Abstract
LPS-induced TNF-α factor (LITAF) mediates cytokine expression in response to endotoxin challenge. Previously, we reported that macrophage-specific LITAF-deficient (macLITAF−/−) mice exposed to LPS have a delayed onset in the serum levels of proinflammatory cytokines and prolonged persistence of anti-inflammatory cytokines, but only partial protection from endotoxic shock. We postulated that greater protection might be achieved if LITAF were deleted from all LITAF-producing cells, including macrophages. Using a Cre-loxP system, we engineered a tamoxifen-induced recombination mouse [tamLITAF(i)−/−] that resulted in whole-body LITAF deficiency. Our findings demonstrate that (i) tamLITAF(i)−/− mice are more resistant to systemic Escherichia coli LPS-induced lethality than our previous macLITAF−/− mice, providing evidence that LITAF-producing cells other than LysMCre-positive cells play an important role in mediating endotoxic shock; (ii) tamLITAF(i)−/− mice show a similar pattern of cytokine expression with decreased proinflammatory and prolonged anti-inflammatory mediators compared with WT mice; and (iii) tamLITAF(i)−/− mice, compared with WT mice, display a significant reduction in bone resorption and inflammation associated with a local chronic inflammatory disease—namely, collagen antibody-induced arthritis. Our findings offer a unique model to study the role of LITAF in systemic and chronic local inflammatory processes, and pave the way for anti-LITAF therapeutic approaches for the treatment of TNF-mediated inflammatory diseases.
Keywords: septic shock, multiplex
Inflammation is an innate response to tissue injury, pathogen insults, or trauma; it is highly deleterious to the host and, as such, warrants better understanding (1–4). When inflammatory responses are uncontrolled, detrimental—sometimes even lethal—outcomes can occur. Systemic conditions, such as sepsis and septic shock, which exhibit a high mortality rate, and local conditions, such as chronic rheumatoid arthritis, which plague over 5.3 million individuals worldwide, are caused by a dysfunction of the inflammatory system (5, 6). Traditional treatments have been shown to have deleterious long-term consequences (7–9). Therapeutics such as anti–TNF-α drugs are useful, but their safety and efficacy can still be improved (10).
In 1998, we isolated and characterized LPS-induced TNF-α factor (LITAF) with activity on TNF-α transcriptional regulation (11, 12). TNF expression is tightly linked to proinflammatory and proapoptotic pathways, and its overproduction can be lethal, as seen in septic shock. LITAF, in its phosphorylated form, translocates to the nucleus where it binds to the promoter regions of TNF-α, MCP-1, and other cytokines, thus regulating their expression (13, 14). LITAF expression can be induced by either Escherichia coli or Porphyromonas gingivalis LPS engaging either TLR-4 or TLR-2, respectively (15). Previously, we demonstrated that mice with LITAF-deficient macrophages (macLITAF−/−) are more resis-tant to the deleterious effects of the LPS-induced proinflammatory cascade and endotoxic shock than wild-type (WT) mice. In these mice, serum levels of proinflammatory cytokines adopt a slower yet gradual increase compared with WT mice, whereas anti-inflam-matory cytokines just as in WT mice remain significantly elevated for a longer time (13). Nonetheless, only partial protection from LPS-induced lethal endotoxic shock was achieved. We questioned whether an extension of LITAF deficiency to other LITAF-producing cells in addition to macrophages would improve protection against the deleterious effects of inflammation (16).
We generated a mouse with whole-body LITAF deficiency to investigate the impact of broader LITAF deletion in inflammatory diseases. Our findings indicate that whole-body excision of LITAF has dramatic effects on systemic and chronic local inflammatory responses. These data highlight the potential benefit of targeted approaches aimed at interfering with overall LITAF function to develop better therapeutics for the treatment of systemic and local forms of inflammation.
Results
Whole-Body Tamoxifen-Inducible LITAF−/− Mice.
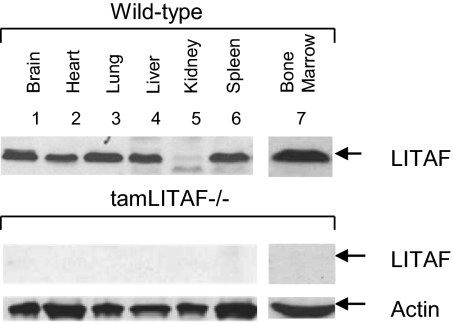
A tamoxifen-inducible LITAF−/−mouse [tamLITAF(i)−/−] was selected for experimental use over a constitutive complete LITAF deletion because it was readily available, time-saving, and proven successful in pilot studies. LITAF was successfully ablated, and its excision was confirmed in bone marrow, brain, heart, lung, liver, kidney, spleen, and skeletal muscle after tamoxifen stimulation, providing a reliable model for studying the role of LITAF in different inflammatory processes. The WT LITAF bands were consistent with our previous studies with a notable absence of LITAF in the kidneys. The tamLITAF(i)−/− tissues showed no LITAF protein compared with the WT (Fig. 1).
Fig. 1.
Confirmation of LITAF excision from tamLITAF(i) knockout mice by Western blot analysis using antibodies against murine LITAF or actin as control. Brain, heart, lung, liver, spleen, kidney, and skeletal muscle tissue were collected from tamLITAF(i)−/− mice after 5 d of tamoxifen injections. The same organ tissues were collected from WT and used as controls.
