitial infiltration by d 1 and 2 after LPS inhalation in FXR−/− mice, with increased interstitial thickening and mixed cellular infiltration on d 2 in the lungs of FXR−/− mice compared with the control WT. Arrow indicates increased interstitial thickening and mixed cellular infiltration. E and F, Representative micrographs of terminal deoxynucleotidyl transferase 2-deoxyuridine, 5-triphosphate nick end labeling (TUNEL) staining of WT and FXR−/− lung tissue after LPS challenge. Lung sections of WT (E) and FXR−/− (F) mice collected 24 h after LPS challenge were stained with FITC-conjugated TUNEL to identify apoptotic cells. G, Number of the TUNEL-positive cells on D1 after LPS treatment. H, Total BAL cell counts of WT and FXR−/− mice after treatment with LPS (cellular infiltration in the airways after exposure to LPS). *, P < 0.05. TUNEL staining revealed there were approximately twice as many positive cells in lung tissue from FXR−/− mice (n = 4) compared with WT control mice (n = 4). The number of cells is the average of at least three different fields from each mouse (E–G). In FXR−/− animals, LPS treatment also triggered an altered profile of leukocyte influx into the airspace, with a marked increased of BAL cells at d 1 (D1) (n = 6) and 2 (D2) (n = 6), as compared with the WT controls (H). H&E, Hematoxylin and eosin.
As seen in the acute phase of ALI in humans, endothelial injury induced by LPS is characterized by EC apoptosis and increased endothelial permeability. We therefore assessed these responses in FXR−/− lungs. On the first day after LPS challenge, there was significant difference in the levels of cell apoptosis between lung sections from FXR−/− and WT mice (Fig. 2, E–G). TUNEL staining revealed that there were approximately twice as many positive cells in lung tissue from FXR−/− mice compared with WT control mice (Fig. 2, E–G). In FXR−/− animals, LPS treatment also triggered an altered profile of leukocyte influx into the airspace, with a marked increased of BAL cells at d 1 and 2, as compared with WT controls (Fig. 2H).
Increased inflammatory response in FXR−/− lung after LPS treatment
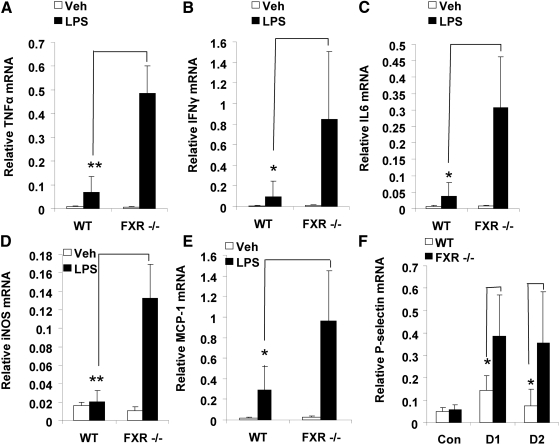
LPS inhalation is accompanied by an increased expression of intrapulmonary cytokines (18). Consistent with this, we observed increased mRNA levels of typical inflammatory mediators such as TNFα, IFNγ, IL-6, iNOS, and MCP1 on d 1 after LPS injection in both WT and FXR−/− mice. However, induction of these mediators was much higher in FXR−/− mice than in the WT control mice (Fig. 3, A–E).
Fig. 3.
FXR displays antiinflammatory activities in the lung after LPS treatment. Quantitative real-time PCR analysis of the expression of proinflammatory genes in lungs from WT and FXR−/− mice that were treated with a single dose of LPS (4 μg/10 g of BW) or PBS [as controls (Con) (Veh)]. RNA expression levels of TNFα (A), IFNγ (B), IL-6 (C), iNOS (D), and MCP1 (E) were analyzed on d 1 (D1) after LPS or PBS treatments. F, RNA levels of P-selectin expression were analyzed on d 1 (D1) and d 2 (D2) after LPS treatment. *, P < 0.05; **, P < 0.01. Con, Control; Veh, vehicle.
