Abstract
Attention and memory are typically studied as separate topics, but they are highly intertwined. Here we discuss the relation between memory and two fundamental types of attention: perceptual and reflective. Memory is the persisting consequence of cognitive activities initiated by and/or focused on external information from the environment (perceptual attention) and initiated by and/or focused on internal mental representations (reflective attention). We consider three key questions for advancing a cognitive neuroscience of attention and memory: To what extent do perception and reflection share representational areas? To what extent are the control processes that select, maintain, and manipulate perceptual and reflective information subserved by common areas and networks? During perception and reflection, to what extent are common areas responsible for binding features together to create complex, episodic memories and for reviving them later? Considering similarities and differences in perceptual and reflective attention helps integrate a broad range of findings and raises important unresolved issues.
Different research traditions tend to emphasize either attentional phenomena (Posner et al., 2007) or memory phenomena (Eichenbaum et al., 2007; Squire and Wixted, 2011). Although this “divide and conquer” approach has been extremely useful for advancing knowledge about cognition, there is increasing recognition that fully understanding each may entail understanding the other. Here we focus on the similarities and differences across these domains and an emerging picture of how they interact. We build, in particular, on two previous theoretical frameworks: the external/internal taxonomy of attention (Chun et al., 2011) and the Multiple-Entry-Modular (MEM) model of cognition, which views memory as traces of perceptual and reflective processing (Johnson and Hirst, 1993). Drawing on these ideas, and empirical findings that they incorporate, we propose an integrative Perceptual/Reflective Attention/Memory (PRAM) framework, which serves to organize current findings and theoretical ideas regarding the relation between attention and memory, and which highlights key unresolved questions.
Cognition can be broadly divided into perceptual processes initiated by and/or directed at external sensory information from the environment, and reflective processes initiated by and/or directed at internal mental representations. Perceptual processes operate on “incoming”, external stimuli (e.g., reading text, listening to a song). Reflective processes are directed at internal representations, such as thoughts, memories, imagery, decision options, problem solving, and self-directed processes. That is, reflective processes can operate on representations in the absence of corresponding external stimuli or independent of current external input (e.g., thinking about what to have for dinner, remembering a friend’s remark).
At any given moment, not all features, objects and events in the environment or in the mind can be processed equally (Marois and Ivanoff, 2005). Both perception and reflection are inherently selective, requiring mechanisms of attention – modulating, sustaining, and manipulating the information that is most relevant for current and/or future behavior (Chun et al., 2011). The byproducts of these perceptual and reflective attentional processes are registered as changes or records in the cognitive system, changes that we call “memory”.
Although the border between perceiving and reflecting can be fuzzy, there are meaningful differences. Logically, perceiving and thinking are unlikely to engage exactly the same neural hardware or have exactly the same memorial consequences. That would produce an epistemological quagmire in which we could not tell fact from fantasy in perceiving, thinking or remembering (Johnson, 2006). On the other hand, if there were no interaction between perception and reflection, we would not be able to constructively and creatively cumulate knowledge across experiences of perceiving and thinking. To what extent do perception and reflection activate the same representational and processing regions? To what extent do they have similar and different memorial consequences? Under what conditions do they operate independently and by what mechanisms do they interact?
Our review and PRAM framework lead us to several hypotheses that invite further testing. (1) Perception and reflection engage some of the same areas (e.g., posterior sensory areas) for representing information (e.g., concrete items such as objects, faces and scenes). However, the extent to which they engage the same or different representations within these areas is an open question. The degree of overlap should predict the extent to which perception and reflection influence each other and how likely they are to be confused, for example, in source memory tasks. (2) Perception and reflection both involve frontal and parietal regions that control the direction/focus of attention. However, whether they engage the same regions for similar cognitive functions is an open question, and should dictate when perception and reflection interfere with or facilitate each other (3) It is well accepted that the hippocampus/medial temporal lobe region associates attended information with other existing representations throughout the brain (Davachi, 2006; Ranganath, 2010). The resulting configural representations bind multiple features (e.g., perceptual, spatial, temporal, semantic, emotional details) together and give representations an episodic quality (i.e., they have source or contextual information). Are there differences in configural processing active during both perception and reflection?
Perceptual attention
Sensory information (e.g., sights, sounds, smells) arrives from different locations in space and points in time. Perceptual attention selects and modulates this information according to current task goals. In the PRAM framework, such processing yields persisting records (traces or memories). Because most research has used visual stimuli, our review will focus on the visual modality.
Limited processing capacity prevents equal attention to all items, but when cues direct selective attention to specific locations, perceptual performance is enhanced for cued items (Posner et al., 1980), as is memory for these attended items (Eger et al., 2004; Uncapher et al., 2011). Perceptual attention can also select on the basis of features. In a classic study, Rock and Gutman (1981) showed participants two abstract shapes that spatially overlapped on each trial. One shape was red and one was green, and each participant was told to attend to shapes in only one of the colors. Shapes in the other color were poorly remembered later, even though they spatially overlapped with attended shapes that were remembered. Even with only brief exposures, we appear to store a great deal of detailed perceptual information about selected information (Hollingworth and Henderson, 2002; Potter, 1976). For example, in one study (Brady et al., 2008), participants saw 2,500 pictures of objects, each for 3 sec, with instructions to try to remember them. On a later forced-choice recognition test, participants selected the correct previously seen item 92% of the time; even more remarkable, performance was still 87% when participants were required to discriminate between an original picture and the same object in a different state or orientation. In priming studies using even briefer presentations, and no instructions to remember, participants show memory for quite specific representations, although participants do not consciously recognize the items (repetition priming; e.g., Tulving and Schacter, 1990; Wiggs and Martin, 1998; Henson and Rugg, 2003). For example, if participants saw an item for 1 sec, they were subsequently better able to identify it under degraded stimulus conditions, even when they did not remember having seen the item before (e.g., Jacoby and Dallas, 1981). A neural measure of such behavioral priming measures of implicit memory is provided by repetition suppression or attenuation during fMRI: Compared to the first exposure to a stimulus, repeating a stimulus results in less activity in representational areas that are active when stimuli of that class are perceived (Grill-Spector et al., 1999; Schacter and Buckner, 1998). For example, object repetition attenuates activity in the lateral occipital complex (LOC, Grill-Spector et al., 1999; Buckner et al., 1998), while face repetition and scene repetition attenuation effects are found in the fusiform face area (FFA, Jiang et al., 2006) and the parahippocampal place area (PPA, Epstein et al, 2000), respectively.
Perceptual attention enhances stimulus-specific representations, as measured with fMRI repetition attenuation. An object appearing in a cued location shows more repetition attenuation than an object appearing in an uncued location (Eger et al., 2004; Chee et al., 2007). In one study, participants were presented on each trial with a face and scene that overlapped spatially and were cued to attend either to the face or the scene. Repetition attenuation was observed in PPA when scenes were repeated on a subsequent trial only when participants were instructed to attend to the scene on both the first and second presentation (Yi and Chun, 2005). Thus attention gates the learning that occurs incidentally during perception, and the expression of learning on implicit tests.
Reflective attention
Component processes of reflection (Johnson and Hirst, 1993) are the cognitive elements of what is often referred to as controlled/executive processing or working memory (Baddeley, 1992; Smith and Jonides, 1999). Refreshing is the act of briefly thinking of, and thereby foregrounding, a percept or thought that was activated moments earlier. Rehearsing maintains information, typically several items, in a phonological loop, over longer intervals of several seconds (Baddeley, 1992). (Ranganath et al., 2005 make a similar distinction between early and late maintenance processes.) Selectively refreshing an activated representation of a perceptual stimulus that has just disappeared, a thought that just became active, or an item that is currently in an active rehearsal set, boosts the strength of that item relative to other active items, making it the focus of attention (Cowan, 2001) and giving it a competitive advantage for additional processing. Thus, refreshing and rehearsing, individually and together, constitute reflective attention that selects, maintains, and manipulates the contents of working memory.
Reviving representations that are not currently active involves the component processes of reactivating or retrieving. Once revived, these longer-term memory representations can be further extended briefly by refreshing and/or rehearsing them. Other reflective processes include noting and/or discovering relations among active representations (processes underlying, for example, elaboration and organization of information), and initiating or shifting between representations, features, tasks or goals (processes needed, for example, to control or manipulate the sequence of mental events).
Thus, reflective attention selects, maintains, and manipulates information from working memory and long-term memory, and promotes long-lasting memories (Craik and Lockhart, 1972; Roediger and Karpicke, 2006; Tulving, 1962). For example, in one study comparing refreshing to perceptual repetition, participants viewed and read aloud words as they appeared one at a time. Some words appeared and were read aloud only once, some words appeared and were read aloud twice in succession (repeated – perceptual processing), and other words were read once and followed by a cue that signaled participants to think of (refresh) the immediately preceding word and say it out loud. A surprise test at the end of the experiment revealed greater recognition memory for words that had been refreshed than words that had been read once or read twice (Johnson et al., 2002). Even greater effects on long-term memory are yielded when information is reactivated and retrieved on different occasions over time (Roediger & Karpicke, 2004). If accurate source features are revived, reflectively reviving events can protect against memory distortion (Henkel, 2004).
To What Extent do Perception and Reflection Share Representations?
Do representations that are the outcome of perceptual attention also serve as targets for reflective attention? Reflection modulates activity in many of the same representational areas as perceptual attention. For example, both refreshing and rehearsing modulate activity in posterior areas involved in perception (Curtis and D’Esposito, 2003; Harrison & Tong, 2009; Johnson et al., 2009; Ranganath et al., 2005). Johnson et al. (2009) directly compared selective perceptual and reflective attention and found similar effects on sensory representations (Figure 1). Participants were shown a scene and a face on each trial and either cued in advance to attend perceptually to the scene or face, or cued after the stimulus was removed to refresh the scene or the face. Both perception (attend) and reflection (refresh) showed comparable enhancement and suppression effects relative to a passive viewing condition.
Figure 1. Comparing reflective (refresh) and perceptual (orient) attention.
