Abstract
The appropriate regulation of neutrophil activation is critical for maintaining host defense and limiting inflammation. Neutrophils (PMNs) express a number of cytoplasmic tyrosine kinases that regulate signaling pathways leading to activation. One of the most highly expressed, but least studied, kinases in PMNs is proline rich kinase 2 (Pyk2). By analogy to the related FAK kinase, Pyk2 has been implicated in regulating PMN adhesion and migration, however its physiologic function has yet to be described. Using pyk2−/− mice, we found that this kinase was required for integrin-mediated degranulation responses, but was not involved in adhesion-induced cell spreading or activation of superoxide production. Pyk2-deficient PMNs also manifested reduced migration on fibrinogen-coated surfaces. The absence of Pyk2 resulted in a severe reduction in paxillin and Vav phosphorylation following integrin ligation, which likely accounts for the poor degranulation and cell migration. Pyk2−/− mice were unable to efficiently clear infection with Staphylococcus aureus in a skin abscess model due in part to poor release of granule contents at the site of infection. However, Pyk2-deficient PMNs responded normally to soluble agonists, demonstrating that this kinase functions mainly in the integrin pathway. These data demonstrate the unrealized physiologic role of this kinase in regulating adhesion-mediated release of PMN granule contents.
Introduction
Neutrophils (PMNs) are an essential component of the innate host defense response and are often the first cells to respond to an infection (1). A primary means of PMN activation is through integrin ligation (2–4). Integrins are heterodimeric receptors on the PMN surface that when engaged by ligands lead to the effector responses resulting in host defense through the process of outside-in signaling (4–6). The absence of integrins or impaired PMN activation results in diminished responses to a number of pathogens, including bacteria such as Staphylococcus aureus (S. aureus), fungal infections such as Aspergillus fumigatus, or protozoan infection by Toxoplasma gondii (7–10). Thus, PMN activation via integrin ligation is a necessary step in the innate host defense response.
The process of PMN activation via integrin ligation has been closely studied. Resting PMNs circulate through the peripheral blood, but upon integrin activation following exposure to inflammatory cytokines or chemoattractants, PMNs are able to attach, spread out and adhere to endothelium (1, 11). After adhesion, PMNs then migrate through the vascular endothelium to the site of the infection, following gradients of chemokines (11, 12). During this process, extracellular matrix/integrin interactions lead to progressive PMN activation. Upon reaching the site of the infection, PMNs phagocytose Ig-opsonized foreign particles, such as bacteria or fungi, via Fc receptors or complement-opsonized particles through integrins for degradation in lysosomes (1, 13–15). Alternatively, PMNs destroy pathogens through production of superoxide radicals or release of antimicrobial proteins via degranulation (1, 16).
The release of antimicrobial products can also damage host tissue. Therefore, regulation of PMN activation leading to superoxide production and degranulation is tightly controlled (3). Since the integrins themselves do not possess any intrinsic catalytic activity, the activation of downstream non-receptor tyrosine kinases is a key event leading to the control of integrin-mediated activation (3, 17). Signaling via these tyrosine kinases promotes localization of scaffolding proteins, such as paxillin, α-actinin, and talin to the site of integrin binding (18). The scaffolding proteins both serve as a framework upon which actin and microtubules can polymerize, as well as recruiting other kinases (19). Key signaling pathways that are activated downstream of integrin ligation are the MAP kinase pathway and the Rho GTPase signaling pathways (3). Activation of these pathways results in adhesion, migration, superoxide production and degranulation (1). However, the specific mechanisms by which these pathways are activated and controlled to lead to superoxide production and degranulation remain to be elucidated.
One such non-receptor tyrosine kinase activated downstream of integrin ligation is Syk. Syk is phosphorylated following integrin ligation (3, 17). Syk deficiency leads to impaired integrin and Fc receptor mediated PMN activation, which is manifest by complete blocks in superoxide production, degranulation and adhesion (20). Absence of Syk has been found to impair the host defense response in PMN-dependent in vivo models of infection (8). However, Syk is not the sole kinase activated downstream of integrin ligation. Pyk2 is a non-receptor tyrosine kinase expressed primarily in hematopoietic and neural tissue (21). Pyk2 undergoes autophosphorylation following integrin ligation, allowing its association with the Src-family kinases (19, 22, 23). The Src-family kinases then phosphorylate Pyk2 at a number of other tyrosines allowing it to achieve an active conformation (24–27). Following activation, both Pyk2 and the Src kinases function tandemly to activate downstream signaling molecules and scaffolding proteins (28).
The function of Pyk2 in innate immune cells is not clear. Previous work has shown that Pyk2 is important in macrophage activation. Pyk2-deficient macrophages exhibit impaired adhesion, migration due to decreased Rho GTPase and PI3K activation following integrin ligation (29). Previous work using Pyk2 inhibitors has suggested that this enzyme is critical for superoxide production in human PMNs stimulated with TNF-α (TNF) (28, 30). However, the role of Pyk2 in integrin-mediated activation of PMNs in vivo remains to be determined. In this paper, we present evidence indicating that Pyk2 functions primarily in the integrin-mediated signaling pathway and that Pyk2 deficiency results in reduced adhesion-mediated degranulation which results in impaired host defense.
Materials and Methods
Mice
Pyk2−/− mice (29), backcrossed onto the C57Bl.6 background for 8 generations, were used with WT (pyk2+/+) controls. Pyk2-deficient mice were identified using PCR-based genotyping. Mice lacking Hck, Fgr or Hck, Fgr and Lyn kinases have previously been described (20). Syk−/− cells were obtained from bone marrow chimeras using Syk-deficient fetal liver cells in lethally irradiated WT recipients as described (20).
PMN Preparation
Bone marrow PMNs were isolated as previously described (20). Briefly, bone marrow cells were exposed to 30 second hypertonic lysis and then the PMNs were separated on a Percoll gradient. Cells were washed in HBSS and used within 2 hours for experiments.
Integrin-mediated adhesion
PMNs in HBSS were plated onto size 12 glass coverslips (Fisher) coated with poly-RGD (pRGD; Sigma) at 20 Ug/mL at 4°C for 15 minutes. Cells were then warmed to 37°C for 2, 5 or 10 minutes and then fixed in 4% PFA solution (2% paraformaldehyde, 60mM dibasic sodium phosphate, 14mM sodium phosphate) for 15 minutes at 37°C. Following fixation, cells were washed and then permeabilized in a 1% saponin PBS solution (3% BSA) for 30 minutes. Cells were then stained with Alexa-546 phalloidin (Invitrogen) for 30 minutes and the coverslips mounted using Prolong-Gold (Invitrogen). PMN adhesion was imaged using Nikon spinning disk confocal with 100× objective. Images were processed using Metamorph Software. To assess static adhesion, PMNs loaded with 2.3 μg/mL Calcein AM (Invitrogen) at 2×106/mL in HBSS were plated onto wells pre-coated with fibrinogen (165 μg/mL) or pRGD (20 μg/mL) and incubated for 15 minutes at 37°C. To obtain the initial number of cells loaded into each well, the baseline level of fluorescence was read on the Spectramax M5 plate reader with excitation at 485 nm and emission at 538 nm. Following the baseline read, cells were spun at 60g then washed with warm HBSS and following each wash step, the fluorescence was measured indicating the number of adherent cells.
Superoxide production
PMNs at a concentration of 5×106/mL resuspended in 50 nM luminol in HBSS were plated in 96 well dishes (type, source) coated either with pRGD (20 μg/mL), fibrinogen (165 μg/mL), or fibronectin (1:1000) with 20 U/mL exogenous HRP and with or without 100 ng/mL of TNF. Following plating, increase in luminescence was measured at 37°C for 2 hours on a Spectramax M5 plate reader and resultant data plotted as relative light units (RLUs) over time. To measure complement-mediated superoxide production, complement-opsonized particles were generated by opsonizing 1 micron polystyrene beads with IgM (Bangs Laboratories), following by blocking in 1% BSA and incubating with 10% serum from RAG-deficient mice (Jackson Laboratories). PMNs were loaded into wells that had previously been blocked with 0.5% milk in PBS. Complement-opsonized beads were added to the cells at an MOI of 10 and the resultant superoxide production measured as described above.
