Abstract
The Caenorhabditis elegans class A and B synthetic multivulva (synMuv) genes redundantly antagonize an EGF/Ras pathway to prevent ectopic vulval induction. We identify a class A synMuv mutation in the promoter of the lin-3 EGF gene, establishing that lin-3 is the key biological target of the class A synMuv genes in vulval development and that the repressive activities of the class A and B synMuv pathways are integrated at the level of lin-3 expression. Using FISH with single mRNA molecule resolution, we find that lin-3 EGF expression is tightly restricted to only a few tissues in wild-type animals, including the germline. In synMuv double mutants, lin-3 EGF is ectopically expressed at low levels throughout the animal. Our findings reveal that the widespread ectopic expression of a growth factor mRNA at concentrations much lower than that in the normal domain of expression can abnormally activate the Ras pathway and alter cell fates. These results suggest hypotheses for the mechanistic basis of the functional redundancy between the tumor-suppressor-like class A and B synMuv genes: the class A synMuv genes either directly or indirectly specifically repress ectopic lin-3 expression; while the class B synMuv genes might function similarly, but alternatively might act to repress lin-3 as a consequence of their role in preventing cells from adopting a germline-like fate. Analogous genes in mammals might function as tumor suppressors by preventing broad ectopic expression of EGF-like ligands.
Author Summary
Extracellular signals that drive cells to divide must be carefully restricted so that only the correct cells receive those signals. Failure to properly control the expression of signaling molecules can lead to aberrant development and cancer. Studies of vulval development in the nematode Caenorhabditis elegans have helped define various multi-step signaling pathways involved in cancer. Here we report that two groups of proteins that control the EGF/Ras/MAP kinase pathway of vulval development act by tightly repressing the spatial expression of the gene lin-3, which encodes an EGF-like signaling molecule. Using a technique that detects single mRNA molecules, we show that inactivation of these proteins causes a low ectopic expression of lin-3 in many cells. In response, the EGF/Ras/MAP kinase pathway is activated in cells normally not exposed to the lin-3 signal, and vulval development is abnormal. This process is analogous to the cancerous growth that occurs in humans when mutations cause both tumor cells and the microenvironment surrounding the tumor cells to ectopically express factors that drive cellular proliferation. We propose that mammalian genes analogous to those that repress lin-3 expression in C. elegans vulval development act as tumor suppressors by preventing broad ectopic expression of EGF-like ligands.
Introduction
Signaling by epidermal growth factor (EGF) family ligands and EGF receptor (EGFR) family tyrosine kinases controls many aspects of mammalian development and can drive cancers: EGFRs are commonly overexpressed or constitutively activated by mutations in tumor cells [1], and EGF-family ligands can be misregulated in cancer. For example, the EGF-family ligands heparin-binding EGF-like growth factor, amphiregulin, and TGF-α are upregulated in cancer cells from many different cancer types [2], [3], and TGF-α overexpression causes widespread epithelial hyperplasia in mice [4], [5]. Growth factors often signal through a Ras pathway, and approximately 20% of tumors carry a constitutively active Ras mutation [6].
In the nematode Caenorhabditis elegans the EGF-family ligand LIN-3 acts through the EGFR LET-23 and the Ras protein LET-60 to control many aspects of development, including the induction of the hermaphrodite vulva [7]–[10]. In wild-type animals, of a set of six equipotent cells, three (P5.p, P6.p and P7.p) adopt vulval cell fates, while the other three (P3.4, P4.p, and P8.p) adopt non-vulval fates [11]. The expression of vulval cell fates requires EGF/Ras signaling, and mutations that reduce EGF/Ras signaling cause a vulvaless (Vul) phenotype in which none of the six cells adopts vulval cell fates [7]–[10]. The anchor cell, located closest to P6.p, is the only cell that both expresses LIN-3 EGF and is located near the six Pn.p cells [10], and laser ablation of the anchor cell results in a Vul phenotype [12] like that seen in mutants defective in lin-3 EGF or let-23 EGFR. Overactivation of the EGF/Ras pathway, by overexpression of lin-3 EGF or by an activating mutation in either let-23 EGFR or let-60 Ras, causes a multivulva (Muv) phenotype in which all six Pn.p cells adopt vulval cell fates [8]–[10],[13].
In vulval development, EGF/Ras signaling is antagonized by the synthetic multivulva (synMuv) genes. The synMuv genes define two classes, A and B [14], [15]. In synMuv single mutants or in class A double mutants or class B double mutants, vulval development is mostly normal. By contrast, animals mutant in both a class A synMuv gene and a class B synMuv gene exhibit a strong Muv phenotype. Many class B synMuv genes have homologs that function in histone modification, chromatin remodeling, and transcriptional repression. For example, the class B synMuv genes encode a DP/E2F/Rb complex [16], [17], a nucleosome remodeling and deacteylase (NuRD) complex [18], [19], two histone methyltransferases [20], [21] and a heterochromatin protein 1 homolog [22]. Of the three molecularly-characterized class A synMuv genes, two encode proteins with a zinc-finger-like THAP domain [23]–[25]. The expression patterns of three class A synMuv proteins have been studied, and all three are localized to the nucleus, suggesting that class A synMuv proteins regulate transcription [25], [26].
The synMuv genes function at least in part by repressing expression of lin-3 EGF. Loss-of-function mutations in either let-23 EGFR or lin-3 EGF can suppress the synMuv phenotype [16], [27], [28], indicating that the synMuv genes act upstream of or in parallel to lin-3. Furthermore, lin-3 mRNA levels are increased in synMuv double mutants but not in synMuv single mutants [27], and overexpression of lin-3 EGF causes a Muv phenotype [10].
Laser ablation of the anchor cell, the source of LIN-3 in wild-type vulval development, does not fully suppress the Muv phenotype of synMuv double mutants [28], indicating that synMuv genes cannot simply prevent overexpression of lin-3 from the anchor cell. Mosaic analyses of the class B synMuv gene lin-37 and the lin-15 locus, which contains both a class A and a class B synMuv gene, did not identify a single site of action. Both experiments indicated that lin-15 and lin-37 do not act cell-autonomously in the Pn.P cells and suggested that lin-15 and lin-37 might function in the syncytial hypodermal cell hyp7 [29], [30]. Heterologous expression experiments showed that the class B synMuv gene lin-35 functions in hyp7 to antagonize vulval cell fates, and tissue-specific RNAi of lin-3 in hyp7 can suppress the synMuv phenotype, indicating that repression of lin-3 in the hypoderm is an important function of the synMuv genes [27], [31]. Another study using the same heterologous promoters found that the class B synMuv gene hpl-2 functions in both hyp7 and the Pn.p cells [32]. However, it is not known where lin-3 is overexpressed in synMuv mutants, how the synMuv genes control lin-3 expression, or if the synMuv genes control targets other than lin-3 important for vulval development.
Here we report the identification of a lin-3 EGF promoter mutation that causes a dominant class A synMuv phenotype. The effect of this mutation reveals that the only major role of the class A synMuv genes in vulval development is to repress lin-3. We find that lin-3 mRNA is ectopically expressed throughout the animal in synMuv mutants. Our results show that low levels of ectopic lin-3 expression outside the cells that normally produce and respond to lin-3 can adversely alter the development of C. elegans, and we propose that the class A and class B synMuv genes might prevent ectopic lin-3 expression by distinct mechanisms.
Results
n4441 causes a dominant class A synMuv phenotype
During a screen for new class A synMuv mutations, we identified a Muv animal in the F1 generation after ethyl methanesulfonate (EMS) mutagenesis of the class B synMuv mutant lin-52(n771). We named the mutation that caused this defect n4441. To seek additional mutations that like n4441 dominantly cause a class A synMuv phenotype, we screened approximately 492,000 F1 progeny of lin-52(n771) animals mutagenized by EMS and approximately 89,000 progeny of animals mutagenized by N-ethyl-N-nitrosourea (ENU), but we did not identify any additional class A synMuv mutants. As a single mutant, n4441 animals are wild-type at 20°C and exhibit a low penetrance Muv defect at 25°C (Table 1), comparable to that of most class A synMuv mutants [15]. Double mutants between n4441 and the class B synMuv mutations lin-15B(n744), lin-52(n771), or lin-61(n3447) exhibit a strong synMuv phenotype. n4441 causes a fully penetrant Muv defect as a heterozygote in the class B synMuv mutant background lin-15B(n744), indicating that n4441 dominantly causes a class A synMuv phenotype. n4441 causes a 97% penetrant synMuv defect in the weak class B synMuv mutant background lin-61(n3447) at 22.5°C, comparable to the previously reported phenotype of double mutants between lin-61(n3447) and the strong class A synMuv mutations lin-15A(n767) or lin-38(n751) [15].
