Abstract
Tetrapods evolved from sarcopterygian fishes in the Devonian and were the first vertebrates to colonize land. The locomotor component of this transition can be divided into four major events: terrestriality, the origins of digited limbs, solid substrate-based locomotion, and alternating gaits that use pelvic appendages as major propulsors. As the sister group to tetrapods, lungfish are a morphologically and phylogenetically relevant sarcopterygian taxon for understanding the order in which these events occurred. We found that a species of African lungfish (Protopterus annectens) uses a range of pelvic fin-driven, tetrapod-like gaits, including walking and bounding, in an aquatic environment, despite having a derived limb endoskeleton and primitively small, muscularly supported pelvis. Surprisingly, given these morphological traits, P. annectens also lifts its body clear of the substrate using its pelvic fins, an ability thought to be a tetrapod innovation. Our findings suggest that some fundamental features of tetrapod locomotion, including pelvic limb gait patterns and substrate association, probably arose in sarcopterygians before the origin of digited limbs or terrestriality. It follows that the attribution of some of the nondigited Devonian fossil trackways to limbed tetrapods may need to be revisited.
The vertebrate water-to-land transition initiated in the Devonian was a key event in the history of life. The ability to move in a variety of terrestrial and semiterrestrial environments opened up a range of new ecosystems for colonization and led to the diversity of tetrapod clades we see today. One essential adaptation in the evolution of tetrapods was the ability to locomote on land, a trait that required significant morphological and functional changes in the appendicular system. Many of these morphological changes can be observed in the fossil record (1–14). Work on basal tetrapod fossils has revealed that even those with digited limbs can be aquatic (5, 7). Some major questions these findings raised were how tetrapod-like limbs functioned in aquatic habitats, and in what sequence the characteristics of terrestrial tetrapod locomotion were acquired. These characteristics include the broad use of alternating limb movements, as in walking, the use of pelvic appendages as major propulsors, and the use of a bottom substrate (i.e., the solid surface underlying the fluid environment) to generate propulsive force.
Whereas body fossils cannot directly inform the order in which these functional traits were acquired, fossil trackways can give us valuable information on how animals moved. Many fossil trackways from the Devonian are indicative of quadrupedal and bipedal gaits very similar to those used by modern terrestrial tetrapods (1, 3, 9, 13, 14). That tracks were preserved demonstrates that these animals were walking on a substrate. That these trackways have a cosmopolitan distribution implies that the animals responsible for generating them were themselves widespread, and likely abundant and diverse. Because we cannot unambiguously associate fossil trackways with body fossils, we must look to other data sources to inform how the earliest tetrapods, both finned and limbed, used their appendages underwater.
We aimed in this study to examine fin-based locomotion of Protopterus annectens, one of the few species of extant finned sarcopterygians. Lungfishes, including P. annectens, are the sister group to living tetrapods (2, 4, 15), and as such are important for understanding the evolution of movement patterns and limb function in this group. P. annectens is clearly specialized relative to other sarcopterygians in that its fins are longer and more slender than all other known examples (16, 17). Its postcranial skeleton is cartilaginous (18), resulting in a less “heavy-bodied” morphology than known early tetrapods. However, this fish serves as a useful model for understanding locomotion in sarcopterygians, including fin-bearing stem-group tetrapods, because they share features that are functionally important. These features include the presence of lungs, the absence of a sacrum, living in a predominantly aquatic habitat, and monobasal fins that are not organized into the three functional regions characteristic of a digited tetrapod limb but rather contain a greater number of serially arranged elements. Although lungfish fins are slender and contain many elements, the potential presence of functional subdivisions has not been explored previously. The buoyancy provided by aquatic environments, coupled with the presence of lungs, would allow even heavy-bodied animals such as early tetrapods to be propelled underwater with small limbs. Because of these morphological similarities, studies of modern sarcopterygian fishes such as P. annectens can complement what is known from modern tetrapod locomotion.