Bactericidal activity
To analyze complement-mediated phagocytosis, PMNs were exposed to complement-opsonized beads that were labeled with FITC at an MOI of 10 for varying time points. As previously described, following phagocytosis, PMNs were placed on ice and washed with PBS mixed with trypan blue, to quench extracellular fluorescence (8). PMNs were analyzed on a FacsScan for increase in FITC fluorescence. The degree of phagocytosis was plotted as increase in median FITC fluorescence. Alternatively, PMNs were exposed to FITC-labelled S. aureus as previously described to measure phagocytosis of complement-opsonized S. aureus (8).
In vitro bactericidal activity was measured by opsonizing S. aureus with 10% serum from RAG-deficient mice and adding the opsonized bacteria to PMNs at an MOI of 5, as previously described (8). The PMNs were allowed to internalize bacteria for 15 minutes at 37C. Following internalization, the PMNs were treated with gentamycin for 15 minutes at 4°C to lyse any extracelluar bacteria. The PMNS were then warmed back to 37°C and allowed to kill internalized bacteria for varying time points. To assess the number of bacteria killed, the PMNs were lysed in H20, pH 11, and the surviving bacteria plated to obtain live CFU.
Degranulation
Protocols were followed as previously described (20, 31). Briefly, PMNs at a concentration of 2×106/mL were plated on wells coated with 20 μg/mL of pRGD in the presence or absence of 100 ng/mL TNF-α. As a control, PMNs were also loaded onto wells that had been blocked with 0.5% milk. After a 1 hour incubation at 37°C, cells were spun and the supernatants were removed for measurement of release of Matrix Metalloproteinase 9 (MMP-9) and lactoferrin. MMP-9 release was measured by mixing of supernatant with non-reducing sample buffer and separation by zymogram gel electorphoresis (Invitrogen), rentauration in 2.5% Triton X-100 buffer and development in zymography buffer (200 mM NaCl, 5 mM CaCl2, 50 mM Tris pH 7.4) overnight. Gels were stained with Coomassie blue dye and quantitated using an Alphaimager. Lactoferrin release was analyzed using an ELISA-based method of detection. Supernatants from the activated PMNs were plated onto a 96 well high protein-binding plate (Nunc) along with differing concentrations of purified lactoferrin to establish a standard curve. Lactoferrin in the supernatant was detected via anti-lactoferrin antibodies and the amount was calculated using the standard curve. Myeloperoxidase (MPO) release was measured by combining the supernatants from activated cells with o-dianisidine reaction buffer (0.165 mg/mL o-dianisidine, 100 mM potassium phosphate, 0.1% H2O2) and MPO read as change in OD at 460 nm over 30 minutes.
In vitro migration assays
To measure migration, PMNs at a concentration of 5×106 cells/mL in RPMI with 1% FBS were loaded onto 3 micron pore transwells (Costar). To measure integrin-mediated migration, the transwells were previously coated with fibrinogen (165 μg/mL) (20). Cells were plated at 37°C and allowed to migrate towards 0.5 μM fMLF or 10 ng/mL MIP2 for 30 minutes. Following incubation, wells were spun to release any migrated PMNs that were adhering to the membrane and the migrated cells lysed in 0.15M sodium acetate buffer, pH 5.6 containing 0.1% Triton X-100 with 5 mM p-nitrophenol (Sigma) for 20 min at RT. The reaction was stopped by adding 2N NaOH. The amount of p-nitrophenol converted was measured on the Spectramax M5 plate reader at 405 nm. The number of cells migrated was quantitated as fraction of input control (total number of cells originally loaded into each well).
Immunoblot and antibodies
All blots shown used IR-labeled secondary antibodies (LI-COR Biosciences) and detection with Odyssey Infrared Imaging System (31). 5×106 PMNs were plated onto 20 μg/mL pRGD coated wells for indicated times. To analyze levels of signal activation following complement-mediated phagocytosis, 5×106 PMNs were exposed to complement-opsonized S. aureus at a MOI of 5 for varying timepoints. For analysis of phosphotyrosine levels in activated PMNs (4G10 antibody) cells were lysed in RIPA buffer (20 mM Tris, pH 7.5, 150 mM NaCl, 1%. Triton X-100 buffer, 1% sodium dexoycholate, 0.1% SDS, 1 mM Na3VO4, 50 mM NaF, 2 mM EDTA, 1 mM Pefabloc, 10 μg/mL of leupeptin, 2 μg/mL of aprotinin, 1 mM diothreitol, 1 Ug/mL of pepstatin and 1 mM di-isopropyl fluorophosphate) with the insoluble component spun out. For analysis of phosphorylated Vav (Santa Cruz), Pyk2 (Bioscience), and paxillin (Cell Signaling Technology), PMNs were lysed directly into 1× sample dilution buffer. Antibodies for paxillin (Cell Signaling Technology), Pyk2 (Santa Cruz) and Vav (Santa Cruz) were used as loading controls.
Bacterial infection model
WT or pyk2−/− mice were injected with 5 cc of air in the central back region on Day 0. On Day 3, the air pouches were re-inflated with 3 cc of sterile air. On Day 6, Staphylococcus aureus were grown to an OD of 0.5 and then washed and diluted in sterile PBS to an OD of 0.2 (at 600 nm). Air pouches were injected with 0.5 cc of bacterial culture. At the indicated time points, the infected mice were sacrificed and the air pouches were lavaged with HBSS fortified with 0.15 M sodium citrate. Cells were counted and stained with CD11b-FITC (Pharmingen), Ly6G-PE (Pharmingen), and propidium iodide for analysis on a FacsScan. The amount of CD11b upregulation on live (PI negative) neutrophils (Ly6G positive) was measured as median fluorescence intensity. The live bacteria from the air pouch were plated to quantitate the number of live CFU, normalized to the initial number of bacteria put into the air pouch. In addition, supernatants from the air pouch lavage were used to measure MPO and MMP-9 release, as described above.
Statistical Analysis
Statistical analysis was conducted using the Students T-test, assuming unequal variances.
Results
Pyk2 deficiency in PMNs and macrophages
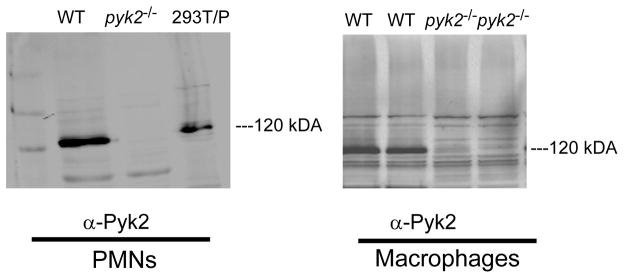
To validate that the pyk2 mutation resulted in complete loss of Pyk2 protein expression we examined PMNs and macrophages from pyk2−/− mice. Pyk2 protein was readily detected in WT PMNs and macrophages by immunoblotting of total cell lysates and was completely absent in cells derived from pyk2−/− animals (Fig. 1).
FIGURE 1.
Pyk2 expression is lost in PMNs and macrophages from pyk2−/− mice. WT or pyk2−/−PMNs and macrophages were lysed and probed with antibodies to Pyk2. 293T cells transfected with Pyk2 (indicated as 293T/P) served as a positive control. Data shown are representative of three independent experiments.
