Abstract
Homeotic selector (Hox) proteins often bind DNA cooperatively with cofactors such as Extradenticle (Exd) and Homothorax (Hth) to achieve functional specificity in vivo. Previous studies identified the Hox YPWM motif as an important Exd interaction motif. Using a comparative approach, we characterize the contribution of this and additional conserved sequence motifs to the regulation of specific target genes for three Drosophila Hox proteins. We find that Sex combs reduced (Scr) uses a simple interaction mechanism, where a single tryptophan-containing motif is necessary for Exd-dependent DNA-binding and in vivo functions. Abdominal-A (AbdA) is more complex, using multiple conserved motifs in a context-dependent manner. Lastly, Ultrabithorax (Ubx) is the most flexible, in that it uses multiple conserved motifs that function in parallel to regulate target genes in vivo. We propose that using different binding mechanisms with the same cofactor may be one strategy to achieve functional specificity in vivo.
Understanding the molecular processes by which gene expression is regulated remains at the core of many biological questions. The predominant model of eukaryotic gene regulation emphasizes the role of site-specific transcription factors in target gene selection. The initial binding of these transcription factors anchors the rest of the transcriptional regulatory complex, or enhanceosome, to the target site. Recruitment of additional proteins is often required to determine the regulatory sign, whether a gene is activated or repressed, and if this state will be maintained. In some cases, the DNA sequence can provide considerable insight into which other proteins are recruited (1). However, enhanceosome formation also requires protein-protein interactions: Mutational analyses of transcription factors demonstrate that sequences outside of the DNA-binding domain can influence regulatory activity, in part, by influencing the assembly of DNA-bound protein complexes (2, 3).
The homeotic selector (Hox) proteins provide a powerful system in which to study the role of protein-protein interactions in enhanceosome formation and transcription factor function. Best known for their role in anterior-posterior patterning, Hox proteins contain a highly conserved DNA-binding domain, termed the homeodomain (4). Although most homeodomains bind similar AT-rich sequences in vitro (5), each Hox protein displays a high level of functional specificity in vivo (6). These observations imply that residues outside of the DNA-binding domain influence specificity in vivo. One way Hox proteins achieve higher specificity is through cooperative interactions with DNA-binding cofactors, such as Extradenticle [Exd in Drosophila, Pre-B-cell leukemia homeobox (Pbx) in vertebrates] and Homothorax [Hth in Drosophila, Myeloid ecotropic viral integration site (Meis) in vertebrates] (7, 8). Exd and Hth, both members of the three-amino acid loop extension (TALE) family of homeodomain proteins, are obligate dimer partners for both nuclear translocation and transcriptional activity in vivo (9, 10). Previous genetic analyses highlight the importance of exd and hth for Hox function during embryogenesis (10–12). In addition to Exd and Hth, the abdominal Hox proteins Ultrabithorax (Ubx) and AbdominalA (AbdA) have the ability to bind cooperatively with another homeodomain protein, Engrailed (En) (1). As with Exd-Hth-Hox interaction, En–Hox–DNA complex formation has been shown to be critical for Hox-mediated gene regulation in vivo (1).
Biochemical and X-ray crystallography studies identified the highly conserved Hox motif called YPWM as one mode by which Hox proteins interact with Exd-Hth. However, despite being evolutionarily conserved and present in most Hox proteins, the importance of the YPWM motif appears to vary (2). For example, although vertebrate Hoxa1 and Deformed (Dfd) require the YPWM motif for Pbx/Exd-dependent functions (13, 14), Ubx and AbdA do not require YPWM for some Exd-dependent functions (15–19). In the case of Ubx and AbdA, a shared six-amino acid motif C-terminal to the homeodomain, termed UbdA, has also been suggested to contribute to interactions with Exd (17, 18, 20). Interestingly, in addition to UbdA, both Ubx and AbdA have other evolutionarily conserved residues in the C terminus that may also be important for mediating Hox functions in vivo (16, 21–23). These C-terminal sequences, which are distinct in Ubx and AbdA, could modify UbdA-dependent interactions so that its function may not be equivalent in both proteins. Further, the presence of multiple Exd interaction motifs poses the question of what determines which mode of interaction is most relevant for a given in vivo function. In the case of Ubx, both the protein context and the target site have been suggested to influence how individual motifs are used (18).
Currently, it is generally unknown how different modes of cofactor interaction influence Hox specificity. In the case of Sex combs reduced (Scr), structural studies demonstrated that the YPWM-Exd interaction helps position amino acids of the Hox linker region, which separates YPWM from the homeodomain, to make critical contacts in the minor groove of a specific DNA-binding site (24). These observations raise the possibility that other modes of Exd-Hox interaction could also alter the way in which these protein complexes recognize and bind to their target DNA sequences. In addition, alternate modes of protein–DNA complex formation may have an impact on the recruitment of coactivators and corepressors, as has been suggested for the glucocorticoid receptor (25).
In the current study, we use a comparative approach to characterize conserved sequence motifs for three Hox proteins and analyze their requirement for different in vivo functions. We demonstrate that the YPWM motif is critical for Scr to carry out Exd-dependent functions in vivo. In contrast to the single Exd interaction motif of Scr, AbdA and Ubx are more complex. In addition to the previously described YPWM and UbdA motifs, both AbdA and Ubx have other conserved motifs that contribute to cooperative complex formation with Exd-Hth in vitro. However, the in vivo requirements for conserved motifs vary according to the Hox protein. AbdA uses motifs in a context-dependent manner, whereas Ubx is more flexible, with some motifs apparently acting in a redundant manner for certain readouts. Our results suggest that having multiple sequence motifs adds complexity to the assembly and function of Hox complexes in vivo.
Results
Scr Requires Its YPWM Motif for Exd-Dependent Functions.
