Abstract
Sexual reproduction can promote genetic diversity in eukaryotes, and yet many pathogenic fungi have been labeled as obligate asexual species. It is becoming increasingly clear, however, that cryptic sexual programs may exist in some species, and that efficient mating requires the necessary developmental switch to be triggered. In this study we investigate Candida tropicalis, an important human fungal pathogen that has been reported to be asexual. Significantly, we demonstrate that C. tropicalis uses a phenotypic switch to regulate a cryptic program of sexual mating. Thus, diploid a and α cells must undergo a developmental transition to the mating-competent form, and only then does efficient cell-cell conjugation take place resulting in the formation of stable a/α tetraploids. We show that both the phenotypic switch and sexual mating depend on the conserved transcriptional regulator Wor1, which is regulated by temperature in other fungal species. In contrast, C. tropicalis mating occurs efficiently at both 25 °C and 37 °C, suggesting that it could occur in the mammalian host and have direct consequences for the outcome of an infection. Transcriptional profiling further reveals that ≈400 genes are differentially expressed between the two phenotypic states, including the regulatory factor Wor1. Taken together, our results demonstrate that C. tropicalis has a unique sexual program, and that entry to this program is controlled via a Wor1-mediated, metastable switch. These observations have direct implications for the regulation and evolution of cryptic sexual programs in related fungal pathogens.
Keywords: epigenetic, recombination, bistability
The prevalence of sexual reproduction among eukaryotic organisms indicates that sex brings significant benefits despite the costs associated with the sexual lifestyle (1, 2). Benefits include increased rates of recombination and accelerated adaptation, which may be particularly advantageous during exposure to hostile environments (3, 4). Sexual reproduction may also help organisms maintain a fitness advantage against coevolving pathogens, a tenet of the Red Queen hypothesis (5). In this model, sexual outcrossing is necessary if an organism is to generate sufficient genetic variation to outpace infectious agents. Recent experiments have provided support for this hypothesis, because populations of Caenorhabditis elegans are better suited to survive bacterial infection if able to undergo sexual outbreeding (6, 7).
Many fungal species have also been investigated for their ability to undergo sexual reproduction, and the yeast Saccharomyces cerevisiae has served as an excellent model system for studies into sexual development. Fungal sex in this hemiascomycete species is regulated by genes encoded at the mating-type (MAT) locus. MATa-containing cells are able to mate with MATα-containing cells to generate MATa/α mating products. MATa/α cells are unable to mate but are competent to undergo meiosis under conditions of nutrient starvation. Transcription factors encoded at the MAT locus regulate the characteristics of the three cell types, a, α, and a/α, that together define the sexual program of S. cerevisiae and many related fungi (8, 9).
Genome sequencing has revealed the presence of mating-type-like (MTL) loci in other hemiascomycete species, including those with apparently obligate asexual lifestyles (10, 11). For example, Candida species were originally classified as yeasts that propagate by asexual budding and form hyphae or pseudohyphae, yet lack sexual cycles and ascospore production (12). The Candida genus is now recognized as containing a large number (>150) of diverse species because of its broad classification (12). Many Candida species are medically relevant, and together represent the most common cause of opportunistic fungal infections in humans (13). Although complete sexual cycles have been described for several Candida species, the most prevalent human pathogens either lack sexual mating or have restricted sexual cycles, which may indicate that limiting sexual reproduction can benefit fungal pathogenesis (14–16). In particular, rare sexual reproduction could allow propagation of a well-adapted clonal lineage in the host, while retaining the ability to undergo sexual recombination in response to stressful environments. Further understanding of the lifestyles of pathogenic fungi is therefore important for establishing how mating and virulence have evolved in these species.
The Candida species most commonly encountered in the clinic are Candida albicans, Candida tropicalis, Candida parapsilosis, and Candida glabrata, which together are responsible for >85% of invasive infections (13). Strikingly, of these species, only C. albicans has been shown to undergo sexual mating (17, 18), and entry to the sexual program is regulated by a unique phenotypic switch (19). Thus, C. albicans a and α cells must undergo a heritable switch from the white form to the opaque form to be mating competent. The phenotypic switch is regulated by the Wor1 transcription factor, because high expression of this factor is necessary and sufficient for formation of the opaque state (20–23). Homologs of Wor1 are found across the entire fungal lineage (24), although the white-opaque switch is thought to be unique to C. albicans (and its sister species C. dubliniensis), and has not been described in any other hemiascomycete species (25–27). In contrast to C. albicans, sexual mating has never been observed in C. tropicalis, C. parapsilosis, or C. glabrata, despite extensive efforts to find laboratory conditions conducive to mating (28–32). Sequence analysis reveals that “asexual” Candida species contain MAT/MTL loci resembling those of sexual species, and they encode many of the genes necessary for mating and meiosis (10, 11, 31). It is therefore an open question whether these species have lost the ability to undergo sex or whether the developmental signals necessary for mating have yet to be uncovered (28, 33).
In this study, we address the potential for diploid C. tropicalis strains to undergo a cryptic sexual program. Surprisingly, our experiments reveal that C. tropicalis exhibits a unique form of white-opaque phenotypic switching, and that this switch regulates an extant program of sexual reproduction. Thus, only cells in the opaque state undergo efficient cell-cell conjugation. Mating occurs efficiently at 37 °C, making it likely that sexual recombination can occur during colonization and infection of the mammalian host. We further show that the phenotypic switch in C. tropicalis is regulated by the conserved transcription factor, Wor1. However, transcriptional regulation of the C. tropicalis phenotypic switch shows fundamental differences to that of the white-opaque switch in C. albicans, which may account for the distinct switching characteristics of the two species. We discuss these findings as they reveal the regulation of the hitherto undiscovered sexual program in C. tropicalis and propose that cryptic sexual cycles remain to be discovered in related Candida pathogens.
