Abstract
Integrins are the principle cell adhesion receptors that mediate leukocyte migration and activation in the immune system. These receptors signal bidirectionally through the plasma membrane in pathways referred to as “inside-out” and “outside-in” signaling. Each of these pathways is mediated by conformational changes to the integrin structure. Such changes allow high affinity binding of the receptor with counter-adhesion molecules on the vascular endothelium or extracellular matrix, and lead to association of the cytoplasmic tails of the integrins with intracellular signaling molecules. Leukocyte functional responses resulting from outside-in signaling include migration, proliferation, cytokine secretion and degranulation. Here we review the key signaling events that occur in the inside-out versus outside-in pathways, highlighting recent advances in our understanding of how integrins are activated by a variety of stimuli and how they mediate a diverse array of cellular responses.
Keywords: inside-out, outside-in, kinases, Rap GTPase, ITAM
INTRODUCTION
At sites of inflammation or infection, leukocytes exit the vasculature through a cascade of events that involve a series of adhesion and homing receptors (1). Integrins play a central role in this cascade, mediating firm arrest of leukocytes on the inflamed endothelium and coordinating transmigration through the basement membrane to allow homing to the site of infection or inflammation.
Integrins are cell surface receptors composed of α and β chain heterodimers of type I transmembrane glycoproteins with short cytoplasmic tails. There are 18 different α and 8 different β subunits, which associate in pairs to form at least 24 distinct αβ receptors (2). Common integrins expressed on leukocytes include leukocyte function-associated antigen 1 (LFA-1 or αLβ2), Mac-1 (αMβ2) and very late antigen 4 (VLA-4 or α4β1). The ligands (or counter- receptors) for LFA-1 are the intercellular adhesion molecules (ICAMs 1–5) that are expressed primarily on vascular endothelial cells. Mac-1 recognizes a more diverse set of ligands including extracellular matrix proteins such as fibrinogen and fibronectin as well as activated complement proteins such as iC3b. VLA-4 recognizes the vascular intercellular adhesion molecule, VCAM-1, while the major integrin on platelets, αIIbβ3, binds primarily fibrinogen. In leukocytes these integrins are involved in slow rolling, adhesion strengthening, transendothelial migration and transmit signals that facilitate respiratory burst, complement-mediated phagocytosis, cytokine production, proliferation, survival, differentiation, degranulation and cellular polarization. In platelets, integrins are required for stable clot formation and hemostasis. From a cell biologic point of view, the primary function of integrins is to serve as a link between the actin cytoskeleton of the cell (or platelet) and the extracellular matrix.
The key role integrins play in immune function is evidenced by several human conditions where defects in integrin expression or function occur. These conditions are collectively known as leukocyte adhesion deficiencies (LAD) and summarized in Table 1. In mice, all the leukocyte integrins have been knocked out, and several floxed alleles have been reported. In addition, mice containing specific point mutations in different integrins have been engineered either to study the role of specific pathways in vivo, or to try and mimic human disease (those discussed in the text are summarized in Table 2).
Table 1.
Human genetic mutations associated with leukocyte adhesion deficiency.
| Syndrome | Phenotype | Defect | Mutations | References |
|---|---|---|---|---|
| LADI | recurrent bacterial infections, impaired wound healing, defects in multiple cell types | loss or reduced β2 on cell surface, defects in β2 signaling; mutations in β2 | http://bioinf.uta.fi/ITGB2base/ | (107) |
| LADII | infections (periodontitis), decreased chemotaxis and neutrophilia | mutations in selectin glycosylation (absence of SLeX) | mutations found in Golgi guanosine diphosphate (GDP)-fucose transporter | (108) |
| LADIII (variant LADI) | recurrent bacterial infections, impaired wound healing, defects in multiple cell types | defects in GPCR-mediated activation of multiple integrins | mutations in CalDAG GEFI identified | (109) |
| Glanzmanns Thrombasthenia | autosomal recessive bleeding disorder | mutations in αIIb or β3, platelet defects | http://sinaicentral.mssm.edu/intranet/research/glanzmann | (110) |
| Wiskott Aldrich Syndrome | increased susceptibility to pathogens, defects in various immune cell types | mutations in WASp | http://homepage.mac.com/kohsukeimai/wasp/WASPbase.html | (104) |
Table 2.
Engineered mouse mutants used to study integrin function as discussed in the text.
| Integrin | Pairs with | Mouse model | Phenotype | References |
|---|---|---|---|---|
| β1 | α1–11, αV | Knockout | Embryonic lethal | (111) |
| Floxed-β1 × Mx-Cre | normal hematopoietic development, mild delay in platelet responses | (26) | ||
| Y763A, Y775A | phenocopies β1 knockout | (43, 44) | ||
| Y763F, Y775F | no gross phenotype, mild defect in outside-in integrin signaling in platelets | (43, 44) | ||
| D739A | no phenotype | (43) | ||
| β2 | αL, M, X, D | Gene targeting, hypermorph | viable, fertile, mild granulocytosis | (112) |
| Knockout | viable but chronic dermatitis, T cell defects, increased neutrophil number, leukocytosis, increased susceptibility to bacterial infections | (113) | ||
| β3 | αV, αIIb | Knockout | viable but platelet defects and osteosclersosis, pathological bleeding | (114) |
| Y747F, Y759F | selectively disrupts outside-in signaling | (45) | ||
| Y747A | disrupts binding of talin, filamin and other proteins, disrupts inside-out signaling | (34) | ||
| L746A | disrupts binding with talin specifically, disrupts inside-out signaling, protection from pathological bleeding | (34) | ||
| α4 | β1, 7 | Knockout | embryonic lethal | (115) |
| Y991A | disrupts binding with paxillin, mice viable and fertile, impaired mononuclear cell recruitment in thioglycollate-induced peritonitis | (20) | ||
| Floxed-α4 × Mx-Cre | defects in hematopoiesis | (116) | ||
| αIIb | α3 | no KO made but treatment with blocking antibodies that don’t block αVβ3 show same phenotype as β3 null | (117) | |
| αL (CD11a) | β2 | Knockout | viable, defect in leukocyte adhesion and migration (decrease in airpouch model) | (118) |
| Deletion of GFFKR | constitutively activated integrin, impaired de-adhesion and migration, impaired T cell activation and neutrophil chemotaxis | (119) | ||
| αM (CD11b) | β2 | Knockout | defect in neutrophil binding and degranulation, but no effect on neutrophil emigration | (120) |
INSIDE-OUT SIGNALING
Inside-out signaling is defined as those events that induce conformational changes in the integrin leading to increased ligand binding affinity (“integrin activation”) and clustering of integrins in the membrane, which together result in “avidity modulation” allowing cell attachment. Circulating leukocytes generally maintain their integrins in a non-adhesive state in which the integrin ectodomains are held in a bent or folded conformation that restricts their ability to bind ligands. Chemoattractants or cytokines stimulate receptors leading to rapid integrin activation and clustering. In lymphocytes, integrin avidity is also modulated by stimulation through the B cell receptor (BCR) and T cell receptor (TCR). Signaling through other receptors, such as CD14 on monocytes and CD40 on B cells, also affects integrin-mediated adhesion. The first step in integrin activation is separation of the cytoplasmic tails. This ultrastructural change is relayed throughout the length of the integrin and results in unfolding of the ectodomain of the receptor (2). The extended conformation of the integrin becomes a high affinity receptor for its ligand and facilitates clustering of the extended receptors at the cell surface. This process enables leukocytes to rapidly adhere and de-adhere to vascular endothelium during inflammatory cell responses. Experimentally, inside-out signaling leading to integrin activation can be measured by binding of activation state-specific antibodies or attachment to ligands such as ICAM. Integrin affinity modulation is also involved with the initial stages of leukocyte rolling on vascular endothelium (1).
Analysis of integrin mutants that either cause constitutive integrin activation or failure to bind ligands, along with structures obtained by x-ray crystallography and electron microscopy, have defined regions important in integrin activation (2, 3). It is clear from FRET analysis of αL and β2 that the cytoplasmic domains are close together in the resting inactive state, but spatial separation is detected upon integrin activation by a variety of intracellular signals including activation of protein kinase C (PKC), G-protein coupled receptors (GPCRs) or transfection of the talin head domain.
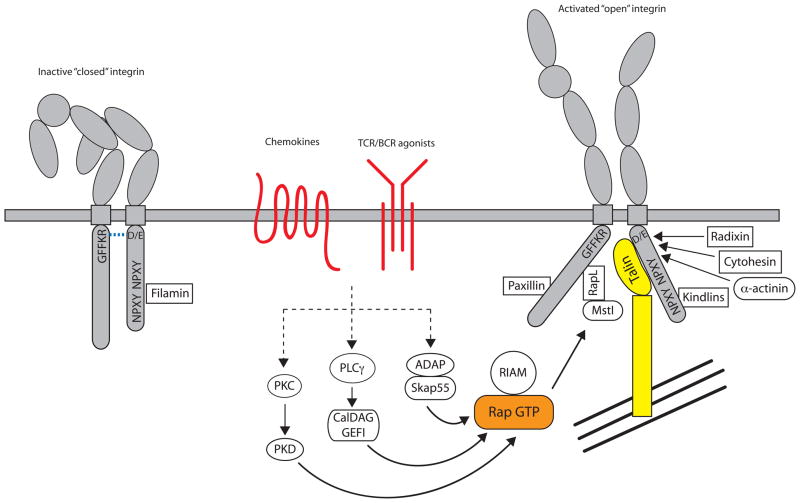
How do signals from divergent cell surface receptors converge to mediate these structural changes in integrins? The cytoplasmic tails of integrins are fairly short (Figure 1). α integrin cytoplasmic tails contain a conserved GFFKR amino acid sequence that is critical for a salt bridge-mediated interaction with the β chain; deletion or mutations of the GFFKR sequence lead to impaired association with the β chain and constitutive integrin activation both in vitro and in vivo (2). The β taiIs also contain amino acids important for the salt bridge with the α chain, and two NPXY/F motifs that mediate protein binding. Several phosphorylation sites have been identified in α and β tails and a number of proteins specifically associate with either tail (Figure 2).
Figure 1.
Alignment of transmembrane and cytoplasmic regions of leukocyte integrins. Data is taken from UniProt (www.uniprot.org), and amino acid numbering does not include the signal sequence. The solid orange box indicates the transmembrane domain predicted by the Hidden Markov model (121). The open red box indicates residues that were experimentally determined to be within the lipid bilayer (122). Residues boxed in yellow are important for the salt bridge linking the α and β integrin tails. The purple boxes indicate the two NPXY/F motifs in the β integrin tails.
Figure 2.
Integrin inside-out signaling. The figure outlines the key signaling events that occur downstream of chemokine, T and B cell receptors that lead to integrin activation. Inactive integrins exist in a bent conformation and the α and β cytoplasmic tails are held in close proximity by a salt bridge between residues found in the membrane proximal region of the tail. Activation of a variety of signaling pathways results in the recruitment of GTP-bound Rap1 and activated talin to the integrin, leading to tail separation. The conformational change in the cytoplasmic region is transmitted through the integrin transmembrane domain and results in structural changes in the extracellular region, leading to an open conformation that can bind ligand with high affinity. The C-terminal rod domain of talin interacts with the actin cytoskeleton, to provide physical coupling of the integrin to the actin network of the cell. Many other molecules have been shown to interact with integrin cytoplasmic tails, but exactly how these interactions are coordinated with integrin activation is unclear.
Rap GTPases in the inside-out pathway
The Rap GTPases have been implicated as major regulators of the inside-out pathway in lymphocytes (4). Rap1 and Rap2 are activated downstream of TCR/BCR or chemokine signaling, leading to LFA-1 and α4 integrin-dependent adhesion (5–7). Activation of either Rap1 or 2 in myeloid cells has not been reported. Sebzda et al made a transgenic mouse expressing a constitutively active form of Rap1 in T cells that leads to unregulated activation of LFA-1 (8). Expression of dominant negative Rap1 inhibits TCR-mediated LFA-1 activation in Jurkat T cells (9), while overexpression of the Rap1-specific GAP, SPA1, inhibits rapid ICAM-dependent adhesion of primary T cells to vascular endothelium (7). In B cells, expression of constitutively active Rap2 enhances B cell adhesion, whereas expression of a Rap-specific GAP leads to a reduction in LFA-1 and VLA-4 dependent adhesion (5). Mice that lack Rap1b show embryonic and perinatal lethality due to bleeding disorder, which may be attributable to poor activation of αIIbβ3 on platelets (10). Despite high similarity between Rap1a and Rap1b, mice that lack Rap1a are viable, but both lymphoid and myeloid cells manifest integrin-dependent adhesion defects (11, 12). Several GEFs that activate Rap GTPases have been identified; in particular modulation of CalDAG-GEF1 activity leads to changes in cell adhesion and migration (13). These GEFs are activated by a variety of upstream stimuli such as PKC or PKD activation, cAMP and calcium suggesting that multiple pathways can converge on Rap GTPases.
