Abstract
We have used chemical protein synthesis and advanced physical methods to probe dynamics-function correlations for the HIV-1 protease, an enzyme that has received considerable attention as a target for the treatment of AIDS. Chemical synthesis was used to prepare a series of unique analogues of the HIV-1 protease in which the flexibility of the “flap” structures (residues 37–61 in each monomer of the homodimeric protein molecule) was systematically varied. These analogue enzymes were further studied by X-ray crystallography, NMR relaxation, and pulse-EPR methods, in conjunction with molecular dynamics simulations. We show that conformational isomerization in the flaps is correlated with structural reorganization of residues in the active site, and that it is preorganization of the active site that is a rate-limiting factor in catalysis.
Keywords: enzyme catalysis, HIV protease, protein dynamics, protein NMR
The dynamics of the HIV-1 protease protein molecule have attracted a great deal of attention because of the critical role that this enzyme plays in the maturation of the AIDS virus (1). NMR studies of the HIV-1 protease have identified regions of the protein molecule with enhanced mobility (2, 3), and attempts have been made with the help of molecular dynamics (MD) simulations to utilize knowledge of the dynamic properties of the HIV-1 protease protein molecule for improved drug design and to rationalize drug resistant mutations (4–7). Particularly important are two highly mobile regions in the HIV-1 protease molecule, the pair of so-called “flaps” (Fig. 1A), which are seen to close over the substrate or substrate-derived inhibitor in the crystal state (8–11). The conventional view is that the role of the flaps is to provide a gating mechanism for substrate binding and for product release, and to help to orient the substrate within the enzyme-substrate complex in a conformation suitable for catalysis (2–7). In the work reported here, we have reinvestigated the role of the HIV-1 protease flaps in enzyme catalysis by employing advanced biophysical methods in conjunction with a series of unique analogue enzymes prepared by total chemical synthesis.
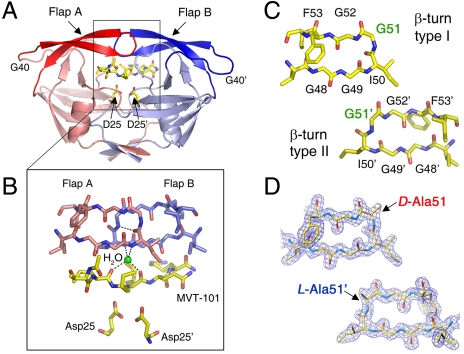
Fig. 1.
Structural features of HIV-1 protease. (A) Homodimeric catalytically active form of HIV-1 protease (2 × 99 amino acids) complexed with the peptidomimetic reduced isostere-containing MVT-101 inhibitor. (B) The β-turn structures (residues 37–61) from each monomer, known as “flaps,” donate hydrogen bonds to the substrate (or inhibitor) through a structural water (water 301) molecule (in green). (C) Flaps with β-turn type I (Upper Left) and β-turn type II (Lower Right) conformations depicted separately for clarity. Residue Gly51 has D-amino acid conformation in the β-turn type I structure and L-amino acid conformation in the β-turn type II structure. (D) Flap X-ray structures in chemically synthesized [L-Ala51; D-Ala51′] covalent dimer HIV-1 protease molecule; 2Fo - Fc electron density contoured in blue at 1.5σ level for the 1.6-Å resolution crystal structure [Protein Data Bank (PDB) ID code 3FSM; see SI Appendix].
Results
Design and Chemical Synthesis of Flap Analogues of HIV-1 Protease.
In X-ray structures of the HIV-1 protease complexed with a peptide substrate or substrate-derived inhibitor, the tips of the flaps adopt two different β-turn conformations, type I or type II (Fig. 1 B and C) (8, 9). The differences between these two flap structures are most pronounced for the highly conserved residues Gly51 and Gly51′ in the middle of the β-turns, where the Gly residue in one flap adopts an L-amino acid conformation (φ = -103°, ψ = -3°) whereas the Gly residue in the other flap has a D-amino acid conformation (φ = 97°, ψ = -13°). Previous NMR studies of HIV-1 protease enzyme/inhibitor complexes have demonstrated the isomerization of these two different β-turn conformations, on both subnanosecond and microsecond-to-millisecond time scales (2, 3). To evaluate whether such isomerizations have any functional significance, we introduced conformational constraints by preparing a series of protein analogues in which residues Gly51/Gly51′ were substituted with L-Ala, D-Ala, or Aib (α-aminoisobutyric acid) in one or both of the flaps of the homodimeric HIV-1 protease protein molecule. In addition, we synthesized chemical analogues of the HIV-1 protease in which individual α-amino acid residues 50–52 at the tips of both flaps were systematically substituted with isosteric α-hydroxy acids, replacing the amide N–H of the peptide bond with an ester moiety.