Table 1. lin-3(n4441) causes a dominant class A synMuv phenotype.
| genotype | % multivulva ± s.d. (n)a | |||
| 20°C | 25°C | |||
| N2 | 0±0 | (1422) | 0±0 | (1368) |
| lin-3(n4441) | 0±0 | (954) | 1±0.4 | (1105) |
| lin-3(n4441)/+ b | 0±0 | (621) | 1±2 | (830) |
| lin-15B(n744) | 0±0 | (1058) | 0±0 | (642) |
| lin-3(n4441); lin-15B(n744) | 100±0 | (729) | 100±0 | (248) |
| lin-3(n4441)/+; lin-15B(n744) c | 100±0 | (795) | 100±0 | (152) |
| lin-52(n771) | 0±0 | (1029) | 0±0 | (1233) |
| lin-3(n4441); lin-52(n771) | 98±0.1 | (974) | 100±0 | (1244) |
| lin-61(n3447) | 0±0 | (1039) | 0±0 | (986) |
| lin-61(n3447); lin-3(n4441) | 17±13 | (778) | 100±0 | (1201) |
| mys-1(n3681) | 0.4±0.4 | (833) | Lvad | |
| mys-1(n3681); lin-3(n4441) | 56±9 | (640) | Lvad | |
| lin-8(n2731) | 0±0 | (902) | 0±0 | (910) |
| lin-8(n2731); lin-3(n4441) | 0.4±0.5 | (1093) | 1±2 | (788) |
| lin-15A(n767) | 0.1±0.2 | (1022) | 2±1 | (1009) |
| lin-3(n4441); lin-15A(n767) | 5±3 | (831) | 60±17 | (609) |
| lin-38(n751) | 0±0 | (947) | 1±1 | (1307) |
| lin-38(n751); lin-3(n4441) | 1±2 | (1028) | 12±3 | (889) |
| lin-56(n2728) | 0±0 | (932) | 1±1 | (733) |
| lin-56(n2728); lin-3(n4441) | 0.3±0.3 | (930) | 6±4 | (843) |
Animals were scored as Muv if any ventral ectopic protrusions were observed. For each strain, animals were scored on three separate days, and % multivulva shown is the average for those three days. SD, standard deviation. n, total number of animals scored.
These animals were also heterozygous for dpy-17(e164) and unc-32(e189) and descended from lin-3(n4441) homozygous parents.
These animals were also heterozygous for dpy-5(e61) and descended from dpy-5(e61); lin-3(n4441); lin-15B(n744) parents.
Lva, larval arrest. Animals arrested as larvae, precluding the assaying of vulval development.
To determine how n4441 interacts with other class A synMuv mutations, we built double mutants between n4441 and an allele of each known class A synMuv gene. We used the putative null alleles lin-8(n2731), lin-15A(n767), and lin-56(n2728) and the missense allele lin-38(n751), since a null allele of lin-38 causes lethality (A.M.S and H.R.H., unpublished results). At 20°C and 25°C, the double mutants n4441; lin-15A(n767), lin-38(n751); n4441, and lin-56(n2728); n4441 were enhanced for the Muv phenotype when compared to their respective single mutants (Table 1). The lin-8(n2731); n4441 double mutant was roughly comparable to n4441 alone when scored at 25°C and also exhibited a low penetrance Muv defect at 20°C, which neither n4441 or lin-8(n2731) did on their own (Table 1). Thus, mutations in all known class A synMuv genes can enhance the Muv phenotype of n4441, but the enhancement is much weaker than the enhancement caused by class B synMuv mutations.
Several members of a Tip60/NuA4 histone acetyltransferase complex were previously identified as class C synMuv genes [33]. Class C synMuv genes are strongly Muv in combination with class A synMuv mutations and weakly Muv in combination with class B synMuv mutations and can be considered a subset of the class B synMuv genes [15]. To test if n4441 might be a class C synMuv gene, we built a double mutant between n4441 and the partial loss-of-function class C synMuv mutation mys-1(n3681), as null mutants of mys-1 cannot be maintained as homozygous strains [33]. The mys-1(n3681); n4441 double mutant exhibited a 56% penetrant Muv defect at 20°C, which is much stronger than the 5% penetrant Muv defect of the n4441; lin-15A(n767) strain at 20°C, despite the fact that lin-15A(n767) is a null mutation. We conclude that n4441 is not a class C synMuv mutation.
n4441 is an allele of lin-3
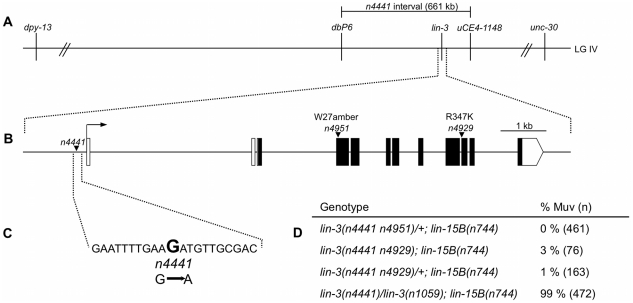
By performing SNP mapping experiments using the CB4856 polymorphic strain of C. elegans, we mapped the n4441 mutation to a 661 kb region containing approximately 170 genes between SNPs dbP6 and uCE4-1148 (Figure 1A). n4441 dominantly causes a synMuv phenotype and thus might well be a gain-of-function mutation, so we sought loss-of-function mutations in the gene affected by n4441. n4441/nT1[qIs51]; lin-15B(n744) animals, which display a fully penetrant Muv defect, were mutagenized with EMS. The nT1[qIs51] translocation causes inviability when homozygous and suppresses recombination across an interval that includes lin-3 [34]. Approximately 6,800 F1 progeny were screened, and two animals were identified that were non-Muv and produced only non-Muv progeny, indicating that they contained a suppressor mutation tightly linked to n4441. We named these mutations n4929 and n4951. n4441 n4929; lin-15B(n744) animals were sterile and exhibited a very low penetrance Muv defect (Figure 1D). n4441 n4951/nT1[qIs51] animals were superficially wild-type with no Muv defect, and n4441 n4951 homozygotes died as L1 larvae with a rod-like appearance (Figure 1D). The rod-like lethal phenotype is characteristic of loss-of-function mutations in genes in the EGF/Ras pathway required for vulval induction [35]. The only known gene in the EGF/Ras pathway in the genetic interval containing n4441 is lin-3, which encodes the EGF ligand. Strong loss-of-function alleles of lin-3 cause a rod-like lethal phenotype, and lin-3 mutations can also cause sterility [36], [37]. The n4929 mutant carries a G-to-A transition in the first nucleotide of exon 8 of lin-3 and is predicted to mutate an arginine to a lysine at amino acid 347 of LIN-3 (Figure 1B). The n4951 mutant carries a G-to-A transition that results in a nonsense mutation predicted to truncate LIN-3 after only 26 amino acids, before the EGF domain (Figure 1B). The lin-3(n1059) nonsense mutation failed to complement the sterility caused by n4929 and the lethality caused by n4951, proving that n4929 and n4951 are alleles of lin-3. Since the lin-3(n4951) nonsense mutation suppressed the n4441 synMuv defect in cis, but the lin-3(n1059) nonsense mutation did not suppress the n4441 synMuv defect in trans (Figure 1D), a lin-3 loss-of-function mutation is a cis dominant suppressor of n4441, indicating that n4441 is a gain-of-function allele of lin-3.
Figure 1. n4441 is an allele of lin-3.
(A) Genetic map showing n4441 on LGIV. n4441 was localized between dpy-13 and unc-30 by three-factor mapping. SNP mapping using polymorphisms present in the CB4856 strain further localized n4441 to a 661 kb region between the SNPs dbP6 at 10909553 and uCE4-1148 at 11570158 of LGIV (data not shown). (B) The lin-3 locus. The lin-3a isoform (Wormbase web site, http://www.wormbase.org, release WS200, Mar 20 2009) is shown. Solid boxes, exons; open boxes, UTRs. The start of the transcript is indicated by an arrow. Arrowheads indicate the locations of mutations. n4951 is a nonsense mutation that truncates LIN-3 after 26 amino acids, and n4929 is a missense mutation that converts an arginine to a lysine at amino acid 347. (C) n4441 is a G-to-A mutation at nucleotide 30904 of cosmid F36H1, 211 bp upstream of the lin-3 transcript. No other mutations were present in n4441 mutants in the region shown in (B). (D) A lin-3 loss-of-function mutation suppresses the n4441 synMuv phenotype in cis but not in trans. lin-3(n1059) is a nonsense mutation in lin-3 [37]. lin-3(n4441 n4951) and lin-3(n4441 n4929) heterozygotes also carried the nT1[qIs51] translocation. All animals were grown at 20°C. Animals were scored as Muv if any ventral ectopic protrusions were observed. n, total number of animals scored.