Proteins that interact with integrin α chains during inside-out signaling
The Rap effector RapL binds to a site consisting of two lysine residues following the GFFKR motif in the αL chain (14). As shown in Figure 1, only αL contains these lysine residues, so the role of RapL in activation of other integrins is unclear, although reduction of RapL protein affects VLA-4-mediated adhesion (15). Lymphocytes and dendritic cells isolated from RapL-deficient mice display reduced adhesion in response to chemokines, and homing and migration defects in vivo (16). RapL forms a complex with the serine/threonine kinase Mst1. Rap1 activation leads to recruitment of both RapL and Mst1 to LFA-1 and activation of Mst1 kinase activity. Mst1 activation is not seen in RapL-deficient cells. When Mst1 expression is knocked down using siRNA, integrin activation in response to TCR or chemokine stimulation is reduced (17).
Paxillin binds to a sequence within the α4 cytoplasmic tail; Y991A or E983A mutations within the α4 tail abolish this interaction (18). Binding is also blocked by phosphorylation of S988 within this sequence; mutation of this residue to a phosphomimetic amino acid, S988D, disrupts paxillin binding and causes increased spreading and decreased migration. Conversely, mutation of this residue to S988A results in enhanced binding of paxillin to α4 and reduced spreading (19). Mice expressing α4 that lacks the paxillin binding site due to a Y991A mutation are viable and fertile, in contrast to mice that lack α4, however they show impaired mononuclear cell recruitment in thioglycollate-induced peritonitis (20). These observations are consistent with an inhibitory role for paxillin association with α4 integrin.
Phosphorylation of integrin α chains can affect integrin activation, however the residues involved are found in human but not mouse integrins. Phosphorylation of the αL cytoplasmic tail occurs on S1140 in T cells stimulated either by antibody crosslinking of the TCR, or by treatment with phorbol ester. A mutant S1140A αL chain cannot be activated in T cells by chemokines or expression of constitutively active Rap1 (21). Integrin αM is constitutively phosphorylated on S1126 in human neutrophils, and when this site is mutated neutrophils can no longer be activated to bind ICAM-1 or 2 and fail to migrate into target organs in vivo (22).
Proteins that interact with integrin β chains during inside-out signaling
Talin is a large cytoskeletal protein consisting of head and rod domains that are separated by a calpain cleavage site. In human T cells, siRNA knockdown of talin leads to defects in TCR-mediated LFA-1 activation (23), while in CHO cells that express integrin αIIbβ3 reduced binding of an activation state-specific antibody is observed (24). In phagocytes, talin is required for complement-mediated, but not FcRγ-mediated phagocytosis; lack of talin impairs particle binding (25). Mice deficient in talin die during embryogenesis but platelets that lack talin (generated using a talinloxP strain, crossed with the Mx-cre line) fail to activate integrins in response to many different ligands; these mice are resistant to arterial thrombosis mediated by platelet β1 and β3 integrins (26, 27).
The head domain of talin contains a FERM domain that interacts with β1, β2, and β3 integrin cytoplasmic tails. The FERM domain contains three subdomains F1, F2 and F3. The F3 subdomain contains a phosphotyrosine binding (PTB)-like domain that binds to the membrane proximal NPXY/F motif found in β integrin cytoplasmic tails (28). Expression of the talin N-terminal head domain leads to increased ligand binding of several integrins including αMβ2 and αIIbβ3 (25, 29). However, the talin-integrin interaction is complex and requires “activation” of full length talin via mechanisms such as calpain-mediated cleavage, binding of phosphoinositol phosphates and phosphorylation (29).
Recent work has tried to accurately determine the nature of the talin-integrin interaction through structural studies. It has been suggested that talin constitutively associates with inactive integrins but its cleavage alters the binding in such a way that integrin tail unfolding occurs, initiating integrin activation. Talin then dissociates from the β integrin tail and α-actinin binds in its place (30). β3 integrins that contain mutations in the talin binding site fail to activate, although some of these mutants will also prevent binding of other proteins such as filamin (24). Association of any PTB-containing protein to the integrin NPXY motif alone is not sufficient to cause activation. Other regions of the talin head domain associate with more membrane proximal residues in the integrin tail with a lower affinity, and these disrupt the salt bridge between the α and β tails allowing integrin activation (31). These other requirements for talin binding may be distinct for different integrins, for example the F3 domain of talin by itself will activate β3 but not β1 integrins, where portions of the F1 head domain are also required (32). Hato et al found that a chimera consisting of β3 integrin with a β1 tail is constitutively active. Two mutations in β3 also lead to constitutive activation (I719M and E749S), and conversely reverse mutations in β1 inhibit its activation. The activity of the mutants is dependent on the presence of talin (33).
Confirmation that the talin interaction with β integrin tails controls inside-out signaling was demonstrated in knock-in mice expressing β3 integrin that contains point mutations in the cytoplasmic tail. A Y747A mutation disrupts binding of talin, filamin and several other binding proteins, while L746A disrupts binding of specifically talin (34). Platelets from both strains of mice show impaired agonist-induced fibrinogen binding and platelet aggregation, hallmarks of integrin inside-out signaling. When integrins are activated exogenously by addition of Mn2+, platelets are able to spread and initiate outside-in signals. In in vivo models of thrombosis, the Y746A mouse is resistant to pulmonary thromboembolism and ferric-chloride-induced vascular injury. Furthermore, this mouse does not show the anemia and gastrointestinal bleeding seen in β3-null mice suggesting that different functions of integrins could be targeted therapeutically.
Cytohesin-1, a GEF for ARF-GTPases, is predominantly expressed in hematopoietic cells where it interacts with the cytoplasmic tail of β2 integrin and regulates adhesion (35). Mutation of membrane proximal residues WKA723-725TRG of the β2 cytoplasmic tail abolishes binding. Expression of this mutant in a T cell line that lacks endogenous β2 integrins results in reduced adhesion to ICAM when compared to expression of wild type β2. The exchange activity of cytohesin-1 is not required for integrin activation as measured by binding of an integrin activation state-specific antibody, but is required for spreading suggesting that cytohesin plays different roles in inside-out versus outside-in integrin signaling (see below). Overexpression of cytohesin-1 leads to LFA-1-dependent arrest of T cells stimulated by SDF1α.
The actin-binding protein, α-actinin, binds to β2 integrin tails in neutrophils following activation (30). Experiments suggest that following talin activation, tail displacement opens up the α-actinin binding site in a membrane proximal region of the β2 tail, which may stabilize the integrin open conformation allowing for firm adhesion. α-actinin binding is not mediated by a PTB/NPXY interaction. Activation state-specific antibodies have demonstrated that α-actinin co-localizes with intermediate affinity extended integrins found at the leading edge of migrating T cells rather than high affinity integrins found at firm adhesion points (36). These results suggest there may be a cascade of protein interactions with integrin tails that stabilize different conformations during inside-out signaling.
Kindlin family members are FERM domain-containing proteins that interact with integrin β1 and β3 tails at the membrane distal NPXY motif i.e. a site distinct from the talin interaction domain. The FERM domain of the kindlins is most closely related to that of talin, however expression of the kindlin-2 FERM domain alone cannot activate integrins in the same way that talin does (37). Kindlins instead seem to work as coactivators of integrins with talin because co-expression of the talin and kindlin-2 FERM domains has a synergistic effect on integrin activation (38). Kindlin-1 and 2 are ubiquitously expressed whereas kindlin-3 is restricted to hematopoietic cells. Mutations in kindlin-1 are associated with skin disease; perhaps mutations in other kindlins could cause LAD. Kindlin-3-null mice die shortly after birth due to severe bleeding from a platelet dysfunction; inside-out αIIbβ3 integrin signaling in platelets is completely defective in these animals (39). Loss of kindlin-2 in mice results in peri-implantation lethality, but embryonic stem cells from these mice show decreased adhesion on a variety of integrin ligands (38).
Several other FERM domain-containing proteins have been described that bind to β integrin tails. Radixin, like talin, is held in a closed conformation by an intramolecular interaction. Expression of the head domain alone leads to increased adhesiveness of CHO cells containing αMβ2 and increased binding of an activation state-specific antibody. The interaction does not require either of the β2 tail NPXF motifs, instead it binds to a more membrane proximal region that overlaps with the cytohesin-1 binding site (40). Other FERM domain-containing proteins that interact with integrin β chains have been identified. These proteins may act in the same way as kindlins to promote integrin activation through talin or inhibit integrin activation by competing with talin. Examples include Myosin X, ICAP-1a, Dok1 and Numb (41, 42).
Several phosphorylation sites have been reported in β integrin tails. The tyrosine residues found in both NPXY motifs of β1 and β3 integrins are obvious targets. While binding of talin to integrins is phosphorylation-independent, other proteins show preference for phosphorylation of the tyrosine contained within the NPXY motif. Genetically engineered mice have been generated to address the role of the tyrosine residues contained within the NPXY motifs of the integrin β tail (Table 2). Mutation of the tyrosines to phenylalanines prevents phosphorylation of the tail but would not be expected to interfere with proteins that bind in a phosphorylation-independent manner, whereas mutation of the tyrosines to alanines would be expected to interfere with all NPXY motif-binding proteins. Mice that express β1 containing both Y783A and Y795A mutations phenocopy the β1 null phenotype implying that the NPXY motifs are critical for β1 integrin function. Mice that express β1 containing both Y783F and Y795F mutations do not show any obvious phenotype suggesting that tyrosine phosphorylation of these motifs is not required (43, 44). However, platelets from these mice do have mild spreading defects suggesting that tyrosine phosphorylation of the integrin β tails is important for outside-in signaling. Similar results were seen in mice containing analogous mutations in β3 integrin (Y772F,Y784F); platelets showed no defect in inside-out signaling but failed to aggregate (45). A Y772A mutation in the β3 cytoplasmic tail disrupts talin binding to the proximal NPXY motif and prevents integrin activation, but also disrupts binding of other PTB-containing proteins such as filamin. It is interesting to note that β2 integrins contain phenylalanine residues instead of tyrosine in both their NPXY/F motifs, and therefore would not be regulated by tyrosine phosphorylation. β2 chain phosphorylation on T758, which is the first of three threonine residues found between the two NPXF motifs, occurs in response to TCR stimulation and phorbol ester treatment and creates a binding site for 14-3-3 proteins (21). Mutation of this residue to alanine seems to affect cell spreading which may suggest an outside-in signaling defect, possibly linking integrins to Rac and Cdc42-mediated actin cytoskeletal changes. Filamin also binds to a region of the β2 tail that includes T758, but only when the threonine is unphosphorylated. Talin and filamin, and therefore 14-3-3 proteins, can all compete for the same binding site on β2 integrin tails (46).
The sequence of events in inside-out signaling
While it is clear that many proteins associate with integrin cytoplasmic tails and impact both inside-out and outside-in signaling, the kinetics of binding of all these proteins and the cascade of protein associations that lead to integrin conformational change and phosphorylation are poorly understood. Moreover, the pathways regulating inside-out signaling can be common to several integrins or specific to others depending on cellular context. Han et al used CHO cells expressing αIIb and β3 to reconstruct integrin inside-out signaling (47). They observed increased binding of an αIIbβ3 activation state-specific antibody in response to phorbol 12-myristate 13-acetate (PMA) if cells also expressed levels of talin and PKCα equivalent to levels found in platelets. Although talin is a substrate for PKC, phosphorylation of talin by PKC is not required for PMA-stimulated αIIbβ3 activation. The authors also found that activated Rap1 could substitute for PMA and PKCα. PKC (activated by PMA or through TCR stimulation) can activate and relocalize Rap1 by phosphorylating PKD1; PKD1 associates with Rap1 via its PH domain, which leads to membrane relocalization and association with the cytoplasmic tail of β1 integrin (48). Rap1 is activated by DAG and cytosolic calcium therefore is likely to be downstream of PLC. Indeed, TCR-mediated activation of LFA-1 and Rap1 is dependent on PLCγ (6). Strong evidence for a role of the Rap1 GEF, CalDAG-GEFI, in the activation of Rap1 has come from in vivo mouse and human data. Platelets from CalDAG-GEFI null mice showed greatly reduced activation of αIIbβ3 as measured by activation state-specific antibodies, and fail to aggregate (49). Neutrophils from these mice also show defects in Rap1 activation, and consequently β1 and β3 activation (13). These mice show similar defects as patients with LAD type III where mutations in CalDAG-GEFI were found (50).