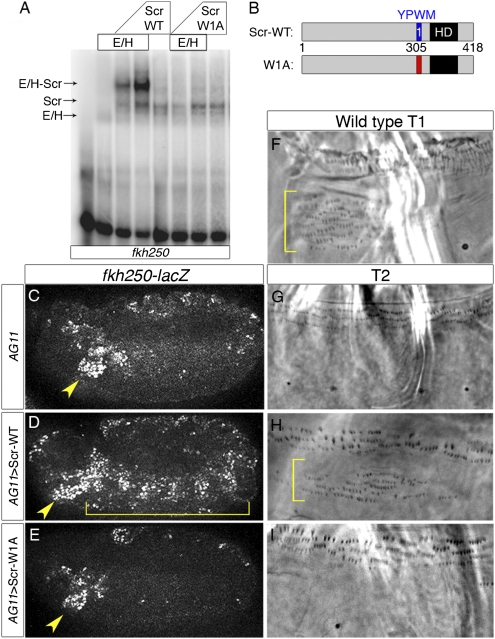
Previous studies demonstrated that the YPWM motif of Scr makes direct contacts within the Exd homeodomain and contributes to Scr function in vivo (24, 26). Because, as shown below, some Hox proteins have multiple YPWM-like motifs, we rename them here as W motifs and number them according to their proximity to the homeodomain. To test the requirement of Scr's single W motif, we constructed a mutant variant in which this sequence (YPWM) was mutated to four alanines (Scr-W1A; Fig. 1B). This mutant was assayed both for its ability to bind cooperatively with Exd to DNA in vitro and to perform Exd-dependent functions in vivo. One well-characterized readout is the Scr-specific target gene forkhead (fkh), which uses a Scr-Exd DNA-binding site, fkh250, for in vivo regulation (27). Although strong cooperative binding was observed for Scr-WT and Exd to fkh250, Scr-W1A was unable to bind cooperatively with Exd to this site (Fig. 1A). In contrast, both Scr-WT and Scr-W1A were able to bind to DNA as monomers in vitro (Fig. 1A), demonstrating that these proteins are still competent to bind DNA.
Fig. 1.
Scr requires the W motif for Exd-dependent functions. (A) EMSA of Scr proteins with Exd-HthHM on fkh250. HthHM is a homeodomainless isoform of Hth that is sufficient for fkh250-lacZ regulation in vivo (12). Complexes are indicated with arrows. (B) Schematics of WT and mutant Scr proteins. The homeodomain (HD) is shown in black. Blue designates the W-motif. Red indicates residues mutated to alanines (YPWM→AAAA). (C–E) fkh250-lacZ expression. AG11-Gal4 is a ubiquitous Gal4 driver controlled by armadillo. Embryos were stained for β-galactosidase to monitor fkh250-lacZ expression (white). Yellow arrowheads indicate areas of WT lacZ expression. Brackets indicate induction of ectopic lacZ expression. Images are approximately 480 μm wide. (F) Phase contrast image of a WT T1 ventral denticle pattern normally controlled by Scr. Phase contrast images depicting ventral T2 cuticle patterns for WT larvae (G) or animals ectopically expressing WT Scr (H) or Scr-W1A (I) using the AG11-Gal4 driver. Cuticle images are approximately 140 μm wide.
To test both WT and mutant proteins for in vivo function, we used the Gal4-upstream activation sequence (UAS) system to misexpress WT and mutant versions of Scr in vivo (28). In WT embryos, endogenous expression of Scr activates fkh250-lacZ only in parasegment 2 (Fig. 1C). When Scr was ubiquitously expressed throughout the embryo, ectopic activation of the fkh250-lacZ reporter gene was observed (Fig. 1D). In contrast, Scr-W1A was unable to activate this reporter (Fig. 1E). Scr-W1A was also analyzed for its ability to induce homeotic transformations of the larval cuticle. When WT Scr was ubiquitously expressed throughout the embryo, ectopic hairs similar to those found in the first thoracic (T1) segment (called the T1 beard, Fig. 1F) were observed in additional segments (Fig. 1H compared with Fig. 1G). In contrast, ubiquitous expression of Scr-W1A did not induce any ectopic beard (Fig. 1I). However, both Scr-WT and Scr-W1A were able to repress the Exd-independent target spalt (sal) in the wing imaginal disk, demonstrating that Scr-W1A is still an active transcription factor (Fig. S1). From these results, we conclude that Scr's W motif is crucial to form cooperative complexes with Exd in vitro and to execute Exd-dependent but not Exd-independent functions in vivo. These observations are consistent with the finding that severely truncated forms of Scr that retain its W motif and homeodomain retain the ability to generate Scr-like transformations in vivo (26).
Multiple Conserved Motifs Contribute to Exd-Dependent Functions of AbdA in a Context-Dependent Manner.
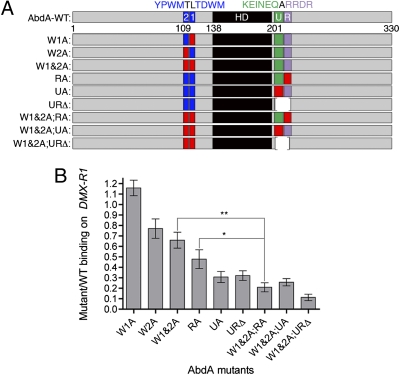
In addition to its classic YPWM motif (AbdA-W2, Fig. 2A), AbdA has another putative cofactor interaction motif C-terminal to its homeodomain, UbdA (AbdA-U, Fig. 2A) (29). Based on sequence conservation among arthropods, we identified two additional sequence motifs: the W motif TDWM (AbdA-W1) and RRDR (AbdA-R), C-terminal to UbdA (Fig. S2). To test the role of each motif, both individually and in combination, we made a series of AbdA mutants (Fig. 2A). In the abdomen of the developing embryo, AbdA normally represses the limb-forming gene Distalless (Dll) to restrict leg development to the thorax (30). Previous studies characterized the DNA-binding sites that abdominal Hox proteins and their cofactors use directly to repress Dll in the abdomen (1, 31). We analyzed the contribution of the conserved motifs of AbdA for cooperative complex formation with Exd/Hth on the DMX-R–binding site (Fig. 2B). All these motifs individually, except for W1, contribute to AbdA's ability to form complexes with Exd-Hth on the DMX-R1–binding site (Fig. 2B). Moreover, we often observed an additional reduction in complex formation when multiple motifs were mutated, suggesting that they contribute in an additive manner to complex formation with Exd-Hth. For example, the W1&2A;RA mutant (in which W1, W2, and RRDR motifs are mutated) was significantly impaired in complex formation compared with the W1&2A or the RA mutant individually (Fig. 2B). In contrast, when binding was assayed on a different site (rhoA), where the functional interaction is between AbdA and Hth (32, 33), complex formation was only minimally affected, suggesting that these motifs contribute specifically to Exd-dependent DNA binding (Fig. S3).
