Abstract
Enhancing bone morphogenetic protein (BMP) signaling increases bone formation in a variety of settings that target bone repair. However, the role of BMP in the maintenance of adult bone mass is not well understood. Targeted disruption of BMP3 in mice results in increased trabecular bone formation, whereas transgenic overexpression of BMP3 in skeletal cells leads to spontaneous fracture, consistent with BMP3 having a negative role in bone mass regulation. Here we investigate the importance of BMP3 as a mediator of BMP signaling in the adult skeleton. We find that osteoblasts (OBL) and osteocytes are the source of BMP3 in adult bone. Using in vitro cultures of primary bone marrow stromal cells, we show that overexpression of BMP3 suppresses OBL differentiation, whereas loss of BMP3 increases colony-forming unit fibroblasts and colony-forming unit OBL. The ability of BMP3 to affect OBL differentiation is due to its interaction with activin receptor type 2b (Acvr2b) because knockdown of endogenous Acvr2b in bone marrow stromal cells reduces the suppressive effect of BMP3 on OBL differentiation. These findings best fit a model in which BMP3, produced by mature bone cells, acts to reduce BMP signaling through Acvr2b in skeletal progenitor cells, limiting their differentiation to mature OBL. Our data further support the idea that endogenous BMPs have a physiological role in regulating adult bone mass.
Bone morphogenetic proteins (BMP) were identified as signals that induce ectopic bone formation through effects on skeletal progenitor cells present in adult animals (1). In rodents, cellular targets for BMP include periosteal cells, muscle satellite cells, vessel pericytes, and bone marrow stromal cells (BMSC) (2–6). BMP signaling is initiated when dimeric ligands associate with type I and type II receptors that are transmembrane serine/threonine kinases, forming a multimeric receptor ligand complex. Within this complex, the constitutively activated type II receptors serve a regulatory function by phosphorylating type I receptors. The importance of type II receptor control of BMP signaling is reinforced by the recent finding that fibrodysplasia ossificans progressiva, a genetic disorder of uncontrolled endochondral ossification in soft tissue, is caused by a mutation that renders Alk2, a type I BMP receptor, constitutively active and removed from regulation by type II receptor (7, 8). Once activated, type I receptors propagate BMP signals by phosphorylating BMP-specific R-Smad1, -5, and -8 (canonical BMP signaling), and also by phosphorylating MAPK such as Erk, Jnk, and p38 (noncanonical BMP signaling) (9). Phosphorylated R-Smad form heteromeric complexes with Smad4 and directly activate the transcription of BMP-responsive genes (10, 11).
Although BMP have been successfully developed as clinical tools for enhancing bone formation during tissue regeneration, the potential of endogenous BMP signaling to regulate bone mass is less well understood (12). Recent studies employing administration of neutralizing antibodies or soluble receptor decoys found reducing the availability of TGF-β or activin leads to significant gains in bone formation, identifying these factors as negative regulators of adult bone mass (13–16). In contrast, enhanced BMP signaling appears to play a positive role, because reducing the levels of the BMP antagonists noggin or gremlin enhances bone formation in the adult skeleton (17, 18). BMP3, a receptor antagonist specific for the type II receptor activin receptor type 2b (Acvr2b), appears to serve a unique role in the adult skeleton. Adult mice lacking BMP3 have increased bone mass, whereas mice with increased BMP3 levels in bone show delayed endochondral ossification with spontaneous rib fractures (19, 20). However, the cellular targets of BMP3 and the molecular mechanisms used by BMP3 to affect bone formation remain to be completely elucidated. In the present study, we use a LacZ knock-in allele to the BMP3 locus (BMP3 LacZ knock-in) to identify osteoblasts (OBL) and osteocytes (OCY) as the bone cells responsible for BMP3 production in the adult skeleton. We show that BMP3 targets osteoprogenitor cells where it inhibits BMP signaling. We define the interaction of BMP3/Acvr2b as a key event in BMP-mediated OBL differentiation by demonstrating that knockdown of endogenous Acvr2b diminishes the suppressive effect of BMP3 on osteoprogenitor cells. In addition, we find that loss of BMP3 increases colony-forming unit fibroblasts (CFU-F) and colony-forming unit OBL (CFU-OB) in BMSC cultures. Taken together, these data confirm that BMP3, secreted by OBL and OCY, regulates trabecular bone formation by regulating the differentiation of BSMC, thus identifying BMP3 as a novel target for bone mass augmentation.