To enable asymmetric incorporation of different α-amino acids or α-hydroxy acids in the flaps of a single HIV-1 protease enzyme molecule, the two 99-residue monomers were covalently joined through a short linker (five amino acids). The asymmetric analogues were prepared by a fully convergent total synthesis of the 203-amino acid residue protein, based on a combination of native chemical ligation (12) and kinetically controlled ligation (13), giving a “covalent dimer” HIV-1 protease enzyme molecule with full catalytic activity (14). The wild-type enzyme and nine enzyme analogues prepared in this way are listed in Table 1.
Table 1.
Steady-state kinetics of proteolysis by chemically synthesized HIV-1 protease and its analogues
| Flap | Flap′ | kcat, s-1; mean ± SD | Km, μM; mean ± SD | kcat/Km; s-1 μM-1 | PDB ID code | |
| 1* | Gly51 | Gly51 | 23.4 ± 0.4 | 25.1 ± 1.2 | 0.93 | 3HAU |
| 2† | L-Ala51 | D-Ala51 | 17.6 ± 0.3 | 26.1 ± 1.4 | 0.67 | 3FSM |
| 3 | L-Ala51 | Gly51 | 22.2 ± 0.6 | 47.4 ± 2.9 | 0.47 | 3KA2 |
| 4 | Gly51 | D-Ala51 | 6.2 ± 0.2 | 43.8 ± 2.8 | 0.14 | 3HLO |
| 5 | L-Ala51 | L-Ala51 | 3.7 ± 0.1 | 50.1 ± 2.6 | 0.073 | 3HAW |
| 6 | Gly51 | Aib51 | 4.04 | 104.6 | 0.042 | 3IAW |
| 7 | D-Ala51 | D-Ala51 | 4.9 | 434.2 | 0.011 | 3HBO |
| 8 | Aib51 | Aib51 | n/a | n/a | 0.001 | 3HDK |
| 9 | Ile50 | O-Ile50 | 4.2 ± 0.2 | 41.2 ± 3.2 | 0.1 | 3NXE |
| 10 | O-Ile50 | O-Ile50 | 0.94 | 99.6 | 0.009 | 3NWQ |
The PDB ID code of the corresponding X-ray structure with MVT-101 inhibitor is included in the rightmost column.
*The wild-type enzyme 1 was prepared both as a homodimer and as a covalent dimer, and the catalytic properties were found to be identical within experimental uncertainty.
†In a control experiment, the 99-residue L-Ala51 HIV-1 protease polypeptide was folded by dialysis in the presence of an equimolar amount of the 99-residue D-Ala51 HIV-1 protease polypeptide. We observed kcat 9.1 s-1 (one half of the value for analogue 2) and Km 25.6 μM, which corresponded to the properties of the statistical 0.5-molar ratio of (L-Ala51, D-Ala51′) heterodimer enzyme in the mixture; this result precluded the possibility that either dimerization equilibrium or the five-amino acid interdomain linker peptide sequence inserted in covalent dimers affected the observed enzyme kinetics.
Steady-State Kinetics of HIV-1 Protease Flap Analogues.
Steady-state proteolysis kinetics of the analogue enzymes were measured with the fluorogenic substrate Abz-NF6*. The results are shown in Table 1. The values of kcat/Km varied over a wide range, where the least active enzyme was analogue 8 containing Aib51/Aib51′ (approximately 1,000 times lower activity than wild-type enzyme 1). Interestingly, the introduction of L-Ala or D-Ala in either symmetric or asymmetric fashion had distinct effects on catalytic efficiencies as measured by steady-state kinetics. Substitution of both Gly51/51′ with L-Ala led to analogue 5 in which proteolytic activity was found to be approximately 10 times lower than for wild-type enzyme 1, whereas insertion of D-Ala at position 51/51′ in both flaps (enzyme 7) gave an enzyme that had approximately 100 times lower activity. Substituting Gly at position 51 in one flap only with L-Ala (enzyme 3) enhanced the measured Km almost two times with no effect on kcat, whereas substituting with D-Ala in one flap only (enzyme 4) led both to a 2-fold higher Km and an approximately 4-fold reduction of kcat. In contrast to these results, the asymmetric enzyme molecule 2 with L-Ala51 in one flap and D-Ala51′ in the other flap had proteolytic activity similar to the wild-type enzyme 1, suggesting that the asymmetric conformations of the Gly51 and Gly51′ residues seen in the X-ray structure of the wild-type enzyme may be functionally relevant.