Pyk2-deficient PMNs display reduced integrin-mediated migration in vitro
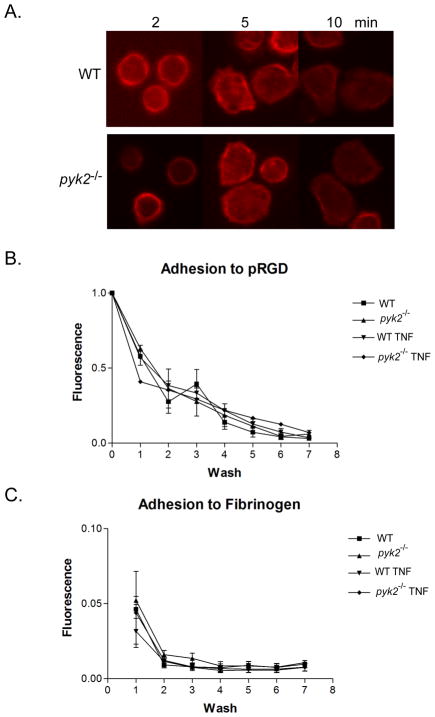
Since Pyk2 has been implicated in integrin signaling in fibroblasts, we began by examining integrin mediated adhesion and cell spreading, which is characterized by actin polymerization at cell lamellipodia (18, 19, 32), in pyk2−/− PMNs. Cells from WT or Pyk2-deficient mice were plated onto coverslips coated with pRGD, an artificial fibronectin-like molecule that ligates both β1 and β2 integrins (20, 31), then stained with phalloidin. No differences were observed between WT and Pyk2-deficient PMNs in their ability to spread and polymerize actin, as measured with confocal fluorescence microscopy (Fig. 2A). In concert with their ability to spread on integrin-coated surfaces, pyk2−/− PMNs also displayed normal adhesion to both pRGD and fibrinogen-coated microtiter wells in a static adhesion assay (Fig. 2B). These data suggested that Pyk2 deficiency did not affect the ability of PMNs to attach and adhere to integrin coated surfaces. In accordance with the adhesion results, the ability of the surface integrins to induce high affinity ligand binding was intact in the absence of Pyk2. When WT or pyk2−/− PMNs were stimulated with PMA, both PMNs populations showed similar levels of increased binding to fluorescently-labelled soluble dimeric ICAM (Supplemental Fig. 1). Similar results were observed with fMLF-stimulated PMNs from WT or pyk2−/− animals (data not shown).
FIGURE 2.
Analysis of integrin-mediated adhesion in pyk2−/− PMNs. A, PMNs from WT or pyk2−/− mice were allowed to adhere to pRGD-coated coverslips and fixed at the indicated time points. The fixed cells were stained for actin (red). Images shown are representative of three independent experiments. B, Fluorescently-labelled bone marrow PMNs from WT or pyk2−/−mice were plated on pRGD coated wells and then exposed to a series of washes in a static adhesion assay. The decrease in fluorescence corresponding to the decrease in cell number was measured by fold-change from the baseline value was plotted over the series of washes. C, Following the assay design in B, PMNs from WT or pyk2−/− mice were allowed to adhere to fibrinogen coated wells for 15 minutes. Error bars represent ± SEM of triplicate samples. Data shown are pooled from 3 independent experiments.
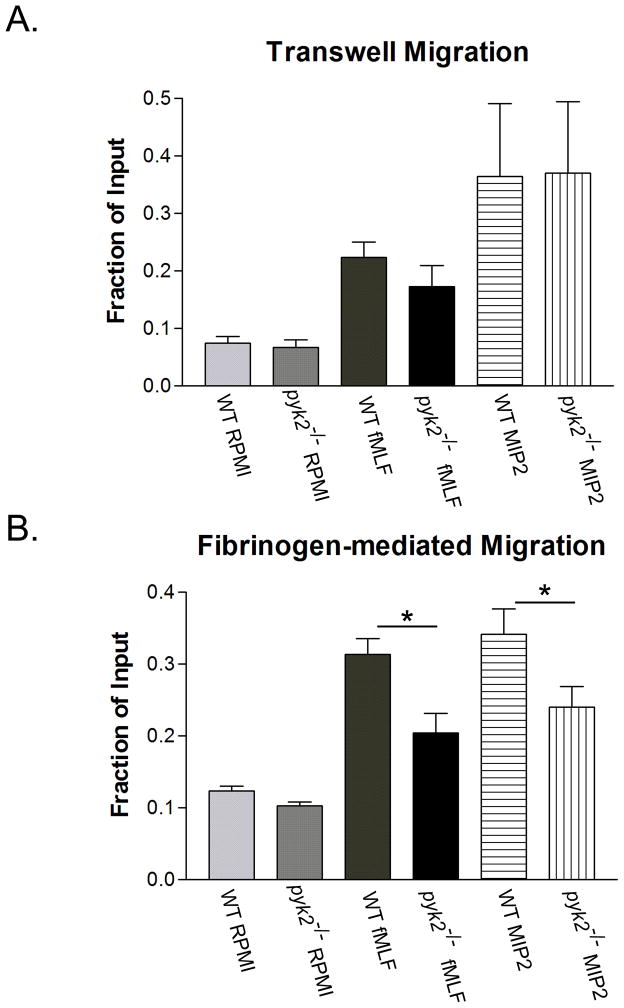
To examine the role of Pyk2 in PMN motility, we performed transwell chemotaxis assays. Using both fMLF and MIP2 as chemottractants, we found no difference in the ability of pyk2−/− PMNs to migrate across uncoated 3 micron pore sized filters (Fig 3A). However, when the transwells were coated with fibrinogen to engage β2 integrins, pyk2−/− PMNs were unable to migrate as efficiently as WT PMNs in response to both fMLF and MIP2 (Fig. 3B). The reduced migration was not the result of direct impairment of fMLF signaling per se, since suspended pyk2−/− PMNs showed normal Ca2+ signaling responses and granule release when stimulated with this agonist (Supplemental Fig. 2). Therefore, Pyk2 deficiency impairs integrin signaling required for maximal migratory response in vitro without dramatically reducing cell adhesion.
FIGURE 3.
In vitro migration in pyk2−/− PMNs. A, WT or pyk2−/− PMNs were plated upon transwells and allowed to migrate toward 0.1 μM fMLF or 10 ng/mL MIP2, with RPMI used as a control. After 30 minutes, the cells that had migrated through the pores were lysed and stained for phosphatase. The amount of phosphatase remaining on the wells was quantitated through absorbance and plotted as a percentage of the total number of cells seeded originally on the transwell. B, Following the methodology described in A, PMNs from WT or pyk2−/− mice were plated onto transwells that had been previously coated with fibrinogen. * = p< 0.05. Error bars represent ± SEM of duplicate samples from data averaged between three independent experiments.
Pyk2-deficient neutrophils display impaired integrin stimulated degranulation
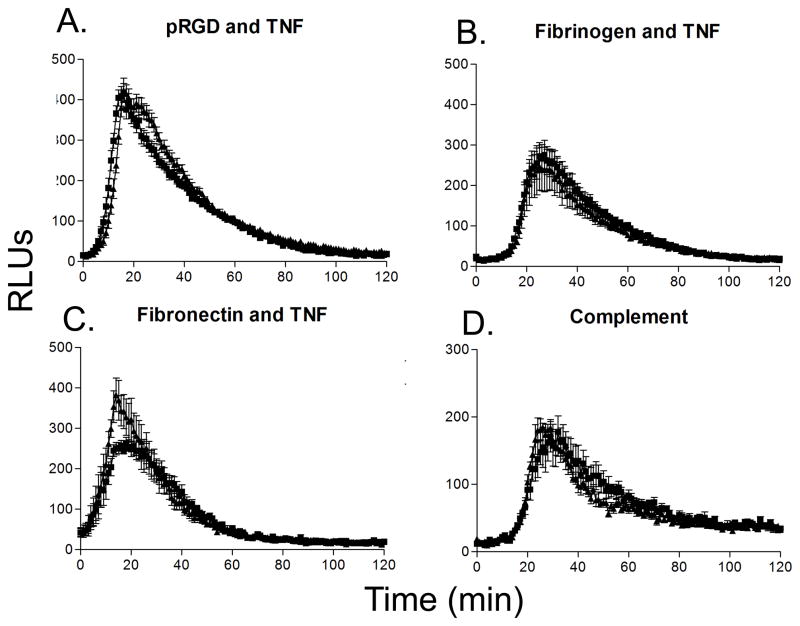
In addition to the functional events of adhesion and migration, integrin ligation leads to potent antibacterial responses, including superoxide production and degranulation in PMNs (33). Thus, we examined the ability of pyk2−/− PMNs to produce and release superoxide following integrin ligation using a luminol reduction assay. To our surprise, Pyk2-deficient PMNs showed no defect in superoxide production following plating on pRGD, fibrinogen or fibronectin in the presence of TNF (Fig. 4A – C). Pyk2−/− cells responded normally when plated on pRGD alone, which is a sufficiently high affinity integrin ligand that it induces superoxide production in the absence of the TNF co-stimulus (data not shown). Pyk2-deficient PMNs also produced normal levels of superoxide when stimulated with complement opsonized polystyrene beads (Fig. 4D). These observations conflict with reports from Fuortes et al (28) and Han et al (30), which suggest a role for Pyk2 in integrin mediated superoxide production using either tyrosine kinase inhibitors (tyrphostin A9) or an inhibitory tat fusion protein.