Results
BMP3 is expressed by OBL and OCY
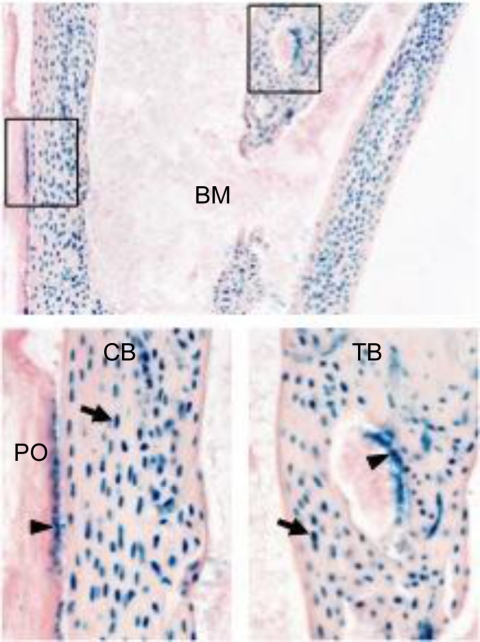
Using Velocigene technology (21), a BMP3-null mouse was generated where the first exon (encoding the N-terminal part of the precursor region) was replaced in frame with the lacZ reporter gene (BMP3 LacZ knock-in). To examine skeletal expression of BMP3, we analyzed heterozygous mice at 4 wk of age and found that OBL and OCY are the source of BMP3 in adult bone (Fig. 1). These data are in agreement with profiling data from Paic et al. (21) who reported that BMP3 as well as Acvr2b transcripts were present in OBL and OCY. We did not find BMP3 expression in cartilage, osteoclasts, or skeletal progenitor cells including those of the bone marrow stroma or the outer layers of the periosteum, consistent with the idea that BMP3 is a product of mature OBL lineage cells (Fig. 1 and data not shown).
Fig. 1.
OBL and OCY express BMP3. Tibia from BMP3 4-wk-old LacZ knock-in mice stained for β-galactosidase. Diaphyseal region is shown in the top panel. The boxed areas in the upper panel are shown as magnified images (×20) of cortical bone (CB) and trabecular bone (TB) in lower panels. Arrowheads and arrows indicate OBL and OCY, respectively. BM, Bone marrow; PO, periosteum.
BMP3 inhibits Smad signaling and suppresses OBL differentiation
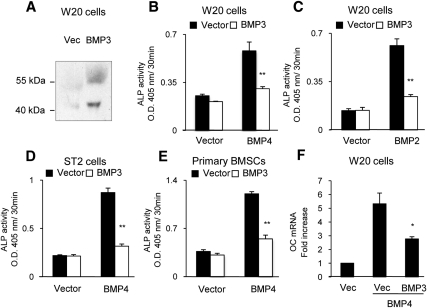
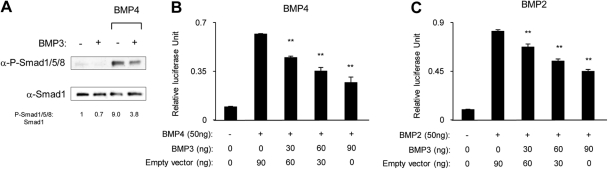
We next wanted to determine the effects of BMP3 on skeletal progenitor cell differentiation in vitro. As a source for BMP3 protein, we transfected W20-17 cells with BMP3 expression vectors. W20-17 cells are an osteoprogenitor cell line that requires BMP signaling for OB differentiation (2). Conditioned medium from W20-17 cells transfected with control or BMP3 containing pcDEF3 expression vectors were analyzed by Western blot, and BMP3 expression was verified (Fig. 2A). We then examined the effects of overexpression of BMP3 in skeletal progenitor cells and found that it suppressed the osteogenic activity of BMP4 and BMP2 in all cells examined including W20-17 (Fig. 2, B and C); ST2, a mouse bone marrow stromal cell line (Fig. 2D); and primary BSMC (Fig. 2E). Furthermore, BMP3 also suppressed expression of osteocalcin, a marker of mature OBL in cells treated with osteogenic BMP (Fig. 2F). To determine whether the inhibitory effects of BMP3 target canonical BMP signaling, we analyzed phosphorylation of endogenous Smad1/5/8 by Western blotting and activation of the IdWTF4 BMP-responsive reporter in W20-17 cells. Although transfection of cells with BMP4 or BMP2 resulted in increased p-Smad1/5/8 levels and robustly activated IdWTF4-luc, coexpression of BMP3 reduced BMP4-induced phosphorylation of Smad1/5/8 (Fig. 3A) and also suppressed IdWT4F-luc reporter activity induced by coexpression of BMP4 (Fig. 3B) or BMP2 (Fig. 3C) in a dose-dependent manner. BMP3 appeared to directly suppress BMP signaling and not act by changing levels of BMP antagonists, because transcript levels for chordin, noggin, and gremlin were not altered in mouse BSMC treated with BMP3-conditioned media (data not shown).
Fig. 2.
BMP3 suppresses OBL differentiation induced by BMP in osteoprogenitor cells. A, Western blot of conditioned medium from W20-17 cells transfected with empty vector or BMP3 verifies BMP3 expression. B and C, W20-17 cells were transfected with empty vector or BMP3, and then ALP activity was stimulated by cotransfection with BMP2 or BMP4. BMP3 significantly reduced the BMP-induced ALP activity. D and E, ST2 cells and primary BSMC that had been transfected with BMP4 to stimulate ALP activity were treated with control (vector) or BMP3-conditioned medium from W20-17 cells. BMP3-conditioned medium reduced ALP activity in both cell types. F, W20-17 cells cotransfected with BMP3 and BMP4 expressed reduced levels of osteocalcin (OC) on d 3, as determined by quantitative real time PCR analysis. Data are shown as the mean ± sd (n = 3). *, P < 0.05; **, P < 0.01 in comparison with cells transfected or treated with an empty vector (Vec) or conditioned medium from empty vector-transfected cells in each group.