Fig. 2.
AbdA has multiple motifs that mediate cooperative complex formation with Exd/Hth. (A) Schematics of WT and mutant AbdA proteins. Diagrams are drawn approximately to scale. The homeodomain (HD) is shown in black. Blue designates W-motifs. Green designates the UbdA motif (U). Purple designates the RRDR motif (R). Red indicates residues mutated to alanines (YPWM→AAAA, TDWM→AAAA, KEINEQ→AAAAAA, and RRDR→AAAA). (B) Average binding of different AbdA mutants to the DMX-R1 probe. The bar graph represents the mean of n ≥ 3 ratios from independent experiments for each mutant. Error bars represent the SEM. To determine if the difference in cooperative binding is significant for a subset of mutants, t tests were used (**P = 0.004; *P = 0.031).
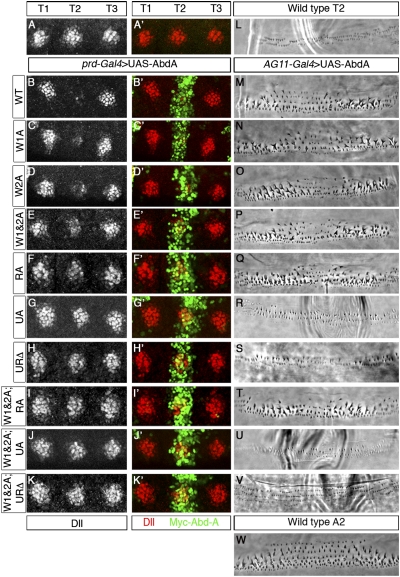
We next compared the ability of each mutant to repress Dll in vivo using prdGal4 to drive expression of AbdA variants in the second thoracic segment (T2). WT Dll expression in the first and third thoracic segments, where prdGal4 is not expressed, was used as reference (T1 and T3, respectively; Fig. 3 A–K). These data parallel the in vitro results: Most motifs contribute to Dll repression to some extent and often show additive affects when tested in combination. For example, additional loss of Dll repression was observed for the W1&2A mutant compared with the two single mutants (Fig. 3E compared with Fig. 3 C and D), as well as for the W1&2A;RA mutant compared with W1&2A and RA (Fig. 3I compared with Fig. 3 E and F). Unlike the W motifs, UbdA is necessary for all the in vivo functions of AbdA analyzed (Fig. 3 G and R). For example, mutation of UbdA (UA) alone abolished AbdA's ability to repress Dll (Fig. 3G). Therefore, no additive effects were observed in compound mutants containing mutations in UbdA (Fig. 3 H, J, and K). However, although necessary, UbdA is not sufficient to impart WT repressive ability: The AbdA-W1&2A;RA mutant, in which UbdA is still intact, did not repress Dll (Fig. 3I). As with the Scr-W1A mutant, all the AbdA mutants were able to repress sal in the wing imaginal disk (Fig. S4), demonstrating that these proteins are still active transcription factors and that the motifs mapped here are required for Exd-dependent functions in vivo.
Fig. 3.
AbdA uses conserved motifs in a context-dependent manner. (A–K) Thoracic region of a WT embryo (A) or embryos expressing AbdA proteins in T2 via the prd-Gal4 driver, stained for Dll (white or red) and myc-AbdA (green). The protein variant used is indicated on the left. Images are approximately 140 μm wide. Phase contrast images depicting ventral T2 cuticle patterns for WT larvae (L) or animals ectopically expressing WT AbdA (M) or mutant variants (N–V) using the AG11-Gal4 driver. (W) WT A2 ventral denticle pattern normally controlled by AbdA. Cuticle images are approximately 140 μm wide.
AbdA also patterns the denticle belts in the second through eighth abdominal segments (A2–A8) of the Drosophila larva (34) (WT A2 shown in Fig. 3W). Ectopic expression of AbdA causes homeotic transformations to A2 in segments anterior to the WT A2 segment (16) (Fig. 3M compared with Fig. 3L). By ubiquitously misexpressing AbdA variants throughout the embryo, we tested the effect that different mutations have on the ability to confer segment identity (Fig. 3 M–V). Similar to loss of Dll repression, mutation of UbdA alone or in combination with other motifs prevented abdominal-like transformations (Fig. 3 R, S, U, and V). However, AbdA mutants in which UbdA was intact were able to induce strong abdominal transformations (Fig. 3 N–Q and T). Comparing the contribution of each motif for different AbdA functions therefore suggests that motif use is target-dependent. For example, although W1&2A;RA, which has an intact UbdA motif, still induced AbdA-like cuticle transformations, it did not repress Dll (Fig. 3 I and T).
Ubx Has a Different Requirement for Motifs Shared with AbdA.
