Abstract
Some prion protein mutations create anchorless molecules that cause Gerstmann–Sträussler–Scheinker (GSS) disease. To model GSS, we generated transgenic mice expressing cellular prion protein (PrPC) lacking the glycosylphosphatidyl inositol (GPI) anchor, denoted PrP(ΔGPI). Mice overexpressing PrP(ΔGPI) developed a late-onset, spontaneous neurologic dysfunction characterized by widespread amyloid deposition in the brain and the presence of a short protease-resistant PrP fragment similar to those found in GSS patients. In Tg(PrP,ΔGPI) mice, disease onset could be accelerated either by inoculation with brain homogenate prepared from spontaneously ill animals or by coexpression of membrane-anchored, full-length PrPC. In contrast, coexpression of N-terminally truncated PrP(Δ23–88) did not affect disease progression. Remarkably, disease from ill Tg(PrP,ΔGPI) mice transmitted to mice expressing wild-type PrPC, indicating the spontaneous generation of prions.
Keywords: protein misfolding, amyloidosis, neurodegenerative disease, proteinopathies
Protein aggregation and amyloid formation are central pathologic events in a wide range of neurodegenerative illnesses, including Alzheimer's, Parkinson's, and prion diseases (1). Perhaps the best understood of these disorders are the prion diseases, in which the host-encoded, cellular prion protein (PrPC) undergoes structural isomerization to an infectious form, termed PrPSc (2). The process is autocatalytic, with PrPSc serving as a template for refolding of PrPC.
Though the requirement of PrPC in disease pathogenesis is known, the function of PrPC in nondiseased tissue remains enigmatic because Prnp0/0 mice exhibit only subtle or disputed phenotypic deficits, arguing that PrPC is largely dispensable for proper neuronal function (3). PrPC is a glycoprotein that is expressed predominantly in the central nervous system (CNS) and is attached to the external surface of the plasma membrane by a glycosylphosphatidyl inositol (GPI) anchor (4). Secreted forms of PrPC lacking a GPI anchor are generated in the brain by sheddase cleavage of membrane-anchored PrPC (5); the physiological role of such unanchored PrP isoforms, termed PrP(ΔGPI), remains unclear. In earlier studies, transgenic (Tg) mice expressing low levels of PrP(ΔGPI) did not develop signs of spontaneous neurologic illness, but harbored large amyloidogenic PrP(ΔGPI) aggregates in the brain after exposure to prions (6, 7). These mice were used to demonstrate that the anchor was not required for conversion of PrP into a protease-resistant isoform, a result first demonstrated in cell culture (8). Mutations in human PrP leading to C-terminally truncated polypeptides lacking the GPI signal sequence, which cause the inherited prion disorder Gerstmann–Sträussler–Scheinker (GSS) disease, are known to result in the production of secreted, highly amyloidogenic forms of PrP (9). Other mutations that do not lead to truncated PrP but cause GSS, such as the F198S mutation, result in the production of anchorless PrP upon expression in cell culture (10). Importantly, GSS patients with mutations in PrP that result in the production of nearly full-length anchorless PrPs (11) suggest that anchorless PrP might be a cause of GSS-like neuropathology and clinical disease.
To investigate the role of the GPI anchor in the biology of PrPC and its relation to GSS, we established several lines of Tg mice expressing various levels of PrP(ΔGPI). In agreement with earlier investigations (6), mice expressing low levels of PrP(ΔGPI) remained healthy and exhibited little or no neuropathologic changes. In contrast, neuronal expression of PrP(ΔGPI) at 1.7× levels compared with PrPC expression in wt mice resulted in late-onset, spontaneous neurologic illness accompanied by a profound CNS amyloidosis resembling GSS. This PrP amyloidosis was transmissible to mice of the same line and, in 75% of our transmission attempts, to mice expressing GPI-anchored PrPC. Tg(PrP,ΔGPI) mice were crossed with mice expressing wt PrP to generate Tg mice expressing PrP(ΔGPI) and wt PrPC; these mice showed hastened onset of neurologic illness in a PrPC dose-dependent manner. Neurologic illness was not hastened if Tg mice coexpressed PrP(ΔGPI) and N-terminally truncated PrP(Δ23–88), arguing that the N terminus has an important role in disease pathogenesis. These studies demonstrate that PrP(ΔGPI) spontaneously forms prions, and that membrane-bound wt PrPC modulates the kinetics of PrP(ΔGPI) amyloid formation.
Results
Production of Tg Mice Expressing PrP(ΔGPI).
Tg mice were generated as previously reported (SI Materials and Methods). Lines were developed from three founders with the highest copy numbers. PrP(ΔGPI) was expressed at levels between 0.3× and 1.7× compared with that of PrPC in wt FVB mice (Table 1). These expression levels were considerably higher than those in lines expressing an analogous construct reported previously by others (7). In agreement with previous results, we found that PrP(ΔGPI) in the brain was mostly unglycosylated with a minor fraction of monoglycosylated PrP(ΔGPI) as revealed by PNGaseF digestion (Fig. 1A).
Table 1.
Development of spontaneous disease in transgenic mice expressing PrP(ΔGPI) with or without coexpression of membrane-anchored PrP
| Expression level* (-fold) |
|||||
| Line | PrP(ΔGPI) | wt PrP | PrP (Δ23–88) | Time to disease onset (d)† | n/n0‡ |
| Tg(PrP,ΔGPI)8446/Prnp0/0 | 0.3 | — | — | >675 | 0/7 |
| Tg(PrP,ΔGPI)8423/Prnp0/0 | 0.3 | — | — | >600 | 0/8 |
| Tg(PrP,ΔGPI)8423/Prnp0/+ | 0.3 | 0.5 | — | >600 | 0/10 |
| Tg(PrP,ΔGPI:PrP)8423/4053 | 0.3 | 4 | — | >600 | 0/12 |
| Tg(PrP,ΔGPI)8015/Prnp0/0 | 1.7 | — | — | 597 ± 17 | 16/30 |
| Tg(PrP,ΔGPI)8015/Prnp0/+ | 1.7 | 0.5 | — | 211 ± 6 | 18/18 |
| Tg(PrP,ΔGPI)8015/Prnp+/+ | 1.7 | 1 | — | 154 ± 7 | 8/8 |
| Tg(PrP,ΔGPI:PrP)8015/4053 | 1.7 | 4 | — | 121 ± 3 | 18/18 |
| Tg(PrP,ΔGPI:PrP,Δ23–88)8015/9949 | 1.7 | — | 16 | 572 ± 24 | 4/9 |
| Prnp0/+ | — | 0.5 | — | >600 | 0/20 |
| Prnp+/+ | — | 1 | — | >600 | 0/11 |
| Tg(PrP)4053/Prnp0/0 | — | 4 | — | >600 | 0/20 |
| Tg(PrP,Δ23–88)9949/Prnp0/0§ | — | — | 16 | 617 ± 7 | 62/96 |
*Expression levels compared with PrPC in the brains of wt FVB mice.
