Abstract
p23 is a chaperone with multiple heat shock protein 90 dependent and independent cellular functions, including stabilizing unliganded steroid receptors and modulating receptor-DNA dynamics. p23 protein is also up-regulated in several cancers, notably breast cancer. We previously demonstrated that higher expression of p23 in the estrogen-dependent breast cancer line MCF-7 (MCF-7+p23) selectively increased estrogen receptor (ER) target gene transcription and ER recruitment to regulatory elements, promoted cell invasion, and predicted a poor prognosis in breast cancer patients. To probe the impact of p23 on ER binding throughout the human genome, we compared ER occupancy in MCF-7+p23 cells relative to MCF-7-control cells by using chromatin immunoprecipitation followed by ultrahigh-throughput DNA sequencing in the absence and presence of 17β-estradiol (E2) treatment. We found that increased expression of p23 resulted in a 230% increase in the number of E2-induced ER-binding sites throughout the genome compared with control cells and also increased ER binding under basal conditions. Motif analysis indicated that ER binds to a similar DNA sequence regardless of p23 status. We also observed that ER tends to bind closer to genes that were induced, rather than repressed by either E2 treatment or p23 overexpression. Interestingly, we also found that the increased invasion of MCF-7+p23 cells was not only p23 dependent but also ER dependent. Thus, a small increase in the expression of p23 amplifies ER-binding genome wide and, in combination with ER, elicits an invasive phenotype. This makes p23 an attractive target for combating tumor cell metastasis in breast cancer patients.
The chaperone p23 is traditionally thought of as impacting regulatory signaling cascades and steroid hormone ligand binding in the cytoplasm (1–3), yet recent studies have alluded to important effects of p23 on protein-DNA dynamics in the nucleus (4–6). However, the impact of p23 on transcription factor binding to DNA on a genome-wide scale has not been investigated.
We previously identified p23 as a modulator of estrogen receptor (ER)α-dependent transcriptional activity (7, 8). Gene expression profiling of MCF-7 cells with a small (<2-fold) increase in p23 protein expression relative to endogenous levels uncovered p23-dependent changes in the mRNA expression of a subset of genes under both basal and 17β-estradiol (E2)-treatment conditions, including genes involved in metastasis and drug resistance (9). In addition, higher p23 expression converted MCF-7 cells from a noninvasive to invasive phenotype in a matrigel invasion assay, and this phenotype correlated with findings in breast cancer patients where higher p23 expression was associated with increased lymph node metastases, elevated disease recurrence, and higher mortality (8, 9). Thus, p23 elicits changes in gene expression and cellular responses that promote a more aggressive cancer phenotype.
Although ER binding throughout the genome of MCF-7 cells has been examined (10–12), the effect of p23 expression on ER-binding genome wide has not been explored. Using ultrahigh-throughput sequencing of chromatin immunoprecipitated (ChIP) DNA fragments (ChIP-seq), we have determined the ER-bound sites as a function of p23 protein levels under basal conditions and in response to E2. We found that higher p23 expression resulted in a large increase in the number of sites bound by ER genome wide, and the proximity of this enhanced binding was associated more with E2-induced, rather than E2-repressed, genes. Surprisingly, the effect of p23 overexpression in promoting cellular invasion also required ER. Thus, higher p23 expression resulted in greater ER-binding genome wide and facilitates cell invasion that is ER dependent.
Results
Genome-wide binding of ER as a function of p23
We used ChIP-seq to identify the genomic locations bound by ER upon overexpression of p23 in the MCF-7 breast cancer cell line in response to E2. We isolated DNA from ER immunoprecipitated chromatin samples from MCF-7-control cells and MCF-7+p23 cells treated for 45 min with either 10 nm E2 or ethanol as a control. We sequenced the ChIP-isolated DNA with an Illumina Genome Analyzer IIx and aligned the sequence reads to the human genome by using the ELAND program (Illumina, San Diego, CA). We then used the model-based analysis of ChIP-seq (MACS) algorithm (13) with a P value cutoff of 10−10 to identify peaks in the aligned sequence data.
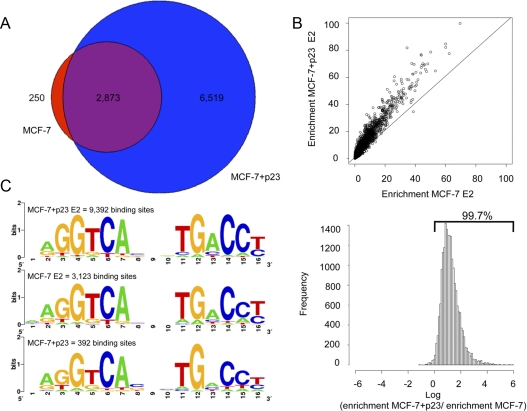
In addition to comparing genome-wide ER binding from MCF-7-control and MCF-7+p23 cells upon E2 treatment, we evaluated ER occupancy under low hormone (basal) conditions from two independent biological replicates. Only binding sites determined to be significant in both experiments are reported and represent high confidence ER-binding sites under the conditions of our assay. In MCF-7-control cells, we identified 3123 genomic positions that became occupied by ER upon E2 treatment. Significantly, in MCF-7+p23 cells, there were 9392 genomic sites occupied by ER. Of the sites bound by ER in MCF-7-control cells, 2873 (92%) overlapped with those from the MCF-7+p23 cells, whereas we identified 6519 additional ER-binding sites in MCF-7+p23 cells compared with control cells (Fig. 1A). This represents a 230% increase in the number of ER-binding sites in MCF-7 cells that have higher p23 expression. In addition, we observed 250 sites in the MCF-7-control cells that were not occupied by ER in the MCF-7+p23 cells (Fig. 1A), suggesting that p23 expression not only enhances ER binding to chromatin but also promotes, either directly or indirectly, ER removal from a small number of genomic sites (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org).
