Abstract
Relationships between primates and snakes are of widespread interest from anthropological, psychological, and evolutionary perspectives, but surprisingly, little is known about the dangers that serpents have posed to people with prehistoric lifestyles and nonhuman primates. Here, we report ethnographic observations of 120 Philippine Agta Negritos when they were still preliterate hunter–gatherers, among whom 26% of adult males had survived predation attempts by reticulated pythons. Six fatal attacks occurred between 1934 and 1973. Agta ate pythons as well as deer, wild pigs, and monkeys, which are also eaten by pythons, and therefore, the two species were reciprocally prey, predators, and potential competitors. Natural history data document snake predation on tree shrews and 26 species of nonhuman primates as well as many species of primates approaching, mobbing, killing, and sometimes eating snakes. These findings, interpreted within the context of snake and primate phylogenies, corroborate the hypothesis that complex ecological interactions have long characterized our shared evolutionary history.
Our reactions to snakes range from disgust, horror, and ophidiophobia to curiosity, consumption, and deification. Those responses have been widely discussed by anthropologists, herpetologists, primatologists, psychologists, and philosophers (1–7), often from ecological and evolutionary perspectives; we know little, however, about the dangers that snakes actually posed to extinct hominins or contemporary humans with prehistoric lifestyles. Serpents swallow prey intact and thus, unlike crocodilians and mammalian carnivores (8), have left no trace fossils of predation on australopithecines; also, their few fossilized stomach contents have not included primates (9, 10). Moreover, although there have been quantitative assessments of snakebite to rural and forest-dwelling humans (11), evidence for attacks by constrictors is anecdotal, is often of doubtful provenance, and refers to people with relatively modern lifestyles (3, 12). Snakes are uncommonly cited as killing nonhuman primates (1, 3, 6, 13), predation on and competition with snakes typically is not mentioned with respect to primate evolution (1, 14), and these reciprocal relationships have not been evaluated in comprehensive natural history and phylogenetic frameworks.
Here, we show that 20th century hunter–gatherers had intense, complex interactions with giant serpents and then evaluate our findings in the context of the natural history and phylogenetic relationships of primates and snakes. Until recently, Negritos were widespread in the Philippines and elsewhere in Southeast Asia; however, by 1990, transition to a sedentary peasant lifestyle was complete, and now they are threatened with extinction (15). T.N.H. began working with Agta Negritos in 1962, at Casiguran, in the Sierra Madre of Aurora Province, Luzon, when they were preliterate, lived in small kin-related groups, slept in tiny temporary shelters, foraged in old-growth rainforest, and ate wild meat daily. Adult male Agta in a large demographic sample averaged 44.2 kg (16). Reticulated pythons (Python reticulatus) live throughout much of Southeast Asia and the Indo-Australian Archipelago; males reach a total length (TL) of ≤5 m and mass of 20 kg, whereas females achieve >7 m and 75 kg (17) but rarely reach 10 m (12).
We (i) report ethnographic evidence for high incidence of predatory python attacks on Philippine Agta, (ii) compare our data with published records for python attacks on rural humans in Indonesia and Sarawak, (iii) document that Agta ate pythons as well as several mammal species also consumed by those snakes, (iv) summarize field observations of nonhuman primate interactions with snakes, and (v) evaluate these findings in the context of the phylogenetic relationships of snakes and of primates.
Results
Pythons had attacked 15 of 58 Agta men (25.9%) and 1 of 62 women (1.6%), with the significant sex difference (χ2 = 15.249, P < 0.01) presumably because men spent more time in the forest than women (18). Giant serpents had attacked 14 Agta once each and 2 Agta men twice for a total of 18 nonfatal attacks. Fifteen (81.3%) of the Agta attacked had sustained python bites; 11 exhibited substantial scars on the lower limbs or less frequently, hands and torso (Table 1). Men generally were struck while walking in dense rainforest seeking game and useful plants, and they thwarted attacks by dispatching snakes with a large bolo knife or homemade shotgun. Nineteen (15.8%) respondents had known at least one Agta killed by a python for a total of six specified fatalities (Table 2). Fifteen respondents recalled that a python entered a thatched dwelling at sunset (not yet dark), killed two of three sibling children, and was coiled around and swallowing one of them headfirst when the father returned and killed the snake with his bolo; the third child, a 6-mo-old girl, was uninjured. In 2007, a python killed a dog and bit a 22-mo-old Agta child, who survived after his father dispatched the snake with a bolo.
Table 1.
Unsuccessful attacks by reticulated pythons on 16 Agta (includes two men each attacked twice)
| Victim's name | Sex | Born | Bitten | Visible scar | Bite location |
| Didog | Male | 1922 | No | N/A | N/A |
| Sinabuyon (first) | Male | 1927 | No | N/A | N/A |
| Sinabuyon (second) | Male | 1927 | No | N/A | N/A |
| Maninting | Male | 1929 | No | N/A | N/A |
| Pekto | Male | 1902 | Yes | No | Lower right calf |
| Kulut | Male | 1950 | Yes | No | Back of upper right calf |
| Madyaning | Male | 1921 | Yes | Yes | Back of right knee |
| Bilyesa (first) | Male | 1924 | Yes | Yes | Buttocks |
| Bilyesa (second) | Male | 1924 | Yes | Yes | Middle of back |
| Duduyan | Male | 1926 | Yes | Yes | Left calf |
| Kandeg | Male | 1927 | Yes | Yes | Outer right calf |
| Balonse | Male | 1928 | Yes | Yes | Left calf |
| Eleden | Male | 1931 | Yes | Yes | Right elbow |
| Liminida | Female | 1935 | Yes | Yes | Calf |
| Abdon | Male | 1943 | Yes | Yes | Front of left ankle |
| Mario | Male | 1943 | Yes | Yes | Lower left calf |
| Hakob | Male | 1944 | Yes | Yes | Right calf and right little finger (both left scars) |
| Ending | Male | 1944 | Yes | Yes | Four bites to left lower thigh |
Table 2.