Whereas little is known of finned locomotion in lungfishes, locomotion of the coelacanth (Latimeria chalumnae), the only other extant sarcopterygian fish species, has been examined. Coelacanths were long assumed to use benthic, fin-driven gaits based on the similarity between their fin morphology and that of early tetrapods (10, 19, 20). The coelacanth does not use its appendages to move against the substrate (19, 21). However, Latimeria does use alternating gaits similar to those used by terrestrial tetrapods for slow swimming (19, 21). This suggests that tetrapod-like motor patterns were present in the sarcopterygian lineage before the evolution of tetrapods, and may be general to sarcopterygians (19, 21). The use of these gaits for locomotion on a hard substrate, however, remains equivocal: Field studies suggest that lungfish, especially Protopterus, use benthic, quadrupedal locomotion, but these claims have never been investigated or quantified (22, 23). Our goals were to test the hypothesis that lungfish use substrate-based locomotion and to explore the diversity of limb movement patterns they use during locomotion underwater. In addition, we investigated whether aquatic fishes, even with simple fin morphology and a pleisiomorphic pelvis, could have produced movements that were consistent with early fossil trackways.
Results
In all observed behavior that included fin movement, lungfish used paired fins for benthic locomotion against the solid substrate (Figs. 1 and 2 and Movies S1 and S2). The pelvic fins alone were capable of producing movement. From our lateral-view videos it is clear that the pelvic fins were contacting the substrate during these movements (Fig. 3 and Movies S3 and S4). Whereas in many locomotor events, pectoral fins and axis both appeared to be propelling the animal, in a subset of trials (7 of 20 trials, ventral-view video), locomotion was driven by only the pelvic fins. We also observed that when there was no grid on the bottom of the tank, there was considerable slippage of the pelvic fins on the glass bottom and little forward propulsion. Together, these data and observations indicate that during routine locomotion the pelvic fins propel the body by pushing off the bottom substrate.
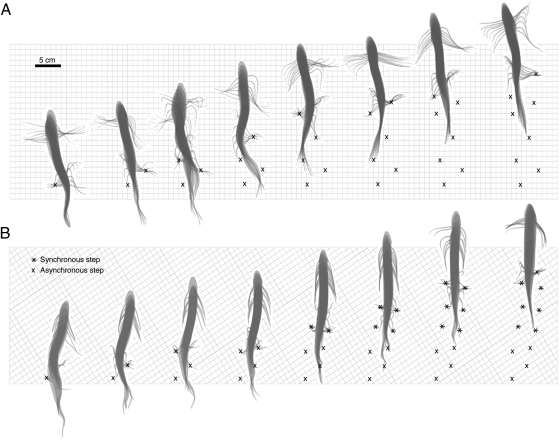
Fig. 1.
Alternating and synchronous pelvic fin gaits in P. annectens. A and B illustrate two bouts of bipedal locomotion. Angles are 2D and are relative to the body wall. An angle of 0° indicates that the fin is adducted in the direction of the head; an angle of 180° indicates adduction in the direction of the tail; an angle of 90° indicates that the fin is perpendicular to the body wall. Fin contacts with the substrate are indicated by an x or *. (A) Alternating pelvic fin gait (x steps); duration 8.48 s. (B) Alternating pelvic fin gait, with a discrete transition to a synchronous pelvic fin gait (* steps); duration 11.18 s.
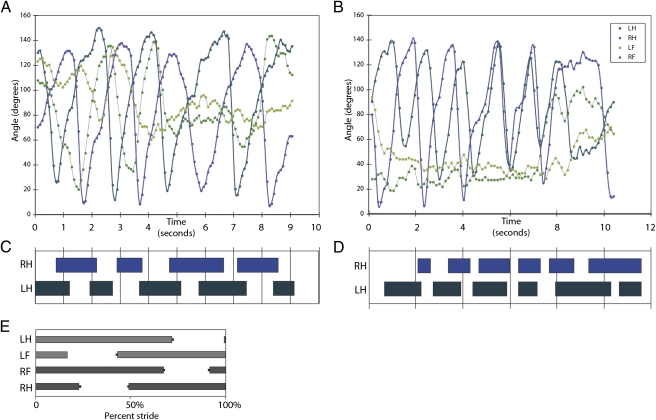
Fig. 2.