Results
Identification of a Phenotypic Switch in C. tropicalis.
To address the potential for sexual mating in C. tropicalis, we first generated isogenic MTLa and MTLα strains from the diploid a/α parental strain ATCC34139 (Materials and Methods). Mixing of a and α cells on a variety of media did not, however, result in either the formation of polarized mating projections or mating zygotes, as typically seen during mating of many hemiascomycete species (Fig. S1). We subsequently examined whether C. tropicalis a and α strains may undergo phenotypic switching, in which there is a heritable change either in the cell or colony phenotype. A phenotypic switch between white and opaque states was shown to regulate the sexual program in C. albicans, because efficient cell-cell conjugation depends on cells being in the opaque state in this species (19, 34).
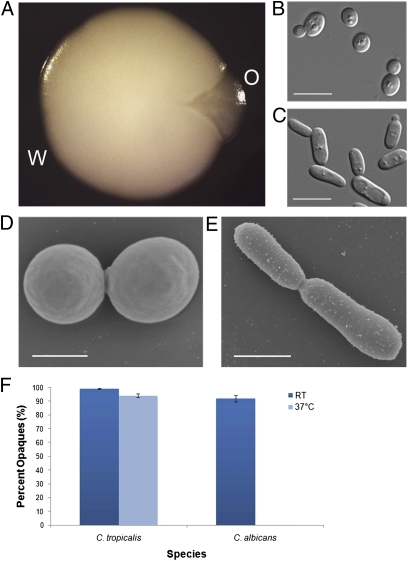
C. tropicalis strains were analyzed under a variety of in vitro culture conditions and occasional sectoring of colonies was observed, indicative of phenotypic switching (Fig. 1A). Cells from sectored colonies were restreaked and found to represent a stable, heritable state distinct from that of the parental cells. Analysis of cells from the two phenotypic states also revealed differences at the cellular level. Cells from the lighter-colored parental colonies appeared round and had a smooth cell surface, whereas cells from the darker-colored colonies were elongated and exhibited a spotted cell surface (Fig. 1 B–E and Fig. S2). Cells in both states appeared to be metastable; cell types were heritable for multiple generations, with stochastic switching observed between states. On average, we observed white-to-opaque switching at a frequency of ≈0.3% in a strains and ≈1% in α strains, whereas no white-opaque switching was seen in the a/α parental strain. Because of the superficial similarities between the C. tropicalis phenotypes and those previously described in C. albicans, we have termed the light and dark states of C. tropicalis white and opaque, respectively. It should be noted, however, that several characteristic features of C. albicans opaque cells are not seen in C. tropicalis, such as red staining with the chemical phloxine B (35) (Fig. S3) and switching of opaque cells en masse to white cells at 37 °C (36, 37) (Fig. 1F).
Fig. 1.
Phenotypic switching in C. tropicalis. Colony and cellular morphologies of C. tropicalis “white” and “opaque” states. (A) A white (W) colony of C. tropicalis (CAY1504) that has a sector in which cells have switched to opaque (O). (B and C) Cells from white colonies of C. tropicalis exhibit a characteristic round shape (B), whereas cells from opaque colonies exhibit an elongated cell shape (C). (D and E) Scanning electron micrographs of white and opaque cells. White cells (CAY1504) exhibit a smooth cell surface, whereas opaque cells (CAY2275) typically exhibit a spotted cell surface. (F) Comparison of opaque stability in C. tropicalis (CAY2054) and C. albicans (RBY1179). Cells were grown for 1 d on YPD medium at room temperature or at 37 °C and replated and grown at room temperature to determine their phenotype. (Scale bars: B and C, 10 μm; D, 2.5 μm; E, 5 μm.)
Mating in C. tropicalis.
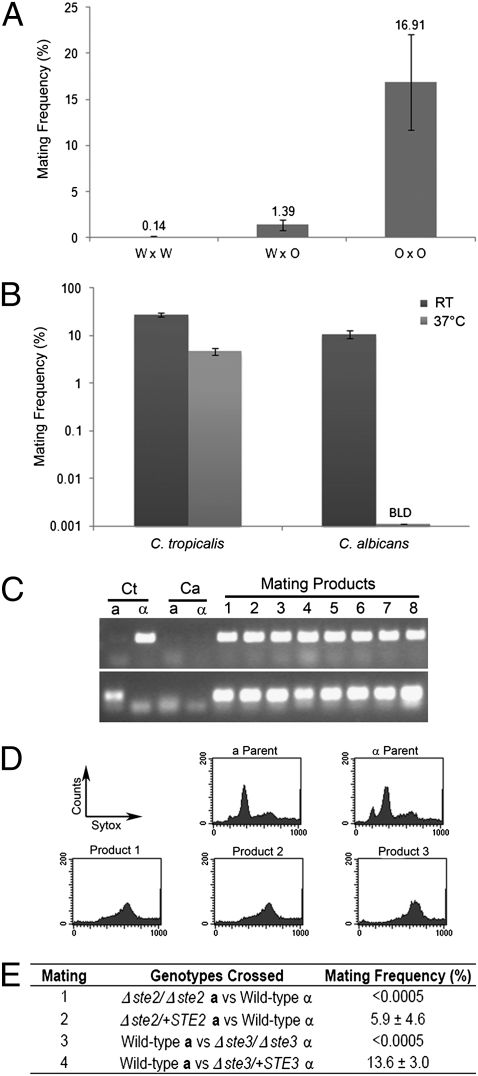
To test for C. tropicalis mating in both the white and opaque states, we constructed auxotrophic a and α strains defective in histidine (Δhis1/Δhis1) or arginine (Δarg4/Δarg4) metabolism. Successful mating events could subsequently be monitored by selection for His+ Arg+ prototrophs. As shown in Fig. 2A, mating between a and α cells was observed by using both white and opaque forms of the species, but the frequency of mating increased >100-fold when both partners were in the opaque form. When white cells were mated with opaque cells, the mating efficiency was intermediate to that of white-white and opaque-opaque crosses (Fig. 2A). These results indicate that mating is most efficient when cells are in the opaque state in both mating types. Same-sex mating was not detected between different auxotrophic strains, indicating that mating takes place specifically between a and α partners. Surprisingly, mating in C. tropicalis was also highly efficient at 37 °C, whereas the instability of C. albicans opaque cells prevents mating at this temperature in vitro (Fig. 2B). PCR analysis of the MTL locus confirmed that a/α cells were generated from a and α parental strains (Fig. 2C). In addition, DAPI (4′,6′-diamidino-2-phenylindole) staining and flow cytometry indicated that the majority of mating products were mononuclear, tetraploid cells (Fig. 2D and Fig. S4).