The ability of Rap1 to activate αIIbβ3 is dependent on talin interacting with the β3 tail. Rap1 induces the formation of an “integrin activation complex” containing talin and the Rap1 effector, RIAM (Rap1-GTP-interacting adaptor molecule), which binds and activates αIIbβ3 (51). RIAM-mediated recruitment of talin unmasks the β3 integrin binding site in the talin F3 domain. RIAM also links Rap1 to ADAP and Skap55, which are essential for TCR-induced integrin activation (52). Downregulation of Skap55 by siRNA in T cells impairs TCR-mediated LFA-1 clustering and T cell-APC conjugation (53). T cells from Skap55-deficient mice show defects in β1 and β2 integrin adhesion, clustering and downstream functions. A similar phenotype is seen in mice lacking ADAP, which also lack Skap55 expression (54). Membrane targeting ADAP and Skap55 can increase T cell adhesiveness in the absence of TCR stimulation indicating that this complex may help to recruit active Rap1 to the membrane (55). These observations suggest that, in T cells, formation of a Rap1/RIAM/ADAP/Skap55 complex at the membrane is an early step in the pathway leading to talin activation and integrin tail unfolding. How this pathway intersects with PKC/PKD-mediated activation of Rap1 is unclear. Related molecules, such as the ADAP/Skap55 homologs PRAM-1 and SkapHom, may function in a similar fashion in myeloid cells.
Open questions in the inside-out pathway
In summary, it is clear that a pathway involving Rap GTPases and talin is critical for inside-out signaling but there are many unanswered questions. The requirement for the different pathway components downstream of classical immunoreceptors such as the TCR and BCR compared to GPCRs still needs to be clarified, for example the role of Rap GTPases and ADAP-RapL-RIAM proteins in myeloid cells downstream of chemokine receptors is unknown. Whether these pathways differ in their ability to activate different integrins is also unclear. For example, in human T cells, inactivation of Rap1 blocks SDF1α stimulated LFA-1-mediated binding to ICAM1, but not binding of VLA-4-mediated binding to VCAM. Similarly, silencing of CalDAG-GEFI inhibits SDF1α and PMA stimulated adhesion to ICAM but not VCAM. This data suggests that different pathways are used to activate LFA-1 and VLA-4 (56). However in B cells, inactivation of Rap2 decreases both LFA-1 and VLA-4-mediated adhesion to ligands (5). Experiments have suggested that different chemokines show specificity for activation of different integrins. Neutrophil chemotaxis to fMLP is dependent on Mac-1 and mediated by p38MAPKs, whereas IL-8 is dependent on LFA-1 and mediated by phosphatidylinositol-3-kinase (PI3K). In the presence of both chemokines, fMLP is dominant unless p38MAPKs are inhibited (57). Are both these pathways activating Rap GTPases to the same extent? There is recent evidence that Rap GTPases are also involved in the maintenance of basal integrin activity. Intermediate affinity VLA-4 is found on resting eosinophils, allowing attachment to the endothelium under shear flow, and this requires Rap GTPase activity, PLC and homeostatic calcium concentrations, but not p38MAPK (58). Lastly, if a similar pathway is activating integrins downstream of chemokines and TCR or BCR stimulation, is the signaling additive? This prompts the question that, in general, chemokines promote migration whereas TCR or BCR stimulation requires stable cell-cell contacts found in immunological synapses, so how are these two outcomes achieved? Imaging the localization of integrins at different stages of activation upon treatment with diverse stimuli could help to address this.
The pathway to clustering
Inside-out signaling pathways are known to stimulate integrin clustering, which contributes towards increased integrin avidity. It appears that this is particularly important for LFA-1, which undergoes dramatic relocalization in T and B cells during the formation of immunological synapses allowing cells to respond to much lower concentrations of ligand (59). It is unclear whether clustering is stimulated by the same signaling pathways as changes in integrin affinity, although stimuli that affect either clustering or affinity selectively have been observed (60). Luo et al identified mutations in the transmembrane domains of αIIb and β3 that selectively affect either affinity upregulation or clustering (61); molecules such as Skap55 are clearly required for clustering only (53). Upon PMA stimulation of T cells, LFA-1 is known to become more mobile within the lipid bilayer and is clustered in rafts. Clustering of LFA-1 into rafts alone using crosslinking antibodies against cholera toxin or raft-associated CD24 increases binding to ICAM, and this effect is dependent on PI3K activity (62). Cholesterol depletion prevents cholera toxin antibody- or PMA-mediated increases in integrin ligand binding, but importantly does not affect increased ligand binding mediated by extracellular Mg2+ (62, 63). More recently, single particle tracking has been used to determine that different integrin conformations have distinct diffusion profiles within the membrane implying that different activation states are uniquely tethered (64). LFA-1 may exist in clusters on some cells allowing them to respond more rapidly to stimuli. Cambi et al suggest that the presence of LFA-1 in nanoclusters allows monocytes to bind ICAM-1 whereas DCs cannot (65). Clearly, how integrin affinity modulation by inside-out signaling affects integrin clustering remains to be defined. Clustering represents the final steps in the inside-out pathway leading to a transition to outside-in signaling events that are more associated with leukocyte functional activation.
OUTSIDE-IN SIGNALING
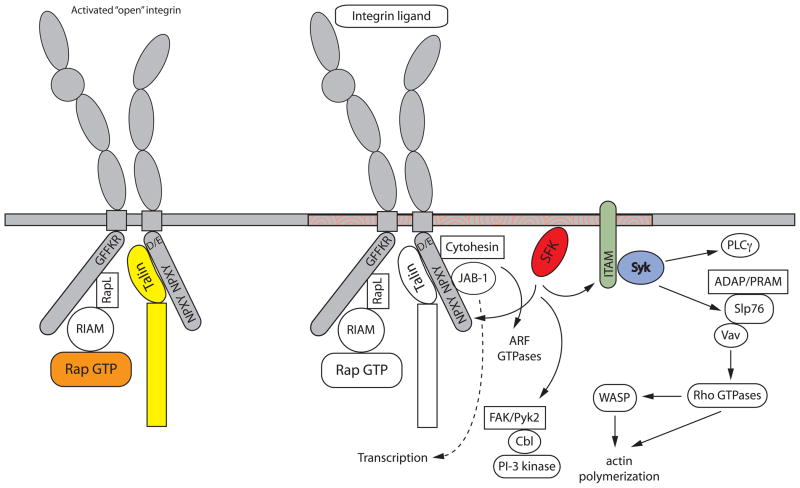
The signaling steps that occur following ligand-induced clustering of leukocyte integrins are referred to as the outside-in pathway (Figure 3). In leukocytes, much of the signaling from integrins themselves occurs in the context of other stimulatory events involved in inside-out pathways, such as engagement of the TCR or activation of GPCRs. Hence, it can be difficult to separate outside-in signaling events unique to the integrin versus those that are also induced by additional stimuli. In this regard, especially in lymphocytes, we often think of the outside-in pathway as being mainly an amplifier, or “co-stimulus” to other signaling reactions. However, it is now well defined in lymphocytes, myeloid cells and especially platelets that there are specific signaling pathways induced by integrin engagement that lead to well-defined functional responses of cells. Using agents that bypass inside-out signaling such as PMA or Mn2+ investigators can isolate outside-in signaling. For the sake of discussion, we will define outside-in signaling responses from integrins as those events that lead to firm cell adherence, cell spreading due to actin cytoskeletal rearrangement and aspects of cell migration. These cellular responses in turn lead to functional leukocyte activation. In T cells, integrin outside-in signaling responses include activation of proliferation, IL-2 secretion and stabilization of T cell/APC contacts. In neutrophils, integrin-induced signaling results in degranulation and activation of the NADPH oxidase, leading to ROS production, while in macrophages integrin signaling induces stabilization of cytokine messages, in particular IL-1β, and differentiation. Outside-in integrin signaling responses in platelets are classically studied by examination of filopodial and lamellipodial extensions that produce full spreading and firm adhesion, leading in vivo to thrombus formation (66).
Figure 3.
Integrin outside-in signaling. The figure details integrin-mediated signaling events that occur downstream of ligand binding. Zhu et al have shown that outside-in signaling requires structural changes with the cytoplasmic region of integrin tails (87). Activation of Src family kinases is a key step, although the exact mechanism by which this occurs is unclear, and results in phosphorylation of a variety of downstream molecules. These include ITAM-containing adapters that, when phosphorylated, lead to the recruitment and activation of Syk or ZAP-70 kinases. These kinases in turn phosphorylate various substrates including SLP76 and Vav. This lead us to propose that integrin outside-in signaling is analogous to signaling downstream of immunoreceptors, as indicated by the molecules in bold (85, 86). Vav activates Rho GTPases leading to actin cytoskeletal reorganization. Src family kinases can also activate FAK and Pyk2 kinases leading to Cbl phosphorylation and recruitment, as well as PI3K activation. Association of other molecules such as JAB and cytohesin with the integrin cytoplasmic tails activates other downstream signaling pathways. In this figure, signaling is depicted as happening in lipid rafts (indicated as orange swirls in the membrane), although the role of rafts in integrin signaling differs in various cell types.
Initiating events in the outside-in pathway
Perhaps the earliest biochemical event in the outside-in signaling response is activation of tyrosine kinases, particularly those of the Src and Syk families. Rapid activation of these enzymes has repeatedly been demonstrated in lymphocytes, myeloid cells and platelets following β1, β2 and β3 integrin engagement (67). The requirement for these kinases in mediating outside-in signaling responses has been most clearly defined using knockout mouse models, in particular in studies of myeloid cells and platelets where integrin signaling events can be clearly distinguished from other signaling events. Deficiency of the major Src-family kinases expressed in myeloid cells (Hck, Fgr and Lyn) produces a complete block in neutrophil degranulation and ROS production following engagement of β2 and β3 integrins. Similarly, firm adhesion, cell spreading and phosphorylation of specific downstream substrates (such as Vav, Cbl and other molecules described below) are defective in macrophages lacking Src-family kinases. Platelets isolated from src−/−hck−/−fgr−/−lyn−/− combinatorial mutant mice show complete defects in spreading responses following plating on fibrinogen-coated surfaces, which are mediated by the β3 integrin (66). That deficiency of these kinases affects primarily the outside-in versus inside-out integrin signaling pathways has been somewhat difficult to prove. Indeed, there are many examples in the literature of Src-family kinases being implicated in integrin activation events. In part, this distinction depends on the assays being used – for example classic leukocyte adhesion assays, which are generally assumed to reflect integrin affinity modulation required for attachment, may also reflect changes in outside-in pathways such as cell spreading and actin cytoskeletal rearrangement. Deficiency of Src-family kinases appears not to affect cell attachment following stimulation by fMLP, P- or E-selectin (68, 69). Instead, post-integrin receptor events leading to cell spreading, firm adhesion and resistance to shear force (i.e. sustained adhesion) are primarily lost in neutrophils isolated from mice lacking Hck, Fgr and/or Lyn kinase demonstrating that these kinases are involved in the outside-in pathway. In Jurkat T cells, it is easier to distinguish signaling events that are specifically mediated by β2 integrins using mAbs that directly crosslink and cluster the integrin. Such experiments result in formation of a focal area of polymerized actin (referred to as the actin cloud), which contains many of the adapters and downstream signaling molecules implicated in outside-in integrin signaling (70). Formation of this structure is dependent on the Src-kinase Lck in Jurkat cells suggesting that Lck functions predominately in the outside-in pathway since these experiments are done in the absence of agonists that would affect integrin affinity modulation. Ultimately, the question of whether loss of Src-family kinases affects the inside-out versus outside-in pathway will necessitate development of conformation-specific mAbs that recognize activated or clustered versions of the murine β2 or β3 integrins – such antibodies have been invaluable in the study of integrin activation in human leukocytes.