†Mean ± SEM, for ill mice; this may be an underestimate when not all mice become ill.
‡n, number of ill mice; n0, number of monitored mice.
§These mice develop spontaneous neurological dysfunction that is nontransmissible and different from typical prion disease and the disease described in the present work. Data from ref. 27.
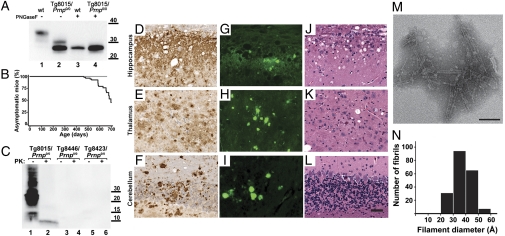
Fig. 1.
Characterization of Tg(PrP,ΔGPI) mice. (A) Western blot of brain homogenates prepared from wt FVB (lanes 1 and 3) or Tg8015/Prnp0/0 (lanes 2 and 4) mice with (+) or without (–) pretreatment with PNGaseF. PrP(ΔGPI) is predominantly expressed as mono- and unglycosylated forms (lanes 2 and 4) compared with wt PrPC, which is predominantly diglycosylated (lane 1). (B) Kaplan–Meier survival curves for three Tg(PrP,ΔGPI)/Prnp0/0 lines: Tg8015 (black line), Tg8423, and Tg8446 (gray line). (C) Western blot of insoluble PrP(ΔGPI), following ultracentrifugation at 100,000 × g, from the brains of ill Tg8015/Prnp0/0 mice (lanes 1 and 2), compared with healthy, age-matched Tg8446/Prnp0/0 (lanes 3 and 4) and Tg8423/Prnp0/0 (lanes 5 and 6) mice, with (+) or without (–) PK treatment (20 μg/mL for 1 h at 37 °C). For A and C, molecular masses are shown in kilodaltons. PrP was detected with Fab HuM-P. (D–L) Neuropathologic analysis of the hippocampus (Top), thalamus (Middle), and cerebellum (Bottom) from spontaneously ill Tg8015/Prnp0/0 mice. (D–F) Immunostaining with the anti-PrP antibody HuM-R2 showed large PrP deposits in all brain regions. (G–I) ThioS staining confirmed that many of these aggregates contain PrP(ΔGPI) amyloid. (J–L) H&E staining demonstrated some age-related vacuolation predominantly in the hippocampus. The mouse shown developed symptoms after 589 d. (Scale bar: D–L, 50 μm.) (M and N) Ultrastructural characterization of PrP(ΔGPI) from the brains of Tg8015/Prnp0/0 mice. (M) Electron micrograph of PrP amyloid purified from the brain of an ill Tg8015/Prnp0/0 mouse at 589 d shows fibrillar aggregates upon negative staining with 2% ammonium molybdate. (Scale bar: 100 nm.) (N) Diameter distribution of ∼200 individual fibrils viewed by negative-stain TEM, recorded at a magnification of 75,750×.
Mice from the three Tg lines were monitored for spontaneous neurologic disease. All of the Tg(PrP,ΔGPI)8423/Prnp0/0 and Tg(PrP,ΔGPI)8446/Prnp0/0 mice expressing PrP(ΔGPI) at ∼0.3× remained well for more than 600 d. In contrast, the Tg(PrP,ΔGPI)8015/Prnp0/0 line, hereafter referred to as Tg8015/Prnp0/0, expressing PrP(ΔGPI) at ∼1.7×, developed spontaneous disease beginning at ∼500 d of age (Table 1 and Fig. 1B). Older Tg8015/Prnp0/0 mice generally presented with ataxia, circling behavior, and proprioceptive deficits.
Spontaneously Ill Mice Accumulate Insoluble PK-Resistant PrP(ΔGPI).
Brain homogenates from spontaneously ill Tg8015/Prnp0/0 mice contained high levels of insoluble PrP collected in the pellet after ultracentrifugation at 100,000 × g for 1 h (Fig. 1C, lane 1). Following limited digestion with proteinase K (PK), all of the insoluble PrP was hydrolyzed except for a ∼10-kDa fragment (Fig. 1C, lane 2 and Fig. S1A).
We were unable to resolve the band with an anti-myc antibody (Fig. S1B), suggesting that the C terminus was truncated. We then used a panel of antibodies with epitopes directed against different regions of the PrP sequence. Of these antibodies, only HuM-P and 12B2 recognized the ∼10-kDa fragment in all Tg8015/Prnp0/0 mice (Fig. S1 C–G), suggesting that the ∼10-kDa fragment spans PrP residues ∼40 to ∼130. This part of the sequence precedes the glycosylation sites at residues 180 and 196, and the fragment is therefore unglycosylated.
In all reviewed samples of ill Tg8015/Prnp0/0 mice, we could detect the ∼10-kDa, PK-resistant fragment. In contrast, the two Tg lines expressing PrP(ΔGPI) at 0.3× showed neither signs of CNS dysfunction nor insoluble PrP (Fig. 1C, lanes 3 and 5); we were also unable to detect any PK-resistant PrP fragments (lanes 4 and 6).