Fig. 2.
Quantitative mating of C. tropicalis white and opaque cells. (A) Mating of C. tropicalis strains at room temperature. White (W) or opaque (O) versions of auxotrophic strains were coincubated on Spider medium for 1 d, and cells subsequently were plated on selective media to determine the efficiency of mating. Data shows mating between a/a (CAY1503 or CAY1504) and α/α (CAY1505) strains. The difference in mating frequency between white-white and opaque-opaque crosses, as well as that between white-opaque and opaque-opaque crosses, was significant when analyzed with a two-tailed t test (P < 0.01). (B) Mating of C. tropicalis and C. albicans opaque cells at room temperature and 37 °C. Mating mixes were incubated on Spider medium for 1 d and plated to selective media to determine the mating frequency. C. albicans strains used were RBY1119 and RBY1179 (65). BLD, below limit of detection (0.0005%). Error bars represent SE. (C) PCR confirms that C. tropicalis mating products are a/α cells. Upper, PCR of the MTLα2 gene; Lower, PCR of the MTLa2 gene. (D) Flow cytometric analysis confirms that C. tropicalis mating products are tetraploid. (E) Table of mating frequencies for different mating crosses. Crosses 2 and 4 use Δste2 and Δste3 mutant strains (CAY2200/CAY2202 and CAY2244/CAY2245, respectively) with a wild-type copy of the receptor added back to the native locus (CAY2246/CAY2247 and CAY2285, respectively). Values are listed as frequency ± SD.
Genetic analysis of C. tropicalis mating was used to address the specificity of cell-cell conjugation. In hemiascomycete yeast, STE2 encodes the receptor for α pheromone, whereas STE3 encodes the receptor for a pheromone (38). Loss of STE2 therefore prevents mating of a cells, whereas loss of STE3 prevents mating of α cells. Gene deletions of STE2 or STE3 were generated in auxotrophic C. tropicalis strains and subsequently tested for the ability to undergo mating. Deletion of STE2 in a cells or STE3 in α cells resulted in a complete block in C. tropicalis mating, whereas reintegration of the deleted gene successfully restored mating competency (Fig. 2E). These results confirm that a/α cells are products of cell-cell conjugation and that mating depends on the canonical pheromone signaling pathways conserved among hemiascomycetes.
Cell Biology of Mating in C. tropicalis.
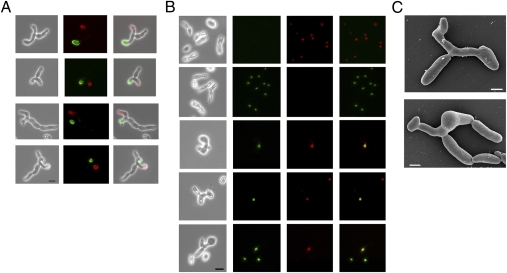
To visualize the process of mating in C. tropicalis, we used several complementary microscopy techniques. First, the two mating types were differentially labeled with FITC-ConA and Rhodamine-ConA, which labels the cell walls of Candida cells green and red, respectively (39). Labeled cells were then coincubated on Spider medium to induce mating and subsequently analyzed for cell-cell conjugants. Within several hours of coincubation, mixtures of opaque a and α cells showed polarized projections, indicative of cell “shmooing” in response to pheromone. In addition, mating zygotes were readily observed, in which a and α cells had fused and daughter cells started to bud from the center of the fusant cell (Fig. 3A). Similar experiments using white a and α cells showed only rare fusant cells, confirming that opaque cells are necessary for efficient cell-cell conjugation.
Fig. 3.
Cell microscopy of mating between C. tropicalis opaque a and α cells. (A) Mating zygotes formed by fusion between opaque a and α cells. MTLa and MTLα cells were incubated with FITC-ConA or Rhodamine-ConA to label cell walls green or red, respectively. Labeled cells were coincubated on Spider medium for 8 h, and zygote cells were identified by microscopy. (B) Nuclear fusion during mating between opaque a and α cells. Nuclei in parental MTLa and MTLα cells were labeled by expression of a histone-mCherry or histone-GFP fusion protein, respectively. Nuclear-labeled strains were coincubated on Spider medium for 5–24 h, and zygotes were identified by microscopy. (C) Scanning electron micrographs of C. tropicalis zygotes. (Scale bars: A and B, 10 μm; C, 4 μm.)
The process of nuclear fusion (karyogamy) during C. tropicalis mating was examined by generating strains with fluorescently labeled nuclei. Histone proteins fused to GFP or mCherry demonstrated that nuclei from opaque a and α cells had undergone fusion within a subset of mating zygotes, as revealed by the presence of a single nucleus showing red/green fluorescence (Fig. 3B). In addition, scanning electron micrographs of zygote cells were taken that provide high resolution images of mating cells (Fig. 3C and Fig. S2). Taken together, these experiments demonstrate that even in the absence of genetic selection, mating between opaque a and α cells leads to the formation of mononuclear, tetraploid C. tropicalis mating products.