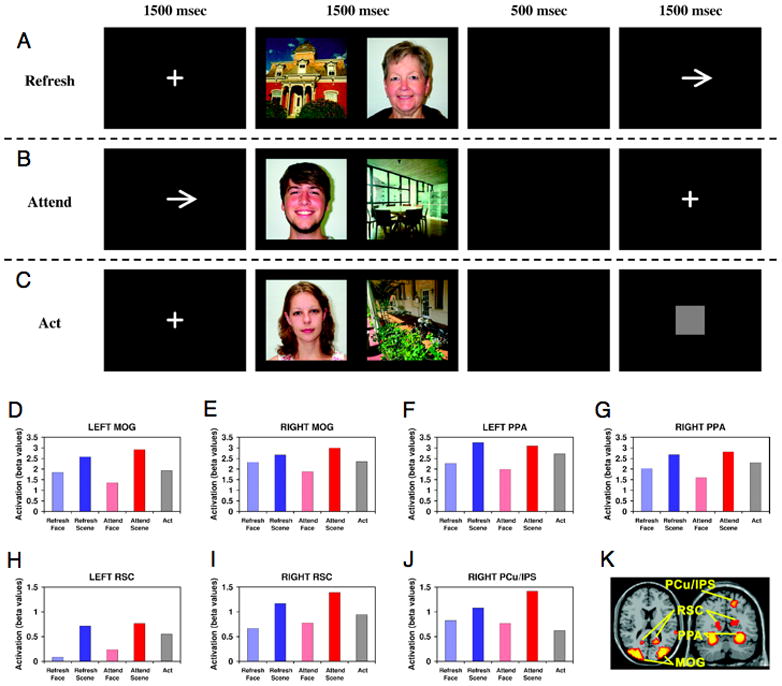
Task design. (A) Refresh condition: Participants first saw a fixation cross, then a face and a scene picture, followed by an arrow cueing them to briefly think back to, or visualize, either the face or the scene, as indicated by the direction of the arrow. (B) Orient condition: Participants first saw an arrow, cueing them to look only at the picture on the left or right side of the screen. This was followed by a face and a scene picture, and then a fixation cross. (C) Act (control) condition: Participants first saw a fixation cross, then a face and a scene picture, followed by a gray square cueing them simply to press a button (and not think about either picture). (D–J) Activation for each of seven scene-selective regions of interest across the five conditions of the task. (K) Locations of these regions overlaid on the MNI single-subject template brain (Adapted from Johnson and Johnson, 2009, Journal of Cognitive Neuroscience).
Although perceptual representations and refreshed representations in working memory may engage the same brain areas, long-term memory could reside in areas different from those of the processes that gave rise to them (Barsalou et al., 2008). However, fMRI evidence suggests that long-term memory often involves reactivation of the same areas engaged during encoding. Retrieving visual events during long-term memory tasks activates visual cortex, while retrieving auditory events from memory activates auditory cortex (Wheeler et al., 2000). Importantly, the extent to which encoding activity is reinstated during long-term remembering, depends in part on what reflective agenda is engaged during remembering (McDuff et al., 2009).
Further evidence that perception and reflection may each later re-engage the same representations comes from a study in which Turk-Browne et al. (2006) examined how repetition attenuation in scene-selective regions was related to measures of explicit subsequent memory. Each scene was repeated somewhere in the study sequence and the experimenters sorted the data according to whether or not each scene was later recognized. Repetition attenuation in PPA (and behavioral priming) on the second presentation were only significant for repeated items that were later remembered (see also Gonsalves et al., 2005; Chee et al., 2007), consistent with the idea that repetition attenuation (a perceptual effect) draws on the same level of representation (PPA) as does the phenomenal experience of remembering. Other evidence that reflection and perception can operate on the same representations comes from an fMRI study that measured repetition suppression to assess representational strength of previously viewed and previously refreshed scenes. There were similar levels of repetition suppression in PPA for items seen and refreshed once, as for items seen twice (Yi et al., 2008). The impact on long-term memory from viewing an item once and refreshing it was equivalent to having seen the item twice. This provides strong evidence that refreshing active representations of perceptual events engages the same representation (not simply the same representational area), and that the consequences last beyond a few seconds. These findings also support the idea that perception and reflection interact to influence memory through the engagement of common representations. Other evidence that perception and reflection can share common representations is that a reflective representation may serve as a template for perceptual attention (Olivers et al., 2011). Additional research is needed to clarify to what extent individual memories can be decoded from brain activity at test. Currently, decoding category-specific activity within ventral cortex during recall, using multi-voxel pattern analysis (MVPA, Polyn et al., 2005), can signal the class of an item one is likely remembering (e.g., scene, face, object). Also, the ability to discriminate more specifically what a person is remembering is starting to show promise. In a face recognition task, MVPA reliably decoded whether or not participants said they had seen faces but not whether they had actually seen them (Rissman et al., 2010). This is consistent with behavioral and fMRI evidence that true and false memories are attributions about mental experiences based on their qualitative characteristics (Johnson, 2006; Mitchell et al., 2009). Mental imagery of specific visual orientations can be decoded above chance from low-level visual cortex (Kamitani and Tong, 2005), and mental imagery of a small set of well-learned, scenes can be decoded above chance in scene-sensitive cortex (Johnson, 2011) MVPA of the hippocampus can differentiate episodic memories of three film clips of everyday actions (Chadwick et al., 2010). Furthermore, perceptual representations of more diverse natural images can be reconstructed using quantitative receptive-field models that characterize the relationship between visual input and fMRI activity in early visual cortex (Naselaris et al., 2009; Nishimoto et al., 2011). Collectively, these promising findings (see Danker and Anderson, 2010), suggest that decoding of more specific memory representations, at least of visual images, may be possible within the next few years.
There is utility in having both perceptual representations that are more specific, and reflective representations that are more abstract and global. PRAM posits that classifiers should transfer across perceptual and reflective tasks more successfully for more abstract, global representations. Different brain regions represent different types of information in perception (Bar, 2004; Epstein et al., 2007; Park et al., 2007; Park & Chun, 2009) and we would expect people to be differentially successful in representing such information during reflection. For example, PPA represents scene details whereas retrosplenial cortex (RSC) represents less viewpoint specific, more global information that relates a scene to the larger environment (Epstein and Higgins, 2007; Bar, 2004; Park et al., 2007). In a direct comparison of perceiving and refreshing stimuli across several areas of visual cortex (perception showed greater activity in middle occipital gyrus and PPA than did refreshing, but there was little difference between perception and refreshing in RSC and precuneus Johnson et al., 2007),. At least for the hierarchy of visual processing, PRAM predicts that perceptual and reflective representations should be more confusable in high-level areas than in mid-level or low-level visual areas. Indeed, in subsequent memory tasks, precuneus activity during imagery is associated with later false memory for the imagined items (Gonsalves et al., 2004). Thus, understanding similarities and differences in how different brain regions represent perceptual and reflective information may help explain cases where the distinction between perception and reflection breaks down, such as in schizophrenia (Simons et al., 2006) or false memories for childhood events (Loftus, 2003).
Because even a simple stimulus such as a face or scene is not represented only in one area, the relative contribution of different regions to perceptual and reflective representations is a potential way we discriminate between them. Cross-validation of classification on brain activity engaged during perception and reflection would be interesting not only for explicit memory tasks, but also for implicit memory tasks. At what level of specificity can we decode representations, even those not giving rise to the subjective experience of recollection or familiarity? And using combined information from multiple brain areas, can a classifier do better than participants in discriminating between real and false memories?
Control of Perceptual and Reflective Attention
For both perception and reflection, control mechanisms select, and sustain processing of target information, and combat interference from perceptual or mnemonic distraction.
Attention to perceptual events recruits frontal and parietal areas that modulate and maintain activity in other brain areas. For example, changes in activity in posterior representation areas as a function of attention are accompanied by increased activation in frontal eye fields (FEF) and dorsal (SPL, IPS) and ventral (IPL, SMG, TPJ, AG] parietal cortex (Corbetta et al., 2000; Hopfinger et al., 2000; Kastner et al., 1999). Such activity supports perceptual awareness (e.g., Asplund et al., 2010; Dehaene et al., 2006).
Reflective processes also depend heavily on frontal and parietal mechanisms. Refreshing typically activates left dorsolateral prefrontal cortex and left parietal regions (SMG and PCu) (Raye et al., 2002). Refreshing one among several active representations (Johnson et al., 2005) also recruits anterior cingulate cortex (ACC, an area associated with competition, Carter et al., 1998) and left ventrolateral PFC (BA 45, an area associated with resolving interference, D’Esposito et al., 1999; Thompson-Schill et al., 1997). Initiating refreshing, or shifting between refreshing and another task agenda, recruits left rostrolateral PFC (BA 10, Raye et al., 2007), an area associated with task switching, engaging subgoals, and attending to internal representations (Braver and Bongiolatti, 2002; Burgess et al., 2007; Henseler et al., 2011). In contrast, rehearsing information tends to recruit left ventrolateral PFC (BA 44), premotor, pre-SMA and parietal cortex (SMG) (Chein and Fiez, 2010; D’Esposito et al., 1999; Raye et al., 2007; Smith and Jonides, 1999;). Tasks requiring both maintenance and manipulation typically show both VLPFC and DLPFC activity (Cohen et al., 1997).
The frontal and parietal areas active during refreshing and rehearsing are typically found in more complex tasks requiring executive function (Duncan and Owen, 2000; Smith and Jonides, 1999). That is, the foregrounding (refreshing) of task-relevant information within working memory is important for most executive tasks that involve selective attention, task maintenance, task switching, or manipulation of information (Miller and Cohen, 2001; Duncan and Owen, 2000; Smith and Jonides, 1999). Furthermore, encoding activity in regions associated with component processes of reflective attention predicts long-term memory. Greater activity in DLPFC during refreshing at encoding is associated with better subsequent long-term recognition memory (Raye et al., 2002). Rote (phonological) rehearsal is associated with activity in left ventrolateral PFC, as well as supplementary motor area (SMA) (Jonides et al., 1998). Amount of activation in these regions when participants are instructed to rehearse predicts subsequent recognition memory (Davachi et al., 2001). Relational processing at encoding is associated with increased activity and subsequent memory effects in ventrolateral PFC and, especially, dorsolateral PFC (e.g., Blumenfeld and Ranganath, 2007; Staresina and Davachi, 2006).
Neural activity that occurs during remembering has also been vigorously investigated. Many studies show activity in DLPFC and VLPFC during recognition and recall in long-term memory tasks, and there are increasing efforts to differentially associate different PFC areas with subprocesses involved in reviving and/or evaluating information (e.g., Mitchell et al., 2009). For example, there is evidence that rostrolateral PFC maintains memory-relevant goals or specific agendas to look for a particular type of information (e.g., Dobbins and Han, 2006).
Dissociable Control Mechanisms of Perceptual and Reflective Attention
The review above indicates that frontal and parietal regions are engaged during both perceptual and reflective attention. This similarity likely reflects the fact that they are serving related functions. However, according to PRAM, perceptual and reflective attention should be dissociable at the neural level. A growing body of work makes distinctions similar to that between perceptual and reflective attention: stimulus-oriented vs. stimulus independent attending (Burgess et al., 2007), selective attention vs. memorial selection (Nee and Jonides, 2009), attentional orienting in the perceptual domain vs. the working memory domain (Lepsien and Nobre, 2006), and attentional modulation of sensory information and information in working memory (Awh et al., 2006). Although the literature directly comparing perception and reflection is still quite small, recent studies are beginning to advance our understanding of the relation between perception and reflection and their consequences for memory.
In one direct comparison of perceptual attention and reflective attention to word stimuli (Roth et al., 2009), regions more active for perceptual attention included right frontal cortex and bilateral posterior visual cortex. Activity more specific to refreshing was recorded in left dorsolateral frontal cortex, left temporal cortex, and bilateral inferior frontal cortex. Another comparison between perceptual selection and reflective selection found that the superior parietal lobule and frontal eye fields were more specific to perceptual selection, while left ventrolateral prefrontal cortex was more specific to reflective selection (see Figure 2, Nee and Jonides, 2009). Attention to locations within mental representations revealed stronger activations in frontal cortex compared to when attending to locations in perceptual arrays (Nobre et al., 2004). Furthermore, rostromedial PFC was more active during perceptual attention, while rostrolateral PFC was more active during reflective attention (Henseler et al., 2011). Burgess et al. (2007) note that the region of rostral medial PFC that was more active for perceptual and reflective cognitive tasks is anterior to the medial PFC area that is typically found when participants are not engaged in a task (“rest”) or when they are specifically instructed to engage in self-referential thinking (see below).
