Abstract
Spontaneous eye blinking serves a critical physiological function, but it also interrupts incoming visual information. This tradeoff suggests that the inhibition of eye blinks might constitute an adaptive reaction to minimize the loss of visual information, particularly information that a viewer perceives to be important. To test this hypothesis, we examined whether the timing of blink inhibition, during natural viewing, is modulated between as well as within tasks, and also whether the timing of blink inhibition varies as a function of viewer engagement and stimulus event type. While viewing video scenes, we measured the timing of blinks and blink inhibition, as well as visual scanning, in a group of typical two-year-olds, and in a group of two-year-olds known for attenuated reactivity to affective stimuli: toddlers with Autism Spectrum Disorders (ASD). Although both groups dynamically adjusted the timing of their blink inhibition at levels greater than expected by chance, they inhibited their blinking and shifted visual fixation differentially with respect to salient onscreen events. Moreover, typical toddlers inhibited their blinking earlier than toddlers with ASD, indicating active anticipation of the unfolding of those events. These findings indicate that measures of blink inhibition can serve as temporally precise markers of perceived stimulus salience and are useful quantifiers of atypical processing of social affective signals in toddlers with ASD.
Keywords: autonomic function, child development, eye-tracking, social engagement
When we blink, the flow of visual information between the world and one's retina is temporarily interrupted. In that instant of blinking, visual stimulation from the external world is lost for 150–400 ms (1, 2). As a result, the average adult, in the course of a single waking day, will spend ∼44 min with his or her eyelids closed, missing visual information. During those moments, an exquisite choreography of neural systems—encompassing movement of the oculomotor muscles (3); activity in supplementary and frontal eye fields (4); and widespread activity in visual, parietal, and prefrontal cortical areas (5, 6)—works together to suppress the actual visual signal of an occluding eyelid. These systems create the illusion of perceptual continuity (6, 7), but if new visual information is presented in that instant of blinking, it will be missed (8, 9).
From the standpoint of physiology, blinks exist primarily to protect: They keep the eyes hydrated and protect against foreign objects (10, 11). Average individual rates of blinking increase with age (12, 13) and are correlated with dopamine levels in human and nonhuman primates (14, 15). However, blinking also relates, like other autonomic processes (e.g., heart rate, perspiration), to cognitive states beyond physiological function alone (16): Blink rate has been observed to vary as a function of several cognitive tasks (17–21), and blink rates decrease during activities that require greater attention [as when reading vs. sitting in a waiting room (22)]. Studies have also shown that the timing of blinks is related to both explicit (20, 21) and implicit (23, 24) attentional pauses in task content. Together, these observations highlight a key difference between blinking and other autonomic reactions: Blinking sets a physical limit on visual attention because of its profound interruption of incoming visual information (25).
This evidence also suggests another possibility: that although in everyday situations we remain largely unaware of our blinking, it would be highly adaptive if we dynamically adjusted the exact timing of when we do or do not blink. More specifically, if the inhibition of blinking ensures that the flow of critical visual information remains undisrupted, then measurements of the precise timing of when individuals inhibit their blinking might serve as markers of the subjective assessment of perceived stimulus salience: that is, moment-by-moment, unconscious appraisals of what is or is not important enough to warrant the inhibition of blinking.
Paradoxically, in most experimental studies of visual scanning and eye movements, even those that focus on participants’ response to scene content, blink data are commonly discarded as artifacts or noise (26, 27). If the timing of blink inhibition is an adaptive reaction to minimize the loss of critical information, then discarding these data may mean losing a measure of not simply what a person is looking at but of how engaged that person is with what is being looked at.
While filtering blinks from our own data, we made an informal initial observation: The blink rate of participants appeared to decrease during the presentation of video scenes and then to increase during intertrial intervals before and after the videos. This observation gave way to the current experiment: We hypothesized that the timing of blink inhibition might vary, not only before or after an entire video trial but on a moment-by-moment basis within the video scenes themselves, in relation to viewers’ subjective perceptions of the relative importance of what they were fixating on. In the current experiment, we tested the hypothesis that blinking is inhibited at moments in natural viewing that are perceived as more important or engaging. To test this hypothesis, we measured blinking and visual scanning in 93 viewers.
Results
Experimental Design.
We hypothesized that viewers’ blink inhibition could vary (i) on the basis of content (with some categories of content being more engaging than others) and (ii) as a function of individual interests (with a given category of content being more important to some viewers but relatively less important to others). In each case, we measured viewers’ visual scanning and tested whether the likelihood of blink inhibition was modulated in relation to those factors. The study design was 2 × 2: two groups of viewers (with varying interests, described below) and two categories of content.
Ninety-three children with a mean (M) chronological age of 2.3 y (SD = 0.55) participated in the study, all with the written informed consent of their parents and/or legal guardians. The video the children watched consisted of unscripted interaction between a boy and a girl playing together in a toy wagon (still images from the video are presented in Fig. 1). None of the participants had previously seen the video. To operationalize the two categories of content, in unscripted scenes of natural interaction, the video included both physical movements of an object (a door on the toy wagon) as well as affectively charged interactions (an argument between the boy and the girl). Although these physical movements and affective interactions were not mutually exclusive (e.g., angry facial expressions from the boy could be followed by a movement of the wagon door), the locations of greatest affect were spatially discrete from those of most movement, with affectively charged facial expressions separated from the physical location of the wagon door.