We also measured the expression of P-selectin mRNA in lung tissues from LPS-treated mice. P-selectin is a cell adhesion molecule on the surfaces of activated EC that line the inner surface of blood vessels and activated platelets. P-selectin plays an essential role in the initial recruitment of leukocytes to the site of injury during inflammation. We measured the P-selectin mRNA expression levels and found that P-selectin levels in FXR−/− mice were significantly greater than those in WT mice after LPS challenge (Fig. 3F).
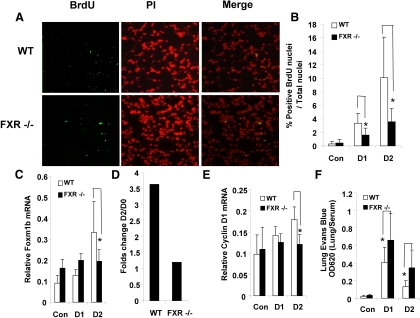
Defective lung regeneration in FXR−/− mice after LPS treatment
FXR has antiinflammation activity in vivo. Moreover, it has been reported that, after LPS-induced vascular injury, lungs from EC-restricted Foxm1-deficient mice (Foxm1 CKO) have impaired cell proliferation, and we have reported that Foxm1b is one of FXR's direct targets during tissue regeneration (5, 6). Therefore, we asked whether the defect in LPS-induced lung repair in FXR−/− mice was attributed to decreased lung EC proliferation. Mice were challenged with LPS, and cell proliferation was measured by BrdU incorporation. Lungs from WT mouse exhibited increased cellular proliferation (Fig. 4, A and B), with a similar time course of Foxm1b mRNA induction (Fig. 4, C and D). In contrast, FXR−/− mice showed fewer BrdU-positive nuclei in the first 2 d after LPS challenge compared with WT mice (Fig. 4, A and B). Furthermore, real-time PCR analysis revealed that Foxm1b gene transcription was induced in WT mice over the first 2 d after LPS treatment (Fig. 4C). On d 2 in particular, there was a 3-fold increase of Foxm1b mRNA levels in WT as compared with d 0, whereas FXR−/− mice did not show an increase in Foxm1b expression (Fig. 4D). Cyclin D1 expression was also greater on d 1 and 2 in the WT mice but not in FXR−/− mice (Fig. 4E). Finally, loss of FXR function increased the vascular permeability of FXR−/− lungs (Fig. 4F), further indicating a role of FXR in promoting lung repair.
Fig. 4.
Defective lung regeneration in FXR−/− mice after LPS-induced ALI. A, Representative micrographs of BrdU immunostaining of WT and FXR−/− lungs after LPS-induced lung microvascular injury. Sections (5 μm) of lungs collected 48 h after LPS challenge were stained with FITC-conjugated anti-BrdU antibody to identify proliferating cells; nuclei were counterstained with propidium iodide. B, Quantification of BrdU-positive nuclei of the lung sections described in A at indicated time points after LPS challenge [d 1 (D1), n = 4; and d 2 (D2), n =4]. C, QRT-PCR analysis of Foxm1b mRNA levels in lungs collected from WT or FXR−/− mice at the indicated time points after LPS exposure. D, Fold change in Foxm1b gene transcription induction (D2/D0). E, Quantitative analysis of Cyclin D1 mRNA levels after treatment of WT or FXR−/− mice with LPS. F, Pulmonary permeability of WT or FXR−/− mice as a function of Evans blue dye extravasation at the indicated time points after treatment with LPS [d 1 (D1), n = 4 and d 2 (D2), n = 4]. *, P < 0.05. Con, Control; PI, propidium iodide.