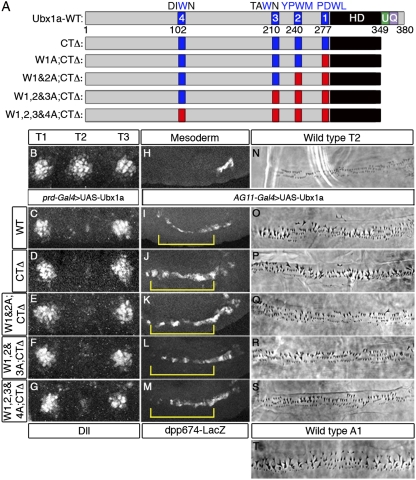
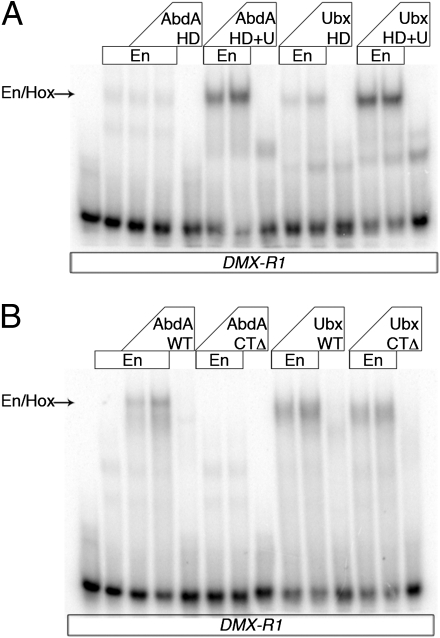
Based on the knowledge gained from the AbdA analyses and sequence conservation (Fig. S5), we performed a similar structure-function study of another abdominal Hox protein, Ubx, which is a repressor of Dll like AbdA. Also like AbdA, Ubx has multiple conserved W motifs and a UbdA motif, as well as a previously identified QA motif in an analogous location to the RRDR motif of AbdA (Fig. 4A). However, unlike AbdA, point mutations in Ubx's UbdA motif (to AALVAV) did not hinder cooperative complex formation with Exd-Hth on DMX-R1 in vitro or the ability to repress Dll in vivo (Fig. S6 A–D). Previous studies have shown that a different set of point mutations in the UbdA motif (to VVLIVA) prevented cooperative complex formation with Exd-Hth on DMX-R1 (17) (Fig. S6A). However, we find that this set of point mutations (but not the AALVAV mutant) resulted in a decrease in the ability of Ubx to bind DNA as a monomer (Fig. S6 A and E). Therefore, any decrease in cooperative complex formation observed with the VVLIVA mutant may not be attributable to a loss of interaction with Exd/Hth but, instead, to the compromised ability of this mutant to bind any DNA sequence. Moreover, and in agreement with the AALVAV UbdA mutant, deletion of the entire C terminus (Ubx-CTΔ), including the UbdA motif, did not affect its ability to repress Dll or induce homeotic transformations in vivo (Fig. 4 D and P). Thus, although the UbdA motif is critical for AbdA to execute its in vivo functions, it is dispensable in Ubx.
Fig. 4.
Ubx does not require C-terminal or W motifs for in vivo functions. (A) Schematics of WT and mutant Ubx proteins. Diagrams are drawn approximately to scale. Blue designates W-motifs. Green designates the UbdA motif (U). Purple designates the QA motif (Q). Red indicates residues mutated to alanines (PDWL→AAAA, YPWM→AAAA, TAWN→TAAN, and DIWN→DIAN). (B–G) Thoracic region of a WT embryo (B) or embryos expressing Ubx proteins in T2 via the prd-Gal4 driver, stained for Dll (white; here and in Fig. 5 the ectopic expression of Hox proteins was robust and nuclear but the images were omitted due to space constraints). The protein variant is indicated on the left. Images are approximately 140 μm wide. (H–M) Visceral mesoderm region of a WT embryo (H) or embryos expressing Ubx proteins via the AG11-Gal4 driver, stained for β-galactosidase to monitor dpp674-lacZ expression (white). Yellow brackets indicate ectopic lacZ activation. Images are approximately 270 μm wide. Phase contrast images depicting ventral T2 cuticle patterns for WT larvae (N) or animals ectopically expressing WT Ubx (O) or mutant Ubx variants (P–S). (T) WT A1 ventral denticle pattern normally specified by Ubx. Cuticle images are approximately 140 μm wide.
Starting with the Ubx-CTΔ mutant, we then mutated each of four potential W motifs (Fig. 4). Surprisingly, all these mutants, even when all putative W motifs were mutated in the context of Ubx-CTΔ, were able to repress Dll (Fig. 4 D–G). Moreover, all these Ubx mutants were also able to activate dpp674-lacZ, an Exd-dependent Ubx target in the visceral mesoderm (20, 35) (Fig. 4 J–M). Ubx also controls the formation of a specific pattern of denticles in the first abdominal segment (A1) of Drosophila larvae (36) (Fig. 4T). Ectopic expression of Ubx causes homeotic transformations to A1 in segments anterior to the WT A1 segment (37, 38) (Fig. 4O compared with Fig. 4 N and T). Remarkably, all mutants in the CTΔ series were also able to transform T2 to an A1-like identity (Fig. 4 P–S). Similar results for Dll repression, dpp674-lacZ activation, and cuticle transformation were obtained when the same series of mutations was tested in the context of a shorter isoform of Ubx, UbxIVa, in which a small portion of the linker region is removed by alternative splicing (39) (Fig. S7). Unfortunately, truncation mutants aimed at identifying essential sequences in the N terminus were uninformative because of a lack of nuclear localization and/or instability of the truncated proteins in vivo. However, it is unlikely that the Ubx N terminus has additional non-W motifs for interacting with Exd because mutating the W motifs in Ubx-CTΔ effectively eliminated cooperative complex formation with Exd on the DMX-R1–binding site (Figs. S7B and S8). Therefore, these results raise the possibility that Ubx may execute these in vivo functions, at least in part, in an Exd-independent manner.