FIGURE 4.
Superoxide production in pyk2−/− PMNs. A, PMNs from WT or pyk2−/− mice were plated on pRGD coated surfaces for 2 hours in the presence of 100 ng/mL of TNF-α. The amount of superoxide released by the cells was measured via luminol reduction. B, PMNs from the indicated mice were plated on fibrinogen-coated surfaces for 2 hours in the presence of 100 ng/mL of TNF. C, PMNs from WT or pyk2−/− mice were plated on fibronectin-coated surfaces for 2 hours in the presence of 100 ng/mL of TNF-α. D, PMNs from the indicated mice were exposed to complement-coated beads for 2 hours in the presence of 50 ng/mL of TNF-α. Data are the mean of at least 3 independent experiments each with n = 3. Error bars represent ± SEM.
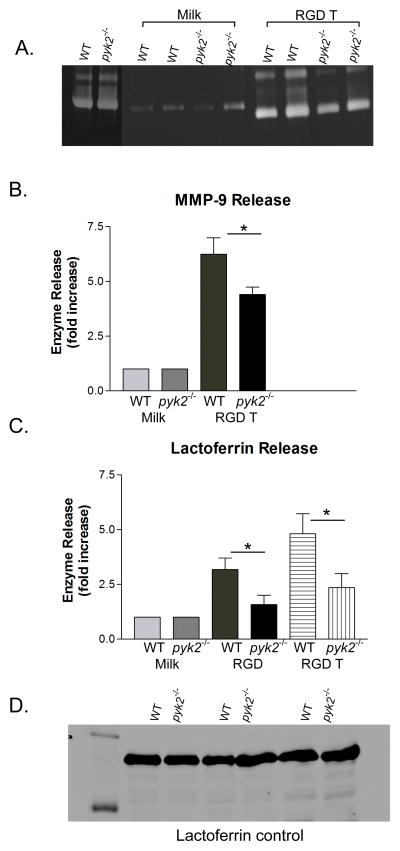
Integrin ligation in the presence of TNF also induces robust PMN degranulation, which can be assessed by following release of granule constituent proteins, such as MMP-9 or lactoferrin, into the extracellular milieu (16, 34). Following plating on pRGD in the presence of TNF, pyk2−/− PMNs showed a significant reduction, but not a complete block, in MMP-9 release as determined by protein zymography (Fig. 5A and B). Similarly, pyk2−/− PMNs exhibited reduced release of the secondary granule component lactoferrin, as determined by ELISA assay in cell supernatants, following plating on pRGD either in the presence or absence of TNF co-stimulation (Fig. 5C). The reduced release of MMP-9 and lactoferrin was not caused by poor production of these proteins in pyk2−/− versus WT cells, since intracellular amounts of both were equivalent in both PMN types, as determined by immunoblotting (Fig. 5A and D). Adhesion to pRGD was insufficient to stimulate a significant amount of primary (azurophilic) granule release, hence in this in vitro assay we could not assess differences between WT versus Pyk2-deficient PMNs. Exocytosis of MMP-9, lactoferrin and MPO were normal in pyk2−/− cells stimulated in suspension with fMLF, thus ruling out a general defect in the degranulation machinery per se (Supplemental Fig. 2). We conclude that Pyk2 deficiency in PMNs resulted in impairment of release of at least two different granule subtypes (MMP-9 and secondary granules) following integrin-mediated stimulation.
FIGURE 5.
Degranulation response in pyk2−/− PMNs. To measure integrin-mediated degranulation responses, PMNs from WT or pyk2−/− mice were isolated and plated onto pRGD-coated surfaces in the presence or absence of 100 ng/mL TNF-α for 1 hour. Supernatants from the stimulated PMNS were isolated for analysis. A, The ability of the PMNs to release MMP-9 upon integrin ligation was measured on a zymogram gel. B, Levels of MMP-9 were normalized to the level released by PMNs plated on milk-blocked wells. C, The amount of lactoferrin released into the supernatant by PMNs from WT or pyk2−/− mice was measured via Ab plate-bound ELISA (n=18). D, Control immunoblot probing for levels of lactoferrin in WT or pyk2−/− PMNs. Data are the mean of at least 3 independent experiments. Error bars represent ± SEM. * = p<0.05.
Pyk2-deficient PMNs show normal complement-mediated bacterial phagocytosis and killing in vitro
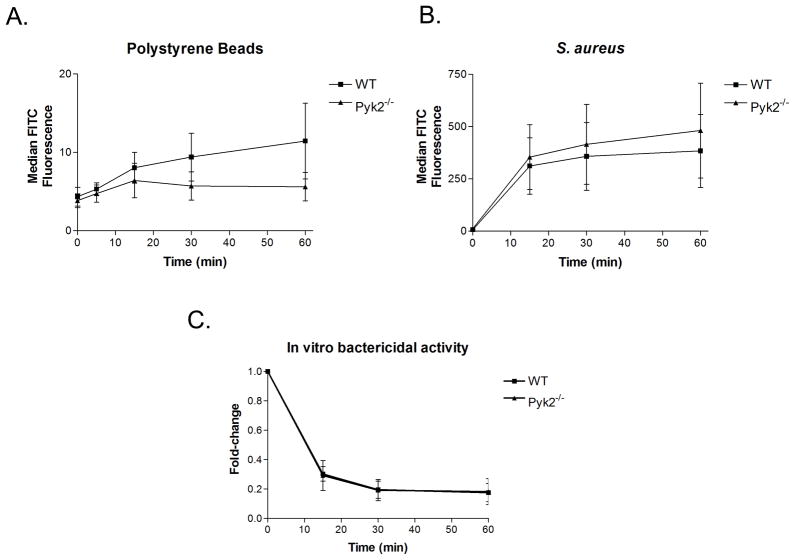
Integrins play a significant role in host defense by both directing leukocyte adhesion and migration to sites of pathogen invasion but also by directly activating PMNs through engagement of complement-opsonized bacteria. To determine if Pyk2 deficiency resulted in impairment of anti-bacterial responses in vitro we stimulated WT versus pyk2−/− cells with complement-opsonized S. aureus. As shown in Fig. 6A–B, using a flow cytometric assay we found no apparent difference in the phagocytosis of complement-opsonized polystyrene beads or S. aureus between WT and pyk2−/− PMNs, which was consistent with the normal actin polymerization responses seen in Pyk2-deficient cells following integrin mediated adhesion. In addition, pyk2−/−PMNs were able to internalize and kill similar numbers of live complement-opsonized S. aureus as measured in an in vitro bactericidal assay (Fig. 6C). The lack of a defect in bactericidal activity corresponds to the previous findings with Pyk2 inhibitors in human PMNs (28, 30).
FIGURE 6.
Antibacterial responses in pyk2−/− PMNs. A, B, Rates of complement-mediated phagocytosis were compared in WT and pyk2−/− PMNs using FITC-labelled complement-opsonized polystyrene beads, A, or complement-opsonized S. aureus, B. The rate of phagocytosis in PMNs was measured via FACs analysis using median FITC fluorescence as the degree of uptake over time. Data are representative of 3 independent experiments. C, The in vitro bactericidal activity of WT and pyk2−/− PMNs was measured over time. Complement-opsonized S. aureus was fed to PMNs and then the amount of live bacteria over time was measured as fold-change from CFUs at timepoint zero. Data averaged from 3 independent experiments. Error bars represent ± SEM.