To determine the relationship between the PK-resistant, ∼10-kDa band and the neuropathologic observations (amyloid staining and PrP deposition in the brain), we analyzed the brains of asymptomatic Tg8015/Prnp0/0 mice collected between ∼200 and 700 d (Table S1). The ∼10-kDa, PK-resistant PrP fragment was detected in 9 of 10 mice that were 300 d of age or older; the intensity of this protein fragment on immunoblots increased with age. Neuropathologic analysis of these mouse brains also revealed Thioflavin S (ThioS) staining and PrP deposits in mice older than 300 d. Similar to the ∼10-kDa PrP fragment on Western blots, older mice showed greater immunohistological staining compared with younger animals.
Characterization of PrP(ΔGPI) Amyloid.
Immunohistochemical analysis of the brains from spontaneously ill Tg8015/Prnp0/0 mice revealed the presence of abundant PrP deposits in all brain regions examined (Fig. 1 D–F). Many of these PrP deposits consisted of amyloid, as demonstrated by ThioS staining (Fig. 1 G–I). Vacuolation was always present in ill Tg8015/Prnp0/0 mice (Fig. 1 J–L), but it was similar to that found in age-matched FVB control mice, and thus could not be attributed to either the PrP(ΔGPI) expression or the amyloidosis. In asymptomatic aged Tg(PrP,ΔGPI)8423/Prnp0/0 mice, we observed sparse ThioS staining in the subcallosal region (Fig. S2), arguing that the amyloidosis observed in ill Tg8015/Prnp0/0 mice cannot be solely attributed to supraphysiologic levels of protein expression, and demonstrating the intrinsic property of PrP(ΔGPI) to aggregate into amyloid.
We enriched PrP(ΔGPI) amyloid by phosphotungstic acid (PTA) precipitation following limited digestion with PK. Biochemical analysis of the enriched samples (n = 7) showed substantial levels of the PK-resistant, insoluble, ∼10-kDa PrP fragment (Fig. S1A, lane 5). The samples were examined by transmission electron microscopy (TEM). Micrographs of the purified PrP aggregates revealed a fibrillar morphology with no discernable helical periodicity (Fig. 1M). The fibers were reminiscent of the rods found in purified preparations of prions but had a significantly smaller diameter (36 ± 7 Å; Fig. 1N) compared with fibrils obtained from FVB mice infected with the Rocky Mountain Laboratory (RML) prion strain (48 ± 8 Å, P < 0.001) (12). The extent to which the smaller diameter of these fibrils reflects the absence of the GPI anchor and glycosylation as well as the truncation of the polypeptide chain remains to be established.
Spontaneous Generation of Anchorless Prions in Tg8015/Prnp0/0 Mice.
To determine whether Tg8015/Prnp0/0 mice spontaneously generate prions, we prepared brain homogenates from ill Tg8015/Prnp0/0 mice and injected them intracerebrally into weanling Tg8015/Prnp0/0 mice or Tg(PrP)4053 mice overexpressing wt mouse PrP.
Brain homogenates from two Tg8015/Prnp0/0 mice, spontaneously ill at 585 d, both transmitted to all Tg8015/Prnp0/0 mice with mean incubation periods of 202 d and 205 d, respectively (Table 2). All 16 mice exhibited the ∼10-kDa, PK-resistant fragment (Fig. 2A), and all six mice examined neuropathologically showed extensive amyloid deposition and vacuolation (Fig. 2 E, I, and M), similar to the considerably older mice from which the two inocula were prepared (Fig. 1). Two additional brains from Tg8015/Prnp0/0 mice that became spontaneously ill at 521 d and 659 d transmitted to Tg8015/Prnp0/0 mice within 169 ± 4 d and 275 ± 16 d, respectively (Table 2). However, these inocula resulted in slightly different biochemical phenotypes. Brains from these mice contained the ∼10-kDa, PK-resistant fragment, in addition to a ∼17- to 19-kDa fragment, with extensive PrP deposition that was also identified as amyloid; representative brains from Tg8015/Prnp0/0 mice ill at 275 d after injection with the 659-d-old inoculum are shown in Fig. 2 B, F, J, and N.
Table 2.
Transmission of anchorless amyloids from spontaneously ill Tg8015/Prnp0/0 mice into Tg8015/Prnp0/0 and Tg4053 mice
| Inoculum |
Host |
|||
| Tg(PrP,ΔGPI)8015/Prnp0/0 |
Tg(PrP,ΔGPI)8015/Prnp0/0 |
Tg(PrP)4053 |
||
| Age of spontaneous disease onset, d | Incubation time, d* | n/n0† | Incubation time, d* | n/n0† |
| 469‡ | n.d. | — | 107 ± 6 | 10/18 |
| 521 | 169 ± 4 | 7/7 | 99, 109 (>308) | 2/7§ |
| 585 | 202 ± 2 | 8/8 | 450 ± 23 | 7/7 |
| 585 | 205 ± 3 | 23/23 | >540 | 0/8 |
| 630 | n.d. | — | 83 ± 3 | 7/7 |
| 659 | 275 ± 16 | 8/8 | 80 ± 2 | 7/8 |
| 668 | n.d. | — | >509 | 0/6 |
| 697 | n.d. | — | 167 (>252) | 1/6§ |
*Mean ± SEM, for ill mice; when fewer than half the mice became ill, individual incubation periods are given in italics. n.d., not determined.
†n, number of ill mice; n0, number of inoculated mice.
‡Brain sample collected before showing clinical signs of disease.
§Ongoing experiment.
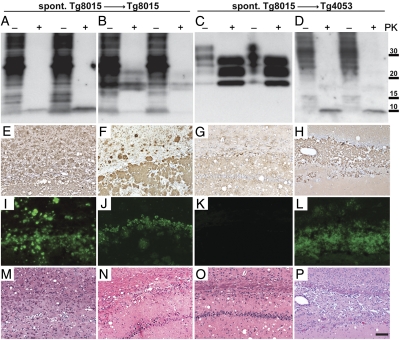
Fig. 2.