Fig. 3.
BMP3 suppresses BMP-Smad signaling. A, W20-17 cells were cotransfected with empty vector or BMP3 along with BMP4. At 24 h after transfection, protein levels were determined by Western blot. The protein levels were quantified by using Image J software. Smad1 was used for protein normalization. BMP3 reduced the amount of phospho- (P-)Smad1/5/8 induced by BMP4. B and C, W20-17 cells were cotransfected with 0, 30, 60, or 90 ng BMP3 expression vector or 0, 30, 60, or 90 ng empty vector along with 45 ng IdWT4F-luc reporter and 15 ng phRL-SV40 internal control vector. Luciferase activity was then stimulated by transfection of 50 ng BMP4 expression vector (B) or 50 ng BMP2 expression vector (C). BMP3 was able to reduce the luciferase activity induced by both BMP4 and BMP2. Data are shown as the mean ± sd (n = 3). **, P < 0.01 in comparison with cells transfected with an empty vector and BMP4 or BMP2 in each group.
Suppressive effects of BMP3 on OBL differentiation require Acvr2b
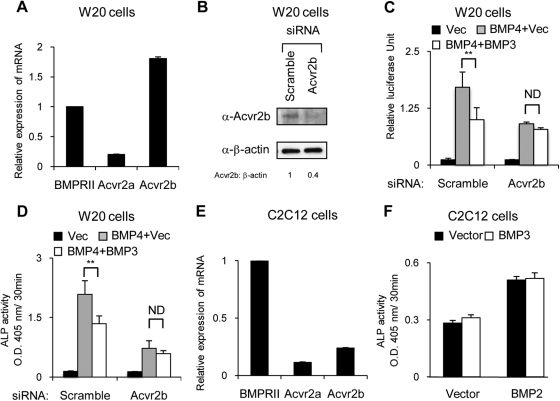
We previously reported that BMP3 interferes with BMP signaling by binding to the multifunctional type II BMP/activin receptor Acvr2b (22). Allendorph et al. (23) confirmed these data by showing that BMP3 receptor specificity for Acvr2b is controlled through the interaction of lysine 30 of BMP3 and glutamic acid 76 of Acvr2b. To investigate whether BMP3 suppresses OB differentiation via its specific interaction with Acvr2b, we examined the effects of BMP3 on cells in which endogenous Acvr2b had been knocked down using small interfering RNA (siRNA). As a first step, we confirmed that W20-17 cells express Acvr2b and found that Acvr2b is the most abundant type II BMP receptor in these cells (Fig. 4A). We next confirmed that transfection of W20-17 cells with siRNA specific for Acvr2b significantly reduced protein levels of endogenous Acvr2b in Western blots (60% knockdown) when compared with control/scrambled RNA oligonucleotides (Fig. 4B). We then tested the ability of BMP3 to reduce BMP4-mediated IdWT4F-luc and alkaline phosphatase (ALP) activation in the Acvr2b knockdown cells. We found that BMP3 could no longer block BMP4 activation of the BMP luciferase reporter (Fig. 4C) or induction of ALP activity (Fig. 4D), consistent with the finding that BMP3 binds and acts specifically through Acvr2b (24, 25). To confirm our RNA interference (RNAi) studies, we used C2C12 cells, which have been reported to lack expression of Acvr2b and should prevent them from being targeted by BMP3 (23–25). Although we detected low levels of Acvr2b by quantitative PCR in our C2C12 cells (Fig. 4E), we found that BMP3 was unable to block ALP activity induced by BMP treatment (Fig. 4F). Taken together, these findings confirm that the suppressive effect of BMP3 on OBL differentiation requires Acvr2b.
Fig. 4.
BMP3 suppresses BMP signaling via Acvr2b. A, Transcript levels of BMPR-II, Acvr2a, and Acvr2b were determined in W20-17 cells using quantitative real-time PCR. B–D, W20-17 cells were transfected with siRNA specific for Acvr2b or a scrambled siRNA. B, Western blot analysis on d 1 shows the level of endogenous Acvr2b was reduced 60% using siRNA for Acvr2b. The protein levels were quantified by using Image J software. β-Actin was used for protein normalization. The siRNA specific for Acvr2b reduced the suppressive effect of BMP3 on IdWT4F-luc activity (C) and ALP activity (D) induced by BMP4 in W20-17 cells. E, Transcript levels of BMPR-II, Acvr2a, and Acvr2b were determined in C2C12 cells by quantitative real-time PCR. F, Cotransfection of BMP3 did not suppress ALP activity induced by cotransfection of BMP2 in C2C12 cells. Data are shown as the mean ± sd (n = 3). *, P < 0.05; **; P < 0.01.