We determined the sequences of all exons and introns of lin-3 and of approximately 11 kb of upstream DNA in lin-3(n4441) mutants. The only mutation was a G-to-A transition at nucleotide 30904 of cosmid F36H1, approximately 200 bp upstream of the lin-3 transcript F36H1.4a (http://www.wormbase.org, release WS200, 20 Mar 2009) (Figure 1C). To show that the F36H1(30904) mutation is required for the class A synMuv phenotype caused by lin-3(n4441), we sought recombinants between lin-3(n4441) and the lin-3(n4951) nonsense mutation, which is 5.3 kb downstream of F36H1(30904). We screened approximately 90,000 progeny from lin-3(n4441 n4951)/+; lin-15B(n744) animals, identified five independent Muv animals and established homozygous lines. None of the five lines contained the lin-3(n4951) mutation, and all five carried the F36H1(30904) G-to-A mutation. Thus, the lin-3(n4441) mutation that causes the class A synMuv phenotype must be to the left of lin-3(n4951), because if it were to the right then the recombinants would not carry the F36H1(30904) mutation. The 5.3 kb between F36H1(30904) and lin-3(n4951), as well as 10.8 kb of DNA upstream of F36H1(30904), carried no additional mutations in lin-3(n4441) animals. If the mutation that causes the lin-3(n4441) synMuv phenotype is not the F36H1(30904) mutation, then the lin-3(n4441) mutation must be at least 10.8 kb to the left of the F36H1(30904) mutation. However, in that case, assuming a constant recombination rate throughout the lin-3 interval, the likelihood that all five recombination events would have occurred between F36H1(30904) and lin-3(n4951) is ((5.3)/(5.3+10.8))5, or <0.004. We conclude that the G-to-A mutation at nucleotide 30904 of cosmid F36F1 is necessary for the class A synMuv phenotype caused by lin-3(n4441). However, we cannot rule out the possibility there is a second mutation more than 11 kb upstream of lin-3 that is also required along with the F36H1(30904) mutation to cause a class A synMuv phenotype. There are no known consensus transcription factor binding sites that include the site of the lin-3(n4441) mutation (Transfac database of known transcription binding sites; http://www.gene-regulation.com). The region surrounding the lin-3(n4441) mutation is moderately conserved in the related nematodes C. briggsae and C. remanei (data not shown).
lin-3(n4441) might be a class A synMuv specific allele of lin-3. Alternatively, lin-3(n4441) might cause weak overexpression of lin-3 if weak overexpression of lin-3 behaves like a class A synMuv mutation. To differentiate between these alternatives, we overexpressed lin-3 weakly using the syIs12 integrated transgene. syIs12 expresses the EGF domain of lin-3 under the control of a heat-shock promoter [38]. At 20°C in the absence of heat-shock, syIs12 did not cause a Muv phenotype (Table 2). syIs12; lin-15B(n744) animals were mostly wild-type, with only a 1% penetrant Muv defect, whereas syIs12; lin-15A(n767) animals exhibited a Muv defect with 40% penetrance (Table 2). Thus, weak overexpression of lin-3 from the syIs12 transgene was enhanced by a class A synMuv mutation but not by a class B synMuv mutation. By contrast, lin-3(n4441) was enhanced much more strongly by class B synMuv mutations than by class A synMuv mutations (Table 1). We conclude that lin-3(n4441) is a class A synMuv specific allele of lin-3 and does not simply cause weak overexpression of lin-3.
Table 2. lin-3 overexpression is enhanced more strongly by a class A synMuv mutation than by a class B synMuv mutation.
| genotype | % multivulva (n)a |
| syIs12 b | 0 (92) |
| syIs12; lin-15A(n767) | 38 (105) |
| syIs12; lin-15B(n744) | 1 (100) |
Animals were scored as Muv if any ventral ectopic protrusions were observed. n, total number of animals scored.
syIs12 is an integrated transgene expressing the lin-3 EGF domain under the control of a heat-shock promoter [38]. Animals were assayed in the absence of heat-shock.
lin-3(n4441) specifically prevents repression of lin-3
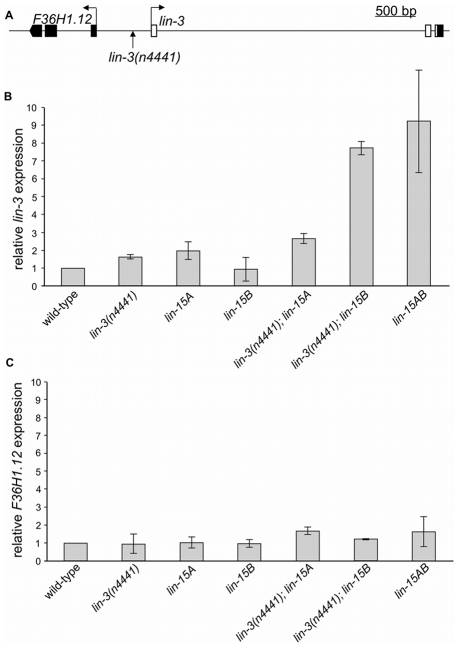
The class A and B synMuv genes redundantly repress expression of lin-3 mRNA [27]. To test if the lin-3(n4441) mutation affects lin-3 mRNA levels similarly to other class A synMuv mutations, we assayed lin-3 mRNA levels using real-time RT-PCR. As previously reported, the class B synMuv mutant lin-15B(n744) has wild-type lin-3 levels (Figure 2B). The class A synMuv mutants lin-15A(n767) and lin-3(n4441) both had slightly increased levels of lin-3 mRNA. The synMuv double mutants lin-15AB(e1763) and lin-3(n4441); lin-15B(n744) had substantially increased lin-3 mRNA levels (Figure 2B). Therefore, the lin-3(n4441) mutation behaves as a class A synMuv mutation with respect to lin-3 mRNA repression.
Figure 2. The lin-3(n4441) mutation specifically prevents repression of lin-3.
(A) The lin-3(n4441) mutation is located 211 bp upstream of the lin-3 transcript. The gene F36H1.12 is upstream of lin-3 in the opposite orientation, and lin-3(n4441) is located 465 bp from the predicted F36H1.12 transcript. Solid boxes, exons; open boxes, UTRs. (B) lin-3 mRNA levels in lin-3(n4441) single and double mutants. As reported previously [27], lin-3 mRNA levels are substantially increased in lin-15AB double mutants but not in lin-15A or lin-15B single mutants. Like other class A synMuv mutations, lin-3(n4441) caused a substantial increase in lin-3 mRNA levels only in a class B synMuv mutant background. Realtime RT-PCR experiments were performed using RNA harvested at the late L2 or early L3 stage from each strain shown. Relative lin-3 mRNA levels were normalized to the levels of mRNA encoding the ribosomal protein subunit rpl-26 using the ΔΔCt method [52]. The means and standard deviations of relative lin-3 mRNA levels from two independent trials are shown. The lin-15A(n767), lin-15B(n744) and lin-15AB(e1763) alleles were used in this experiment. (C) F36H1.12 mRNA levels in synMuv single and double mutants. No combination of lin-3(n4441), lin-15A, and lin-15B mutations affected F36H1.12 mRNA levels. Realtime RT-PCR experiments were performed using RNA harvested at the late L2 or early L3 stage from each strain shown. Relative F36H1.12 mRNA levels were normalized to the levels of mRNA encoding the ribosomal protein subunit rpl-26 using the ΔΔCt method. The means and standard deviations of relative F36H1.12 mRNA levels from two independent trials are shown.
The lin-3(n4441) mutation is located 211 bp upstream of lin-3 and is also 465 bp upstream of F36H1.12, which is upstream of lin-3 in the opposite orientation (Figure 2A). To determine if lin-3(n4441) or other synMuv mutations also affect expression of F36H1.12, we assayed F36H1.12 mRNA levels by real-time RT-PCR. F36H1.12 mRNA levels were roughly equivalent to those of the wild type in all possible single and double mutant combinations involving lin-15A(n767), lin-3(n4441), and lin-15B(n744) (Figure 2C). Therefore, the synMuv proteins specifically repress lin-3 and do not establish a broad domain of repression.