Characterization of spontaneously generated PrP(ΔGPI) prions upon passage in Tg8015/Prnp0/0 and Tg4053 mice. Different phenotypes were observed biochemically following parallel transmission of spontaneously generated prions into both Tg8015/Prnp0/0 and Tg4053 mice. (A–D; two examples shown in each case) Brain homogenates from spontaneously ill Tg8015/Prnp0/0 mice transmitted completely to Tg8015/Prnp0/0 mice within ∼200–300 d. We observed two different phenotypes: brains of two mice contained only the ∼10-kDa, PK-resistant band (A), whereas transmissions from two other brains resulted in a mixture of ∼10-kDa and 17- to 19-kDa PK-resistant fragments (B). In parallel experiments, we inoculated the same brain homogenates from Tg8015/Prnp0/0 mice into Tg4053 mice (C and D). Those brains that contained PK-resistant PrP of 10 kDa and 17–19 kDa upon inoculation to Tg8015/Prnp0/0 (B) transmitted efficiently to Tg4053 mice and resulted in a typical three-band PrPSc pattern (C). Conversely, a brain homogenate that resulted in only the ∼10-kDa fragment in Tg8015/Prnp0/0 mice (A) also resulted in only the ∼10-kDa fragment in Tg4053 mice (D) following long incubation periods. Samples were left undigested (–) or digested with PK (+). Blots were probed with the HuM-P antibody. Molecular masses of protein standards are shown in kilodaltons. (E–P) Neuropathologic analysis of ill Tg8015/Prnp0/0 (E, F, I, J, M, and N) and Tg4053 mice (G, H, K, L, O, and P); representatives of the biochemical phenotype shown at the top of each column. Micrographs of brain sections were taken from the hippocampus, stained for PrP with the HuM-R2 antibody (E–H), for amyloid by ThioS (I–L), and for vacuolation by H&E (M–P). Mice harboring the 10-kDa, protease-resistant PrP fragment showed extensive PrP deposition (E, F, and H) that stained as amyloid (I, J, and L). Amyloid staining was less abundant in the Tg8015/Prnp0/0 mice that showed both the 10-kDa fragment and a band at 17–19 kDa (J). In the Tg4053 mice that did not harbor the 10-kDa fragment but showed the three-band PrP pattern upon treatment with PK, PrP deposition was apparent (G) but was not amyloid (K). All ill mice showed vacuolation (M–P). These results suggest that at least two different strains were spontaneously generated. (Scale bars: E–P, 100 μm.)
Next, we asked whether these anchorless prions could also transmit to mice expressing wt membrane-anchored PrPC. Brain homogenates from eight Tg8015/Prnp0/0 mice were inoculated into Tg4053 mice. Transmissions were observed with six of eight inocula (Table 2); however, transmission efficiency and incubation time varied widely. Two inocula produced rapid transmissions with mice developing disease in ∼80 d. A third inoculum transmitted disease to all seven inoculated mice with a mean incubation period of 450 d. Three other brain homogenates led to incomplete transmissions with mice exhibiting neurologic dysfunction at ∼100 d.
Western blotting for PrP in the brains of 16 Tg4053 mice ill at ∼100 d postinoculation from five different inocula showed a three-band pattern of un-, mono-, and diglycosylated PK-resistant PrP (Fig. 2C). Six ill mice exhibited PrP deposition and vacuolation in their brains but did not show any PrP amyloid plaques (Fig. 2 G, K, and O). In contrast, Tg4053 mice that developed illness 450 d after inoculation with a 585-d-old Tg8015 /Prnp0/0 mouse brain showed the ∼10-kDa, PK-resistant PrP fragment (Fig. 2D) and abundant amyloid deposition (Fig. 2 H and L). The subcallosal region was filled with amyloid, which thinned the corpus callosum and led to severe nerve cell loss in the hippocampal CA1 region (Fig. 2P).
We serially passaged brain homogenates from four ill Tg4053 mice into Tg4053 mice (Table S2). All four inocula produced disease in Tg4053 mice within 60 d. Three mice from each inoculum were examined biochemically and showed similar banding patterns as well as similar conformational stability (GdnHCl1/2 values of 1.4–1.8 M). In one mouse, PK-resistant PrP had a different GdnHCl1/2 stability of 3.4 M (Table S2).
Coexpression of PrP(ΔGPI) and wt PrPC Hastens Disease.
To measure the influence of coexpression of wt PrPC, Tg8015/Prnp0/0 and Tg8423/Prnp0/0 mice were crossed with FVB and Tg4053 mice (Table 1). Western blotting confirmed that expression of PrPC was not affected by coexpression of anchorless PrP compared with their founder lines (Fig. 3A). Similarly, expression levels of PrP(ΔGPI), determined using an anti-myc tag antibody, were unaffected by coexpression of wt GPI-anchored PrP (Fig. 3A).
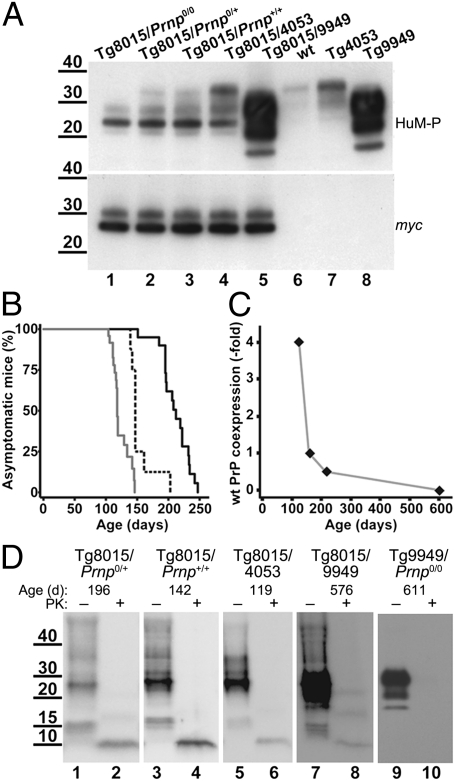
Fig. 3.