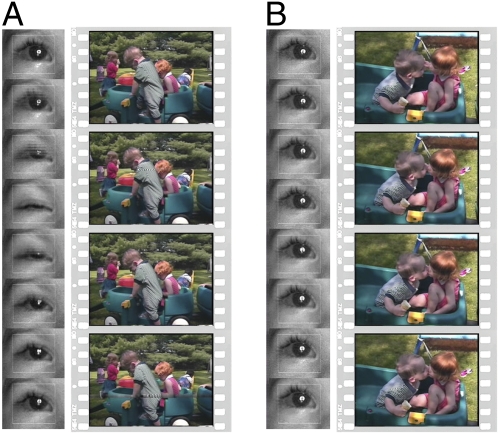
Fig. 1.
Blinking and statistically significant blink inhibition while watching scenes of peer interaction. Example still images from videos of peer interaction, together with viewer eye images during blinking (A) and statistically significant blink inhibition (B). Example eye images were sampled at 100-ms intervals. Example video stills were sampled at corresponding 200-ms intervals. Eye-tracking data were collected at 60 Hz.
The distinction between affective and physical events was important to the experimental design because the children who watched the video were divided into two groups that vary in their response to affective and physical cues (28, 29). The video was shown to 41 two-year-olds with autism spectrum disorders (ASD) as well as 52 typical two-year-olds (full clinical characterization data and procedures are provided in Table S1 and the SI Materials and Methods, Participants).
Here, the children with ASD provide the critical comparison group because these children have been shown previously to display atypical patterns of visual attention to social interaction (30, 31), attenuated reactivity to varying social affect (32), and lack of differential response to social attentional cues (28) but also intact response to physical attentional cues (28, 29) and intact ability to predict and attend to physical events (31, 33, 34). In the current experimental paradigm, we tested blink inhibition as a marker of perceived stimulus salience, varying by group membership.
Physiological Controls.
We first examined overall blink rate and blink duration to test for physiological differences in eye-blink behavior between toddlers with ASD and typical toddlers. Eye movement data were collected at the rate of 60 Hz, and blinks were recorded as events with a measurable duration, identified by an automated algorithm, supplemented and verified by simultaneous video recording in all participants, and separately verified by simultaneous electromyography recordings in one adult viewer [complete details are provided in SI Materials and Methods, Data Acquisition and Analysis and are described in the study by Jones et al. (30)].
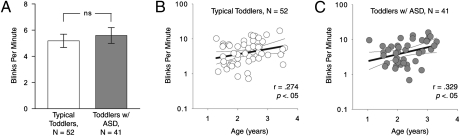
No difference was found in blinks per minute (bpm) between toddlers with ASD (M = 5.58 bpm, SD = 3.88) and typical toddlers (M = 5.18 bpm, SD = 3.66) [t(91) = 0.519, P = 0.60] (Fig. 2A) (analysis performed on log-transformed data, M and SD are untransformed data). In addition, no difference in blink duration was found between toddlers with ASD (M = 300.0 ms, SD = 98.7) and typical toddlers (M = 301.3 ms, SD = 98.0) [t(91) = −0.23, P = 0.82]. Consistent with previous research on the ontogeny of blinking (12), individual blink rates (bpm) were positively correlated with chronological age in both groups (r = 0.33, P < 0.05 for the toddlers with ASD and r = 0.27, P < 0.05 for typical toddlers; Fig. 2 B and C). There was no between-group difference in the strength or direction of this correlation (z = 0.28, P > 0.05) (35).
Fig. 2.
Mean blink rate and blink rate in relation to age. (A) No difference was found in blink rate (bpm) between toddlers with ASD and typical toddlers (analysis performed on log-transformed data; bars are untransformed data, error bars are SEM). Consistent with previous research on chronological change in blink rate, individual blink rates were positively correlated with chronological age in both typical toddlers (B) and toddlers with ASD (C), with no significant difference in correlation between groups (P = 0.28).
Blink Rate Before, During, and After Task.
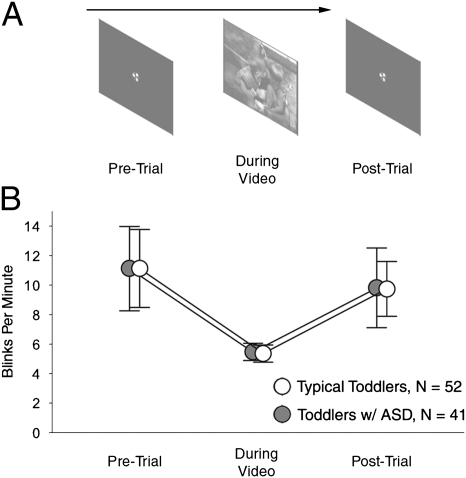
We next tested explicitly our anecdotal observation of variation in blink rate during the intertrial intervals before and after each experimental trial (the video scene) (Fig. 3A). During these intervals, a centering cue was presented on an otherwise blank screen to draw the attention of viewers to a common fixation location. Based on our earlier observations, we predicted that blink rate would decrease during the experimental trial relative to intertrial intervals.