Figure 2. Comparing perceptual attention and reflective attention.

(A) Regions active for high versus low selection in a perceptual selection task (top), memory selection task (middle), and the conjunction of both tasks (bottom). (B) Regions unique to perceptual selection (left) and memorial selection (right). (C) Differential activation of the rostromedial and rostrolateral PFC during attentional orientation to external and internal information, respectively. Center: Brain activation map showing significantly stronger activation of the anterior rostromedial PFC during the orientation of attention to external as compared to internal information (blue), and significantly stronger activation of the rostrolateral PFC during the orientation of attention to internal as compared to external information (red). The scale below shows the color-coding of the displayed T-values. Periphery: Parameter estimates extracted from the rostromedial PFC (left side) and rostrolateral PFC (right side) color coded for the different tasks (blue: Externally Oriented position task; purple: Externally Oriented target task; red: Internally Oriented task). [(A) and (B) from Nee and Jonides (2009, Neuroimage); (C) from Henseler et al. (2011, Neuroimage).
Sestieri, Shulman & Corbetta (2010) compared a perceptual attention task in which participants looked for specific targets in a video (e.g., Can you detect a man standing on the street wearing red pants?) with a reflective attention task in which they answered recognition questions about videos seen previously (“Richard mentioned his problem with alcohol before his intimacy problem.”). For the perceptual task they found activity in SPL and posterior IPS, regions commonly found in perceptual attention tasks. For the memory task, they found areas of AG, SMG, lateral IPS, and medial areas (PCu, PCC, RSC). These findings suggest a dissociation of regions engaged during perceptual and reflective attention. However, this study did not equate items across perceptual and reflective conditions. Furthermore, as noted by Sestieri et al., the memory retrieval task likely involved an “ensemble of processes” (p. 8453) and thus was not designed to contrast specific component processes of perceptual and/or reflective attention.
Functional connectivity analyses help to segregate functionally different networks (Fox et al., 2006; Corbetta et al., 2008; Chadick & Gazzaley, 2011). PRAM predicts different patterns of connectivity between representational areas and frontal and/or parietal cortex for perceptual vs. reflective tasks. Also, the timing of activity between frontal and parietal control mechanisms may yield differences between perceptual and reflective attention. For example, frontal activity occurs before parietal activity during top-down perceptual attention, while parietal activity precedes frontal activity during bottom up perceptual attention (e.g., Buschman and Miller, 2007). It would be useful to see if such findings extend to reflective attention tasks.
Dissociations between patterns of enhancement and suppression also show differences between perceptual and reflective attention. During encoding of multiple items presented in a sequence, Older adults showed intact enhancement but disrupted suppression effects relative to young adults, suggesting that enhancement and suppression are dissociable processes (Gazzaley et al., 2005). Although it provided evidence regarding overall enhancement and suppression effects during encoding, the design of the Gazzaley et al. study did not separately assess effects of perceptual and reflective attention. Evidence that perceptual and reflective attention are also dissociable comes from a study finding that older adults showed disrupted suppression during refreshing, but not during perceptual attention, while enhancement effects in both perceptual and reflective attention were preserved (Mitchell et al., 2009). Large-scale brain networks for enhancement and suppression appear dissociable Functional connectivity analysis during a perceptual attention task revealed that visual cortical areas that process target information coupled with right MFG and bilateral IFJ during enhancement, and with mPFC and PCC during suppression (Chadick & Gazzaley, 2011). The differences between enhancement and suppression in connectivity suggest that on-task perceptual attention contributed to enhancement effects and that off-task, self-referential attention (activating the “default network” [see below]) contributed to suppression effects. Additional studies are needed to compare such network effects for perception and reflection.
Bottom-up and Top-down Orienting in Perception or Reflection
Perceptual attention is controlled by two orienting systems (Corbetta and Shulman, 2002; Corbetta et al., 2008). A dorsal system includes the frontal eye fields (FEF) and intraparietal cortex (IPS, SPL) and is involved in goal-directed, top-down attention to stimuli. A ventral network includes inferior frontal cortex and IPL (TPJ and is specialized for bottom-up detection of salient or unexpected events. The ventral network has a right hemisphere bias and mediates the ability to “reorient” quickly to salient events that are potentially rewarding or dangerous to an observer. Reorienting involves interruption and resetting of ongoing activity in the dorsal network, which otherwise suppresses the ventral network during focused and sustained attention to an ongoing task. One would expect that perceptual attention would be important for encoding events for LTM and indeed, this is the case. For example, Uncapher et al. (2011) found that cuing top-down perceptual attention to an upcoming target location engaged the IPS and was associated with better subsequent memory, while cuing participants to an invalid non-target location engaged TPJ and was associated with poorer subsequent memory. Presumably, activity in TPJ reflected perceptual capture and/or reorienting necessary when the cued location did not contain a target. These findings provide important evidence of the role during encoding of top-down and bottom-up perceptual attention, but the study did not compare perceptual and reflective attention.
Whether a simple dorsal/ventral distinction applies to remembering is a subject of current debate. Lateral parietal activity is commonly found to be associated with correct recognition memory for old items (Vilberg and Rugg, 2008). It has been proposed that the dorsal/ventral distinction in perceptual attention may generalize to the kind of reflective attention processes engaged during remembering. Cabeza et al (2008) and Ciaramelli et al. (2008) suggested that superior parietal cortex supports retrieval search, monitoring and verification, similar to its role in the top-down, voluntary control of perceptual attention, and that inferior parietal cortex is active when there is clear and more detailed recollection, similar to the exogenous capture of attention by salient, bottom-up perceptual events. However, a comparison by Hutchinson et al. (2009) of their meta-analysis of regions involved in top-down and bottom up attention, with previously published analyses of top-down and bottom up effects in episodic remembering (Ciaramelli et al., 2008; Vilberg and Rugg, 2008), did not support the idea of overlap between perceptual attention and memory processes, especially for ventral parietal cortex. And, as noted above, Sestieri et al. (2010) found different parietal areas associated with their perceptual and memory search tasks.
Nevertheless, as Wagner et al., 2005) suggested, parietal activity is associated with a number of factors important for memory judgments, including a subjective sense that the relevant information is old or new (independent of the memory’s veracity, Johnson, 2006), level of detail that the memory supports, and retrieval orientation – the type of detail that participants are asked to retrieve about target memories. That is, parietal mechanisms may be involved in attending to internal, mnemonic representations, act as a buffer to integrate details that have been activated, reflect the overall strength of memories, and/or play a role in the evaluation of the task relevance of what is remembered (Wagner et al., 2005; Cabeza et al., 2008; Vilberg and Rugg, 2008; Shimamura, 2011).
Importantly, the PRAM framework assumes that the distinction between perceptual and reflective attention is orthogonal to the distinction between top-down and bottom-up attention (Chun et al., 2011; Corbetta and Shulman, 2002). Thus, efforts to compare control mechanisms for perceptual and reflective information should attempt to equate whether attention is directed to the task stimuli in a top-down or bottom-up manner. Studies to date typically relied on top-down manipulations (Nee and Jonides, 2009; Henseler et al., 2011; Roth et al., 2009). It would be helpful to introduce stimuli that capture attention in a bottom-up manner to assess the extent to which a common ventral network is engaged in both perceptual and reflective tasks. That is, it would be useful to directly compare four conditions: top-down and bottom-up attentional conditions in both perceptual and reflective tasks.
Resolving Interference within Perceptual and Reflective Attention
Perception and reflection both need selective mechanisms to resolve interference. Perception requires focusing on task-relevant information from among perceptually present task-irrelevant information. Perceptual competition makes it more difficult to find a T among Ls than among Os in visual search, and can even produce quite dramatic examples of blindness to unattended information (Simons and Chabris, 1999; reviewed in Marois and Ivanoff, 2005). Resolution of competition (successful selection) occurs when goals bias activation in favor of goal relevant features (Desimone and Duncan, 1995).
During perceptual identification, the strength of sensory evidence for a target can be measured by the strength of activity within a cortical region for the target category. Competing perceptual hypotheses, as under conditions of perceptual noise and degradation, arise from similar levels of activity within different cortical representational regions. For example, when trying to discriminate a face from a house under near-threshold conditions of degradation, successful recognition is dependent on FFA activity being greater than baseline and greater than PPA activity. Frontal and parietal mechanisms presumably evaluate the sensory evidence, and to suppress competing information. In particular, the findings shown in Figure 3 are consistent with the idea that DLPFC (left superior frontal sulcus) evaluates sensory evidence from posterior sensory areas to make perceptual discrimination decisions for target stimuli degraded with noise; stronger activity was associated with better psychophysical performance (Heekeren et al., 2004). Other regions including IPS and frontal eye field were more active during perceptually difficult conditions.
Figure 3. Perceptual and reflective resolution of competition.

(A) Activity in posterior DLPFC (left SFS) during perceptual discrimination of faces vs. houses showing a higher response to suprathreshold images of faces and houses relative to perithreshold images, and a correlation with the difference in activity in face vs. house selective areas, suggesting that this region integrates evidence from sensory processing areas to make perceptual decisions. (B) Signal changes in the posterior portion of the DLPFC predicted task performance. Points represent average BOLD change and performance for each condition (suprathreshold face, perithreshold face, perithreshold house and suprathreshold house) and participant. (C) Frontoparietal regions that were more active during memory retrieval for trials on which a classifier showed less activity for a target category under conditions of A–B, A–C interference (B and C were faces and scenes). (D) Responses in several regions of interest was characterized by marked activation for AC trials associated with low-fidelity reactivation. (Panels A and B adapted from Heekeren et al., 2004, Nature; C and D adapted from Kuhl et al., 2011, PNAS).
Reflection also involves the resolution of interference—competition from representations cued because they share features (e.g., spatial or temporal context, objects or people, perceptual or emotional qualities) with a target event. Examples of retrieval competition include proactive interference, when older memories interfere with the ability to retrieve more recent information (e.g., where did I park my car today?), or retroactive interference, when newer memories interfere with older ones (e.g., What was my former address? Wixted 2004). Resolution of such competition or interference is an act of selective reflective attention analogous to perceptual attention (Kuhl et al., 2011; Heekeren et al., 2004).
Patients with frontal lobe damage show greater deficits under conditions of increased mnemonic competition (Shimamura et al., 1995; Smith et al., 1995). Functional neuroimaging helps to specify the relative roles of different areas of PFC. For example, left ventrolateral PFC is more active in the face of interference, both during encoding (Dolan et al., 1997; Fletcher et al., 2000) and retrieval (Badre et al., 2005; Thompson-Schill et al., 1997). After learning person-location pairs (e.g., “The hippie is in the park”), subsequent retrieval of any association (hippie-park, yes/no?) is slower when more facts are associated with the same concept, producing slower response times and greater activity in left VLPFC (BA 44/45) (Sohn et al., 2003). As noted above, left VLPFC is also more active as interference accumulates over trials during working memory tasks, and may play a role in overcoming proactive interference (Jonides and Nee, 2006).