High-Resolution X-Ray Structures of Chemical Analogues of HIV-1 Protease.
High-resolution X-ray structures of the HIV-1 protease protein molecule and its chemical analogues were obtained in complexes with the reduced isostere containing substrate-derived inhibitor MVT-101 (SI Appendix, Tables S3–S7). The enzyme protein crystals were all found to be isomorphous (space group P212121), with the geometry and H-bond network at the protein-inhibitor interface highly preserved for different enzyme analogues (see SI Appendix, Table S2). In most of the structures, the flaps were observed to close over the inhibitor, adopting asymmetric βI/βII turn conformations at the flap tips as seen in the wild-type HIV-1 protease. Exceptions were the [L-Ala51/51′]HIV-1 protease where both flaps close over the MVT-101 inhibitor in the symmetric βII/βII conformation, and the [Gly51/Aib51′]HIV-1 protease-MVT-101 complex, where the asymmetric βI/βII conformation is switched to βII/βI relative to the N-to-C orientation of the MVT-101 substrate-derived inhibitor (SI Appendix, Fig. S11). In addition, in the case of the [Aib51/51′]HIV-1 protease complex with MVT-101, one flap does not fully close over the inhibitor. It was not possible to rationalize the very different enzyme kinetics observed for these analogue proteins by looking only at the structures of the flaps.
Interestingly, despite identical growth conditions (pH 6.0) and isomorphous crystal forms for all the protein-inhibitor complexes, the “O—O” distances for the carboxyl oxygens distal from the inhibitor in the catalytic residues Asp25 and Asp25′ were found to vary over the range 2.34–2.55 Å for different chemical analogues (SI Appendix, Table S3). These O—O distances are significantly shorter than the sum of the van der Waal’s contact radii (approximately 3.0–3.1 Å), indicating that a proton must be present between the two oxygens in all these complexes. At the same time, these distances are too short in order to accommodate two protons (i.e., the diprotonated state), as illustrated in the crystal structure of the diprotonated wild-type HIV-1 protease with DMP-323 inhibitor (15) where the corresponding distance was found to be 3.4 Å (16).
Interflap Distance Measurements.
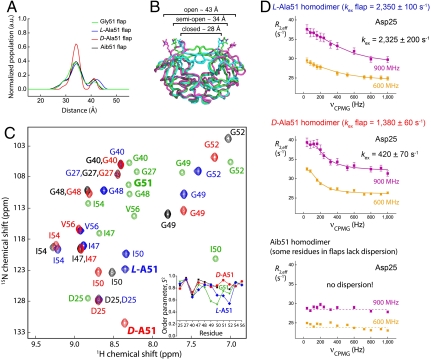
In order to measure how substitutions at position Gly51 affect large-scale motions of the flaps, we incorporated nitroxide spin labels at amino acid positions 55/55′ and measured the nitroxide-to-nitroxide distance distribution profiles for symmetric analogues using the pulse-EPR double electron–electron resonance (DEER) method (Fig. 2 A and B) (17). The experiments were performed with approximately 45 μM solutions of unliganded enzymes, frozen in the glass capillary to 55 K with helium gas, by recording the dipolar spin-echo evolution responses and converting the obtained data into distance snapshots of all conformers present in a given sample at the moment of flash-freezing (18). As was reported earlier (18), three major conformers were observed for all samples—so-called “closed,” “semiopen,” and “open.” We found significant differences in the nitroxide-to-nitroxide distance distributions for the D-Ala51/51′ symmetric analogue, which became much narrower, with the semiopen conformer being more populated here than in all other analogues. This observed predominance of the semiopen conformer for the D-Ala51/51′ symmetric analogue is in agreement with the conformations previously observed in the crystal structure of the unliganded form of HIV-1 protease, where both Gly51 and Gly51′ have a D-amino acid backbone conformation and the flaps adopt a semiopen conformation and are symmetrically related via a twofold axis (19).
Fig. 2.