Expression of a constitutively active FXR in FXR−/− mice rescues the defects in lung injury repair
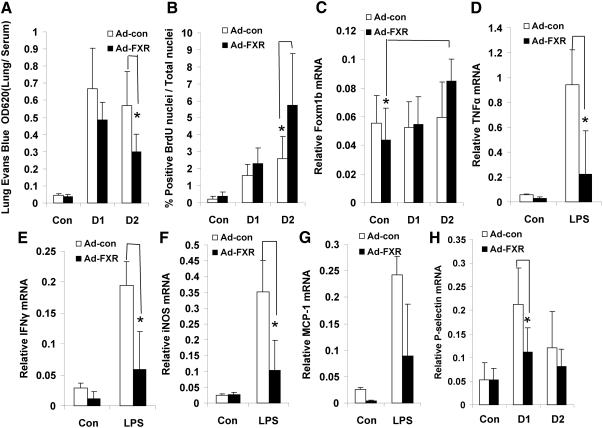
We next conducted rescue experiments using a constitutively active form of FXR, VP16-FXR, as described previously (7, 19). Overexpression of VP16-FXR in LPS-treated FXR−/− mice resulted in lower lung microvascular permeability (Fig. 5A) and greater lung BrdU incorporation levels (Fig. 5B) than did control adenovirus, suggesting that expression of an active FXR improved the lung repair induced by LPS injection. Furthermore, forced expression of VP16-FXR markedly reduced expression of proinflammatory cytokines on d 1 after LPS treatment (Fig. 5, D–G). Moreover, P-selectin was repressed (Fig. 5H), and Foxm1b expression was induced in the FXR-VP16 transfected mice compared with the control mice (Fig. 5C). Taken together, these data clearly demonstrate that activation of FXR suppressed the lung permeability and promoted lung repair.
Fig. 5.
Expression of a constitutively active FXR rescues promotes lung repair in FXR−/− mice. FXR−/− mice were infected by iv injection with either a control VP-16 adenovirus or an adenovirus that expresses FXR-VP16. After 4 d, the mice were treated with vehicle control (Con) or LPS (4 μg/10 g of BW). A, Lung vascular permeability was measured at the indicated time points [d 1 (D1), and d 2 (D2)] after LPS treatment. B, Quantification of BrdU-positive nuclei. C–H, Relative mRNA levels of Foxm1b (C), TNFα (D), IFNγ (E), iNOS (F), MCP1 (G), and P-selectin (H) as measured by RT-QPCR using total RNA prepared from the whole lung. *, P < 0.05. Con, Control.
FXR ligands suppress P-selectin expression induced by TNFα and stimulate lung EC proliferation in vitro
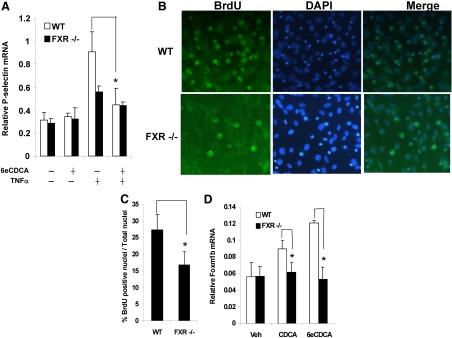
The reduced lung cell proliferation and the greater permeability found in FXR−/− lungs suggest that FXR in the EC may play a role during recovery from ALI. Treatment of WT mouse lung EC with 20 ng/ml TNFα resulted in up-regulation of P-selectin mRNA expression (Fig. 6A). In contrast, TNFα-induced P-selectin mRNA expression was attenuated when cells were pretreated with 6eCDCA, a synthetic FXR ligand. However, this suppressive effect of 6eCDCA on P-selectin induction was nearly abolished in FXR−/− EC (Fig. 6A).
Fig. 6.
