An emerging hallmark of cancer cells is the reprogramming of energy metabolism pathways (1). By using the aerobic glycolysis pathway, cancer cells metabolize glucose at higher rates than normal tissues and convert it to lactate, a phenomenon known as the Warburg effect (2). Additionally, cancer cells have been reported to have increased glutamine metabolism, which exceeds the metabolic use of other nonessential amino acids (2). This altered metabolism fuels the growth and proliferation of cancer cells by providing energy and macromolecular building blocks, and it also contributes to the maintenance of redox balance (3, 4). A report in PNAS (5) provides evidence for how glucose and glutamine metabolism are regulated during cell cycle progression.
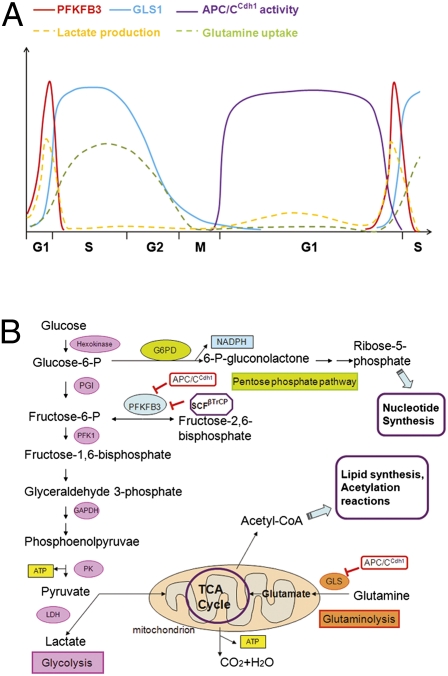
Metabolic activity is a major determinant of a cell's “decision” to proliferate or exit the cell cycle to enter into a quiescent state, and accumulating evidence now suggests that crosstalk occurs between cell cycle transition regulators and metabolism regulators (4, 6). The study by Colombo et al. (5) demonstrates that the levels of two enzymes, PFKFB3 (6-phosphfructo-2-kinase/fructose-2,6-bisphosphatase, isoform 3) and GLS1 (glutaminase 1), which play key roles in the glycolysis and glutaminolysis pathways, respectively, are controlled by two ubiquitin ligase complexes: APC/CCdh1 and SCFβTrCP. APC/CCdh1 mediates the degradation of both PFKFB3 and GLS1 as cells exit mitosis and during the G1 phase, whereas SCFβTrCP specifically targets PFKFB3 during S phase. The oscillation in protein levels of these two enzymes coincides with their respective metabolic activities, in terms of lactic acid generation and glutamine utilization (Fig. 1A). Experiments coupling siRNA knockdowns and cell cycle synchronization further reveal that depletion of either PFKFB3 or GLS1 interferes with the G1/S transition, whereas only GLS1, but not PFKFB3, is required to complete S phase. This study (5), together with previous findings by Moncada and coworkers (7–9), suggests that the metabolism of glucose and glutamine is tightly controlled at distinct phases of the cell cycle through the activity of APC/CCdh1 and SCFβTrCP (Fig. 1B).
Fig. 1.
Cell cycle regulation of glycolysis and glutaminolysis via the APC/CCdh1 and SCFβTrCP ubiquitin ligases. (A) Model of the temporal expression patterns for PFKFB3 and GLS1 proteins (and consequently the utilization of glucose and glutamine) during cell cycle progression. PFKFB3 and GLS1 accumulate in mid- to late G1 phase, when APC/CCdh1 activity ceases. The level of PFKFB3, but not GLS1, decreases in S phase and is kept low during the remainder of the cell cycle. The rate of lactate production and glutamine utilization at different times during the cell cycle is also shown. (B) Crosstalk between metabolic pathways and the APC/CCdh1 and SCFβTrCP ubiquitin ligases.