Impaired integrin stimulated paxillin and Vav phosphorylation in pyk2−/− PMNs
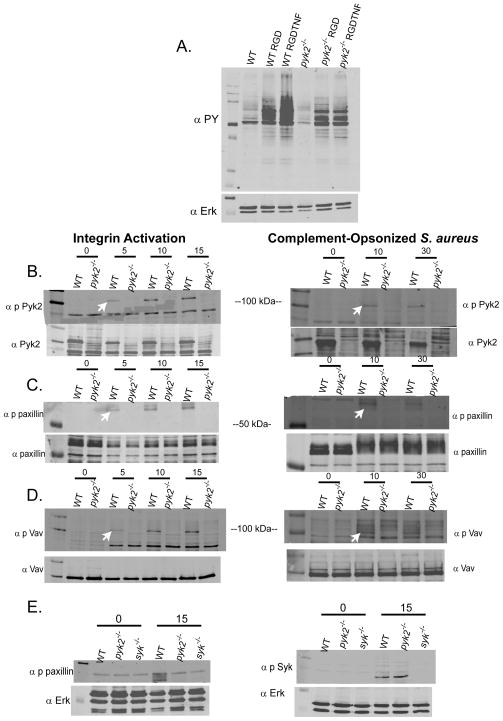
To begin to map out where in the integrin signaling pathway Pyk2 is functioning, we examined signaling responses in PMNs following plating on pRGD or during stimulation with complement opsonized bacteria. As shown in Fig. 7A, adhesion of PMNs to pRGD induced robust increase in overall protein tyrosine phosphorylation, which was further increased with TNF co-stimulation. The overall protein tyrosine phosphorylation response was blunted in pyk2−/− cells, though not nearly as severely reduced as seen in the absence of Src-family or Syk kinases (20). One of these newly tyrosine phosphorylated proteins was Pyk2 itself which progressively increased over time following either adhesion to pRGD in the presence or absence of TNF or during phagocytosis of complement-opsonized S. aureus (Fig. 7B). Paxillin is also known to become tyrosine phosphorylated following integrin ligation and it associates directly with Pyk2 (28, 35, 36). Paxillin tyrosine phosphorylation was completely blocked in pyk2−/− cells following either plating on pRGD or following stimulation with complement opsonized S. aureus (Fig. 7C). Paxillin phosphorylation has been implicated in activation of both the MAPK and Rac signaling pathways (37, 38). Vav is a major GEF of the Rac and Rho GTPases and is activated via tyrosine phosphorylation following integrin ligation (39). Like paxillin, Vav phosphorylation was completely lost in pyk2−/− cells following integrin engagement either by pRGD or complement opsonized S. aureus (Fig. 7D). In contrast, downstream activation of the Erk and AKT pathways following integrin ligation were normal in pyk2−/− cells (Supplemental Fig. 3A and B), indicating that only certain aspects of the integrin signaling response were affected by Pyk2 deficiency.
FIGURE 7.
Signal activation following integrin ligation in pyk2−/− PMNs. A, WT or pyk2−/−bone marrow-derived PMNs were plated on pRGD in the presence or absence of 100 ng/mL TNF for 15 minutes. PMNs in suspension were used as control. Lysates were immunoblotted for phosphotyrosine (4G10 antibody) with Erk1 used as an equal loading control. B–D, WT or pyk2−/− PMNs were activated on pRGD for 0, 5, 10 or 15 minutes (left panels) or by exposure to complement-opsonized S. aureus for 0, 10 or 30 minutes (right panels) and then lysed. Lysates were immunoblotted with Abs specific for phospho-Pyk2, phospho-paxillin or phospho-Vav. Arrows indicate the appropriate bands in each gel. Lower panels show the same lysates probed for total Pyk2, paxillin, and Vav as loading controls. Arrows indicate specific bands. Data are representative of 2–6 different experiments for each panel. E, Lysates from WT, pyk2−/− and syk−/− PMNs activated on pRGD for 15 minutes were probed for phospho-paxillin and phospho-Syk as indicated with Erk1 serving as an equal loading control.
In fibroblasts, phosphorylation of Pyk2 following integrin ligation is mediated by Src-family kinases suggesting that Pyk2 is downstream of Src-family members (19). Similarly, Syk kinase activation following integrin ligation occurs downstream of Src-family kinases (17). However, the relationship between Syk and Pyk2 activation is less clear. Previous work in our lab has shown that phosphorylation of Pyk2 is blocked in Syk-deficient PMNs following integrin ligation (20). To further examine the relationship between these two kinases, we determined Syk phosphorylation in pyk2−/− PMNs following plating on pRGD. As shown in Fig. 7E, Syk phosphorylation was normal in pyk2−/− cells, suggesting that like Src-family kinases, Syk is upstream of Pyk2 in the integrin signaling pathway. Consistent with Src-family kinases being upstream of Pyk2, we also observed reduced paxillin phosphorylation in PMNs derived from hck−/−fgr−/−lyn−/− mice, as well as from fgr−/− single mutant animals, suggesting that Fgr is the dominant Src kinase signaling to Pyk2/paxillin (Supplemental Fig. 3C). Likewise, paxillin phosphorylation was also lost in syk−/− PMNs (Fig. 7E).
Pyk2−/− mice have impaired clearance of S. aureus in vivo
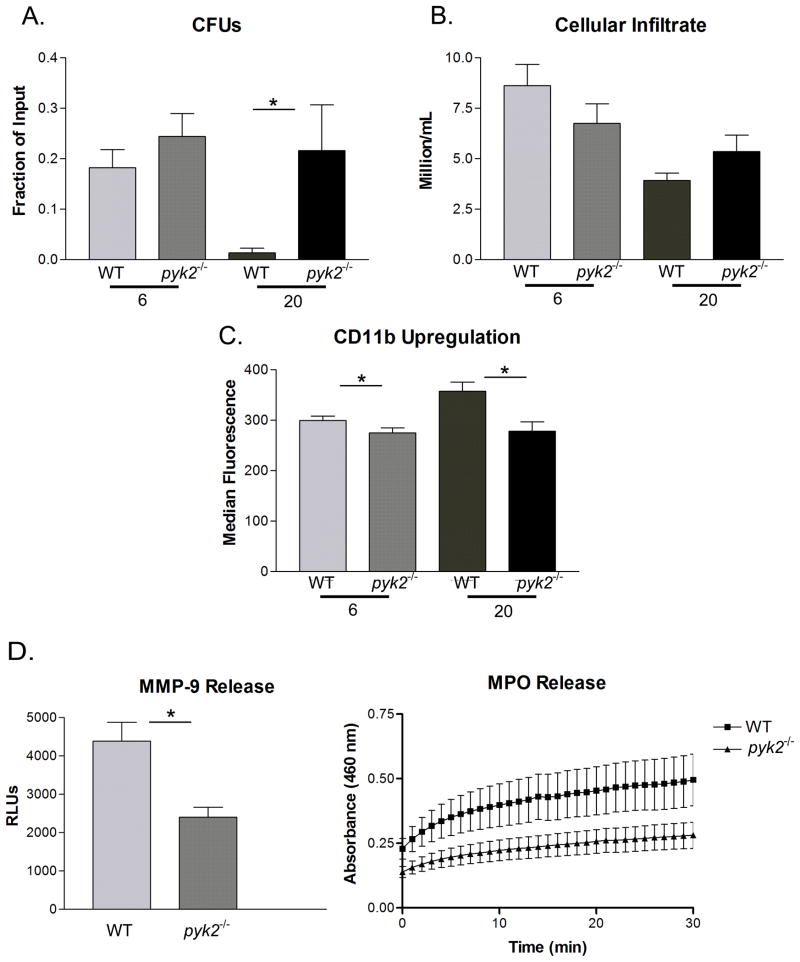
Given the reduced degranulation and migration responses of pyk2−/− PMNs, as well as the impaired signaling responses, we examined the ability of pyk2−/− mice to mount host defense responses in vivo. We utilized a non-lethal skin abscess model of S. aureus infection by generation of air filled pouches in the dermis of mice into which bacteria were introduced. Use of the air pouch approach allowed sampling of infection site for leukocyte recruitment and bacterial loads (measured as colony forming units – CFUs). In this model, S. aureus clearance is mediated predominantly by PMNs, which are the dominant inflammatory cell type recruited to the air pouch over the first 48 hours of infection (40). Within 6 hours following infection, bacteria were readily found within the air pouches of both WT and pyk2−/− mice, however by 20 hours WT mice had nearly cleared the infection while the S. aureus titer remained high in the Pyk2 deficient animals (Fig. 8A). At both the 6 and 20 hour time points, pyk2−/− PMNs were recruited to the infected air pouch as efficiently as WT cells, with a trend to even higher PMN recruitment in the pyk2−/− mice at 20 hours likely because of higher bacterial load (Fig. 8B). However, the pyk2−/− PMNs within the air pouch showed reduced evidence of activation at both time points, as indicated by lower expression of CD11b compared to WT cells (Fig. 8C). More importantly, by 20 hours post infection, the amount of primary granule constituent myeloperioxidase (MPO) and the tertiary granule component MMP-9 were dramatically lower in the infected air pouches of pyk2−/− mice compared to WT animals (Fig. 8D), which directly correlates with the reduced degranulation responses seen in Pyk2 deficient PMNs in vitro. Other cell types, such as macrophages, mast cells or lymphocytes could also play a role in the clearance of S. aureus from the airpouch. However, cytospins of the airpouch lavage revealed predominantly PMN infiltrate with some macrophages (Supplemental Fig. 5A). Flow cytometry confirmed that the vast majority of infiltrating cells were PMNs, as previously reported (40), which was equivalent in WT versus pyk2−/− mice. Moreover, pyk2−/− macrophages showed no defect in MPO release following integrin ligation compared to WT cells (Supplemental Fig. 5B) suggesting the degranulation defect is more pronounced in PMNs. Additionally, pyk2−/−macrophages showed no defects in phagocytosis or TLR responses (to a number of agonists – data not shown). Thus, these data demonstrate that Pyk2 deficient mice show reduced host defense to S. aureus infection in vivo, likely in part to diminished activation and degranulation responses from PMNs.