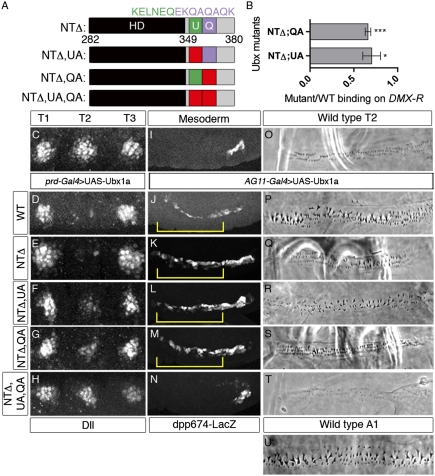
Ubx Homeodomain and C Terminus Are Sufficient for Several in Vivo Functions.
Although the sequences C-terminal to the Ubx homeodomain are dispensable for Dll repression, sequences N-terminal to the homeodomain are also dispensable: A severely truncated form of Ubx that begins at the homeodomain (Ubx-NTΔ, Fig. 5A) was a potent repressor of Dll (Fig. 5E). This mutant, which has no W motifs, was also able to activate dpp674-lacZ in the visceral mesoderm (Fig. 5K) but was compromised in its ability to generate a T2-to-A1 homeotic transformation (Fig. 5Q). The activity of this protein is particularly striking because it only consists of the homeodomain, the UbdA motif, the QA motif, a polyalanine stretch, and eight C-terminal residues that are not conserved (Fig. S7). When either UbdA or QA was mutated in the NTΔ context, we observed a partial reduction in the ability to repress Dll (Fig. 5 F and G). This reduction in repression correlated with a reduction in the ability of these mutants to bind cooperatively with Exd-Hth to DMX-R1 in vitro (Fig. 5B). Additionally, NTΔ;UA and NTΔ;QA induced very weak homeotic transformations (Fig. 5 R and S), but both were able to activate dpp674-lacZ (Fig. 5 L and M). When both UbdA and QA motifs were mutated in the NTΔ context, the protein was completely unable to repress Dll, activate dpp674-lacZ, and induce Ubx-like homeotic transformations (Fig. 5 H, N, and T). Significantly, all these proteins, even NTΔ;UA;QA, were able to repress sal in the wing imaginal disk, demonstrating that they are all still functional transcription factors in vivo (Fig. S9). Together, these data reveal that Ubx is remarkably flexible in its ability to use different motifs to execute its various functions in vivo. Moreover, despite many overt similarities with AbdA, Ubx uses a unique mechanism for regulating Exd-dependent targets in vivo.
Fig. 5.
C-terminal motifs are sufficient for Ubx function. (A) Schematics of NTΔ Ubx proteins. Diagrams are drawn approximately to scale. Red indicates residues mutated to alanines or valines (KELNEQ→AALVAV and EKQAQAQK→AAVAVAVA). (B) Average binding of Ubx NTΔ mutants to the DMX-R probe. The bar graph represents the mean of n ≥ 3 ratios from independent experiments for each mutant. Error bars represent the SEM. To determine if the cooperative binding is significantly different from 1.0, t tests were used (***P = 0.0005; *P = 0.043). Because of cleavage of the protein in bacteria, the NTΔ;UA;QA mutant could not be purified and analyzed by EMSA. (C–H) Thoracic region of a WT embryo (A) or embryos expressing Ubx proteins in T2 via the prd-Gal4 driver, stained for Dll (white). The protein variant is indicated on the left. Images are approximately 140 μm wide. (I–N) Visceral mesoderm region of a WT embryo (I) or embryos expressing Ubx proteins via the AG11-Gal4 driver, stained for β-galactosidase to monitor dpp674-lacZ expression (white). Yellow brackets indicate ectopic lacZ activation. Images are approximately 270 μm wide. Phase contrast images depicting ventral T2 cuticle patterns for WT larvae (O) or animals ectopically expressing WT Ubx (P) or mutant variants (Q–T). (U) WT A1 ventral denticle pattern normally specified by Ubx. Cuticle images are approximately 140 μm wide.
UbdA Mediates Cooperative Complex Formation with En.
Previous studies demonstrated that Exd and Hth are not the only homeodomain proteins that Hox factors can bind DNA cooperatively with: Both Ubx and AbdA also bind cooperatively with En to the Dll DMX-R element (1). Using in vitro DNA-binding assays, we find that in addition to mediating cooperative complex formation with Exd-Hth (Fig. 2B and Fig. S8), the UbdA peptide contributes to cooperative DNA binding with En (Fig. 6). However, there are interesting differences in the way in which these two Hox proteins interact with En. For both Ubx and AbdA, the homeodomain plus UbdA peptide were sufficient to form cooperative complexes with En in vitro (Fig. 6A). However, Ubx but not AbdA bound cooperatively with En in the absence of all sequences C-terminal to the homeodomain (Fig. 6B), suggesting that Ubx has additional sequences in its N terminus or homeodomain that are sufficient to mediate cooperative binding with En. Together with the Exd-binding experiments described above, these results demonstrate that UbdA can mediate complex formation with more than one cofactor. Moreover, they also highlight that the same evolutionarily conserved motif, UbdA, is essential in one context (AbdA) but dispensable in another context (Ubx).
Fig. 6.
UbdA motif mediates interaction with En. (A and B) EMSAs of AbdA and Ubx proteins with En on DMX-R1. The positions of Hox–En cooperative complexes are indicated with arrows. (A) Truncations include the homeodomain (HD) and/or the UbdA motif (U).
Discussion
Cooperative DNA binding with cofactors increases the specificity of many transcription factors, including Hox proteins. In the case of Hox and Exd, physical interactions between these factors are critical for complex formation: The conserved YPWM motif, present in nearly all Hox proteins, directly contacts Exd's homeodomain (2). However, additional analysis of Hox proteins and their target genes has indicated that the W-Exd interaction is not the only mode of Hox-Exd interaction (13–18). Indeed, we demonstrate that there are a surprisingly large number of different ways in which Hox proteins functionally interact with cofactors, even when regulating the same target gene.