Fig. 3.
Task-dependent modulation of blinking. (A) We measured individual blink rates before, during, and after experimental trials. (B) Mean blink rate of both toddlers with ASD and typical toddlers decreased during the experimental trial relative to pre- and posttrial periods (error bars are SEM).
As shown in Fig. 3B, the mean blink rate of both toddlers with ASD and typical toddlers decreased during the experimental trial relative to pre- and posttrial periods. Given the positive skew of the dependent variable (bpm), with larger variance than mean, we performed a repeated measures ANOVA [diagnostic group (2 levels) × trial type (3 levels: pretrial, during trial, and posttrial)] with underlying negative binomial distributions assumed (36, 37). The ANOVA yielded a significant main effect of trial type (Wald X2 = 18.70, df = 2, P < 0.001). Post hoc comparisons indicated that mean bpm pre- and posttrial were not significantly different from one another (Wald X2 = 0.64, df = 1, P = 0.42) but that blink rate during each of those conditions was significantly greater than blink rate during the experimental trial (Wald X2 = 20.58, df = 1, P < 0.001 and Wald X2 = 14.57, df = 1, P < 0.001, respectively). There was no main effect of diagnosis (Wald X2 = 0.002, df = 1, P = 0.97) and no significant interaction of diagnosis by condition (Wald X2 = 0.003, df = 2, P = 0.99).
Instantaneous Blink Rate and Periods of Intratask Blink Inhibition.
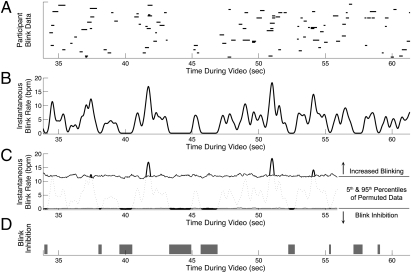
We next tested whether instantaneous blink rate was significantly modulated during the video itself (Fig. 4A). Individual data were recorded as 60-Hz time series (with binary values at each point in the series indicating whether a given individual was blinking or not). Instantaneous blink rate was computed across all individuals for each group (complete details are provided in SI Materials and Methods, Instantaneous Blink Rate). To test the null hypothesis that the timing of blink inhibition was unrelated to scene content, we used permutation testing (38). In each of 1,000 iterations, for each group, the binary times series blink data for each child were permuted by circular shifting (39), with shift size for each child drawn independently from a random number generator with uniform distribution. Instantaneous blink rate was then calculated across the shifted individual data. Because each individual's data had been shifted independently, the timing of each shifted blink time series was random in relation to the actual time line of video content and random in relation to the timing of other participants’ blinking (details are provided in SI Materials and Methods, Permutation Test). By this approach, in the permuted data, the mean blink rate of participants during the entire task remains unchanged (and task-specific) but the timing of when instantaneous blink rate is increased or decreased is made random.
Fig. 4.
Instances of statistically significant blink inhibition during natural viewing of a video scene. (A) Raster plot of eye blinks made by typical toddlers. (B) Instantaneous blink rate (bpm) of typical toddlers while viewing the movie. (C) Fifth and 95th percentiles of permuted blink data. Periods of statistically significant blink inhibition were identified as times when the actual blink rate was less than the fifth percentile of permuted data. (D) Time line showing periods of significant blink inhibition in gray (P < 0.05). (A–D) Twenty-eight-second excerpt from video data is shown.
This enabled a basic permutation test with exact probabilities (38): At each time point, the fifth percentile across all permuted data served as a statistical threshold (P = 0.05) for identifying periods of statistically significant blink inhibition (Fig. 4 C and D). If the timing of actual measured blinks was random with respect to ongoing video content, we would expect that the measured instantaneous blink rate for each group would differ from that of the permuted data no more than 5% of the time. In contrast, in the actual data, we found that the blink rate for typical toddlers was significantly inhibited (exhibiting values less than the 0.05 threshold of permuted data) during 8.8% of video viewing time and that the blink rate for the ASD group was significantly inhibited during 7.0% of video viewing time (Fig. S1). We tested this difference between observed blink rates and permuted data for each group by two-sample Kolmogorov–Smirnov tests, finding significant differences for each (D = 0.22, P < 0.001 for typical toddlers and D = 0.28, P < 0.001 for toddlers with ASD).
Blink Inhibition Relative to Affective and Physical Events.
Having confirmed that blinking was inhibited at levels greater than expected by chance and inhibited at specific times during unconstrained viewing of natural scenes, we next tested whether blink inhibition varied selectively with respect to video content, visual fixation, and viewer group. As described above, the experimental paradigm presented two categories of content (affective and physical events) to two populations of children known for differential attention to those categories (children with ASD and typical toddlers). In the video shown to participants, the boy in the video desires to leave the wagon door open, whereas the girl wants it to be closed; this scenario conveniently created varying levels of affective content (the discord between the boy and the girl) and a repeated physical action (the closing or opening of the wagon door).