Absence of BMP3 increases CFU-F and CFU-OB from BMSC
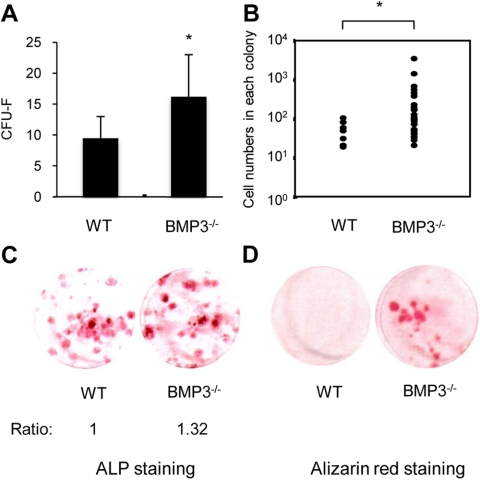
If endogenous BMP3 functions to regulate OBL differentiation from BMSC, then mice lacking BMP3 should have an enhanced basal level of OBL differentiation. To investigate this idea, we harvested BMSC from BMP3-null mice and wild-type littermates and tested these cells in standard CFU-F and CFU-OB assays. BSMC that reside in a BMP3-null environment show an increase in CFU-F colony number and colony size (Fig. 5, A and B). In addition, the number of ALP-positive colonies produced by BMSC from mice lacking BMP3 and their spontaneous differentiation into OBL-lineage cells was significantly increased in the absence of exogenous osteogenic signals when compared with wild-type controls (Fig. 5, C and D). These data confirm that loss of BMP3 results in increased differentiation of early OBL precursors into mature OBL and further suggest a negative role for BMP3 in adult bone.
Fig. 5.
Absence of BMP3 increases CFU-F and CFU-OB in BMSC cultures. BMSC were plated in six-well plates at two different densities (1.0 × 106 or 1.5 × 106 cells per well). CFU-F (A) and cell number per colony (B) were evaluated after 10 d culture using methylene green staining. BSMC from BMP3−/− mice had increased CFU-F colony number and colony size when compared with wild-type (WT) controls. The appearance of CFU-OB was assessed by ALP (C) and alizarin red staining (D) after 10 or 28 d, respectively. The density of ALP staining was quantified by using Image J software. The number of ALP- and alizarin red-positive colonies was increased in BSMC from BMP3−/− mice when compared with wild-type control mice. *, P < 0.05.
Discussion
In the present study, we examined the cellular targets and molecular mechanisms that underlie the inhibition of BMP-induced OBL differentiation by BMP3, leading to the high-bone-mass phenotype in the BMP3 knockout mice (19, 22, 23). We demonstrate that this inhibition is due to the interaction of BMP3 with the type II receptor, Acvr2b, present on skeletal progenitor cells. BMP3/Acvr2b binding occurs upstream of type I receptor function, an idea confirmed by the observation that overexpression of BMP3 could not suppress IdWT4F-luc activity induced by a constitutively active Smad1 (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org) and in agreement with results obtained previously in Xenopus embryo studies (22). These data suggest an important regulatory role for type II BMP receptors in the process of bone formation in the adult skeleton, an idea supported by the pathological endochondral ossification that occurs when type II receptor control is bypassed in fibrodysplasia ossificans progressiva (7, 8).
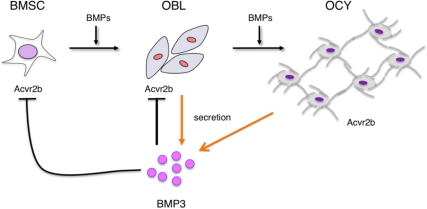
Our results show that in adult bone, BMP3 is produced by OBL and OCY. Removing BMP3 from the bone environment increased CFU-F colony number and colony size as well as the spontaneous differentiation of osteoprogenitors into bone-forming cells. These data suggest a model of BMP3 activity in the skeleton whereby BMP3 produced by mature bone cells acts to limit the differentiation of Acvr2b-positive osteoprogenitors residing in the bone marrow microenvironment (Fig. 6). Osteoprogenitors not only express Acvr2b but also have other type II receptors (Acvr2a, BMPR-II, and transforming growth factor beta type II receptor) that are used by BMP/TGF-β/activin ligands to regulate signal transduction in bone. This fact is highlighted by recent data showing that systemic administration of a TGF-β neutralizing antibody to adult mice increased bone mass and bone density, suggesting that TGF-β signaling via transforming growth factor beta type II receptor on BMSC limits osteogenesis (13, 14, 26). In addition, systemic administration of AR2AFc (soluble activin type II A receptor) and AR2BFc (soluble activin type II B receptor) type II activin receptor decoys also increases bone mass in vivo, and administration of AR2AFc to BMP3-null mice further increases bone mineral density (15, 16, 27). Taken as a whole, these data point to the potential therapeutic benefits of increasing bone formation through modulating the interactions between type II receptors and BMP/TGF-β/activins and suggest that BMP3 and its specific interaction with Acvr2b may be a novel means to enhance osteogenesis.
Fig. 6.
Model for BMP3 control of BMSC differentiation in the bone marrow microenvironment. BMP3 produced by OBL and OCY interacts with Acvr2b present on BMSC to suppress OBL differentiation and maturation induced by BMP. In addition, because OBL and OCY express Acvr2b, BMP3 may also effect BMP signaling and gene expression in these cells as a means of regulating adult bone mass.