Increasing Specificity Using a Shared Cofactor.
Considering the sequence similarities between the Hox homeodomains and YPWM motifs, it is surprising that the same cofactor can increase DNA binding and functional specificity for Hox family members. As a counterexample, the Sry-related HMG box (SOX) family of transcription factors uses different cofactors in different developmental contexts to regulate specific target genes (40). Hox proteins are unusual in that they all have the ability to use the same cofactor, Exd, when executing many of their in vivo functions (7). One way to increase specificity using a single cofactor may be through changing the mode of interaction. Accordingly, we find that of the three Hox proteins analyzed, each uses a different repertoire of binding mechanisms for regulating Exd-dependent target genes.
Based on our data and previous studies, we suggest that the W motif provides Hox proteins with a basal, shared mechanism for interacting with Exd. Interestingly, other non-Hox proteins, such as En and Myogenic differentiation (MyoD), also use tryptophan residues to interact with Exd and Pbx (41, 42). In the case of Scr, a single W motif is necessary for Exd-dependent functions. However, the abdominal Hox proteins Ubx and AbdA use more complex binding mechanisms. AbdA has an additional W motif, TDWM, as well as sequences in its C terminus that contribute, in a context-dependent manner, to the regulation of Exd-dependent targets. The observation that motifs are differentially required depending on the in vivo function suggests that motif utilization may play a role in target site recognition and gene regulation. Previous studies demonstrated that for Scr, interaction of its W motif with the Exd homeodomain helps position paralog-specific residues of the linker region in the minor groove of a specific binding site (24). Although additional structural studies are needed to address this question fully, we speculate that the alternative modes of Hox-Exd interaction described here may function in an analogous manner by directly affecting the way Hox proteins interact with DNA. It is also possible that Exd-Hox interactions alter the way in which the homeodomain docks onto DNA. In support of this idea, previous studies have shown that sequences immediately C-terminal to the homeodomain can play a role in homeodomain structure and specificity (43, 44). Consistently, we find that mutating the UbdA motif in Ubx can adversely affect the ability of the homeodomain to bind DNA, even in the absence of Exd (Fig. S6).
Our results also reveal that despite sharing binding sites and having several similar conserved sequence motifs, Ubx uses a more complex binding mechanism compared with AbdA. Ubx displays impressive flexibility, in that neither sequences N- nor C-terminal to its homeodomain are required for executing some of its functions in vivo (Figs. 4 and 5). Ubx's complexity is further enhanced by the possibility of an Exd-independent mechanism based on the ability of some mutants to function in vivo despite their inability to bind cooperatively with Exd/Hth in vitro (Fig. 4 and Fig. S8). However, because our readout for Dll expression is protein expressed from the native Dll locus (as opposed to a reporter construct), we cannot rule out the possibility that Ubx has the ability to bind cooperatively with Exd to other, as yet unidentified, DNA-binding sites using sequences still present in the W1,2,3&4A;CTΔ mutant.
In addition to the possibility of altering Hox-DNA recognition, the fact that some Hox proteins have more and qualitatively distinct cofactor interaction motifs may be relevant to phenotypic suppression (also called posterior dominance), where more posterior Hox proteins posttranslationally suppress anterior Hox functions (37, 45, 46). Consistent with this notion, the presence of additional posterior-specific cofactor interaction motifs, such as AbdA's UR motif, endows posterior Hox proteins with the ability to outcompete and phenotypically suppress more anterior Hox proteins (47).
Navigating Multiple Cofactors.
Lastly, our data suggest that multiple interaction motifs may help Hox proteins facilitate interactions with other cofactors. In the case of AbdA and Ubx, cooperative complexes on DMX-R include both Exd and En. Using in vitro DNA-binding assays, we found that the UbdA peptide is necessary and sufficient for AbdA to form cooperative complexes with En. Additional structural studies will be necessary to understand the exact mechanism for how a single motif can mediate interaction with multiple cofactors; however, we speculate that having additional Exd-interaction motifs leaves UbdA free to interact with En. Alternatively, UbdA could act as a bridge between the two cofactors, helping to anchor both to the DMX-R–binding site. Interestingly, UbdA is not required for Ubx to form cooperative complexes with En, again suggesting that the same motif has distinct properties in different Hox proteins. In addition, these results suggest that Ubx has other mechanisms that further enhance its flexibility. Additional homeodomain-containing proteins, such as Hth (and Meis in vertebrates), have been suggested to interact with Hox proteins (reviewed in 8). Although specific interaction motifs have yet to be identified, our data suggest, at least in the case of AbdA-Hth-Exd on the rhoA enhancer, that the motifs examined here are not critical for these interactions. It is curious that although Hth is also a TALE class homeodomain, the tryptophan-containing motifs are not playing a measurable role in complex formation on this target in vitro (Fig. S3). From the data presented here, it is clear that the relationship between Hox proteins and their cofactors is not only complex but critical for how functional specificity is achieved.
Methods
Genetic Manipulations and Immunohistochemistry.
Additional information regarding cloning and generation of transgenic UAS lines is provided in SI Methods. UAS lines were chosen to express similar levels of tagged Hox proteins. Either AG11-GAL4 or prd-GAL4 was used for ectopic expression as indicated. Flip-out clones were generated using hs-flp; act<y<Gal4, UAS-GFP. Antibodies used are described in SI Methods. Z-series of embryos were collected on a Leica SP5 confocal microscope. Cuticle images were collected on a Zeiss Axioplan 2 microscope.
EMSAs.
EMSAs were carried out as previously described (31). Cooperative DNA binding was calculated as a ratio of the amount of mutant Exd–Hth complex to the amount of WT Exd–Hth complex in the same gel. Proteins were all purified after their expression in Escherichia coli. Details regarding expression constructs, production and purification from E. coli, and specific EMSA conditions are provided in SI Methods.