Fin angles and duty factors for benthic gaits in P. annectens. A and C correspond to Fig. 1A, and B and D correspond to Fig. 1B. (A) Alternating pelvic fin gait and (D) corresponding duty factor. (B) Alternating pelvic fin gait with discrete transition to a synchronous pelvic fin gait and (C) corresponding duty factor. In A and B, note the lack of rhythmic movement in the pectoral fin angles. (E) Duty factor summary for a single step cycle in a terrestrial tetrapod (Dicamptodon tenebrosus); error bars are SEM. LH, left hindlimb; LF, left forelimb; RH, right hindlimb; RF, right forelimb. Reproduced with permission from the Journal of Experimental Biology (24).
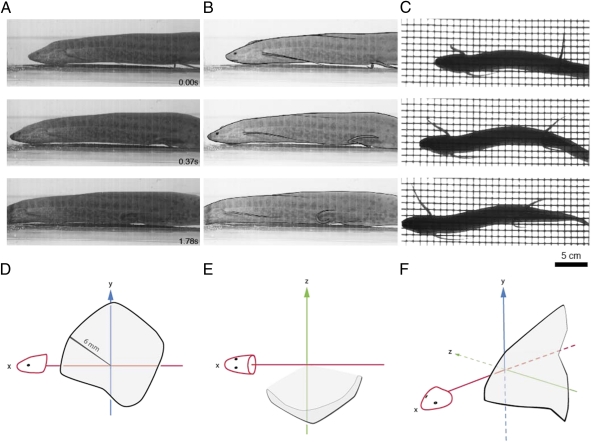
Fig. 3.
Lifting of body and fins clear of the substrate and range of rotation of pelvic fins in P. annectens. (A) Movie stills in lateral view. (B) Outline of lateral-view movie stills to emphasize the location of the fins and body. (C) Corresponding ventral view. Note that P. annectens can lift its body clear of the substrate, and that the pelvic fin moves dorsally and rostrally relative to the articulation with the body. Here we show a generalized step cycle: (D) lateral view, (E) ventral view, and (F) three-quarter view. See text for peak angles in each direction. Note that these angles were measured at the base of the fin, and do not represent the trajectory of the entire fin. The range of movement of the pelvic fins allows the animal to lift both its fins and body clear of the substrate during benthic movement.
P. annectens is a pelvic fin biped under our experimental conditions. The pelvic fins were the only paired appendages that used rhythmic, alternating movements. The pectoral fins were occasionally used for support (Fig. 1A), but we saw no evidence for the close coordination of pectoral and pelvic appendages that characterize the lateral sequence walk typical of many terrestrial tetrapods (e.g., ref. 24). The pelvic fins were capable of both symmetrically alternating (walking/running) and synchronous (bounding) pelvic fin gaits, with discrete transitions between these gaits (Figs. 1 and 2). However, P. annectens also used a wide range of asymmetrical gaits, with a mean phase relationship of 174.1° ± 49.6° (SD; n = 48 fin cycles), and ranging from 0° to 226.7° out of phase. This might be because the animals were allowed to vary both the direction and the speed of their movements in our experiments, in contrast to locomotion recorded in many kinematic studies that constrain both speed and direction of movement by using a flow tank or a treadmill. For all gaits combined, the mean pelvic fin frequency was 0.50 ± 0.17 Hz (SD; n = 87 fin cycles); the left- and right-fin beat frequencies were not significantly different (P = 0.99). Axial movements were also recorded in a subset of trials (13 of 20 trials; mean frequency 1.48 ± 0.77 Hz; n = 37 tail beats, SD), but these, too, were not coordinated with pelvic fin movements, and instead occurred uniformly throughout pelvic fin cycles (P = 0.7765; Rayleigh test) under our experimental conditions. It is possible that under some conditions or at certain speeds the axis may coordinate with fin movement.