Materials and Methods
Animals
BMP3−/− mice were previously generated and described elsewhere (19). BMP3 LacZ knock-in mice were generated at Regeneron Pharmaceuticals using Velocigene technology (28). The region of the BMP3 gene containing exon 1 was deleted and replaced with a transmembrane-lacZ/neo cassette using bacterial homologous recombination. All studies performed were approved by the Institutional Animal Care and Use Committee at Harvard Medical School (Boston, MA).
X-gal staining
Tibias were collected from 4-wk-old BMP3 LacZ knock-in mice and littermate controls. Tissues were fixed for 24 h at 4 C in 4% paraformaldehyde/PBS (pH 8), decalcified at 4 C in 0.5 m EDTA (pH 7.4) for 10–14 d, equilibrated in 15% sucrose for 1 h, 30% sucrose overnight at 4 C, and cryo-embedded. For X-gal staining, 20-μm sections were postfixed in 4% paraformaldehyde, washed three times for 15 min in 2 mm MgCl2, 0.01% sodium deoxycholate, 0.02% Nonidet P-40 in PBS, stained overnight in 0.1% X-gal, 5 mm potassium ferrocyanide, 5 mm potassium ferricyanide in PBS (pH 8) overnight at 37 C, and counterstained with eosin/floxin (29).
Plasmids
Plasmids encoding wild-type human BMP3 (accession number NM_001201.2) were obtained by a standard RT-PCR technique using PrimeSTAR HS DNA polymerase (TaKaRa, Ohtsu, Japan) and cloned into a pcDEF3 expression vector (30). The human BMP2, BMP4, mouse constitutively active Smad1, and IdWT4F-luc reporter plasmids have been previously described (10, 31).
Cell culture and transfection
W20-17 cells were cultured and maintained as described previously (2). ST2 cells were purchased from the RIKEN Cell Bank (Tsukuba, Japan). ST2 cells were cultured and maintained in α-MEM supplemented with 10% fetal bovine serum. Primary BSMC were collected from femurs and tibias of 6-wk-old wild-type C57BL/6J mice or BMP3−/− mice. After epiphyses were removed, bone was flushed with 15 ml α-MEM using a 20-ml syringe fitted with a 23-gauge needle. Flushed bone marrow cells were cultured with α-MEM containing 15% fetal bovine serum for 4 d (32). Cells were transfected with plasmids using Lipofectamine 2000 (Invitrogen) (33). Conditioned media were collected from W20-17 cells 3 d after transfection with empty vector, BMP3, or BMP4 for each assay.
ALP and luciferase assays
ALP activity was measured as a marker of OBL differentiation on d 2. Cells treated with an acetone/ethanol (50:50) mixture were incubated with a substrate solution composed of 0.1 m diethanolamine, 1 mm MgCl2, and 1 mg/ml p-nitrophenylphosphate. The reaction was terminated by adding 3 m NaOH, and absorbance values were measured at 405 nm (34, 35). Luciferase assays were performed using IdWT4F-luc, phRL-SV40 (Promega, Madison, WI) with the Dual-Glo Luciferase Assay System (Promega) (36).
CFU-F and CFU-OB assay
Primary BSMC were plated in triplicate cultures (six-well plates) at two different densities (1.0 × 106 or 1.5 × 106 cells per well). The formation of CFU-F and CFU-OB was evaluated after 10 and 28 d of culture. At the end of each experiment, cultures were washed twice with PBS and fixed with cold ethanol, and CUF-F were stained with methylene green, and colonies with more than 50 cells were counted. For CFU-OB assays, formation of OBL progenitors and OBL was detected using ALP and alizarin red staining (37). For ALP staining, cells were incubated for 20 min with a mixture of 0.1 mg/ml naphthol AS-MX phosphate (Sigma-Aldrich Chemicals, St. Louis, MO), 0.5% N,N-dimethylformamde, 2 m MgCl2, and 0.6 mg/ml fast red TR salt (Sigma-Aldrich) in 0.1 m Tris-HCl (pH 8.5) at room temperature. The density of ALP staining was quantified by using Image J (National Institutes of Health, Bethesda, MD) software. For alizarin red staining, the cells were immersed in a 40 mm alizarin (Sigma-Aldrich) solution (pH 4.2) for 20 min at room temperature with gentle agitation. The solution then was removed, and the mineralized matrices were washed with water.
Trichloroacetic acid (TCA) precipitation
To concentrate the BMP3 protein, TCA precipitation was performed on medium collected from W20-17 cells that were transfected with BMP3 expression vectors. A 25-ml volume of medium was collected, and 10 times the sample volume of 10% TCA was added and incubated on ice for 1.5 h. This was followed by centrifugation at 4000 × g for 15 min at 4 C. The TCA was aspirated, 400 μl acetone was added, and the sample was vortexed vigorously and then centrifuged at 12000 × g for 10 min at 4 C. The acetone was aspirated, and the sample was dried at room temperature for 5 min. Finally, lysis buffer was added, and the sample incubated at 37 C for 5 min before Western blot analysis (38).