Expression pattern of lin-3 in wild-type animals and synMuv mutants
Although lin-3 is overexpressed in synMuv double mutants [27] (also, Figure 2), it is not known where this overexpression occurs. GFP- and LacZ-tagged lin-3 repetitive transgene arrays have been used as reporters for lin-3 expression [10], [39], [40], but these reporters might not be appropriate for determining lin-3 expression in synMuv mutants: first, the level of ectopic lin-3 expression might be too low to visualize using a GFP reporter; second, many synMuv mutations affect the expression of repetitive transgene arrays, potentially confounding interpretation of the expression pattern of such reporters [41]. Instead, we assayed lin-3 expression using a fluorescence in situ hybridization (FISH) technique that has sufficient sensitivity to detect single mRNA molecules [42]. We used 48 non-overlapping probes against lin-3 (Table S1), each conjugated to a single fluorophore, to label individual mRNA molecules brightly enough to be visible as distinct fluorescent spots. Because there are 48 probes that bind independently to the target mRNA, any single probe that binds non-specifically should not cause a false-positive signal. The distribution of intensities of the spots in any given animal was unimodal, consistent with each spot's representing a single mRNA molecule (Figure S1). Furthermore, by comparing the spot intensities in different tissues and mutants, we found that the level of expression in a given cell or tissue was independent of the intensity of the spots in that cell or tissue, and if the number of spots in a cell was altered then the average intensity of spots in that cell was unchanged (Figure S2). If each spot represented multiple mRNA molecules, then as the expression level in a given cell increased the average number of mRNA molecules in each of those spots would also be expected to increase, leading to greater intensity. Because the intensity of each spot was independent of the level of expression, we conclude that each spot is likely to represent a single mRNA molecule. We also found that all tissues are accessible to FISH probes, as probes directed against ama-1 and eft-2 robustly detected mRNA in all cells (data not shown). However, we cannot know if we are detecting every mRNA molecule; it is possible that some mRNA molecules are not accessible to the oligonucleotide probes or are not detected for some other reason.
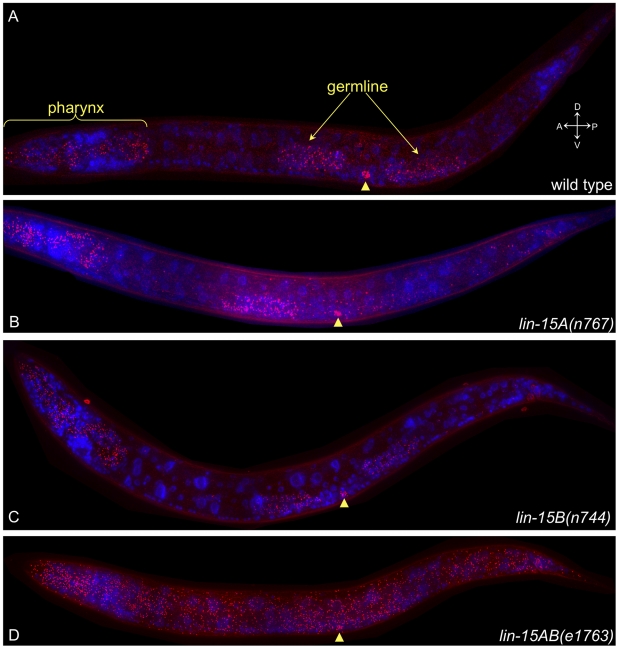
We first determined the expression pattern of lin-3 in wild-type animals at the late L2 to early L3 stage when vulval induction occurs. Previous studies found that at the early L3 stage lin-3 is expressed in the anchor cell and in the pharynx [10], [40]. We indeed observed robust expression of lin-3 in the anchor cell and throughout the pharynx. We also saw expression of lin-3 in the germline (Figure 3A and Figure S3). In some wild-type animals we also observed a few copies of lin-3 mRNA in one or more cells in the tail, on the ventral side slightly anterior to the anus. In addition, a few copies of lin-3 mRNA were seen on the ventral side of the animal, slightly behind the posterior gonad arm. We imaged several animals that were slightly older, in the late L3 stage, and observed expression of several copies of lin-3 mRNA in the region where P6.p and its descendants are located (data not shown), consistent with previous reports of expression of lin-3 in the descendants of P6.p by the L4 stage [39]. We did not consistently detect any lin-3 mRNA in other tissues, although in some animals we observed a single lin-3 mRNA molecule elsewhere. For example, in the animal shown in Figure 3A a single lin-3 mRNA molecule was observed in or near an intestinal cell close to the anchor cell. Overall, other than for those tissues that highly expressed lin-3 there was very tight repression of lin-3. The numbers of copies of lin-3 mRNA we observed in each tissue in individual animals are listed in Table S2 and are summarized in Table 3. The expression pattern we observed for lin-3 is consistent with that seen using GFP- and LacZ-tagged lin-3 reporters [10], [39], [40] and with functional studies of lin-3 [43], indicating that most if not all of the mRNA spots identified by this technique are likely to represent actual lin-3 mRNA molecules.
Figure 3. The synMuv genes prevent widespread ectopic expression of lin-3 mRNA.
FISH of lin-3 mRNA in late L2 to early L3 animals. Each dot represents a single mRNA molecule [42]. lin-3 mRNAs are shown in red, and 4′,6-diamidino-2-phenylindole (DAPI) staining of nuclei is shown in blue. The images shown are maximum intensity projections of a z-stack of images. The anchor cell (AC) is indicated by an arrowhead in each panel. (A) Wild type. lin-3 mRNA is expressed in the pharynx, anchor cell, and germline and is tightly repressed elsewhere. (B) lin-15A(n767). There is a low level of ectopic lin-3 expression, with approximately 60 ectopic copies of lin-3 mRNA seen outside the pharynx, anchor cell, and germline. (C) lin-15B(n744). There is a very low level of ectopic lin-3 expression, with approximately 10 ectopic copies of lin-3 mRNA seen outside the pharynx, anchor cell, and germline. The large bright spot at the edge of the head is outside the animal. (D) lin-15AB(e1763). lin-3 is ectopically expressed throughout the animal, with approximately 900 ectopic copies of lin-3 mRNA seen outside the pharynx, anchor cell, and germline.
Table 3. Quantification of lin-3 expression.
| genotype | na | Anchor cell | Germline | Pharynx | Leakyb |
| N2 | 6 | 29±5 | 314±69 | 284±48 | 1±1 |
| lin-15A(n767) | 7 | 23±5 | 279±46 | 451±92 | 64±21 |
| lin-15B(n744) | 6 | 21±4 | 199±57 | 380±134 | 6±2 |
| lin-15AB(e1763) | 7 | 22±4 | 231±54 | 503±154 | 1087±211 |
| lin-3(n4441) | 8 | 26±4 | 340±62 | 387±68 | 71±28 |
| lin-3(n4441); lin-15B(n744) | 5 | 21±7 | 360±109 | 578±70 | 1156±111 |
The average number of copies of lin-3 mRNA observed in each tissue and the standard deviation between animals is shown.
n, total number of animals assayed.
lin-3 mRNA observed outside of the normal domain of expression of lin-3.
lin-15AB(e1763) animals expressed lin-3 in the pharynx, germline, and anchor cell at levels grossly similar to those of wild-type animals (Figure 3D and Table 3). In addition there was widespread ectopic expression of lin-3, with an average of approximately 1100 ectopic copies of lin-3 mRNA observed per animal (Figure 3D and Table 3). This ectopic expression was much weaker than the normal expression in the anchor cell; whereas an average of 29 copies of lin-3 mRNA was seen in the anchor cell in wild-type animals (Table 3), only one or a few copies of lin-3 mRNA were observed in most cells in lin-15AB(e1763) mutants. Because we could not see cell boundaries, we could not determine if every cell ectopically expressed lin-3, but there were no tissues that appeared to lack ectopic lin-3 mRNA (Figure S4). Cells around the perimeter of the animal expressed lin-3 in the lin-15AB(e1763) mutant, consistent with ectopic expression in the hypodermis (Figure 3D and Figure S4). There were also many ectopic lin-3 mRNA copies that clearly were not in the hypodermis (Figure S4).
We also determined lin-3 expression in lin-15A(n767) and lin-15B(n744) single mutants. lin-15B(n744) animals had a lin-3 expression pattern similar to that of wild-type animals (Figure 3C). In lin-15B(n744) mutants there was an extremely low level of ectopic lin-3 expression, with an average of six ectopic lin-3 mRNA molecules detected per animal (Table 3), but lin-3 was still tightly repressed outside of the germline, anchor cell, and pharynx. lin-15A(n767) animals exhibited broad ectopic expression of lin-3, but at a much lower level than that of lin-15AB(e1763) animals (Figure 3B). An average of 64 copies of lin-3 mRNA were seen outside of the pharynx, germline, and anchor cell in lin-15A(n767) animals (Table 3). Unlike in lin-15AB(e1763) animals, in any given lin-15A(n767) animal most cells did not display ectopic lin-3 expression. However, we observed no obvious cell or tissue specificity to the ectopic expression among several lin-15A(n767) animals. Rather, it appeared that in lin-15A(n767) animals lin-3 is globally derepressed, but at a very low level.