APC/CCdh1 and SCFβTrCP are known to be major regulators of cell cycle progression (10, 11). However, accumulating evidence now suggests additional roles in regulating cell metabolism. Colombo et al. (5) report that the decrease in APC/CCdh1 activity that occurs in late G1 leads to the accumulation of PFKFB3 and GLS1, and, consequently, elevated glycolysis and glutaminolysis. This observation supports their previous finding that overexpression of Cdh1 largely prevents the increase in glycolysis and reduces the proportion of cells in S phase (7). APC/CCdh1 is active during late anaphase, telophase, and G1, and it is an important contributor to G1 maintenance by promoting the degradation of many positive regulators of cell proliferation (10, 11). Inactivation of APC/CCdh1 in late G1 is required for cells to efficiently proceed into S phase and divide (10, 11). The study by Colombo et al. (5) suggests that the inactivation of APC/CCdh1 also plays a role in satisfying metabolic needs during the G1/S transition.
Interestingly, the authors also found that SCFβTrCP is responsible for PFKFB3 degradation and decreased glycolysis activity in S phase. This scenario is reminiscent of the “dual mode” regulation of other proteins (including CDC25A and Claspin) by APC/CCdh1 and SCFβTrCP, the former targeting them in G1 and the latter in other phases (CDC25A in S phase and Claspin in G2) (12, 13). Notably, SCFβTrCP has been implicated in regulating other pathways that control cellular metabolism. DEPTOR is a recently identified SCFβTrCP substrate that directly regulates the activity of mammalian target of rapamycin kinase (mTOR), a key player in cell growth and metabolism (14–17). By mediating the degradation of DEPTOR, SCFβTrCP enhances mTOR activity and regulates the response to nutrient stimuli, such as glucose or serum (15–17).
Degradation via SCF ligases often requires posttranslational modifications of the substrates (12), and Colombo et al. show that phosphorylation of human PFKFB3 on Ser273, which is present in a conserved SCFβTrCP recognition motif (268DSGxxS273), is required for degradation (5). However, the kinase(s) responsible for this modification and, presumably, for the second serine in the degradation motif (Ser269), remain(s) unknown. It is also noteworthy that the levels of PFKFB3 and GLS1 are already low in G2 phase, when APC/CCdh1 is still inactive, suggesting the involvement of additional regulatory mechanisms. Further investigation of the signaling pathways controlling the levels of PFKFB3 and GLS1 will provide deeper insight into this cell cycle-dependent degradation.
Significantly, APC/CCdh1 is also active in postmitotic cells (10) and, in fact, PFKFB3 was originally reported to be targeted by APC/CCdh1 in neurons (18). Attenuation of glycolysis in these terminally differentiated cells appears to prevent oxidative stress and apoptosis caused by glucose oxidation. It would be interesting to understand whether GLS1 is also down-regulated in postmitotic cells.
The accelerated utilization of glucose and glutamine in cancer cells goes beyond the need for energy. This metabolic reprogramming also contributes to the rapid production of biosynthetic precursors, such as nucleotides, carbohydrates, amino acids, and fatty acids, which are required for cell proliferation (19). To maximize the rate of anabolic growth, the individual pathways controlling glycolysis, glutaminolysis, oxidative phosphorylation, and the pentose phosphate pathway, as well as others, must be interconnected and temporally controlled. The study by Colombo and colleagues shows that PFKFB3 levels and, consequently, glycolysis, are elevated in mid- to late G1 and decrease during S phase. One possible explanation for this behavior is that, by switching off the glycolysis pathway, glucose is diverted into the pentose phosphate pathway and
PFKFB3 levels and, consequently, glycolysis, are elevated in mid- to late G1 and decrease during S phase.
converted to ribose-5-phosphate for nucleotide synthesis. Alternatively, it may be used to provide carbons for fatty acid synthesis. Unlike glucose, glutamine is required for the G1/S transition and throughout S phase (5). These observations suggest different roles for the metabolic pathways at distinct phases of the cell cycle. It will be interesting to determine whether nonmalignant cells also use these mechanisms to regulate energy production and macromolecular precursor biosynthesis.
An emerging theme in cancer is that that oncogenic gene alterations reprogram the metabolic network, thus enabling tumorigenesis (20). Cdh1 displays tumor suppressor activity, as supported by its down-regulation or the inactivation of APC/CCdh1 observed in human cancers (10, 13). It will be worthwhile to test if deregulation or mutations of Cdh1 or certain substrates contributes to alterations in cell metabolism pathways, leading to malignant transformation of the cells.