Characterization of Tg8015 mice coexpressing wt PrP. (A) Analysis of total PrP levels by Western blot of brain homogenates obtained from Tg mice expressing only PrP(ΔGPI) (lane 1) or coexpressing different levels of wt PrPC (lanes 2–4) or N-terminally truncated PrP (lane 5). PrP expression levels of Tg mouse lines that were used to create the coexpressors are shown as controls (lanes 6–8). (Upper) Probed using the anti-PrP Fab HuM-P, shows the total PrP content; (Lower) detected using anti-myc tag antibody, shows only PrP(ΔGPI) expression. Neither wt PrP nor PrP(ΔGPI) expression level was affected by coexpression. (B) Kaplan–Meier survival curves of three different mouse lines coexpressing PrP(ΔGPI) at 1.7× and PrPC at various levels: Tg8015/Prnp0/+ (0.5× wt PrP; solid black line), Tg8015/Prnp+/+ (1× wt PrP; dashed black line), and Tg8015/4053 (4× wt PrP; solid gray line). (C) Plot of the relationship between wt PrPC coexpression level and age of onset of spontaneous disease. (D) Western blots of insoluble PrP isolated from brain homogenates of ill Tg8015/Prnp0/+ (lanes 1 and 2), Tg8015/Prnp+/+ (lanes 3 and 4), Tg8015/4053 (lanes 5 and 6), Tg8015/9949 (lanes 7 and 8), and Tg9949/Prnp0/0 (lanes 9 and 10) mice before (–) and after (+) PK digestion. The age of mice when clinical symptoms manifested is indicated above each panel. A ∼10-kDa, PK-resistant PrP fragment is apparent in the brains of all mice expressing PrP(ΔGPI). PrP was detected with the Fab HuM-P. In A and D, molecular masses are shown in kilodaltons.
Coexpression of wt PrPC and PrP(ΔGPI) hastened the onset of spontaneous disease in mice. We observed an inverse relationship between the level of wt PrPC expression and the age at which CNS dysfunction manifested (Table 1 and Fig. 3 B and C). The coexpression of wt PrPC and PrP(ΔGPI) in Tg8015/Prnp0/+ mice reduced the mean age of disease onset from ∼600 d to 211 d. Doubling the expression of wt PrPC in Tg8015/Prnp+/+ mice reduced the age of onset to 154 d; another fourfold increase in the expression of wt PrPC reduced the age of onset to 121 d (Table 1 and Fig. 3B). All spontaneously ill mice coexpressing wt PrPC and PrP(ΔGPI) showed an insoluble, PK-resistant, ∼10-kDa PrP fragment (Fig. 3D and Table S3), which by epitope mapping was indistinguishable from the fragment found in Tg8015/Prnp0/0 mice with spontaneous disease (Fig. S3). In contrast, coexpression of wt PrPC in Tg mice expressing low levels of PrP(ΔGPI) did not result in spontaneous disease. Tg(PrP,ΔGPI)8423/Prnp0/+ and Tg(PrP,ΔGPI)8423/4053 mice remained well for more than 600 d (Table 1).
To inquire if N-terminally truncated PrP(Δ23–88) could also hasten disease in Tg mice expressing high levels of PrP(ΔGPI), we established Tg(PrP,ΔGPI:PrP,Δ23–88)8015/9949 mice. These Tg8015/9949 mice developed CNS dysfunction at 572 d, which was similar to that of Tg8015/Prnp0/0 mice at 597 d (Table 1). Even though the level of PrP(Δ23–88) expression in Tg(PrP,Δ23–88)9949/Prnp0/0 mice is 16-fold higher than that of wt PrPC in FVB mice, this truncated PrP was unable to hasten disease. The result argues that PrP residues 23–88 or some subset thereof are required for acceleration of neurologic dysfunction in Tg(PrP,ΔGPI) mice. Western blotting of brain homogenates from spontaneously ill Tg8015/9949 mice showed a PK-resistant, ∼10-kDa PrP fragment (Fig. 3D, lane 8) in contrast to spontaneously ill Tg9949 mice that exhibited no PK-resistant PrP (Fig. 3D, lanes 9 and 10).
Neuropathologic Analysis of Mice Coexpressing PrP(ΔGPI) and wt PrPC.
In a Tg(PrP,ΔGPI:PrP)8015/4053 mouse coexpressing PrP(ΔGPI) at 1.7× and wt PrP at 4× that developed spontaneous disease at 143 d, PrP deposits were prevalent in the hippocampus, sparse in the thalamus, and absent in the cerebellum (Fig. 4 A–C). Some of these deposits were identified as amyloid by staining with ThioS (Fig. 4 D–F), whereas little or no vacuolation was seen in the examined brain areas (Fig. 4 G–I). As shown by ThioS and anti-myc tag double staining, anchorless PrP was incorporated into the plaques (Fig. 4 J–L). Similar neuropathologic changes were observed in Tg8015 lines coexpressing lower levels of wt PrP (Fig. S4 A–I). Despite developing spontaneous illness with shorter onset times, Tg8015 mice coexpressing wt PrP exhibited milder neuropathologic changes compared with spontaneously ill Tg8015/Prnp0/0 mice expressing only PrP(ΔGPI). However, numerous plaques containing PrP(ΔGPI) amyloid were observed in mice coexpressing wt PrP younger than 200 d, suggesting that wt PrPC modulates the kinetics of PrP(ΔGPI) amyloid formation.
Fig. 4.
Neuropathologic analysis of spontaneously ill Tg(PrP,ΔGPI:PrP)8015/4053 mice. (A–I) Brain sections taken from the hippocampus (Left), thalamus (Center), and cerebellum (Right) were examined for neuropathologic changes. (A–C) Immunostaining with anti-PrP Fab R2 showed that PrP deposits were abundant in the hippocampus, sparse in the thalamus, and absent in the cerebellum. (D–F) ThioS staining demonstrated that some of the PrP deposits could be classified as amyloid. (G–I) H&E staining showed only little or no vacuolation. Overall, the neuropathology was less severe than that observed in ill Tg8015/Prnp0/0 mice. (Scale bar: A–I, 50 μm.) (J–L) Double-fluorescence labeling of brain sections with ThioS (J) and anti-myc tag antibody (K) showed coincidence (L), indicating that amyloid plaques from Tg mice coexpressing PrP(ΔGPI) and PrPC contain PrP(ΔGPI). (Scale bar: J–L, 100 μm.)