The second class of kinases implicated in the outside-in pathway are the Syk/ZAP-70 kinases – Syk in myeloid cells and platelets and ZAP-70 in T cells. Again, the strongest evidence implicating Syk/ZAP-70 comes from genetic studies. Deficiency of Syk kinase results in a complete block in β1, β2 and β3 integrin signaling events in neutrophils and macrophages (71). Similarly, Syk-deficient platelets manifest a complete block in firm adhesion and spreading over fibrinogen-coated surfaces (66, 72). In contrast, deficiency of this enzyme does not block GPCR signaling events in neutrophils or mast cells, supporting the notion that the integrin signaling defect resides within the outside-in pathway (73). Deficiency of ZAP-70 in T cells also abrogates signaling events from the β1 integrin in Jurkat T cells and can, in part, be separated from the signaling defects in CD3/TCR-mediated events caused by deficiency of this kinase (74).
While there is strong evidence that these kinases are required for leukocyte integrin signaling events leading to cellular activation (especially in myeloid cells), the role of these kinases in leukocyte migration, which is classically thought to be the primary function of integrins, is somewhat confused (67). While monocytes and macrophages lacking Src-family kinases or Syk display significant migratory defects both in vitro and in vivo, kinase-deficient neutrophils seem to migrate normally in both standard transwell chemotaxis assays and during migration into the inflamed peritoneum. In contrast, migration of neutrophils or eosinophils into the lung during inflammatory or allergic reactions is dependent on Src-family kinase activity (67). Similar differences seem to exist for Syk-deficient neutrophils. In the inflamed cremaster muscle model, syk−/− cells show profound defects in adhesion to the vessel wall and transmigration (75). In contrast, in the Schwartzman skin hemorrhagic vasculitis model, Syk-deficient (and Src-family kinase-deficient) neutrophils readily enter the inflammatory site, but fail to undergo Mac-1-mediated activation and hence cause very little tissue damage (76). Thus one is left with the conclusion that the requirement for integrin outside-in signaling during leukocyte migration is dependent on both the cell type and the inflammatory tissue site. Indeed, the recent report from Lammermann et al suggests that leukocyte integrins are not required at all for dendritic cell migration both in 3D collagen gel matrices in vitro as well as into lymph nodes and skin in vivo (77). Of course, it has long been recognized that at least β2 integrins are essential for neutrophil migration, since both mice and humans lacking this receptor present with LAD (Table 1 and 2). Hence, the notion that integrin outside-in signaling is a required component of leukocyte migration is too broad and needs to be assessed in the context of the specific type of inflammatory response involved.
The principle other tyrosine kinases implicated in leukocyte integrin signaling are FAK and its homolog Pyk2. Based on models in fibroblasts, these kinases are generally thought to act downstream of Src-family members – FAK has a number of tyrosine residues that are phosphorylated by Src and are required for FAK function. Since FAK deficiency results in early embryonic lethality, FAKloxP mice crossed to the LysM-cre strain have been used to study the effect of FAK deletion in myeloid cells. Macrophages lacking FAK fail to form stable lamellipodia and as a result have marked defects in directional chemotaxis and impaired migration into the peritoneum in vivo (78). Macrophages derived from pyk2−/− mice also exhibit altered cell polarization and diminished chemokine-induced motility, similar to the FAK-deficient cells (79), suggesting that these kinases may have partially overlapping functions in macrophage motility. In neutrophils, Pyk2 is implicated in integrin outside-in signaling using pharmacologic inhibitors or by transduction of inhibitory Tat-Pyk2 fusion peptides into human cells (80). Macrophages and neutrophils lacking Src-family or Syk kinases show impaired activation/phosphorylation of FAK and Pyk2 following integrin engagement, while the reverse tends not to be the case, supporting the notion that FAK and Pyk2 act further downstream in the integrin outside-in pathway (81). Further studies of the role of these kinases using newly available knockout mouse models are needed.
Coupling kinases to the integrins
As in other signaling cascades, it is believed that physical clustering of the integrins leads to clustering of associated Src and Syk kinases, which can phosphorylate each other and initiate the outside-in pathway. This raises the unanswered question of how the kinases become coupled to integrins. Most leukocyte integrins do not have any obvious interaction motifs for the various protein domains (such as SH2 or SH3) involved in protein kinase associations. However, direct interaction between both Src-family kinases and Syk kinase and the cytoplasmic domain of β2 and β3 integrins has been reported (82). In most cases, these interactions have been defined either by co-immunoprecipitation or by analysis of binding by recombinant proteins encompassing domains of the kinases and integrins. However, in CHO cells it is possible to observe physical interactions between the β3 tail and Src using FRET reporter methods (83). The interaction between the kinase and the integrin cytoplasmic tail is found in resting cells, and in the case of Syk, occurs independently of kinase activation. This supports the general model that resting, inactive integrin tails have low levels of associated Src and Syk kinases, and following integrin activation/clustering, the enzymes become activated (often recruiting more kinase molecules to the complex) and downstream signaling is initiated (66).
More recently, studies from several other groups have suggested that molecules containing immunoreceptor tyrosine-based activation motifs (ITAMs) are also involved in the coupling of kinases to integrins (72, 84). Neutrophils isolated from mice lacking the two ITAM adapter proteins FcRγ (the ITAM-containing signaling chain of the IgG and IgE Fc receptors) and DAP-12 (which is associated with a number of activating receptors in NK and myeloid cells) are deficient in β2 and β3 integrin signaling. The function of ITAM-containing molecules in classical immunoreceptor signaling pathways, such as the TCR, BCR or FcR, has been well defined; these proteins are phosphorylated by Src-family kinases and serve as docking sites for Syk or ZAP-70 which in turn initiate downstream signaling responses (85). Using retroviral-mediated gene transduction of hematopoietic stem cells, the two studies demonstrate that Syk SH2 domain binding to phosphorylated ITAMs is critical for outside-in signaling from β2 and β3 integrins in neutrophils and platelets, respectively. In this regard, initiation of the integrin outside-in pathway seems to mimic the steps involved in initiation of immunoreceptor signaling (86).
While at first glance it may appear that these models of initiation of outside-in signaling are conflicting, it is possible that both models represent different phases of the signal. Integrin-associated kinase activity may be involved in helping the integrin cytoplasmic tails open up, allowing association of ITAM adapters such as DAP-12 and FcRγ, which in turn amplify the signal leading to actin cytoskeletal rearrangement, cell spreading and activation of effector function. An obvious question in the ITAM model is how the signaling adapter becomes associated with the cytoplasmic tail of β2 or β3. Is the association constitutive in resting cells or is it induced in activated cells? Integrins lack transmembrane charged residues that are generally required for receptor association with DAP-12 or FcRγ and to date there is no genetic or biochemical evidence for direct association of the adapters with the integrins. An intriguing recent study from Zhu et al demonstrates that physical separation of the α and β tails of the αIIbβ3 integrin is required for initiation of intracellular outside-in signaling events (87). In this study, the investigators introduced a disulfide bond between the α and β chains which locks the transmembrane regions together. When expressed in CHO cells, this mutant integrin displays normal inside-out signaling responses in that it adopts an extended conformation and allows cell attachment to fibrinogen-coated surfaces. However, subsequent outside-in signaling steps of cell spreading, actin polymerization and focal adhesion formation are blocked. Other mutations in the integrin β3 tail have been defined which impair outside-in signaling specifically, though it is unclear if they affect structural changes in the cytoplasmic tails (88). It is interesting to speculate that this tail separation is required for association of ITAM-containing adapters with the integrin tail, to allow propagation and expansion of the outside-in signal.
Another potential mechanism of co-association of ITAM-containing proteins and kinases with leukocyte integrins may be through their joint recruitment to lipid raft membrane domains following adhesion. Disruption of lipid raft structures inhibits T cell adhesion via both LFA-1 and VLA-4 (63). The reverse is also true – enforced clustering of T cell lipid rafts will induce LFA-1 mediated adhesion (62). In contrast, lipid rafts seem not to be involved in αIIbβ3 integrin outside-in signaling in platelets (89) while treatment of neutrophils with methyl-β-cyclodextrin primes the cells for enhanced adhesion and integrin signaling (90). Clearly, the details in this area remain to be explored. Since the Src-family kinases are well known components of lipid raft domains, it is likely that some co-localization of ITAM adapters, integrins and potentially other downstream signaling molecules within these domains is contributing to the outside-in signal.
Recent observations have shown that these ITAM-containing adapters also play a role in signaling through the P/E-selectin receptor PSGL-1 to modulate integrin affinity states allowing the neutrophil to respond more rapidly to endothelial-expressed chemokines (91). The homology between the signaling events involved in PSGL-1-mediated integrin activation and subsequent integrin-mediated functional responses in leukocytes is perhaps the best example of the overlap between inside-out and outside-in signaling pathways.
Downstream proteins in the outside-in pathway
A number of adapter proteins that serve as scaffolds for protein-protein interactions have been implicated in integrin outside-in signaling. Perhaps the most well studied example is SLP-76. Deficiency of SLP-76 leads to a severe block in β2 and β3 integrin outside-in signaling events in neutrophils, dendritic cells and platelets (92). Mice lacking SLP-76 specifically within the myeloid lineage (using SLP-76loxP mutants crossed to the LysM-cre strain) are completely protected in the Schwartzman skin hemorrhagic vasculitis model, similar to mice lacking the myeloid Src-family kinases or Syk, demonstrating the in vivo significance of SLP-76 for integrin outside-in signaling. While SLP-76 is also implicated in immunoreceptor signaling, it is interesting that specific mutations within SLP-76, which affect its ability to bind downstream proteins such as Gads, can impair T cell receptor signaling without affecting integrin signaling. Likewise, targeting SLP-76 to membrane lipid rafts restores the ability of this adapter protein to function in the immunoreceptor pathway but does not restore normal β2 integrin signaling events in SLP-76−/− neutrophils (72). This suggests that differential association of downstream signaling molecules with SLP-76 may serve to distinguish immunoreceptor from integrin signaling responses in leukocytes.
The adapter protein ADAP and its myeloid specific homolog PRAM-1 have also been implicated in outside-in integrin signaling. The major function of ADAP in T cells is the activation of β1 and β2 integrins following engagement of the TCR, as described above. In neutrophils however, ADAP seems to play a role in the outside-in pathway, since ADAP- deficient cells show defects in adhesion-induced superoxide production (92). Similar results have been observed with PRAM-1-deficient neutrophils, suggesting an overlapping role for these two adapters in myeloid integrin outside-in pathways (93). The dual role of SLP-76, ADAP and its associated molecules in inside-out pathways in T cells, versus outside-in events in myeloid cells, illustrates the cell specificity of these signaling cascades.
Cytohesin-1 also appears to serve a dual function in T cell integrin signaling. Though initially studied in the inside-out pathway of integrin activation, as described above, direct involvement of cytohesin-1 in the outside-in pathway is suggested by the observation that engagement of LFA-1 in Jurkat T cells leads to threonine phosphorylation of cytohesin-1 while signaling through the TCR does not. Expression of a dominant negative form of cytohesin-1 blocks LFA-1-mediated ERK activation and impairs IL-2 production following costimulation of LFA-1 and the TCR (94).
JAB1, a co-activator of the c-Jun transcription factor, is implicated in LFA-1 outside-in signaling. JAB1 is found in both the nucleus and cytoplasm, where a fraction of the JAB1 pool associates with LFA-1. Engagement of LFA-1 causes dissociation of JAB1 from LFA-1 with a concomitant increase in the nuclear pool of JAB1, leading to formation of c-Jun complexes and activation of AP-1 transcriptional responses (94). Expression of dominant negative forms of JAB1 specifically block c-Jun phosphorylation and result in impaired IL-2 production, similar to the effects of cytohesin-1 inhibition.
In myeloid cells, the dual adapter and ubiquitin ligase protein c-Cbl has been implicated in integrin outside-in signaling responses through study of Cbl-deficient macrophages (95). Following engagement of integrin β2 in neutrophils or β1 in macrophages, c-Cbl is rapidly tyrosine phosphorylated by Src-family kinases and becomes associated with PI3K where it may assist in translocation of a fraction of intracellular PI3K to the membrane (96). Macrophages from c-Cbl−/− mice display reduced adhesion, poor podosome formation and impaired migratory responses (95). Hence, part of the role of Cbl in the outside-in pathway may involve its ability to bring PI3K to the membrane to generate lipid products needed to activate downstream signaling components.