We also measured duty factor, the fraction of time that a limb spends in contact with the substrate per step cycle (25). A step cycle is the amount of time between the initial contact of an appendage with the substrate and its next contact. The duty factor can be used to define a gait: Bipedal walking gaits have a duty factor of more than 0.5 per limb (25). Like the phase relationships, the duty factor for P. annectens was highly variable, ranging from 0.06 to 0.82, with a mean of 0.46 ± 0.20 (SD; n = 88 steps). Duty factors for the left and right fins were not significantly different (P = 0.2). The mean duty factor for both pelvic fins is <0.5, indicating that, on average, P. annectens does not use a walking gait in the strict sense. However, an examination of individual trials shows that they use gaits both with and without overlap between fin contacts (Fig. 2), meaning that although the average pattern is not technically a walk, these lungfish use a range of alternating gaits that includes walking.
The body was lifted clear of the bottom during locomotion, as were the appendages during the swing phase of movement. In the lateral-view videos, there is a clear separation between the body and underlying substrate (Fig. 3 and Movies S3 and S4). The pelvic fins also rotate dorsally and rostrally relative to their articulation with the pelvis, thus lifting them above the substrate as well during the swing phase of the step cycle. The mean peak angle of the pelvic fin relative to the articulation with the body in the rostral direction was 19.36° ± 15.48° (SD; n = 16 trials), in the caudal direction was 150.2° ± 16.8° (SD; n = 16 trials), in the dorsal direction was 39.44° ± 7.34° (SD; n = 16 trials), and in the ventral direction was 117.5° ± 9.2° (SD; n = 16 trials; Fig. 3).
Discussion
Our experiments show that P. annectens, an African lungfish, can be bipedal and uses substrate-dependent locomotion during routine movement, with gaits that range from walking to bounding. Pectoral fins play a major role in the propulsion of most fishes (but see refs. 26–28), whereas pelvic appendages are the major propulsors for most living tetrapods (29). Taken with data and observations on the coelacanth L. chalumnae (19, 21), our results suggest that the use of pelvic appendages for propulsion may be a general condition of sarcopterygian fishes. That P. annectens uses its paired appendages for substrate-associated locomotion provides evidence for this trait arising in sarcopterygians before the evolution of tetrapods, and before the evolution of digited limbs.
The ability to lift the body and appendages clear of the substrate during pelvic fin-driven locomotion in P. annectens is surprising, as it demonstrates the presence of a supposed tetrapod innovation in a basal sister taxon that has a highly autapomorphic fin and a pleisiomorphically slight pelvis (13). Nontetrapod sarcopterygian fishes such as P. annectens do not have a sacrum, the skeletal connection found in tetrapods that is associated with transfer of support and thrust from the hind limbs to the axial skeleton during locomotion. We propose that the ability to lift the appendages and body above the substrate is possible in lungfish in part because of the lungs. The lungs, which run the length of the body cavity and end just anterior to the pelvic fin base, might provide additional buoyancy (Fig. 4). This would augment the contribution from the pelvic appendages in lifting the body above the substrate. This mechanism has been proposed by others (10, 30); the bipedal ability of P. annectens suggests that it may be used in general by members of the sarcopterygian clade.
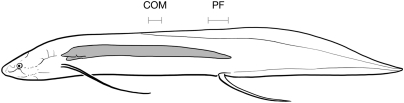
Fig. 4.
Location of lungs and center of mass in P. annectens. The location of the lungs relative to the pelvic fins (PF) and center of mass (COM) suggests that the air-filled lungs would allow the pelvic fins to both propel the fish and lift its body clear from the bottom. The center of mass was located at 35.8 ± 1.7% of total body length, and the pelvic fins were located at 54.0 ± 2.5% of total body length. Lung location was redrawn from Owen (18). Center of mass and pelvic fin location (means and SD) were taken from n = 9 individuals.