Dynamic properties of chemically synthesized Gly51 analogues of HIV-1 protease. (A) “Snapshots” of the conformational states (open, semiopen, and closed overlaid in B) of HIV-1 protease chemical analogues labeled with nitroxide-spin label, measured at 55 K using pulse-EPR spectroscopy. Populations of conformers are depicted for Gly51, L-Ala51, D-Ala51 and Aib51 homodimers in green, blue, red, and black, respectively. In the case of the D-Ala51 homodimer analogue, the semiopen conformer is overpopulated. (C) 15N-HSQC spectra overlaid for L-Ala51, D-Ala51, and Aib51 homodimers with the same color coding as in A. Peaks for the wild-type HIV-1 protease (in green) for corresponding residues were reconstructed from a previous study (21). Order parameters S2, obtained by model-free analysis of R1, R2 and 1H-15N NOE values, versus residue number are depicted as Inset. (D) CPMG 15N relaxation dispersion data for catalytic residue Asp25 in three HIV-1 protease symmetric homodimers obtained at two magnetic fields (600 and 900 MHz). Remarkably, the chemical exchange rates for the three analogue enzyme molecules are drastically different, and are correlated with the dynamic properties of the flaps and with the catalytic rates for proteolysis (Table 1; entries 5, 7, and 8).
Protein Dynamics on the Subnanosecond Time Scale.
In order to characterize how substitutions at position 51/51′ of the HIV-1 protease affect the conformational properties of the flaps in these unique enzyme analogues, we performed NMR studies on the unliganded enzymes using site-specifically 15N-labeled protein molecules. Key residues in the flaps were site-specifically 15N-labeled; in addition, the residue Gly40 in the “elbow” regions (see Fig. 1A) and residues Asp25 and Gly27 in the catalytic site were also 15N-labeled (see Fig. 1 A and B and SI Appendix). For these NMR studies, all measurements were performed with symmetrically substituted chemical analogues (homodimers) in order to simplify interpretation of the data (Fig. 2C).
Order parameters (S2) for the protein backbone amides were derived from model-free analysis of measured R1 (spin-lattice relaxation rate), R2 (spin-spin relaxation rate), and heteronuclear 1H-15N NOE (nuclear Overhauser effect) values (20). The order parameter (S2) represents the degree of spatial restriction of internal fluctuation of the amide bond on the subnanosecond time scale, ranging from 0 (completely unrestricted motions) to 1 (completely rigid). S2 values measured for unliganded enzymes were found to be significantly higher for the D-Ala51-containing flap (“D-flap”) and Aib51-containing flap (“Aib-flap”) than for the L-Ala51-containing flap (“L-flap”) (Fig. 2C, Inset). The higher order parameters for the tips of the flaps (residues 48–52) of D-flap and Aib-flap enzyme molecules indicate greater rigidity on the subnanosecond time scale. Remarkably, the L-Ala51-containing flap enzyme molecule had S2 values and hence subnanosecond flexibility comparable to those of the corresponding wild-type Gly51-containing flaps (21). This is consistent with our observation that, of the symmetrically substituted enzyme analogues, the L-Ala51/51′ homodimer had proteolytic activity closest to that of the wild-type enzyme (Table 1; see entries 1, 5, 7, and 8, respectively).
Protein Dynamics on the Microsecond–Millisecond Time Scale.
To elucidate the dynamics of the enzyme analogues in the microsecond–millisecond time regime, 15N Carr–Purcell–Meiboom–Gill (CPMG) relaxation dispersion measurements were performed on the unliganded enzyme analogues (Fig. 2D and SI Appendix) (22). In the three symmetric analogues containing L-flaps, D-flaps, or Aib-flaps, we observed a systematic decrease in mobility on the microsecond–millisecond time scale; the previously observed higher subnanosecond mobility of the L-flap enzyme analogue (above) was also reflected in faster microsecond–millisecond regime dynamics, and a greater subnanosecond rigidity corresponding to slower mobility on the microsecond–millisecond time scale was observed for the D-flap and Aib-flap enzyme analogues. In the L-flap and D-flap enzyme analogues, all labeled residues showed coherently matched chemical exchange rates in these NMR measurements; after fitting the data on a residue-by-residue basis, they were further fitted globally with a three-site exchange model (23), yielding kex (fast) 2,350 ± 100 s-1 (mean ± SD), kex (slow) 38 ± 2 s-1 (mean ± SD) for the L-flap enzyme, and kex (fast) 1,380 ± 60 s-1 (mean ± SD), kex (slow) 80 ± 5 s-1 (mean ± SD) for the D-flap enzyme (see SI Appendix, Figs. S12 and S13, Table S12, and Scheme S3). In the Aib-flap enzyme analogue, however, it was not possible to perform global fitting because of substantial noncoherent variations in exchange rates for different residues, indicating either greater motional complexity or a shift in the principal isomerization rate constant for the flaps as global structures. In fact, several residues actually lost their R2 dispersion in the Aib-flap enzyme analogue (see SI Appendix, Figs. S12 and S13).