Western blot analysis
The following antibodies were used for Western blot analysis: anti-BMP3 polyclonal antibody, anti-Acvr2b polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA), anti-phosphorylated Smad1/5/8 polyclonal antibody, anti-Smad1 polyclonal antibody (Cell Signaling, Beverly, MA), and anti-β-actin mouse monoclonal antibody (Sigma-Aldrich). The target proteins were detected using a horseradish peroxidase-conjugated antimouse, antirabbit IgG antibody (Cell Signaling), or anti-goat IgG antibody (Santa Cruz). Protein levels were quantified using Image J software.
Reverse transcription and quantitative PCR analysis
Total RNA was isolated from W20-17 cells using Trizol (Invitrogen, Carlsbad, CA) and then reverse transcribed into cDNA using Superscript III (Invitrogen). The cDNA was amplified by PCR using specific primers for murine osteocalcin (primer sequences: forward, agactccggcgctacctt; reverse, ctcgtcacaagcagggttaag), BMPR-II (primer sequences: forward, gagccctcccttgacctg; reverse, gtatcgaccccgtccaatc), Acvr2a (primer sequences: forward, ccctcctgtacttgttcctactca; reverse, gcaatggcttcaaccctagt), Acvr2b (primer sequences: forward, tggctgttcggtttgagc; reverse, ggccatgtaccgtctggt), and β-actin. Quantitative real-time PCR was performed using the Roche (Indianapolis, IN) Light Cycler 480 Real-time PCR system with probe-based detection (Universal Probe Library; Roche). Values were normalized to β-actin using the 2-ΔΔCt method (39).
RNAi transfection
RNAi Stealth oligonucleotides were designed against murine Acvr2b (no. MSS201683; Invitrogen), and a scrambled RNAi was used as a negative control (Invitrogen). Cells were transfected with siRNA using N-TER Nanoparticle siRNA Transfection System according to the manufacturer's instructions (Sigma-Aldrich).
Statistical analysis
Comparisons were made using an unpaired Student's t test; the results are shown as means ± sd. Statistical significance is indicated as P < 0.05 or P < 0.01.
Acknowledgments
This work was supported by Grant AR50174 from National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), National Institutes of Health (NIH) (to V.R.).
Disclosure Summary: S.K., L.G., K.C., J.L., K.T., T.K., and V.R. have nothing to declare. R.R. was previously employed by Regeneron Pharmaceuticals and had equity interests in Regeneron Pharmaceuticals. A.E. is employed by Regeneron Pharmaceuticals and has equity interests in Regeneron Pharmaceuticals.
Footnotes
- Acvr2b
- Activin receptor type 2b
- ALP
- alkaline phosphatase
- BMP
- bone morphogenetic protein
- BMSC
- bone marrow stem cell
- CFU-F
- colony-forming unit fibroblast
- CFU-OB
- colony-forming unit osteoblast
- OBL
- osteoblast
- OCY
- osteocyte
- RNAi
- RNA interference
- siRNA
- small interfering RNA
- TCA
- trichloroacetic acid.
References
- 1. Urist MR. 1965. Bone: formation by autoinduction. Science 150:893–899 [DOI] [PubMed] [Google Scholar]
- 2. Thies RS, Bauduy M, Ashton BA, Kurtzberg L, Wozney JM, Rosen V. 1992. Recombinant human bone morphogenetic protein-2 induces osteoblastic differentiation in W-20-17 stromal cells. Endocrinology 130:1318–1324 [DOI] [PubMed] [Google Scholar]
- 3. Yamaguchi A, Ishizuya T, Kintou N, Wada Y, Katagiri T, Wozney JM, Rosen V, Yoshiki S. 1996. Effects of BMP-2, BMP-4, and BMP-6 on osteoblastic differentiation of bone marrow-derived stromal cell lines, ST2 and MC3T3-G2/PA6. Biochem Biophys Res Commun 220:366–371 [DOI] [PubMed] [Google Scholar]
- 4. Tsuji K, Bandyopadhyay A, Harfe BD, Cox K, Kakar S, Gerstenfeld L, Einhorn T, Tabin CJ, Rosen V. 2006. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet 38:1424–1429 [DOI] [PubMed] [Google Scholar]
- 5. Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, Rosen V, Wozney JM, Fujisawa-Sehara A, Suda T. 1994. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol 127:1755–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bianco P, Robey PG, Saggio I, Riminucci M. 2010. “Mesenchymal” stem cells in human bone marrow (skeletal stem cells): a critical discussion of their nature, identity, and significance in incurable skeletal disease. Hum Gene Ther 21:1057–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho TJ, Choi IH, Connor JM, Delai P, Glaser DL, LeMerrer M, Morhart R, Rogers JG, Smith R, Triffitt JT, Urtizberea JA, Zasloff M, Brown MA, Kaplan FS. 2006. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet 38:525–527 [DOI] [PubMed] [Google Scholar]