Fig. 1.
Global ChIP-seq analysis of ER binding in MCF-7 cells as a function of p23 expression. A, Venn Diagram showing the number of ER-binding sites in MCF-7-control (red) and MCF-7+p23 cells (blue) treated with E2; the overlap is shown in purple. B, Scatter plot (top panel) and histogram (bottom panel) showing the relative enrichment of ER-binding sites in E2-treated MCF-7-control and MCF-7+p23 cells. C, Consensus motif analysis of ER-binding sites in MCF-7-control cells with E2 treatment and MCF-7+p23 cells with and without E2 treatment.
Up-regulation of p23 also resulted in an increase in the number of regions bound by ER under basal conditions. Although there are no significant ER-binding sites identified in MCF-7-control cells in the absence of E2 treatment, we observed 392 regions bound by ER in MCF-7+p23 cells under basal conditions (Supplemental Fig. 2). There was a 42% overlap between ER binding in MCF-7+p23 in the absence of E2 and MCF-7-control cells in the presence of E2 (Supplemental Fig. 2), suggesting that increased p23, even in the absence of hormone treatment, is promoting ER binding to the same regions occupied upon E2 treatment. Interestingly, over half of the sites bound by ER under basal conditions did not overlap the ER-binding regions in the MCF-7-control cells upon E2 treatment but did significantly overlap (∼80%) with the sites bound by ER in the MCF-7+p23 cells (Supplemental Fig. 3). Thus, increased p23 expression modulates ER binding to chromatin in both the absence and presence of E2.
We next wanted to determine whether the additional approximately 6000 ER-binding regions in response to E2 in the MCF-7+p23 cells are new regions bound by ER or correspond to sites weakly bound by ER in MCF-7-control cells that were below the threshold used to “call” ER binding. To test this, we lowered the threshold for ER binding in the MCF-7-control cells from a P value of 10−10 to a P value of 10−5. Using this less stringent parameter, we observed 6636 binding sites bound by ER compared with 3123 ER-binding sites obtained with a more stringent cutoff in MCF-7-control cells. Of the 6636 ER-binding sites, 4980 regions (∼75%) overlap with the sites bound by ER in MCF-7+p23 cells (Supplemental Fig. 4). Additional ER-binding sites are also observed in MCF-7+p23 cells compared with control cells, suggesting that p23 has multiple modes of regulating ER binding to chromatin. However, overall, these results suggest that a majority of the ER sites occupied in MCF-7+p23 cells are also bound, albeit much more weakly in MCF-7-control cells upon E2 treatment. Consistent with this observation, we plotted the enrichment of ER-binding signal in MCF-7-control vs. MCF-7+p23 cells and observed that p23 overexpression caused an overall increase in ER-binding signal in response to E2 (Fig. 1B, top panel). In fact, more than 99.7% of ER-binding sites in the MCF-7-control cells displayed more ER binding in MCF-7+p23 cells (Fig. 1B, bottom panel). These results indicate that increased p23 expression results in a stronger binding response of ER to chromatin.
We also examined whether ER sites bound upon p23 overexpression would be associated with ER-mediated long-range interactions identified in an assay capable of interrogating three-dimensional chromosomal interactions, termed ChIA-PET (chromatin interaction analysis using paird end tag sequencing), in MCF-7 cells (14). We compared the locations of ER-binding sites from the published ChIA-PET database with the ER sites bound in MCF-7+p23 cells. There appears to be several binding sites involved in long-range interactions that are in common with the ER sites bound upon p23 overexpression (Supplemental Fig. 5), suggesting that p23 is contributing, either directly or indirectly, to DNA chromatin looping.
Next we compared our ER-interaction sites from MCF-7-control [3123 (P < 10−10) and 6636 (P < 10−5)] and MCF-7+p23 cells [9392 (P < 10−10)] with genome-wide profiles determined in MCF-7 cells using either a ChIP-chip platform from Carroll et al. (15) [3665 (P < 10−5)] or a ChIP-seq approach from Welboren et al. (16) [7713 sites (P < 10−5)]. As shown in Table 1, when we compared ER-bound sites from our MCF-7-control cell dataset using a P value cutoff of 10−10, we found a substantial 57% overlap with ChIP-seq targets as well as a 43% overlap with the target from the ChIP-chip study. Reducing the stringency of ER binding to a P value cutoff of 10−5 in our MCF-7-control cells increased the total number of ER-interaction sites but reduced the overlap with ChIP-seq and ChIP-chip targets to 41 and 30%, respectively. This likely reflects the emergence of less specific ER-interaction sites when the threshold for ER binding is reduced. We also compared ER interactions from MCF-7+p23 cells to ChIP-seq and ChIP-chip datasets and found an overlap of 39 and 26%, respectively. This is similar to the overlap of ER-interaction sites found in MCF-7-control cells with a P value cutoff of 10−5 and is consistent with the finding that p23 overexpression enhances ER binding to sites weakly occupied in MCF-7-control cells. Variations in cells, culture conditions, and sample handling likely account for some of the differences in the quantity and overlap of ER-binding sites between our study and the other studies. In fact, such differences are also apparent when comparing ER-binding sites between the Carroll et al. (15) and Welboren et al. (16) studies, in which ER-binding sites overlap by 42%. Thus, evaluating datasets from multiple ChIP studies has been highly informative by revealing what are likely to comprise the highest affinity ER-binding sites.