To operationalize the designation of affective and physical events in a video of unscripted natural interaction, 10 adult viewers rated the level of affect throughout the entire video, identifying eight segments within the video in which facial expressions and/or vocalizations showed heightened emotional affect (e.g., time periods when the boy or the girl in the video became visibly angry). The coefficient of concordance for interrater affective ranking was highly significant (Kendall's W = 0.879, X2 = 123.02, df = 14, P < 0.0001) (40). Physical events were operationalized as times when the wagon door was moving (complete details of all rating procedures are provided in SI Materials and Methods, Ratings of Affective and Physical Events). The two event types were not mutually exclusive but, per the independent raters, overlapped less than 25.18% of the time.
The remaining segments of the video were classified as nonaffective nonphysical events. We predicted that viewers would inhibit their blinking during moments perceived to be particularly important to process and would increase their blinking during moments perceived to be less important.
To examine how the timing of blink inhibition varied with respect to affective and physical events, we used peristimulus (or “peri-event”) time histograms (PSTHs) (41). PSTHs were constructed by aligning segments of individual time series blink data to the onset of events and by then computing counts of an individual's blinks occurring in 33.3-ms bins in a surrounding 2,000-ms window. Bin counts were computed for each participant across all events and then averaged across all participants to obtain group means.
To test whether the observed changes in blink rate differed from those expected by chance, we computed a second set of PSTHs from permuted blink data. As before, individual blink sequences were permuted by circular shifting of individual data 1,000 times (42). PSTHs were then computed on each of those permuted datasets. The mean instantaneous blink rate, during each bin, across all 1,000 PSTHs from permuted data quantified the blink rate one would observe if blink rate were random with respect to onscreen events. If, on the other hand, blink rate were time-locked to onscreen events and not random, one would expect to see significant deviations from the permuted data distribution. The 5th and 95th percentiles of instantaneous blink rate across all PSTHs from permuted data served as a P = 0.05 confidence level against which to compare blink rates in the actual data (one-tailed comparisons). To test for between-group differences, we computed confidence intervals (CIs) of bootstrapped data for each group (42).
Blink Inhibition Dissociates Perceived Stimulus Salience.
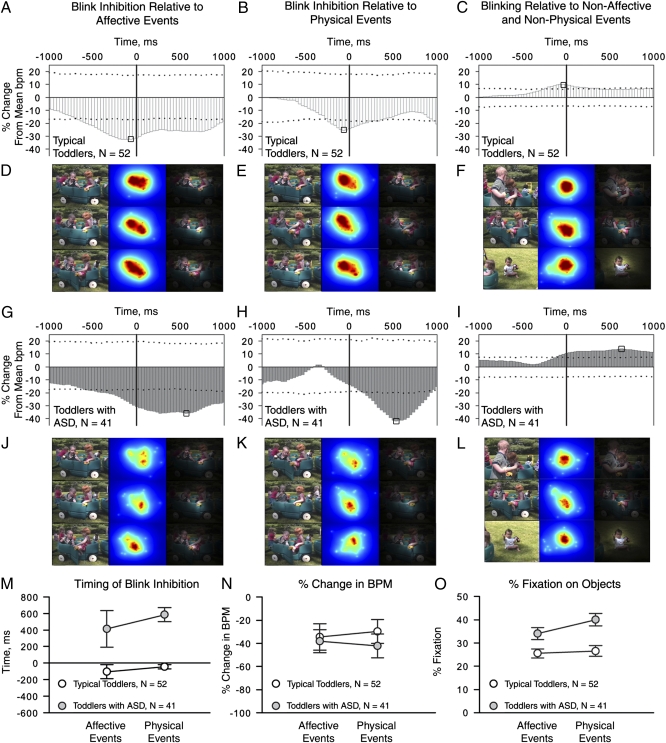
As shown in Fig. 5A, the PSTH for typical toddlers reveals a 32.4% reduction in blink rate for affective events, reaching its minimum 66 ms prior to the zero lag. This indicates statistically significant blink inhibition in typical toddlers (P < 0.05), time-locked to the occurrence of events with high affective valence. Toddlers with ASD also show a reduction in blink rate (35.8%), but that reduction is greatest 599 ms after the zero lag of affective events (Fig. 5G).
Fig. 5.