Survival Rates of LPS-induced Endotoxin Challenge in TamLITAF(i)−/− and WT Mice.
Two models were tested:
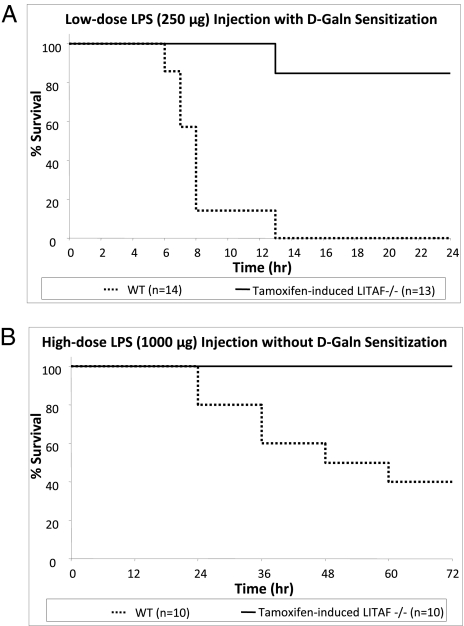
(i) WT and tamLITAF(i)−/− mice were subjected to a model of lethal TNF-α sensitization by pretreatment with d-GalN, a known factor that sensitizes mice to the lethal effects of LPS and causes death due to TNF-α toxicity (16, 17). The d-GalN was followed by an i.p. injection of a lethal dose of E. coli LPS (25 μg). Most deaths in the WT group occurred between 5 and 8 h after injection, whereas the tamLITAF(i)−/− group did not have any observed deaths until hour 12. After 13 h, 11 of the 13 (85%) tamLITAF(i)−/− mice survived, and none of the 14 WT mice survived. There were no deaths in the tamLITAF(i)−/− mice during the remaining 11 h of the study or in the 3 d following (Fig. 2A). These data show that complete excision of LITAF provides greater protection from the lethal effects of LPS-induced TNF-α–mediated septic shock compared with WT mice.
(ii) WT and tamLITAF(i)−/− mice were injected i.p. with a lethal dose of E. coli LPS (1,000 μg) in the absence of d-galactose (d-Gal) sensitization to simulate a more pathophysiological septic shock. Deaths in the WT group occurred between 24 and 48 h after LPS injection, whereas the tamLITAF(i)−/− group did not have any observed deaths during the study period. At the end of the 72-h study period, 10/10 mice injected with tamLITAF(i)−/− remained alive, whereas only 4 of 10 (40%) WT mice survived (Fig. 2B). Nonsensitized tamLITAF(i)−/− mice injected with a high dose of LPS show a significant increase of survival compared with WT mice.
Fig. 2.
Comparison of survival rates between WT and tamLITAF(i)−/− mice in response to two models of endotoxic challenge. tamLITAF(i)−/− animals were more resistant to LPS-induced septic shock than the WT controls. (A) Age-matched LITAF (n = 13) and WT (n = 14) mice were injected i.p. with E. coli LPS (25 μg per mouse). Mortality was assessed every hour for 24 h. At the end of 24 h, 85% of LITAF(i)−/−mice had survived, whereas there was no survival in the WT animals. (B) Age-matched LITAF (n = 10) and WT (n = 10) mice were injected i.p. with E. coli LPS (1,000 μg per mouse). Mortality was assessed in 12-h intervals up to 72 h. At the end of 72 h, 100% of LITAF−/−mice had survived, whereas only 40% of WT animals survived. All experiments were performed in triplicate.
Analysis of Serum Levels of Inflammatory Mediators in TamLITAF(i)−/− and WT Mice After a Lethal Dose of LPS.
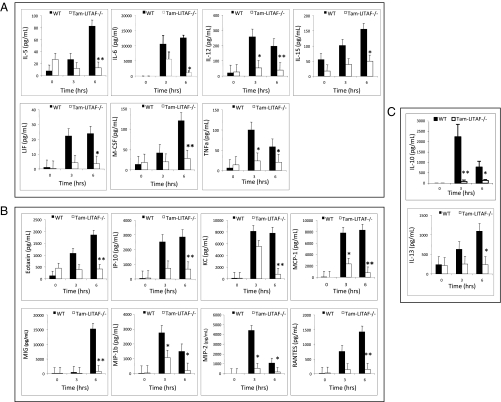
In the d-GalN model, 3 h after the LPS injection, serum samples from tamLITAF(i)−/− mice showed no statistically significant differences compared with serum from WT mice, However, 6 h after injection, compared with the WT mice, serum from tamLITAF(i)−/− mice demonstrated statistically significant (P < 0.05) reductions in the proinflammatory cytokines IL-5, IL-12p40, IL-15, LIF, M-CSF, and TNF-α; IL-6 and IL-10 were significant to P < 0.01 (Fig. 3A). Likewise, significant (P < 0.05) reductions were also observed in the proinflammatory chemokines KC, MIP-1b, and MIP-2; eotaxin, IP-10, MCP-1, MIG, and RANTES had a significance of P < 0.01 (Fig. 3B). There was also a significant (P < 0.05) reduction in the anti-inflammatory cytokine IL-13 (Fig. 3C).