We also performed neuropathologic analysis on the brains of Tg mice coexpressing PrP(ΔGPI) at 1.7× and N-terminally truncated PrPC at 16×. In Tg8015/9949 mice that developed spontaneous neurologic illness at ∼600 d of age, large numbers of PrP-immunopositive and ThioS-positive plaques were found, indistinguishable from the Tg8015/Prnp0/0 mice that also developed neurologic symptoms at ∼600 d (Fig. S4 J–R).
Bioassays Failed to Detect Prion Infectivity in Coexpressing Mice.
To test for the generation of infectivity in mice coexpressing wt PrPC and PrP(ΔGPI), we prepared brain homogenates from clinically ill Tg8015/Prnp0/+ and Tg8015/4053 mice, and injected them intracerebrally into Tg4053 mice. None of the inoculated mice developed disease after >600 d (Table S4). The brains from 10 aged, asymptomatic animals were analyzed biochemically, and two were found to contain PrP deposits and a ∼10-kDa, PK-resistant PrP fragment (Fig. S5). Therefore, we conclude that the initial process of PrPC hastening neurotoxicity in Tg8015 mice is independent of its conversion to PrPSc.
Discussion
In the present study, we developed Tg mouse lines expressing PrP lacking the GPI membrane anchor, one of which developed spontaneous neurologic illness. Neuropathologic and biochemical analysis of these animals showed deposition of PrP(ΔGPI) amyloid, which contained a unique prion strain with a PK-resistant, N- and C-terminally truncated, ∼10-kDa PrP core. This finding highlights the importance of the GPI anchor in preventing PrP from following its intrinsic propensity to form infectious aggregates in vivo.
A previous study of mice expressing a similar construct at low levels showed that the GPI anchor is not required for the structural conversion of PrPC into PrPSc upon prion inoculation. These animals did not show a spontaneous phenotype (6), comparable to the Tg8423 and Tg8446 lines described here, which is likely to be a consequence of lower levels of PrP(ΔGPI) expression (7).
Spontaneously Generated PrP Amyloids Are Prions.
Tg8015/Prnp0/0 mice expressing anchorless PrP at almost twice the level of PrPC in wt FVB mice developed a cerebral PrP amyloidosis accompanied by the accumulation of insoluble PrP. Most important, six independently prepared homogenates from the brains of ill Tg8015/Prnp0/0 mice were serially transmissible to Tg4053 mice expressing GPI-anchored, full-length wt PrP (Table 2).
We surmise that PrP(ΔGPI) can fold into multiple infectious conformations, some of which transmit a CNS disease characterized by a ∼10-kDa, PK-resistant PrP fragment and other conformations that can more rapidly induce disease in Tg4053 mice.
Prion infectivity from Tg8015/Prnp0/0 mice transmitted not only to Tg4053 mice but also to Tg8015/Prnp0/0 mice. The serial passage of spontaneously ill Tg8015/Prnp0/0 brains resulted in neurologic dysfunction 300–400 d earlier than the spontaneous onset of illness in mice used as the inoculum (Table 2). Moreover, the brains of the inoculated Tg8015/Prnp0/0 mice contained insoluble PrP, which produced the same ∼10-kDa fragment found in the brains of older, spontaneously ill Tg8015/Prnp0/0 mice (Fig. 2). Numerous PrP amyloid plaques were found in the brains of ill Tg8015/Prnp0/0 mice whether they developed spontaneous disease or were inoculated with homogenates prepared from the brains of ill Tg8015/Prnp0/0 mice (Fig. 2). It is likely that the ∼10-kDa, PK-resistant fragment represents the structural core of PrP amyloids, and is clearly different from RML prions, as shown by Western blot and comparison of fiber diameters by TEM (Fig. 1).
Although amyloidogenic forms of PrP(ΔGPI) were detected in the brains of asymptomatic Tg8015/Prnp0/0 mice as early as 305 d of age, as revealed by pathologic examination and Western blot (Table S1), it is unclear when detectable prion infectivity was first generated. By the time the mice developed clinical illness, large amounts of PrP amyloid and a PK-resistant PrP fragment were detectable in the brain (Fig. 1). That Tg8015 mice developed spontaneous illness over a broad time range (500–700 d) suggests that the initiation of sustained PK-resistant PrP amyloid deposition in these mice results from a stochastic process. Together, these data show that the spontaneous prion formation in Tg8015/Prnp0/0 mice is transmissible to mice expressing wt PrP, but sometimes resulted in different incubation periods, neuropathology, and biochemical properties of PrP, suggesting that multiple strains may have been formed.
Coexpression of PrP(ΔGPI) and wt PrPC Accelerates Spontaneous Disease.
Although widespread amyloid deposition was not observed in ill Tg8015 mice coexpressing wt PrP, the age at which CNS dysfunction appeared was inversely proportional to the brain levels of wt PrPC (Fig. 3B and Table 1). Because it was suggested that membrane-anchored PrPC is necessary to mediate the neurotoxic effects of PrPSc (13), spontaneously misfolded PrP(ΔGPI) may interact directly with PrPC molecules to cause perturbations in neuronal homeostasis in a PrPC concentration-dependent manner. This process did not depend on the conversion of wt PrPC into PrPSc because neither transmissibility, as measured by four independent bioassays in Tg4053 mice (Table S4), nor PK-resistant PrPSc by Western blotting (Fig. S5 A and D), were found.
Although the mechanism by which PrPC accelerates disease in mice expressing PrP(ΔGPI) is currently unknown, it is tempting to speculate that wt PrPC may function as a molecular switch for misfolded and toxic PrP species. In this scenario, a wt PrPC/PrP(ΔGPI) complex may promote cell death in a manner similar to the hippocampal cell death observed following antibody-mediated dimerization of PrPC (14). It is interesting to note that PrP has also been suggested to be a receptor and mediator of toxicity for Aβ and other β-sheeted peptides (15, 16). Future studies in these bigenic mouse models might reveal mechanistic insights and consequences of interaction between PrP aggregates and membrane-anchored PrPC. Blocking this particular interaction may reveal a novel strategy for a therapeutic intervention in prion and other amyloid diseases.
