Abstract
Two frequently debated aspects of hominin evolution are the development of upright bipedal stance and reduction in body hair. It has long been argued, on the basis of heat-balance models, that thermoregulation might have been important in the evolution of both of these traits. Previous models were based on a stationary individual standing in direct sunlight; here we extend this approach to consider a walking hominin, having argued that walking is more thermally challenging than remaining still. Further, stationary activities may be more compatible with shade seeking than activities (such as foraging) involving travel across the landscape. Our model predictions suggest that upright stance probably evolved for nonthermoregulatory reasons. However, the thermoregulatory explanation for hair loss was supported. Specifically, we postulate progressive hair loss being selected and this allowing individuals to be active in hot, open environments initially around dusk and dawn without overheating. Then, as our ancestors’ hair loss increased and sweating ability improved over evolutionary time, the fraction of the day when they could remain active in such environments extended. Our model suggests that only when hair loss and sweating ability reach near-modern human levels could hominins have been active in the heat of the day in hot, open environments.
Two frequently debated aspects of hominin evolution are the development of upright stance and the reduction in body hair. Indeed, a good case can be made that upright stance was “the first novelty of our lineage” (1), and lack of hair is one of the visually most obvious aspects of modern humans compared with other similar-sized mammals (2). One line of reasoning posits that thermoregulation may have been important in the evolutionary development of both of these traits. Highly supportive of such discussions has been a series of papers by Wheeler that used simple heat-balance models to quantify the potential effects of such adaptations (3–10). Recently, we presented a revised version of Wheeler's model to explore thermoregulatory aspects of putative endurance running in extinct hominids (11). The key modification in our revised model was a change from a stationary individual, as considered by Wheeler, to a moving one. Movement both adds to the thermal challenge on an individual by increasing their metabolically generated heat production and allows greater heat loss to the air by forced convection. Here we apply a further development of this model to the issues in which Wheeler was originally interested: bipedalism and hair loss.
We believe that consideration of a walking individual should be particularly informative, because it is easier to envisage ecological reasons why an individual would be required to walk through areas that offer no shade compared with situations where an individual would stand still for long periods in direct sunlight. Vision is likely to have been an important sense in both food finding (e.g., searching for and pursuing mobile animal prey) and predator avoidance, so much travel and activity likely took place during the day, and the locations of food may have required movement into areas that offered no shade (and thus made overheating a greater risk). In contrast, activities that require an individual to be stationary (resting, food handling, vigilance for predators) may often have allowed choice of microhabitat that minimizes risk of overheating (primarily shade seeking). As well as allowing for a moving individual, we took advantage of recent developments in thermal biology to update some parameter values and expressions from Wheeler's original model (11). In Methods, we set out the key further developments to the model of ref. 11 needed to explore the issues of bipedality and hair loss.
Model Predictions
With the modifications described in Methods, we use the model detailed in SI Text, Section 1 to calculate the amount of heat that must be lost through sweating to achieve heat balance as a function of the time of day for an early hominin. For the parameters of interest in our models (leg length and body mass), Australopithicus africanus has very similar reconstructed values to A. afarensis and to Homo habilis; thus, these are grouped together as “early hominins.” We consider the two sexes separately, as most observers have concluded that these species were sexually dimorphic (12). We consider the consequences of whether the individual had modern levels of hair loss or not, and was quadrupedal or bipedal in a factorial design, giving four different modeled situations. Like Wheeler, we consider a situation equivalent to a hot, cloudless day in an open equatorial region: The air temperature reaches a maximum of 40 °C and the maximum intensity of solar radiation is 865 watts per square meter (W⋅m−2).
We assume that females had a leg length (L) of 0.52 m and a mass (M) of 30 kg. These predict a walking speed of 1.2 m⋅s−1 and a metabolic rate while walking at this speed of 209 W (SI Text, Section 1). We use the same equation as ref. 11 to calculate skin surface area in m2: 0.11 M0.67, which gives a value of 1.07 m2. The calculated maximal amounts of heat that can be lost by sweating are then 107 W for a hair-covered individual and 473 W with reduced hair (see SI Text, Section 1 for details of these calculations).