PKC family members also function in both inside-out and outside-in pathways. Recently, both PKCβ and PKC θ have been implicated in αIIbβ3 outside-in signaling responses (97, 98). Platelets deficient in PKCβ or PKCθ do not spread on fibrinogen-coated surfaces, although agonist-induced binding of soluble fibrinogen occurs normally (indicating normal β3 integrin activation via inside-out pathways). Both PKC isoforms associate with β3 integrin; PKCβ following integrin ligand binding and PKCθ constitutively. The substrates of these PKC isoforms are unknown, and their roles in inside-out or outside-in pathways remains to be explained.
Connecting integrin outside-in signaling to the actin cytoskeleton
Following integrin clustering, a number of biochemical events are induced downstream that culminate in modulation of the actin cytoskeletal network. Many of these reactions function to modulate Rho GTPases. Activation of the Vav family of Rho-GEFs is a key step downstream of integrin clustering (99) and the most definitive studies have come from examination of Vav-family-deficient mice. Neutrophils lacking all three Vav family members show profound defects in β2 integrin signaling, including poor adhesion, spreading, and activation of oxidative burst, which mimics the phenotype of Src-family kinase or Syk-deficient cells (100). As predicted, β2-induced activation of Cdc42, Rac1, and RhoA is defective in Vav1/3-deficient neutrophils. Like Src or Syk kinase-deficient cells, chemokine-induced attachment and initial adhesion of Vav-deficient neutrophils to inflamed endothelium is unaffected, arguing that integrin affinity modulation is intact in these cells. However, because of impaired signaling events to the Rho GTPases and poor cell spreading, Vav-deficient neutrophils show an inability to maintain firm adhesion under shear flow conditions. The defective activation of oxidative burst activity as well as other integrin signaling events likely contributes to the severely compromised host defense against bacterial pneumonia seen in Vav-deficient mice (100). In a similar fashion, platelets lacking Vav1 and Vav3 show poor adhesion and spreading responses following engagement of αIIbβ3 (101).
Another major function of Vav family members is regulation of PLCγ isoforms and downstream Ca2+ signaling, particularly in immunoreceptor pathways in T cells and B cells (99). Recent studies in neutrophil integrin signaling show a similar role for Vav proteins; Vav-deficient neutrophils fail to activate PLCγ2, leading to poor Ca2+ responses which likely contribute to impaired activation of oxidative burst (100). This same phenotype is seen in Vav1/3-deficient platelets (101). These observations are supported by the fact that PLCγ2-deficient neutrophils and platelets show similar defects in integrin-mediated activation events as Vav-deficient cells (100, 101). The mechanism by which PLC isoforms modulate integrin signaling is unclear, but likely involves associations with SLP-76 and Vav proteins. As in the immunoreceptor pathway, integrin ligation induces a SLP-76/Vav/PLCγ complex that is required for PLCγ phosphorylation and activation. Mutations in SLP-76 that block this association also block integrin outside-in responses (92). An equally likely role for PLCγ is production of the lipid mediator IP3, which is known to have direct effects on Rho GTPase activation.
The primary effectors of the integrin outside-in signaling pathway leading to the actin cytoskeletal rearrangements needed for cell spreading and firm adhesion are the RhoGTPase family members – Rac, Rho and Cdc42. The literature on these GTPases in integrin signaling is vast, primarily in non-hematopoietic cells. Most studies of the RhoGTPases in leukocyte integrin signaling involve their role in polarization and migration following adhesion (102). A recent example involves phosphorylation of the α4 chain following VLA4 ligand binding. This releases paxillin and the GTPase ARF6 from the membrane, leading to accumulation of active Rac at the leading edge (103). Ultimately, the establishment of cell polarity and directed migration will be the sum of chemoattractant, mechanical (caused by shear stress in the vasculature) and integrin signals, which will obviously vary depending on the migratory site and the inflammatory stimulus involved.
Of the many effector molecules downstream of the RhoGTPases, the Wiskott-Aldrich syndrome protein (WASp) has been defined as playing a major role in integrin signaling in lymphocytes, myeloid cells and platelets (104). WASp is activated by a number of stimuli, such as association with GTP-bound Cdc42 and tyrosine phosphorylation. Activated WASp assumes an unfolded conformation that allows the molecule to interact with the Arp2/3 complex to nucleate actin polymerization. Lack of WASp, in both humans and mice, results in impaired leukocyte (and platelet) adhesion, poor spreading responses, reduced migration and impaired lymphocyte activation. WASp-deficiency results in poor clustering of β2 integrins in macrophages and dendritic cells, resulting in impaired podosome formation and reduced migration both in vitro and in vivo. Both mouse and human neutrophils lacking WASp show similar defects in β2 integrin clustering, leading to poor polarization following adhesion, impaired transendothelial migration in shear flow and reduced activation of degranulation/respiratory burst (105). At face value, reduced integrin clustering may sound more like an inside-out signaling impairment, however given that WASp is downstream of Cdc42 GTPase activation, we interpret this finding to indicate that the defective actin polymerization responses in the WASp-deficient leukocytes results in a reduced ability to form secondary adhesion structures (e.g. podosomes in mononuclear cells) needed for firm adhesion and spreading. In other words, loss of this distal step in outside-in signaling leads to a reduction of subsequent inside-out steps as leukocyte adhesion progresses suggesting that integrin signaling is really a circular pathway.
CONCLUSION
The bidirectional nature of integrin signaling, first envisioned by Richard Hynes (106), is now a firmly established fact. The conformational changes that β2 and β3 integrins undergo following activation by inside-out signals are becoming well understood at a molecular level. The diversity of signals originating from these receptors, when clustered by their respective ligands, is also being unraveled, cell type by cell type. In reality, the two phases of integrin signaling are almost certainly overlapping and it is often difficult to separate them experimentally. It is clear that some molecules are involved in the activation of different integrins in different cell types, such as talin and Rap GTPases, but inside-out pathways leading to LFA-1 activation in T cells are different from those that activate αIIbβ3 in platelets. Likewise, it is clear that many molecules appear to be involved in both inside-out and outside-in pathways; in many cases this results in a circular pathway that propagates signaling which is likely needed for rapid adhesion and cell spreading, particularly in response to intravascular shear stress. This type of regulation has recently been coined as an adhesion-strengthening function of integrins (1). In a general sense, for leukocytes, this dynamic regulation makes sense. Leukocyte adhesion needs to be carefully regulated to allow rapid migration to sites of infection/inflammation, to modulate immune responses in secondary lymph nodes, or in the case of platelets to quickly establish hemostasis. So given this multitude of functional requirements, it is not surprising that integrin signaling is complex. Yet general themes do exist in all integrins, such as the conformational changes in the cytoplasmic domains induced by binding of cytoplasmic proteins such as talin or the activation of tyrosine kinases as initiators of outside-in responses. If we are ever going to design therapeutics that target integrin signaling events (and given some of the initial positive clinical results with inhibitors that block integrin/ligand interactions, therapeutic modulation of integrin function may hold great clinical promise - see Text Box), then a greater understanding of how these events are regulated will be an area of active research in the future.
TEXT BOX. Integrin-directed therapies in the clinic.
There are several integrin antagonists used in clinical studies. These fall into three groups; humanized antibodies, synthetic peptides and non-peptide small molecules (reviewed in 123, 124). All target the extracellular region of integrins and interfere with ligand binding. Antibodies have been made to target several different integrins including Efalizumab (Raptiva®), which antagonizes αLβ2, and is used to treat psoriasis, and Natalizumab (Tysabri or Antegren®), which binds to the α4 chain and antagonizes α4β1 and α4b7 integrins, and is used to treat multiple sclerosis and Crohn’s disease. The antibodies MEDI-522 (Vitaxin or Abegrin) and CNTO95 target integrin αV and are currently begin tested as inhibitors of angiogenesis in several cancer trials (125). Abciximab (ReoPro®) is a Fab fragment that binds αIIbβ3 and is used to inhibit platelet aggregation. Cilengitide and Eptifibatide (Integrilin®) are cyclic RGD peptide mimetics that inhibit either αVβ3 and αVβ5 or αIIbβ3 respectively. Non-peptide inhibitors include Tirofiban (Aggrostat®) that targets αIIbβ3 and is also used to inhibit platelet aggregation, and Valategrast, a small molecule inhibitor of α4β1 for treatment of asthma. With the increased understanding of integrin conformational changes, other modulators of integrin function have been generated and are being evaluated in preclinical models. Whether inhibitors could be designed that target the intracellular portion of integrins, potentially distinguishing between different signaling pathways, has not yet been explored.
Acknowledgments
This work was supported by NIH grants AI068150 and AI065495. We apologize to all colleagues whose work we could not cite due to space limitations.
Definitions
- LFA1
leukocyte function-associated antigen 1, integrin αLβ2, CD11a/CD18
- Mac1
integrin αMβ2, CD11b/CD18
- VLA4
very late antigen 4, integrin α4β1
- LAD
leukocyte adhesion deficiency, encompasses a variety of human syndromes detailed in Table 1
- GEF
guanine nucleotide exchange factor, activates GTPases by catalyzing exchange of GDP for GTP
- Integrin affinity
alterations in integrin conformation from bent inactive form to intermediate and high affinity active ligand binding forms
- Integrin avidity
combination of affinity changes and clustering that lead to increased ligand binding and signaling
- Integrin clustering
changes in local densities of integrin molecules within the membrane
- FRET
fluorescence resonance energy transfer
- GPCR
G protein coupled receptor
- TCR
T cell receptor
- BCR
B cell receptor
- FERM domain
named after domain found in 4.1 protein, ezrin, radixin and moesin. Molecules containing this domain act as linkers between the membrane and the actin cytoskeleton
- PTB
phosphotyrosine binding domain, typically binds NPXY motifs and can be dependent or independent of tyrosine phosphorylation
- Src family kinases
a family of cytoplasmic tyrosine kinases containing an N terminal unique domain, followed by SH3 and SH2 domains. Src, Hck, Fgr, Lyn, Blk, Fyn and Lck are expressed in hematopoietic cells
- Syk
predominantly hematopoietic expressed cytoplasmic tyrosine kinase containing two N terminal SH2 domains, related to ZAP-70 which is restricted to T and NK cells