Retrieval competition in LTM is often studied with an A–B/A–C paradigm. A list of A–B pairs is studied, with the goal of being able later to recall B (target) when A (cue) is presented. Later in the experiment, some of the A cues are paired with new targets (A–C) for new learning. Relative to other completely new D–E cue-target pairs, subsequent memory for A–C pairs is impaired (proactive interference from the earlier A–B pairs), while memory for A–B pairs is impaired (retroactive interference from the more recent A–C pairs). For example, Kuhl et al. (2011) asked participants to associate cue words with faces or scenes, and a given cue was associated with both a face and a scene. Since faces and scenes have distinguishable representations in ventral-occipito-temporal cortex (including FFA and PPA), Kuhl et al. used MVPA to decode the relative strength of face and scene activation during memory retrieval to investigate how recall for an A–C pairing was affected by the earlier A–B pairing. Competition between associates B and C (from opposing face-scene categories) was assessed by the degree to which the classifier favored either face or scene activity. Compared to control items without competition, classifier performance was poorer for items with face/scene competition, suggesting that target and competing memories were being simultaneously reactivated. Furthermore when the classifier indicated more conflict, frontal and parietal areas were more strongly engaged, suggesting a role for these areas in resolving mnemonic conflict between target and competing memories (see Figure 3). Active regions included dorsolateral prefrontal cortex, medial prefrontal cortex, and lateral and medial parietal cortex. Overall, the results support a model in which multiple representations are reactivated in sensory areas, and control mechanisms in frontal and parietal lobes serve to resolve the interference and select a representation.
What is the fate of competing memories that are not selected during remembering? When goal-relevant memories are consistently and repeatedly retrieved, competing memories are often forgotten. That is, retrieval competition appears to at least sometimes be resolved through inhibition of competing memories, mediated by PFC mechanisms (Anderson et al., 2004). Furthermore, over time, forgetting is accompanied by reduced involvement of cognitive control mechanisms required for detecting (anterior cingulate cortex) and resolving (dorsolateral and ventrolateral prefrontal cortex) mnemonic competition (Kuhl et al., 2007). Thus forgetting has the adaptive benefit of reducing the burden on cognitive control mechanisms (Anderson, 2003).
Fluctuations in Perceptual and Reflective Attention
As a series of items appears at the focus of perceptual attention, an observer may try to sustain attention equally to every item but, typically, some items are encoded and retrieved better than others. Considering variations in perceptual and reflective attention can help explain this variability. Emotional significance or perceptual salience can draw more attention to some items (“attentional capture”), enhancing memory (Mather, 2007; Phelps, 2006). People may be more successful in noting associations or using elaborative strategies that facilitate encoding for some items than others (Craik and Lockhart, 1972). Distraction or mind wandering will reduce memory (Weissman et al., 2006). Both perceptual and reflective attention have limited capacity, and the fact that they often trade-off suggests that they are not entirely independent.
Researchers have identified some of the neural changes associated with trial to trial fluctuations in attention and their consequences for memory, and PRAM attempts to explain subsequent memory according to variation in either perceptual or reflective attention to different items. As described above, in studies of LTM using simple materials (e.g., words, pictures) various lateral PFC, lateral parietal and MTL regions show greater activity for subsequently remembered than forgotten items (Blumenfeld and Ranganath, 2007; Diana et al., 2007; Kim, 2011; Uncapher and Wagner, 2009). In contrast, greater activity for subsequently forgotten than remembered items is often found in medial PFC and medial parietal cortex (posterior cingulate, precuneus, TPJ; Kim et al., 2010; Otten & Rugg, 2001; Park et al., 2008; Turk-Browne et al., 2006; Uncapher et al., 2011). Interestingly, these same regions are active when participants are cued to engage in reflective, self-referential, internally-oriented processing (e.g., Gusnard et al., 2001; Ochsner et al., 2005), such as thinking about their aspirations or obligations (Johnson et al., 2006). This suggests that forgotten items in non-self-referential cognitive tasks were ones for which the participant’s attention was momentarily diverted from the task and focused on more personal concerns. In fact, these same anterior and posterior medial regions are spontaneously more active during “rest”, when no task at all is specified, than during many cognitive tasks (and are part of what is known as the “default network”, Raichle et al., 2001; Buckner et al., 2008). It should be noted that when self-referential processing is relevant to a later memory task, activation in medial PFC is positively related to later memory (e.g., Macrae et al., 2004; Kim and Johnson, 2010).
Limited attention is not necessarily allocated in an all-or-none manner but may be distributed between primary (target) and other (non-target or task irrelevant) information (Pashler, 1998). For example, according to load theory (Lavie, 2005), the amount of processing that nominally “unattended” stimuli receive depends on how much processing is devoted to the primary, attended target task. Difficult primary tasks (high load) consume attention; easy primary tasks (low load) do not fully consume attention which thus spills over to process “unattended” stimuli. Consistent with load theory, increasing the perceptual difficulty of a target task can eliminate negative priming from unattended distractors (Lavie and Fox, 2000). In contextual learning tasks, increasing the difficulty of the search through attended arrays decreases implicit learning of unattended arrays (Jiang and Chun, 2001). In repetition attenuation studies, increasing the perceptual difficulty of a primary task reduced repetition attenuation for repeated cycles of task-irrelevant background images (Yi et al., 2004). Interestingly, there is some evidence that in reflective tasks (e.g., recall), the negative impact of a concurrent reflective task (e.g., recognition) depends at encoding on whether the two tasks engage similar processes and, at retrieval, depends on whether the two tasks engaged similar representations (Fernandes and Moscovitch, 2000).
Our distinction between perceptual and reflective attention relates to how Lavie (2005) distinguished between perceptual and central (e.g., working memory, executive control) difficulty. This helps predict when perceptual and reflective attention trade off with each other or when they are independent. In some situations, reflection and perception clearly interfere with each other. For example, carrying on a conversation on a cell phone dramatically reduces perception and memory for stimuli encountered in a driving task (Strayer, Dreews & Johnston, 2003). Perceptual distraction (visual or auditory) disrupts reflective memory for visual details of pictures (Wais & Gazzaley, 2011; Wais, Rubens, Boccanfuso & Gazzaley, 2010). In other situations, there is little or no evidence of interference between perception and reflection. For example, in one study, Yi et al. (2004) manipulated working memory load (central/reflective processing), and found no impact on processing or implicit memory for an unattended, repeating background. Importantly, perceptual load manipulations of comparable difficulty did affect background processing. Another case where reflection did not disrupt perceptual learning comes from a study by Watanabe et al. (2001). Participants were given a primary task that required them to detect and be able to report target stimuli in a series of rapidly changing visual stimuli (a rapid serial visual presentation or RSVP task). In RSVP tasks, rapidly presented stimuli are perceptually processed to a level at which they are identified, but memory for the target depends on more than perceptual processing—it depends on central (reflective) processes that encode the target into working memory (Chun & Potter, 1995). One possibility is that this is accomplished via briefly refreshing the target. In the Watanabe et al. study, the RSVP task occurred against a background display of coherently moving dot stimuli embedded in enough random noise that their trajectory could not be consciously perceived or guessed above chance levels. Perceptual learning occurred for this unconscious but coherent background motion in spite of the perceptual and reflective demands of the primary RSVP task.
These examples highlight the question of what kinds of perceptual processes are and are not affected by reflective demands and vice/versa. At least part of the answer should depend on whether perceptual and reflective attention similarly or differentially engage the same or different brain areas and networks. Furthermore, the answer to this should in turn depend on exactly which perceptual and which reflective processes are being compared. Insofar as different reflective processes (e.g., refreshing, rehearsing, retrieving) differentially engage specific frontal and parietal regions, we would expect them to differentially interact with specific perceptual attention tasks. For example, some types of perceptual learning show effects in a very early visual processing area (V1) but not in other visual areas (V2, V3) nor in parietal or frontal cortex (Yotsumoto, Watanabe & Sasaki, 2008). V1 is an area relatively unlikely to be activated during reflection.
Thus, more work is needed to clarify the relations between perceptual load and reflective load (or central load, Lavie, 2005). These two types of load have been dissociated in some prior studies, but not all. For example, active manipulation of information in working memory (e.g., counting backwords), which involves reflective processing, impairs concurrent visual search efficiency (Han and Kim, 2004). Perceptual secondary tasks frequently disrupt reflective processing as well. For example, when making categorical decisions about visual stimuli, participants can be asked to concurrently perform an easy or difficult auditory monitoring task. Dividing attention with a difficult secondary task engaged DLPFC and superior parietal regions, impairing both visual task performance and subsequent memory for the stimuli (Uncapher & Rugg, 2005).
While both inferior frontal gyrus (IFG) and hippocampus show subsequent memory effects, these areas are affected by divided attention (dual task) in some experiments (Kensinger et al., 2003) but not others (Uncapher & Rugg, 2005). Differences in experimental outcomes may be explained by how well participants can share processing across dual tasks, that is, whether or not the two overlapping tasks recruit the same type of attentional processing (Uncapher & Rugg, 2008) or involved the same general representational areas (Fernandes and Moscovitch, 2000). In addition, different encoding conditions yield qualitatively different types of memory experiences. For example, Kensinger et al. (2003) found that words encoded under difficult divided attention conditions yielded a sense of familiarity, while words encoded with easier concurrent tasks yielded a more detailed (“recollective”) experience. Overall, whether two tasks interfere with each other should depend on whether common processes are important for the task and the type of representations involved. In sum, because perceptual load and reflective (central) load interfere differently with perceptual tasks (Lavie and De Fockert, 2005; Yi et al., 2004), they will likely have different effects on reflective tasks. Thus, dual-task studies sensitive to the distinction between perceptual and reflective attention will be important for conclusions about the presence or absence of divided attention costs. Furthermore, task switching studies would produce revealing findings regarding the important question of the mechanisms by which the cognitive system switches between perceptual and reflective attention (e.g., Burgess et al., 2007).
The brain is active even when at rest, and investigators have begun to explore the functional connectivity between areas when participants are not given an explicit task (Fox and Raichle, 2007). Early interest focused on the relation between a general “task positive network” including regions often found in cognitive tasks and a “task negative network” including regions that often deactivate during cognitive tasks and activate during rest (Fox and Raichle, 2007). These networks are also evident during sleep and anesthesia, consistent with the idea that they originate from intrinsic connectivity rather than uncontrolled, spontaneous cognition. Investigators are beginning to identify other “resting state networks” (RSNs) that are similar to networks found during explicit task manipulations (Smith et al., 2009). Thus, a potential direction for future research is whether dissociable intrinsic networks can be identified that are associated with differences in perceptual vs. reflective attention (when the content is held constant).