The most remarkable differences observed for this series of flap analogues of the HIV-1 protease involved the active-site residues. Our NMR measurements on the flap analogue-containing unliganded enzyme molecules showed that the microsecond–millisecond dynamics of the catalytic residues Asp25/25′ as well as the nearby Gly27/27′ residues were correlated with the dynamics of the flaps. For the L-flap enzyme analogue, both Gly27 and Asp25 in the catalytic region have microsecond–millisecond chemical exchange constants (kex 2,325 ± 200 s-1, mean ± SD) similar to the fast exchange constants observed for the flap region; for the D-flap enzyme analogue, the slower microsecond–millisecond dynamics observed in the flap were found to be correlated to the reduced microsecond–millisecond mobility of the Asp25 and Gly27 residues (kex 420 ± 70 s-1, mean ± SD). And, for the Aib-flap-containing enzyme, both the catalytic Asp25 and residue Gly27 were found to be lacking R2 dispersion as was the case for some residues in the flaps in this enzyme analogue (Fig. 2D, Bottom and SI Appendix, Figs. S12 and S13), meaning that the time scale window probed with our experimental approach was not suitable for this particular chemical analogue.
Molecular Basis of a Protein Dynamics Feedback Catalytic Mechanism.
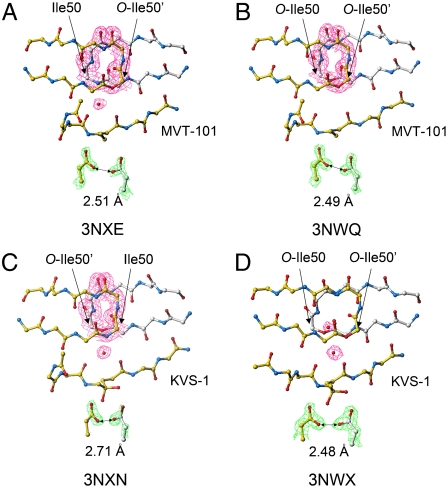
To explore the role of flap backbone hydrogen bonds in catalysis, we synthesized both a monoester [Ile50, O-Ile50′]HIV-1 protease (enzyme 9) and the corresponding diester [O-Ile50,O-Ile50′]HIV-1 protease (enzyme 10), and obtained high-resolution X-ray structures for their complexes with substrate-based inhibitors [for catalytic activities see Table 1; values obtained were generally comparable with previous experimental data (24, 25)]. Interestingly, in the X-ray structures of the ester analogue enzymes complexed with the reduced isostere MVT-101 inhibitor, electron density for the nonnucleophilic water molecule (water 301) bridging the flaps of the enzyme and the carbonyls of the inhibitor was found to be much less intense (Fig. 3A); moreover, for the complex of diester enzyme 10 with MVT-101, we could not locate clear electron density for water 301 (Fig. 3B). This was to be expected, because at least one hydrogen-bond-donating amide—NH—is replaced by an ester oxygen atom in these enzyme analogues, thus knocking out the possibility for a water 301 H2O—HN-Ile50 hydrogen bond. However, the structure of the MVT-101 inhibitor in these complexes did not deviate significantly from the complex with the wild-type enzyme molecule (for enzyme 9, rmsd backbone 0.13 Å, rmsd all atoms 0.44 Å; for enzyme 10, rmsd backbone 0.13 Å, rmsd all atoms 0.84 Å), and both flaps adopted a closed conformation rather similar to that found in the wild-type HIV-1 protease complexed with the same inhibitor.
Fig. 3.
X-ray structures of HIV-1 protease and its ester chemical analogues 9 and 10 complexed with substrate-based inhibitors. (A and B) In complexes of enzymes 9 and 10 with MVT-101 inhibitor, the electron density for structural water 301 molecule is significantly diffused—most strikingly in the enzyme 10 complex (see B), where we could not locate clear density for a water molecule at the flaps/inhibitor interface. (C and D) Interestingly, with the mechanistically based hydrated ketomethylene inhibitor KVS-1 (9), water 301 is well-populated in structures of enzymes 9 and 10. Moreover, in the complex of enzyme 10, there is second structural water molecule. [In all structures, side chains are deleted for clarity except the residues of interest. The 2Fo - Fcelectron density map was contoured at 1.0 σ level (in magenta) for selected flap residues and water molecules, and at a level of 3σ (in green) for residues Asp25 and Asp25′. Distances between distal oxygens of two catalytic Asp25 and Asp25′ and PDB codes are specified for each structure].