Fatal attacks on six Agta by reticulated pythons
| Victim's name | Sex | Year of death | Age | Comments |
| Dinsiweg | Male | ∼1940 | Adult | Ayogyog said the man was killed at Dinapgigi (16° 31′ N, 122° 06' E); the next day, the man's son Sinayatan found the snake, cut it open, and found the body, which Ayogyog helped bury. |
| Pasing | Female | ∼1973 | Adult | Pasing was the wife of Piping Karbunel, who died 2 mo after attack from a leg infection where the python bit her; Karbunel remarried to Rosita in 1976. |
| Saldag | Male | ∼1934 | Old adult | Grandfather of Pirente. |
| Diladeg | Male | 1935 ± 2 y | ∼25 y | Victim did not return from hunting and was found the next day in the coils of a python, which was killed by informant Pidela's father Mahew. |
| Mardyi | Female | March 23, 1973 | 4 y | One of two children of mother Berhinya and father Teteng killed by the same python. |
| Totoy | Male | March 23, 1973 | 3 y | Other child of Berhinya and Teteng killed by the same python. |
Of 19 rural Asians attacked by reticulated pythons (12) (Methods), 8 of 16 men and all 3 women died. Seven cases involved ambush, whereas one victim was killed when a foraging python entered a dwelling; 16 attacks were terrestrial (including one man in water struck from a drainage pipe), whereas pythons struck two victims from trees. Among eight survivors, one was bitten on the head, and four were bitten on a leg; five escaped, because the snake was killed with a knife; and one python knocked over a man without biting or constricting. Eleven of the pythons ranged from 4.5 to 10 m in TL (x = 6.45 m).
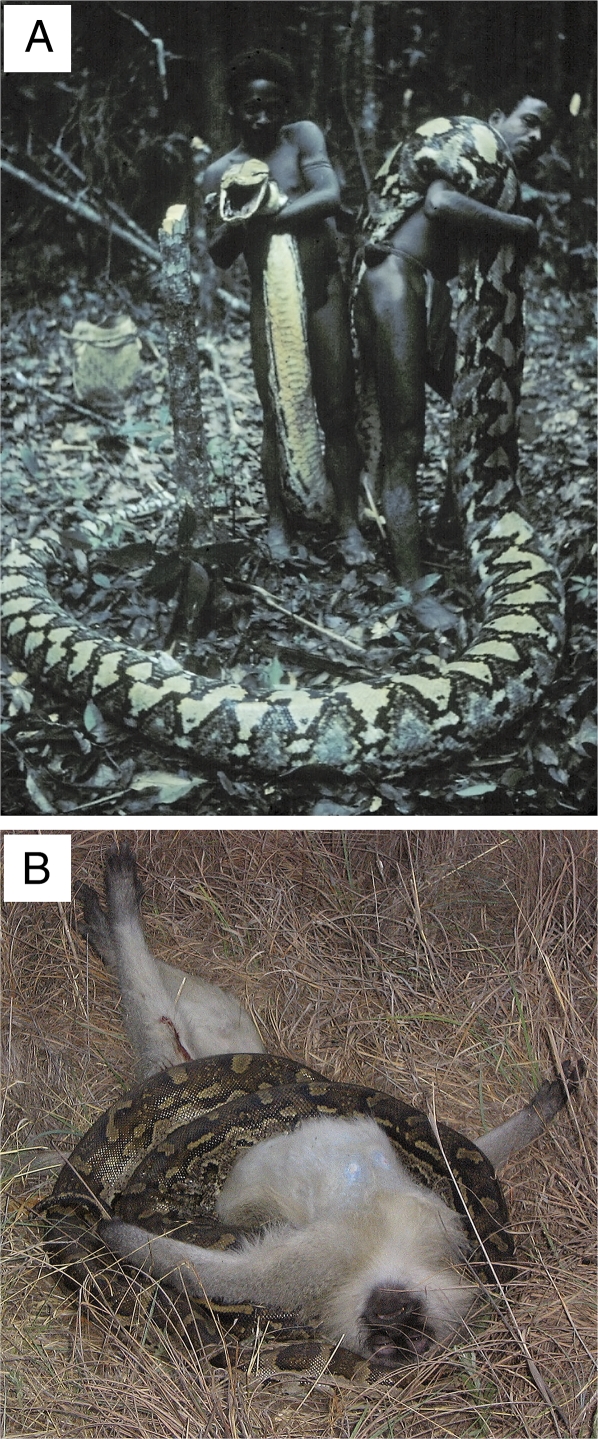
Until the 1970s, Agta routinely hunted and consumed Philippine deer (Rusa marianna), Philippine warty pigs (Sus philippensis), long-tailed macaques (Macaca fascicularis), and pythons but not other snakes (18); all Agta men at that time had probably killed small pythons (TL = 1–2 m) at least one time during their lives, and they occasionally killed large individuals. T.N.H. examined a python immediately after an Agta hunter shot it on June 9, 1970, that was 6.9 m in TL, 65 cm in maximum circumference (Fig. 1), and female based on size (17). Three men skinned and butchered the snake in less than 1 h (Fig. 2) and obtained at least 25 kg of meat, assuming average mass for a snake of that length (17) and estimating butchering efficiency at 33%. The Agta killed a 6.4-m python in Palanan, Isabela Province, on May 27, 1982, that contained a 14.51-kg pig.