Time-locked blinks and blink inhibition during natural viewing, together with example visual fixation data. We measured time-locking of blinks and blink inhibition relative to affective events (A and G), physical events (B and H), and nonaffective nonphysical events (C and I) by constructing PSTHs. PSTHs show the percent change in bpm relative to the mean of permuted blink data. Dashed horizontal lines mark 0.05 and 0.95 CIs; the percent change in bpm beyond these levels represents a change in bpm greater than expected by chance (one-tailed, P < 0.05). CIs scale inversely with the number of events (with approximately double the number of events in the nonaffective nonphysical category). Absolute minimum and maximum changes in bpm are highlighted by black squares in each plot. Example visual fixation data during change in blink rate for affective (D and J), physical (E and K), and nonaffective nonphysical (F and L) events. Data from typical toddlers show greater density of fixations on people during both affective (D) and physical (E) events, whereas data from toddlers with ASD show greater density of fixations on the wagon door (J and K). Three column plots show a still frame from the video (first column, sampled at the absolute minimum decrease in bpm); kernel density plot of fixation data at the same moment (second column, with hotter colors denoting greater density); and the same kernel density plot scaled from black to transparent, overlaid on the original frame (third column). The color of fixation density plots is scaled relative to the sample size of each group, such that maximum and minimum possible densities have the same color values for each group despite differences in sample size. (M) Timing of blink inhibition for affective vs. physical events. (N) Percent decrease in bpm for affective vs. physical events. (O) Percent fixation on objects for affective vs. physical events. Error bars are SEM.
The between-group difference in timing is highly significant, because the CIs of bootstrapped lag data for each group are nonoverlapping (Fig. 5M, lag time for blink rate minimum in typical toddlers: CI5 = −230 ms, CI95 = 0 ms; lag time for blink rate minimum in toddlers with ASD: CI5 = 33 ms, CI95 = 700 ms). The observed difference in timing was not attributable to a more general delay in speed or frequency of eye movements, because we found no between-group differences in latency to shift gaze [typical toddler: M = 1.09 s (SE = 0.20), toddlers with ASD: M = 0.96 s (SE = 0.28); t(91) = 0.40, P = 0.69, measured as reaction time to initiate a first saccade following the onset of the movie] or in duration or frequency of fixations [duration for typical toddlers: M = 442 ms (SE = 16.4), duration for toddlers with ASD: M = 492 (SE = 29.4); t(91) = −1.57, P = 0.12 and frequency for typical toddlers: M = 2.04 fixations per second (SE = 0.09), frequency for toddlers with ASD: M = 1.93 (SE = 0.11); t(91) = 0.85, P = 0.40].
Each group shows a numerical, although not statistically significant, reduction in blink rate by event type (Fig. 5N): Typical toddlers exhibit greater reduction in blink rate during affective than physical events (32.4% vs. 25.4%, Fig. 5 A and B), whereas toddlers with ASD exhibit the reverse pattern, with a 41.7% reduction for physical events and a 35.8% reduction for affective events (Fig. 5 G and H). Both groups of toddlers show a significant increase in blink rate relative to nonaffective nonphysical events (Fig. 5 C and I).
Helping to disambiguate the question of differential engagement is the pattern of each group's visual fixations during the two event types (Fig. 5 D–F, J–L, and O). Typical toddlers spent significantly less time looking at objects than toddlers with ASD during both event types [F1,91 = 12.01, P = 0.001, repeated measures ANOVA with diagnosis (2 levels) × event (affective vs. physical)], and the interaction between diagnosis and event type was significant (Fig. 5O) (F1,91 = 5.99, P = 0.016). Paired-samples t tests confirmed that typical toddlers showed no difference in percentage of fixation on objects during affective vs. physical events (t1,51 = 0.85, P = 0.4; Maffective = 25.5%, SD = 14.21 vs. Mphysical = 26.5%, SD = 16.7) but that toddlers with ASD increased fixation on objects, such as the moving wagon door, during physical events (Fig. 5O) [M(SD) = 33.9(16.7) for affective vs. 40.0(17.2) for physical; t1,40 = 3.57, P = 0.001].
In sum, blink inhibition for typical toddlers was (i) most reduced just prior to the zero lag of events, (ii) numerically greater for affective rather than physical events, and (iii) unrelated to level of fixation on objects (marked instead by greater than 73% fixation on people during both event types). In contrast, for toddlers with ASD, blink inhibition was (i) most reduced after the zero lag of events, (ii) numerically greater for physical rather than affective events, and (iii) marked by a significant increase in fixation on objects during physical events.
Discussion
In the present study, we show that inhibition of eye blinking during natural viewing can be used as a quantifiable metric of viewers’ moment-by-moment engagement with visual content. These data indicate that children as young as 2 y of age inhibit their blinking to maximize access to visual information that they perceive to be important. Although previous research has shown that blinks are aligned with dynamic changes or “breaks” in visual information (23), the present results suggest that the key cognitive metric may not be blinking, per se, but rather the inhibition of blinking—an adaptive reaction to minimize possible information loss, which can also be used to index level of engagement with visual content.
Of particular interest to our laboratory, the patterns of blink inhibition and the distribution of visual fixations map onto well-established between-group differences (30, 31, 33) but also reveal more subtle differences in the subjective assessment of stimulus salience. When the data were time-aligned to scenes of heightened affective content (Fig. 5A), typical toddlers showed a persistent inhibition of blinking that peaked before the zero event lag. Toddlers with ASD, in contrast, exhibited a peak in blink inhibition that occurred more than 0.5 s after the zero event lag.