Table 1.
Comparison of genome-wide ER binding
To determine whether ER binding to its response elements [estrogen response element (ERE)] was affected by p23 overexpression, we performed in silico binding motif identification on the significant ER-binding sites determined by the MACS peak caller from: 1) MCF-7-control in the presence of E2 (3123 binding sites); 2) MCF-7+p23 in presence of E2 (9392 binding sites); and 3) MCF-7+p23 in absence of E2 (392 binding sites). The half-site AGGTCA sequence motifs identified in each set did not change as a function of p23 or E2 treatment, agreeing with earlier studies (Fig. 1C) (10, 17). Thus, increased p23 expression enables ER to bind to more ERE sites genome wide without changing the DNA sequence preference of ER.
ER binding to E2 activated and repressed genes as a function of p23
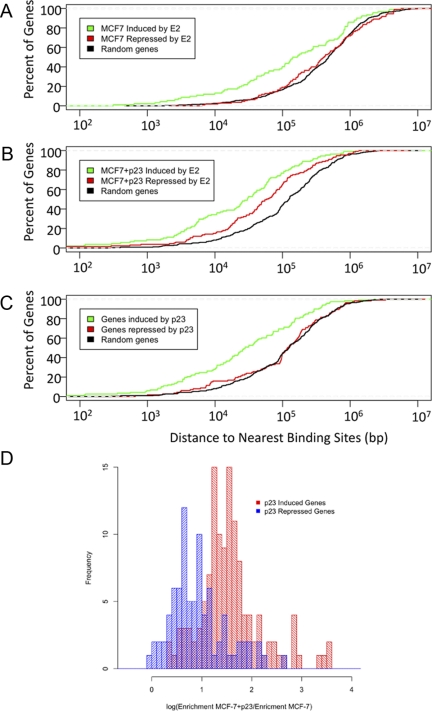
To investigate the role of ER binding in E2-mediated gene expression, we searched for ER-bound sites within 107 bp upstream and downstream of the transcriptional start site (TSS) of E2-responsive genes from MCF-7-control and MCF-7+p23 cells using a previously published microarray dataset (9). For the E2-induced genes in MCF-7-control and MCF-7+p23 cells, the fraction of genes with ER bound within 102-103 bp of the TSS is significant but low (Fig. 2, A and B), which is consistent with previous reports (14, 15, 18, 19). As the distance from the TSS is extended, the fraction of E2-induced genes bound by ER increases for MCF-7-control and MCF-7+p23 cells, with a median distance from the TSS of 1.4 × 105 and 3.0 × 104 bp, respectively (Fig. 2, A and B, and Table 2).
Fig. 2.
p23 overexpression in MCF-7 cells increases ER recruitment to EREs both distal and proximal to p23 and E2-regulated genes. Percentage of E2-responsive (A and B) or p23-responsive (C) genes (y-axis) that have ER binding within a given distance from the transcription start site (x-axis) in (A) MCF-7-control cells, (B) MCF-7+p23 cells, and (C) p23-dependent ER-binding sites in MCF-7 cells. Green line, p23-induced genes; red line, p23-repressed genes; black line, random set of genes. D, Histogram showing an enrichment of ER binding in the presence of E2 treatment in MCF-7+p23 cells relative to MCF-7-control cells at p23-induced genes (red) but not at p23-repressed genes (blue).
Table 2.
Median distance between ER-binding sites and transcription start site of affected genes (base pairs)
| MCF-7-control E2-dependent | MCF-7-control E2-dependent | MCF-7+p23 E2-dependent | |
|---|---|---|---|
| Induced | 1.42 × 105 | 3.03 × 104 | 2.27 × 104 |
| Repressed | 4.02 × 105 | 6.23 × 104 | 1.18 × 105 |
| Random | 4.74 × 105 | 1.24 × 105 | 1.24 × 105 |
However, the proximity of ER recruitment to the TSS differs for the E2-repressed genes compared with E2-induced genes. For example, in MCF-7-control cells, the fraction of E2-repressed genes with ER bound at 103 bp is insignificant, whereas a significant fraction of genes with ER bound is observed at 104 bp, and the fraction increases at 105 bp from the TSS (median distance of 4.02 × 105 from the TSS) (Fig. 2A). For the E2-repressed genes in MCF-7+p23 cells, there is a small but increasing fraction of genes bound by ER between 103 and 104 bp, and a substantial fraction of E2-repressed genes now displays ER binding at 105 bp upstream of the TSS (median distance of 6.23 × 104) (Fig. 2B and Table 2). We also observed a large difference in ER binding in the vicinity of genes affected by p23 overexpression (Fig. 2C). The median distance from the TSS of genes induced by p23 overexpression to ER-binding sites identified in MCF-7+p23 cells treated with E2 was 2.8 × 104, whereas the median distance to genes repressed by p23 overexpression was 1.2 × 105.