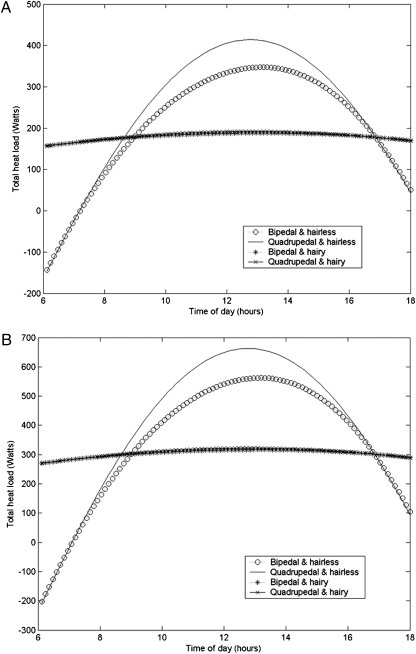
Fig. 1A gives model predictions for the amount of heat that must be dissipated by sources other than normal respiration (such as sweating) to maintain heat balance. Let us consider the predictions for hair-covered individuals first. Notice that for these individuals there is very little variation over the day in this heat load. This occurs because the dominant factor in these individuals’ heat balance is their own metabolism while active (209 W), which does not change with time of day. There is relatively little heat gain from the environment because hair is a good thermal insulator. This means that as the heat load on the surface of the hair increases so the temperature of the outer portion of the pelt rises, and this aids heat loss by both radiation and convection. We next note that there is almost no effect of stature (bipedal or quadrupedal) on thermoregulation for such hair-covered individuals; this again follows from internal heat production being the dominant contributor to heat load. Last, we note that the rate at which excess heat energy needs to be lost (around 150–170 W) is significantly in excess of the maximal rate at which we expect that heat can be lost by sweating (107 W). Thus, our model predicts that hair-covered female hominins could not have maintained heat balance while walking for sustained periods of time under such sunny and hot environmental conditions (even if they traveled near dusk and dawn). We suggest that either long-range movements were confined to cooler conditions (e.g., when there was cloud cover), or movement was curtailed after short intervals to allow heat dumping during rest periods. An increase in core body temperature of 1 °C is sufficient to trigger the onset of heat stroke in modern humans (13). Human specific heat capacity of 3,470 W⋅kg-1⋅oC−1 (14) suggests a 50-W excess in heat production would raise the temperature of a 30-kg individual like that modeled here by 1 °C in around 30 min.
Fig. 1.
Model predictions for the amount of heat that must be dissipated by sources other than normal respiration (such as sweating) to maintain heat balance, as a function of time of day; (A) for a female hominin and (B) for a male hominin. In each case, we model four situations involving all combinations of quadrupedal versus bipedal stance and full body hair versus loss of hair to near-modern human levels.
Turning to hairless female individuals (still in Fig. 1A), we first note the much greater daily variation in predicted heat load. This comes about because the thermal buffering effect of hair has been lost over the hairless parts of the body. Next, we observe that the excess heat load on these individuals is actually higher than that of hair-covered individuals in the middle of the day (total heat load being 350–400 W at midday). This gain compared with hair-covered individuals can be understood because skin temperature is assumed to remain constant throughout, and so there is not the enhanced radiation and convection that arise when the surface of the hair is heated and rises in temperature. Such lack of variation in skin temperature is seen in modern humans—even in sunbathing individuals and those taking vigorous exercise, skin temperature is often lower than core temperature and very rarely more than 1 or 2 degrees higher (15–17). Notice that at the beginning and end of the day, these individuals are predicted to lose heat even when not sweating. These losses are relatively modest and could be counteracted, for example, by increasing the speed of movement. If the individuals were assumed to be running at their aerobic optimum speed then (according to equation 15 of ref. 11), this would cost them an added 130 W over the cost of walking, and return them to a positive energy balance. Next, notice that even in the heat of the day, the maximum excess heat load is less than the maximum that we predict could be shed by sweating at a rate similar to the maximal levels shown by modern humans (473 W). Thus, we predict that (providing such sweating was physiologically possible and sufficient water was available to replace that lost in sweat) such prolonged walking would be thermally sustainable. Last, we note that the excess heat load that must be dumped through sweating is higher toward the middle of the day for quadrupeds than bipeds; this is driven by the higher fraction of a quadruped's surface area exposed to direct sunlight when the sun is high in the sky.