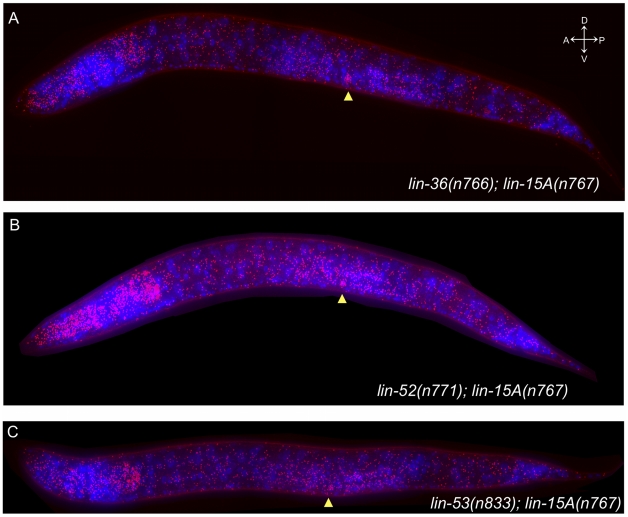
The numerous class B synMuv genes have highly similar although not identical effects on vulval development [15]. However, the class B synMuv genes have widely differing effects on other aspects of growth and development. For example, PGL-1, which is normally expressed in the germline, is misexpressed in the somatic cells of mutants of many class B synMuv genes, including lin-15B, but not in mutants of some other class B synMuv genes, including lin-36, lin-52, and lin-53 [44], [45]. We therefore investigated the role of the class B synMuv genes lin-36, lin-52, and lin-53 in controlling lin-3 expression. We determined the expression pattern of lin-3 in lin-36(n766); lin-15A(n767), lin-52(n771); lin-15A(n767), and lin-53(n833); lin-15A(n767) mutants. All three double mutants exhibited ubiquitous ectopic expression of lin-3, with lin-3 mRNA observed in most if not all tissues (Figure 4). There were no obvious differences in the spatial pattern of lin-3 expression in lin-36(n766); lin-15A(n767), lin-52(n771); lin-15A(n767), and lin-53(n833); lin-15A(n767) double mutants as compared to lin-15AB(e1763) mutants.
Figure 4. Multiple class B synMuv mutations have similar effects on lin-3 mRNA expression.
FISH of lin-3 mRNA in late L2 to early L3 animals. Each dot represents a single mRNA molecule [42]. lin-3 mRNAs are shown in red, and 4′,6-diamidino-2-phenylindole (DAPI) staining of nuclei is shown in blue. The images shown are maximum intensity projections of a z-stack of images. The anchor cell (AC) is indicated by an arrowhead in each panel. Each mutant displayed ectopic lin-3 mRNA expression throughout the animal in most if not all tissues. (A) lin-36(n766); lin-15A(n767). (B) lin-52(n771); lin-15A(n767). (C) lin-53(n833); lin-15A(n767).
The lin-3(n4441) mutation could cause global derepression of lin-3 similarly to lin-15A(n767), or it could affect lin-3 expression in a subset of tissues. We examined the expression of lin-3 mRNA in lin-3(n4441) and lin-3(n4441); lin-15B(n744) animals. lin-3(n4441) animals had widespread but weak ectopic expression of lin-3, similar to lin-15A(n767) animals (Figure 5A and Table 3). lin-3(n4441); lin-15B(n744) animals exhibited ectopic lin-3 expression in most cells and were indistinguishable from lin-15AB(e1763) animals (Figure 5B and Table 3).
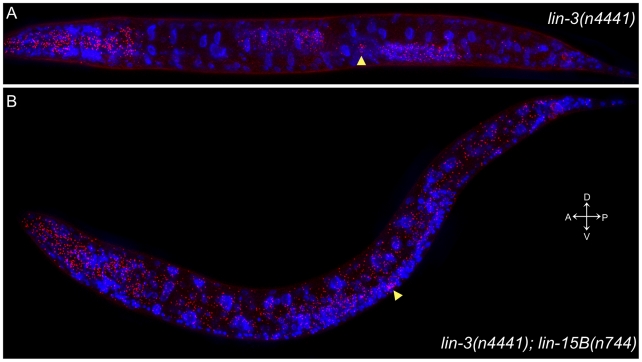
Figure 5. The lin-3(n4441) mutation causes widespread ectopic expression of lin-3 mRNA.
FISH of lin-3 mRNA in late L2 to early L3 animals. Each dot represents a single mRNA molecule [42]. lin-3 mRNAs are shown in red, and 4′,6-diamidino-2-phenylindole (DAPI) staining of nuclei is shown in blue. The images shown are maximum intensity projections of a z-stack of images. The anchor cell (AC) is indicated by an arrowhead in each panel. (A) lin-3(n4441). there is a low level of ectopic lin-3 expression, with approximately 45 ectopic copies of lin-3 mRNA seen outside the pharynx, anchor cell, and germline. (B) lin-3(n4441); lin-15B(n744). lin-3 is ectopically expressed throughout the animal, with approximately 1000 ectopic copies of lin-3 mRNA seen outside the pharynx, anchor cell, and germline.
Discussion
Identifying the biologically relevant targets of transcriptional regulators that control development is a challenging problem. The synMuv genes encode putative transcriptional repressors that prevent ectopic vulval development. Mutating a synMuv binding site in a target gene might relieve repression of that target, and if that repression were essential to prevent ectopic vulval development could cause a dominant synMuv phenotype. We isolated a mutation in the lin-3 EGF gene that derepresses lin-3 transcription and causes a dominant class A synMuv phenotype. This finding establishes that lin-3 is a functionally important target of the class A synMuv genes, consistent with a previous report that lin-3 expression is repressed by the synMuv genes and that double-stranded RNA directed against lin-3 can suppress the synMuv phenotype [27]. Importantly, the lin-3(n4441) mutation fully recapitulates the class A synMuv phenotype with regard to vulval development and lin-3 expression and causes a class A synMuv phenotype equivalent to that caused by strong alleles of class A synMuv genes. If the class A synMuv genes repressed multiple targets to prevent ectopic vulval development, then a mutation that abolished class A synMuv-mediated repression of lin-3 would recapitulate only partially the class A synMuv phenotype. We conclude that lin-3 is likely to be the only key biologically relevant target of the class A synMuv genes in vulval development.
The simplest interpretation of the effect of the lin-3(n4441) mutation is that this mutation abolishes a binding site for a transcriptional repressor consisting of or controlled by class A synMuv proteins. However, the effect of the lin-3(n4441) mutation is slightly enhanced by mutations in all other class A synMuv genes. If the lin-3(n4441) mutation completely inactivated a binding site that responds to only one of the known class A synMuv proteins, then mutation of that class A synMuv gene should not enhance the synMuv phenotype caused by lin-3(n4441). One possibility is that a complex consisting of multiple class A synMuv proteins binds to the lin-3 promoter, the lin-3(n4441) mutation strongly reduces but does not completely eliminate that binding, and removing any one class A synMuv protein does not fully abrogate the ability of the complex to bind to the lin-3 locus and repress transcription. This model is consistent with the observation that most class A synMuv mutations, including lin-3(n4441), are enhanced by class A synMuv mutations in other genes [15]. Alternatively, the class A synMuv genes might indirectly repress lin-3 by regulating the expression or activity of or by binding to another protein that binds to the lin-3 promoter to prevent ectopic transcription. Because a mutation in the lin-3 promoter can cause a class A synMuv phenotype, the class A and class B synMuv pathways must be integrated at the point of lin-3 repression, and hence it is unlikely that the class A and B synMuv genes redundantly control a transcriptional regulator which in turn controls lin-3 expression.