Analysis of the C. tropicalis White-Opaque Switch.
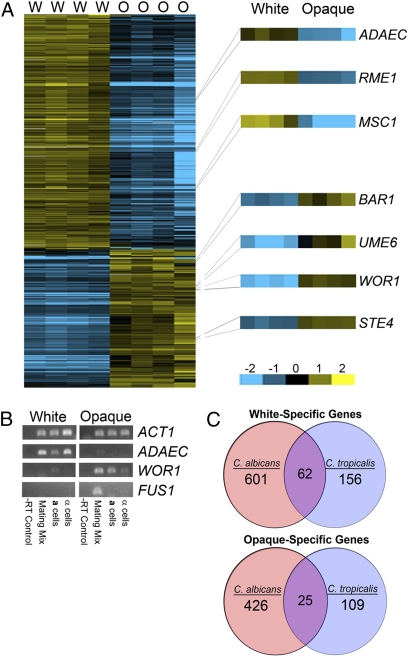
To compare the transcriptional profiles of C. tropicalis white and opaque cells, custom microarrays were hybridized with cDNA derived from both cell states. These experiments revealed that distinct sets of genes are expressed in the two phenotypic states, with 250 genes up-regulated in white cells and 146 genes up-regulated in opaque cells (Fig. 4A and Tables S4 and S5). Comparison with C. albicans white-opaque cell types (37, 40–43) revealed that most phase-specific genes were species-specific. Thus, of the 134 opaque-specific genes in C. tropicalis that have homologs in C. albicans, only 25 (19%) were opaque-specific in both species. In fact, 15 genes that were expressed in the opaque state in C. tropicalis were white-specific genes in C. albicans. Conversely, 218 of the C. tropicalis white genes have C. albicans counterparts, and of these, 62 (28%) were white-specific in C. albicans (Fig. 4C and SI Results). Again, a number of genes were expressed in the opposite cell type in the other species, because 31 white-specific genes in C. tropicalis were opaque-specific in C. albicans. Despite apparent large-scale differences in gene expression, analysis of white- and opaque-specific genes in C. tropicalis and C. albicans indicated that there was a statistically significant overlap of phase-specific genes between the two species (SI Results).
Fig. 4.
Transcriptional profiling of white and opaque genes in C. tropicalis. (A) Gene expression differences between C. tropicalis white and opaque cells. cDNA was prepared from white and opaque cells of RBY1504 (a/a) in four independent experiments and hybridized against a universal reference (cDNA from all eight samples). Up-regulated genes are shown in yellow, and down-regulated genes are shown in blue. A full list of phase-specific genes is shown in Tables S4 and S5. (B) RT-PCR showing expression of a white-specific gene (ADAEC) and an opaque-specific gene (WOR1). The FUS1 gene is only expressed only in mating mixes of opaque a and α cells (Bottom), and is a mating-specific gene in C. albicans and S. cerevisiae. (C) Comparison of white-specific and opaque-specific genes between C. tropicalis and C. albicans. Data for C. albicans was obtained from refs. 37 and 40–43.
C. tropicalis Wor1 Regulates Phenotypic Switching and Mating.
One notable gene found to be up-regulated in opaque cells of both C. tropicalis and C. albicans was WOR1. WOR1 is the master regulator of the white-opaque phenotypic switch in C. albicans, and its expression is both necessary and sufficient for formation of the opaque state (20–23). Expression of WOR1 is low in C. albicans white cells but is high in opaque cells. We show here that C. tropicalis WOR1 is also preferentially expressed (more than fivefold) in the opaque form (Fig. 4A). Up-regulation of the WOR1 gene in C. tropicalis opaque cells was verified by using RT-PCR (Fig. 4B). To analyze the potential role of Wor1 in C. tropicalis switching, WOR1 was deleted in a and α strain backgrounds. Significantly, no detectable switching to the opaque form was observed in Δwor1/Δwor1 strains, indicating that Wor1 function is critical for promoting opaque cell formation in both C. tropicalis and C. albicans.
The role of WOR1 in regulating C. tropicalis mating was investigated by deletion of the WOR1 gene in auxotrophic a and α strain backgrounds. Strikingly, crossing of these strains revealed that mating was essentially abolished between Δwor1 a and Δwor1 α cells (limit of detection <10−7). Mating between a Δwor1 white strain and a wild-type white strain was also extremely low (in the range of 10−5 to 10−6). Reintroduction of the WOR1 gene into Δwor1 strains restored mating ability, because WOR1 complemented strains mated with wild-type opaque strains at 2.7% efficiency, confirming the key role of this gene in mating. These results establish that deletion of WOR1 eliminates even the low frequency mating exhibited by white cells. Two models could account for this result. First, WOR1 is required for white cells to switch to opaque, and these switched cells then undergo mating. Alternatively, Wor1 expression in white cells could be directly relevant for the appreciable mating seen in these cell types.
Wor1 regulation of the C. albicans white-opaque switch involves interlocking transcriptional feedback loops with three additional factors, Czf1, Wor2, and Efg1. Czf1 and Wor2 promote formation of the opaque state, whereas Efg1 promotes formation of the white state (23). Surprisingly, expression of C. tropicalis CZF1 and WOR2 did not change significantly between white and opaque cells. In addition, the EFG1 gene is absent from C. tropicalis, and expression levels of a related APSES transcription factor, EFH1 (44), were similar between white and opaque forms. In C. albicans, the Mcm1–Ahr1 complex also regulates the white-opaque switch, yet the motif recognized by this complex is absent from other Candida species including C. tropicalis (27, 45, 46). Taken together, these findings establish that the transcriptional circuitry regulating the phenotypic switch in C. tropicalis is fundamentally distinct from that regulating the white-opaque switch in C. albicans.