To determine whether this shift toward more proximal ER binding at the repressed genes by p23 is genuine, or a by-product of the increased ER binding upon p23 overexpression, we compared the fraction of genes bound by ER at E2-repressed genes vs. a random set of genes not regulated by E2. Although there was a small enrichment over random genes of ER binding at sites proximal to the TSS of repressed genes, it was still marginal compared with the amount of ER binding proximal and distal to induced genes (Fig. 2C). In fact, when plotted as a histogram, there is enrichment in ER binding in MCF-7+p23 vs. MCF-7-control cells at induced as compared with repressed genes (Fig. 2D). Thus, genes induced by p23 have more ER-binding sites nearby than genes repressed by p23.
Increased ER binding and expression of SERPINA1 by p23
To validate our ChIP-seq experiments, we selected a representative gene, SERPINA1 [serpin peptidase inhibitor clade A (alpha-1 antiproteinase, antitrypsin), member 1], which exhibited a large increase in ER binding in the MCF-7+p23 cells compared with control cells. SERPINA1 is a protease inhibitor and has been shown to play important roles in promoting carcinogenesis. Although protease inhibitors are generally thought to be antimalignant, SERPINA1, by contrast, correlates with tumor progression in lung and pancreatic cancers and is up-regulated in liver metastasis from pancreatic tumors (20, 21).
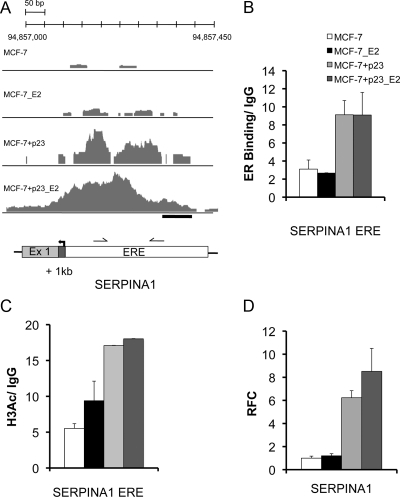
In ChIP-seq experiments of MCF-7-control cells, SERPINA1 showed a small enrichment of sequence tags over a narrow range near a promoter proximal ERE in the absence of ligand, and this was slightly increased upon E2 treatment (Fig. 3A). By contrast, in MCF-7+p23 cells, there was a vast enrichment of tags over the ERE in the absence of E2 treatment and more upon E2 treatment (Fig. 3A). ChIP-quantitative real-time PCR (qPCR) validated the binding of ER to the SERPINA1 ERE upon p23 overexpression (Fig. 3B). This increased ER binding was also associated with a significant increase of histone H3 lysine 9 acetylation (Fig. 3C) and enhanced mRNA expression (Fig. 3D) in MCF-7+p23 cells compared with control cells especially under basal conditions. Thus, overexpression of p23 enhances ER binding and histone H3 lysine 9 acetylation to the SERPINA1 promoter, which in turn facilitates gene expression under both basal and E2-dependent conditions.
Fig. 3.
ER binding and mRNA regulation of SERPINA1 by E2 and p23. A, The number of overlapping sequence tags represented as peak height is shown for SERPINA1. ER peaks are detected at the promoter proximal ERE upon E2 treatment in MCF-7-control cells, whereas only residual binding is observed in the absence of ligand. In MCF-7+p23 cells, bound ER is increased in both the absence and presence of E2. B, Validation ChIP assays were performed at ChIP-seq-predicted ER-binding sites for SERPINA1, a gene made more responsive to E2 by p23 overexpression. Shown is a schematic representation of SERPINA1 loci. Arrows indicate TSS; gray boxes, exons; white boxes, predicted ERE; black bars, regions amplified by qPCR to determine the level of ER binding. ChIP assays were performed using specific antibodies against ER or negative control IgG. Relative levels of ER binding in the presence and absence of E2 were determined using qPCR. Data are averages from replicate experiments, normalized to input, and presented as relative fold-enrichment over IgG control. Error bars, se. C, Increased basal ER recruitment in p23 overexpressing MCF-7 cells correlates with increased histone H3K9/14 acetylation. Directed ChIP were performed at the SERPINA1 ERE using specific antibodies against acetylated histone H3K9/14. Error bars, se. Note the error bars in the last two columns are so small and therefore do not show up readily on graph. D, The p23 and E2 responsiveness of SERPINA1 was examined in MCF-7-control and MCF-7+p23 cells using qPCR. Data are means from three independent experiments, normalized to glyceraldehyde-3-phosphate dehydrogenase, and presented as relative fold-change (RFC) of E2-treated MCF-7-control, E2-treated MCF-7+p23, or untreated MCF-7+p23 cells from that of untreated MCF-7-control cells, set to 1. Error bars, se.
p23 overexpression induces cell invasion via ER
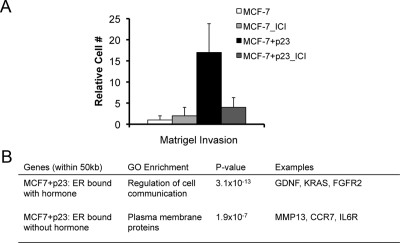
Our previous work demonstrated that p23 overexpression converts the noninvasive MCF-7 cells to an invasive phenotype under both basal and E2-treated conditions (8). This was substantiated in breast cancer patient samples, where high levels of p23 were associated with increased lymph node metastasis (9). To test the requirement for ER in the enhanced invasion upon p23 overexpression, we performed matrigel invasion assays in MCF-7-control and MCF-7+p23 cells treated with the pure ER antagonist ICI 182,780 (ICI) (Fulvestrant), which extinguishes ER action by promoting ER degradation (22, 23). MCF-7+p23 cells displayed increased invasion through matrigel under basal conditions (Fig. 4A). Importantly, this p23-dependent invasion was largely blocked by ICI treatment (Fig. 4A). Thus, the invasive phenotype of MCF-7+p23 cells requires both ER and p23.