- SH2
Src homology 2 domain, binds phosphotyrosine residues
- SH3
Src homology 3 domain, binds polyproline motifs
- ITAM
immunoreceptor tyrosine-based activation motif
- Lipid raft
cholesterol- and sphingolipid-enriched microdomain found in the plasma membrane which allows compartmentalization of different signaling molecules
- PLC
phospholipase C, recruited to plasma membrane through lipid binding pleckstrin homology domain and converts PIP2 into two important signaling second messengers, DAG and IP3
- PKC
Protein kinase C has many different isoforms that are activated (variably depending on isoform) by DAG and Ca2+; serine/threonine kinases, key signaling modulators
LITERATURE CITED
- 1.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–89. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 2.Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–47. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnaout MA, Goodman SL, Xiong JP. Structure and mechanics of integrin-based cell adhesion. Curr Opin Cell Biol. 2007;19:495–507. doi: 10.1016/j.ceb.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kinashi T. Intracellular signalling controlling integrin activation in lymphocytes. Nat Rev Immunol. 2005;5:546–59. doi: 10.1038/nri1646. [DOI] [PubMed] [Google Scholar]
- 5.McLeod SJ, Shum AJ, Lee RL, Takei F, Gold MR. The Rap GTPases regulate integrin-mediated adhesion, cell spreading, actin polymerization, and Pyk2 tyrosine phosphorylation in B lymphocytes. J Biol Chem. 2004;279:12009–19. doi: 10.1074/jbc.M313098200. [DOI] [PubMed] [Google Scholar]
- 6.Katagiri K, Shimonaka M, Kinashi T. Rap1-mediated lymphocyte function-associated antigen-1 activation by the T cell antigen receptor is dependent on phospholipase Cγ1. J Biol Chem. 2004;279:11875–81. doi: 10.1074/jbc.M310717200. [DOI] [PubMed] [Google Scholar]
- 7.Shimonaka M, Katagiri K, Nakayama T, Fujita N, Tsuruo T, Yoshie O, Kinashi T. Rap1 translates chemokine signals to integrin activation, cell polarization, and motility across vascular endothelium under flow. J Cell Biol. 2003;161:417–27. doi: 10.1083/jcb.200301133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sebzda E, Bracke M, Tugal T, Hogg N, Cantrell DA. Rap1A positively regulates T cells via integrin activation rather than inhibiting lymphocyte signaling. Nat Immunol. 2002;3:251–8. doi: 10.1038/ni765. [DOI] [PubMed] [Google Scholar]
- 9.Katagiri K, Hattori M, Minato N, Irie S, Takatsu K, Kinashi T. Rap1 is a potent activation signal for leukocyte function-associated antigen 1 distinct from protein kinase C and phosphatidylinositol-3-OH kinase. Mol Cell Biol. 2000;20:1956–69. doi: 10.1128/mcb.20.6.1956-1969.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chrzanowska-Wodnicka M, Smyth SS, Schoenwaelder SM, Fischer TH, White GC., 2nd Rap1b is required for normal platelet function and hemostasis in mice. J Clin Invest. 2005;115:680–7. doi: 10.1172/JCI22973. Genetic evidence that Rap1 GTPases are required for integrin inside-out signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Yan J, De P, Chang HC, Yamauchi A, Christopherson KW, 2nd, Paranavitana NC, Peng X, Kim C, Munugulavadla V, Kapur R, Chen H, Shou W, Stone JC, Kaplan MH, Dinauer MC, Durden DL, Quilliam LA. Rap1a null mice have altered myeloid cell functions suggesting distinct roles for the closely related Rap1a and 1b proteins. J Immunol. 2007;179:8322–31. doi: 10.4049/jimmunol.179.12.8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duchniewicz M, Zemojtel T, Kolanczyk M, Grossmann S, Scheele JS, Zwartkruis FJ. Rap1A-deficient T and B cells show impaired integrin-mediated cell adhesion. Mol Cell Biol. 2006;26:643–53. doi: 10.1128/MCB.26.2.643-653.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergmeier W, Goerge T, Wang HW, Crittenden JR, Baldwin AC, Cifuni SM, Housman DE, Graybiel AM, Wagner DD. Mice lacking the signaling molecule CalDAG-GEFI represent a model for leukocyte adhesion deficiency type III. J Clin Invest. 2007;117:1699–707. doi: 10.1172/JCI30575. Genetic evidence that mutation of CalDAG-GEFI leads to symptoms of LADIII. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tohyama Y, Katagiri K, Pardi R, Lu C, Springer TA, Kinashi T. The critical cytoplasmic regions of the αL/β2 integrin in Rap1-induced adhesion and migration. Mol Biol Cell. 2003;14:2570–82. doi: 10.1091/mbc.E02-09-0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parmo-Cabanas M, Garcia-Bernal D, Garcia-Verdugo R, Kremer L, Marquez G, Teixido J. Intracellular signaling required for CCL25-stimulated T cell adhesion mediated by the integrin α4β1. J Leukoc Biol. 2007;82:380–91. doi: 10.1189/jlb.1206726. [DOI] [PubMed] [Google Scholar]
- 16.Katagiri K, Ohnishi N, Kabashima K, Iyoda T, Takeda N, Shinkai Y, Inaba K, Kinashi T. Crucial functions of the Rap1 effector molecule RAPL in lymphocyte and dendritic cell trafficking. Nat Immunol. 2004;5:1045–51. doi: 10.1038/ni1111. [DOI] [PubMed] [Google Scholar]
- 17.Katagiri K, Imamura M, Kinashi T. Spatiotemporal regulation of the kinase Mst1 by binding protein RAPL is critical for lymphocyte polarity and adhesion. Nat Immunol. 2006;7:919–28. doi: 10.1038/ni1374. [DOI] [PubMed] [Google Scholar]
- 18.Liu S, Ginsberg MH. Paxillin binding to a conserved sequence motif in the α4 integrin cytoplasmic domain. J Biol Chem. 2000;275:22736–42. doi: 10.1074/jbc.M000388200. [DOI] [PubMed] [Google Scholar]
- 19.Han J, Rose DM, Woodside DG, Goldfinger LE, Ginsberg MH. Integrin α4β1-dependent T cell migration requires both phosphorylation and dephosphorylation of the α4 cytoplasmic domain to regulate the reversible binding of paxillin. J Biol Chem. 2003;278:34845–53. doi: 10.1074/jbc.M304691200. [DOI] [PubMed] [Google Scholar]
- 20.Feral CC, Rose DM, Han J, Fox N, Silverman GJ, Kaushansky K, Ginsberg MH. Blocking the α4 integrin-paxillin interaction selectively impairs mononuclear leukocyte recruitment to an inflammatory site. J Clin Invest. 2006;116:715–23. doi: 10.1172/JCI26091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fagerholm SC, Hilden TJ, Nurmi SM, Gahmberg CG. Specific integrin α and β chain phosphorylations regulate LFA-1 activation through affinity-dependent and -independent mechanisms. J Cell Biol. 2005;171:705–15. doi: 10.1083/jcb.200504016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fagerholm SC, Varis M, Stefanidakis M, Hilden TJ, Gahmberg CG. α-chain phosphorylation of the human leukocyte CD11b/CD18 (Mac-1) integrin is pivotal for integrin activation to bind ICAMs and leukocyte extravasation. Blood. 2006;108:3379–86. doi: 10.1182/blood-2006-03-013557. [DOI] [PubMed] [Google Scholar]
- 23.Simonson WT, Franco SJ, Huttenlocher A. Talin1 regulates TCR-mediated LFA-1 function. J Immunol. 2006;177:7707–14. doi: 10.4049/jimmunol.177.11.7707. [DOI] [PubMed] [Google Scholar]
- 24.Tadokoro S, Shattil SJ, Eto K, Tai V, Liddington RC, de Pereda JM, Ginsberg MH, Calderwood DA. Talin binding to integrin β tails: a final common step in integrin activation. Science. 2003;302:103–6. doi: 10.1126/science.1086652. [DOI] [PubMed] [Google Scholar]
- 25.Lim J, Wiedemann A, Tzircotis G, Monkley SJ, Critchley DR, Caron E. An essential role for talin during αMβ2-mediated phagocytosis. Mol Biol Cell. 2007;18:976–85. doi: 10.1091/mbc.E06-09-0813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nieswandt B, Moser M, Pleines I, Varga-Szabo D, Monkley S, Critchley D, Fassler R. Loss of talin1 in platelets abrogates integrin activation, platelet aggregation, and thrombus formation in vitro and in vivo. J Exp Med. 2007;204:3113–8. doi: 10.1084/jem.20071827. Genetic evidence that talin is required for platelet inside-out integrin signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petrich BG, Marchese P, Ruggeri ZM, Spiess S, Weichert RA, Ye F, Tiedt R, Skoda RC, Monkley SJ, Critchley DR, Ginsberg MH. Talin is required for integrin-mediated platelet function in hemostasis and thrombosis. J Exp Med. 2007;204:3103–11. doi: 10.1084/jem.20071800. Genetic evidence that talin is required for platelet inside-out integrin signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-Alvarez B, de Pereda JM, Calderwood DA, Ulmer TS, Critchley D, Campbell ID, Ginsberg MH, Liddington RC. Structural determinants of integrin recognition by talin. Mol Cell. 2003;11:49–58. doi: 10.1016/s1097-2765(02)00823-7. [DOI] [PubMed] [Google Scholar]
- 29.Calderwood DA, Yan B, de Pereda JM, Alvarez BG, Fujioka Y, Liddington RC, Ginsberg MH. The phosphotyrosine binding-like domain of talin activates integrins. J Biol Chem. 2002;277:21749–58. doi: 10.1074/jbc.M111996200. [DOI] [PubMed] [Google Scholar]
- 30.Sampath R, Gallagher PJ, Pavalko FM. Cytoskeletal interactions with the leukocyte integrin β2 cytoplasmic tail. Activation-dependent regulation of associations with talin and α-actinin. J Biol Chem. 1998;273:33588–94. doi: 10.1074/jbc.273.50.33588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wegener KL, Partridge AW, Han J, Pickford AR, Liddington RC, Ginsberg MH, Campbell ID. Structural basis of integrin activation by talin. Cell. 2007;128:171–82. doi: 10.1016/j.cell.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 32.Bouaouina M, Lad Y, Calderwood DA. The N-terminal domains of talin cooperate with the phosphotyrosine binding-like domain to activate β1 and β3 integrins. J Biol Chem. 2008;283:6118–25. doi: 10.1074/jbc.M709527200. [DOI] [PubMed] [Google Scholar]
- 33.Hato T, Yamanouchi J, Tamura T, Yakushijin Y, Sakai I, Yasukawa M. Cooperative role of the membrane-proximal and -distal residues of the integrin β3 cytoplasmic domain in regulation of talin-mediated IIbβ3 activation. J Biol Chem. 2008;283:5662–8. doi: 10.1074/jbc.M707246200. [DOI] [PubMed] [Google Scholar]
- 34.Petrich BG, Fogelstrand P, Partridge AW, Yousefi N, Ablooglu AJ, Shattil SJ, Ginsberg MH. The antithrombotic potential of selective blockade of talin-dependent integrin αIIbβ3 (platelet GPIIb-IIIa) activation. J Clin Invest. 2007;117:2250–9. doi: 10.1172/JCI31024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolanus W. Guanine nucleotide exchange factors of the cytohesin family and their roles in signal transduction. Immunol Rev. 2007;218:102–13. doi: 10.1111/j.1600-065X.2007.00542.x. [DOI] [PubMed] [Google Scholar]
- 36.Stanley P, Smith A, McDowall A, Nicol A, Zicha D, Hogg N. Intermediate-affinity LFA-1 binds α-actinin-1 to control migration at the leading edge of the T cell. Embo J. 2008;27:62–75. doi: 10.1038/sj.emboj.7601959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma YQ, Qin J, Wu C, Plow EF. Kindlin-2 (Mig-2): a co-activator of β3 integrins. J Cell Biol. 2008;181:439–46. doi: 10.1083/jcb.200710196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montanez E, Ussar S, Schifferer M, Bosl M, Zent R, Moser M, Fassler R. Kindlin-2 controls bidirectional signaling of integrins. Genes Dev. 2008;22:1325–30. doi: 10.1101/gad.469408. First demonstration of role for kindlin family members in integrin inside-out signaling in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moser M, Nieswandt B, Ussar S, Pozgajova M, Fassler R. Kindlin-3 is essential for integrin activation and platelet aggregation. Nat Med. 2008;14:325–30. doi: 10.1038/nm1722. First demonstration of role for kindlin family members in integrin inside-out signaling in vivo. [DOI] [PubMed] [Google Scholar]