Discussion
In this paper, we demonstrate that C. tropicalis, one of the most prevalent human fungal pathogens, has a hitherto unrecognized sexual program, and that sexual reproduction is regulated by a unique phenotypic switch. These findings provide additional support for the theory that many “asexual” fungal pathogens are actually sexual species when the appropriate conditions can be established. In the case of C. tropicalis, diploid MTLa and MTLα cells must undergo a developmental transition from the white form to the opaque form before efficient cell conjugation can take place. After the formation of mating zygotes, karyogamy can ensue between parental nuclei resulting in stable, mononuclear, tetraploid a/α cells. This discovery has important ramifications for the lifestyle of this pathogenic fungus, because it indicates that recombinant forms of the species can be generated via cell conjugation. C. tropicalis mating is efficient even at 37 °C, making it likely that strains can undergo sexual exchange during colonization and infection of the mammalian host. In support of mating in natural populations, an analysis of clinical isolates has revealed that the population structure of C. tropicalis is consistent with it being a sexually active species (47). The discovery of sexual reproduction is significant, because mating could potentially result in recombinant strains with increased virulence. This phenomenon has been reported in other human fungal pathogens such as Cryptococcus gattii, which is responsible for an ongoing outbreak in the Pacific Northwest of the United States and Canada (48–50).
The discovery that a phenotypic switch regulates C. tropicalis mating indicates that this mechanism is used to control sexual reproduction in multiple pathogenic Candida species. Previous studies established that C. albicans, the most common human fungal pathogen, undergoes a cryptic sexual program that is controlled by a white-opaque phenotypic switch (19). In this system, only cells in the opaque state are competent for efficient mating. It was originally thought that this switch was unique to C. albicans (and its closely related sister species C. dubliniensis), in part because a similar switch had not been observed in any other hemiascomycete yeast (26). The results described here now require revision of this model, because they indicate that a white-opaque switch also exists in C. tropicalis and, likewise, acts to regulate the program of sexual reproduction.
Despite physical similarities between white-opaque switching in C. albicans and C. tropicalis, transcriptional regulation of the switch is significantly different in the two lineages. For example, white-opaque switching in C. albicans is regulated by the Mcm1–Ahr1 complex, yet the motif recognized by this complex is absent from species other than C. albicans/C. dubliniensis (22, 46, 47). In addition, the Efg1 transcription factor that is essential for formation of white cells in C. albicans, and is conserved in most hemiascomycete yeast, has been lost from the C. tropicalis genome (10). Although the current data does not allow us to formally distinguish between a model for convergent or divergent evolution of the white-opaque switch in C. albicans and C. tropicalis, our data are consistent with the ancestor of these species evolving a WOR1-regulated switch, which has undergone transcriptional rewiring events since these two species diverged. Further analysis now is required to fully define how the white-opaque switching mechanisms evolved and the means by which they regulate sexual reproduction.
The most notable aspect of white-opaque switching shared between C. tropicalis and C. albicans is regulation by Wor1. Wor1 is the master regulator of white-opaque switching in C. albicans, and its expression is both necessary and sufficient for formation of the opaque state (20–23). Interestingly, Wor1 is also recognized as being the founding member of a family of DNA binding proteins that are conserved across the fungal lineage and serve as key regulators of developmental transitions (24). Wor1 orthologs have been studied in distantly related fungi including Histoplasma capsulatum, where the Ryp1 protein regulates a morphologic switch (51, 52), and Fusarium oxysporum, where Sge1 is necessary for parasitic growth in the plant (53). Curiously, C. albicans Wor1 and H. capsulatum Ryp1 are associated with temperature-induced changes in cell fate. In the case of C. albicans, opaque cells are unstable at 37 °C, because Wor1 expression is compromised and cells rapidly convert en masse to the white state (36, 37). In contrast, C. tropicalis opaque cells are stable at 37 °C, thereby allowing efficient mating at the body temperature of the mammalian host. Differences in the transcriptional regulation of the switch may account, at least in part, for the differential stability of the opaque states in C. albicans and C. tropicalis. Deletion of Ahr1 (also called Zcf37) in C. albicans increased the temperature stability of opaque cells (46), and it is therefore possible that the loss of this regulatory factor in C. tropicalis contributes to the increased thermal stability of the opaque state in this species.
Although several studies have begun to dissect the mechanism of phenotypic switching, it remains an open question as to the precise role of this switch in Candida biology (54–56). One hypothesis is that white and opaque cells evolved differential responses to pheromone to promote mating in the host. This model is based on the observation that whereas C. albicans opaque cells are the mating-competent form, white cells can also respond to sexual pheromones resulting in biofilm formation (57, 58). The resulting biofilm matrix allows stable pheromone gradients to be generated between opaque cells, thereby potentially enhancing mating in the host. Biofilms are also an important first step in medical device-associated infections, making an understanding of these processes important for fungal pathogenesis (59, 60). The discovery of a white-opaque switch in C. tropicalis will now allow further testing of this model and will reveal whether biofilm formation is also regulated by pheromone signaling and white-opaque switching in this species.
Finally, it is becoming apparent that highly specialized sexual cycles are the norm rather than the exception for human fungal pathogens. Discovering the developmental signals necessary for activation of cryptic sexual programs is therefore a key goal for the field. The results presented here establish Wor1 as a critical regulator of sexual activity in Candida species, because Wor1 is required for phenotypic switching and sexual mating in both C. albicans and C. tropicalis. This result was particularly striking in C. tropicalis, where the loss of Wor1 further reduced the mating efficiency of white cells by several orders of magnitude. It will therefore be revealing to see whether engineered expression of Wor1 can promote mating in related asexual species such as C. parapsilosis (61), as we predict that cryptic sexual programs remain to be discovered in related human fungal pathogens.