Fig. 1.
Reticulated python, TL = 6.9 m, shot by Kekek Aduanan, the adult male Agta on the right, on June 9, 1970, at the headwaters of the Koso River (16° 15′ N, 122° 10′ E) in the Sierra Madre of Aurora Province, Luzon, Philippines. Note the snake's girth and head size relative to the size of the men holding her carcass (photo by J. Headland).
Fig. 2.
Skin of the same python as in Fig. 1 after the two hunters and T.N.H. butchered it, thereby providing ∼25 kg meat to the men's families and fellow group members (photo by J. Headland).
A winnowing of the natural history literature supports three generalizations about snake–primate interactions.
(i) Snakes have killed 2 species of tree shrews (17, 19); at least 6 species of strepsirrhines, including 3 species of lemurs (20–22), 2 species of galagos (23–25), and a slow loris (Nycticebus coucang) (26); and 20 species of nonhuman haplorhines, including a spectral tarsier (Tarsius spectrum) (27), 8 species of New World monkeys (13, 28–35), 10 species of Old World monkeys (17, 36–45), and a siamang (Hylobates syndactylus) (46). Primates have been ambushed as they descended from trees [e.g., boa constrictor (Boa constrictor) on a white-faced capuchin (Cebus capucinus)] (28), as they passed over water on vegetation [e.g., green anaconda (Eunectes murinus) on a black-chested mustached tamarin (Saguinus mystax)] (29), and from trailside or overhead in trees (reticulated pythons on humans); juveniles have been snatched from their mothers [Madagascan ground boa (Acrantophis madagascariensis) on Verraux's sifaka (Propithecus verreauxi)] (21) or eaten with them (reticulated python on long-tailed macaque) (44) as well as taken by foraging into shelters (reticulated pythons on humans). Although venomous snakes sometimes kill primates in defense (43), mangrove snakes (Boiga dendrophila) (19), black-necked spitting cobras (Naja nigricollis) (25), mambas (Dendroaspis) (24, 45), white-tailed lanceheads (Bothrops leucurus) (34), and Gaboon adders (Bitis gabonica) (45) occasionally consume tree shrews, strepsirrhines, and haplorhines.
No living serpents specialize on primates, but several species of constrictors regularly prey on them. Reticulated pythons frequently eat long-tailed macaques (Fig. 3) and silvered leaf monkeys (Trachopithecus cristatus) (17, 42) as well as lorises (26) and tarsiers (27). Northern (P. sebae) and southern (P. natalensis) African pythons eat diverse vertebrates, including galagos (25), chacma baboons (Papio hamadryas) (37), red colobus monkeys (Procolobus badius) (40), mona monkeys (Cercopithecus mona) (41), and vervets (Chlorocebus pygerythrus) (42). Multiple records exist of predation by Madagascan ground and tree (Sanzinia madagascariensis) boas on lemurs (20–22), and New World boa constrictors have attacked white-tailed titis (Callicebus discolor) (13), black-chested mustached tamarins (29, 31), white-eared marmosets (Callithrix aurita) (30), bearded sakis (Chiropotes satanus) (32), two species of capuchins (13, 28, 33), and lion tamarins (Leontopithecus rosalia) (35).
Fig. 3.
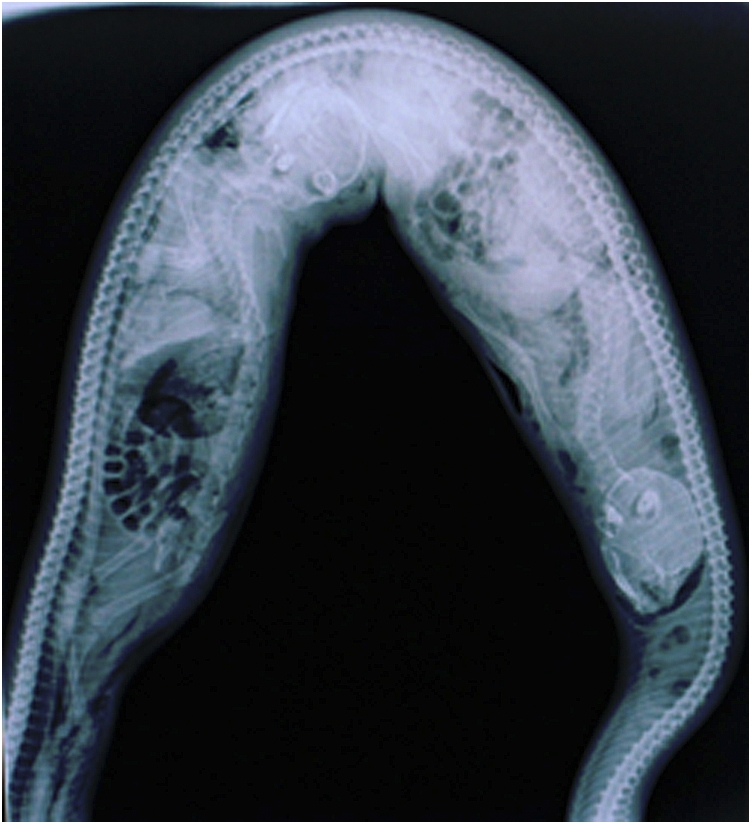
Radiograph of two juvenile long-tailed macaques, swallowed headfirst, inside the stomach of a reticulated python (TL = 2.24 m) in Singapore (photo by A. Devan-Song).