Supplementary Material
Acknowledgments
We thank Tiffany Cook for the anti-Spalt antibody and members of the R.M. laboratory, Laura Johnston, and Gary Struhl for suggestions and comments. We also thank Gary Struhl for generous use of his confocal microscope. This work was supported by National Institutes of Health Grant GM54510 (to R.M.) and training Grant 5T32DK07328.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1114118109/-/DCSupplemental.
References
- 1.Gebelein B, McKay DJ, Mann RS. Direct integration of Hox and segmentation gene inputs during Drosophila development. Nature. 2004;431:653–659. doi: 10.1038/nature02946. [DOI] [PubMed] [Google Scholar]
- 2.Mann RS, Lelli KM, Joshi R. Hox specificity unique roles for cofactors and collaborators. Curr Top Dev Biol. 2009;88:63–101. doi: 10.1016/S0070-2153(09)88003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Georges AB, Benayoun BA, Caburet S, Veitia RA. Generic binding sites, generic DNA-binding domains: Where does specific promoter recognition come from? FASEB J. 2010;24:346–356. doi: 10.1096/fj.09-142117. [DOI] [PubMed] [Google Scholar]
- 4.McGinnis W, Krumlauf R. Homeobox genes and axial patterning. Cell. 1992;68:283–302. doi: 10.1016/0092-8674(92)90471-n. [DOI] [PubMed] [Google Scholar]
- 5.Noyes MB, et al. Analysis of homeodomain specificities allows the family-wide prediction of preferred recognition sites. Cell. 2008;133:1277–1289. doi: 10.1016/j.cell.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes CL, Kaufman TC. Hox genes and the evolution of the arthropod body plan. Evol Dev. 2002;4:459–499. doi: 10.1046/j.1525-142x.2002.02034.x. [DOI] [PubMed] [Google Scholar]
- 7.Mann RS, Chan SK. Extra specificity from extradenticle: The partnership between HOX and PBX/EXD homeodomain proteins. Trends Genet. 1996;12:258–262. doi: 10.1016/0168-9525(96)10026-3. [DOI] [PubMed] [Google Scholar]
- 8.Moens CB, Selleri L. Hox cofactors in vertebrate development. Dev Biol. 2006;291:193–206. doi: 10.1016/j.ydbio.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 9.Stevens KE, Mann RS. A balance between two nuclear localization sequences and a nuclear export sequence governs extradenticle subcellular localization. Genetics. 2007;175:1625–1636. doi: 10.1534/genetics.106.066449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rieckhof GE, Casares F, Ryoo HD, Abu-Shaar M, Mann RS. Nuclear translocation of extradenticle requires homothorax, which encodes an extradenticle-related homeodomain protein. Cell. 1997;91:171–183. doi: 10.1016/s0092-8674(00)80400-6. [DOI] [PubMed] [Google Scholar]
- 11.Peifer M, Wieschaus E. Mutations in the Drosophila gene extradenticle affect the way specific homeo domain proteins regulate segmental identity. Genes Dev. 1990;4:1209–1223. doi: 10.1101/gad.4.7.1209. [DOI] [PubMed] [Google Scholar]
- 12.Noro B, Culi J, McKay DJ, Zhang W, Mann RS. Distinct functions of homeodomain-containing and homeodomain-less isoforms encoded by homothorax. Genes Dev. 2006;20:1636–1650. doi: 10.1101/gad.1412606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green NC, Rambaldi I, Teakles J, Featherstone MS. A conserved C-terminal domain in PBX increases DNA binding by the PBX homeodomain and is not a primary site of contact for the YPWM motif of HOXA1. J Biol Chem. 1998;273:13273–13279. doi: 10.1074/jbc.273.21.13273. [DOI] [PubMed] [Google Scholar]
- 14.Joshi R, Sun L, Mann R. Dissecting the functional specificities of two Hox proteins. Genes Dev. 2010;24:1533–1545. doi: 10.1101/gad.1936910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galant R, Walsh CM, Carroll SB. Hox repression of a target gene: Extradenticle-independent, additive action through multiple monomer binding sites. Development. 2002;129:3115–3126. doi: 10.1242/dev.129.13.3115. [DOI] [PubMed] [Google Scholar]
- 16.Merabet S, et al. The hexapeptide and linker regions of the AbdA Hox protein regulate its activating and repressive functions. Dev Cell. 2003;4:761–768. doi: 10.1016/s1534-5807(03)00126-6. [DOI] [PubMed] [Google Scholar]
- 17.Merabet S, et al. A unique Extradenticle recruitment mode in the Drosophila Hox protein Ultrabithorax. Proc Natl Acad Sci USA. 2007;104:16946–16951. doi: 10.1073/pnas.0705832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saadaoui M, et al. Selection of distinct Hox-Extradenticle interaction modes fine-tunes Hox protein activity. Proc Natl Acad Sci USA. 2011;108:2276–2281. doi: 10.1073/pnas.1006964108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tour E, Hittinger CT, McGinnis W. Evolutionarily conserved domains required for activation and repression functions of the Drosophila Hox protein Ultrabithorax. Development. 2005;132:5271–5281. doi: 10.1242/dev.02138. [DOI] [PubMed] [Google Scholar]
- 20.Chan SK, Jaffe L, Capovilla M, Botas J, Mann RS. The DNA binding specificity of Ultrabithorax is modulated by cooperative interactions with extradenticle, another homeoprotein. Cell. 1994;78:603–615. doi: 10.1016/0092-8674(94)90525-8. [DOI] [PubMed] [Google Scholar]
- 21.Galant R, Carroll SB. Evolution of a transcriptional repression domain in an insect Hox protein. Nature. 2002;415:910–913. doi: 10.1038/nature717. [DOI] [PubMed] [Google Scholar]
- 22.Ronshaugen M, McGinnis N, McGinnis W. Hox protein mutation and macroevolution of the insect body plan. Nature. 2002;415:914–917. doi: 10.1038/nature716. [DOI] [PubMed] [Google Scholar]