Materials and Methods
A complete description of all materials and methods can be found in SI Materials and Methods.
Media and Strains.
Media was prepared as described (62, 63). Yeast extract peptone dextrose (YPD) plates containing 200 μg/mL nourseothricin was used for selection of strains that were resistant to nourseothricin (SATR strains) (64). Strains are listed in Table S1.
Mating Assays.
Quantitative mating analyses of C. tropicalis strains were performed essentially as described for C. albicans (19). Briefly, His− and Arg− mating strains in the white or opaque phase were taken from plates and resuspended in water, and ≈2 × 107 cells of each strain mixed and pipetted onto 0.8-μm pore-size nitrocellulose filters. Filters were grown on the surface of Spider media for 1 d at room temperature. Cells were collected from the filters and plated at different dilutions onto His− Arg− media to select for mating products and onto His− and Arg− plates to monitor each parent population. The limiting parent was used to calculate mating frequencies as follows: mating efficiency = conjugants/(limiting parent + conjugants) = the greater of (Arg− His−)/Arg− or (Arg− His−)/His−. Cell ploidies were determined by flow cytometry as described (17). PCR to determine mating type was performed by using primers 47/48 directed at MTLa2 and primers 49/50 directed at MTLα2.
RT-PCR.
RNA was isolated from ≈2 × 108 cells plated onto Spider media for 4 h. Cells were removed from the media, and RNA was isolated by using the Ribopure-Yeast Kit (Ambion). RNA was treated with Turbo DNase (Ambion), and 500 ng of RNA was used for subsequent cDNA generation by using the GoScript enzyme (Promega). PCR was then performed by using gene specific primers listed in Table S2.
Supplementary Material
Acknowledgments
We thank Brian Tuch [University of California, San Francisco (UCSF)], Sandy Johnson (UCSF), and Aaron Hernday (UCSF) for the gift of C. tropicalis strains and plasmids; Marlowe Tessmer and Anita Sil for comments on the manuscript; and Geoff Williams in the Leduc BioImaging Facility at Brown University for help with microscopy. Work in the author's laboratory (R.J.B.) was supported by National Institutes of Health Grants R21AI081560, AI081560, AI081704 (to R.J.B.), T32GM007601 (to A.M.P.), and F31DE019752 (to K.A.). M.P.H. was supported by Graduate Assistance in Areas of National Need Training Grant P200A100100. R.J.B. also holds an Investigator in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1112076109/-/DCSupplemental.
References
- 1.Maynard Smith J. The Evolution of Sex. Cambridge, UK: Cambridge University Press; 1978. [Google Scholar]
- 2.Williams GC. Sex and Evolution. Princeton, NJ: Princeton University Press; 1975. [Google Scholar]
- 3.Goddard MR, Godfray HC, Burt A. Sex increases the efficacy of natural selection in experimental yeast populations. Nature. 2005;434:636–640. doi: 10.1038/nature03405. [DOI] [PubMed] [Google Scholar]
- 4.Grimberg B, Zeyl C. The effects of sex and mutation rate on adaptation in test tubes and to mouse hosts by Saccharomyces cerevisiae. Evolution. 2005;59:431–438. [PubMed] [Google Scholar]
- 5.Bell G. The Masterpiece of Nature: The Evolution and Genetics of Sexuality. London: Croom Helm; 1982. [Google Scholar]
- 6.Brockhurst MA. Evolution. Sex, death, and the Red Queen. Science. 2011;333:166–167. doi: 10.1126/science.1209420. [DOI] [PubMed] [Google Scholar]
- 7.Morran LT, Schmidt OG, Gelarden IA, Parrish RC, 2nd, Lively CM. Running with the Red Queen: Host-parasite coevolution selects for biparental sex. Science. 2011;333:216–218. doi: 10.1126/science.1206360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herskowitz I, Rine J, Strathern J. Mating type determination and mating-type interconversion. In: Pringle JR, Jones EW, Broach JR, editors. Saccharomyces cerevisiae. The Molecular and Cellular Biology of the Yeast Saccharomyces. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1992. pp. 583–657. [Google Scholar]
- 9.Johnson AD. Molecular mechanisms of cell-type determination in budding yeast. Curr Opin Genet Dev. 1995;5:552–558. doi: 10.1016/0959-437x(95)80022-0. [DOI] [PubMed] [Google Scholar]
- 10.Butler G, et al. Evolution of pathogenicity and sexual reproduction in eight Candida genomes. Nature. 2009;459:657–662. doi: 10.1038/nature08064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dujon B, et al. Genome evolution in yeasts. Nature. 2004;430:35–44. doi: 10.1038/nature02579. [DOI] [PubMed] [Google Scholar]
- 12.Reedy JL, Heitman J. In: Evolution of MAT in the Candida Species Complex: Sex, Ploidy, and Complete Sexual Cycles in C. lusitaniae, C. guilliermondii, and C. krusei. Sex in Fungi. Heitman J, editor. Washington: Am Soc Microbiol; 2007. pp. 235–245. [Google Scholar]
- 13.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: A persistent public health problem. Clin Microbiol Rev. 2007;20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heitman J. Evolution of eukaryotic microbial pathogens via covert sexual reproduction. Cell Host Microbe. 2010;8:86–99. doi: 10.1016/j.chom.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SC, Ni M, Li W, Shertz C, Heitman J. The evolution of sex: A perspective from the fungal kingdom. Microbiol Mol Biol Rev. 2010;74:298–340. doi: 10.1128/MMBR.00005-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nielsen K, Heitman J. Sex and virulence of human pathogenic fungi. Adv Genet. 2007;57:143–173. doi: 10.1016/S0065-2660(06)57004-X. [DOI] [PubMed] [Google Scholar]