Fig. 3.
Comparison of the serum levels of inflammatory mediators in WT (n = 4) and tamLITAF(i)−/− (n = 5) mice in response to a lethal dose of E. coli LPS after pretreatment with d-GalN. Serum samples were collected at 0 h, 3 h, and 6 h and analyzed with 32-plex mouse cytokine kits (Millipore). The data are means ± SEM; *P < 0.05, **P < 0.01. (A) Proinflammatory cytokines. (B) Chemokines. (C) Anti-inflammatory cytokines. All experiments were performed in triplicate.
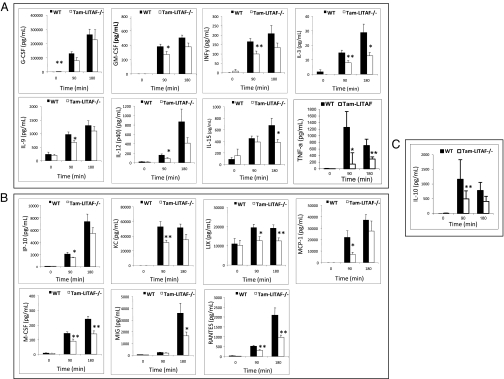
Similarly, in the high-dose LPS challenge, WT and tamLITAF(i)−/− mice were administered a lethal dose of E. coli LPS. Ninety minutes after injection, compared with the WT animals, tamLITAF(i)−/− mice serum demonstrated statistically significant reductions in the proinflammatory cytokines GM-CSF, INF-y, IL-9, IL-10, IL-12, IL-3, M-CSF, and TNF-α (Fig. 4A). A trend in the reduction of the anti-inflammatory cytokines IL-5, IL-6, and IL-13 was demonstrated, but statistical significance was not achieved. Three hours after injection, compared with the WT mice, the serum levels of the proinflammatory cytokines IL-3, IL-15(i)−/− mice demonstrated statistically significant reductions (Fig. 4A). Significant reductions were also observed in the proinflammatory chemokines IP-10, LIX, MCP-1, KC, and RANTES (Fig. 4B). The serum levels of the chemokines MIG, LIX RANTES, and M-CSF that were reduced at 90 min were also reduced at 3 h (Fig. 4B).
Fig. 4.
Comparison of inflammatory cytokines levels in WT (n = 10) and tamLITAF(i)−/− (n = 10) mice in response to a lethal high-dose of E. coli LPS. Serum samples were collected at 0 min, 90 min, and 180 h and analyzed with 32-plex mouse cytokine kits (Millipore). The data are means ± SEM; *P < 0.05, **P < 0.01. (A) Proinflammatory cytokines. (B) Chemokines. (C) Anti-inflammatory cytokines. All experiments were performed in triplicate.
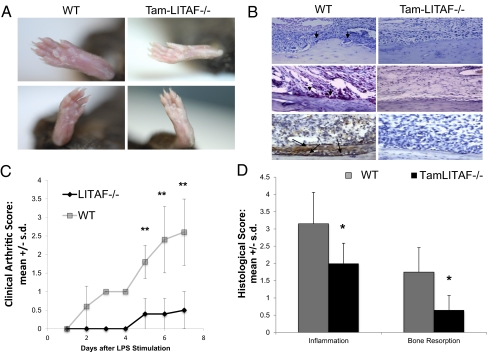
Comparison of Clinical and Histological Effects of Arthritis-Inducing Antibody Mixture in WT and TamLITAF(i)−/− Mice.
To explore how whole-body excision of LITAF might affect a local model of inflammation, WT and tamoxifen-induced LITAF−/− mice were both subjected to collagen antibody-induced arthritis (CAIA). The severity of the clinical presentation of arthritis after LPS stimulation was graded. A greater degree of inflammation in metatarsophalangeal and interphalangeal joints along with edema was observed macroscopically in paws of WT mice compared with tamLITAF−/− mice (Fig. 5A). Three days after LPS stimulation, significantly more inflammation was observed in the WT compared with the tamLITAF(i)−/− mice. This response became more exaggerated and lasted throughout the remainder of the study (Fig. 5 A and C). To substantiate these macroscopic findings, histological analysis was performed. The WT mice had a higher presentation of pannus, synovitis, and inflammation compared with the tamLITAF(i)−/− mice (P < 0.05). Furthermore, the tamLITAF(i)−/− animals demonstrated a decrease in bone resorption compared with the WT (P < 0.05; Fig. 5 B and D). The histological evidence, in concert with the clinical presentation, shows that the whole-body excision of LITAF can effectively decrease the deleterious effects of CAIA disease. In addition to a reduction in inflammation and bone loss, tamLIFAF(i) paws stained with an antibody against TNF-α displayed a substantial reduction in of TNF expression compared with WT paws (LITAF+/+; Fig. 5B).
Fig. 5.