Fig. 1B shows the equivalent model predictions for a male hominin with leg length of 0.72 m and mass of 55 kg. For such an individual, the predicted walking speed is 1.4 m⋅s−1 and the rate of metabolic heat generation is 334 W. The skin surface area is predicted to be 1.6 m2, and the maximum rate of heat dissipation through sweating is 160 W for a hair-covered individual and 710 W for an individual with greatly reduced hair. Essentially, the predictions are qualitatively identical to that of females. One issue of note is that for hair-covered individuals the excess heat is less than the metabolic heat production, because the thermal insulation of the hair gives excellent protection from external heat load, thus the surface of the pelt remains warmer than the skin and so heat is more effectively radiated and convected away, and because the faster walking speed of longer-legged males allows them to shed more heat by forced convection. For hair-covered individuals, the heat load is around 290 W, whereas the predicted heat dissipation is only 160 W; such an excess heat gain of 130 W would be predicted to cause a 1-°C rise in core body temperature of such a 55-kg individual after ∼25 min. The assumed body mass of 55 kg represents a relatively “robust” individual; however, our qualitative conclusions are unchanged if we assume a mass of 45 kg more appropriate to a gracile morph (18) (see SI Text, Section 2 and Figure S1 for details).
Discussion
Our model predicts that while early hominins remained hair-covered they would have struggled with overheating if active in hot, sunny, open environments. Such species would have been at no thermoregulatory advantage in evolving bipedality. However, our model also suggests that if bipedality evolved for other reasons, then there would have been no thermoregulatory opposition to this adaptation. Our predictions stand in contrast to those of Wheeler (3, 8, 9), who demonstrated that (for an individual standing at rest) bipedalism reduces external heat load and thus would have reduced thermal challenge. The key difference is that for our model (of an exercising individual), external heat challenge is much less important than internally generated heat, and so the effect described by Wheeler makes a trivial difference to heat balance. As we argue in the introduction, we believe that it is much easier to envisage scenarios whereby a walking individual would be unable to take advantage of available shade without compromising their activity than a standing individual. Hence, we believe that prolonged exposure to hot, sunny conditions is more biologically plausible for a walking than a standing individual, and so our model is likely to be of more biological relevance. We predict that while early hominins remained hair-covered, there was no evolutionary pressure through thermoregulation under hot conditions selecting for bipedalism. Conversely, however, if bipedalism was selected through other mechanisms, then the need for thermoregulation under hot conditions would not have opposed such evolutionary change.
Unlike previous works in this field, we have been able to make quantitative predictions about the behavioral consequences of failure to thermoregulate. Assuming early hominins had similar reactions to hyperthermia as modern humans, we calculate that hair-covered individuals would only have been able to walk in full bright sunshine in hot weather (we assume cloudless skies and a maximum air temperature near the ground of 40 °C) for 10–20 min before overheating was sufficient to induce symptoms of heat stroke. This estimated duration was very similar for male and female individuals and for both gracile and robust body forms.
In contrast to the situation for bipedalism, our work is in agreement with previous predictions (for example, those of ref. 5) that enhanced heat loss might select for reduced hair cover in early hominins. Specifically, our model predicts that once hair loss and sweating ability have evolved to near-modern human levels, a hominin could thermoregulate even under hot, sunny conditions and even when involved in something as energetically demanding as brisk walking. Further, our calculations make predictions as to how the evolution of such hair loss and sweating ability might be linked to daily behavioral patterns. Specifically, it is clear from the extremes of hairiness that we simulated that progressive hair loss (in the absence of specialist adaptations to maximize sweating ability) would have led to even greater risk of overheating in the heat of the day (compared with a hairier individual), but would have made it easier to have been active nearer dusk and dawn. This could have provided selection pressure for hair loss, combined with behavioral adaptations such that long-distance travel under hot, sunny conditions was constrained to occur only early and late in the day. Once hair loss and sweating ability have evolved increasingly toward near-modern human levels, this temporal restriction would have progressively decreased, until eventually such exercise was even possible (providing sufficient water was available to allow replenishment of reserves) in the hottest part of the day.