- 23.Hittinger CT, Stern DL, Carroll SB. Pleiotropic functions of a conserved insect-specific Hox peptide motif. Development. 2005;132:5261–5270. doi: 10.1242/dev.02146. [DOI] [PubMed] [Google Scholar]
- 24.Joshi R, et al. Functional specificity of a Hox protein mediated by the recognition of minor groove structure. Cell. 2007;131:530–543. doi: 10.1016/j.cell.2007.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meijsing SH, et al. DNA binding site sequence directs glucocorticoid receptor structure and activity. Science. 2009;324:407–410. doi: 10.1126/science.1164265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Papadopoulos DK, et al. Function and specificity of synthetic Hox transcription factors in vivo. Proc Natl Acad Sci USA. 2010;107:4087–4092. doi: 10.1073/pnas.0914595107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryoo HD, Mann RS. The control of trunk Hox specificity and activity by Extradenticle. Genes Dev. 1999;13:1704–1716. doi: 10.1101/gad.13.13.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 29.Chan SK, Mann RS. The segment identity functions of Ultrabithorax are contained within its homeo domain and carboxy-terminal sequences. Genes Dev. 1993;7:796–811. doi: 10.1101/gad.7.5.796. [DOI] [PubMed] [Google Scholar]
- 30.Vachon G, et al. Homeotic genes of the Bithorax complex repress limb development in the abdomen of the Drosophila embryo through the target gene Distal-less. Cell. 1992;71:437–450. doi: 10.1016/0092-8674(92)90513-c. [DOI] [PubMed] [Google Scholar]
- 31.Gebelein B, Culi J, Ryoo HD, Zhang W, Mann RS. Specificity of Distalless repression and limb primordia development by abdominal Hox proteins. Dev Cell. 2002;3:487–498. doi: 10.1016/s1534-5807(02)00257-5. [DOI] [PubMed] [Google Scholar]
- 32.Li-Kroeger D, Witt LM, Grimes HL, Cook TA, Gebelein B. Hox and senseless antagonism functions as a molecular switch to regulate EGF secretion in the Drosophila PNS. Dev Cell. 2008;15:298–308. doi: 10.1016/j.devcel.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uhl JD, Cook TA, Gebelein B. Comparing anterior and posterior Hox complex formation reveals guidelines for predicting cis-regulatory elements. Dev Biol. 2010;343:154–166. doi: 10.1016/j.ydbio.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sánchez-Herrero E, Vernós I, Marco R, Morata G. Genetic organization of Drosophila bithorax complex. Nature. 1985;313:108–113. doi: 10.1038/313108a0. [DOI] [PubMed] [Google Scholar]
- 35.Capovilla M, Brandt M, Botas J. Direct regulation of decapentaplegic by Ultrabithorax and its role in Drosophila midgut morphogenesis. Cell. 1994;76:461–475. doi: 10.1016/0092-8674(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 36.Duncan I. The bithorax complex. Annu Rev Genet. 1987;21:285–319. doi: 10.1146/annurev.ge.21.120187.001441. [DOI] [PubMed] [Google Scholar]
- 37.Mann RS, Hogness DS. Functional dissection of Ultrabithorax proteins in D. melanogaster. Cell. 1990;60:597–610. doi: 10.1016/0092-8674(90)90663-y. [DOI] [PubMed] [Google Scholar]
- 38.González-Reyes A, Morata G. The developmental effect of overexpressing a Ubx product in Drosophila embryos is dependent on its interactions with other homeotic products. Cell. 1990;61:515–522. doi: 10.1016/0092-8674(90)90533-k. [DOI] [PubMed] [Google Scholar]
- 39.O'Connor MB, Binari R, Perkins LA, Bender W. Alternative RNA products from the Ultrabithorax domain of the bithorax complex. EMBO J. 1988;7:435–445. doi: 10.1002/j.1460-2075.1988.tb02831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kondoh H, Kamachi Y. SOX-partner code for cell specification: Regulatory target selection and underlying molecular mechanisms. Int J Biochem Cell Biol. 2010;42:391–399. doi: 10.1016/j.biocel.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 41.Peltenburg LT, Murre C. Engrailed and Hox homeodomain proteins contain a related Pbx interaction motif that recognizes a common structure present in Pbx. EMBO J. 1996;15:3385–3393. [PMC free article] [PubMed] [Google Scholar]
- 42.Knoepfler PS, et al. A conserved motif N-terminal to the DNA-binding domains of myogenic bHLH transcription factors mediates cooperative DNA binding with pbx-Meis1/Prep1. Nucleic Acids Res. 1999;27:3752–3761. doi: 10.1093/nar/27.18.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.LaRonde-LeBlanc NA, Wolberger C. Structure of HoxA9 and Pbx1 bound to DNA: Hox hexapeptide and DNA recognition anterior to posterior. Genes Dev. 2003;17:2060–2072. doi: 10.1101/gad.1103303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin L, McGinnis W. Mapping functional specificity in the Dfd and Ubx homeo domains. Genes Dev. 1992;6:1071–1081. doi: 10.1101/gad.6.6.1071. [DOI] [PubMed] [Google Scholar]
- 45.González-Reyes A, Urquia N, Gehring WJ, Struhl G, Morata G. Are cross-regulatory interactions between homoeotic genes functionally significant? Nature. 1990;344:78–80. doi: 10.1038/344078a0. [DOI] [PubMed] [Google Scholar]
- 46.Bachiller D, Macías A, Duboule D, Morata G. Conservation of a functional hierarchy between mammalian and insect Hox/HOM genes. EMBO J. 1994;13:1930–1941. doi: 10.1002/j.1460-2075.1994.tb06462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noro B, Lelli K, Sun L, Mann RS. Competition for cofactor-dependent DNA binding underlies Hox phenotypic suppression. Genes Dev. 2011;25:2327–2332. doi: 10.1101/gad.175539.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.