Fig. 4.
Ablation of ER through ICI (Fulvestrant) treatment decreases invasiveness of MCF-7+p23 cells. A, MCF-7-control and MCF-7+p23 cells were plated in invasion chambers in the presence of 100 nm ICI. Cells were harvested and counted 48 h after ICI treatment. Results are presented as the mean of three independent experiments. B, GO classes of genes with ER binding within 50,000 bp of the TSS in the presence and absence of E2 treatment.
Given that p23 overexpression promotes cell invasion under basal as well as E2-stimulated conditions, we examined the gene sets associated with ER binding within 5 × 104 bp of a gene's TSS in both the absence and presence of E2. Our previous analysis of gene expression profiles of MCF-7-control and MCF-7+p23 cells revealed that p23 protein levels modulate the expression of genes misregulated in advanced breast cancers, including those involved in transcriptional regulation, migration and invasion, and metabolism (9). Consistent with our previous transcription profiling analysis, in the presence of E2, we found enrichment of genes in the gene ontology class representing “regulation of cell communication” (P < 3.09 × 10−13). These genes include glial cell-derived neurotrophic factor (GDNF), a ligand for the rearranged during transfection (RET) oncogene; Kirsten rat sarcoma viral oncogene homolog (KRAS), a member of the small GTPase family implicated in various malignancies; and fibroblast growth factor receptor 2 (FGFR2), a receptor for mitogenic signaling molecules. Without hormone treatment for genes with ER bound within 50 kb, we observed enrichment in “plasma membrane proteins” (P < 1.9 × 10−7). These included matrix metallopeptidase 13 (MMP13), a matrix metalloproteinase family member involved in the breakdown of extracellular matrix and tumor initiation; chemokine receptor 7 (CCR7), a chemokine receptor important in cell migration; and IL-6 receptor, a cytokine receptor that regulates cell growth and differentiation and implicated in carcinogenesis. Thus, genes bound by ER with E2 treatment are enriched for regulators of cellular communication, whereas genes bound by ER without hormone treatment are associated with cell adhesion and migration. This suggests that p23 differentially modulates ER binding to select genes in absence and presence of E2 that influence distinct pathways of cell movement and cell communication, respectively.
Discussion
We have examined the impact of higher p23 expression on ER-binding genome wide in MCF-7 cells. This revealed a large increase in the number of sites bound by ER throughout the genome under both basal and E2-induced conditions. This was not a result of alterations in ER protein, because we have previously shown that MCF-7-control and MCF-7+p23 cells have similar levels of ER protein expression (8). We also observed p23-dependent eviction of ER at a small number (250) of sites such that less ER binding was evident at the targets in MCF-7+p23 compared with MCF-7-control cells (Fig. 1A and Supplemental Fig. 4). Thus, increasing p23 levels modulates ER occupancy at genomic sites.
Our findings also indicate that p23 is making ERE in the genome more accessible to ER, rather than altering ER DNA binding specificity. In addition, it does not appear that this enhanced ER binding with higher p23 is a result of variations in the neighboring transcription factor-binding sites (so called pioneer factors), because forkhead box protein A1 (FOXA1) is the most prevalent binding motif adjacent to the ER-binding sites irrespective of p23 status (data not shown).
p23 overexpression enhanced ER binding to the SERPINA1 promoter, increased histone H3 lysine 9 acetylation to this same region, and increased SERPINA1 mRNA expression under basal and E2-stimulated conditions (Fig. 3). This suggests that if enough ER is driven onto DNA, even in the absence of added E2, it is capable of eliciting a transcriptional response at certain target genes. This is reminiscent of what is observed when the intracellular concentration of ER is raised in MCF-7 cells, which results in ER-dependent gene activation under basal conditions (24). Thus, it appears that higher p23 levels modulate the specific activity of ER for chromatin.
So how is p23 affecting ER binding to chromatin? Given the recent elegant study from John et al. (25) demonstrating the propensity for GR to bind to open chromatin, we hypothesize that higher p23 expression in MCF-7 cells is influencing chromatin architecture, perhaps inducing a more open state, which would allow ER to bind more efficiently to DNA.
In fact, a recent study from the Freeman laboratory has demonstrated that p23 plays important roles in transcription independent of heat shock protein 90, including a synthetic lethal interaction in yeast between p23 and the histone acetyl transferase general control of amino acid synthesis protein 5 (GCN5) (6). In addition, we find that higher p23 expression also resulted in greater p23 levels in the nucleus of MCF-7 cells to control gene expression (9). Although Freeman and Yamamoto (26) and Stavreva et al. (27) have been able localize p23 to glucocorticoid response elements in cells by ChIP and by immunofluorescence, respectively, we have been unable to ChIP p23 to specific genomic regions in MCF-7 cells. However, we have demonstrated that p23 binds chromatin in MCF-7 cells by isolating a nuclear fraction that contains DNA and proteins tightly bound to chromatin, treating with micrococcal nuclease to release chromatin-bound proteins (28) and blotting for p23 (Supplemental Fig. 6). Thus, we speculate that the gene-specific effects of higher p23 expression occur in response to p23 control of general control of acid synthesis protein 5 (GCN5) activity, which through changes in histone acetylation alter chromatin accessibility and ER DNA binding. Consistent with this is the enhanced histone H3 lysine 9 acetylation observed at p23-enhnaced ER-target genes, such as SERPINA1 (Fig. 3C).