- 40.Tang P, Cao C, Xu M, Zhang L. Cytoskeletal protein radixin activates integrin αMβ2 by binding to its cytoplasmic tail. FEBS Lett. 2007;581:1103–8. doi: 10.1016/j.febslet.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 41.Zhang H, Berg JS, Li Z, Wang Y, Lang P, Sousa AD, Bhaskar A, Cheney RE, Stromblad S. Myosin-X provides a motor-based link between integrins and the cytoskeleton. Nat Cell Biol. 2004;6:523–31. doi: 10.1038/ncb1136. [DOI] [PubMed] [Google Scholar]
- 42.Calderwood DA, Fujioka Y, de Pereda JM, Garcia-Alvarez B, Nakamoto T, Margolis B, McGlade CJ, Liddington RC, Ginsberg MH. Integrin β cytoplasmic domain interactions with phosphotyrosine-binding domains: a structural prototype for diversity in integrin signaling. Proc Natl Acad Sci U S A. 2003;100:2272–7. doi: 10.1073/pnas.262791999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Czuchra A, Meyer H, Legate KR, Brakebusch C, Fassler R. Genetic analysis of beta1 integrin “activation motifs” in mice. J Cell Biol. 2006;174:889–99. doi: 10.1083/jcb.200604060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen H, Zou Z, Sarratt KL, Zhou D, Zhang M, Sebzda E, Hammer DA, Kahn ML. In vivo β1 integrin function requires phosphorylation-independent regulation by cytoplasmic tyrosines. Genes Dev. 2006;20:927–32. doi: 10.1101/gad.1408306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Law DA, DeGuzman FR, Heiser P, Ministri-Madrid K, Killeen N, Phillips DR. Integrin cytoplasmic tyrosine motif is required for outside-in αIIbβ3 signalling and platelet function. Nature. 1999;401:808–11. doi: 10.1038/44599. [DOI] [PubMed] [Google Scholar]
- 46.Kiema T, Lad Y, Jiang P, Oxley CL, Baldassarre M, Wegener KL, Campbell ID, Ylanne J, Calderwood DA. The molecular basis of filamin binding to integrins and competition with talin. Mol Cell. 2006;21:337–47. doi: 10.1016/j.molcel.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 47.Han J, Lim CJ, Watanabe N, Soriani A, Ratnikov B, Calderwood DA, Puzon-McLaughlin W, Lafuente EM, Boussiotis VA, Shattil SJ, Ginsberg MH. Reconstructing and deconstructing agonist-induced activation of integrin αIIbβ3. Curr Biol. 2006;16:1796–806. doi: 10.1016/j.cub.2006.08.035. Expression of components of integrin signaling in CHO cells defines an inside-out pathway. [DOI] [PubMed] [Google Scholar]
- 48.Medeiros RB, Dickey DM, Chung H, Quale AC, Nagarajan LR, Billadeau DD, Shimizu Y. Protein kinase D1 and the β1 integrin cytoplasmic domain control β1 integrin function via regulation of Rap1 activation. Immunity. 2005;23:213–26. doi: 10.1016/j.immuni.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 49.Crittenden JR, Bergmeier W, Zhang Y, Piffath CL, Liang Y, Wagner DD, Housman DE, Graybiel AM. CalDAG-GEFI integrates signaling for platelet aggregation and thrombus formation. Nat Med. 2004;10:982–6. doi: 10.1038/nm1098. [DOI] [PubMed] [Google Scholar]
- 50.Pasvolsky R, Feigelson SW, Kilic SS, Simon AJ, Tal-Lapidot G, Grabovsky V, Crittenden JR, Amariglio N, Safran M, Graybiel AM, Rechavi G, Ben-Dor S, Etzioni A, Alon R. A LAD-III syndrome is associated with defective expression of the Rap-1 activator CalDAG-GEFI in lymphocytes, neutrophils, and platelets. J Exp Med. 2007;204:1571–82. doi: 10.1084/jem.20070058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Watanabe N, Bodin L, Pandey M, Krause M, Coughlin S, Boussiotis VA, Ginsberg MH, Shattil SJ. Mechanisms and consequences of agonist-induced talin recruitment to platelet integrin αIIbβ3. J Cell Biol. 2008;181:1211–22. doi: 10.1083/jcb.200803094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Menasche G, Kliche S, Chen EJ, Stradal TE, Schraven B, Koretzky G. RIAM links the ADAP/SKAP-55 signaling module to Rap1, facilitating T-cell-receptor-mediated integrin activation. Mol Cell Biol. 2007;27:4070–81. doi: 10.1128/MCB.02011-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jo EK, Wang H, Rudd CE. An essential role for SKAP-55 in LFA-1 clustering on T cells that cannot be substituted by SKAP-55R. J Exp Med. 2005;201:1733–9. doi: 10.1084/jem.20042577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang H, Liu H, Lu Y, Lovatt M, Wei B, Rudd CE. Functional defects of SKAP-55-deficient T cells identify a regulatory role for the adaptor in LFA-1 adhesion. Mol Cell Biol. 2007;27:6863–75. doi: 10.1128/MCB.00556-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kliche S, Breitling D, Togni M, Pusch R, Heuer K, Wang X, Freund C, Kasirer-Friede A, Menasche G, Koretzky GA, Schraven B. The ADAP/SKAP55 signaling module regulates T-cell receptor-mediated integrin activation through plasma membrane targeting of Rap1. Mol Cell Biol. 2006;26:7130–44. doi: 10.1128/MCB.00331-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ghandour H, Cullere X, Alvarez A, Luscinskas FW, Mayadas TN. Essential role for Rap1 GTPase and its guanine exchange factor CalDAG-GEFI in LFA-1 but not VLA-4 integrin mediated human T-cell adhesion. Blood. 2007;110:3682–90. doi: 10.1182/blood-2007-03-077628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heit B, Colarusso P, Kubes P. Fundamentally different roles for LFA-1, Mac-1 and α4-integrin in neutrophil chemotaxis. J Cell Sci. 2005;118:5205–20. doi: 10.1242/jcs.02632. [DOI] [PubMed] [Google Scholar]
- 58.Ulfman LH, Kamp VM, van Aalst CW, Verhagen LP, Sanders ME, Reedquist KA, Buitenhuis M, Koenderman L. Homeostatic intracellular-free Ca2+ is permissive for Rap1-mediated constitutive activation of α4 integrins on eosinophils. J Immunol. 2008;180:5512–9. doi: 10.4049/jimmunol.180.8.5512. [DOI] [PubMed] [Google Scholar]
- 59.Carrasco YR, Fleire SJ, Cameron T, Dustin ML, Batista FD. LFA-1/ICAM-1 interaction lowers the threshold of B cell activation by facilitating B cell adhesion and synapse formation. Immunity. 2004;20:589–99. doi: 10.1016/s1074-7613(04)00105-0. [DOI] [PubMed] [Google Scholar]
- 60.Kim M, Carman CV, Yang W, Salas A, Springer TA. The primacy of affinity over clustering in regulation of adhesiveness of the integrin αLβ2. J Cell Biol. 2004;167:1241–53. doi: 10.1083/jcb.200404160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luo BH, Carman CV, Takagi J, Springer TA. Disrupting integrin transmembrane domain heterodimerization increases ligand binding affinity, not valency or clustering. Proc Natl Acad Sci U S A. 2005;102:3679–84. doi: 10.1073/pnas.0409440102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Krauss K, Altevogt P. Integrin leukocyte function-associated antigen-1-mediated cell binding can be activated by clustering of membrane rafts. J Biol Chem. 1999;274:36921–7. doi: 10.1074/jbc.274.52.36921. [DOI] [PubMed] [Google Scholar]
- 63.Marwali MR, Rey-Ladino J, Dreolini L, Shaw D, Takei F. Membrane cholesterol regulates LFA-1 function and lipid raft heterogeneity. Blood. 2003;102:215–22. doi: 10.1182/blood-2002-10-3195. [DOI] [PubMed] [Google Scholar]
- 64.Cairo CW, Mirchev R, Golan DE. Cytoskeletal regulation couples LFA-1 conformational changes to receptor lateral mobility and clustering. Immunity. 2006;25:297–308. doi: 10.1016/j.immuni.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 65.Cambi A, Joosten B, Koopman M, de Lange F, Beeren I, Torensma R, Fransen JA, Garcia-Parajo M, van Leeuwen FN, Figdor CG. Organization of the integrin LFA-1 in nanoclusters regulates its activity. Mol Biol Cell. 2006;17:4270–81. doi: 10.1091/mbc.E05-12-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kasirer-Friede A, Kahn ML, Shattil SJ. Platelet integrins and immunoreceptors. Immunol Rev. 2007;218:247–64. doi: 10.1111/j.1600-065X.2007.00532.x. [DOI] [PubMed] [Google Scholar]
- 67.Baruzzi A, Caveggion E, Berton G. Regulation of phagocyte migration and recruitment by Src-family kinases. Cell Mol Life Sci. 2008 doi: 10.1007/s00018-008-8005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Giagulli C, Ottoboni L, Caveggion E, Rossi B, Lowell C, Constantin G, Laudanna C, Berton G. The Src family kinases Hck and Fgr are dispensable for inside-out, chemoattractant-induced signaling regulating β2 integrin affinity and valency in neutrophils, but are required for beta 2 integrin-mediated outside-in signaling involved in sustained adhesion. J Immunol. 2006;177:604–11. doi: 10.4049/jimmunol.177.1.604. [DOI] [PubMed] [Google Scholar]
- 69.Totani L, Piccoli A, Manarini S, Federico L, Pecce R, Martelli N, Cerletti C, Piccardoni P, Lowell CA, Smyth SS, Berton G, Evangelista V. Src-family kinases mediate an outside-in signal necessary for β2 integrins to achieve full activation and sustain firm adhesion of polymorphonuclear leucocytes tethered on E-selectin. Biochem J. 2006;396:89–98. doi: 10.1042/BJ20051924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suzuki J, Yamasaki S, Wu J, Koretzky GA, Saito T. The actin cloud induced by LFA-1-mediated outside-in signals lowers the threshold for T-cell activation. Blood. 2007;109:168–75. doi: 10.1182/blood-2005-12-020164. [DOI] [PubMed] [Google Scholar]
- 71.Mocsai A, Zhou M, Meng F, Tybulewicz VL, Lowell CA. Syk is required for integrin signaling in neutrophils. Immunity. 2002;16:547–58. doi: 10.1016/s1074-7613(02)00303-5. [DOI] [PubMed] [Google Scholar]
- 72.Abtahian F, Bezman N, Clemens R, Sebzda E, Cheng L, Shattil SJ, Kahn ML, Koretzky GA. Evidence for the requirement of ITAM domains but not SLP-76/Gads interaction for integrin signaling in hematopoietic cells. Mol Cell Biol. 2006;26:6936–49. doi: 10.1128/MCB.01040-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mocsai A, Zhang H, Jakus Z, Kitaura J, Kawakami T, Lowell CA. G-protein-coupled receptor signaling in Syk-deficient neutrophils and mast cells. Blood. 2003;101:4155–63. doi: 10.1182/blood-2002-07-2346. [DOI] [PubMed] [Google Scholar]
- 74.Epler JA, Liu R, Chung H, Ottoson NC, Shimizu Y. Regulation of β1 integrin-mediated adhesion by T cell receptor signaling involves ZAP-70 but differs from signaling events that regulate transcriptional activity. J Immunol. 2000;165:4941–9. doi: 10.4049/jimmunol.165.9.4941. [DOI] [PubMed] [Google Scholar]
- 75.Frommhold D, Mannigel I, Schymeinsky J, Mocsai A, Poeschl J, Walzog B, Sperandio M. Spleen tyrosine kinase Syk is critical for sustained leukocyte adhesion during inflammation in vivo. BMC Immunol. 2007;8:31. doi: 10.1186/1471-2172-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hirahashi J, Mekala D, Van Ziffle J, Xiao L, Saffaripour S, Wagner DD, Shapiro SD, Lowell C, Mayadas TN. Mac-1 signaling via Src-family and Syk kinases results in elastase-dependent thrombohemorrhagic vasculopathy. Immunity. 2006;25:271–83. doi: 10.1016/j.immuni.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 77.Lammermann T, Bader BL, Monkley SJ, Worbs T, Wedlich-Soldner R, Hirsch K, Keller M, Forster R, Critchley DR, Fassler R, Sixt M. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453:51–5. doi: 10.1038/nature06887. Surprising study showing that integrins may not be required for some aspects of leukocyte migration. [DOI] [PubMed] [Google Scholar]