- 8. Fukuda T, Kohda M, Kanomata K, Nojima J, Nakamura A, Kamizono J, Noguchi Y, Iwakiri K, Kondo T, Kurose J, Endo K, Awakura T, Fukushi J, Nakashima Y, Chiyonobu T, Kawara A, Nishida Y, Wada I, Akita M, Komori T, Nakayama K, Nanba A, Maruki Y, Yoda T, Tomoda H, et al. 2009. Constitutively activated ALK2 and increased SMAD1/5 cooperatively induce bone morphogenetic protein signaling in fibrodysplasia ossificans progressiva. J Biol Chem 284:7149–7156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Katagiri T, Suda T, Miyazono K. 2008. The bone morphogenetic proteins. New York: Cold Spring Harbor Press [Google Scholar]
- 10. Katagiri T, Imada M, Yanai T, Suda T, Takahashi N, Kamijo R. 2002. Identification of a BMP-responsive element in Id1, the gene for inhibition of myogenesis. Genes Cells 7:949–960 [DOI] [PubMed] [Google Scholar]
- 11. Hollnagel A, Oehlmann V, Heymer J, Rüther U, Nordheim A. 1999. Id genes are direct targets of bone morphogenetic protein induction in embryonic stem cells. J Biol Chem 274:19838–19845 [DOI] [PubMed] [Google Scholar]
- 12. Alam N, St-Arnaud R, Lauzier D, Rosen V, Hamdy RC. 2009. Are endogenous BMP necessary for bone healing during distraction osteogenesis? Clin Orthop Relat Res 467:3190–3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Qiu T, Wu X, Zhang F, Clemens TL, Wan M, Cao X. 2010. TGF-β type II receptor phosphorylates PTH receptor to integrate bone remodelling signalling. Nat Cell Biol 12:224–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Edwards JR, Nyman JS, Lwin ST, Moore MM, Esparza J, O'Quinn EC, Hart AJ, Biswas S, Patil CA, Lonning S, Mahadevan-Jansen A, Mundy GR. 2010. Inhibition of TGF-β signaling by 1D11 antibody treatment increases bone mass and quality in vivo. J Bone Miner Res 25:2419–2426 [DOI] [PubMed] [Google Scholar]
- 15. Pearsall RS, Canalis E, Cornwall-Brady M, Underwood KW, Haigis B, Ucran J, Kumar R, Pobre E, Grinberg A, Werner ED, Glatt V, Stadmeyer L, Smith D, Seehra J, Bouxsein ML. 2008. A soluble activin type IIA receptor induces bone formation and improves skeletal integrity. Proc Natl Acad Sci USA 105:7082–7087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Perrien DS, Akel NS, Edwards PK, Carver AA, Bendre MS, Swain FL, Skinner RA, Hogue WR, Nicks KM, Pierson TM, Suva LJ, Gaddy D. 2007. Inhibin A is an endocrine stimulator of bone mass and strength. Endocrinology 148:1654–1665 [DOI] [PubMed] [Google Scholar]
- 17. Dickman S. 1998. Growing joints use their noggins. Science 280:1350. [DOI] [PubMed] [Google Scholar]
- 18. Khokha MK, Hsu D, Brunet LJ, Dionne MS, Harland RM. 2003. Gremlin is the BMP antagonist required for maintenance of Shh and Fgf signals during limb patterning. Nat Genet 34:303–307 [DOI] [PubMed] [Google Scholar]
- 19. Daluiski A, Engstrand T, Bahamonde ME, Gamer LW, Agius E, Stevenson SL, Cox K, Rosen V, Lyons KM. 2001. Bone morphogenetic protein-3 is a negative regulator of bone density. Nat Genet 27:84–88 [DOI] [PubMed] [Google Scholar]
- 20. Gamer LW, Cox K, Carlo JM, Rosen V. 2009. Overexpression of BMP3 in the developing skeleton alters endochondral bone formation resulting in spontaneous rib fractures. Dev Dyn 238:2374–2381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Paic F, Igwe JC, Nori R, Kronenberg MS, Franceschetti T, Harrington P, Kuo L, Shin DG, Rowe DW, Harris SE, Kalajzic I. 2009. Identification of differentially expressed genes between osteoblasts and osteocytes. Bone 45:682–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gamer LW, Nove J, Levin M, Rosen V. 2005. BMP-3 is a novel inhibitor of both activin and BMP-4 signaling in Xenopus embryos. Dev Biol 285:156–168 [DOI] [PubMed] [Google Scholar]
- 23. Allendorph GP, Isaacs MJ, Kawakami Y, Izpisua Belmonte JC, Choe S. 2007. BMP-3 and BMP-6 structures illuminate the nature of binding specificity with receptors. Biochemistry 46:12238–12247 [DOI] [PubMed] [Google Scholar]
- 24. Yeh LC, Tsai AD, Zavala MC, Lee JC. 2004. Cartilage-derived morphogenetic proteins enhance the osteogenic protein-1-induced osteoblastic cell differentiation of C2C12 cells. J Cell Physiol 201:401–408 [DOI] [PubMed] [Google Scholar]