In the X-ray structures of these ester analogue enzyme molecules with the ketomethylene isostere KVS-1 inhibitor (which closely mimics a tetrahedral intermediate) (9), the occupancy of water 301 was not so drastically affected as in the case of complexes with MVT-101 inhibitor (Fig. 3 C and D). In the complex of the monoester enzyme 9 with KVS-1 inhibitor, water 301 occupancy (see Fig. 3C) was comparable to that observed for the wild-type HIV-1 protease in the same complex (9). However, in the case of the diester enzyme 10, the H-bonding network was significantly perturbed, and an additional water molecule has been located at the flaps-inhibitor interface (Fig. 3D).
These observations suggest to us that the hydrogen-bonding network at the flaps—inhibitor interface mediated by the nonnucleophilic water molecule may be responsible for a protein dynamics feedback mechanism in HIV-1 protease catalysis. In the case of the ester analogues, the mediating role of the amide hydrogen bond(s) present in the native NH-Ile50 is eliminated, thus destabilizing the rigid closed flaps conformational state, which then would lead to higher flexibility of the corresponding flap structure. Thus, the dynamics of the catalytic site could never adjust to achieve the structure necessary for efficient electrostatic stabilization of the transition state of the catalyzed reaction as happens in the wild-type enzyme. In the wild-type HIV-1 protease, such stabilization is achieved by hydrogen bonds mediated by water 301 that rigidify the flap structures, lock the substrate in a productive conformation, and contribute to preorganization of the structure of the catalytic site. The flaps and water 301 serve to sense the progress of the proteolytic reaction through hydrogen bonding to two carbonyl groups of the substrate (from the amide bond preceding and the amide bond following the scissile amide bond in the polypeptide substrate sequence, respectively), and to thus properly attenuate the dynamics of the catalytic aspartic acid residues. The distance between the aforementioned substrate carbonyl groups increases with the progress of the amide bond cleavage (formation of tetrahedral intermediate, breakdown of tetrahedral intermediate concomitant with product formation and stepwise protein/products dissociation) as was shown by recent crystallographic work (10, 11).
The Active-Site Structure of the HIV-1 Protease Is Preorganized for Catalysis and Is Asymmetric.
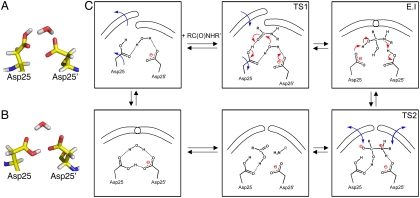
In silico experiments were performed to get a better picture of how the dynamics of the flap structures correlate with the state of the catalytic residues in the HIV-1 protease-substrate complex. All-atom explicit water MD simulations of symmetric L-flap and D-flap enzyme analogues gave results in agreement with interflap distance distributions observed by pulse-EPR measurements (SI Appendix, Fig. S15). In the active site of these two analogue enzymes, MD simulations showed a symmetric cyclic structure consisting of two catalytic Asp25 and Asp25′ residues and the nucleophilic water molecule as the most predominant conformer (Fig. 4B). Because proton transfer between the two catalytic aspartates Asp25 and Asp25′ cannot be treated adequately by the classical molecular mechanics approach employed in the MD simulation, for the case of the asymmetric [L-Ala51; D-Ala51] heterodimer, we performed calculations on two boundary states, with either the L-flap-containing domain Asp25 or the D-flap-containing domain Asp25′ ionized and the other aspartate in the same molecule protonated [note: the monoprotonated state for the two catalytic aspartates is invoked for general acid-general base catalysis (26, 27)]. For the case in which the L-flap-containing domain has a charged Asp25 carboxylate and D-flap-containing domain Asp25′ has a protonated side chain, we observed the same cyclic hydrogen-bonded structure that we found as the most populous state in symmetric homodimers. Strikingly, if we reversed protonation states (i.e., with the L-flap-containing domain Asp25 now being protonated and the D-flap-containing domain Asp25′ being charged), MD simulations showed an enhanced population (approximately 100-fold) of the structure composed of Asp25, Asp25′ residues, and the nucleophilic water molecule hydrogen-bonded asymmetrically and with a geometry preorganized for catalysis (Fig. 4A) (28).
Fig. 4.