Despite similarities to terrestrial vertebrates in movement patterns and motor control, the limbs of P. annectens have a very different morphology from those of tetrapods, with numerous serially repeated elements along the length of the limb rather than few, specialized segments. In terrestrial vertebrates, the pes (foot) is the region with which the organism generally contacts the substrate during movement; however, lungfishes appear to have no analogous distal limb morphology. We found that the region of the pelvic fins that contacts the substrate in P. annectens is not anatomically constrained as it is in tetrapod limbs. As the pelvic fins contact the substrate, they bend along their length to form regions of support that function as a “foot.” The anatomical locations of these regions, which change from step to step, likely depend on properties of the substrate. For example, support regions were formed where the fins contacted the plastic grid used to provide traction on the tank bottom (Fig. 1). Rather than a stereotypical location serving as the anatomical foot, the location of this foot was translated from proximal to distal along the fin as the body moved forward (Fig. S1). This organization appears to be a novel mechanism for locomotor force generation, and perhaps is more similar to the limbs of invertebrate taxa (31) than to those of other extant vertebrates; however, fossil sarcopterygians (8, 10) also have similar, serially repeated limb segmentation, suggesting that the locomotor system of extant lungfish may have at one time been more prevalent. Future work on morphology, mechanical modeling, and force production by these limbs will be necessary to understand how lungfish limbs function and may have evolved.
The behavior described in this study provides a model for the minimum morphology necessary for substrate-based locomotion. This model includes the presence of lungs in an aquatic environment, the use of pelvic-driven bipedal gaits, and long slender limbs that lack any obvious morphological specialization for walking. The study also illustrates the difficulty of predicting the movements used by an animal solely using morphological comparisons; although the pectoral and pelvic fins are superficially very similar, their movements during locomotion are very different. Even if the information provided by this study cannot tell us with certainty that the water-to-land transition began with benthically moving animals, it does draw another line of evidence to this possibility. Although it is unclear how representative Protopterus is of the primitive condition in the sarcopterygian clade, as the extant sister group to the tetrapods, lungfish provide one of the most phylogenetically relevant models for understanding locomotor behavior in sarcopterygian fishes.
We have shown here that gaits similar to those indicated by some early fossil trackways attributed to early tetrapods (1, 3, 9, 14) can be produced by nontetrapod sarcopterygians. Trackways lacking unambiguous evidence of digits, particularly those from the Devonian, when sarcopterygian fishes were diverse and abundant (8, 32), are now open to question. Examples include Valentia Island tracks (14), Kap Graah tracks (1), and Glen Isla tracks (3). We have shown that benthic sarcopterygian fishes, even with very minimal appendages, can lift their bodies above the substrate. These data from Protopterus suggest that some of the early fossil trackways may not be evidence of tetrapods with limbs, but might be interpreted as evidence of substrate-based locomotion originating earlier in the sarcopterygian lineage. The shapes and sizes of trackways that would result from Protopterus have not yet been studied, but are of interest in the context of Devonian tracks.
These findings, interpreted along with data from trace and body fossils, add to the current understanding of the vertebrate water-to-land transition, and expand our understanding of locomotion in fossil and recent members of the sarcopterygian clade. This work also demonstrates that a careful and discriminating approach to the use of trackways as markers of the temporal and spatial distribution of taxa in the fossil record is appropriate. We found that few of the morphological features traditionally associated with land-based locomotion, such as robust, digited limbs and a sacrum, were necessary for producing similar gaits underwater. If small, “whip-like” fins are sufficient for submerged substrate-based locomotion, why did aquatic tetrapods evolve robust appendages? Animals using aquatic, substrate-based locomotion were probably more common than currently thought among the finned sarcopterygians, especially given the extensive sarcopterygian diversity before the advent of limbed tetrapods in the Devonian (8, 32).