Another clear prediction of our model is that once hair loss and sweating ability evolved to near-modern human levels, there becomes an added thermoregulatory advantage to bipedality. This allows us to make predictions about the relative timing of hair loss and development of bipedalism. If both of these changes occurred primarily through thermoregulatory evolutionary pressures, then we predict that hair loss would occur before bipedalism. However, if physical evidence suggests a different relative timing, then this would suggest that the initial evolution of bipedalism was not driven by thermoregulatory selection pressures. Currently, the earliest “unambiguous” evidence for bipedalism in hominins comes from around 4 million y ago—although there is more ambiguous evidence for bipedalism at least 2 million y before this—with 6–9 million y being suggested by many as the likely time period for the evolution of upright stance (19) (reviewed in ref. 12). For obvious reasons, evidence for loss of functional body hair is hard to come by. Probably the best currently available evidence comes from the evolutionary history of hominin ectoparasites; this suggests a loss of hair by around 3 million y ago (20). So currently it appears that upright stance may have evolved earlier than hair loss; however, such a conclusion must be very tentative.
Upright walking occurred in hominins in earlier species than those modeled here (such as Ardipithecus ramidus). However, Ar. ramidus still retained anatomical features (e.g.,, of the foot and pelvis) equally suited to arboreal than terrestrial movement, and thus likely had both slower and less energetically efficient walking than that considered in our models (19). Further, although there is some current dispute over the habitat exploited by Ar. ramidus, there seems agreement that it was not open savanna grassland, with some researchers favoring closed wooded habitat (21) and some arguing for “tree or bush savanna” in which shade would still generally have been readily available (22). Hence, it seems unlikely that hominid bipedalism was selected for the thermoregulatory reasons explored in our paper. However, extensive open grasslands spread across Africa around 3–2 million y ago and thus would have been ecologically relevant to the hominins considered in this paper that showed much fuller anatomical adaptations to a terrestrial (rather than arboreal) habitat.
Other than hominins, the other diurnally active primate line that has extensively colonized the African plains are the papionon baboons, which have an extensive fossil record in such environments, and whose extant member (Papio hamadryas) is ubiquitous in African open habitats (23). However, activity is strongly influenced by shade attraction in times of high temperature and solar radiation in this species (see ref. 24 for a review). The skin of baboons has an unusually high density of sweat glands (25), and sweating seems an important aspect of thermoregulation (23). As such, the foraging range of baboons is strongly constrained by proximity to sources of drinking water (26). It may be that hominin bipedalism was an important precondition for the greatly reduced hair and increased heat loss through sweating seen in hominins; our model suggests a thermoregulatory advantage to bipedalism only once hair loss and high sweat rates had evolved. In addition, various behavioral advantages are possible. Mitchell et al. (23) speculate that bipedalism may have freed the hands to carry drinking water or water-rich plants on extended journeys across hot plains without shade. It may also be that tool use allowed access to sources of water (e.g., tubers) during a journey across shadeless hot plains that allowed thermoregulation through sweating to be more important in the hominid lineage.
Our previous modeling work (11) suggested that a hominin would need the locomotive efficiency, sweating rates, and areas of hairless skin similar to modern humans to be highly physically active running for long periods in open savanna during the heat of the day. H. erectus sensu lato (e.g., H. ergaster) is often reconstructed as such an animal (26), and there is considerable evidence for more open vegetation, driven by climate change, at this time (27). Our modeling suggests that, on thermoregulatory grounds, these later hominins should be reconstructed as predominantly hairless and with sweating rates close to those of modern humans even if they were only indulging in sustained walking (rather than endurance running) during the day. However, it is important to remember that there may have been other important selective pressures acting on hair coverage, such as ectoparasite load (28), and that a full understanding of the evolution and maintenance of the predominantly hairless condition in modern humans will require consideration of the interaction of these pressures, as well as sexual selection (29).