lin-3 expression in the germline had not been previously observed, likely because the reporters used to assay lin-3 expression were either silenced in the germline [46] or lacked distant regulatory regions necessary to drive germline expression. Mutations in the FOG and FBF translational inhibitor RNA-binding proteins cause a germline-dependent Muv phenotype, and the FBF proteins can bind to the 3′ UTR of lin-3 in vitro, suggesting that germline lin-3 mRNA is translationally repressed during the larval stage when vulval induction occurs [43]. In many class B synMuv mutants, somatic cells express normally germline-specific genes [19], [44], [45]. Given our finding that lin-3 is normally expressed in the germline, one possibility is that the class B synMuv genes repress ectopic lin-3 expression in somatic cells as a consequence of their role in ensuring that somatic cells do not inappropriately adopt germline-like fates. The class B synMuv genes might all directly repress lin-3 in somatic cells. Alternatively, as there are a large number of class B synMuv genes and their effects on vulval development are not identical, perhaps at least some class B synMuv genes indirectly repress lin-3 by preventing the ectopic adoption of germline-like fates. In class B synMuv single mutants, the somatic cells adopt a more germline-like fate that would include lin-3 expression except that the class A synMuv genes still tightly repress lin-3, mostly preventing ectopic lin-3 expression. In class A synMuv single mutants, lin-3 is not tightly repressed, but most somatic cells are not fated to express lin-3, so there is only a low level of leaky ectopic lin-3 expression. However, in class AB synMuv double mutants, the somatic cells adopt a germline-like fate that includes lin-3 expression, and there is no class A synMuv mechanism that tightly represses lin-3, resulting in widespread and substantial ectopic lin-3 expression. In short, we suggest that the synthetic Muv phenotype caused by mutations in the synMuv genes might be a consequence of two distinct functions of the class A and class B synMuv genes: the class A synMuv genes either directly or indirectly tightly repress ectopic lin-3 transcription, and the class B synMuv genes prevent somatic expression of germline-expressed genes, which include lin-3; only if both functions are lost will somatic cells ectopically express sufficient lin-3 mRNA to cause ectopic vulval induction. These findings raise the possibility that the development of some human tumors might require the loss of one tumor suppressor gene that prevents cells from adopting a fate that is permissive for the expression of a growth factor and the loss of a second tumor suppressor gene that specifically represses the expression of that growth factor.
A subset of the class B synMuv genes is required to prevent the somatic misexpression of normally germline-restricted P-granule proteins such as PGL-1 [44], [45]. We found that lin-15B mutants, which do exhibit somatic PGL-1 expression, and lin-36, lin-52, and lin-53 mutants, which do not exhibit somatic PGL-1, all have highly similar effects on lin-3 expression. These results indicate that different germline genes are broadly repressed in the soma by different sets of transcriptional repressors. The class B synMuv genes define one such group of repressors and are classified together because they have comparable effects on the germline gene lin-3, resulting in similar vulval phenotypes. Many such partially-overlapping groups of transcriptional repressors, including various subsets of the class B synMuv genes, are likely to be required for the repression in the soma of other germline-restricted genes.
Whereas lin-3 expression in most cells is tightly repressed by the synMuv genes, the anchor cell and germline exhibit robust lin-3 expression that is not substantially affected by the synMuv genes. While it has not been reported whether or not any synMuv genes are expressed in the anchor cell, several synMuv genes are expressed in the germline [17], [22], [26], [33], and we are not aware of studies that have conclusively shown any synMuv genes not to be expressed in the germline. In most cells, the synMuv genes reduce lin-3 expression from an average of one to two copies per cell to nearly zero copies per cell. The synMuv genes clearly do not have a similar fold effect on lin-3 expression in the anchor cell and germline. However, it is possible that the synMuv genes repress a similar absolute number of leaky lin-3 mRNA molecules in all cells; given the animal-to-animal variability in lin-3 expression we likely would not have been able to detect such a small increase in the anchor cell or germline. Alternatively, the synMuv genes might not repress lin-3 in the anchor cell or germline. The strong activator(s) of lin-3 that drive expression in those tissues could override the activity of the synMuv genes, or one or more synMuv genes might not be expressed in those tissues, thereby compromising synMuv repression of lin-3.
In lin-15AB mutants, lin-3 is ectopically expressed throughout the animal in a broad range of cells and tissues. Site-of-action experiments have shown that the synMuv genes function at least in large part in the hyp7 hypodermal syncytium to prevent ectopic vulval development [31]. The expression pattern of lin-3 in synMuv mutants does not directly identify the site-of-action of synMuv genes in regulating vulval development but does show that the synMuv genes function throughout the animals to keep lin-3 very tightly repressed in numerous cells and tissues. lin-3 EGF regulates non-vulval cell fates in C. elegans development, and at least some of these fates, such as the P11/P12 fate, are also regulated by the synMuv genes in a manner analogous to that of vulval development [47]. In short, the synMuv genes act throughout the animal to prevent ectopic lin-3 expression, which can cause a variety of developmental abnormalities. Mutants with a displaced anchor cell show that lin-3 can act at a distance [48], so a cell ectopically expressing lin-3 could affect fates in both nearby and distant cells. We suggest that for any given cell-fate decision, the site of action of the synMuv genes is likely to be spread across multiple cells and determined by the size and proximity of those cells to the cell being regulated by lin-3. In the case of vulval development, hyp7 plays the major role, given its large size and close proximity to the Pn.p cells, with likely lesser contributions from many other cells. The site at which the synMuv genes repress lin-3 to ensure proper vulval development is therefore probably a combination of the Pn.p cells themselves and neighboring cells that do not normally either express or respond to lin-3. This situation is similar to that in which both tumor cells and the microenvironment surrounding the tumor provide factors that drive tumor development [49]. We suggest that analogously to the synMuv genes some tumor suppressor genes function by repressing growth factor expression in both tumor cells and the surrounding microenvironment.
In synMuv double mutants, lin-3 was ectopically expressed but at a much lower level than at its major normal site of function, the anchor cell. synMuv double mutants might ectopically express as few as one to two copies of lin-3 mRNA per cell. Thus, normal C. elegans development requires lin-3 to be exceedingly tightly repressed outside of a few cells, and only slight expression of lin-3 throughout the animal can cause abnormal cell-fate transformations. Such low levels of ectopic expression would likely be missed by most techniques used to assay gene expression. We suggest it could be important to examine the expression of EGF-family ligands in tumors using highly sensitive techniques with single-molecule resolution to determine if broad low-level misexpression of EGF-family ligands plays a role in oncogenic growth. In C. elegans, the tight repression of lin-3 EGF requires both the class A synMuv gene pathway and the class B gene synMuv pathway, which includes homologs of known tumor suppressor genes, such as lin-35 Rb. Therefore, some tumor suppressor genes in mammals might function by tightly repressing low-level ectopic expression of EGF-family ligands in many cells, possibly in both the tumor and the microenvironment surrounding the tumor.
Materials and Methods
Strains and genetics
C. elegans strains were cultured by standard methods on OP50 bacteria [50]. All animals were grown at 20°C, except where otherwise noted. The wild-type strain was N2, except in SNP mapping experiments in which the polymorphic CB4856 strain was also used [51]. The following mutations were used in this study:
LGI: dpy-5(e61), lin-61(n3447), lin-53(n833)
LGII: lin-8(n2731), lin-56(n2728), lin-38(n751), syIs12
LGIII: dpy-17(e164), lin-36(n766), unc-32(e189), lin-52(n771)
LGIV: lin-3(n4441), lin-3(n4929), lin-3(n4951), lin-3(n1059)
LGX: lin-15A(n767), lin-15B(n744), lin-15(e1763)
The balancer strain nT1[qIs51] IV∶V [34] was used; qIs51 is a GFP-expressing transgene integrated onto the nT1 translocation. Table S3 lists all strains used in this study.
Quantitative PCR assay
Synchronized animals were harvested at or near the L2-to-L3 larval transition, when vulval induction occurs. N2 animals were harvested 33 hours after starved L1 larvae were placed on plates with food. Some mutants grew more slowly and were harvested after 39 hours. Quantitative RT-PCR for lin-3 was performed as previously described [15]. lin-3 was amplified using the primers CGCATTTCTCATTGTCATGC and CTGGTGGGCACATATGACTC.
Fluorescence in situ hybridization
Animals were grown to the L2-to-L3 transition as in the quantitative RT-PCR experiments. Fixation and hybridization were performed as described previously [42], except that worms were fixed for one hour instead of 45 minutes. The lin-3 probes (Biosearch Technologies, Inc) were conjugated to the fluorophore Cy5 using the Amersham Cy5 Mono-reactive Dye pack (GE Healthcare). DNA was visualized using 4′,6-diamidino-2-phenylindole (DAPI). The probe sequences used are shown in Table S1. Figure 2 and Figure 3 are maximum intensity projections of a Z-stack of images processed with the Find Edges and Smooth operations in ImageJ. lin-3 mRNA spots were computationally identified with manually determined thresholds as previously described [42]. The number of molecules within each tissue were then manually counted. The anchor cell was identified based on position. lin-3 mRNA expression in the anchor cell appeared as a tight cluster of spots; molecules within that cluster were considered to be in the anchor cell. To determine which lin-3 mRNA molecules were in the pharynx, we noted the outline of the pharynx that was clearly visible as a dark boundary in the Cy5 channel of the image stacks (see Figure S3 and Figure S4). The boundaries of the germline were estimated from the positions of DAPI-labeled germline nuclei.