Methods
Data Collection and Trial Selection.
Adult P. annectens were obtained commercially and housed under University of Chicago Institutional Animal Care and Use Committee guidelines (protocol 71589 to M.E.H.). Individuals of P. annectens were filmed in either a 61 × 61-cm still tank in ventral view only, or in a 25.5 × 91.5-cm tank in simultaneous ventral and lateral views. Fish were allowed to behave freely, and were moved to the center of the tank every 5 min to ensure that the behavior would be visible to the camera. Most trials were filmed with a Photron APX-RS high-speed digital video camera at 1,024 × 1,024-pixel spatial resolution and at 250 or 125 frames/s. Photron videos were used for their high spatial resolution and light sensitivity. A subset of trials was filmed in ventral view with a Panasonic PV-GS320 digital video camera at 720 × 480-pixel spatial resolution and at 60 frames/s. The Panasonic camera was used for preliminary data collection and allowed for hour-long filming episodes. A plastic grid with squares measuring 1 × 1 cm was placed on the tank bottom for traction and was used to scale images. After all data were recorded, trials in ventral view in which all four fins and the tail were visible and which contained at least three step cycles were chosen for analysis, with a final sample size of five trials each from four individuals. Trials in simultaneous lateral and ventral views in which the pelvic fin underwent one step cycle, and the same pelvic fin was clearly visible in both views were chosen for analysis, with a total of four trials each from four individuals.
Fin Angle/Frequency Analysis.
Data for fin angles were collected using a custom macro in ImageJ (33); points were recorded at the base of each fin (as visible in ventral view), and 1 cm from the base of the fin on the body wall and the fin itself. Angles were calculated in Microsoft Excel (2007). Mean averages and SDs were calculated in Microsoft Excel; a two-tailed Student's t test (α level 0.05) was performed using MATLAB (MathWorks). Due to the variation in behavior, pelvic fin frequencies were calculated for each fin cycle rather than averaging across single or multiple trials. Axial frequency was calculated by recording the time points at which the tip of the tail was at maximum lateral displacement for the subset of trials with axial movement (n = 13 trials in four individuals). To determine whether the axial fin beats were coordinated with the pelvic fin cycles, the times at which axial maxima occurred were calculated as a percentage of a left pelvic fin step cycle. These percentages were plotted on a polar plot, and a Rayleigh test (α level 0.05) for circular uniformity was performed using the CircStat toolbox in MATLAB (34). Circular uniformity would indicate that the axial fin beats occurred uniformly throughout pelvic fin cycles and were not coordinated with a particular stage of the pelvic fin cycle.
Duty Factor Analysis.
Regions of the fin held stationary during movement were assumed to be contacting the substrate during walking. To identify these stationary regions of contact, 10 points on the leading edge of each pelvic fin for each frame of a trial were recorded using a custom macro in ImageJ. These points were not uniformly distributed along the length of the fin, but were intended to capture the overall shape of the fin. These 10 points were then imported into MATLAB, and the spline function was used to fit a spline to the points, with an interpolation of that spline at 1-pixel intervals, thus filling in all pixel coordinates along the leading edge of the fin. The location and duration of fin contact were determined by calculating the Euclidian distance between each point on the leading edge of the pelvic fin from one frame to the next. When the distance a point on the fin had traveled from one frame to the next was less than 3 pixels, that point was considered stationary. These stationary regions were recorded and used to calculate duty factor. The timing of the formation of these stationary regions was verified using the video data. Although it is standard to calculate duty factor using the left hindlimb as the point at which a step cycle begins, we calculated step cycles based on the first limb to contact the substrate in each trial.
3D Angle Analysis.