The Role of Attention in Binding Features and in Consolidation
It was once thought that the hippocampus was the memory region and that frontal and parietal cortex served other functions (cognition, attention). However, as noted above, the specific roles of frontal and parietal cortex in both attention and memory are under active investigation. It is also now recognized that other structures in the MTL (entorhinal cortex, perirhinal cortex and parahippocampal cortex) are important for memory (Ranganath, 2010). Although some maintain that evidence that various MTL structures have different functions in memory is weak (Squire et al., 2004), others have concluded they play differential roles in either item vs. relational memory, the types of features they process (e.g., object vs. spatial), or the level of representation at which binding occurs (Davachi, 2006; Eichenbaum et al., 2007; Shimamura, 2010). Nevertheless there is common agreement that the hippocampus (and perhaps other MTL structures, Shimamura, 2010) mediates binding among features (e.g., location, color, time) and of features with prior knowledge (e.g., schemas, Tse et al., 2007).
The importance of the hippocampus for long-term episodic memory is beyond debate based on patient and lesion data (Squire and Wixted, 2011; Eichenbaum et al., 2007). Consistent with patient data are neuroimaging findings of hippocampal activity during long-term memory tests, especially during source memory tasks (Weis et al., 2004) and correlations between hippocampal activity and the subjective experience of remembered details (Addis et al., 2004). Neuroimaging data from studies of long-term memory have also made it clear that the hippocampus is engaged not only during remembering, but also during encoding. For example, hippocampal activity during encoding predicts better source memory on a later test (Davachi, Mitchell & Wagner., 2003) further suggesting a major role for the hippocampus in initial feature binding.
Although most research on the MTL has focused on its role in long-term memory, it is increasingly evident that the hippocampus plays a much broader role in perception and reflection.. With respect to short-term memory, MTL damage impairs working memory for visual objects across delays as short as 4 s (Olson et al., 2006). Furthermore, object-location conjunction information can be impaired across delays as short as 8 s with MTL damage (Hannula et al., 2006; Olson et al., 2006). During perception, contextual representations mediated by the hippocampus/MTL can facilitate object recognition (Bar, 2004), guide the focus of attention (Chun and Phelps, 1999; Summerfield et al., 2006) and generate perceptual anticipation (Turk-Browne et al., 2010). Differences in eye movement patterns when processing a particular stimulus that has been seen before vs. one that has not provide an implicit measure of memory, and hippocampal activity and its connectivity with lateral PFC predicts eye movement measures of memory for relational information (Hannula and Ranganath, 2009). Furthermore, MTL damage can also impair perceptual tasks requiring difficult object discriminations (Baxter, 2009; but see Suzuki, 2009) or visual associations (Degonda et al., 2005; Chun and Phelps, 1999). These findings of hippocampal involvement in long-term memory, working memory, and perception make clear that the hippocampus is engaged in an on-going fashion during cognition. Is there a general function being served in these various situations? One possibility is that the hippocampus helps bridge temporal and spatial gaps between features of experience so that information that is not strictly contiguous can be bound together (Johnson & Chalfonte, 1994; Staresina & Davachi, 2009). Of course, the hippocampus may bind whatever features are contiguous (perceptually or reflectively) and other regions (e.g., frontal and parietal) may actually do the bridging, for example, via refreshing (Park et al., 2009).
From the PRAM perspective, a critical issue is how perceptual and reflective attention affect MTL function. Assuming that attention modulates MTL regions, are different frontal, parietal, and/or MTL regions engaged during perceptual and reflective attention? Do attentional networks that include MTL depend on the type of perception (e.g., focal, peripheral) or the type of reflection (e.g., refreshing, reactivating) or the type of target (scenes vs. objects vs. faces)?
Intriguing recent work demonstrates that hippocampal-cortical interactions occur not only during encoding, but also during retention intervals during which participants have no explicit task (“rest”). For example, functional connectivity between the hippocampus and the lateral occipital complex (LOC) during a rest period predicts subsequent memory for face-object pairs, presumably reflecting a consolidation process during the retention interval (Tambini et al., 2010). Tambini et al. concluded that evidence of hippocampal-LOC connectivity during resting was unlikely to reflect active rehearsal of the target information during rest.
Can patterns of incidental functional connectivity after an experience be distinguished according to whether they were initiated by perceptual encoding or by reflective encoding? Does reflection during encoding contribute to memory not only via, for example, elaborated encoding, but also by jumpstarting critical consolidation processes during rest? Another important question is whether incidental functional connectivity during rest can be distinguished from spontaneous reflection (reactivating, retrieving) during rest. Can we discriminate reactivations that have functional significance but (a) do not yield a subjective experience of target information coming to mind; (b) yield the phenomenal experience of a target coming to mind, but not attributed to memory; (c) yield the phenomenal experience of remembering; (c) are the result of active attempts to remember?
Another function that both perception and reflection share is binding multiple features into coherent objects or event representations. Failure to bind correctly can result in illusory conjunctions during perception (Treisman and Schmidt, 1982), or source memory errors during recall (Johnson, 2006). The intraparietal sulcus serves to bind different features together, important for feature integration in perceptual tasks (Corbetta et al., 1995; Friedman-Hill et al., 1995), and episodic memory tasks (Uncapher et al., 2006; Mitchell et al., 2009). That is, greater activity in IPS is correlated with successful encoding and recall of combinations of features.
What are the respective roles of parietal cortex and hippocampus in binding? Two potential differences require further investigation. The first difference concerns the type of information that undergoes binding (reviewed in Mitchell et al., 2009). The hippocampal system can bind and associate objects and events with each other and across domains (e.g., associating two different faces, or a face and a place, Mayes et al., 2007), while the parietal cortex might be more focused on binding multiple features into coherent objects or episodes (e.g., the screen location and font color of words, Uncapher et al., 2006).
Related to this difference in the level of binding, the second potential difference may be one of time scale. The function of the hippocampal system for long-term, durable binding seems uncontroversial. Parietal cortex was originally considered to be primarily involved in binding features during perception.. However, Shimamura (2011) recently highlighted how episodic retrieval (reflective attention in PRAM) contributes to feature binding, and proposes that ventral parietal cortex is a convergence zone for integrating episodic information (the cortical binding of relational activity [CoBRA] model).
Necessity of brain regions for perception and reflection
Although there is increasing evidence that different brain areas cooperate and interact during both perceptual attention and reflective attention, whether such activity is necessary for a particular function is indeterminate from brain imaging data alone. To simplify greatly, damage to the parietal cortex impairs spatial attention, but memory less so. In contrast, damage to the hippocampus and other medial temporal lobe regions impair explicit memory, but perception less so. However, newer work challenges this simplification, as parietal damage can result in memory impairments in specific situations such as free recall, but not recognition (Berryhill et al., 2007), and produces deficits in perceptual binding (Friedman-Hill et al., 1995), but not associative learning (Simons et al., 2008). Conversely, hippocampus/MTL damage can impair perceptual/attentional tasks (Murray et al., 2007; Chun and Phelps, 1999). Thus, more neuropsychological work is needed to investigate to what extent parietal mechanisms are necessary for reflective processes, and to what extent hippocampus and medial temporal lobe structures are necessary for perception. For disrupting both frontal and parietal function in humans, transcranial magnetic stimulation studies are promising (Miller et al., 2008; Zanto et al., 2011; Morishima et al., 2009).
Conclusion
The fields of attention and memory are beneficiaries of an increasingly vast amount of research in cognitive neuroscience, each complex and rich in its own right. The goal of a framework is to synthesize available evidence and suggest new directions for systematic analysis (Johnson, 2007). The PRAM framework and related empirical findings suggest that considering the similarities and differences between perception and reflection can help clarify and integrate the study of attention and memory to advance understanding of each in a symbiotic way, and points to potentially fruitful areas of additional research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguirre GK, Detre JA, Alsop DC, D’Esposito M. The parahippocampus subserves topographical learning in man. Cerebral Cortex. 1996;6:823–29. doi: 10.1093/cercor/6.6.823. [DOI] [PubMed] [Google Scholar]
- Anderson MC. Rethinking interference theory: Executive control and the mechanisms of forgetting. J Mem Lang. 2003;49:415–445. [Google Scholar]
- Anderson MC, Ochsner KN, Kuhl B, Cooper J, Robertson E, Gabrieli SW, Glover GH, Gabrieli JDE. Neural Systems Underlying the Suppression of Unwanted Memories. Science. 2004;303:232–35. doi: 10.1126/science.1089504. [DOI] [PubMed] [Google Scholar]
- Asplund CL, Todd JJ, Snyder AP, Marois R. A central role for the lateral prefrontal cortex in goal-directed and stimulus-driven attention. Nat Neurosci. 2010;13:507–512. doi: 10.1038/nn.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awh E, Vogel EK, Oh S. Interactions between attention and working memory. Neuroscience. 2006;139:201–08. doi: 10.1016/j.neuroscience.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory. Science. 1992;255:556–59. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Badre D, Poldrack RA, Paré-Blagoev EJ, Insler RZ, Wagner AD. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47:907–918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Bar M. Visual objects in context. Nature Reviews Neuroscience. 2004;5:617–629. doi: 10.1038/nrn1476. [DOI] [PubMed] [Google Scholar]
- Barsalou LW, Fiske F, Schacter S, Sternberg S. Grounded cognition. Annu Rev Psychol. 2008;59:617–645. doi: 10.1146/annurev.psych.59.103006.093639. [DOI] [PubMed] [Google Scholar]