Active-site structures revealed by MD simulations, and a scheme for the mechanism of HIV-1 protease catalysis taking into account the dynamics of the flaps. (A) An asymmetric structure of catalytic Asp25 and Asp25′ and the nucleophilic water molecule dominated in the MD trajectory for the asymmetric [L-Ala51; D-Ala51′] chemical analogue of HIV-1 protease with the L-domain having a protonated Asp25 and the D-domain having a charged Asp25′ side chain. (B) Symmetric structure for catalytic residues and nucleophilic water was observed as the most populous state in the MD simulation for the symmetric [L-Ala51; L-Ala51′] and [D-Ala51; D-Ala51′] chemical analogues. (C) Scheme for the mechanism of HIV-1 protease catalysis. Asymmetric conformers are preorganized for catalysis. Conformational isomerizations are depicted by blue arrows, and electron rearrangements are depicted by red arrows. TS1 and TS2 are earlier and later transition states, respectively, and E.I is the enzyme complex with the tetrahedral intermediate.
The computational prediction of a greater concentration of conformers preorganized for catalysis being present in the unliganded [L-Ala51, D-Ala51′] heterodimeric enzyme molecule was supported by surface plasmon resonance (SPR) binding experiments (see SI Appendix, Fig. S16). In the SPR measurements on the HIV-1 protease flap analogues, using a reduced isostere inhibitor to mimic the earlier transition state of the enzyme-catalyzed proteolysis, the [L-Ala51; D-Ala51′] asymmetric enzyme analogue showed tighter binding affinity toward inhibitor than any other studied enzyme analogues, including the wild-type HIV-1 protease (Kd value of 100 nM, four times lower than the Kd value of 420 nM observed for the wild-type enzyme).
Discussion
The accepted chemical mechanism for the aspartyl proteases involves general acid–general base catalysis, where one catalytic aspartate side chain carboxylate (COO-) acts as a general base to remove a proton from the water molecule nucleophile, while another aspartic acid side chain carboxyl (COOH) general acid donates a proton to the carbonyl oxygen atom of the scissile peptide bond (26, 27). Our results are in agreement with the general acid–general base mechanism, with a nucleophilic water molecule preorganized for catalysis in the asymmetric environment of the two aspartates, one being protonated and one being ionized (Fig. 4C). Thus, our data suggest that in the case of the HIV-1 protease, the so-called “L domain” (which contains the L-Gly51 conformation flap) is more mobile and bears the general acid at the Asp25 COOH, whereas the less mobile “D domain” (containing the D-Gly51 flap) is more rigid and bears the general base Asp25 COO- in the active site.
We recently reported that flap structures in HIV-1 protease—complexed with three different inhibitors that mimic, respectively, an early transition state, the tetrahedral intermediate, and a late transition state—display significantly different equilibrium populations of conformers; the flaps are least mobile at the earlier stages of the reaction, and attain more flexibility in the course of the reaction en route to product release (18). The results obtained from the combination of experiments used in the work reported here show that the glycine residue at position 51 in each monomer serves as a surrogate for both the L- and D-amino acids required at that position in each domain of the homodimeric HIV-1 protease molecule preorganized for catalysis. Our data suggest that catalysis is not rate-limited by opening and closing events of flaps, but rather by the emergence of catalytically preorganized asymmetric β-turn type I/β-turn type II conformers in the Gly51/51′-containing wild-type HIV-1 protease. If substrate binding/product release were rate-limiting, we would expect the [L-Ala51, D-Ala51′] covalent dimer enzyme to posses catalytic efficiency lower than the more flexible [L-Ala51, L-Ala51′] homodimer and greater than more rigid [D-Ala51, D-Ala51′] homodimer. However, as illustrated in Fig. 5, the [L-Ala51, D-Ala51′] heterodimer enzyme is approximately 10 times and 100 times, respectively, more efficient as a catalyst than the above mentioned two homodimer enzymes (see also Table 1, entries 2, 5, and 7).
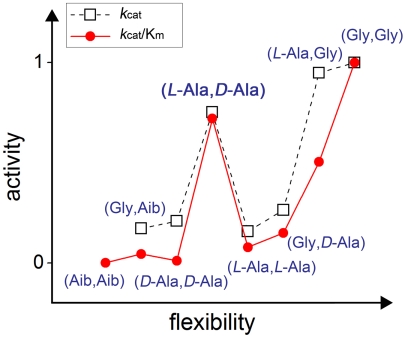
Fig. 5.