Role of the N Terminus of PrPC in Accelerated Disease.
Insight into understanding the interaction between anchorless PrP and membrane-anchored PrP was achieved by crossing Tg8015/Prnp0/0 mice to Tg mice expressing an N-terminally truncated form of PrP. In contrast to full-length wt PrPC, coexpression of GPI-anchored but N-terminally truncated PrP(Δ23–88) with PrP(ΔGPI) did not produce an accelerated neurologic illness despite very high levels (∼16×) of PrP(Δ23–88) expression. Either of two scenarios might explain this observation. First, the highly charged, octarepeat-containing N-terminal region (residues 23–88) of PrP may mediate the interaction of PrP(ΔGPI) amyloid with PrPC. Interestingly, PrP residues 23–30 bind tightly to PrPSc (17), and similarly, PrP residues 23–27 were found to be crucial for the binding of Aβ oligomers to PrPC (18). A second possibility is that PrP(Δ23–88)/PrP(ΔGPI) complexes may be incapable of transducing neurotoxic signals. Deletion of residues 23–28 or residues 51–90 from PrP has been shown to reduce or eliminate the endocytosis of PrPC (19). Thus, PrP(Δ23–88) may be unable to transport anchorless PrP leading to a lack of toxicity.
Pathogenic Relevance of Anchorless PrP to Human PrP Amyloidoses.
The neuropathologic and biochemical signatures of ill Tg8015/Prnp0/0 mice present interesting parallels with GSS in humans. GSS is a familial prion disease caused by several different mutations in the PRNP gene (9). Neuropathologically, GSS is characterized by the deposition of amyloid plaques that show N- and C-terminally truncated, low-molecular-mass (7–11 kDa) PrP fragments upon PK treatment. These fragments are similar in size to those reported here for ill Tg8015/Prnp0/0 mice. Five different PrP mutations, which generate C-terminally truncated PrP molecules lacking the GPI-anchor attachment site as a result of a premature stop codon, are known to cause GSS (9, 11). Two recently discovered patients expressing almost full-length wt PrP without the GPI-anchor moiety (stop mutations occurring at either residue 226 or 227) died of GSS and were found to have small, PK-resistant PrP fragments as well as PrP amyloid plaques in their brains (11). The F198S mutation causing GSS is located within the globular domain of PrP but resulted in the expression of PrP(ΔGPI) in cell culture (10). Also, the variable transmissibility of spontaneous infectivity in Tg8015/Prnp0/0 mice to Tg4053 mice might reflect the variable transmissibility of confirmed GSS cases (20). Taken together, the findings in GSS of humans and in Tg8015/Prnp0/0 mice suggest that impaired GPI anchoring may be responsible for several forms of GSS.
Inoculation of Tg8015/Prnp0/0 mice with brain homogenates from aged, spontaneously ill Tg8015/Prnp0/0 animals accelerated the formation of PK-resistant PrP and onset of disease from ∼600 d to ∼200 d. A similar phenomenon has also been observed in mice expressing PrP containing the GSS(P102L) mutation (21). Presumably, the acceleration of disease was caused by PrP(ΔGPI) amyloids in the inoculum that seed the polymerization of PrP(ΔGPI) in the recipient mice. Prion-mediated dysfunction of the CNS has been reported for other neurodegenerative diseases, including models of Alzheimer's disease (22), Parkinson's disease (23), and the tauopathies (24). These findings argue that prion-mediated spread of neuronal dysfunction in the CNS represents a general biological phenomenon in amyloidosis that leads to neurodegeneration.
Materials and Methods
Production of Tg Mice Expressing PrP(ΔGPI).
The wt mouse PrP sequence was modified by substituting a myc tag (EQKLISEEDL) for the C-terminal GPI anchor signal sequence following PrP residue 231; a stop codon followed the myc tag. This anchorless PrP(ΔGPI) construct was amplified by PCR and then inserted into the SalI site within the cos.Tet vector (25), which drives neuronal expression of proteins using the hamster Prnp promoter (SI Materials and Methods).
Solubility Analysis and PK Digestion of PrP.
Brain homogenates (10% wt/vol) were prepared in Ca2+- and Mg2+-free PBS. The brain homogenate was further diluted 1:10 into lysis buffer [10 mM Tris⋅HCl (pH 8.0); 150 mM NaCl; 0.5% (wt/vol) sodium deoxycholate; 0.5% (vol/vol) Nonidet P-40]. Limited protease digestion was initiated by adding 20 μg/mL of PK (Fermentas) at 37 °C for 1 h, with constant agitation, stopped by the addition of 1 mM PMSF followed by ultracentrifugation at 100,000 × g for 1 h at 20 °C using a TLA-55 rotor in an Optima TLX ultracentrifuge (Beckman-Coulter). The supernatant was discarded and the pellet was resuspended in SDS loading buffer and heated to 95 °C for 5 min before SDS/PAGE and Western blotting.
PrP Amyloid Purification.
PrP amyloid was purified from the brains of spontaneously ill animals using a modified version of a purification protocol for PrPSc (12). We introduced a Benzonase digestion step (150 U/mL of Benzonase for 1 h at 37 °C) instead of a clearance spin before PK digestion.
TEM and Negative Staining.
Conventional ammonium molybdate-negative staining was carried out with PTA-purified samples as described previously(12). For fibril diameter distribution, a set of ∼200 individual fibrils at a magnification of 75,750× was measured using ImageJ software (26).
Histological Analysis.
For each genotype, four coronal brain regions (caudate nucleus, hippocampus/thalamus, hippocampus/midbrain, and cerebellum/pons) from at least three mice were analyzed to provide a comprehensive neuropathologic examination. For the semiquantitative analysis in Table S1, we analyzed 12 brains (cortex, hippocampus, and thalamus) by immunostaining and ThioS staining from asymptomatic mice between 200 and 660 d of age. Severity of immunostaining was judged in comparison with the 200-d-old control (no ThioS/immunostaining signal) and the samples at 660 d with the most intense ThioS staining and immunostaining.