- Baxter MG. Involvement of Medial Temporal Lobe Structures in Memory and Perception. Neuron. 2009;61:667–677. doi: 10.1016/j.neuron.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Berryhill ME, Phuong L, Picasso L, Cabeza R, Olson IR. Parietal lobe and episodic memory: Bilateral damage causes impaired free recall of autobiographical memory. J Neurosci. 2007;27:14415–423. doi: 10.1523/JNEUROSCI.4163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld RS, Ranganath C. Prefrontal cortex and long-term memory encoding: An integrative review of findings from neuropsychology and neuroimaging. Neuroscientist. 2007;13:280–291. doi: 10.1177/1073858407299290. [DOI] [PubMed] [Google Scholar]
- Brady TF, Konkle T, Alvarez GA, Oliva A. Visual long-term memory has a massive storage capacity for object details. Proc Natl Acad Sci U S A. 2008;105:14325–29. doi: 10.1073/pnas.0803390105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Bongiolatti SR. The role of frontopolar cortex in subgoal processing during working memory. NeuroImage. 2002;15:523–536. doi: 10.1006/nimg.2001.1019. [DOI] [PubMed] [Google Scholar]
- Brewer JB, Zhao Z, Desmond JE, Glover GH, Gabrieli JDE. Making memories: Brain activity that predicts how well visual experience will be remembered. Science. 1998;281:1185–87. doi: 10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL, Kingstone K, Miller M. The brain’s default network: Anatomy, function, and relevance to disease. Ann New York Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Goodman J, Burock M, Rotte M, Koutstaal W, Schacter D, Rosen B, Dale AM. Functional-anatomic correlates of object priming in humans revealed by rapid presentation event-related fMRI. Neuron. 1998;20:285–295. doi: 10.1016/s0896-6273(00)80456-0. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Dumontheil I, Gilbert SJ. The gateway hypothesis of rostral prefrontal cortex (area 10) function. Trends Cogn Sci. 2007;11:290–98. doi: 10.1016/j.tics.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–64. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, Moscovitch M. The parietal cortex and episodic memory: An attentional account. Nat Rev Neurosci. 2008;9:613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–49. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Chadick JZ, Gazzaley A. Differential coupling of visual cortex with default or frontal parietal network based on goals. Nature Neuroscience. 2011;14:830–832. doi: 10.1038/nn.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick MJ, Hassabis D, Weiskopf N, Maguire EA. Decoding individual episodic memory traces in the human hippocampus. Current Biology. 2010;20:544–7. doi: 10.1016/j.cub.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MWL, Tan JC. Inter-relationships between attention, activation, fMR adaptation and long-term memory. NeuroImage. 2007;37:1487–495. doi: 10.1016/j.neuroimage.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Chun MM, Phelps EA. Memory deficits for implicit contextual information in amnesic subjects with hippocampal damage. Nature Neuroscience. 1999;2:844–47. doi: 10.1038/12222. [DOI] [PubMed] [Google Scholar]
- Chun MM, Potter MC. A Two-Stage Model for Multiple Target Detection in Rapid Serial Visual Presentation. J Exp Psychol Hum Percept Perform. 1995;21:109–127. doi: 10.1037//0096-1523.21.1.109. [DOI] [PubMed] [Google Scholar]
- Chun MM, Golomb JA, Turk-Browne NB. A taxonomy of external and internal attention. Annual Review of Psychology. 2011;62:73–101. doi: 10.1146/annurev.psych.093008.100427. [DOI] [PubMed] [Google Scholar]
- Ciaramelli E, Grady CL, Moscovitch M. Top-down and bottom-up attention to memory: A hypothesis (AtoM) on the role of the posterior parietal cortex in memory retrieval. Neuropsychologia. 2008;46:1828–851. doi: 10.1016/j.neuropsychologia.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386:604–08. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci. 2000;3:292–7. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: From environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL, Miezin FM, Petersen SE. Superior parietal cortex activation during spatial attention shifts and visual feature conjunction. Science. 1995;270:802–05. doi: 10.1126/science.270.5237.802. [DOI] [PubMed] [Google Scholar]
- Cowan N. The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behav Brain Sci. 2001;24:87–114. doi: 10.1017/s0140525x01003922. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Lockhart RS. Levels of processing: A framework for memory research. Journal of Verbal Learning and Verbal Behavior. 1972;11:671–684. [Google Scholar]
- Curtis CE, D’Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci. 2003;7:415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR, Jonides J, Smith EE. The neural substrate and temporal dynamics of interference effects in working memory as revealed by event-related functional MRI. Proc Natl Acad Sci U S A. 1999;96:7514–19. doi: 10.1073/pnas.96.13.7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danker JF, Anderson JR. The ghosts of brain states past: Remembering reactivates the brain regions engaged during encoding. Psychol Bull. 2010;136(1):87–102. doi: 10.1037/a0017937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Curr Opin Neurobiol. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Davachi L, Maril A, Wagner AD. When keeping in mind supports later bringing to mind: Neural markers of phonological rehearsal predict subsequent remembering. J Cogn Neurosci. 2001;13:1059–070. doi: 10.1162/089892901753294356. [DOI] [PubMed] [Google Scholar]
- Degonda N, Mondadori CRA, Bosshardt S, Schmidt CF, Boesiger P, Nitsch RM, Hock C, Henke K. Implicit associative learning engages the hippocampus and interacts with explicit associative learning. Neuron. 2005;46:505–520. doi: 10.1016/j.neuron.2005.02.030. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Changeux JP, Naccache L, Sackur J, Sergent C. Conscious, preconscious, and subliminal processing: a testable taxonomy. Trends Cogn Sci. 2006;10:204–211. doi: 10.1016/j.tics.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural Mechanisms of Selective Visual-Attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Han S. Cue- versus probe-dependent prefrontal cortex activity during contextual remembering. J Cogn Neurosci. 2006;18:1439–452. doi: 10.1162/jocn.2006.18.9.1439. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Fink GR, Rolls E, Booth M, Holmes A, Frackowiak RS, Friston KJ. How the brain learns to see objects and faces in an impoverished context. Nature. 1997;389:596–99. doi: 10.1038/39309. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Eger E, Henson RN, Driver J, Dolan RJ. BOLD repetition decreases in object-responsive ventral visual areas depend on spatial attention. J Neurophysiol. 2004;92:1241–47. doi: 10.1152/jn.00206.2004. [DOI] [PubMed] [Google Scholar]
- Eich E. Memory for unattended events: remembering with and without awareness. Mem Cognit. 1984;12:105–111. doi: 10.3758/bf03198423. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007 doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. The parahippocampal place area: A cortical representation of the local visual environment. Journal of Cognitive Neuroscience. 1998:20–0. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Epstein R, Stanley D, Harris A, Kanwisher N. The Parahippocampal Place Area: Perception, Encoding, or Memory Retrieval? Neuron. 2000;23:115–25. doi: 10.1016/s0896-6273(00)80758-8. [DOI] [PubMed] [Google Scholar]
- Epstein RA, Higgins JS. Differential parahippocampal and retrosplenial involvement in three types of visual scene recognition. Cereb Cortex. 2007;17:1680–693. doi: 10.1093/cercor/bhl079. [DOI] [PubMed] [Google Scholar]
- Fernandes MA, Moscovitch M. Divided attention and memory: Evidence of substantial interference effects at retrieval. Journal of Experimental Psychology: General. 2000;129:155–176. doi: 10.1037//0096-3445.129.2.155. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Shallice T, Dolan RJ. ‘Sculpting the response space’ - An account of left prefrontal activation at encoding. NeuroImage. 2000;12:404–417. doi: 10.1006/nimg.2000.0633. [DOI] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A. 2006;103:10046–051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience. 2007;7:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Friedman-Hill SR, Robertson LC, Treisman A. Parietal contributions to visual feature binding: Evidence from a patient with bilateral lesions. Science. 1995;269:853–55. doi: 10.1126/science.7638604. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, Rissman J, D’Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nat Neurosci. 2005;8:1298–1300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- Gonsalves B, Reber PJ, Gitelman DR, Parrish TB, Mesulam M, Paller KA. Neural evidence that vivid imagining can lead to false remembering. Psychol Sci. 2004;15:655–660. doi: 10.1111/j.0956-7976.2004.00736.x. [DOI] [PubMed] [Google Scholar]
- Gonsalves BD, Kahn I, Curran T, Norman KA, Wagner AD. Memory strength and repetition suppression: multimodal imaging of medial temporal cortical contributions to recognition. Neuron. 2005;47:751–761. doi: 10.1016/j.neuron.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kushnir T, Edelman S, Avidan G, Itzchak Y, Malach R. Differential processing of objects under various viewing conditions in the human lateral occipital complex. Neuron. 1999;24:187–203. doi: 10.1016/s0896-6273(00)80832-6. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kushnir T, Edelman S, Itzchak Y, Malach R. Cue-invariant activation in object-related areas of the human occipital lobe. Neuron. 1998;21:191–202. doi: 10.1016/s0896-6273(00)80526-7. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:4259–264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Kim M. Visual search does not remain efficient when executive working memory is working. Psychol Sci. 2004;15:623–28. doi: 10.1111/j.0956-7976.2004.00730.x. [DOI] [PubMed] [Google Scholar]
- Hannula DE, Ranganath C. The Eyes Have It: Hippocampal Activity Predicts Expression of Memory in Eye Movements. Neuron. 2009;63:592–99. doi: 10.1016/j.neuron.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula DE, Tranel D, Cohen NJ. The long and the short of it: Relational memory impairments in amnesia, even at short lags. J Neurosci. 2006;26:8352–59. doi: 10.1523/JNEUROSCI.5222-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SA, Tong F. Decoding reveals the contents of visual working memory in early visual areas. Nature. 2009;458:632–35. doi: 10.1038/nature07832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heekeren HR, Marrett S, Bandettini PA, Ungerleider LG. A general mechanism for perceptual decision-making in the human brain. Nature. 2004;431:859–862. doi: 10.1038/nature02966. [DOI] [PubMed] [Google Scholar]
- Henseler I, Krüger S, Dechent P, Gruber O. A gateway system in rostral PFC? Evidence from biasing attention to perceptual information and internal representations. NeuroImage. 2011;56:1666–676. doi: 10.1016/j.neuroimage.2011.02.056. [DOI] [PubMed] [Google Scholar]
- Henson RN, Rugg MD. Neural response suppression, haemodynamic repetition effects, and behavioural priming. Neuropsychologia. 2003;41:263–270. doi: 10.1016/s0028-3932(02)00159-8. [DOI] [PubMed] [Google Scholar]