(ii) Snakes are potentially valuable prey after discovered, because they lack toxic flesh, often rely on crypsis and confrontation rather than locomotor escape, and are easily dispatched with simple weapons (1). Prehistoric Amerindians and modern humans have killed and eaten boas, pythons, and harmless colubrids as well as venomous elapids and viperids (12, 47–53). Nonhuman primates also dispatch relatively large venomous species, which was exemplified by a Bornean tarsier (Tarsius bacanus) that ate a long-glanded coral snake (Calliophis intestinalis) (14), a patas monkey (Erythrocebus patas) that killed a mamba (Dendroaspis sp.) (54), and white-faced capuchins that used branches to slay a terciopelo (Bothrops asper) (49). Black lemurs (Eulemur cacaco) and Coquerel's dwarf lemur (Mirza coquereli) mob Madagascan ground boas (55), and several species of haplorhines, including black-chested mustached tamarins, saddle-backed tamarins (Saguinus fuscicolis), white-eared marmosets, white-faced capuchins, vervets, and chimpanzees (Pan troglodytes), respond to snakes with curiosity, mobbing, group defense, social learning, and unease at the scene of fatal encounters (6, 30, 31, 49, 56–58).
(iii) Lorises (59), tarsiers (60), New World monkeys (61), and Old World monkeys (62) feed on diverse smaller animals, including small vertebrates. They, thus, broadly overlap many snakes in diet (1, 63) and are potential competitors.
Discussion
Our Agta data address the incidence of snake feeding attacks on humans, are among the few quantitative estimates of hazards confronting hunter–gatherers from any predators (3, 64, 65), and therefore are noteworthy in several respects.
(i) Herpetologists have long claimed that giant serpents eat humans only under exceptional circumstances (66), but indigenous people living where large boas and pythons occur (Africa, Asia and nearby archipelagos, Australia, and South America) are well within the prey size range of those species; an adult male Agta would constitute ≤60% of the mass of a large female P. reticulatus, which is not a heavy meal by snake standards (1, 63) and especially not for a species whose natural diet includes pigs weighing up to 60 kg (17). Plio-Pliestocene hominins before divergence of Homo erectus and reaching back to Australopithecus afarensis and Ardipithecus ramidus averaged 30–52 kg (67, 68) and thus, would have been comparably susceptible to giant snake predation.
(ii) Although T.N.H. could only directly confirm fatal attacks on two unarmed children, his sample total of 24 unsuccessful and fatal attacks amounts to one traumatic python incident every 2–3 y. Furthermore, most unsuccessful attacks resulted in bites, and therefore, fatalities would have been more common in the absence of iron weapons, which was the case until a few centuries ago. If the incidence of fatalities had approached the incidence of contemporary unsuccessful attacks, Agta deaths from python predation might have exceeded the 4% of overall mortality that Ecuadorian Waorani sustained from venomous snakebites (64) and 8% that Paraguayan Aché (65) incurred from jaguars, both also groups of forest dwelling foragers.
(iii) Granting that mid-20th century Agta were in no general sense primitive, our data quantify a high potential for snake predation on people similar in size and hunter–gatherer lifestyle to prehistoric hominins. The threat of being killed by a python must have significantly influenced the lives of precontact Agta, and because they learned during butchering that those snakes also eat deer, pigs, and macaques, they would have accurately regarded pythons as ecological competitors.
(iv) The rural Asian dataset undoubtedly suffers from sampling bias and is not statistically comparable with the survey by T.N.H., but the two are similar in terms of snake predatory behavior, attack sites on victims, and means by which humans escape python attacks. The higher number of fatalities in the Asian dataset likely reflects unreported escapes from python attacks, and perhaps women are more often alone when attacked and/or unarmed, thus less likely to survive.
(v) Because some living members of most primate lineages eat snakes and are killed by them, Greene (ref. 1, p. 293) proposed that an ancient heritage of reciprocal predator–prey relationships influenced our polarized ambivalence to these reptiles. Others have argued that vigilance and defense against predators, including giant snakes, played important, even dominant, roles in human evolution (2, 3, 6). Observations summarized here show that Agta hunter–gatherers had dangerous ecological relationships with giant serpents as well as document nonhuman primate predation on snakes and vice versa in core lineages of each group. To the extent that omnivorous early primates ate vertebrates, as with the Agta, they would have overlapped the diets of and potentially competed with sympatric snakes.
Placed in a phylogenetic context (Methods), these findings corroborate the view that snakes have had significant relationships with primates, including hominins, throughout their shared evolutionary history—the early diversification of serpents that could have eaten primates antedates the latter's origin by ∼20 myr—and all core primate lineages are eaten by and eat or persecute diverse constricting and venomous snakes. Our results also emphasize a central role for the natural history of hunter–gatherers, their nonhuman relatives, and their potential predators and competitors in the emerging synthesis of anthropology and biology (3, 6, 69–72).