FXR ligands suppress TNFα-induced P-selectin gene expression and stimulate DNA synthesis in primary lung EC. A, Primary lung EC from WT and FXR−/− mice were treated with TNFα or vehicle control for 5 h after pretreatment with or without 6eCDCA for 19 h. The mRNA levels of P-selectin were measured by RT-QPCR. B, Representative micrographs of BrdU immunostaining of 6eCDCA-treated primary lung EC. Nuclei were counterstained with DAPI. Cells were isolated from WT and FXR−/− lungs with an CD31 antibody and cultured for 5 d in M199 plus 20% FBS. Then the medium was changed to 5% resin-charcoal-superstripped serum for 2 d. Cells were then treated with 2 μm 6eCDCA or vehicle (dimethylsulfoxide) for 24 h before being subjected to immunostaining. C, Quantification of the BrdU-positive nuclei in WT and FXR−/− EC. The number of nuclei is the average of at least three different fields. D, CDCA and 6eCDCA-mediated up-regulation of Foxm1b mRNA expression in WT EC compared with FXR−/− cells. *, P < 0.05. Veh, Vehicle.
We then isolated microvascular EC from lungs of WT and FXR−/− mice and determined whether FXR ligands could promote proliferation of lung EC in vitro. EC isolated from FXR−/− lungs incorporated markedly less BrdU than WT lung cells after 6eCDCA treatment (Fig. 6, B and C). In addition, Foxm1b was induced in WT cells treated with CDCA or 6eCDCA but was not induced in FXR−/− cells (Fig. 6D). These results demonstrate that FXR activation stimulates proliferation of lung vascular EC probably by up-regulating Foxm1b expression.
Discussion
Our results indicate that FXR plays a protective role in inflammation-induced lung injury. FXR−/− mice displayed exacerbated lung pathology as compared with WT mice after LPS treatment. We propose that FXR suppresses lung injury through its antiinflammatory function. Previously, we show that FXR has antiinflammatory properties in the liver in a rodent LPS-induced inflammation model (11, 14), suggesting a role for FXR in suppressing the hepatic inflammatory response (20, 21). Furthermore, FXR is highly expressed in the vascular smooth muscle of normal and atherosclerotic blood vessels (9), and FXR is expressed in rat and human pulmonary EC. FXR is functional in EC, as demonstrated by induction of its target genes, including small heterodimer partner, after ligand treatments (10). FXR induces EC motility and angiogenesis, and FXR agonists induce cell motility and the tube formation capacity of EC in an FXR-matrix metalloproteinase 9 (MMP-9)-dependent manner (22). Mice deficiency in MMP-9 is associated with enhanced cell recruitment to the lung after allergen challenge, because MMP-9 is involved in the recruitment of leukocytes to an inflammatory site either by affecting development of allergen-specific T-cell responses or by influencing expression of inflammatory mediators, such as cytokines and chemokines (23).
FXR may also suppress lung injury by inhibiting P-selectin expression. P-selectin is found in both platelets and EC, which mediates leukocyte rolling, the initial step in leukocyte recruitment from the vascular lumen to the extravascular tissue. Loss of P-selectin has been reported to be protective during acute inflammatory settings, such as ischemia/reperfusion injury, sepsis, allergic inflammation, and contact hypersensitivity, as well as in chronic inflammation, such as atherosclerosis (15, 24, 25). Rapid translocation of P-selectin from cytoplasmic granules to the cell membrane of EC promotes adhesive interactions with neutrophils, which, when activated, damage the endothelium. Antihuman P-selectin reacts with a pulmonary vascular antigen in rats, and this antigen is essential for the full expression of lung injury (26). As a therapeutic approach, blocking P-selectin with antibodies after induction of ALI reduces the formation of platelet-neutrophil aggregates and development of ALI and leads to prolonged survival (27). Our results indicate that FXR ligands can suppress P-selectin expression in both LPS- induced lung injury model and in cell culture, which suggests that FXR ligands maybe used in the future as potential therapeutic agents to treat lung injury. But how FXR influences P-selectin expression still needs more investigation.