Comparison of the clinical and histological reactions of WT and tamLITAF(i)−/− mice after injection of arthritis-inducing antibody mixture. (A) Photographs of the right rear paw of WT and tamLITAF(i)−/− mice 8 d after arthritis-inducing mixture (ArthroMAB) injection. (B) (Top) H&E-stained sections of WT and tamLITAF(i)−/− 8 d after mixture treatment. (Middle) TRAP staining. Arrows depict osteoclasts. (Bottom) Antibody against mouse TNF-α staining. Arrows depict TNF-α protein expression. (Magnification: 200×.) (C) Clinical arthritis severity in mixture-treated WT (n = 5) and tamLITAF(i)−/− mice (n = 5); graded daily in a nonblinded fashion after LPS stimulation (50 μg). Data are means ± SD. (D) Semiquantitative analysis of inflammation and bone resorption of WT and tamLITAF(i)−/− animals exposed to the arthritis-inducing mixture graded in a blinded fashion. Data are means ± SD; *P < 0.05.
Repeated-measures ANOVA was performed on clinical arthritis scores (CAS) for values observed from day 2 to day 7 in the experimental and control groups (day 1 was omitted because all values in both groups were zero). The model included treatment group (group), time (day), and a group × day interaction. Highly significant effects were observed by repeated-measures ANOVA for tamLITAF(i) (P = 0.0002), reflecting the overall mean differences between the tamLITAF(i) and WT animals, for day (P < 0.0001), reflecting the increasing scores over time, and for the group × day interaction (P = 0.0002), reflecting the progressively greater difference in mean scores over time between the two groups. By Wilcoxon rank-sum tests, mean CAS for WT animals were marginally higher (P = 0.05) at day 2 and substantially higher at days 3–7 (all P ≤ 0.01) relative to tamLITAF(i).
Discussion
Using this model of whole-body excision of LITAF, we demonstrated that both systemic and local forms of inflammation are significantly reduced. Our results indicate the importance of LITAF in cells (i.e., macrophages, lymphocytes, or fibroblasts) other than LysMCre-positive cells, as evidenced by a reduced responsiveness to endotoxic challenge obtained in tamLITAF(i)−/− mice than previously reported in our macLITAF−/− (15). However, other cells, in addition to macrophages, are involved in systemic inflammation and associated inflammatory pathways (18), yet no studies have examined the effect of whole-body LITAF deficiency on the response to inflammatory stimulants.
We show that the complete excision of LITAF has myriad ramifications on innate and acquired immunity. In our previous study using the d-GalN model, removing LITAF only from macrophages led to a survival rate of 53%. In the present study, whole-body removal of LITAF led to a survival rate of 83%. Although previously debated, there is now agreement that the d-GalN model yields lethality due to TNF-α production targeting the liver and subsequently leading to hepatic necrosis and apoptosis (19). Marino and colleagues (20) demonstrated that even at a high dose of LPS, TNF-α knockout (−/−) mice suffered no deaths, whereas all of the controls succumbed to the effects of TNF-α. Our results using the d-GalN lethality model indicate that excision of LITAF ameliorated these adverse effects in the liver and subsequently increased survival in the tamLITAF(i)−/− animals. However, to ensure that the systemic endotoxic shock involves multiple organ systems, high-dose LPS injection without prior d-GalN pretreatment was used. This method yielded no mortality among the tamLITAF(i)−/− mice, yet only 40% of the WT animals survived. This increased resistance to endotoxic shock illustrates that the full deletion of LITAF, targeting all cells, is important to achieve greater protection to LPS challenge (21).
Our data from WT mice is consistent with observations that an important release of proinflammatory mediators is produced at the onset of sepsis followed by anti-inflammatory mediators to limit proinflammatory deleterious effects (22). However, tamLITAF(i)−/− mice exhibited reduced levels of pro- and the anti-inflammatory mediators, demonstrating that LITAF may have transcriptional regulation on pro- as well as anti-inflammatory mediators. Although tamLITAF(i)−/− mice exposed to LPS had a greater reduction of pro- and the anti-inflammatory mediators than macLITAF−/− mice, the general pattern of cytokine expression was comparable. More specifically, tamLITAF and macLITAF mice both exhibited significant reductions in the production of inflammatory cytokines TNF- α, IL-6, and IL-13, and proinflammatory chemokines eotaxin, KC, and MCP-1, 6 h postinjection compared with WT. However, even with full excision of LITAF, a number of mediators remain unaffected and follow the established septic response, suggesting that the endotoxic shock triggers multiple pathways in addition to LITAF-mediated responses that warrant further investigation. Together, these findings further support the observation that whole-body deletion of LITAF leads to a reduced systemic inflammatory response, providing the animals (when exposed to lethal LPS doses) greater control over the adverse effects of septic shock.