Methods
In 1976, T.N.H., who by then, spoke fluent Agta, used standard ethnographic techniques to interview 120 adult Negritos. The 58 men and 62 women ranged in age from 16 to 75 y (mean = 38.6 y, SD ± 12.9), and therefore, the survey encompassed approximately seven decades of memories. Because of the potential human tendency to exaggerate violent incidents (ref. 73, p. 103), each python fatality was corroborated by multiple informants during separate interviews, always in answer to questions like “What did your uncle die from?” rather than “Did a python kill your uncle?” When in subsequent questioning, T.N.H. asked, “Do you know anyone who has been killed by a python?,” the same six Agta names were repeatedly mentioned. In 1976, T.N.H. interviewed the parents of the two children killed by a python and questioned the surviving sibling in 2000 as well as A. Rieger, a US Catholic priest who officiated at the burial and confirmed that the deceased children bore numerous bite marks. Conversely, among hundreds of Agta in the demographic sample (16) who were asked about family members’ deaths, none alluded to three other violent potential causes—venomous snakebite, crocodile predation, and murder by Japanese soldiers in World War II.
We refer to 20 case histories in a chronology of reticulated python attacks in Indonesia and Sarawak (12) as pertaining to rural Asians, because although some of the victims were forest dwellers, none were small group hunter–gatherers like the Agta. We discarded case number 15 as unsubstantiated, and for the other 19 cases, we extracted, whenever possible, information on snake length, sex of victim, outcome (including in the case of survival, where the victim was struck and how escape was facilitated), mode of attack as ambush or active foraging, and attack site as terrestrial or arboreal.
To address the evolutionary chronicle of primate–snake interactions, we combined ethnographic and natural history data in the context of three predictions. (i) Although ecological interactions themselves are not heritable (74), diet preferences are to a varying extent (75), and therefore, if ancient snakes ate ancestral primates, then living members of core primate lineages should occur in the diets of serpentine lineages that are at least as old as those mammals. (ii) If ancestral primates ate and otherwise negatively affected snakes, living members of core lineages should as well. (iii) If ancestral primates and snakes were potential food competitors, at least some living members of their core lineages should exhibit diet overlap. Extensive failure to confirm these predictions would falsify scenarios of prominent roles for snakes in primate evolution.
In terms of evolutionary history, Dermoptera (flying lemurs) and Scandentia (tree shrews) are the successive living sister lineages of primates, the latter having diverged ∼80 mya into strepsirrhines (galagos, lemurs, and lorises) and haplorhines. Among haplorhines, ∼70 mya, anthropoids split from tarsiers, and ∼45 mya, they diverged into platyrrhine (New World monkeys) and catarrhine lineages; ∼30 mya, the latter split into cercopithecoids (Old World monkeys) and hominoids (gibbons and apes), with hominins diverging from the chimpanzee and bonobo lineage ≥7 mya (76, 77).
Tropidophiidae (dwarf boas), Boidae (boas and anacondas), and Pythonidae (pythons) are the most basal macrostomatan snakes with prey size ranges that encompass at least some fossil and living primates [e.g., the largest living tropidophiid (Tropidophis melanurus) eats birds and rodents larger than the smallest primates] (9, 78). Those lineages originated ∼100 mya, whereas highly venomous Viperidae (vipers) and Elapidae (cobras and relatives) diversified ∼35 mya (79); Miocene and Pliocene fossils of large pythons, cobras, and Gaboon adder relatives (e.g., Bitis olduvaiensis) confirm that a diverse and dangerous serpent fauna was in place at the onset of hominin evolution (80).
Acknowledgments
T.N.H. thanks SIL International and the L. S. B. Leakey Foundation for support, J. Headland for assistance with the fieldwork, and B. Griffin and A. Rieger for corroborating his observations. We thank D. G. Barker, S. T. Emlen, S. B. Hrdy, T. C. LaDuke, J. B. Losos, W. C. McGrew, D. Rodriguez, F. de Waal, D. B. Wake, T. A. Wake, G. T. Woodward, and K. R. Zamudio for feedback. We also thank T. W. Wright and a Lichen Foundation grant (to H.W.G.) for facilitating our collaboration.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Author Summary on page 20865.