The apoptotic pathways are thought to play an important role in the pathogenesis of ALI. Both delayed neutrophil apoptosis and enhanced endothelial/epithelial cell apoptosis have been identified in ALI/ARDS. In the case of neutrophils, which contribute significantly to ALI/ARDS, studies in both animals and ARDS patients suggest that apoptosis is inhibited during the early stages of inflammation. There is also compelling evidence that increased epithelial/EC apoptosis contributes to the endothelial and epithelial injury that is characteristic of ALI/ARDS in humans. Studies have shown that ALI is associated with increased cell death in humans, whereas apoptosis inhibitors increased survival in rodent models of ALI (28, 29). Therefore, a possible explanation for the greater numbers of apoptotic cells in FXR-deficient mice is that FXR suppressed the leukocyte-endothelial adhesion molecules, and thus more BAL cells were released to the alveoli in FXR−/− mice, causing higher production of cytokines, such as TNFα and IFNγ, which induced lung cell death. Another possibility is that FXR can inhibit cell apoptosis directly, as previously reported (19).
FXR has been shown to regulate liver repair by increasing Foxm1b expression, and Foxm1b expression levels are also increased in the LPS-induced lung injury model. This induction is at least partially dependent on FXR, because our results showed that Foxm1b levels are not induced in FXR−/− lung after injury. As a result, the proliferation of the lung cells is significantly greater in WT mice than in FXR−/− mice after injury. Foxm1b also helps to restore the integrity of lung endothelial barrier, probably by stimulating EC proliferation in response to vascular endothelial injury. For example, premature pulmonary expression of the Foxm1b transgene was associated with earlier increases in DNA synthesis and mitosis in regenerating Rosa26-Foxm1b transgene lung (3). Our results showed that FXR contributes to the permeability of the lung, because overexpression of a constitutively active FXR increased the Foxm1b expression levels in injured FXR−/− lung and improved the permeability of the lung. FXR−/− mice and Foxm1b CKO mice display some different response after lung injury. FXR−/− mice have increased cytokine expression, lung permeability, and apoptosis of injured lung cells. However, the extent of EC apoptosis and neutrophil sequestration in the lungs between WT and Foxm1b CKO mice was the same (4). This suggests that, in addition to Foxm1b, FXR may regulate expression of other genes during acute lung repair. We should also note that FXR−/− mice have increased bile acid levels that may be related to certain lung injury (30). Therefore, whether FXR in other organs besides lung also involve in the LPS-induced lung injury needs more investigation.
In summary, FXR and its ligands play important roles in tissue repair after injury. Further elucidation of the mechanism by which FXR promote lung regeneration and inhibit inflammation will help us identify novel approaches to treat ALI diseases and ARDS.
Acknowledgments
We thank Keely Walker for editing and proofreading the manuscript and Dr. Peter A. Edwards at University of California at Los Angeles (CA) for providing adenovirus that expressed VP16 alone (Ad-VP16) or murine FXRα2 fused to VP16 (FXRα2-VP16).
This work was supported by the National Cancer Institute Grant 1R01CA139158-01A2 (to W.H.).
Disclosure Summary: The authors have nothing to disclose.
NURSA Molecule Pages†:
Nuclear Receptors: FXR-α.
Annotations provided by Nuclear Receptor Signaling Atlas (NURSA) Bioinformatics Resource. Molecule Pages can be accessed on the NURSA website at www.nursa.org.
- ALI
- Acute lung injury
- BAL
- bronchoalveolar lavage
- BrdU
- bromodeoxyuridine
- BW
- body weight
- CDCA
- chenodeoxycholic acid
- DAPI
- 4′,6-diamidino-2-phenylindole
- EC
- endothelial cell
- 6eCDCA
- 6-ethylchenodeoxycholic acid
- F
- forward
- FBS
- fetal bovine serum
- FITC
- fluorescein isothiocyanate
- FXR
- farnesoid X receptor
- IFN
- interferon
- iNOS
- inducible nitric oxide synthase
- i.t.
- intranasal instillation
- LPS
- lipopolysaccharide
- MCP
- monocyte chemoattractant protein
- MMP-9
- matrix metalloproteinase 9
- QRT-PCR
- quantitative RT-PCR
- R
- reverse
- TUNEL
- terminal deoxynucleotidyl transferase 2′-deoxyuridine, 5′-triphosphate nick end labeling
- WT
- wild-type.