Methods
Our model is identical to that specified in full detail in ref. 11 except for the changes described below. In introducing these modifications, we also clearly state whether expressions or parameter values are the same as those originally used by Wheeler or have been updated in the light of more recent work. A full description of the model is also given in SI Text. The model estimates heat balance with the environment as a function of individual size, posture, and coverage of hair. This heat balance will also vary over the course of the day. We also model metabolic heat generation, and heat losses through respiration. We finally assume that any net heat gain must be compensated for by evaporative cooling or else hyperthermia will occur.
Thermal Environment.
The model description in ref. 11 began by defining how the temperature of the substrate (Tg) and the temperature of the air at a height of 200 cm (T200) change over the course of the day. We then used a parameter α to specify the characteristic height of the hominid and thus the air temperature (Ta) experienced:
The model in ref. 11 followed Wheeler in assuming the value (α = 0.41) for a biped. This value was calculated from scale models of the size and posture of a putative early hominid (see ref. 3 for details). Using the same methods, Wheeler (3) calculated a value of α = 0.54 for a quadruped. This value is adopted here; clearly, the heightened value of α increases the relative influence of the ground temperature for a quadruped than for a biped.
Speed of Movement Through the Air.
Here we will assume that the air movement past the body is produced by the travel of the individual rather than by wind. For simplicity, we will ignore the acceleration and deceleration of different body parts, and simply characterize movement by a constant velocity. This assumption is supported by a recent modeling study (30) that compared a rigid-structure model like ours with a more complex model of a running human that captured the complex range of movement of the different body parts. Although the two models produced very different predictions for heat exchange between the human and the environment at low air temperatures, at high air temperatures like those considered here the two models agreed to within 1%.
We use the same formula given in ref. 11 for walking. This equation was originally due to Alexander (31) and gives the most energetically efficient walking speed (v⋅m⋅s−1) as 1.7 L0.5, where L is leg length in meters. We use the leg lengths argued in ref. 11 to be approximately characteristic of A. africanus, A. afarensis, and H. habilis: 52 cm for females and 72 cm for males. The corresponding masses used in ref. 11 are 30 kg for females and 55 kg for males. These values were derived from refs. 18, 32, and 33.
Metabolic Heat Generation.
Because no strong and consistent differences in either routine metabolism or cost of transport have been found between bipeds and quadrupeds in cross-taxonomic studies of extant animals (e.g., ref. 34), we make the same assumptions about metabolic heat generation for quadrupeds as was used for bipeds in ref. 11.
Total Environmental Heat Load.
Both posture and hairiness will affect this. For bipeds (11), we used the following equation to describe the fraction (β) of the skin surface exposed to the sun as a function of time (t in hours since midnight):
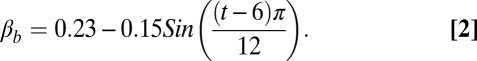 |
This equation comes directly from ref. 8. That same publication also provides an analogous equation for quadrupeds:
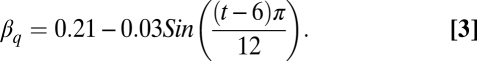 |
These equations indicate that the maximum fraction of the surface area exposed to direct sunlight occurs when the sun is low in the sky (near dusk and dawn), being 23% for bipeds and 21% for quadrupeds at that time. The minimum exposure for both stances occurs when the sun is directly overhead, and is 8% for bipeds and 18% for quadrupeds. Thus, the daily change in exposure to direct sunlight is much more dramatic for bipeds, which reduces their exposure considerably when the sun is high in the sky.
Effect of Heat Loss.
We follow refs. 6 and 11 in assuming that after evolutionary hair loss, only 15% of both the shaded and unshaded portions of the body surface are covered in a thick pelt. For the ancestral hair-covered type, we assume that all of the body is covered in hair with identical thermal properties. This assumption is exactly like that of ref. 6.
Heat Loss Through Sweating.