Supplementary Material
Acknowledgments
The authors thank the staff at the Hunters Point Animal Facility for the mouse studies, and Dr. Jan Langeveld for the kind gift of anti-PrP antibody 12B2. This work was supported by National Institutes of Health Grants AG02132, AG10770, and AG021601; gifts from the G. Harold and Leila Y. Mathers Charitable Foundation, the Hillblom Foundation, and the Sherman Fairchild Foundation; and postdoctoral fellowships from the German Research Foundation, the John Douglas French Alzheimer's foundation (J. Stöhr), and the Canadian Institutes for Health Research (J.C.W.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1117827108/-/DCSupplemental.
References
- 1.Aguzzi A. Cell biology: Beyond the prion principle. Nature. 2009;459:924–925. doi: 10.1038/459924a. [DOI] [PubMed] [Google Scholar]
- 2.Prusiner SB. Prions. In: Knipe DM, et al., editors. Fields Virology. 5th Ed. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 3059–3092. [Google Scholar]
- 3.Watts JC, Westaway D. The prion protein family: Diversity, rivalry, and dysfunction. Biochim Biophys Acta. 2007;1772:654–672. doi: 10.1016/j.bbadis.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Stahl N, Borchelt DR, Hsiao K, Prusiner SB. Scrapie prion protein contains a phosphatidylinositol glycolipid. Cell. 1987;51:229–240. doi: 10.1016/0092-8674(87)90150-4. [DOI] [PubMed] [Google Scholar]
- 5.Taylor DR, et al. Role of ADAMs in the ectodomain shedding and conformational conversion of the prion protein. J Biol Chem. 2009;284:22590–22600. doi: 10.1074/jbc.M109.032599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chesebro B, et al. Anchorless prion protein results in infectious amyloid disease without clinical scrapie. Science. 2005;308:1435–1439. doi: 10.1126/science.1110837. [DOI] [PubMed] [Google Scholar]
- 7.Chesebro B, et al. Fatal transmissible amyloid encephalopathy: A new type of prion disease associated with lack of prion protein membrane anchoring. PLoS Pathog. 2010;6:e1000800. doi: 10.1371/journal.ppat.1000800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogers M, Yehiely F, Scott M, Prusiner SB. Conversion of truncated and elongated prion proteins into the scrapie isoform in cultured cells. Proc Natl Acad Sci USA. 1993;90:3182–3186. doi: 10.1073/pnas.90.8.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghetti B, et al. Hereditary prion protein amyloidosis. In: Brown DR, editor. Neurodegeneration and Prion Disease. New York: Springer; 2005. pp. 83–109. [Google Scholar]
- 10.Kiachopoulos S, Bracher A, Winklhofer KF, Tatzelt J. Pathogenic mutations located in the hydrophobic core of the prion protein interfere with folding and attachment of the glycosylphosphatidylinositol anchor. J Biol Chem. 2005;280:9320–9329. doi: 10.1074/jbc.M412525200. [DOI] [PubMed] [Google Scholar]
- 11.Jansen C, et al. Prion protein amyloidosis with divergent phenotype associated with two novel nonsense mutations in PRNP. Acta Neuropathol. 2010;119(2):189–197. doi: 10.1007/s00401-009-0609-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wille H, et al. Natural and synthetic prion structure from X-ray fiber diffraction. Proc Natl Acad Sci USA. 2009;106:16990–16995. doi: 10.1073/pnas.0909006106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brandner S, et al. Normal host prion protein necessary for scrapie-induced neurotoxicity. Nature. 1996;379:339–343. doi: 10.1038/379339a0. [DOI] [PubMed] [Google Scholar]
- 14.Solforosi L, et al. Cross-linking cellular prion protein triggers neuronal apoptosis in vivo. Science. 2004;303:1514–1516. doi: 10.1126/science.1094273. [DOI] [PubMed] [Google Scholar]
- 15.Laurén J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Resenberger UK, et al. The cellular prion protein mediates neurotoxic signalling of β-sheet-rich conformers independent of prion replication. EMBO J. 2011;30:2057–2070. doi: 10.1038/emboj.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lau AL, et al. Characterization of prion protein (PrP)-derived peptides that discriminate full-length PrPSc from PrPC. Proc Natl Acad Sci USA. 2007;104:11551–11556. doi: 10.1073/pnas.0704260104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen S, Yadav SP, Surewicz WK. Interaction between human prion protein and amyloid-β (Abeta) oligomers: Role of N-terminal residues. J Biol Chem. 2010;285:26377–26383. doi: 10.1074/jbc.M110.145516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sunyach C, et al. The mechanism of internalization of glycosylphosphatidylinositol-anchored prion protein. EMBO J. 2003;22:3591–3601. doi: 10.1093/emboj/cdg344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piccardo P, Manson JC, King D, Ghetti B, Barron RM. Accumulation of prion protein in the brain that is not associated with transmissible disease. Proc Natl Acad Sci USA. 2007;104:4712–4717. doi: 10.1073/pnas.0609241104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsiao KK, et al. Serial transmission in rodents of neurodegeneration from transgenic mice expressing mutant prion protein. Proc Natl Acad Sci USA. 1994;91:9126–9130. doi: 10.1073/pnas.91.19.9126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyer-Luehmann M, et al. Exogenous induction of cerebral beta-amyloidogenesis is governed by agent and host. Science. 2006;313:1781–1784. doi: 10.1126/science.1131864. [DOI] [PubMed] [Google Scholar]
- 23.Mougenot AL, et al. Prion-like acceleration of a synucleinopathy in a transgenic mouse model. Neurobiol Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.06.022. in press. [DOI] [PubMed] [Google Scholar]
- 24.Clavaguera F, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009;11:909–913. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott MR, Köhler R, Foster D, Prusiner SB. Chimeric prion protein expression in cultured cells and transgenic mice. Protein Sci. 1992;1:986–997. doi: 10.1002/pro.5560010804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rasband WS. ImageJ (National Institutes of Health, Bethesda) 1997–2009 [Google Scholar]
- 27.Colby DW, et al. Protease-sensitive synthetic prions. PLoS Pathog. 2010;6:e1000736. doi: 10.1371/journal.ppat.1000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.