Multiple laboratories have previously examined ER binding in MCF-7 cells (10–12, 16). As with any genome-wide study, there are differences in the number of ER-bound regions and the extent of overlap. For example, the overlap in ER-binding sites between the Carroll et al. (10) and Welboren et al. (16) studies was 42%. The apparent discrepancy in the number of ER-bound regions between our study and that of Welboren et al. (16) is in part a result of different P values employed in calling the ER-binding sites using the MACS program. When we compare bound ER using the same algorithm (MACS) and P value (1 × 10−10), we find fewer ER-bound sites [∼3000 compared with ∼7000 for Welboren et al. (16)] and an overlap of 57%. However, if we use the same P value as Welboren et al. (16) (1 × 10−5), a similar number of ER sites bound (∼7000) is observed between studies. Of those sites, 41% overlapped, which represent the highest affinity ER-binding sites. The observed nonoverlapping ER-interaction sites are likely the result of experimental variation, including MCF-7 cell line heterogeneity, culture conditions, the antibody used to ChIP ER, and the length of E2 treatment. Moreover, given our findings that higher p23 expression modulates ER chromatin binding, we would also maintain that subtle differences in p23 expression, even within the same MCF-7 cell type and under the same set of experimental conditions, could influence the amount and location of ER-interaction sites genome wide. Thus, the collection of ER-binding sites derived from a given ChIP-seq study is a function of the methodological and statistical parameters employed, as well as the expression of p23.
Our data showed that the majority of down-regulation occurs independently of proximal ER-DNA interactions. A study on genome-wide binding of GR by Reddy et al. (29) first described this proximity difference in receptor binding between activated vs. repressed genes and suggests that receptor-mediated repression involves long-range cis interactions.
Another important finding from this study is that the invasive phenotype of MCF-7+p23 cells under basal conditions was also ER dependent. Importantly, this ties ER binding in MCF-7+p23 cells to invasion and metastasis, even under low E2 conditions. These findings also have important clinical ramifications in those breast cancer patients whose tumors express ER and higher p23 levels and might benefit from Fulvestrant therapy in early stage disease, such as ductal carcinoma in situ, to prevent local lymph node metastasis. Moreover, finding ways to keep p23 protein levels low could also be an important chemoprevention strategy in breast cancer and delay early stage breast cancers from spreading, even in the postmenopausal setting, where estrogen levels are low. Thus, understanding how p23 protein expression is regulated in breast cancer and identifying ways to maintain low p23 protein expression are important future areas for translational research.
Materials and Methods
Cell lines
MCF-7-control and p23-overexpressing MCF-7 cells (MCF-7+p23) were cultured and hormone starved as previously described (8, 9).
ChIP and analysis
After induction with 10 nm E2 for 45 min, cells were fixed in 1% formaldehyde for 10 min at room temperature. Glycine was then added to a concentration of 125 mm. Cross-linked cells were washed with cold PBS and scraped into Farnham lysis buffer [5 mm PIPES (pH 8.0), 85 mm KCl, and 0.5% Nonidet P-40]. Nuclei were collected by centrifugation for 5 min at 1000 × g. ChIP was performed as previously described (29) using the ERα (HC-20) antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). ChIP interrogating histone H3 K9 acetylation were performed with acetyl-histone H3-K9/14 antibody (Millipore, Inc., Bedford, MA) or equivalent amount of rabbit IgG (I-8140; Sigma, St. Louis, MO) antibody.
For ChIP-seq experiments, DNA purified after ChIP went through Illumina library construction and was sequenced on a Genome Analyzer IIx. Thirty-six base pair sequence reads were aligned to the hg19/GRCh37 assembly of the human genome using ELAND (Illumina). The program MACS (13) was used to find significant binding sites for each experiment with the P value cutoffs described in Results. To determine E2-induced-binding sites in MCF-7+p23 cells, we used the MCF-7+p23 sample treated with ethyl alcohol (EtOH) as the control. To determine E2-induced-binding sites in MCF-7 cells, we used the MCF-7 sample treated with EtOH as the control. To determine ER-binding sites in MCF-7+p23 cells under basal conditions, we used the MCF-7 sample treated with EtOH as the control. Each ChIP was performed in duplicate, and only binding sites that were identified as significant in each replicate were considered.
Motif finding was performed by searching for sequence patterns within 50 base pairs on each side of the summit of each binding site identified by MACS using the program BioProspector (30). To calculate enrichment scores for each binding site, the average number of sequence alignments that mapped to each binding site per 1 million aligned sequence reads was calculated. All comparisons with genes were done to RefSeq coordinates (31).
Directed ChIP-qPCR to validate ER recruitment and histone 3 K9/14 acetylation at the SERPINA1 ERE were preformed as described in Simpson et al. (9). ChIP Primers flanking the SERPINA1 ERE are: SERPINA1_ERE_Forward, 5′-ACAAGTCACCCTCTCCCTTTGAG-3′ and SERPINA1_ERE_Reverse, 5′-CCAGAAACCTGCCAGTTATTGG-3′.