Evaporation of sweat is much easier from bare skin than from under thick hair (35). We follow refs. 3, 4, and 11 in assuming maximum heat loss through sweating from bare skin as 500 W⋅m−2. For areas of skin under thick hair, we assume the same value as refs. 3 and 4, namely 100 W⋅m−2: this is in good agreement with maximum values measured in hair-covered primates (e.g., 36–38). Notice that by assuming such high values, we implicitly assume low levels of atmospheric humidity associated with low surface moisture levels. Higher levels of humidity would make heat loss by sweating even more challenging to achieve than predicted by our simulations.
Supplementary Material
Acknowledgments
We thank Peter Wheeler for useful discussions and two referees and the editor for valuable suggestions.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1113915108/-/DCSupplemental.
References
- 1.Kingdom J. Self-Made Man and His Undoing. London: Simon and Schuster; 1993. [Google Scholar]
- 2.Morris D. The Naked Ape. London: Jonathan Cape; 1967. [Google Scholar]
- 3.Wheeler PE. The thermoregulatory advantages of hominid bipedalism in open equatorial environments: The contribution of increased convective heat loss and cutaneous evaporative cooling. J Hum Evol. 1991;21(2):107–115. [Google Scholar]
- 4.Wheeler PE. The influence of bipedalism on the energy and water budgets of early hominids. J Hum Evol. 1991;21(2):117–136. [Google Scholar]
- 5.Wheeler PE. The thermoregulatory advantages of large body size for hominids foraging in savannah environments. J Hum Evol. 1992;23:351–362. [Google Scholar]
- 6.Wheeler PE. The influence of the loss of functional hair on the water budgets of early hominids. J Hum Evol. 1992;23:379–388. [Google Scholar]
- 7.Wheeler PE. The influence of stature and body form on hominid energy and water budgets: A comparison of Australopithecus and early Homo physiques. J Hum Evol. 1993;24(1):13–28. [Google Scholar]
- 8.Wheeler PE. The evolution of bipedality and loss of functional body hair in hominids. J Hum Evol. 1984;13(1):91–98. [Google Scholar]
- 9.Wheeler PE. The loss of functional body hair in man: The influence of thermal environment, body form and bipedality. J Hum Evol. 1985;14(1):23–28. [Google Scholar]
- 10.Wheeler PE. The environmental context of functional body hair loss in hominids (a reply to Amaral, 1996) J Hum Evol. 1996;30:367–371. [Google Scholar]
- 11.Ruxton GD, Wilkinson DM. Thermoregulation and endurance running in extinct hominins: Wheeler's models revisited. J Hum Evol. 2011;61(2):169–175. doi: 10.1016/j.jhevol.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Klein RG. The Human Career. 3rd Ed. Chicago: Univ of Chicago Press; 2009. [Google Scholar]
- 13.Guyton A. Textbook of Medical Physiology. 5th Ed. Philadelphia: W.B. Saunders; 1976. [Google Scholar]
- 14.Schmidt-Nielsen K. Animal Physiology: Adaptation and Environment. 5th Ed. Cambridge, UK: Cambridge Univ Press; 1997. [Google Scholar]
- 15.Clark RP, Goff MR, Mullen BJ. Skin temperature during sunbathing and some observations on the effect of hot and cold drinks on these temperatures. J Physiol. 1977;267(1):8P–9P. [PubMed] [Google Scholar]
- 16.Clark RP, Mullan BJ, Pugh LGCE. Skin temperature during running—A study using infra-red colour thermography. J Physiol. 1977;267(1):53–62. doi: 10.1113/jphysiol.1977.sp011800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nielsen B. Solar heat load: Heat balance during exercise in clothed subjects. Eur J Appl Physiol Occup Physiol. 1990;60:452–456. doi: 10.1007/BF00705036. [DOI] [PubMed] [Google Scholar]