- Hollingworth A, Henderson JM. Accurate visual memory for previously attended objects in natural scenes. Journal of Experimental Psychology: Human Perception and Performance. 2002;28:113–136. [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. NAT NEUROSCI. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Hutchinson JB, Uncapher MR, Wagner AD. Posterior parietal cortex and episodic retrieval: Convergent and divergent effects of attention and memory. Learn Mem. 2009;16:343–356. doi: 10.1101/lm.919109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby LL, Dallas M. On the relationship between autobiographical memory and perceptual learning. 1981;110:306–340. doi: 10.1037//0096-3445.110.3.306. On the relationship between autobiographical memory and perceptual learning. [DOI] [PubMed] [Google Scholar]
- Jiang X, Rosen E, Zeffiro T, VanMeter J, Blanz V, Riesenhuber M. Evaluation of a Shape-Based Model of Human Face Discrimination Using fMRI and Behavioral Techniques. Neuron. 2006;50:159–172. doi: 10.1016/j.neuron.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Chun MM. Selective attention modulates implicit learning. Q J Exp Psychol A. 2001;54:1105–124. doi: 10.1080/713756001. [DOI] [PubMed] [Google Scholar]
- Johnson JD, McDuff SGR, Rugg MD, Norman KA. Recollection, Familiarity, and Cortical Reinstatement: A Multivoxel Pattern Analysis. Neuron. 2009;63:697–708. doi: 10.1016/j.neuron.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MK. Memory and reality. Am Psychol. 2006;61:760–771. doi: 10.1037/0003-066X.61.8.760. [DOI] [PubMed] [Google Scholar]
- Johnson MK. Memory systems: A cognitive construct for analysis and synthesis. In: Roediger HL, Dudai Y, Fitzpatrick SM, editors. Science of Memory: Concepts. New York: Oxford University Press; 2007. pp. 353–357. [Google Scholar]
- Johnson MK, Hirst W. MEM: Memory subsystems as processes. In: Collins AA, Gathercole SS, Conway MM, Morris PE, editors. Theories of Memory. East Sussex, England: Erlbaum; 1993. [Google Scholar]
- Johnson MK, Nolde SF, De Leonardis DM. Emotional focus and source monitoring. J Mem Lang. 1996;35:135–156. [Google Scholar]
- Johnson MK, Raye CL, Mitchell KJ, Greene EJ, Cunningham WA, Sanislow CA. Using fMRI to investigate a component process of reflection: Prefrontal correlates of refreshing a just-activated representation. Cogn Affective Behav Neurosci. 2005;5:339–361. doi: 10.3758/cabn.5.3.339. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Reeder JA, Raye CL, Mitchell KJ. Second thoughts versus second looks: An age-related deficit in reflectively refreshing just-activated information. Psychol Sci. 2002;13:64–67. doi: 10.1111/1467-9280.00411. [DOI] [PubMed] [Google Scholar]
- Johnson MR. Unpublished Doctoral Dissertation. Yale University; 2011. Component processes, top-down modulation, and interactions between perceptual and reflective processing. [Google Scholar]
- Johnson MR, Mitchell KJ, Raye CL, D’Esposito M, Johnson MK. A brief thought can modulate activity in extrastriate visual areas: Top-down effects of refreshing just-seen visual stimuli. NeuroImage. 2007;37:290–99. doi: 10.1016/j.neuroimage.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonides J, Nee DE. Brain mechanisms of proactive interference in working memory. Neuroscience. 2006;139:181–193. doi: 10.1016/j.neuroscience.2005.06.042. [DOI] [PubMed] [Google Scholar]
- Jonides J, Schumacher EH, Smith EE, Koeppe RA, Awh E, Reuter-Lorenz PA, Marshuetz C, Willis CR. The role of parietal cortex in verbal working memory. Journal of Neuroscience. 1998;18:5026–034. doi: 10.1523/JNEUROSCI.18-13-05026.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamitani Y, Tong F. Decoding the visual and subjective contents of the human brain. Nat Neurosci. 2005;8:679–685. doi: 10.1038/nn1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: A module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22:751–761. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Clarke RJ, Corkin S. What neural correlates underlie successful encoding and retrieval? A functional magnetic resonance imaging study using a divided attention paradigm. J Neurosci. 2003;23:2407–415. doi: 10.1523/JNEUROSCI.23-06-02407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. Neural activity that predicts subsequent memory and forgetting: A meta-analysis of 74 fMRI studies. NeuroImage. 2011;54:2446–461. doi: 10.1016/j.neuroimage.2010.09.045. [DOI] [PubMed] [Google Scholar]
- Kim H, Daselaar SM, Cabeza R. Overlapping brain activity between episodic memory encoding and retrieval: Roles of the task-positive and task-negative networks. NeuroImage. 2010;49:1045–054. doi: 10.1016/j.neuroimage.2009.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KM, Johnson MK. Extended self: Medial prefrontal activity during transient association of self and objects. Social Cognitive and Affective Neuroscience. 2010 doi: 10.1093/scan/nsq096. Advance online publication. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl BA, Dudukovic NM, Kahn I, Wagner AD. Decreased demands on cognitive control reveal the neural processing benefits of forgetting. Nat Neurosci. 2007;10:908–914. doi: 10.1038/nn1918. [DOI] [PubMed] [Google Scholar]
- Kuhl BA, Rissman J, Chun MM, Wagner AD. Fidelity of neural reactivation reveals competition between memories. Proc Natl Acad Sci U S A. 2011;108:5903–08. doi: 10.1073/pnas.1016939108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavie N. Distracted and confused?: Selective attention under load. Trends Cogn Sci. 2005;9:75–82. doi: 10.1016/j.tics.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Lavie N, De Fockert J. The role of working memory in attentional capture. Psychonom Bull Rev. 2005;12:669–674. doi: 10.3758/bf03196756. [DOI] [PubMed] [Google Scholar]
- Lavie N, Fox E. The role of perceptual load in negative priming. Journal of Experimental Psychology-Human Perception and Performance. 2000;26:1038–052. doi: 10.1037//0096-1523.26.3.1038. [DOI] [PubMed] [Google Scholar]
- Lepsien J, Nobre AC. Cognitive control of attention in the human brain: Insights from orienting attention to mental representations. Brain Research. 2006;1105:20–31. doi: 10.1016/j.brainres.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Loftus E. Our changeable memories: Legal and practical implications. Nat Rev Neurosci. 2003;4:231–34. doi: 10.1038/nrn1054. [DOI] [PubMed] [Google Scholar]
- Macrae CN, Moran JM, Heatherton TF, Banfield JF, Kelley WM. Medial prefrontal activity predicts memory for self. Cereb Cortex. 2004;14:647–654. doi: 10.1093/cercor/bhh025. [DOI] [PubMed] [Google Scholar]
- Marois R, Ivanoff J. Capacity limits of information processing in the brain. Trends Cogn Sci. 2005;9:296–305. doi: 10.1016/j.tics.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Mather M. Emotional arousal and memory binding: An object-based framework. Perspectives on Psychological Science. 2007;2:33–52. doi: 10.1111/j.1745-6916.2007.00028.x. [DOI] [PubMed] [Google Scholar]
- Mayes A, Montaldi D, Migo E. Associative memory and the medial temporal lobes. Trends Cogn Sci. 2007;11:126–135. doi: 10.1016/j.tics.2006.12.003. [DOI] [PubMed] [Google Scholar]
- McCandliss BD, Cohen L, Dehaene S. The visual word form area: Expertise for reading in the fusiform gyrus. Trends Cogn Sci. 2003;7:293–99. doi: 10.1016/s1364-6613(03)00134-7. [DOI] [PubMed] [Google Scholar]
- McDuff SGR, Frankel HC, Norman KA. Multivoxel pattern analysis reveals increased memory targeting and reduced use of retrieved details during single-agenda source monitoring. J Neurosci. 2009;29:508–516. doi: 10.1523/JNEUROSCI.3587-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miller BT, Verstynen T, Johnson MK, D’Esposito M. Prefrontal and parietal contributions to refreshing: An rTMS study. NeuroImage. 2007;39:436–440. doi: 10.1016/j.neuroimage.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Mitchell JF, Sundberg KA, Reynolds JH. Spatial Attention Decorrelates Intrinsic Activity Fluctuations in Macaque Area V4. Neuron. 2009;63:879–888. doi: 10.1016/j.neuron.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima Y, Akaishi R, Yamada Y, Okuda J, Toma K, Sakai K. Task-specific signal transmission from prefrontal cortex in visual selective attention. Nat Neurosci. 2009;12:85–91. doi: 10.1038/nn.2237. [DOI] [PubMed] [Google Scholar]
- Murray EA, Bussey TJ, Saksida LM. Visual perception and memory: A new view of medial temporal lobe function in primates and rodents. Annu Rev Neurosci. 2007;30:99–122. doi: 10.1146/annurev.neuro.29.051605.113046. [DOI] [PubMed] [Google Scholar]
- Naselaris T, Prenger RJ, Kay KN, Oliver M, Gallant JL. Bayesian Reconstruction of Natural Images from Human Brain Activity. Neuron. 2009;63:902–915. doi: 10.1016/j.neuron.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee DE, Jonides J. Common and distinct neural correlates of perceptual and memorial selection. NeuroImage. 2009;45:963–975. doi: 10.1016/j.neuroimage.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, Cohen AL, Power JD, Wig GS, Miezin FM, Wheeler ME, Velanova K, Donaldson DI, Phillips JS, Schlagger BL, Petersen SE. A parcellation scheme for human left lateral parietal cortex. Neuron. 2010;67:156–70. doi: 10.1016/j.neuron.2010.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto S, Vu AT, Naselaris T, Benjamini Y, Yu B, Gallant JL. Reconstructing visual experiences from brain activity evoked by natural movies. Current Biology. 2011;21:1641–46. doi: 10.1016/j.cub.2011.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobre AC, Coull JT, Maquet P, Frith CD, Vandenberghe R, Mesulam MM. Orienting attention to locations in perceptual versus mental representations. J Cogn Neurosci. 2004;16:363–373. doi: 10.1162/089892904322926700. [DOI] [PubMed] [Google Scholar]
- O’Craven KM, Kanwisher N. Mental imagery of faces and places activates corresponding stiimulus-specific brain regions. J Cogn Neurosci. 2000;12:1013–023. doi: 10.1162/08989290051137549. [DOI] [PubMed] [Google Scholar]
- O’Craven KM, Downing PE, Kanwisher N. fMRI evidence for objects as the units of attentional selection. Nature. 1999;401:584–87. doi: 10.1038/44134. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Beer JS, Robertson ER, Cooper JC, Gabrieli JDE, Kihsltrom JF, D’Esposito M. The neural correlates of direct and reflected self-knowledge. NeuroImage. 2005;28:797–814. doi: 10.1016/j.neuroimage.2005.06.069. [DOI] [PubMed] [Google Scholar]
- Olivers CNL, Peters J, Houtkamp R, Roelfsema PR. Different states in visual working memory: When it guides attention and when it does not. Trends Cogn Sci. 2011;15:327–334. doi: 10.1016/j.tics.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Olson IR, Moore KS, Stark M, Chatterjee A. Visual working memory is impaired when the medial temporal lobe is damaged. J Cogn Neurosci. 2006;18:1087–097. doi: 10.1162/jocn.2006.18.7.1087. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Rugg MD. When more means less: neural activity related to unsuccessful memory encoding. Curr Biol. 2001;11:1528–530. doi: 10.1016/s0960-9822(01)00454-7. [DOI] [PubMed] [Google Scholar]
- Park H, Uncapher MR, Rugg MD. Effects of study task on the neural correlates of source encoding. Learn Mem. 2008;15:417–425. doi: 10.1101/lm.878908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Intraub H, Yi D, Widders D, Chun MM. Beyond the edges of a view: Boundary extension in human scene-selective visual cortex. Neuron. 2007;54:335–342. doi: 10.1016/j.neuron.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Park S, Chun MM. Different roles of the parahippocampal place area (PPA) and retrosplenial cortex (RSC) in scene perception. Neuroimage. 2009;47:1747–1756. doi: 10.1016/j.neuroimage.2009.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashler HE. The psychology of attention. MIT Press; 1998. [Google Scholar]