Supporting Information
Unimodal distribution of FISH spot intensity. Histograms of FISH spot intensity for six images from six different animals are shown. Each image included the anchor cell and most or all of the germline. Intensities were calculated by taking the maximum intensity of a spot and subtracting the average background intensity of a four-pixel radius surrounding the spot.
(TIFF)
FISH spot intensity is independent of expression level. The mean spot intensity was calculated for mRNAs expressed in the anchor cell, germline, or ectopically. For each animal, the intensity of each spot was normalized to the mean intensity of the spots in the anchor cell, which was set to 1. The mean and standard deviation for the expression in the germline or ectopically from 7–10 animals for each genotype are shown. lin-3(e1417) had roughly wild-type numbers of lin-3 FISH spots in the germline but had approximately 4.5-fold fewer lin-3 FISH spots in the anchor cell (Table S2). In both wild-type and lin-15AB(e1763) animals the density of spots in the anchor cell was noticeably higher than in the germline or elsewhere (e.g. Figure 3A and 3D).
(TIFF)
lin-3 mRNA expression in a wild-type animal. FISH of lin-3 mRNA in a wild-type animal. The animal shown is the same as in Figure 3A. Each frame is a different plane in the Z-axis. lin-3 mRNA was expressed in the pharynx, anchor cell, and germline and was tightly repressed elsewhere.
(AVI)
The synMuv genes prevent widespread ectopic expression of lin-3 mRNA. FISH of lin-3 mRNA in a lin-15AB(e1763) mutant animal. The animal shown is the same as in Figure 3D. Each frame is a different plane in the Z-axis. lin-3 was ectopically expressed throughout the animal, with approximately 900 ectopic copies of lin-3 mRNA seen outside of the pharynx, anchor cell, and germline.
(AVI)
Oligonucleotides in lin-3 FISH probe.
(DOC)
Quantification of lin-3 expression.
(DOC)
List of strains used in this study.
(DOC)
Acknowledgments
We thank Dave Harris for helpful comments concerning this manuscript; Beth Castor, Elissa Murphy, and Rita Droste for technical assistance; and WormBase.
Footnotes
The authors have declared that no competing interests exist.
HRH is the David H. Koch Professor of Biology at MIT and an Investigator of the Howard Hughes Medical Institute. This work was supported by NIH grant GM24663. AvO was supported by an NIH Pioneer Award (1DP1OD003936). Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, et al. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 2006;366:2–16. doi: 10.1016/j.gene.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 2.Massague J. Transforming growth factor-alpha. A model for membrane-anchored growth factors. J Biol Chem. 1990;265:21393–21396. [PubMed] [Google Scholar]
- 3.Yotsumoto F, Yagi H, Suzuki SO, Oki E, Tsujioka H, et al. Validation of HB-EGF and amphiregulin as targets for human cancer therapy. Biochem Biophys Res Commun. 2008;365:555–561. doi: 10.1016/j.bbrc.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Jhappan C, Stahle C, Harkins RN, Fausto N, Smith GH, et al. TGF alpha overexpression in transgenic mice induces liver neoplasia and abnormal development of the mammary gland and pancreas. Cell. 1990;61:1137–1146. doi: 10.1016/0092-8674(90)90076-q. [DOI] [PubMed] [Google Scholar]
- 5.Sandgren EP, Luetteke NC, Palmiter RD, Brinster RL, Lee DC. Overexpression of TGF alpha in transgenic mice: induction of epithelial hyperplasia, pancreatic metaplasia, and carcinoma of the breast. Cell. 1990;61:1121–1135. doi: 10.1016/0092-8674(90)90075-p. [DOI] [PubMed] [Google Scholar]
- 6.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 7.Aroian RV, Koga M, Mendel JE, Ohshima Y, Sternberg PW. The let-23 gene necessary for Caenorhabditis elegans vulval induction encodes a tyrosine kinase of the EGF receptor subfamily. Nature. 1990;348:693–699. doi: 10.1038/348693a0. [DOI] [PubMed] [Google Scholar]
- 8.Beitel GJ, Clark SG, Horvitz HR. Caenorhabditis elegans ras gene let-60 acts as a switch in the pathway of vulval induction. Nature. 1990;348:503–509. doi: 10.1038/348503a0. [DOI] [PubMed] [Google Scholar]
- 9.Han M, Sternberg PW. let-60, a gene that specifies cell fates during C. elegans vulval induction, encodes a ras protein. Cell. 1990;63:921–931. doi: 10.1016/0092-8674(90)90495-z. [DOI] [PubMed] [Google Scholar]
- 10.Hill RJ, Sternberg PW. The gene lin-3 encodes an inductive signal for vulval development in C. elegans. Nature. 1992;358:470–476. doi: 10.1038/358470a0. [DOI] [PubMed] [Google Scholar]
- 11.Sternberg PW, Horvitz HR. Pattern formation during vulval development in C. elegans. Cell. 1986;44:761–772. doi: 10.1016/0092-8674(86)90842-1. [DOI] [PubMed] [Google Scholar]
- 12.Kimble J. Alterations in cell lineage following laser ablation of cells in the somatic gonad of Caenorhabditis elegans. Dev Biol. 1981;87:286–300. doi: 10.1016/0012-1606(81)90152-4. [DOI] [PubMed] [Google Scholar]
- 13.Katz WS, Lesa GM, Yannoukakos D, Clandinin TR, Schlessinger J, et al. A point mutation in the extracellular domain activates LET-23, the Caenorhabditis elegans epidermal growth factor receptor homolog. Mol Cell Biol. 1996;16:529–537. doi: 10.1128/mcb.16.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferguson EL, Horvitz HR. The multivulva phenotype of certain Caenorhabditis elegans mutants results from defects in two functionally redundant pathways. Genetics. 1989;123:109–121. doi: 10.1093/genetics/123.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersen EC, Saffer AM, Horvitz HR. Multiple levels of redundant processes inhibit Caenorhabditis elegans vulval cell fates. Genetics. 2008;179:2001–2012. doi: 10.1534/genetics.108.092197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu X, Horvitz HR. lin-35 and lin-53, two genes that antagonize a C. elegans Ras pathway, encode proteins similar to Rb and its binding protein RbAp48. Cell. 1998;95:981–991. doi: 10.1016/s0092-8674(00)81722-5. [DOI] [PubMed] [Google Scholar]
- 17.Ceol CJ, Horvitz HR. dpl-1 DP and efl-1 E2F act with lin-35 Rb to antagonize Ras signaling in C. elegans vulval development. Mol Cell. 2001;7:461–473. doi: 10.1016/s1097-2765(01)00194-0. [DOI] [PubMed] [Google Scholar]
- 18.Solari F, Ahringer J. NURD-complex genes antagonise Ras-induced vulval development in Caenorhabditis elegans. Curr Biol. 2000;10:223–226. doi: 10.1016/s0960-9822(00)00343-2. [DOI] [PubMed] [Google Scholar]
- 19.Unhavaithaya Y, Shin TH, Miliaras N, Lee J, Oyama T, et al. MEP-1 and a homolog of the NURD complex component Mi-2 act together to maintain germline-soma distinctions in C. elegans. Cell. 2002;111:991–1002. doi: 10.1016/s0092-8674(02)01202-3. [DOI] [PubMed] [Google Scholar]
- 20.Andersen EC, Horvitz HR. Two C. elegans histone methyltransferases repress lin-3 EGF transcription to inhibit vulval development. Development. 2007;134:2991–2999. doi: 10.1242/dev.009373. [DOI] [PubMed] [Google Scholar]
- 21.Poulin G, Dong Y, Fraser AG, Hopper NA, Ahringer J. Chromatin regulation and sumoylation in the inhibition of Ras-induced vulval development in Caenorhabditis elegans. Embo J. 2005;24:2613–2623. doi: 10.1038/sj.emboj.7600726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Couteau F, Guerry F, Muller F, Palladino F. A heterochromatin protein 1 homologue in Caenorhabditis elegans acts in germline and vulval development. EMBO Rep. 2002;3:235–241. doi: 10.1093/embo-reports/kvf051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang LS, Tzou P, Sternberg PW. The lin-15 locus encodes two negative regulators of Caenorhabditis elegans vulval development. Mol Biol Cell. 1994;5:395–411. doi: 10.1091/mbc.5.4.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clark SG, Lu X, Horvitz HR. The Caenorhabditis elegans locus lin-15, a negative regulator of a tyrosine kinase signaling pathway, encodes two different proteins. Genetics. 1994;137:987–997. doi: 10.1093/genetics/137.4.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davison EM, Saffer AM, Huang LS, Demodena J, Sternberg PW, et al. The LIN-15A and LIN-56 Transcriptional Regulators Interact to Negatively Regulate EGF/Ras Signaling in Caenorhabditis elegans Vulval Cell-Fate Determination. Genetics. 2011;187:803–815. doi: 10.1534/genetics.110.124487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davison EM, Harrison MM, Walhout AJ, Vidal M, Horvitz HR. lin-8, which antagonizes Caenorhabditis elegans Ras-mediated vulval induction, encodes a novel nuclear protein that interacts with the LIN-35 Rb protein. Genetics. 2005;171:1017–1031. doi: 10.1534/genetics.104.034173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cui M, Chen J, Myers TR, Hwang BJ, Sternberg PW, et al. SynMuv genes redundantly inhibit lin-3/EGF expression to prevent inappropriate vulval induction in C. elegans. Dev Cell. 2006;10:667–672. doi: 10.1016/j.devcel.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Ferguson EL, Sternberg PW, Horvitz HR. A genetic pathway for the specification of the vulval cell lineages of Caenorhabditis elegans. Nature. 1987;326:259–267. doi: 10.1038/326259a0. [DOI] [PubMed] [Google Scholar]