- 17.Hull CM, Raisner RM, Johnson AD. Evidence for mating of the “asexual” yeast Candida albicans in a mammalian host. Science. 2000;289:307–310. doi: 10.1126/science.289.5477.307. [DOI] [PubMed] [Google Scholar]
- 18.Magee BB, Magee PT. Induction of mating in Candida albicans by construction of MTLa and MTLalpha strains. Science. 2000;289:310–313. doi: 10.1126/science.289.5477.310. [DOI] [PubMed] [Google Scholar]
- 19.Miller MG, Johnson AD. White-opaque switching in Candida albicans is controlled by mating-type locus homeodomain proteins and allows efficient mating. Cell. 2002;110:293–302. doi: 10.1016/s0092-8674(02)00837-1. [DOI] [PubMed] [Google Scholar]
- 20.Huang G, et al. Bistable expression of WOR1, a master regulator of white-opaque switching in Candida albicans. Proc Natl Acad Sci USA. 2006;103:12813–12818. doi: 10.1073/pnas.0605270103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srikantha T, et al. TOS9 regulates white-opaque switching in Candida albicans. Eukaryot Cell. 2006;5:1674–1687. doi: 10.1128/EC.00252-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zordan RE, Galgoczy DJ, Johnson AD. Epigenetic properties of white-opaque switching in Candida albicans are based on a self-sustaining transcriptional feedback loop. Proc Natl Acad Sci USA. 2006;103:12807–12812. doi: 10.1073/pnas.0605138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zordan RE, Miller MG, Galgoczy DJ, Tuch BB, Johnson AD. Interlocking transcriptional feedback loops control white-opaque switching in Candida albicans. PLoS Biol. 2007;5:e256. doi: 10.1371/journal.pbio.0050256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lohse MB, Zordan RE, Cain CW, Johnson AD. Distinct class of DNA-binding domains is exemplified by a master regulator of phenotypic switching in Candida albicans. Proc Natl Acad Sci USA. 2010;107:14105–14110. doi: 10.1073/pnas.1005911107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pujol C, et al. The closely related species Candida albicans and Candida dubliniensis can mate. Eukaryot Cell. 2004;3:1015–1027. doi: 10.1128/EC.3.4.1015-1027.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sahni N, et al. Tec1 mediates the pheromone response of the white phenotype of Candida albicans: Insights into the evolution of new signal transduction pathways. PLoS Biol. 2010;8:e1000363. doi: 10.1371/journal.pbio.1000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tuch BB, Galgoczy DJ, Hernday AD, Li H, Johnson AD. The evolution of combinatorial gene regulation in fungi. PLoS Biol. 2008;6:e38. doi: 10.1371/journal.pbio.0060038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butler G. Fungal sex and pathogenesis. Clin Microbiol Rev. 2010;23:140–159. doi: 10.1128/CMR.00053-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brisse S, et al. Uneven distribution of mating types among genotypes of Candida glabrata isolates from clinical samples. Eukaryot Cell. 2009;8:287–295. doi: 10.1128/EC.00215-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muller H, Hennequin C, Gallaud J, Dujon B, Fairhead C. The asexual yeast Candida glabrata maintains distinct a and alpha haploid mating types. Eukaryot Cell. 2008;7:848–858. doi: 10.1128/EC.00456-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Srikantha T, Lachke SA, Soll DR. Three mating type-like loci in Candida glabrata. Eukaryot Cell. 2003;2:328–340. doi: 10.1128/EC.2.2.328-340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Logue ME, Wong S, Wolfe KH, Butler G. A genome sequence survey shows that the pathogenic yeast Candida parapsilosis has a defective MTLa1 allele at its mating type locus. Eukaryot Cell. 2005;4:1009–1017. doi: 10.1128/EC.4.6.1009-1017.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alby K, Bennett RJ. Sexual reproduction in the Candida clade: Cryptic cycles, diverse mechanisms, and alternative functions. Cell Mol Life Sci. 2010;67:3275–3285. doi: 10.1007/s00018-010-0421-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slutsky B, et al. “White-opaque transition”: A second high-frequency switching system in Candida albicans. J Bacteriol. 1987;169:189–197. doi: 10.1128/jb.169.1.189-197.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson JM, Soll DR. Unique phenotype of opaque cells in the white-opaque transition of Candida albicans. J Bacteriol. 1987;169:5579–5588. doi: 10.1128/jb.169.12.5579-5588.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rikkerink EH, Magee BB, Magee PT. Opaque-white phenotype transition: A programmed morphological transition in Candida albicans. J Bacteriol. 1988;170:895–899. doi: 10.1128/jb.170.2.895-899.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lohse MB, Johnson AD. Temporal anatomy of an epigenetic switch in cell programming: The white-opaque transition of C. albicans. Mol Microbiol. 2010;78:331–343. doi: 10.1111/j.1365-2958.2010.07331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dignard D, El-Naggar AL, Logue ME, Butler G, Whiteway M. Identification and characterization of MFA1; the gene encoding Candida albicans a-factor pheromone. Eukaryot Cell. 2007;6:487–494. doi: 10.1128/EC.00387-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lockhart SR, Daniels KJ, Zhao R, Wessels D, Soll DR. Cell biology of mating in Candida albicans. Eukaryot Cell. 2003;2:49–61. doi: 10.1128/EC.2.1.49-61.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsong AE, Miller MG, Raisner RM, Johnson AD. Evolution of a combinatorial transcriptional circuit: A case study in yeasts. Cell. 2003;115:389–399. doi: 10.1016/s0092-8674(03)00885-7. [DOI] [PubMed] [Google Scholar]