Regarding local chronic inflammatory processes, we tested LITAF deficiency in CAIA (23–25). Rheumatoid arthritis is a condition emerging mainly in response to chronic overexpression of TNF-α partly regulated by LITAF. We hypothesized that whole-body LITAF excision would improve the clinical signs of CAIA. Macroscopic examinations disclosed clear evidence that LITAF intervenes in mediating the deleterious effects of CAIA. tamLITAF(i)−/− mice did not experience any adverse effect on gait due to a significant reduction of paw swelling and redness compared with WT mice. Our histological data confirm these findings that tamLITAF(i)−/− mice exhibit significantly less inflammation and bone resorption compared with WT mice. Given the relationship between inflammation and bone loss (26, 27), the absence of LITAF clearly leads to decreased inflammation in tamLITAF(i)−/− mice as well as decreased bone resorption. Our findings also support the observation that inflammatory cytokines are integral to the development and maintenance of arthritis (26, 27). One way this whole-body LITAF deletion may be beneficial is the reduction of the levels of proinflammatory cytokines involved in the pathogenesis of this condition. There exists a positive feedback loop where the presence of proinflammatory cytokines induces expression of other inflammatory cytokines, and a disruption of this process is possibly mediated via LITAF. It is pertinent to mention that LITAF binding sites have been identified on several promoters of pro- and anti-inflammatory mediators (MCP-1, IL-6, and IL-10) in addition to TNF, suggesting that the reduced LPS-induced lethality and milder clinical and histological inflammatory arthritis (IA) features observed in tamLITAF(i)−/− mice may result from the decrease of activation of mediators regulated by LITAF. Indeed, using anti–IL-6 and anti–IL-1 receptor mABs, Kawane et al. (28) were able to impede the “cytokine storm” and block joint swelling. Furthermore, Maia et al. (29) used ArthroMAB to induce CAIA, and found that removing the cytoplasmic domain of CD248 impaired TNF-α–induced monocyte adhesion, underscoring the importance of regulating TNF-α production in rheumatoid arthritis. Recent clinical trials demonstrated that soluble tumor necrosis factor-receptor immunoglobulin fusion protein (TNFRIg) and anti–TNF-α antibodies can reduce synovitis and serum markers for inflammation. However, there is no data regarding long-term prevention from bone and cartilage destruction (26).
The rationale for using these two inflammatory models in this study stems from the fact that in essence, immunoinflammatory diseases are fueled by cytokines and regulated by cytokine inhibitors, and the role of LITAF would be evaluated simultaneously systemically (LPS-induced sepsis) and locally (IA). Indeed, pathological features associated with LPS-induced sepsis and IA have in common macrophage-activating cytokines TNF, IL-1, IL-6, or MCP-1 (30). However, sepsis is known to result from endotoxemia, whereas rheumatoid arthritis is not triggered by LPS, and our model of IA included LPS stimulation where the lack of LITAF could account for the difference. Though LPS stimulates LITAF, other mediators, such cytokines or p53, can also stimulate LITAF (31–33). It is conceivable that in human rheumatoid arthritis the role of LITAF would remain predominant as being triggered by endogenous TNF, IL-6, or even p53.
There is a pressing need for new pharmacological approaches to reduce the deleterious effects of systemic and local inflammatory responses. The current anti-inflammatory therapies, particularly anti–TNF-α treatments, fall short of expectation. Although a number of these medications have been developed and are presently being prescribed, they can cause serious side effects and are used with discretion (7–10). Novel pharmaceutical approaches are warranted to create compounds that effectively control inflammation and are safe for long-term use for treatment of chronic conditions. Therapeutic methodologies may differ, but we hold that anti-inflammatory molecules acting directly or indirectly on LITAF possess significant promise. Indeed, recently we demonstrated that kavain and related kavalactones treatment of cells suppressed LITAF and reduced TNF-α secretion in macrophages, providing protection in vivo in a TNF-α–driven model of inflammation (34). Compounds interfering with LITAF may prove to be a promising alternative in the treatment of several cytokine-mediated diseases.
Materials and Methods
Generation of TamLITAF−/− Mice.
B6.Cg-Tg(CAG-cre/Esr1)5Amc/J transgenic mice (Jackson Labs) were crossed with C57Bl6/J (Jackson Labs). The resulting Cre heterozygous mice were crossed with LITAFfl/fl (15). The offspring carrying the Cre-ERT allele were backcrossed with LITAFfl/fl to ensure homozygous passage of the LITAF floxed sites. Recombination was induced by the injection of tamoxifen (Sigma T5648) that was suspended in ethanol (10 mg/100 μL) then in autoclaved sunflower oil to a concentration of 1 mg/100 μL. One milligram of tamoxifen was injected i.p. into each mouse for five consecutive days. After 5 d, genotyping for LITAF and Neo was performed. Control mice (LITAF+/+) were Cre-positive littermates bred in house that received oil vehicle in place of tamoxifen. The detection of LITAF or neomycin segments was performed by PCR (iCycler; BioRad) with the following primer pairs: 5′-CTTAAAATACCCTCTCCTACTCCTTCT-3′ and 5′-TGCTTGGTAAGGTCCTGGAG-3′ for generation of a LITAF DNA segment (770 bp) or 5′-TGCTCCTGGCGAGAAAGTATCCATCATGGC-3′ and 5′-CGCCAAGCTCTTCAGCAATATCACGGGTAG-3′ for generation of a neomycin DNA segment (300 bp). All genotyping used tail clips, and QuickGene DNA Tissue Kit S (FujiFilm) was used for DNA extraction. Control mice (LITAF+/+) were littermates of C57B16/J (Jackson Labs), background-generated in house, and screened by PCR and Western blot to confirm presence of LITAF gene and protein, respectively. All animal procedures were approved by the Boston University Institutional Animal Care and Use Committee.
Western Blot Analysis.