To determine the position of the pelvic fins relative to the body in three dimensions, fish were filmed in a tank that allowed for simultaneous viewing of lateral and ventral views. The fins were digitized using the same method as the duty factor analysis in both views. The spline outputs for each view of the fin were combined to create sets of xyz coordinates for each frame of each trial and calculate the angles of fin movement around its base.
Supplementary Material
Acknowledgments
We thank M. Ashley-Ross and J. A. Long for valuable feedback on the manuscript. We thank N. Neubarth and R. Y. Teow for contributions to animal care, M. H. Green and L. Sallan for discussion and comments on the manuscript, J. J. Rowell for comments on the manuscript, and K. Monoyios for illustrations and help with figure design. This material is based upon work supported by the National Science Foundation under Grants DGE-0903637 (an Integrative Graduate Education and Research Traineeship supporting H.M.K.), IBN043977 (to M.E.H.), and EAR0544565 (to N.H.S.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1118669109/-/DCSupplemental.
References
- 1.Friend PF, Alexander-Marrak PD, Nicholson J, Yeats AK. Devonian sediments of East Greenland II: Sedimentary structures and fossils. Medd Gronl. 1976;206:1–91. [Google Scholar]
- 2.Rosen DE, Forey PL, Gardiner BG, Patterson C. Lungfishes, tetrapods, palaeontology, and pleisiomorphy. Bull Am Mus Nat Hist. 1981;167:159–276. [Google Scholar]
- 3.Warren A, Jupp R, Bolton B. Earliest tetrapod trackway. Alcheringa. 1986;10:183–186. [Google Scholar]
- 4.Panchen AL, Smithson TR. Character diagnosis, fossils and the origin of tetrapods. Biol Rev Camb Philos Soc. 1987;62:341–438. [Google Scholar]
- 5.Coates MI, Clack JA. Fish-like gills and breathing in the earliest known tetrapod. Nature. 1991;352:234–236. [Google Scholar]
- 6.Ahlberg PE. Elginerpeton pancheni and the earliest tetrapod clade. Nature. 1995;373:420–425. [Google Scholar]
- 7.Coates MI. The Devonian tetrapod Acanthostega gunnari, Jarvik: Postcranial anatomy, basal tetrapod interrelationships, and patterns of skeletal evolution. Trans R Soc Edinb Earth Sci. 1996;87:363–421. [Google Scholar]
- 8.Janvier P. Early Vertebrates. Oxford: Clarendon; 1996. [Google Scholar]
- 9.Clack JA. Devonian tetrapod trackways and trackmakers; a review of the fossils and footprints. Palaeogeogr Palaeoclimatol Palaeoecol. 1997;130:227–250. [Google Scholar]
- 10.Clack JA. Gaining Ground: The Origin and Evolution of Tetrapods. Bloomington, IN: Indiana Univ Press; 2002. [Google Scholar]
- 11.Shubin NH, Daeschler EB, Coates MI. A Devonian tetrapod-like fish and the evolution of the tetrapod body plan. Science. 2004;304(5667):90–93. doi: 10.1126/science.1094295. [DOI] [PubMed] [Google Scholar]
- 12.Coates MI, Ruta M, Friedman M. Ever since Owen: Changing perspectives on the early evolution of tetrapods. Annu Rev Ecol Evol Syst. 2008;39:571–592. [Google Scholar]
- 13.Niedźwiedzki G, Szrek P, Narkiewicz K, Narkiewicz M, Ahlberg PE. Tetrapod trackways from the early Middle Devonian period of Poland. Nature. 2010;463(7277):43–48. doi: 10.1038/nature08623. [DOI] [PubMed] [Google Scholar]
- 14.Stossel I. The discovery of a new Devonian tetrapod trackway in SW Ireland. J Geol Soc London. 1995;152:407–413. [Google Scholar]