References
- 1.Greene HW. Snakes: The Evolution of Mystery in Nature. Berkeley, CA: University of California Press; 1997. [Google Scholar]
- 2.Öhman A, Mineka S. The malicious serpent: Snakes as a prototypical stimulus for an evolved module of fear. Curr Dir Psychol Sci. 2003;12:5–9. [Google Scholar]
- 3.Hart D, Sussman RW. Man the Hunted: Primates, Predators, and Human Evolution. New York: Westview Press; 2005. [Google Scholar]
- 4.Burghardt GM, Murphy JB, Chiszar D, Hutchins M. In: Snakes: Ecology and Conservation. Mullin SJ, Seigel RA, editors. Ithaca, NY: Cornell University Press; 2009. pp. 262–280. [Google Scholar]
- 5.Emile N, Barros M. Recognition of a 3D snake model and its 2D photographic image by captive black tufted-ear marmosets (Callithrix penicillata) Anim Cogn. 2009;12:725–732. doi: 10.1007/s10071-009-0234-z. [DOI] [PubMed] [Google Scholar]
- 6.Isbell LA. The Fruit, the Tree, and the Serpent: Why We See so Well. Cambridge, MA: Harvard University Press; 2009. [Google Scholar]
- 7.Charlesworth JH. The Good and Evil Serpent: How a Universal Symbol Became Christianized. New Haven, CT: Yale University Press; 2010. [Google Scholar]
- 8.Njau JK, Blumenschine RJ. Crocodylian and mammalian carnivore feeding traces on hominid fossils from FLK 22 and FLK NN 3, Plio-Pleistocene, Olduvai Gorge, Tanzania. J Hum Evol. 2011 doi: 10.1016/j.jhevol.2011.05.008. in press. [DOI] [PubMed] [Google Scholar]
- 9.Greene HW. Dietary correlates of the origin and radiation of snakes. Am Zool. 1983;23:431–441. [Google Scholar]
- 10.Wilson JA, Mohabey DM, Peters SE, Head JJ. Predation upon hatchling dinosaurs by a new snake from the late Cretaceous of India. PLoS Biol. 2010;8:e1000322. doi: 10.1371/journal.pbio.1000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warrell DA. In: Venomous Reptiles of the Western Hemisphere. Campbell JA, Lamar WW, editors. Ithaca, NY: Cornell University Press; 2004. pp. 709–761. [Google Scholar]
- 12.De Lang R. The reticulated python (Broghammerus reticulatus) and man (Homo sapiens) eat each other: Animals, enjoy your meal! Litteratura Serpentium. 2010;30:254–269. [Google Scholar]
- 13.Ferrari SF. In: South American Primates: Comparative Perspectives in the Study of Behavior, Ecology, and Conservation. Garber PA, Estrada A, Bicca-Marques JC, Heymann EK, Strier KB, editors. New York: Springer; 2009. pp. 251–277. [Google Scholar]
- 14.Niemitz C. Tarsius bancanus (Horsfield's Tarsier) preying on snakes. Laboratory Primates Newsletter. 1973;12:18–19. [Google Scholar]
- 15.Headland TN. In: What Place for Hunter-Gatherers in Millennium Three? Headland TN, Blood DE, editors. Dallas: SIL International and International Museum of Cultures; 2002. pp. 25–39. [Google Scholar]
- 16.Headland TN, Headland JD, Uehara RT. Agta Demographic Database: Chronicle of a Hunter-Gatherer Community in Transition, Version 2.0. SIL Language and Culture Documentation and Description. 2011. Available at http://www.sil.org/silepubs/abstract.asp?id=49227. Accessed November 19, 2011.
- 17.Shine R, Harlow PS, Keogh JS, Boeadi NI. The influence of sex and body size on food habits of a giant tropical snake, Python reticulatus. Funct Ecol. 1998;12:248–258. [Google Scholar]
- 18.Headland TN. Ecosytemic change in a Philippine tropical rain forest and its effect on a Negrito foraging society. Trop Ecol. 1988;29:121–135. [Google Scholar]
- 19.Munshi-South J. Boiga dendrophila (Mangrove Snake) Diet Herpetological Rev. 2005;36:188. [Google Scholar]
- 20.Goodman SM, O'Connor S, Langrand O. In: Lemur Social Systems and Their Ecological Basis. Kappeler M, Ganzhorn JU, editors. New York: Plenum; 1993. pp. 51–66. [Google Scholar]
- 21.Burney DA. Sifaka predation by a large boa. Folia Primatol (Basel) 2002;73:144–145. doi: 10.1159/000064793. [DOI] [PubMed] [Google Scholar]
- 22.Gould L, Sauther ML. In: Primate Anti-Predator Strategies. Gursky S, Neckaris KAI, editors. New York: Springer; 2007. pp. 275–288. [Google Scholar]
- 23.Jones C. Notes on ecological relationships of four species of lorisids in Rio Muni, West Africa. Folia Primatol (Basel) 1969;11:255–267. doi: 10.1159/000155274. [DOI] [PubMed] [Google Scholar]
- 24.Isbell LA. Snakes as agents of evolutionary change in primate brains. J Hum Evol. 2006;51:1–35. doi: 10.1016/j.jhevol.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 25.Bearder SK. In: Primate Anti-Predator Strategies. Gursky S, Neckaris KAI, editors. New York: Springer; 2007. pp. 206–221. [Google Scholar]
- 26.Wiens F, Zitzmann A. Predation on a wild slow loris (Nycticebus coucang) by a reticulated python (Python reticulatus) Folia Primatol (Basel) 1999;70:362–364. doi: 10.1159/000021719. [DOI] [PubMed] [Google Scholar]