- 41.Lan CY, et al. Metabolic specialization associated with phenotypic switching in Candida albicans. Proc Natl Acad Sci USA. 2002;99:14907–14912. doi: 10.1073/pnas.232566499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Srikantha T, Soll DR. A white-specific gene in the white-opaque switching system of Candida albicans. Gene. 1993;131:53–60. doi: 10.1016/0378-1119(93)90668-s. [DOI] [PubMed] [Google Scholar]
- 43.Tuch BB, et al. The transcriptomes of two heritable cell types illuminate the circuit governing their differentiation. PLoS Genet. 2010;6:e1001070. doi: 10.1371/journal.pgen.1001070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doedt T, et al. APSES proteins regulate morphogenesis and metabolism in Candida albicans. Mol Biol Cell. 2004;15:3167–3180. doi: 10.1091/10.1091/mbc.E03-11-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Askew C, et al. The zinc cluster transcription factor Ahr1p directs Mcm1p regulation of Candida albicans adhesion. Mol Microbiol. 2011;79:940–953. doi: 10.1111/j.1365-2958.2010.07504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang H, et al. Candida albicans Zcf37, a zinc finger protein, is required for stabilization of the white state. FEBS Lett. 2011;585:797–802. doi: 10.1016/j.febslet.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 47.Jacobsen MD, et al. Molecular phylogenetic analysis of Candida tropicalis isolates by multi-locus sequence typing. Fungal Genet Biol. 2008;45:1040–1042. doi: 10.1016/j.fgb.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 48.Byrnes EJ, 3rd, et al. Emergence and pathogenicity of highly virulent Cryptococcus gattii genotypes in the northwest United States. PLoS Pathog. 2010;6:e1000850. doi: 10.1371/journal.ppat.1000850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fraser JA, et al. Same-sex mating and the origin of the Vancouver Island Cryptococcus gattii outbreak. Nature. 2005;437:1360–1364. doi: 10.1038/nature04220. [DOI] [PubMed] [Google Scholar]
- 50.Ma H, et al. The fatal fungal outbreak on Vancouver Island is characterized by enhanced intracellular parasitism driven by mitochondrial regulation. Proc Natl Acad Sci USA. 2009;106:12980–12985. doi: 10.1073/pnas.0902963106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nguyen VQ, Sil A. Temperature-induced switch to the pathogenic yeast form of Histoplasma capsulatum requires Ryp1, a conserved transcriptional regulator. Proc Natl Acad Sci USA. 2008;105:4880–4885. doi: 10.1073/pnas.0710448105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Webster RH, Sil A. Conserved factors Ryp2 and Ryp3 control cell morphology and infectious spore formation in the fungal pathogen Histoplasma capsulatum. Proc Natl Acad Sci USA. 2008;105:14573–14578. doi: 10.1073/pnas.0806221105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Michielse CB, et al. The nuclear protein Sge1 of Fusarium oxysporum is required for parasitic growth. PLoS Pathog. 2009;5:e1000637. doi: 10.1371/journal.ppat.1000637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lohse MB, Johnson AD. White-opaque switching in Candida albicans. Curr Opin Microbiol. 2009;12:650–654. doi: 10.1016/j.mib.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morschhauser J. Regulation of white-opaque switching in Candida albicans. Med Microbiol Immunol. 2010;199:165–172. doi: 10.1007/s00430-010-0147-0. [DOI] [PubMed] [Google Scholar]
- 56.Soll DR. Why does Candida albicans switch? FEM Yeast Res. 2009;9:973–989. doi: 10.1111/j.1567-1364.2009.00562.x. [DOI] [PubMed] [Google Scholar]
- 57.Daniels KJ, Srikantha T, Lockhart SR, Pujol C, Soll DR. Opaque cells signal white cells to form biofilms in Candida albicans. EMBO J. 2006;25:2240–2252. doi: 10.1038/sj.emboj.7601099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sahni N, et al. Genes selectively up-regulated by pheromone in white cells are involved in biofilm formation in Candida albicans. PLoS Pathog. 2009;5:e1000601. doi: 10.1371/journal.ppat.1000601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ganguly S, Mitchell AP. Mucosal biofilms of Candida albicans. Curr Opin Microbiol. 2011;14:380–385. doi: 10.1016/j.mib.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nobile CJ, Mitchell AP. Genetics and genomics of Candida albicans biofilm formation. Cell Microbiol. 2006;8:1382–1391. doi: 10.1111/j.1462-5822.2006.00761.x. [DOI] [PubMed] [Google Scholar]
- 61.Sai S, Holland L, McGee CF, Lynch DB, Butler G. Evolution of mating within the Candida parapsilosis species group. Eukaryot Cell. 2011;10:578–587. doi: 10.1128/EC.00276-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guthrie C, Fink GR. Guide to Yeast Genetics and Molecular Biology. San Diego: Academic; 1991. [Google Scholar]
- 63.Liu H, Köhler J, Fink GR. Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science. 1994;266:1723–1726. doi: 10.1126/science.7992058. [DOI] [PubMed] [Google Scholar]
- 64.Reuss O, Vik A, Kolter R, Morschhäuser J. The SAT1 flipper, an optimized tool for gene disruption in Candida albicans. Gene. 2004;341:119–127. doi: 10.1016/j.gene.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 65.Schaefer D, Côte P, Whiteway M, Bennett RJ. Barrier activity in Candida albicans mediates pheromone degradation and promotes mating. Eukaryot Cell. 2007;6:907–918. doi: 10.1128/EC.00090-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.