References
- 1. Minamino T, Komuro I. 2006. Regeneration of the endothelium as a novel therapeutic strategy for acute lung injury. J Clin Invest 116:2316–2319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ware LB, Matthay MA. 2000. The acute respiratory distress syndrome. N Engl J Med 342:1334–1349 [DOI] [PubMed] [Google Scholar]
- 3. Kalinichenko VV, Gusarova GA, Tan Y, Wang IC, Major ML, Wang X, Yoder HM, Costa RH, Costal RH. 2003. Ubiquitous expression of the forkhead box M1B transgene accelerates proliferation of distinct pulmonary cell types following lung injury. J Biol Chem 278:37888–37894 [DOI] [PubMed] [Google Scholar]
- 4. Zhao YY, Gao XP, Zhao YD, Mirza MK, Frey RS, Kalinichenko VV, Wang IC, Costa RH, Malik AB. 2006. Endothelial cell-restricted disruption of FoxM1 impairs endothelial repair following LPS-induced vascular injury. J Clin Invest 116:2333–2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang W, Ma K, Zhang J, Qatanani M, Cuvillier J, Liu J, Dong B, Huang X, Moore DD. 2006. Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science 312:233–236 [DOI] [PubMed] [Google Scholar]
- 6. Chen WD, Wang YD, Zhang L, Shiah S, Wang M, Yang F, Yu D, Forman BM, Huang W. 2010. Farnesoid X receptor alleviates age-related proliferation defects in regenerating mouse livers by activating forkhead box m1b transcription. Hepatology 51:953–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang L, Huang X, Meng Z, Dong B, Shiah S, Moore DD, Huang W. 2009. Significance and mechanism of CYP7a1 gene regulation during the acute phase of liver regeneration. Mol Endocrinol 23:137–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meng Z, Wang Y, Wang L, Jin W, Liu N, Pan H, Liu L, Wagman L, Forman BM, Huang W. 2010. FXR regulates liver repair after CCl4-induced toxic injury. Mol Endocrinol 24:886–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bishop-Bailey D, Walsh DT, Warner TD. 2004. Expression and activation of the farnesoid X receptor in the vasculature. Proc Natl Acad Sci USA 101:3668–3673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. He F, Li J, Mu Y, Kuruba R, Ma Z, Wilson A, Alber S, Jiang Y, Stevens T, Watkins S, Pitt B, Xie W, Li S. 2006. Downregulation of endothelin-1 by farnesoid X receptor in vascular endothelial cells. Circ Res 98:192–199 [DOI] [PubMed] [Google Scholar]
- 11. Wang YD, Chen WD, Wang M, Yu D, Forman BM, Huang W. 2008. Farnesoid X receptor antagonizes nuclear factor κB in hepatic inflammatory response. Hepatology 48:1632–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li YT, Swales KE, Thomas GJ, Warner TD, Bishop-Bailey D. 2007. Farnesoid x receptor ligands inhibit vascular smooth muscle cell inflammation and migration. Arterioscler Thromb Vasc Biol 27:2606–2611 [DOI] [PubMed] [Google Scholar]
- 13. Vavassori P, Mencarelli A, Renga B, Distrutti E, Fiorucci S. 2009. The bile acid receptor FXR is a modulator of intestinal innate immunity. J Immunol 183:6251–6261 [DOI] [PubMed] [Google Scholar]
- 14. Mencarelli A, Renga B, Migliorati M, Cipriani S, Distrutti E, Santucci L, Fiorucci S. 2009. The bile acid sensor farnesoid X receptor is a modulator of liver immunity in a rodent model of acute hepatitis. J Immunol 183:6657–6666 [DOI] [PubMed] [Google Scholar]
- 15. Kevil CG. 2003. Endothelial cell activation in inflammation: lessons from mutant mouse models. Pathophysiology 9:63–74 [DOI] [PubMed] [Google Scholar]