- 29.Hedgecock EM, Herman RK. The ncl-1 gene and genetic mosaics of Caenorhabditis elegans. Genetics. 1995;141:989–1006. doi: 10.1093/genetics/141.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herman RK, Hedgecock EM. Limitation of the size of the vulval primordium of Caenorhabditis elegans by lin-15 expression in surrounding hypodermis. Nature. 1990;348:169–171. doi: 10.1038/348169a0. [DOI] [PubMed] [Google Scholar]
- 31.Myers TR, Greenwald I. lin-35 Rb acts in the major hypodermis to oppose ras-mediated vulval induction in C. elegans. Dev Cell. 2005;8:117–123. doi: 10.1016/j.devcel.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 32.Schott S, Ramos F, Coustham V, Palladino F. HPL-2/HP1 prevents inappropriate vulval induction in Caenorhabditis elegans by acting in both HYP7 and vulval precursor cells. Genetics. 2009;181:797–801. doi: 10.1534/genetics.108.089276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ceol CJ, Horvitz HR. A new class of C. elegans synMuv genes implicates a Tip60/NuA4-like HAT complex as a negative regulator of Ras signaling. Dev Cell. 2004;6:563–576. doi: 10.1016/s1534-5807(04)00065-6. [DOI] [PubMed] [Google Scholar]
- 34.Siegfried KR, Kidd AR, 3rd, Chesney MA, Kimble J. The sys-1 and sys-3 genes cooperate with Wnt signaling to establish the proximal-distal axis of the Caenorhabditis elegans gonad. Genetics. 2004;166:171–186. doi: 10.1534/genetics.166.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han M, Aroian RV, Sternberg PW. The let-60 locus controls the switch between vulval and nonvulval cell fates in Caenorhabditis elegans. Genetics. 1990;126:899–913. doi: 10.1093/genetics/126.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferguson EL, Horvitz HR. Identification and characterization of 22 genes that affect the vulval cell lineages of the nematode Caenorhabditis elegans. Genetics. 1985;110:17–72. doi: 10.1093/genetics/110.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu J, Tzou P, Hill RJ, Sternberg PW. Structural requirements for the tissue-specific and tissue-general functions of the Caenorhabditis elegans epidermal growth factor LIN-3. Genetics. 1999;153:1257–1269. doi: 10.1093/genetics/153.3.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katz WS, Hill RJ, Clandinin TR, Sternberg PW. Different levels of the C. elegans growth factor LIN-3 promote distinct vulval precursor fates. Cell. 1995;82:297–307. doi: 10.1016/0092-8674(95)90317-8. [DOI] [PubMed] [Google Scholar]
- 39.Chang C, Newman AP, Sternberg PW. Reciprocal EGF signaling back to the uterus from the induced C. elegans vulva coordinates morphogenesis of epithelia. Curr Biol. 1999;9:237–246. doi: 10.1016/s0960-9822(99)80112-2. [DOI] [PubMed] [Google Scholar]
- 40.Hwang BJ, Sternberg PW. A cell-specific enhancer that specifies lin-3 expression in the C. elegans anchor cell for vulval development. Development. 2004;131:143–151. doi: 10.1242/dev.00924. [DOI] [PubMed] [Google Scholar]
- 41.Hsieh J, Liu J, Kostas SA, Chang C, Sternberg PW, et al. The RING finger/B-box factor TAM-1 and a retinoblastoma-like protein LIN-35 modulate context-dependent gene silencing in Caenorhabditis elegans. Genes Dev. 1999;13:2958–2970. doi: 10.1101/gad.13.22.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raj A, van den Bogaard P, Rifkin SA, van Oudenaarden A, Tyagi S. Imaging individual mRNA molecules using multiple singly labeled probes. Nat Methods. 2008;5:877–879. doi: 10.1038/nmeth.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thompson BE, Lamont LB, Kimble J. Germ-line induction of the Caenorhabditis elegans vulva. Proc Natl Acad Sci U S A. 2006;103:620–625. doi: 10.1073/pnas.0510264103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang D, Kennedy S, Conte D, Jr, Kim JK, Gabel HW, et al. Somatic misexpression of germline P granules and enhanced RNA interference in retinoblastoma pathway mutants. Nature. 2005;436:593–597. doi: 10.1038/nature04010. [DOI] [PubMed] [Google Scholar]
- 45.Petrella LN, Wang W, Spike CA, Rechtsteiner A, Reinke V, et al. synMuv B proteins antagonize germline fate in the intestine and ensure C. elegans survival. Development. 2011;138:1069–1079. doi: 10.1242/dev.059501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kelly WG, Xu S, Montgomery MK, Fire A. Distinct requirements for somatic and germline expression of a generally expressed Caernorhabditis elegans gene. Genetics. 1997;146:227–238. doi: 10.1093/genetics/146.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang LI, Sternberg PW. Interactions of EGF, Wnt and HOM-C genes specify the P12 neuroectoblast fate in C. elegans. Development. 1998;125:2337–2347. doi: 10.1242/dev.125.12.2337. [DOI] [PubMed] [Google Scholar]
- 48.Thomas JH, Stern MJ, Horvitz HR. Cell interactions coordinate the development of the C. elegans egg-laying system. Cell. 1990;62:1041–1052. doi: 10.1016/0092-8674(90)90382-o. [DOI] [PubMed] [Google Scholar]
- 49.Hu M, Polyak K. Microenvironmental regulation of cancer development. Curr Opin Genet Dev. 2008;18:27–34. doi: 10.1016/j.gde.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wicks SR, Yeh RT, Gish WR, Waterston RH, Plasterk RH. Rapid gene mapping in Caenorhabditis elegans using a high density polymorphism map. Nat Genet. 2001;28:160–164. doi: 10.1038/88878. [DOI] [PubMed] [Google Scholar]
- 52.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Unimodal distribution of FISH spot intensity. Histograms of FISH spot intensity for six images from six different animals are shown. Each image included the anchor cell and most or all of the germline. Intensities were calculated by taking the maximum intensity of a spot and subtracting the average background intensity of a four-pixel radius surrounding the spot.
(TIFF)
FISH spot intensity is independent of expression level. The mean spot intensity was calculated for mRNAs expressed in the anchor cell, germline, or ectopically. For each animal, the intensity of each spot was normalized to the mean intensity of the spots in the anchor cell, which was set to 1. The mean and standard deviation for the expression in the germline or ectopically from 7–10 animals for each genotype are shown. lin-3(e1417) had roughly wild-type numbers of lin-3 FISH spots in the germline but had approximately 4.5-fold fewer lin-3 FISH spots in the anchor cell (Table S2). In both wild-type and lin-15AB(e1763) animals the density of spots in the anchor cell was noticeably higher than in the germline or elsewhere (e.g. Figure 3A and 3D).
(TIFF)
lin-3 mRNA expression in a wild-type animal. FISH of lin-3 mRNA in a wild-type animal. The animal shown is the same as in Figure 3A. Each frame is a different plane in the Z-axis. lin-3 mRNA was expressed in the pharynx, anchor cell, and germline and was tightly repressed elsewhere.
(AVI)
The synMuv genes prevent widespread ectopic expression of lin-3 mRNA. FISH of lin-3 mRNA in a lin-15AB(e1763) mutant animal. The animal shown is the same as in Figure 3D. Each frame is a different plane in the Z-axis. lin-3 was ectopically expressed throughout the animal, with approximately 900 ectopic copies of lin-3 mRNA seen outside of the pharynx, anchor cell, and germline.
(AVI)
Oligonucleotides in lin-3 FISH probe.
(DOC)
Quantification of lin-3 expression.
(DOC)
List of strains used in this study.
(DOC)