RNA isolation and qPCR
Total RNA from MCF-7-control and MCF-7+p23 cells was extracted with TRIzol (Invitrogen, Carlsbad, CA) as described by the manufacturer. cDNA was synthesized from 1 μg of RNA using the First-Strand cDNA Synthesis kit for Real-Time PCR (USB, Santa Clara, CA) and random primer hexamers following the manufacturer's instructions. cDNA were amplified with the SYBR Green Taq Ready Mix (USB) using MyiQ Single-Color Real-Time PCR Detection System from Bio-Rad (Hercules, CA). The primers used for qPCR for SERPINA1: forward, 5′-GGAAAATGAACTCACCCACG-3′ and reverse, 5′-AGTTGA CCCAGGACGCTCT-3′.
Cell invasion assay
Invasion assays were performed by using BD BioCoat growth factor reduced matrigel invasion chamber from BD Biosciences (San Diego, CA) according to the manufacturer's instructions. Fibronectin was used as a chemoattractant at a final concentration of 20 μg/ml. After addition of the cell suspension to the matrigel inserts, the invasion chambers were incubated at 37 C for 48 h. The noninvading cells were then removed by using moistened cotton swabs. The invading cells on the lower surface of the membrane were fixed and stained by using the Richard-Allan Scientific three-step stain kit (Thermo Fisher Scientific, Auburn, AL). The membranes were then removed from the insert by using a scalpel and placed on a microscope slide with a drop of immersion oil. The cells were counted under a microscope.
Acknowledgments
We thank Maryem Hussein, Susan Logan, Chaowei Wu, and Chris Gunter for critically reading the manuscript and Henk Stunnenberg for providing the ER ChIP-seq data from the MACS algorithm.
This work was supported by grants from the Vilcek Foundation (N.E.S.) and by the National Human Genome Research Institute Grant 5U54HG004576 (to R.M.M.).
Disclosure Summary: The authors have nothing to disclose.
NURSA Molecule Pages†:
Nuclear Receptors: ER-α;
Ligands: 17β-estradiol.
Annotations provided by Nuclear Receptor Signaling Atlas (NURSA) Bioinformatics Resource. Molecule Pages can be accessed on the NURSA website at www.nursa.org.
- ChIA-PET
- Chromatin interaction analysis using paird end tag sequencing
- ChIP
- chromatin immunoprecipitation
- ChIP-seq
- ultrahigh-throughput sequencing of chromatin immunoprecipitated DNA fragments
- E2
- 17β-estradiol
- ER
- estrogen receptor
- ERE
- estrogen response element
- EtOH
- ethyl alcohol
- ICI
- ICI 182,780
- MACS
- model-based analysis of ChIP-seq
- qPCR
- quantitative real-time PCR
- SERPINA1
- serpin peptidase inhibitor clade A (alpha-1 antiproteinase, antitrypsin), member 1
- TSS
- transcriptional start site.
References
- 1. Felts SJ, Toft DO. 2003. p23, a simple protein with complex activities. Cell Stress Chaperones 8:108–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Picard D. 2006. Chaperoning steroid hormone action. Trends Endocrinol Metab 17:229–235 [DOI] [PubMed] [Google Scholar]
- 3. Pratt WB, Galigniana MD, Harrell JM, DeFranco DB. 2004. Role of hsp90 and the hsp90-binding immunophilins in signalling protein movement. Cell Signal 16:857–872 [DOI] [PubMed] [Google Scholar]
- 4. DeFranco DB, Csermely P. 2000. Steroid receptor and molecular chaperone encounters in the nucleus. Sci STKE 2000:pe1. [DOI] [PubMed] [Google Scholar]
- 5. Freeman BC, Yamamoto KR. 2001. Continuous recycling: a mechanism for modulatory signal transduction. Trends Biochem Sci 26:285–290 [DOI] [PubMed] [Google Scholar]
- 6. Echtenkamp FJ, Zelin E, Oxelmark E, Woo JI, Andrews BJ, Garabedian M, Freeman BC. 2011. Global functional map of the p23 molecular chaperone reveals an extensive cellular network. Mol Cell 43:229–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Knoblauch R, Garabedian MJ. 1999. Role for Hsp90-associated cochaperone p23 in estrogen receptor signal transduction. Mol Cell Biol 19:3748–3759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oxelmark E, Roth JM, Brooks PC, Braunstein SE, Schneider RJ, Garabedian MJ. 2006. The cochaperone p23 differentially regulates estrogen receptor target genes and promotes tumor cell adhesion and invasion. Mol Cell Biol 26:5205–5213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Simpson NE, Lambert WM, Watkins R, Giashuddin S, Huang SJ, Oxelmark E, Arju R, Hochman T, Goldberg JD, Schneider RJ, Reiz LF, Soares FA, Logan SK, Garabedian MJ. 2010. High levels of Hsp90 cochaperone p23 promote tumor progression and poor prognosis in breast cancer by increasing lymph node metastases and drug resistance. Cancer Res 70:8446–8456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, Eeckhoute J, Shao W, Hestermann EV, Geistlinger TR, Fox EA, Silver PA, Brown M. 2005. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell 122:33–43 [DOI] [PubMed] [Google Scholar]