- 25. de Jong DS, van Zoelen EJ, Bauerschmidt S, Olijve W, Steegenga WT. 2002. Microarray analysis of bone morphogenetic protein, transforming growth factor β, and activin early response genes during osteoblastic cell differentiation. J Bone Miner Res 17:2119–2129 [DOI] [PubMed] [Google Scholar]
- 26. Gamer LW, Tsuji K, Cox K, Capelo LP, Lowery J, Beppu H, Rosen V. 2011. BMPR-II is dispensable for formation of the limb skeleton. Genesis 49:719–724 [DOI] [PubMed] [Google Scholar]
- 27. Koncarevic A, Cornwall-Brady M, Pullen A, Davies M, Sako D, Liu J, Kumar R, Tomkinson K, Baker T, Umiker B, Monnell T, Grinberg AV, Liharska K, Underwood KW, Ucran JA, Howard E, Barberio J, Spaits M, Pearsall S, Seehra J, Lachey J. 2010. A soluble activin receptor type IIb prevents the effects of androgen deprivation on body composition and bone health. Endocrinology 151:4289–4300 [DOI] [PubMed] [Google Scholar]
- 28. Valenzuela DM, Murphy AJ, Frendewey D, Gale NW, Economides AN, Auerbach W, Poueymirou WT, Adams NC, Rojas J, Yasenchak J, Chernomorsky R, Boucher M, Elsasser AL, Esau L, Zheng J, Griffiths JA, Wang X, Su H, Xue Y, Dominguez MG, Noguera I, Torres R, Macdonald LE, Stewart AF, DeChiara TM, Yancopoulos GD. 2003. High-throughput engineering of the mouse genome coupled with high-resolution expression analysis. Nat Biotechnol 21:652–659 [DOI] [PubMed] [Google Scholar]
- 29. Colnot C, Huang S, Helms J. 2006. Analyzing the cellular contribution of bone marrow to fracture healing using bone marrow transplantation in mice. Biochem Biophys Res Commun 350:557–561 [DOI] [PubMed] [Google Scholar]
- 30. Goldman LA, Cutrone EC, Kotenko SV, Krause CD, Langer JA. 1996. Modifications of vectors pEF-BOS, pcDNA1 and pcDNA3 result in improved convenience and expression. Biotechniques 21:1013–1015 [DOI] [PubMed] [Google Scholar]
- 31. Nojima J, Kanomata K, Takada Y, Fukuda T, Kokabu S, Ohte S, Takada T, Tsukui T, Yamamoto TS, Sasanuma H, Yoneyama K, Ueno N, Okazaki Y, Kamijo R, Yoda T, Katagiri T. 2010. Dual roles of smad proteins in the conversion from myoblasts to osteoblastic cells by bone morphogenetic proteins. J Biol Chem 285:15577–15586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guo J, Liu M, Yang D, Bouxsein ML, Saito H, Galvin RJ, Kuhstoss SA, Thomas CC, Schipani E, Baron R, Bringhurst FR, Kronenberg HM. 2010. Suppression of Wnt signaling by Dkk1 attenuates PTH-mediated stromal cell response and new bone formation. Cell Metab 11:161–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kokabu S, Nojima J, Kanomata K, Ohte S, Yoda T, Fukuda T, Katagiri T. 2010. Protein phosphatase magnesium-dependent 1A-mediated inhibition of BMP signaling is independent of Smad dephosphorylation. J Bone Miner Res 25:653–660 [DOI] [PubMed] [Google Scholar]
- 34. Kanomata K, Kokabu S, Nojima J, Fukuda T, Katagiri T. 2009. DRAGON, a GPI-anchored membrane protein, inhibits BMP signaling in C2C12 myoblasts. Genes Cells 14:695–702 [DOI] [PubMed] [Google Scholar]
- 35. Fukuda T, Kokabu S, Ohte S, Sasanuma H, Kanomata K, Yoneyama K, Kato H, Akita M, Oda H, Katagiri T. 2010. Canonical Wnts and BMP cooperatively induce osteoblastic differentiation through a GSK3β-dependent and β-catenin-independent mechanism. Differentiation 80:46–52 [DOI] [PubMed] [Google Scholar]
- 36. Kokabu S, Ohte S, Sasanuma H, Shin M, Yoneyama K, Murata E, Kanomata K, Nojima J, Ono Y, Yoda T, Fukuda T, Katagiri T. 2011. Suppression of BMP-Smad signaling axis-induced osteoblastic differentiation by small C-terminal domain phosphatase 1, a Smad phosphatase. Mol Endocrinol 25:474–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gaddy-Kurten D, Coker JK, Abe E, Jilka RL, Manolagas SC. 2002. Inhibin suppresses and activin stimulates osteoblastogenesis and osteoclastogenesis in murine bone marrow cultures. Endocrinology 143:74–83 [DOI] [PubMed] [Google Scholar]
- 38. Hileman SM, Tornøe J, Flier JS, Bjørbaek C. 2000. Transcellular transport of leptin by the short leptin receptor isoform ObRa in Madin-Darby Canine Kidney cells. Endocrinology 141:1955–1961 [DOI] [PubMed] [Google Scholar]
- 39. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]