FIGURE 8.
In vivo host defense response to bacterial infection. WT or pyk2−/− mice with dorsally-located air pouches were infected with S. aureus. Following 6 or 20 hours of infection, the air pouches were lavaged and the number of bacteria or infiltrated PMNs was determined. A, The number of live bacteria (CFU)/mL in the air pouch lavage shown as the fraction of the original amount of CFU loaded into the air pouch. B, The number of PMNs infiltrating the airpouch was quantitated. C, The levels of CDllb upon the surface of PMNs in the lavage (measured on Ly6G+ gated cells) was quantitated by median fluorescence intensity (data representative of at least 3 independent experiments). D, The amount of MMP-9 released into the air pouch following 6 hours of infection was quantitated via zymography (n=8). E, The levels of MPO released into the air pouch following 6 hours of infection was measured via absorbance assay. Data represent average of at least 3 independent experiments with n = 4 or n = 5. Error bars represent ± SEM. *=p<0.05.
Discussion
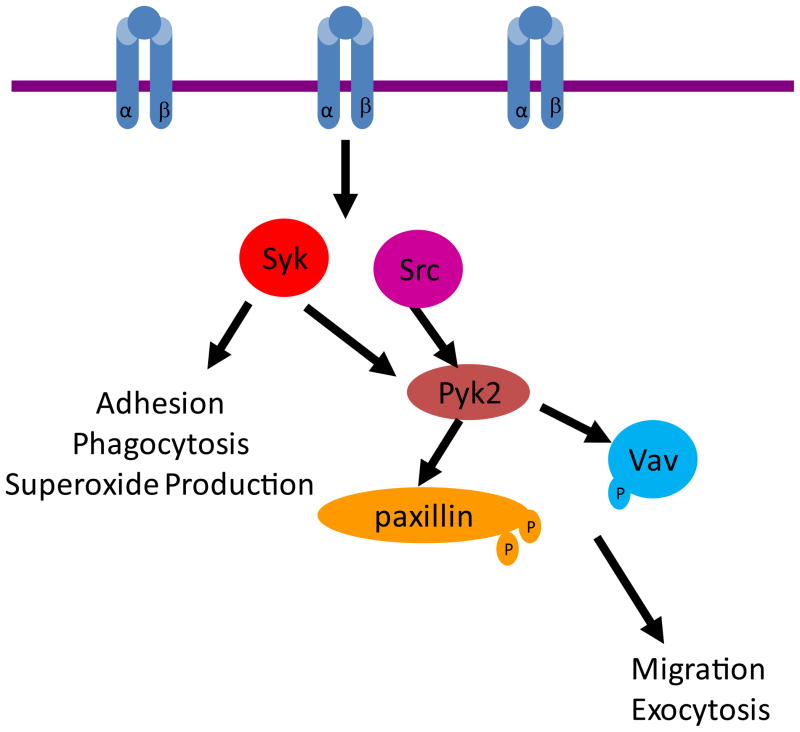
This study demonstrates an important role for the non-receptor tyrosine kinase Pyk2 in the integrin-mediated activation of PMNs that contributes to normal degranulation responses required for efficient host defense to S. aureus infection. Pyk2 deficient PMNs exhibited reduced degranulation responses following integrin ligation both in vitro and during bacterial infection in vivo; however, they responded normally to soluble agonists suggesting that the integrin signaling pathway was the major response affected in the pyk2 mutant cells. It is clear that unlike Src-family or Syk tyrosine kinases, Pyk2 is acting in a more distal step in the integrin signaling pathway since many integrin mediated functions were normal in pyk2−/− PMNs, including attachment, adhesion and integrin mediated activation of superoxide production. These limited impairments correlate with the only partially reduced integrin mediated tyrosine phosphorylation responses, though reduction in phosphorylation of specific substrates such as paxillin and Vav were observed. Our model for the role of Pyk2 in integrin-mediated activation shows it functioning downstream of both Src and Syk kinases to facilitate degranulation and migration responses through pathways involving paxillin and Vav (Supplemental Fig. 5).
It is interesting that pyk2−/− PMNs showed no defects in in vitro responses to bacteria but were compromised in their ability to clear a bacterial infection in vivo. These differences can perhaps be attributed to the artificial nature of in vitro assays in general. In the in vitro bactericidal assays, there is a very large stimulus in the form of complement-opsonized S. aureus, which maximally activates complement receptors, TLRs and GPCRs at levels that are not likely to be present in vivo. Thus, in the in vitro system, only very gross defects are observed. However, more subtle defects, such as those observed with pyk2−/− mice, are revealed in the physiological in vivo system. It was in the in vivo model of infection that the defect in Pyk2-dependent integrin signaling, which resulted in impaired degranulation, impacted the bacterial clearance.
This report, which is the first examination of PMN function in pyk2−/− mice, allows one to compare the effects of genetic deficiency of Pyk2 with chemical or peptide inhibitory approaches to study this enzyme (28, 30). Fuortes et al demonstrated that the tyrphostin A9 inhibitor was the most potent blocker of integrin-mediated activation of PMN superoxide release among a panel of tyrosine kinase inhibitors and that treatment of cells with tyrphostin A9 resulted in loss of Pyk2 phosphorylation. This inhibitor also blocked PMN spreading, leading the investigators to conclude that Pyk2 participates in the signaling cascade leading to respiratory burst in TNF-treated adherent PMNs. However, it is unlikely that tyrphostin A9 is specific for Pyk2 since it was developed to inhibit PDGF receptor tyrosine kinase (41) and also affects Ca2+ entry in CD3 stimulated Jurkat cells (42). Using protein transduction of the COOH terminus of Pyk2 fused to a Tat peptide as a dominant negative inhibitor of Pyk2, Han et al suggested that Pyk2 is required for TNF-mediated superoxide release as well as PMN spreading, confirming prior results with tyrphostin A9 inhibition. In contrast, the COOH-Pyk2/Tat fusion did not affect PMN degranulation in adherent PMNs or alter bacterial killing. These results, done with human PMNs, obviously differ significantly from our observations with Pyk2-deficient murine PMNs. There are many potential explanations for these disparate observations, such as potential compensation for Pyk2 deficiency by other signaling molecules, differences in experimental approaches or differences between human and murine cells. Though difficult to prove, it remains a formal possibility that the pyk2−/− mutation generated in these mice may not be specific to pyk2 alone; ie the ES cells or mice themselves may have acquired other mutations.