After confirmation of LITAF excision through genotyping, mice were taken to confirm excision in various tissues. Bone marrow, brain, heart, lungs, liver, spleen, kidney, and skeletal muscle tissue were surgically removed, flash-frozen with liquid nitrogen, and grinded with a pestle. RIPA buffer containing proteinase mixture inhibitor was added to each tissue sample (3 mL buffer/1 g tissue). Samples were then sonicated, and protein concentrations were recorded using the Bradford Assay (SmartSpec 3000; Bio-Rad). Equal protein concentrations were separated by SDS/PAGE and transferred to a PVDF membrane blocked with 4% nonfat milk power in Tween/TBS. The proteins from the lysed tissues were detected by Western blotting with antibodies to actin (C-11; Santa Cruz) and LITAF (611615; BD Biosciences). Primary antibodies were incubated at room temperature for 1 h, and secondary antibodies were incubated for 30 min. Protein band intensity was analyzed using VersaDoc Imaging System model 4000MP with Quantity One Quantitation Software version 4.6.3 (Bio-Rad). The membrane was incubated with stripping buffer (Fisher Scientific) according to the manufacturer's instructions between antibodies.
Injection of LPS into Mice After d-Gal Sensitization and LPS Lethality Test.
At the age of 8–12 wk, tamLITAF(i)−/− mice along with control animals (LITAF+/+) weighing 20–25 g were i.p. injected with a single dose of d-GalN (25 mg; Acros Organics) followed by an i.p. injection of E. coli (0111:B4) LPS (25 μg; Invivogen) in a total volume of 0.1 mL of PBS containing 1% BSA. All animals were continuously monitored for LPS-induced d-GalN–dependent lethality for 24 h after LPS challenge (WT, n = 14; LITAF−/−, n = 13); no additional deaths occurred after 24 h. Survival graph was produced using the animals’ time of death (up to nearest hour). Additionally, blood was collected immediately at time of injection, and 3 h and 6 h postinjection. The serum was separated and stored in −80° C until cytokine analysis could be performed. The mice were closely monitored and assessed for mortality for 24 h.
Injection of High-Dose LPS into Mice and High-Dose LPS Lethality Test.
At the age of 8–12 wk, tamLITAF(i)−/− mice along with control animals (LITAF+/+) weighing 20–25 g were i.p. injected with E. coli (0111:B4) LPS (1,000 μg; Invivogen) in a total volume of 0.1 mL of PBS containing 1% BSA. All animals were continuously monitored for high-dose LPS-induced lethality for 72 h after LPS challenge (WT, n = 10; LITAF−/−, n = 10); no additional deaths occurred after 72 h. A table was constructed illustrating the number of deaths in each group. Additionally, blood was collected immediately at time of injection, and 90 min and 180 min postinjection. The serum was separated and stored in −80° C until cytokine analysis could be performed. The mice were closely monitored and assessed for mortality for 72 h.
Bio-Plex Analysis.
Cytokine concentrations adjusted according to the recovery rate from the collected serum were determined using the Bio-Plex Protein Array System (Bio-Rad). Cytokine-specific antibody-coated beads (Millipore) were used for these experiments. The assay was performed in triplicate according to the manufacturer's instructions. Cytokine concentrations were automatically calculated with Bio-Plex Manager software by using a standard curve derived from a recombinant cytokine standard. According to previous experiments analyzing five cytokines [IL-1b, IL-6, IL-12(p70), MCP-1, TNF-α] derived from serum samples of tamLITAF(i)−/− as well as from controls, the following 32 cytokines were selected for further analyses: eotaxin, G-CSF, GM-CSF, IFN-γ, IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-6, IL-9, IL-10, IL-12(p40), IL-12(p70), IL-13, IL-15, IL-17, IP-10, KC, LIF, LIX, MCP-1, M-CSF, MIG, MIP-1β, MIP-1α, MIP-2, RANTES, TNF-α, and VEGF.
IA Model.
To simulate IA, a CAIA model was used. Five tamLITAF(i)−/− and five age-matched controls were injected with 7 mg (of 10 mg/mL concentration) of ArthroMAB Monoclonal Antibody Blend (Millipore), which is a mixture of four monoclonal antibodies that bind to the epitopes C11b, J1, D3, and U1 on the collagen II molecule. Four days after ArthroMAB treatment, 50 μg of LPS (E. coli 0111B4) in PBS (Millipore) was administered i.p. to act synergistically in triggering and exacerbating the induction of CAIA. tamLITAF(i)−/− mice were treated with tamoxifen for 5 d before treatment for optimal excision, and additional boosters were given 3× per week to keep LITAF excised. The mice were killed 8 d after the initial ArthroMAB injection. The lower limbs were extracted and fixed in 4% paraformaldehyde overnight at 4 °C. The specimens were then washed with PBS and placed in a decalcification solution consisting of 10% EDTA (pH 7.1) for 10 d.
Tissue sections and staining.