Overall, this study (5) sheds light on how metabolic pathways, such as glycolysis and glutaminolysis, are linked to cell cycle progression. Given their timely and selective targeting of substrates, APC/CCdh1 and SCFβTrCP coordinate global metabolism networks with the cell cycle. Further investigation will provide important information about the contribution of metabolic pathways to cell growth and malignancy, as well as the specific metabolic features coopted by tumor cells.
Footnotes
The authors declare no conflict of interest.
See companion article on page 21069.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 4.Buchakjian MR, Kornbluth S. The engine driving the ship: Metabolic steering of cell proliferation and death. Nat Rev Mol Cell Biol. 2010;11:715–727. doi: 10.1038/nrm2972. [DOI] [PubMed] [Google Scholar]
- 5.Colombo SL, et al. Molecular basis for the differential use of glucose and glutamine in cell proliferation as revealed by synchronized HeLa cells. Proc Natl Acad Sci USA. 2011;108:21069–21074. doi: 10.1073/pnas.1117500108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aguilar V, Fajas L. Cycling through metabolism. EMBO Mol Med. 2010;2:338–348. doi: 10.1002/emmm.201000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Almeida A, Bolaños JP, Moncada S. E3 ubiquitin ligase APC/C-Cdh1 accounts for the Warburg effect by linking glycolysis to cell proliferation. Proc Natl Acad Sci USA. 2010;107:738–741. doi: 10.1073/pnas.0913668107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colombo SL, et al. Anaphase-promoting complex/cyclosome-Cdh1 coordinates glycolysis and glutaminolysis with transition to S phase in human T lymphocytes. Proc Natl Acad Sci USA. 2010;107:18868–18873. doi: 10.1073/pnas.1012362107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tudzarova S, et al. Two ubiquitin ligases, APC/C-Cdh1 and SKP1-CUL1-F (SCF)-beta-TrCP, sequentially regulate glycolysis during the cell cycle. Proc Natl Acad Sci USA. 2011;108:5278–5283. doi: 10.1073/pnas.1102247108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakayama KI, Nakayama K. Ubiquitin ligases: Cell-cycle control and cancer. Nat Rev Cancer. 2006;6:369–381. doi: 10.1038/nrc1881. [DOI] [PubMed] [Google Scholar]
- 11.Skaar JR, Pagano M. Control of cell growth by the SCF and APC/C ubiquitin ligases. Curr Opin Cell Biol. 2009;21:816–824. doi: 10.1016/j.ceb.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frescas D, Pagano M. Deregulated proteolysis by the F-box proteins SKP2 and beta-TrCP: Tipping the scales of cancer. Nat Rev Cancer. 2008;8:438–449. doi: 10.1038/nrc2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bassermann F, et al. The Cdc14B-Cdh1-Plk1 axis controls the G2 DNA-damage-response checkpoint. Cell. 2008;134:256–267. doi: 10.1016/j.cell.2008.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peterson TR, et al. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009;137:873–886. doi: 10.1016/j.cell.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duan S, et al. mTOR generates an auto-amplification loop by triggering the βTrCP- and CK1α-dependent degradation of DEPTOR. Mol Cell. 2011;44:317–324. doi: 10.1016/j.molcel.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao D, et al. mTOR drives its own activation via SCF(βTrCP)-dependent degradation of the mTOR inhibitor DEPTOR. Mol Cell. 2011;44:290–303. doi: 10.1016/j.molcel.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao Y, Xiong X, Sun Y. DEPTOR, an mTOR inhibitor, is a physiological substrate of SCF(βTrCP) E3 ubiquitin ligase and regulates survival and autophagy. Mol Cell. 2011;44:304–316. doi: 10.1016/j.molcel.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herrero-Mendez A, et al. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nat Cell Biol. 2009;11:747–752. doi: 10.1038/ncb1881. [DOI] [PubMed] [Google Scholar]
- 19.Deberardinis RJ, Sayed N, Ditsworth D, Thompson CB. Brick by brick: Metabolism and tumor cell growth. Curr Opin Genet Dev. 2008;18:54–61. doi: 10.1016/j.gde.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones RG, Thompson CB. Tumor suppressors and cell metabolism: A recipe for cancer growth. Genes Dev. 2009;23:537–548. doi: 10.1101/gad.1756509. [DOI] [PMC free article] [PubMed] [Google Scholar]