- 27.Gursky S. Predation on a wild spectral tarsier (Tarsius spectrum) by a snake. Folia Primatol (Basel) 2002;73:60–62. doi: 10.1159/000060422. [DOI] [PubMed] [Google Scholar]
- 28.Chapman CA. Boa constrictor predation and group response in white-faced cebus monkeys. Biotropica. 1986;18:171–172. [Google Scholar]
- 29.Heymann EW. A field observation of predation on a mustached tamarin (Saguinus mystax) by an anaconda. Int J Primatol. 1987;8:193–195. [Google Scholar]
- 30.Corrêa HKM, Coutinho PEG. Fatal attack of a pit viper, Bothrops jararaca, on an infant buffy-tufted ear marmoset (Callithrix aurita) Primates. 1997;38:215–217. [Google Scholar]
- 31.Tello NS, Huck M, Heymann EW. Boa constrictor attack and successful group defence in moustached tamarins, Saguinus mystax. Folia Primatol (Basel) 2002;73:146–148. doi: 10.1159/000064795. [DOI] [PubMed] [Google Scholar]
- 32.Ferrari SF, Pereira WLA, Santos RR, Veiga LM. Fatal attack of a Boa constrictor on a bearded saki (Chiropotes satanas utahicki) Folia Primatol (Basel) 2004;75:111–113. doi: 10.1159/000076272. [DOI] [PubMed] [Google Scholar]
- 33.Perry S, Manson JH, Dower G, Wikberg E. White-faced Capuchins cooperate to rescue a groupmate from a boa constrictor. Folia Primatol (Basel) 2003;74:109–111. doi: 10.1159/000070008. [DOI] [PubMed] [Google Scholar]
- 34.Ferrari SF, Beltrão-Mendes R. Do snakes represent the principal predatory threat to callitrichids? Fatal attack of a viper (Bothrops leucurus) on a common marmoset (Callithrix jacchus) in the Atlantic Forest of the Brazilian Northeast. Primates. 2011;52:207–209. doi: 10.1007/s10329-011-0260-8. [DOI] [PubMed] [Google Scholar]
- 35.Kierulff MC, et al. In: Lion Tamarins: Biology and Conservation. Keiman DG, Rylands AB, editors. Washington DC: Smithsonian Institution Press; 2002. pp. 157–187. [Google Scholar]
- 36.Wall F. Ophidia Taprobanica or the Snakes of Ceylon. Colombo, Sri Lanka: Government Printer; 1921. [Google Scholar]
- 37.Pienar UP. Predator-prey relationships among the larger mammals of the Kruger National Park. Koedoe. 1969;12:108–176. [Google Scholar]
- 38.van Schaik CP, van Noordwijk MA, Warsong B, Sutriono E. Party size and early detection of predators in Sumatran forest primates. Primates. 1983;24:211–221. [Google Scholar]
- 39.Chism J, Rowell TE, Olson DK. In: Female Primates: Studies by Women Primatologists. Small MD, editor. New York: Liss; 1984. pp. 175–190. [Google Scholar]
- 40.Starin ED, Burghardt GM. African rock pythons (Python sebae) in The Gambia: Observations on natural history and interactions with primates. The Snake. 1992;24:50–62. [Google Scholar]
- 41.Luiselli L, Angelici FM, Akani GC. Food habits of Python sebae in suburban and natural habitats. Afr J Ecol. 2001;19:116–118. [Google Scholar]
- 42.Grindley J. Python constricts a vervet monkey. CCA Ecol J. 2003;5:270. [Google Scholar]
- 43.Barrett L, Gaynor DD, Rendall D, Mitchell D, Henzi SP. Habitual cave use and thermoregulation in chacma baboons (Papio hamadryas ursinus) J Hum Evol. 2004;46:215–222. doi: 10.1016/j.jhevol.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 44.Auliya MA. Taxonomy, Life History and Conservation of Giant Reptiles in West Kalimantan. Berlin: Natur und Tier Verlag; 2006. [Google Scholar]
- 45.Foerster S. Two incidents of venomous snakebite on juvenile blue and Sykes monkeys (Cercopithecus mitis stuhlmanni and C. m. albogularis) Primates. 2008;49:300–303. doi: 10.1007/s10329-008-0098-x. [DOI] [PubMed] [Google Scholar]
- 46.Schneider G. Ergebnisse zoologischer Forschungsresien in Sumatra. Zool Jahrb Abt Syst Geogr Biol Tiere. 1906;23:1–172. [Google Scholar]
- 47.Klauber LM. Rattlesnakes: Their Habits, Life Histories, and Influence on Mankind. Berkeley, CA: University of California Press; 1956. [Google Scholar]
- 48.Neil WT, Gut HJ, Brodkorb P. Animal remains from four preceramic sites in Florida. Am Antiq. 1956;21:383–395. [Google Scholar]
- 49.Boinski S. Use of a club by a wild white-faced capuchin (Cebus capucinus) to attack a venomous snake. Am J Primatol. 1988;14:177–179. doi: 10.1002/ajp.1350140208. [DOI] [PubMed] [Google Scholar]
- 50.Lee JC. The Amphibians and Reptiles of the Yucatan Peninsula. Ithaca, NY: Cornell University Press; 1996. [Google Scholar]
- 51.Murphy JR, Henderson RW. Tales of Giant Snakes: A Natural History of Anacondas and Pythons. Malabar, FL: Krieger Publishing Co.; 1997. [Google Scholar]
- 52.Voorhies BD, Kennett J, Jones JG, Wake TA. A Middle Archaic archaeological site on the West Coast of Mexico. Latin Am Antiq. 2002;13:179–200. [Google Scholar]
- 53.Wake TA. In: Maya Zooarchaeology: New Directions in Methods and Theory. Emery KF, editor. Los Angeles: University of California, Los Angeles Insitute of Archaeology Publications; 2004. pp. 209–222. [Google Scholar]