That typical toddlers inhibit their blinking earlier than toddlers with ASD suggests the intriguing possibility that typical toddlers are actively anticipating the unfolding of salient events, and doing so in a time-locked fashion. The visual fixation data tell a similar story: Toddlers with ASD look more at physical objects in the video scene and selectively increase their fixation on those objects when the objects move (that is, during the designated physical events).
Critically, the ASD data show no evidence of more general delays in speed or frequency of eye movements: There are no between-group differences in duration or frequency of fixations, or differences in frequency of saccades or latency to initiate a first saccade at the onset of the movie. Rather than merely being “late” to shift gaze to affective content, toddlers with ASD appear to be reacting, after the fact, to physical events that have already happened in the environment, inhibiting their blinking while increasing their fixation on objects.
In contrast, typical toddlers’ attention to socially relevant cues, such as eye-gaze, facial expression, and body posture, may allow them to anticipate actions that have not yet happened but may be about to happen (as when angry facial expressions precede a yell or the slamming of the wagon door). These cues help typical toddlers generate expectations about how actions in the world will subsequently unfold. For toddlers with ASD, however, blink inhibition, as an after-the-fact reaction, can be seen as reflecting a lack of sensitivity to those environmental (and, in particular, social) cues. It suggests an engagement with affective and physical stimuli separate from the social context in which they are typically perceived: Although typical toddlers may be engaged by the slamming of the car door because of its relevance to the ongoing social interaction between the characters, engagement by toddlers with ASD may be in reaction to the salient physical properties of such events.
These hypotheses regarding between-group differences in how movie events were perceived underscore the point that even though movie events may be classified as affective or physical, it is unlikely that they were perceived as mutually exclusive dualities. One of the main goals of our experiment was to test for blink inhibition using semistructured, naturalistic stimuli. In such situations, categorical boundaries of affective and physical become blurred: Typical toddlers, for instance, are likely to perceive the social significance and affective meaning behind the slamming wagon door. This blurring of affective and physical categories may account for why reductions in blink rate trended in the expected directions but did not reach significance, with typical toddlers showing a larger reduction in response to affective events, whereas toddlers with ASD showed greater reduction to physical events.
The results demonstrate that patterns of blink inhibition can provide an inroad into a critical aspect of social affective experience that has been sorely lacking in the field of autism research and in many neuroethological studies of visual perception in general: a measure of not only what someone is looking at but of how engaged he or she is with what he or she is looking at. Although previous work has shown that children with ASD allocate fewer attentional resources to socially relevant stimuli than their typically developing peers (30), these studies have failed to capture how engaged children are with what they are fixating on. Stated differently, during those times when children with ASD do fixate on “socially relevant” stimuli, are they actually engaged with those stimuli to the same extent as their typical peers? Do children with ASD perceive those stimuli and their adaptive value in the same way as typically developing children? This becomes a cardinal question when one considers that engagement with socially relevant stimuli may be critical for other aspects of neural and behavioral development [such as the acquisition of speech and language skills (43) or specialization of brain function (44)].
From the standpoint of more general research applications, measures of blink inhibition are well suited to providing temporally precise indices of perceived stimulus salience during naturalistic, fast-paced presentations of visual content. In comparison to other autonomic responses traditionally used in psychophysiological studies, such as electrodermal and cardiovascular activity (45), blink inhibition compares well for measuring reactivity to emotional stimuli: Electrodermal and cardiovascular responses are highly multidetermined, preventing strong inferences about their relationship to mental activity; in addition, their latency and refractory periods undermine precise temporal markings of their measurements relative to affective or cognitive state (45, 46). Blink inhibition, in contrast, is intrinsic to the visual system rather than a peripheral function; its on- and off-set parameters are precise and temporally sensitive to ecologically valid, fast-paced presentations of content; and, finally, blink inhibition can be measured by entirely noninvasive, even concealed, eye-tracking cameras, circumventing the need for obtrusive equipment that may alter the ethological validity of other measures.
In sum, measures of blink inhibition provide a promising index of autonomic reactivity and differential engagement, time-locked to salient moments within fast-paced, rapidly changing visual displays. By precisely measuring the timing of blink inhibition relative to unfolding content, one can determine, on a moment-by-moment basis, a viewer's subjective assessment of the importance of what he or she is watching.
Materials and Methods
A complete description of materials and methods can be found in SI Materials and Methods. Details on participant characterization (including age, level of verbal and nonverbal function, and diagnostic procedures) are provided. In addition, data acquisition and analysis, ratings of affective and physical events, calculation of instantaneous blink rate, and permutation testing are described.
Supplementary Material
Acknowledgments
We thank the families and children for their time and participation. We also thank Jennifer Moriuchi, David Lin, Gordon Ramsay, and Kelley Knoch for discussions of concepts and methods; Katelin Carr, Phil Gorrindo, Anna Krasno, Casey Zampella, Jessie Northrup, Laura Edwards, Jennings Xu, Katherine Rice, and Peter Lewis for their help with data collection; and Kasia Chawarska, Rhea Paul, Suzanne Macari, Amy Carney, Tina Goldsmith, Amanda Steiner, Grace Gengoux, Celine Saulnier, Diane Goudreau, Erin Loring, James McGrath, and Abha Gupta for their contributions to the clinical characterization of the samples. This work was supported by Grants U54-MH66494 and P50-MH081756-01 from the National Institute of Mental Health (to W.J. and A.K.) as well as by grants from the Simons Foundation (to W.J. and A.K.) and the Natural Sciences and Engineering Research Council of Canada (to S.S.), and a National Science Foundation Graduate Research Fellowship (to S.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1109304108/-/DCSupplemental.