- 18.Plavcan JM, Lockwood CA, Kimbel WH, Lague MR, Harmon EH. Sexual dimorphism in Australopithecus afarensis revisited: How strong is the case for a human-like pattern of dimorphism? J Hum Evol. 2005;48:313–320. doi: 10.1016/j.jhevol.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Lovejoy CO, Suwa G, Spurlock L, Asfaw B, White TD. The pelvis and femur of Ardipithecus ramidus: The emergence of upright walking. Science. 2009;326:71e1–71e6. [PubMed] [Google Scholar]
- 20.Weiss RA. Apes, lice and prehistory. J Biol. 2009;8:20. doi: 10.1186/jbiol114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White TD, et al. Ardipithecus ramidus and the paleobiology of early hominids. Science. 2009;326(5949):75–86. [PubMed] [Google Scholar]
- 22.Cerling TE, et al. Woody cover and hominin environments in the past 6 million years. Nature. 2011;476(7358):51–56. doi: 10.1038/nature10306. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell D, Fuller A, Maloney SK. Homeothermy and primate bipedalism: Is water shortage or solar radiation the main threat to baboon (Papio hamadryas) homeothermy? J Hum Evol. 2009;56:439–446. doi: 10.1016/j.jhevol.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Hill RA. Thermal constraints on activity scheduling and habitat choice in baboons. Am J Phys Anthropol. 2006;129:242–249. doi: 10.1002/ajpa.20264. [DOI] [PubMed] [Google Scholar]
- 25.Montagna W, Yun JS. The skin of primates. VIII. The skin of the anubis baboon (Papio doguera) Am J Soc Anthropol. 1962;20:131–141. doi: 10.1002/ajpa.1330200214. [DOI] [PubMed] [Google Scholar]
- 26.Elton S. The environmental context of human evolutionary history in Eurasia and Africa. J Anat. 2008;212:377–393. doi: 10.1111/j.1469-7580.2008.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.deMenocal PB. Anthropology. Climate and human evolution. Science. 2011;331:540–542. doi: 10.1126/science.1190683. [DOI] [PubMed] [Google Scholar]
- 28.Rantala MJ. Human nakedness: Adaptation against ectoparasites? Int J Parasitol. 1999;29:1987–1989. doi: 10.1016/s0020-7519(99)00133-2. [DOI] [PubMed] [Google Scholar]
- 29.Rantala MJ. Evolution of nakedness in Homo sapiens. J Zool. 2007;273(1):1–7. [Google Scholar]
- 30.Cross A, Collard M, Nelson A. Body segment differences in surface area, skin temperature and 3D displacement and the estimation of heat balance during locomotion in hominins. PLoS One. 2008;3:e2464. doi: 10.1371/journal.pone.0002464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alexander RM. Principles of Animal Locomotion. Princeton, NJ: Princeton Univ Press; 2006. [Google Scholar]
- 32.Steudel-Numbers KL, Tilkens MJ. The effect of lower limb length on the energetic cost of locomotion: Implications for fossil hominins. J Hum Evol. 2004;47(1-2):95–109. doi: 10.1016/j.jhevol.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Haeusler M, McHenry HM. Body proportions of Homo habilis reviewed. J Hum Evol. 2004;46:433–465. doi: 10.1016/j.jhevol.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 34.Taylor CR, Heglund NC, Maloiy GMO. Energetics and mechanics of terrestrial locomotion. I. Metabolic energy consumption as a function of speed and body size in birds and mammals. J Exp Biol. 1982;97:1–21. doi: 10.1242/jeb.97.1.1. [DOI] [PubMed] [Google Scholar]
- 35.Monteith JL, Unsworth MH. Principles of Environmental Physics. 3rd Ed. New York: Academic; 2008. [Google Scholar]
- 36.Mahoney SA. Cost of locomotion and heat balance during rest and running from 0 to 55 degrees C in a patas monkey. J Appl Physiol. 1980;49:789–800. doi: 10.1152/jappl.1980.49.5.789. [DOI] [PubMed] [Google Scholar]
- 37.Kolka MA, Elizondo RS. Thermoregulation in Erythrocebus patas: A thermal balance study. J Appl Physiol. 1983;55:1603–1608. doi: 10.1152/jappl.1983.55.5.1603. [DOI] [PubMed] [Google Scholar]
- 38.Walters TJ, Ryan KL, Constable SH. Thermoregulation by rhesus monkeys at different absolute humidities. J Comp Physiol B. 2004;174:481–487. doi: 10.1007/s00360-004-0434-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.