- 16. Piqueras L, Sanz MJ, Perretti M, Morcillo E, Norling L, Mitchell JA, Li Y, Bishop-Bailey D. 2009. Activation of PPARβ/δ inhibits leukocyte recruitment, cell adhesion molecule expression, and chemokine release. J Leukoc Biol 86:115–122 [DOI] [PubMed] [Google Scholar]
- 17. Ramirez SH, Heilman D, Morsey B, Potula R, Haorah J, Persidsky Y. 2008. Activation of peroxisome proliferator-activated receptor γ (PPARγ) suppresses Rho GTPases in human brain microvascular endothelial cells and inhibits adhesion and transendothelial migration of HIV-1 infected monocytes. J Immunol 180:1854–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gong H, He J, Lee JH, Mallick E, Gao X, Li S, Homanics GE, Xie W. 2009. Activation of the liver X receptor prevents lipopolysaccharide-induced lung injury. J Biol Chem 284:30113–30121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang YD, Yang F, Chen WD, Huang X, Lai L, Forman BM, Huang W. 2008. Farnesoid X receptor protects liver cells from apoptosis induced by serum deprivation in vitro and fasting in vivo. Mol Endocrinol 22:1622–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang F, Huang X, Yi T, Yen Y, Moore DD, Huang W. 2007. Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid X receptor. Cancer Res 67:863–867 [DOI] [PubMed] [Google Scholar]
- 21. Kim I, Morimura K, Shah Y, Yang Q, Ward JM, Gonzalez FJ. 2007. Spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice. Carcinogenesis 28:940–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Das A, Yaqoob U, Mehta D, Shah VH. 2009. FXR promotes endothelial cell motility through coordinated regulation of FAK and MMP-9. Arterioscler Thromb Vasc Biol 29:562–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McMillan SJ, Kearley J, Campbell JD, Zhu XW, Larbi KY, Shipley JM, Senior RM, Nourshargh S, Lloyd CM. 2004. Matrix metalloproteinase-9 deficiency results in enhanced allergen-induced airway inflammation. J Immunol 172:2586–2594 [DOI] [PubMed] [Google Scholar]
- 24. Subramaniam M, Saffaripour S, Watson SR, Mayadas TN, Hynes RO, Wagner DD. 1995. Reduced recruitment of inflammatory cells in a contact hypersensitive response in p-selectin-deficient mice. J Exp Med 181:2277–2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Singbartl K, Green SA, Ley K. 2000. Blocking p-selectin protects ischemia/reperfusion-induced acute renal failure. FASEB J 14:48–54 [DOI] [PubMed] [Google Scholar]
- 26. Mulligan MS, Polley MJ, Bayer RJ, Nunn MF, Paulson JC, Ward PA. 1992. Neutrophil-dependent acute lung injury. Requirement for P-selectin (GMP-140). J Clin Invest 90:1600–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zarbock A, Singbartl K, Ley K. 2006. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J Clin Invest 116:3211–3219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lucas R, Verin AD, Black SM, Catravas JD. 2009. Regulators of endothelial and epithelial barrier integrity and function in acute lung injury. Biochem Pharmacol 77:1763–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rudkowski JC, Barreiro E, Harfouche R, Goldberg P, Kishta O, D'Orleans-Juste P, Labonte J, Lesur O, Hussain SN. 2004. Roles of iNOS and nNOS in sepsis-induced pulmonary apoptosis. Am J Physiol Lung Cell Mol Physiol 286:L793–L800 [DOI] [PubMed] [Google Scholar]
- 30. Zecca E, De Luca D, Baroni S, Vento G, Tiberi E, Romagnoli C. 2008. Bile acid-induced lung injury in newborn infants: a bronchoalveolar lavage fluid study. Pediatrics 12:e146–e149 [DOI] [PubMed] [Google Scholar]
- 31. Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. 2000. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 102:731–744 [DOI] [PubMed] [Google Scholar]