Paws were sectioned and stained with H&E for inflammatory cell infiltrate assessment. For osteoclast identification, adjacent paw tissue sections were stained for tartarate-resistant acid phosphatase (TRAP). Sections were incubated for 3 h in the TRAP staining solution containing 0.35 mM of naphthol AS-BI phosphate substrate (Sigma), 1% of N,N-dimethylformamide (EM Science), 3.7 mM of fast red violet LB diazonium salt (Sigma), 6.4 mM of tartaric acid (Sigma), and 0.4% MgCl2 in 0.2 M sodium acetate buffer (pH 5.0) at 37 °C in the dark. The slides were then washed with water for 5 min, followed by counterstaining with hematoxylin for 5 s. Multinucleated, TRAP-positive in contact with bone lacuna were considered osteoclasts. Additionally, paws were stained by immunohistochemistry using an antibody against mouse TNF-α (rat monoclonal antibody, clone MP6-xT22; Biolegend) and revealed by HRP secondary antibody to detect TNF-α protein expression.
Semiquantitative analysis.
All sections were evaluated by a blinded investigator (S.A.) who scored inflammation and bone resorption. The right hind paw was macroscopically graded daily, after stimulation with 50 μg of LPS (0, normal; 1, redness and swelling of one finger; 2, swelling of one or more fingers; and 3, swelling of the whole paw), with a maximum score of 3 per animal. Slides were coded and evaluated for the extent of inflammation (synovitis, pannus formation, or bone and/or cartilage destruction) and bone resorption on a scale consisting of grade 0 (no signs of inflammation), grade 1 (mild inflammation with hyperplasia of the synovial lining layer, minimal without cartilage destruction), and grades 2–5 (increasing degrees of inflammatory cell infiltrate, or cartilage and bone destruction by TRAP) as previously described (27).
Statistical analysis.
Data are expressed as mean ± SEM of the results obtained from three independent experiments. Student's two-tailed t test was used for statistical analysis, and P < 0.05 was considered significant. In addition, repeated-measures ANOVA was done on CAS for values observed from days 2–7 in the tamLITAF(i) and WT mice (day 1 was omitted because all values in both groups were zero). The model included the treatment group [tamLITAF(i) vs. WT], time (day), and a group × day interaction. Wilcoxon rank-sum tests mean CAS for WT animals’ tamLITAF(i) KOs were also performed.
Acknowledgments
Support for this work was provided by National Institutes of Health Grant R01 DE14079.
Footnotes
The authors declare no conflict of interest.
References
- 1.Ferrero-Miliani L, Nielsen OH, Andersen PS, Girardin SE. Chronic inflammation: Importance of NOD2 and NALP3 in interleukin-1beta generation. Clin Exp Immunol. 2007;147:227–235. doi: 10.1111/j.1365-2249.2006.03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunt TK, Knighton DR, Thakral KK, Goodson WH, III, Andrews WS. Studies on inflammation and wound healing: Angiogenesis and collagen synthesis stimulated in vivo by resident and activated wound macrophages. Surgery. 1984;96(1):48–54. [PubMed] [Google Scholar]
- 3.Remick DG, Kunkel SL. Pathophysiologic alterations induced by tumor necrosis factor. Int Rev Exp Pathol. 1993;34(Pt B):7–25. doi: 10.1016/b978-0-12-364935-5.50007-9. [DOI] [PubMed] [Google Scholar]
- 4.Neale ML, Williams BD, Matthews N. Tumour necrosis factor activity in joint fluids from rheumatoid arthritis patients. Br J Rheumatol. 1989;28(4):104–108. doi: 10.1093/rheumatology/28.2.104. [DOI] [PubMed] [Google Scholar]
- 5.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 6.Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- 7.Matera MG, Calzetta L, Cazzola M. TNF-alpha inhibitors in asthma and COPD: We must not throw the baby out with the bath water. Pulm Pharmacol Ther. 2010;23(2):121–128. doi: 10.1016/j.pupt.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Dahlström U. Frequent non-cardiac comorbidities in patients with chronic heart failure. Eur J Heart Fail. 2005;7:309–316. doi: 10.1016/j.ejheart.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Wolfe F, Michaud K. Lymphoma in rheumatoid arthritis: The effect of methotrexate and anti-tumor necrosis factor therapy in 18,572 patients. Arthritis Rheum. 2004;50:1740–1751. doi: 10.1002/art.20311. [DOI] [PubMed] [Google Scholar]
- 10.Nakelchik M, Mangino JE. Reactivation of histoplasmosis after treatment with infliximab. Am J Med. 2002;112:78. doi: 10.1016/s0002-9343(01)00945-7. [DOI] [PubMed] [Google Scholar]