- 11. Krum SA, Miranda-Carboni GA, Lupien M, Eeckhoute J, Carroll JS, Brown M. 2008. Unique ERα cistromes control cell type-specific gene regulation. Mol Endocrinol 22:2393–2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Madak-Erdogan Z, Lupien M, Stossi F, Brown M, Katzenellenbogen BS. 2011. Genomic collaboration of estrogen receptor α and extracellular signal-regulated kinase 2 in regulating gene and proliferation programs. Mol Cell Biol 31:226–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, Liu XS. 2008. Model-based analysis of ChIP-Seq (MACS). Genome Biol 9:R137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fullwood MJ, Liu MH, Pan YF, Liu J, Xu H, Mohamed YB, Orlov YL, Velkov S, Ho A, Mei PH, Chew EG, Huang PY, Welboren WJ, Han Y, Ooi HS, Ariyaratne PN, Vega VB, Luo Y, Tan PY, Choy PY, Wansa KD, Zhao B, Lim KS, Leow SC, Yow JS, Joseph R, Li H, Desai KV, Thomsen JS, Lee YK, Karuturi RK, Herve T, Bourque G, Stunnenberg HG, Ruan X, Cacheux-Rataboul V, Sung WK, Liu ET, Wei CL, Cheung E, Ruan Y. 2009. An oestrogen-receptor-α-bound human chromatin interactome. Nature 462:58–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, Wang Q, Bekiranov S, Sementchenko V, Fox EA, Silver PA, Gingeras TR, Liu XS, Brown M. 2006. Genome-wide analysis of estrogen receptor binding sites. Nat Genet 38:1289–1297 [DOI] [PubMed] [Google Scholar]
- 16. Welboren WJ, van Driel MA, Janssen-Megens EM, van Heeringen SJ, Sweep FC, Span PN, Stunnenberg HG. 2009. ChIP-Seq of ERα and RNA polymerase II defines genes differentially responding to ligands. EMBO J 28:1418–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Klinge CM. 2001. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res 29:2905–2919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin CY, Vega VB, Thomsen JS, Zhang T, Kong SL, Xie M, Chiu KP, Lipovich L, Barnett DH, Stossi F, Yeo A, George J, Kuznetsov VA, Lee YK, Charn TH, Palanisamy N, Miller LD, Cheung E, Katzenellenbogen BS, Ruan Y, Bourque G, Wei CL, Liu ET. 2007. Whole-genome cartography of estrogen receptor α binding sites. PLoS Genet 3:e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kininis M, Chen BS, Diehl AG, Isaacs GD, Zhang T, Siepel AC, Clark AG, Kraus WL. 2007. Genomic analyses of transcription factor binding, histone acetylation, and gene expression reveal mechanistically distinct classes of estrogen-regulated promoters. Mol Cell Biol 27:5090–5104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ambrosini-Spaltro A, Potì O, De Palma M, Filotico M. 2009. Pancreatic-type acinar cell carcinoma of the stomach beneath a focus of pancreatic metaplasia of the gastric mucosa. Hum Pathol 40:746–749 [DOI] [PubMed] [Google Scholar]
- 21. Comunale MA, Rodemich-Betesh L, Hafner J, Wang M, Norton P, Di Bisceglie AM, Block T, Mehta A. 2010. A Linkage specific fucosylation of α-1-antitrypsin in liver cirrhosis and cancer patients: implications for a biomarker of hepatocellular carcinoma. PLoS One 5:e12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Howell A. 2000. Faslodex (ICI 182780). an oestrogen receptor downregulator. Eur J Cancer 36(Suppl 4):S87–S88 [DOI] [PubMed] [Google Scholar]
- 23. Howell A, Osborne CK, Morris C, Wakeling AE. 2000. ICI 182,780 (Faslodex): development of a novel, “pure” antiestrogen. Cancer 89:817–825 [DOI] [PubMed] [Google Scholar]
- 24. Fowler AM, Solodin NM, Valley CC, Alarid ET. 2006. Altered target gene regulation controlled by estrogen receptor-α concentration. Mol Endocrinol 20:291–301 [DOI] [PubMed] [Google Scholar]
- 25. John S, Sabo PJ, Thurman RE, Sung MH, Biddie SC, Johnson TA, Hager GL, Stamatoyannopoulos JA. 2011. Chromatin accessibility pre-determines glucocorticoid receptor binding patterns. Nat Genet 43:264–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Freeman BC, Yamamoto KR. 2002. Disassembly of transcriptional regulatory complexes by molecular chaperones. Science 296:2232–2235 [DOI] [PubMed] [Google Scholar]
- 27. Stavreva DA, Müller WG, Hager GL, Smith CL, McNally JG. 2004. Rapid glucocorticoid receptor exchange at a promoter is coupled to transcription and regulated by chaperones and proteasomes. Mol Cell Biol 24:2682–2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wysocka J, Reilly PT, Herr W. 2001. Loss of HCF-1-chromatin association precedes temperature-induced growth arrest of tsBN67 cells. Mol Cell Biol 21:3820–3829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reddy TE, Pauli F, Sprouse RO, Neff NF, Newberry KM, Garabedian MJ, Myers RM. 2009. Genomic determination of the glucocorticoid response reveals unexpected mechanisms of gene regulation. Genome Res 19:2163–2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu X, Brutlag DL, Liu JS. 2001. BioProspector: discovering conserved DNA motifs in upstream regulatory regions of co-expressed genes. Pac Symp Biocomput 127–138 [PubMed] [Google Scholar]
- 31. Pruitt KD, Tatusova T, Maglott DR. 2007. NCBI reference sequences (RefSeq): a curated non-redundant sequence database of genomes, transcripts and proteins. Nucleic Acids Res 35:D61–D65 [DOI] [PMC free article] [PubMed] [Google Scholar]