Given that these studies were done with mice backcrossed for 8 generations, this reduces the probability that other mutations present in the ES cells may be contributing to this phenotype. Similarly, we found no differences in expression level of Src-family kinases, Syk, Vav, paxillin, Akt, Erk, p38, Rac, Rho, Cdc42, PAK1, or myosin light chain kinase (all signaling molecules in the integrin pathway) between WT and pyk2−/− PMNs. Clearly there could be changes in other signaling molecules that could contribute to the PMN phenotype in pyk2−/− mice; additional biochemical studies will be needed to sort out the exact pathways in PMN integrin signaling in which this kinase functions. Given that Pyk2-deficient PMNs showed a defect in degranulation responses, which very likely contributed to poor control of S. aureus infection in vivo, without a major defect in superoxide production, suggests that the pathways leading to these two responses are parallel and are not controlled by the same signals. This then leads to the question of how degranulation and exocytosis are controlled in PMNs following integrin-mediated activation. The complete molecular mechanism underlying control of degranulation by Pyk2 remains to be determined, however the dramatically impaired phosphorylation of paxillin and Vav observed in pyk2−/− cells may provide clues. Both paxillin and Vav activation are associated with the activation and control of the Rho family GTPases. Paxillin can bind to the adaptor Crk which can lead directly to activation of the Rac GTPases following integrin-mediated adhesion (37). Paxillin has also been shown to suppress the activation of RhoA. Similarly, Vav tyrosine phosphorylation is directly associated with activation of both the Rac and Rho GTPases (39). Given the normal production of superoxide in pyk2−/− PMNs following integrin ligation, it is unlikely that Rac2 is affected in these cells, since Rac2 is an important component of the NADPH oxidase in PMNs leading to superoxide production (43). While several studies have suggested that Rac GTPases play roles in PMN degranulation in response to soluble agonists (44, 45), the potential role of Rac and Rho in regulating degranulation downstream of integrin ligation remains unclear. Using PAK-GST pulldown approaches and mAbs designed to recognize GTP-bound activated Rac, we were unable to demonstrate a significant impairment in Rac1 or 2 activation in pyk2−/− deficient cells (data not shown). Examination of activation of downstream targets of Rac and Rho (PAK1 and MLC2, respectively) also showed no differences (data not shown). Hence it is possible that activation of other small GTPases via paxillin and Vav may be contributing to the degranulation phenotype we observed in pyk2−/− cells. Paxillin also serves as an important scaffolding protein and has a direct affect on microtubule assembly (46). Perhaps through paxillin phosphorylation, Pyk2 may contribute to microtubule polarization and assembly during integrin mediated degranulation responses, which remains to be investigated.
In summary, Pyk2 is playing a physiologically significant role primarily in integrin induced responses in PMNs. However, given its more downstream role compared to other non-receptor tyrosine kinases, Pyk2 deficiency does not produce as profound a defect in signaling as Src-family or Syk kinase loss. Hence, therapeutic targeting of Pyk2 may be useful in producing a more mild disruption in integrin function than blockade of Src-family or Syk kinases.
Supplementary Material
FIGURE 9.
Acknowledgments
This work was supported by the National Institutes of Health (AI65495 and AI68150 to C.A.L.).
We thank Anthony DeFranco, Art Weiss and Soren Beinke for valuable advice. We would also like to thank Clare Abram, Chrystelle Lamagna, Allison Miller, Patrizia Scapini, and Jessica Van Ziffle for advice and thoughtful discussion regarding in vitro and in vivo models of PMN integrin activation.
Abbreviations used in this paper
- PMNs
polymorphonuclear leukocytes or neutrophils
- Pyk 2
proline rich kinase 2
- FAK
focal adhesion kinase
- RLUs
relative light units
- MOI
multiplicity of infection
- MMP9
matrix metalloproteinase 9
- MPO
myeloperoxidase
- fMLF
formyl-methionyl-leucyl-phenylalanine peptide
- MIP2
macrophage inflammatory protein-2, also known as CXLC2
- pRGD
poly RGD peptide
Footnotes
Disclosures
The authors have no conflicting financial interests.
References
- 1.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 2.Cullere X, Lauterbach M, Tsuboi N, Mayadas TN. Neutrophil-selective CD18 silencing using RNA interference in vivo. Blood. 2008;111:3591–3598. doi: 10.1182/blood-2007-12-127837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abram CL, Lowell CA. The expanding role for ITAM-based signaling pathways in immune cells. Sci STKE. 2007;2007:re2. doi: 10.1126/stke.3772007re2. [DOI] [PubMed] [Google Scholar]
- 4.Lowell CA, Fumagalli L, Berton G. Deficiency of Src-family kinases p59/61hck and p58c-fgr results in defective adhesion-dependent neutrophil functions. The Journal of cell biology. 1996;133:895–910. doi: 10.1083/jcb.133.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science (New York, NY) 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 6.Miyamoto S, Teramoto H, Coso OA, Gutkind JS, Burbelo PD, Akiyama SK, Yamada KM. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. The Journal of cell biology. 1995;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berton G, Lowell CA. Integrin signalling in neutrophils and macrophages. Cellular signalling. 1999;11:621–635. doi: 10.1016/s0898-6568(99)00003-0. [DOI] [PubMed] [Google Scholar]
- 8.Van Ziffle JA, Lowell CA. Neutrophil-specific deletion of Syk kinase results in reduced host defense to bacterial infection. Blood. 2009;114:4871–4882. doi: 10.1182/blood-2009-05-220806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexander J, Scharton-Kersten TM, Yap G, Roberts CW, Liew FY, Sher A. Mechanisms of innate resistance to Toxoplasma gondii infection. Philos Trans R Soc Lond B Biol Sci. 1997;352:1355–1359. doi: 10.1098/rstb.1997.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mircescu MM, Lipuma L, van Rooijen N, Pamer EG, Hohl TM. Essential role for neutrophils but not alveolar macrophages at early time points following Aspergillus fumigatus infection. J Infect Dis. 2009;200:647–656. doi: 10.1086/600380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 12.Kinashi T. Intracellular signalling controlling integrin activation in lymphocytes. Nat Rev Immunol. 2005;5:546–559. doi: 10.1038/nri1646. [DOI] [PubMed] [Google Scholar]
- 13.Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 14.Brown EJ. Complement receptors and phagocytosis. Curr Opin Immunol. 1991;3:76–82. doi: 10.1016/0952-7915(91)90081-b. [DOI] [PubMed] [Google Scholar]
- 15.Allen LA, Aderem A. Molecular definition of distinct cytoskeletal structures involved in complement- and Fc receptor-mediated phagocytosis in macrophages. The Journal of experimental medicine. 1996;184:627–637. doi: 10.1084/jem.184.2.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faurschou M, Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 2003;5:1317–1327. doi: 10.1016/j.micinf.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Abram CL, Lowell CA. The diverse functions of Src-family kinases in macrophages. Front Biosci. 2008;13:4426–4450. doi: 10.2741/3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rose DM, Alon R, Ginsberg MH. Integrin modulation and signaling in leukocyte adhesion and migration. Immunol Rev. 2007;218:126–134. doi: 10.1111/j.1600-065X.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- 19.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nature reviews. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 20.Mocsai A, Zhou M, Meng F, Tybulewicz VL, Lowell CA. Syk is required for integrin signaling in neutrophils. Immunity. 2002;16:547–558. doi: 10.1016/s1074-7613(02)00303-5. [DOI] [PubMed] [Google Scholar]