- 11.Myokai F, Takashiba S, Lebo R, Amar S. A novel lipopolysaccharide-induced transcription factor regulating tumor necrosis factor alpha gene expression: Molecular cloning, sequencing, characterization, and chromosomal assignment. Proc Natl Acad Sci USA. 1999;96:4518–4523. doi: 10.1073/pnas.96.8.4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang X, Fenton MJ, Amar S. Identification and functional characterization of a novel binding site on TNF-alpha promoter. Proc Natl Acad Sci USA. 2003;100:4096–4101. doi: 10.1073/pnas.0630562100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srinivasan S, Leeman SE, Amar S. Beneficial dysregulation of the time course of inflammatory mediators in lipopolysaccharide-induced tumor necrosis factor alpha factor-deficient mice. Clin Vaccine Immunol. 2010;17:699–704. doi: 10.1128/CVI.00510-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang X, Marciano DL, Leeman SE, Amar S. LPS induces the interaction of a transcription factor, LPS-induced TNF-alpha factor, and STAT6(B) with effects on multiple cytokines. Proc Natl Acad Sci USA. 2005;102:5132–5137. doi: 10.1073/pnas.0501159102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang X, Metzger D, Leeman S, Amar S. LPS-induced TNF-alpha factor (LITAF)-deficient mice express reduced LPS-induced cytokine: Evidence for LITAF-dependent LPS signaling pathways. Proc Natl Acad Sci USA. 2006;103:13777–13782. doi: 10.1073/pnas.0605988103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silverstein R. d-galactosamine lethality model: Scope and limitations. J Endotoxin Res. 2004;10:147–162. doi: 10.1179/096805104225004879. [DOI] [PubMed] [Google Scholar]
- 17.Lehmann V, Freudenberg MA, Galanos C. Lethal toxicity of lipopolysaccharide and tumor necrosis factor in normal and d-galactosamine-treated mice. J Exp Med. 1987;165:657–663. doi: 10.1084/jem.165.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armaka M, et al. Mesenchymal cell targeting by TNF as a common pathogenic principle in chronic inflammatory joint and intestinal diseases. J Exp Med. 2008;205:331–337. doi: 10.1084/jem.20070906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mignon A, et al. LPS challenge in d-galactosamine-sensitized mice accounts for caspase-dependent fulminant hepatitis, not for septic shock. Am J Respir Crit Care Med. 1999;159:1308–1315. doi: 10.1164/ajrccm.159.4.9712012. [DOI] [PubMed] [Google Scholar]
- 20.Marino MW, et al. Characterization of tumor necrosis factor-deficient mice. Proc Natl Acad Sci USA. 1997;94:8093–8098. doi: 10.1073/pnas.94.15.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wipke BT, Allen PM. Essential role of neutrophils in the initiation and progression of a murine model of rheumatoid arthritis. J Immunol. 2001;167:1601–1608. doi: 10.4049/jimmunol.167.3.1601. [DOI] [PubMed] [Google Scholar]
- 22.Osuchowski MF, Welch K, Siddiqui J, Remick DG. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J Immunol. 2006;177:1967–1974. doi: 10.4049/jimmunol.177.3.1967. [DOI] [PubMed] [Google Scholar]
- 23.Khachigian LM. Collagen antibody-induced arthritis. Nat Protoc. 2006;1:2512–2516. doi: 10.1038/nprot.2006.393. [DOI] [PubMed] [Google Scholar]
- 24.Terato K, et al. Induction of arthritis with monoclonal antibodies to collagen. J Immunol. 1992;148:2103–2108. [PubMed] [Google Scholar]
- 25.Fridman JS, et al. Selective inhibition of JAK1 and JAK2 is efficacious in rodent models of arthritis: Preclinical characterization of INCB028050. J Immunol. 2010;184:5298–5307. doi: 10.4049/jimmunol.0902819. [DOI] [PubMed] [Google Scholar]
- 26.Maini RN, et al. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum. 1998;41:1552–1563. doi: 10.1002/1529-0131(199809)41:9<1552::AID-ART5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 27.Deng GM, Zheng L, Chan FK, Lenardo M. Amelioration of inflammatory arthritis by targeting the pre-ligand assembly domain of tumor necrosis factor receptors. Nat Med. 2005;11:1066–1072. doi: 10.1038/nm1304. [DOI] [PubMed] [Google Scholar]
- 28.Kawane K, Tanaka H, Kitahara Y, Shimaoka S, Nagata S. Cytokine-dependent but acquired immunity-independent arthritis caused by DNA escaped from degradation. Proc Natl Acad Sci USA. 2010;107:19432–19437. doi: 10.1073/pnas.1010603107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maia M, et al. CD248 and its cytoplasmic domain: A therapeutic target for arthritis. Arthritis Rheum. 2010;62:3595–3606. doi: 10.1002/art.27701. [DOI] [PubMed] [Google Scholar]
- 30.Fei Y, et al. The combination of a tumor necrosis factor inhibitor and antibiotic alleviates staphylococcal arthritis and sepsis in mice. J Infect Dis. 2011;204(3):348–357. doi: 10.1093/infdis/jir266. [DOI] [PubMed] [Google Scholar]
- 31.Tang X, Molina M, Amar S. p53 short peptide (p53pep164) regulates lipopolysaccharide-induced tumor necrosis factor-alpha factor/cytokine expression. Cancer Res. 2007;67:1308–1316. doi: 10.1158/0008-5472.CAN-06-1600. [DOI] [PubMed] [Google Scholar]
- 32.Tang X, et al. p53 peptide prevents LITAF-induced TNF-alpha-mediated mouse lung lesions and endotoxic shock. Curr Mol Med. 2011;11:439–452. doi: 10.2174/156652411796268731. [DOI] [PubMed] [Google Scholar]
- 33.Zhou J, et al. LITAF and TNFSF15, two downstream targets of AMPK, exert inhibitory effects on tumor growth. Oncogene. 2011;30:1892–1900. doi: 10.1038/onc.2010.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pollastri MP, et al. Identification and characterization of kava-derived compounds mediating TNF-alpha suppression. Chem Biol Drug Des. 2009;74(2):121–128. doi: 10.1111/j.1747-0285.2009.00838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]