- 15.Brinkmann H, Venkatesh B, Brenner S, Meyer A. Nuclear protein-coding genes support lungfish and not the coelacanth as the closest living relatives of land vertebrates. Proc Natl Acad Sci USA. 2004;101:4900–4905. doi: 10.1073/pnas.0400609101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedman M. The interrelationships of Devonian lungfishes (Sarcopterygii: Dipnoi) as inferred from neurocranial evidence and new data from the genus Soederberghia Lehman, 1959. Zool J Linn Soc. 2007;151(1):115–171. [Google Scholar]
- 17.Cavin L, Suteethorn V, Buffetaut E, Tong H. A new Thai Mesozoic lungfish (Sarcopterygii, Dipnoi) with an insight into post-Palaeozoic dipnoan evolution. Zool J Linn Soc. 2007;149(2):141–177. [Google Scholar]
- 18.Owen R. A new species of the genus Lepidosiren. Proc Linn Soc. 1839;1:27–32. [Google Scholar]
- 19.Fricke H, Hissmann K. Locomotion, fin coordination and body form of the living coelacanth Latimeria chalumnae. Environ Biol Fishes. 1991;34:329–356. [Google Scholar]
- 20.Smith JLB. A living fish of Mesozoic type. Nature. 1939;143:455–456. [Google Scholar]
- 21.Fricke H, Reinicke O, Hofer H, Nachtigall W. Locomotion of the coelacanth Latimeria chalumnae in its natural environment. Nature. 1987;329:331–333. [Google Scholar]
- 22.Greenwood PH. The natural history of African lungfishes. J Morphol. 1986;190(Suppl 1):163–179. [Google Scholar]
- 23.Johnels AG, Svensson GSO. On the biology of Protopterus annectens (Owen) Ark Zool. 1954;7:131–164. [Google Scholar]
- 24.Ashley-Ross MA. Hindlimb kinematics during terrestrial locomotion in a salamander (Dicamptodon tenebrosus) J Exp Biol. 1994;193:255–283. doi: 10.1242/jeb.193.1.255. [DOI] [PubMed] [Google Scholar]
- 25.McMahon TA. Muscles, Reflexes, and Locomotion. Princeton, NJ: Princeton Univ Press; 1984. [Google Scholar]
- 26.Koester DM, Spirito CP. Punting: An unusual mode of locomotion in the little skate, Leucoraja erinacea (Chondrichthyes: Rajidae) Copeia. 2003;3:553–561. [Google Scholar]
- 27.Macesic LJ, Kajiura SM. Comparative punting kinematics and pelvic fin musculature of benthic batoids. J Morphol. 2010;271:1219–1228. doi: 10.1002/jmor.10865. [DOI] [PubMed] [Google Scholar]
- 28.Pridmore PA. Submerged walking in the epaulette shark Hemiscyllium ocellatum (Hemiscyllidae) and its implications for locomotion in rhipidistian fishes and early tetrapods. Zoology (Jena) 1995;98:278–297. [Google Scholar]
- 29.Heglunnd NC, Cavagna GA, Taylor CR. Energetics and mechanics of terrestrial locomotion. III. Energy changes of the centre of mass as a function of speed and body size in birds and mammals. J Exp Biol. 1982;79:41–56. doi: 10.1242/jeb.97.1.41. [DOI] [PubMed] [Google Scholar]
- 30.Eaton TH. The aquatic origin of the tetrapods. Trans Kans Acad Sci. 1960;63:115–120. [Google Scholar]
- 31.Kier WM, Stella MP. The arrangement and function of octopus arm musculature and connective tissue. J Morphol. 2007;268:831–843. doi: 10.1002/jmor.10548. [DOI] [PubMed] [Google Scholar]
- 32.Sallan LC, Coates MI. End-Devonian extinction and a bottleneck in the early evolution of modern jawed vertebrates. Proc Natl Acad Sci USA. 2010;107:10131–10135. doi: 10.1073/pnas.0914000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abramoff MD, Magalhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int. 2004;11(7):36–42. [Google Scholar]
- 34.Berens P. CircStat: A MATLAB toolbox for circular statistics. J Stat Softw. 2009;31(10):1–21. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.