- Phelps EA. Emotion and cognition: Insights from studies of the human amygdala. Annu Rev Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Polyn SM, Natu VS, Cohen JD, Norman KA. Neuroscience: Category-specific cortical activity precedes retrieval during memory search. Science. 2005;310:1963–66. doi: 10.1126/science.1117645. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK, Fiske F, Kazdin K, Schacter S. Research on attention networks as a model for the integration of psychological science. Annu Rev Psychol. 2007;58:1–23. doi: 10.1146/annurev.psych.58.110405.085516. [DOI] [PubMed] [Google Scholar]
- Posner MI, Snyder CR, Davidson BJ. Attention and the detection of signals. Journal of Experimental Psychology: General. 1980;109:160–174. [PubMed] [Google Scholar]
- Potter MC. Short-term conceptual memory for pictures. Journal of Experimental Psychology: Human Learning & Memory. 1976;2:509–522. [PubMed] [Google Scholar]
- Puce A, Allison T, Gore JC, McCarthy G. Face-sensitive regions in human extrastriate cortex studied by functional MRI. Journal of Neurophysiology. 1995;74:1192–99. doi: 10.1152/jn.1995.74.3.1192. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. P Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C. A unified framework for the functional organization of the medial temporal lobes and the phenomenology of episodic memory. Hippocampus. 2010;20:1263–290. doi: 10.1002/hipo.20852. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Cohen MX, Brozinsky CJ. Working memory maintenance contributes to long-term memory formation: neural and behavioral evidence. J Cogn Neurosci. 2005;17:994–1010. doi: 10.1162/0898929054475118. [DOI] [PubMed] [Google Scholar]
- Raye CL, Johnson MK, Mitchell KJ, Greene EJ, Johnson MR. Refreshing: A minimal executive function. Cortex. 2007;43:135–145. doi: 10.1016/s0010-9452(08)70451-9. [DOI] [PubMed] [Google Scholar]
- Raye CL, Johnson MK, Mitchell KJ, Reeder JA, Greene EJ. Neuroimaging a single thought: Dorsolateral PFC activity associated with refreshing just-activated information. NeuroImage. 2002;15:447–453. doi: 10.1006/nimg.2001.0983. [DOI] [PubMed] [Google Scholar]
- Rissman J, Greely HT, Wagner AD. Detecting individual memories through the neural decoding of memory states and past experience. Proc Natl Acad Sci U S A. 2010;107:9849–854. doi: 10.1073/pnas.1001028107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock I, Gutman D. The effect of inattention on form perception. Journal of Experimental Psychology: Human Perception & Performance. 1981;7:275–285. doi: 10.1037//0096-1523.7.2.275. [DOI] [PubMed] [Google Scholar]
- Roediger HL., III Implicit memory: Retention without remembering. Am Psychol. 1990;45:1043–056. doi: 10.1037//0003-066x.45.9.1043. [DOI] [PubMed] [Google Scholar]
- Roediger HL, III, Karpicke JD. Test-enhanced learning: Taking memory tests improves long-term retention. Psychol Sci. 2006;17:249–255. doi: 10.1111/j.1467-9280.2006.01693.x. [DOI] [PubMed] [Google Scholar]
- Roth JK, Johnson MK, Raye CL, Constable RT. Similar and dissociable mechanisms for attention to internal versus external information. NeuroImage. 2009;48:601–08. doi: 10.1016/j.neuroimage.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Buckner RL. Priming and the brain. Neuron. 1998;20:185–195. doi: 10.1016/s0896-6273(00)80448-1. [DOI] [PubMed] [Google Scholar]
- Shimamura AP. Hierarchical relational binding in the medial temporal lobe: The strong get stronger. Hippocampus. 2010;20:1206–216. doi: 10.1002/hipo.20856. [DOI] [PubMed] [Google Scholar]
- Shimamura AP. Episodic retrieval and the cortical binding of relational activity. Cogn Affect Behav Neurosci. 2011;11:277–291. doi: 10.3758/s13415-011-0031-4. [DOI] [PubMed] [Google Scholar]
- Shimamura AP, Jurica PJ, Mangels JA, Gershberg FB, Knight RT. Susceptibility to memory interference effects following frontal lobe damage: Findings from tests of paired-associate learning. J Cogn Neurosci. 1995;7:144–152. doi: 10.1162/jocn.1995.7.2.144. [DOI] [PubMed] [Google Scholar]
- Simons DJ, Chabris CF. Gorillas in our midst: sustained inattentional blindness for dynamic events. Perception. 1999;28:1059–074. doi: 10.1068/p281059. [DOI] [PubMed] [Google Scholar]
- Simons JS, Davis SW, Gilbert SJ, Frith CD, Burgess PW. Discriminating imagined from perceived information engages brain areas implicated in schizophrenia. NeuroImage. 2006;32:696–703. doi: 10.1016/j.neuroimage.2006.04.209. [DOI] [PubMed] [Google Scholar]
- Simons JS, Peers PV, Hwang DY, Ally BA, Fletcher PC, Budson AE. Is the parietal lobe necessary for recollection in humans? Neuropsychologia. 2008;46:1185–191. doi: 10.1016/j.neuropsychologia.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Smith ML, Leonard G, Crane J, Milner B. The effects of frontal- or temporal-lore lesions on susceptibility to interference in spatial memory. Neuropsychologia. 1995;33:275–285. doi: 10.1016/0028-3932(94)00120-e. [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, et al. Correspondence of the brain’s functional architecture during activation and during rest. PNAS. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn M, Goode A, Stenger VA, Carter CS, Anderson JR. Competition and representation during memory retrieval: Roles of the prefrontal cortex and the posterior parietal cortex. Proc Natl Acad Sci U S A. 2003;100:7412–17. doi: 10.1073/pnas.0832374100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Wixted JT. The cognitive neuroscience of human memory since H.M. Annu Rev Neurosci. 2011;34:259–288. doi: 10.1146/annurev-neuro-061010-113720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Differential encoding mechanisms for subsequent associative recognition and free recall. J Neurosci. 2006;26:9162–172. doi: 10.1523/JNEUROSCI.2877-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield JJ, Lepsien J, Gitelman DR, Mesulam MM, Nobre AC. Orienting attention based on long-term memory experience. Neuron. 2006;49:905–916. doi: 10.1016/j.neuron.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Suzuki WA. Perception and the Medial Temporal Lobe: Evaluating the Current Evidence. Neuron. 2009;61:657–666. doi: 10.1016/j.neuron.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Tambini A, Ketz N, Davachi L. Enhanced Brain Correlations during Rest Are Related to Memory for Recent Experiences. Neuron. 2010;65:280–290. doi: 10.1016/j.neuron.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proc Natl Acad Sci U S A. 1997;94:14792–97. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper SP. The negative priming effect: inhibitory priming by ignored objects. The Quarterly journal of experimental psychology. A, Human experimental psychology. 1985;37:571–590. doi: 10.1080/14640748508400920. [DOI] [PubMed] [Google Scholar]
- Tootell RB, Reppas JB, Dale AM, Look RB, Sereno MI, Malach R, Brady TJ, Rosen BR. Visual motion aftereffect in human cortical area MT revealed by functional magnetic resonance imaging [see comments] Nature. 1995;375:139–141. doi: 10.1038/375139a0. [DOI] [PubMed] [Google Scholar]
- Treisman A, Schmidt H. Illusory conjunctions in the perception of objects. Cognitive Psychology. 1982;14:107–141. doi: 10.1016/0010-0285(82)90006-8. [DOI] [PubMed] [Google Scholar]
- Tse D, Langston RF, Kakeyama M, Bethus I, Spooner PA, Wood ER, Witter MP, Morris RGM. Schemas and memory consolidation. Science. 2007;316:76–82. doi: 10.1126/science.1135935. [DOI] [PubMed] [Google Scholar]
- Tulving E. Subjective organization in free recall of—unrelated words. Psychological Review. 1962;xx:xx–xx. doi: 10.1037/h0043150. [DOI] [PubMed] [Google Scholar]
- Tulving E, Schacter DL. Priming and human memory systems. Science. 1990;247:301–06. doi: 10.1126/science.2296719. [DOI] [PubMed] [Google Scholar]
- Turk-Browne NB, Yi D, Chun MM. Linking implicit and explicit memory: Common encoding factors and shared representations. Neuron. 2006;49:917–927. doi: 10.1016/j.neuron.2006.01.030. [DOI] [PubMed] [Google Scholar]
- Uncapher MR, Otten LJ, Rugg MD. Episodic Encoding Is More than the Sum of Its Parts: An fMRI Investigation of Multifeatural Contextual Encoding. Neuron. 2006;52:547–556. doi: 10.1016/j.neuron.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uncapher MR, Rugg MD. Effects of divided attention on fMRI correlates of memory encoding. Journal of Cognitive Neuroscience. 2005;17:1923–935. doi: 10.1162/089892905775008616. [DOI] [PubMed] [Google Scholar]
- Uncapher MR, Rugg MD. Fractionation of the component processes underlying successful episodic encoding: A combined fMRI and divided-attention study. Journal of Cognitive Neuroscience. 2008;20:240–54. doi: 10.1162/jocn.2008.20026. [DOI] [PubMed] [Google Scholar]
- Uncapher MR, Hutchinson JB, Wagner AD. A roadmap to brain mapping: Toward a functional map of human parietal cortex. Neuron. 2010;67:5–8. doi: 10.1016/j.neuron.2010.06.025. [DOI] [PubMed] [Google Scholar]
- Uncapher MR, Hutchinson JB, Wagner AD. Dissociable effects of top-down and bottom-up attention during episodic encoding. Journal of Neuroscience. 2011;31:12613–28. doi: 10.1523/JNEUROSCI.0152-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Memory retrieval and the parietal cortex: A review of evidence from a dual-process perspective. Neuropsychologia. 2008;46:1787–799. doi: 10.1016/j.neuropsychologia.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wais PE, Rubins MT, Boccanfuso J, Gazzaley A. Neural mechanisms underlying the impact of visual distraction on retrieval of long-term memory. Journal of Neuroscience. 2010;30:8541–50. doi: 10.1523/JNEUROSCI.1478-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL. Building memories: Remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Nńñez JE, Sasaki Y. Perceptual learning without perception. Nature. 2001;413:844–48. doi: 10.1038/35101601. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nature Neuroscience. 2006;9(7):971–78. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Petersen SE, Buckner RL. Memory’s echo: Vivid remembering reactivates sensory-specific cortex. Proc Natl Acad Sci U S A. 2000;97:11125–29. doi: 10.1073/pnas.97.20.11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggs CL, Martin A. Properties and mechanisms of perceptual priming. Current Opinion in Neurobiology. 1998;8:227–23. doi: 10.1016/s0959-4388(98)80144-x. [DOI] [PubMed] [Google Scholar]
- Wojciulik E, Kanwisher N, Driver J. Covert visual attention modulates face-specific activity in the human fusiform gyrus: fMRI study. Journal of Neurophysiology. 1998;79:1574–78. doi: 10.1152/jn.1998.79.3.1574. [DOI] [PubMed] [Google Scholar]
- Yi D, Turk-Browne NB, Chun MM, Johnson MK. When a thought equals a look: Refreshing enhances perceptual memory. J Cogn Neurosci. 2008;20:1371–380. doi: 10.1162/jocn.2008.20094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi D, Woodman GF, Widders D, Marois P, Chun MM. Neural fate of ignored stimuli: Dissociable effects of perceptual and working memory load. Nat Neurosci. 2004;7:992–96. doi: 10.1038/nn1294. [DOI] [PubMed] [Google Scholar]
- Yi DJ, Chun MM. Attentional modulation of learning-related repetition attenuation effects in human parahippocampal cortex. J Neurosci. 2005;25:3593–3600. doi: 10.1523/JNEUROSCI.4677-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanto TP, Rubens MT, Thangavel A, Gazzaley A. Causal role of the prefrontal cortex in top-down modulation of visual processing and working memory. Nat Neurosci. 2011;14:656–663. doi: 10.1038/nn.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]