References
- 1.Baumstimler Y, Parrot J. Stimulus generalization and spontaneous blinking in man involved in a voluntary activity. J Exp Psychol. 1971;88:95–102. [Google Scholar]
- 2.VanderWerf F, Brassinga P, Reits D, Aramideh M, Ongerboer de Visser B. Eyelid movements: Behavioral studies of blinking in humans under different stimulus conditions. J Neurophysiol. 2003;89:2784–2796. doi: 10.1152/jn.00557.2002. [DOI] [PubMed] [Google Scholar]
- 3.Bour LJ, Aramideh M, de Visser BW. Neurophysiological aspects of eye and eyelid movements during blinking in humans. J Neurophysiol. 2000;83:166–176. doi: 10.1152/jn.2000.83.1.166. [DOI] [PubMed] [Google Scholar]
- 4.Bodis-Wollner I, Bucher SF, Seelos KC. Cortical activation patterns during voluntary blinks and voluntary saccades. Neurology. 1999;53:1800–1805. doi: 10.1212/wnl.53.8.1800. [DOI] [PubMed] [Google Scholar]
- 5.Bristow D, Frith C, Rees G. Two distinct neural effects of blinking on human visual processing. Neuroimage. 2005;27:136–145. doi: 10.1016/j.neuroimage.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 6.Bristow D, Haynes J-D, Sylvester R, Frith CD, Rees G. Blinking suppresses the neural response to unchanging retinal stimulation. Curr Biol. 2005;15:1296–1300. doi: 10.1016/j.cub.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 7.Volkmann FC, Riggs LA, Moore RK. Eyeblinks and visual suppression. Science. 1980;207:900–902. doi: 10.1126/science.7355270. [DOI] [PubMed] [Google Scholar]
- 8.Volkmann FC. Human visual suppression. Vision Res. 1986;26:1401–1416. doi: 10.1016/0042-6989(86)90164-1. [DOI] [PubMed] [Google Scholar]
- 9.O'Regan J, Deubel H, Clark J, Rensink R. Picture changes during blinks: Looking without seeing and seeing without looking. Vis Cogn. 2000;7:191–211. [Google Scholar]
- 10.Evinger C. A brain stem reflex in the blink of an eye. Physiology (Bethesda) 1995;10:147–153. [Google Scholar]
- 11.Evinger C, Manning KA, Sibony PA. Eyelid movements. Mechanisms and normal data. Invest Ophthalmol Vis Sci. 1991;32:387–400. [PubMed] [Google Scholar]
- 12.Zametkin AJ, Stevens JR, Pittman R. Ontogeny of spontaneous blinking and of habituation of the blink reflex. Ann Neurol. 1979;5:453–457. doi: 10.1002/ana.410050509. [DOI] [PubMed] [Google Scholar]
- 13.Bacher LF, Smotherman WP. Spontaneous eye blinking in human infants: A review. Dev Psychobiol. 2004;44:95–102. doi: 10.1002/dev.10162. [DOI] [PubMed] [Google Scholar]
- 14.Karson CN. Spontaneous eye-blink rates and dopaminergic systems. Brain. 1983;106:643–653. doi: 10.1093/brain/106.3.643. [DOI] [PubMed] [Google Scholar]
- 15.Taylor JR, et al. Spontaneous blink rates correlate with dopamine levels in the caudate nucleus of MPTP-treated monkeys. Exp Neurol. 1999;158:214–220. doi: 10.1006/exnr.1999.7093. [DOI] [PubMed] [Google Scholar]
- 16.Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol. 2005;493:154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- 17.Holland MK, Tarlow G. Blinking and thinking. Percept Mot Skills. 1975;41:503–506. [PubMed] [Google Scholar]
- 18.Pivik RT, Dykman RA. Endogenous eye blinks in preadolescents: Relationship to information processing and performance. Biol Psychol. 2004;66:191–219. doi: 10.1016/j.biopsycho.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Fogarty C, Stern JA. Eye movements and blinks: Their relationship to higher cognitive processes. Int J Psychophysiol. 1989;8:35–42. doi: 10.1016/0167-8760(89)90017-2. [DOI] [PubMed] [Google Scholar]
- 20.Orchard LN, Stern JA. Blinks as an index of cognitive activity during reading. Integr Physiol Behav Sci. 1991;26:108–116. doi: 10.1007/BF02691032. [DOI] [PubMed] [Google Scholar]
- 21.Drew GC. Variations in reflex blink-rate during visual-motor tasks. Q J Exp Psychol. 1951;3:73–88. [Google Scholar]
- 22.Bentivoglio AR, et al. Analysis of blink rate patterns in normal subjects. Mov Disord. 1997;12:1028–1034. doi: 10.1002/mds.870120629. [DOI] [PubMed] [Google Scholar]
- 23.Nakano T, Yamamoto Y, Kitajo K, Takahashi T, Kitazawa S. Synchronization of spontaneous eyeblinks while viewing video stories. Proc Biol Sci. 2009;276:3635–3644. doi: 10.1098/rspb.2009.0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrmann A. The interaction of eye blinks and other prosodic cues in German sign language. Sign Lang Linguist. 2010;13:3–39. [Google Scholar]