- 21.Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio JM, Plowman GD, Rudy B, Schlessinger J. Protein tyrosine kinase PYK2 involved in Ca(2+)-induced regulation of ion channel and MAP kinase functions. Nature. 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- 22.Avraham H, Park SY, Schinkmann K, Avraham S. RAFTK/Pyk2-mediated cellular signalling. Cellular signalling. 2000;12:123–133. doi: 10.1016/s0898-6568(99)00076-5. [DOI] [PubMed] [Google Scholar]
- 23.Astier A, Avraham H, Manie SN, Groopman J, Canty T, Avraham S, Freedman AS. The related adhesion focal tyrosine kinase is tyrosine-phosphorylated after beta1-integrin stimulation in B cells and binds to p130cas. The Journal of biological chemistry. 1997;272:228–232. doi: 10.1074/jbc.272.1.228. [DOI] [PubMed] [Google Scholar]
- 24.Sieg DJ, Ilic D, Jones KC, Damsky CH, Hunter T, Schlaepfer DD. Pyk2 and Src-family protein-tyrosine kinases compensate for the loss of FAK in fibronectin-stimulated signaling events but Pyk2 does not fully function to enhance FAK-cell migration. The EMBO journal. 1998;17:5933–5947. doi: 10.1093/emboj/17.20.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qian D, Lev S, van Oers NS, Dikic I, Schlessinger J, Weiss A. Tyrosine phosphorylation of Pyk2 is selectively regulated by Fyn during TCR signaling. The Journal of experimental medicine. 1997;185:1253–1259. doi: 10.1084/jem.185.7.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dikic I, Tokiwa G, Lev S, Courtneidge SA, Schlessinger J. A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature. 1996;383:547–550. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- 27.Duong LT, Lakkakorpi PT, Nakamura I, Machwate M, Nagy RM, Rodan GA. PYK2 in osteoclasts is an adhesion kinase, localized in the sealing zone, activated by ligation of alpha(v)beta3 integrin, and phosphorylated by src kinase. The Journal of clinical investigation. 1998;102:881–892. doi: 10.1172/JCI3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuortes M, Melchior M, Han H, Lyon GJ, Nathan C. Role of the tyrosine kinase pyk2 in the integrin-dependent activation of human neutrophils by TNF. The Journal of clinical investigation. 1999;104:327–335. doi: 10.1172/JCI6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okigaki M, Davis C, Falasca M, Harroch S, Felsenfeld DP, Sheetz MP, Schlessinger J. Pyk2 regulates multiple signaling events crucial for macrophage morphology and migration. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:10740–10745. doi: 10.1073/pnas.1834348100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han H, Fuortes M, Nathan C. Critical role of the carboxyl terminus of proline-rich tyrosine kinase (Pyk2) in the activation of human neutrophils by tumor necrosis factor: separation of signals for the respiratory burst and degranulation. The Journal of experimental medicine. 2003;197:63–75. doi: 10.1084/jem.20021638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mocsai A, Abram CL, Jakus Z, Hu Y, Lanier LL, Lowell CA. Integrin signaling in neutrophils and macrophages uses adaptors containing immunoreceptor tyrosine-based activation motifs. Nature immunology. 2006;7:1326–1333. doi: 10.1038/ni1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu S, Thomas SM, Woodside DG, Rose DM, Kiosses WB, Pfaff M, Ginsberg MH. Binding of paxillin to alpha4 integrins modifies integrin-dependent biological responses. Nature. 1999;402:676–681. doi: 10.1038/45264. [DOI] [PubMed] [Google Scholar]
- 33.Geiszt M, Kapus A, Ligeti E. Chronic granulomatous disease: more than the lack of superoxide? Journal of leukocyte biology. 2001;69:191–196. [PubMed] [Google Scholar]
- 34.Logan MR, Odemuyiwa SO, Moqbel R. Understanding exocytosis in immune and inflammatory cells: the molecular basis of mediator secretion. J Allergy Clin Immunol. 2003;111:923–932. quiz 933. [PubMed] [Google Scholar]
- 35.Li X, Earp HS. Paxillin is tyrosine-phosphorylated by and preferentially associates with the calcium-dependent tyrosine kinase in rat liver epithelial cells. The Journal of biological chemistry. 1997;272:14341–14348. doi: 10.1074/jbc.272.22.14341. [DOI] [PubMed] [Google Scholar]
- 36.Sancho D, Nieto M, Llano M, Rodriguez-Fernandez JL, Tejedor R, Avraham S, Cabanas C, Lopez-Botet M, Sanchez-Madrid F. The tyrosine kinase PYK-2/RAFTK regulates natural killer (NK) cell cytotoxic response, and is translocated and activated upon specific target cell recognition and killing. The Journal of cell biology. 2000;149:1249–1262. doi: 10.1083/jcb.149.6.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deakin NO, Turner CE. Paxillin comes of age. Journal of cell science. 2008;121:2435–2444. doi: 10.1242/jcs.018044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turner CE. Paxillin and focal adhesion signalling. Nature cell biology. 2000;2:E231–236. doi: 10.1038/35046659. [DOI] [PubMed] [Google Scholar]
- 39.Bustelo XR. Regulatory and signaling properties of the Vav family. Molecular and cellular biology. 2000;20:1461–1477. doi: 10.1128/mcb.20.5.1461-1477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clemens RA, Lenox LE, Kambayashi T, Bezman N, Maltzman JS, Nichols KE, Koretzky GA. Loss of SLP-76 expression within myeloid cells confers resistance to neutrophil-mediated tissue damage while maintaining effective bacterial killing. J Immunol. 2007;178:4606–4614. doi: 10.4049/jimmunol.178.7.4606. [DOI] [PubMed] [Google Scholar]
- 41.Levitzki A, Gilon C. Tyrphostins as molecular tools and potential antiproliferative drugs. Trends Pharmacol Sci. 1991;12:171–174. doi: 10.1016/0165-6147(91)90538-4. [DOI] [PubMed] [Google Scholar]
- 42.Marhaba R, Mary F, Pelassy C, Stanescu AT, Aussel C, Breittmayer JP. Tyrphostin A9 inhibits calcium release-dependent phosphorylations and calcium entry via calcium release-activated channel in Jurkat T cells. J Immunol. 1996;157:1468–1473. [PubMed] [Google Scholar]
- 43.Li S, Yamauchi A, Marchal CC, Molitoris JK, Quilliam LA, Dinauer MC. Chemoattractant-stimulated Rac activation in wild-type and Rac2-deficient murine neutrophils: preferential activation of Rac2 and Rac2 gene dosage effect on neutrophil functions. J Immunol. 2002;169:5043–5051. doi: 10.4049/jimmunol.169.9.5043. [DOI] [PubMed] [Google Scholar]
- 44.Glogauer M, Marchal CC, Zhu F, Worku A, Clausen BE, Foerster I, Marks P, Downey GP, Dinauer M, Kwiatkowski DJ. Rac1 deletion in mouse neutrophils has selective effects on neutrophil functions. J Immunol. 2003;170:5652–5657. doi: 10.4049/jimmunol.170.11.5652. [DOI] [PubMed] [Google Scholar]
- 45.Abdel-Latif D, Steward M, Macdonald DL, Francis GA, Dinauer MC, Lacy P. Rac2 is critical for neutrophil primary granule exocytosis. Blood. 2004;104:832–839. doi: 10.1182/blood-2003-07-2624. [DOI] [PubMed] [Google Scholar]
- 46.Efimov A, Schiefermeier N, Grigoriev I, Ohi R, Brown MC, Turner CE, Small JV, Kaverina I. Paxillin-dependent stimulation of microtubule catastrophes at focal adhesion sites. Journal of cell science. 2008;121:196–204. doi: 10.1242/jcs.012666. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.