- 54.Galat-Luong A. Proies inhabituelles pour le patas d'Afrique de l'ouest (Erythrocebus patas patas) Rev Ecol. 1991;46:83–84. [Google Scholar]
- 55.Colquhuon IC. In: Lemur Social Systems and Their Ecological Basis. Kappeler PM, Ganzhorn JU, editors. New York: Plenum; 1993. pp. 11–23. [Google Scholar]
- 56.Goodall J. The Chimpanzees of Gombe. Cambridge, MA: Harvard University Press; 1986. [Google Scholar]
- 57.Bartecki U, Heymann EW. Field observation of snake-mobbing in a group of saddle-back tamarins, Saguinus fuscicollis nigrifrons. Folia Primatol (Basel) 1987;48:199–202. doi: 10.1159/000156296. [DOI] [PubMed] [Google Scholar]
- 58.Cheney DL, Seyfarth RM. How Monkeys See the World: Inside the Mind of Another Species. Chicago: University of Chicago Press; 1992. [Google Scholar]
- 59.Nekaris KAI. Foraging behaviour of the slender loris (Loris lydekkerianus lydekkerianus): Implications for theories of primate origins. J Hum Evol. 2005;49:289–300. doi: 10.1016/j.jhevol.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 60.Niemitz C. The Biology of Tarsiers. New York: Gustav Fischer Verlag; 1984. [Google Scholar]
- 61.Rose LM, et al. Interspecific interactions between Cebus capucinus and other species: Data from three Costa Rican sites. Int J Primatol. 2003;24:759–796. [Google Scholar]
- 62.Gans C. Empathic learning and the mimicry of African snakes. Evolution. 1965;18:705. [Google Scholar]
- 63.Rodríguez-Robles JA, Bell CJ, Greene HW. Gape size and evolution of diet in snakes: Feeding ecology of erycine boas. J Zool. 1999;248:49–58. [Google Scholar]
- 64.Larrick JW, Yost JA, Kaplan J. Snake bite among the Waorani Indians of eastern Ecuador. Trans R Soc Trop Med Hyg. 1978;72:542–543. doi: 10.1016/0035-9203(78)90184-0. [DOI] [PubMed] [Google Scholar]
- 65.Hill K, Hurtado AM. Aché Life History: The Ecology and Demography of a Foraging People. New York: Aldine de Gruyter; 1996. [Google Scholar]
- 66.Pope CH. The Giant Snakes. New York: Alfred A. Knopf; 1961. [Google Scholar]
- 67.McHenry HM. Behavioral ecological implications of early hominid body size. J Hum Evol. 1994;27:77–87. [Google Scholar]
- 68.White TD, et al. Ardipithecus ramidus and the paleobiology of early hominids. Science. 2009;326:75–86. [PubMed] [Google Scholar]
- 69.Lee-Thorp J, Thackeray JF, van der Merwe N. The hunters and the hunted revisited. J Hum Evol. 2000;39:565–576. doi: 10.1006/jhev.2000.0436. [DOI] [PubMed] [Google Scholar]
- 70.McGrew WC, Foley RA. Palaeoanthropology meets primatology. J Hum Evol. 2009;57:335–336. doi: 10.1016/j.jhevol.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 71.Hill KR, et al. Co-residence patterns in hunter-gatherer societies show unique human social structure. Science. 2011;331:1286–1289. doi: 10.1126/science.1199071. [DOI] [PubMed] [Google Scholar]
- 72.Avise JC, Ayala FJ. Colloquium paper: In the light of evolution IV: The human condition. Proc Natl Acad Sci USA. 2010;107(Suppl 2):8897–8901. doi: 10.1073/pnas.1003214107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Early JD, Headland TN. Population Dynamics of a Philippine Rain Forest People. Gainesville, FL: University Press of Florida; 1998. [Google Scholar]
- 74.Grandcolas P, Nattier R, Legendre F, Pellens R. Mapping extrinsic traits such as extinction risk or modeled bioclimatic niches on phylogenies: Does it make sense at all? Cladistics. 2011;27:181–185. doi: 10.1111/j.1096-0031.2010.00324.x. [DOI] [PubMed] [Google Scholar]
- 75.Arnold SJ. In: Foraging Behavior: Ecological, Ethological, and Psychological Approaches. Kamil AC, Sargent TD, editors. New York: Garland STPM Press; 1981. pp. 409–453. [Google Scholar]
- 76.Wilkinson RD, et al. Dating primate divergences through an integrated analysis of palaeontological and molecular data. Syst Biol. 2011;60:16–31. doi: 10.1093/sysbio/syq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jameson NM, et al. Genomic data reject the hypothesis of a prosimian primate clade. J Hum Evol. 2011;61:295–305. doi: 10.1016/j.jhevol.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 78.Cundall D, Greene HW. In: Feeding: Form, Function, and Evolution in Tetrapod Vertebrates. Schwenk K, editor. San Diego: Academic; 2000. pp. 293–333. [Google Scholar]
- 79.Pyron RA, Burbrink FT. Extinction, ecological opportunity, and the origins of global snake diversity. Evolution. 2011 doi: 10.1111/j.1558-5646.2011.01437.x. in press. [DOI] [PubMed] [Google Scholar]
- 80.Rage JC, Bailon S. In: Paleontology and Geology of Laetoli: Human Evolution in Context. Harrison T, editor. New York: Springer; 2011. pp. 467–478. [Google Scholar]