- 25.Kaufman P, Alm A. Adler's Physiology of the Eye: Clinical Applications. Philadelphia: Elsevier; 2003. [Google Scholar]
- 26.Widdel H. In: Theoretical and Applied Aspects of Eye Movement Research. Gale AG, Johnson F, editors. New York: Elsevier; 1984. pp. 21–29. [Google Scholar]
- 27.Gitelman DR. ILAB: A program for postexperimental eye movement analysis. Behav Res Methods Instrum Comput. 2002;34:605–612. doi: 10.3758/bf03195488. [DOI] [PubMed] [Google Scholar]
- 28.Senju A, Tojo Y, Dairoku H, Hasegawa T. Reflexive orienting in response to eye gaze and an arrow in children with and without autism. J Child Psychol Psychiatry. 2004;45:445–458. doi: 10.1111/j.1469-7610.2004.00236.x. [DOI] [PubMed] [Google Scholar]
- 29.Chawarska K, Klin A, Volkmar F. Automatic attention cueing through eye movement in 2-year-old children with autism. Child Dev. 2003;74:1108–1122. doi: 10.1111/1467-8624.00595. [DOI] [PubMed] [Google Scholar]
- 30.Jones W, Carr K, Klin A. Absence of preferential looking to the eyes of approaching adults predicts level of social disability in 2-year-old toddlers with autism spectrum disorder. Arch Gen Psychiatry. 2008;65:946–954. doi: 10.1001/archpsyc.65.8.946. [DOI] [PubMed] [Google Scholar]
- 31.von Hofsten C, Uhlig H, Adell M, Kochukhova O. How children with autism look at events. Res Autism Spectr Disord. 2009;3:556–569. [Google Scholar]
- 32.Klin A, Jones W, Schultz R, Volkmar F. The enactive mind, or from actions to cognition: Lessons from autism. Philos Trans R Soc Lond B Biol Sci. 2003;358:345–360. doi: 10.1098/rstb.2002.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klin A, Lin DJ, Gorrindo P, Ramsay G, Jones W. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature. 2009;459:257–261. doi: 10.1038/nature07868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Falck-Ytter T. Young children with autism spectrum disorder use predictive eye movements in action observation. Biol Lett. 2010;6:375–378. doi: 10.1098/rsbl.2009.0897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen P, Popovich P. Parametric and Nonparametric Measures. Thousand Oaks, CA: Sage Publications; 2002. [Google Scholar]
- 36.Ridout M, Hinde J, Demétrio CG. A score test for testing a zero-inflated Poisson regression model against zero-inflated negative binomial alternatives. Biometrics. 2001;57:219–223. doi: 10.1111/j.0006-341x.2001.00219.x. [DOI] [PubMed] [Google Scholar]
- 37.Bliss CI, Fisher RA. Fitting the negative binomial distribution to biological data. Biometrics. 1953;9:176–200. [Google Scholar]
- 38.Good P. Permutation Test: A Practical Guide to Resampling for Testing Hypotheses. New York: Springer; 2000. [Google Scholar]
- 39.Oppenheim AV, Schafer RW. Digital Signal Processing. Englewood Cliffs, NJ: Prentice Hall; 1975. [Google Scholar]
- 40.Kendall M, Gibbons JD. Rank correlation methods. New York: Oxford University Press; 1990. [Google Scholar]
- 41.Moore GP, Perkel DH, Segundo JP. Statistical analysis and functional interpretation of neuronal spike data. Annu Rev Physiol. 1966;28:493–522. doi: 10.1146/annurev.ph.28.030166.002425. [DOI] [PubMed] [Google Scholar]
- 42.Manly B. Randomization, Bootstrap, and Monte Carlo Methods in Biology. Boca Raton, FL: Chapman & Hall; 2006. [Google Scholar]
- 43.Kuhl PK. Is speech learning ‘gated’ by the social brain? Dev Sci. 2007;10:110–120. doi: 10.1111/j.1467-7687.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- 44.Grelotti DJ, et al. fMRI activation of the fusiform gyrus and amygdala to cartoon characters but not to faces in a boy with autism. Neuropsychologia. 2005;43:373–385. doi: 10.1016/j.neuropsychologia.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 45.Sequeira H, Hot P, Silvert L, Delplanque S. Electrical autonomic correlates of emotion. Int J Psychophysiol. 2009;71:50–56. doi: 10.1016/j.ijpsycho.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 46.Berntson GG, Cacioppo JT, Grossman P. Whither vagal tone. Biol Psychol. 2007;74:295–300. doi: 10.1016/j.biopsycho.2006.08.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.