- 78.Owen KA, Pixley FJ, Thomas KS, Vicente-Manzanares M, Ray BJ, Horwitz AF, Parsons JT, Beggs HE, Stanley ER, Bouton AH. Regulation of lamellipodial persistence, adhesion turnover, and motility in macrophages by focal adhesion kinase. J Cell Biol. 2007;179:1275–87. doi: 10.1083/jcb.200708093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Okigaki M, Davis C, Falasca M, Harroch S, Felsenfeld DP, Sheetz MP, Schlessinger J. Pyk2 regulates multiple signaling events crucial for macrophage morphology and migration. Proc Natl Acad Sci U S A. 2003;100:10740–5. doi: 10.1073/pnas.1834348100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Han H, Fuortes M, Nathan C. Critical role of the carboxyl terminus of proline-rich tyrosine kinase (Pyk2) in the activation of human neutrophils by tumor necrosis factor: separation of signals for the respiratory burst and degranulation. J Exp Med. 2003;197:63–75. doi: 10.1084/jem.20021638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Evangelista V, Pamuklar Z, Piccoli A, Manarini S, Dell’elba G, Pecce R, Martelli N, Federico L, Rojas M, Berton G, Lowell CA, Totani L, Smyth SS. Src family kinases mediate neutrophil adhesion to adherent platelets. Blood. 2007;109:2461–9. doi: 10.1182/blood-2006-06-029082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Arias-Salgado EG, Lizano S, Sarkar S, Brugge JS, Ginsberg MH, Shattil SJ. Src kinase activation by direct interaction with the integrin β cytoplasmic domain. Proc Natl Acad Sci U S A. 2003;100:13298–302. doi: 10.1073/pnas.2336149100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.de Virgilio M, Kiosses WB, Shattil SJ. Proximal, selective, and dynamic interactions between integrin αIIbβ3 and protein tyrosine kinases in living cells. J Cell Biol. 2004;165:305–11. doi: 10.1083/jcb.200402064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mocsai A, Abram CL, Jakus Z, Hu Y, Lanier LL, Lowell CA. Integrin signaling in neutrophils and macrophages uses adaptors containing immunoreceptor tyrosine-based activation motifs. Nat Immunol. 2006;7:1326–33. doi: 10.1038/ni1407. Demonstrates the requirement for ITAM-containing adapters in integrin outside-in signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Abram CL, Lowell CA. The expanding role for ITAM-based signaling pathways in immune cells. Sci STKE. 2007;2007:re2. doi: 10.1126/stke.3772007re2. [DOI] [PubMed] [Google Scholar]
- 86.Abram CL, Lowell CA. Convergence of immunoreceptor and integrin signaling. Immunol Rev. 2007;218:29–44. doi: 10.1111/j.1600-065X.2007.00531.x. [DOI] [PubMed] [Google Scholar]
- 87.Zhu J, Carman CV, Kim M, Shimaoka M, Springer TA, Luo BH. Requirement of α and β subunit transmembrane helix separation for integrin outside-in signaling. Blood. 2007;110:2475–83. doi: 10.1182/blood-2007-03-080077. Demonstrates that integrin conformational changes are required for outside-in signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zou Z, Chen H, Schmaier AA, Hynes RO, Kahn ML. Structure-function analysis reveals discrete β3 integrin inside-out and outside-in signaling pathways in platelets. Blood. 2007;109:3284–90. doi: 10.1182/blood-2006-10-051664. [DOI] [PubMed] [Google Scholar]
- 89.Wonerow P, Obergfell A, Wilde JI, Bobe R, Asazuma N, Brdicka T, Leo A, Schraven B, Horejsi V, Shattil SJ, Watson SP. Differential role of glycolipid-enriched membrane domains in glycoprotein VI- and integrin-mediated phospholipase Cγ2 regulation in platelets. Biochem J. 2002;364:755–65. doi: 10.1042/BJ20020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Solomkin JS, Robinson CT, Cave CM, Ehmer B, Lentsch AB. Alterations in membrane cholesterol cause mobilization of lipid rafts from specific granules and prime human neutrophils for enhanced adherence-dependent oxidant production. Shock. 2007;28:334–8. doi: 10.1097/shk.0b013e318047b893. [DOI] [PubMed] [Google Scholar]
- 91.Zarbock A, Abram CL, Hundt M, Altman A, Lowell CA, Ley K. PSGL-1 engagement by E-selectin signals through Src kinase Fgr and ITAM-adapters DAP12 and FcRγ to induce slow leukocyte rolling. J Exp Med. 2008 doi: 10.1084/jem.20072660. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bezman N, Koretzky GA. Compartmentalization of ITAM and integrin signaling by adapter molecules. Immunol Rev. 2007;218:9–28. doi: 10.1111/j.1600-065X.2007.00541.x. [DOI] [PubMed] [Google Scholar]
- 93.Clemens RA, Newbrough SA, Chung EY, Gheith S, Singer AL, Koretzky GA, Peterson EJ. PRAM-1 is required for optimal integrin-dependent neutrophil function. Mol Cell Biol. 2004;24:10923–32. doi: 10.1128/MCB.24.24.10923-10932.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Perez OD, Mitchell D, Jager GC, South S, Murriel C, McBride J, Herzenberg LA, Kinoshita S, Nolan GP. Leukocyte functional antigen 1 lowers T cell activation thresholds and signaling through cytohesin-1 and Jun-activating binding protein 1. Nat Immunol. 2003;4:1083–92. doi: 10.1038/ni984. [DOI] [PubMed] [Google Scholar]
- 95.Caveggion E, Continolo S, Pixley FJ, Stanley ER, Bowtell DD, Lowell CA, Berton G. Expression and tyrosine phosphorylation of Cbl regulates macrophage chemokinetic and chemotactic movement. J Cell Physiol. 2003;195:276–89. doi: 10.1002/jcp.10236. [DOI] [PubMed] [Google Scholar]
- 96.Meng F, Lowell CA. A β1 integrin signaling pathway involving Src-family kinases, Cbl and PI-3 kinase is required for macrophage spreading and migration. Embo J. 1998;17:4391–403. doi: 10.1093/emboj/17.15.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Buensuceso CS, Obergfell A, Soriani A, Eto K, Kiosses WB, Arias-Salgado EG, Kawakami T, Shattil SJ. Regulation of outside-in signaling in platelets by integrin-associated protein kinase Cβ. J Biol Chem. 2005;280:644–53. doi: 10.1074/jbc.M410229200. [DOI] [PubMed] [Google Scholar]
- 98.Soriani A, Moran B, de Virgilio M, Kawakami T, Altman A, Lowell C, Eto K, Shattil SJ. A role for PKCθ in outside-in αIIbβ3 signaling. J Thromb Haemost. 2006;4:648–55. doi: 10.1111/j.1538-7836.2006.01806.x. [DOI] [PubMed] [Google Scholar]
- 99.Swat W, Fujikawa K. The Vav family: at the crossroads of signaling pathways. Immunol Res. 2005;32:259–65. doi: 10.1385/IR:32:1-3:259. [DOI] [PubMed] [Google Scholar]
- 100.Graham DB, Robertson CM, Bautista J, Mascarenhas F, Diacovo MJ, Montgrain V, Lam SK, Cremasco V, Dunne WM, Faccio R, Coopersmith CM, Swat W. Neutrophil-mediated oxidative burst and host defense are controlled by a Vav-PLCγ2 signaling axis in mice. J Clin Invest. 2007;117:3445–52. doi: 10.1172/JCI32729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pearce AC, McCarty OJ, Calaminus SD, Vigorito E, Turner M, Watson SP. Vav family proteins are required for optimal regulation of PLCγ2 by integrin αIIbβ3. Biochem J. 2007;401:753–61. doi: 10.1042/BJ20061508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ivetic A, Ridley AJ. Ezrin/radixin/moesin proteins and Rho GTPase signalling in leucocytes. Immunology. 2004;112:165–76. doi: 10.1111/j.1365-2567.2004.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nishiya N, Kiosses WB, Han J, Ginsberg MH. An α4 integrin-paxillin-Arf-GAP complex restricts Rac activation to the leading edge of migrating cells. Nat Cell Biol. 2005;7:343–52. doi: 10.1038/ncb1234. [DOI] [PubMed] [Google Scholar]
- 104.Notarangelo LD, Miao CH, Ochs HD. Wiskott-Aldrich syndrome. Curr Opin Hematol. 2008;15:30–6. doi: 10.1097/MOH.0b013e3282f30448. [DOI] [PubMed] [Google Scholar]
- 105.Zhang H, Schaff UY, Green CE, Chen H, Sarantos MR, Hu Y, Wara D, Simon SI, Lowell CA. Impaired integrin-dependent function in Wiskott-Aldrich syndrome protein-deficient murine and human neutrophils. Immunity. 2006;25:285–95. doi: 10.1016/j.immuni.2006.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 107.Anderson DC, Springer TA. Leukocyte adhesion deficiency: an inherited defect in the Mac-1, LFA-1, and p150,95 glycoproteins. Annu Rev Med. 1987;38:175–94. doi: 10.1146/annurev.me.38.020187.001135. [DOI] [PubMed] [Google Scholar]
- 108.Etzioni A, Doerschuk CM, Harlan JM. Of man and mouse: leukocyte and endothelial adhesion molecule deficiencies. Blood. 1999;94:3281–8. [PubMed] [Google Scholar]
- 109.Alon R, Aker M, Feigelson S, Sokolovsky-Eisenberg M, Staunton DE, Cinamon G, Grabovsky V, Shamri R, Etzioni A. A novel genetic leukocyte adhesion deficiency in subsecond triggering of integrin avidity by endothelial chemokines results in impaired leukocyte arrest on vascular endothelium under shear flow. Blood. 2003;101:4437–45. doi: 10.1182/blood-2002-11-3427. [DOI] [PubMed] [Google Scholar]
- 110.Salles, Feys HB, Iserbyt BF, De Meyer SF, Vanhoorelbeke K, Deckmyn H. Inherited traits affecting platelet function. Blood Rev. 2008;22:155–72. doi: 10.1016/j.blre.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 111.Stephens LE, Sutherland AE, Klimanskaya IV, Andrieux A, Meneses J, Pedersen RA, Damsky CH. Deletion of β1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 1995;9:1883–95. doi: 10.1101/gad.9.15.1883. [DOI] [PubMed] [Google Scholar]
- 112.Wilson RW, Ballantyne CM, Smith CW, Montgomery C, Bradley A, O’Brien WE, Beaudet AL. Gene targeting yields a CD18-mutant mouse for study of inflammation. J Immunol. 1993;151:1571–8. [PubMed] [Google Scholar]
- 113.Scharffetter-Kochanek K, Lu H, Norman K, van Nood N, Munoz F, Grabbe S, McArthur M, Lorenzo I, Kaplan S, Ley K, Smith CW, Montgomery CA, Rich S, Beaudet AL. Spontaneous skin ulceration and defective T cell function in CD18 null mice. J Exp Med. 1998;188:119–31. doi: 10.1084/jem.188.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hodivala-Dilke KM, McHugh KP, Tsakiris DA, Rayburn H, Crowley D, Ullman-Cullere M, Ross FP, Coller BS, Teitelbaum S, Hynes RO. β3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest. 1999;103:229–38. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Arroyo AG, Yang JT, Rayburn H, Hynes RO. Differential requirements for α4 integrins during fetal and adult hematopoiesis. Cell. 1996;85:997–1008. doi: 10.1016/s0092-8674(00)81301-x. [DOI] [PubMed] [Google Scholar]
- 116.Scott LM, Priestley GV, Papayannopoulou T. Deletion of α4 integrins from adult hematopoietic cells reveals roles in homeostasis, regeneration, and homing. Mol Cell Biol. 2003;23:9349–60. doi: 10.1128/MCB.23.24.9349-9360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Smyth SS, Reis ED, Vaananen H, Zhang W, Coller BS. Variable protection of β3-integrin--deficient mice from thrombosis initiated by different mechanisms. Blood. 2001;98:1055–62. doi: 10.1182/blood.v98.4.1055. [DOI] [PubMed] [Google Scholar]
- 118.Ding ZM, Babensee JE, Simon SI, Lu H, Perrard JL, Bullard DC, Dai XY, Bromley SK, Dustin ML, Entman ML, Smith CW, Ballantyne CM. Relative contribution of LFA-1 and Mac-1 to neutrophil adhesion and migration. J Immunol. 1999;163:5029–38. [PubMed] [Google Scholar]
- 119.Semmrich M, Smith A, Feterowski C, Beer S, Engelhardt B, Busch DH, Bartsch B, Laschinger M, Hogg N, Pfeffer K, Holzmann B. Importance of integrin LFA-1 deactivation for the generation of immune responses. J Exp Med. 2005;201:1987–98. doi: 10.1084/jem.20041850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lu H, Smith CW, Perrard J, Bullard D, Tang L, Shappell SB, Entman ML, Beaudet AL, Ballantyne CM. LFA-1 is sufficient in mediating neutrophil emigration in Mac-1-deficient mice. J Clin Invest. 1997;99:1340–50. doi: 10.1172/JCI119293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Luo BH, Springer TA, Takagi J. A specific interface between integrin transmembrane helices and affinity for ligand. PLoS Biol. 2004;2:e153. doi: 10.1371/journal.pbio.0020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Armulik A, Nilsson I, von Heijne G, Johansson S. Determination of the border between the transmembrane and cytoplasmic domains of human integrin subunits. J Biol Chem. 1999;274:37030–4. doi: 10.1074/jbc.274.52.37030. [DOI] [PubMed] [Google Scholar]
- 123.Ulbrich H, Eriksson EE, Lindbom L. Leukocyte and endothelial cell adhesion molecules as targets for therapeutic interventions in inflammatory disease. Trends Pharmacol Sci. 2003;24:640–7. doi: 10.1016/j.tips.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 124.Yonekawa K, Harlan JM. Targeting leukocyte integrins in human diseases. J Leukoc Biol. 2005;77:129–40. doi: 10.1189/jlb.0804460. [DOI] [PubMed] [Google Scholar]
- 125.Avraamides CJ, Garmy-Susini B, Varner JA. Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer. 2008;8:604–17. doi: 10.1038/nrc2353. [DOI] [PMC free article] [PubMed] [Google Scholar]