“Activity–flexibility” relationship for a series of chemical analogues of HIV-1 protease. The “activity” axis contains relative values of kcat and kcat/Km for enzyme analogues 1–8 (see Table 1) normalized to those of the wild-type enzyme 1. The “flexibility” axis is built based on the assumption that the flap structure of the enzyme molecule containing Gly at position 51 is most flexible, followed by molecules containing L-Ala, D-Ala, and Aib (α-aminoisobutyric acid) replacing Gly. Most of the data follow a general trend where higher flexibility leads to higher activity. The exception is enzyme analogue 2 with one flap containing L-Ala51 and another D-Ala51′.
These results provide strong experimental support for a chemical mechanism in HIV-1 protease in which the contribution of both flaps to catalysis is critical. First, flap β-turn isomerization attenuates the dynamics of the whole protein molecule, resulting in correlation of the dynamics of the flaps and the catalytic residues; then, hydrogen-bonding interactions of HN-Ile50 and HN-Ile50′ at the tips of the flaps to the carbonyls of the substrate on either side of the scissile bond are mediated by a (nonnucleophilic) water molecule and lock the substrate and enzyme molecule in a productive catalytic conformation. In contrast to the HIV-1 protease and related viral aspartyl proteases, the cell-encoded aspartyl protease enzyme molecules have a single polypeptide chain that folds to form two domains, and a single flap that in crystal structures is seen to form direct hydrogen bonds to the substrate or substrate-derived inhibitor (27, 29). We predict that the dynamic properties of the two domains in eukaryotic aspartic protease molecules will be distinct from one another and reminiscent of those reported here for the artificial [L-Ala51; D-Ala51′] covalent dimer HIV-1 protease prepared by total chemical synthesis.
Materials and Methods
The 99-residue polypeptide chains of symmetric homodimers and the 203-residue polypeptides for asymmetric heterodimers were prepared by total chemical synthesis, folded by dialysis and assayed as described (9, 14).
X-Ray Crystallography.
Crystals were grown at 20 °C by the hanging drop vapor diffusion method within 1–30 d and were frozen in liquid nitrogen using mineral oil as cryoprotectant. Data collection was performed at 100 K at the Advanced Photon Source, Argonne National Laboratory.
Pulse-EPR Experiments.
Dipolar spin-echo evolution measurements were carried out using a constant time version of the four-pulse DEER sequence at a temperature of 55 K. Data were processed and analyzed using DeerAnalysis 2008 software.
NMR Experiments.
R1, R2, and heteronuclear NOE values measured using standard pulse sequences on a 600-MHz spectrometer were used to calculate S2 order parameters. Relaxation dispersion spectra were recorded on 600- and 900-MHz spectrometers using room-temperature probes. Dispersion curves were fitted with either two-state or three-state exchange models.
MD Simulations.
All-atom MD simulations were carried out on the symmetric “L-Ala51/51′-flaps” and “D-Ala51/51′ flaps” analogues of the HIV-1 protease, and on the asymmetric “L-Ala51 flap” and “D-Ala51′ flap” analogues, each for 300 ns. All of the simulations were carried out in a truncated octahedron periodic box of explicit water and neutralizing chloride counterions.
Supplementary Material
Acknowledgments.
We acknowledge D.M. Korzhnev and L.E. Kay (University of Toronto, Toronto, ON, Canada) for providing computer software used for fitting NMR relaxation-dispersion data. This work was supported by the Office of Science, Biological and Environmental Research, US Department of Energy (DE-FG02-07ER64501 to S.B.H.K.). This study made use of the National Magnetic Resonance Facility at Madison, which is supported by National Institute of Health Grants P41RR02301 and P41GM66326. Use of the beamlines 14BM, 22BM, 23ID, and 24ID of the Advanced Photon Source (Argonne National Laboratory, Argonne, IL) was supported by the US Department of Energy (W-31-109-Eng-38, DE-AC02-06CH11357), the National Cancer Institute (Y1-CO-1020), the National Institute of General Medical Sciences (Y1-GM-1104), and the National Center for Research Resources at the National Institutes of Health (RR-15301 and RR007707). D.H. is supported in part by the National Science Foundation CAREER MCB-0953061 and Georgia Cancer Coalition. The MD simulations were carried out using the IBM System p5 supercomputer at Georgia State University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The X-ray structures have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 3HAU, 3FSM, 3KA2, 3HLO, 3HAW, 3IAW, 3HBO, 3HDK, 3NXE, 3NWQ, 3NXN, 3NWX, and 3NYG)
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1111202108/-/DCSupplemental.
References
- 1.Pomerantz RJ, Horn DL. Twenty years of therapy for HIV-1 infection. Nat Med. 2003;9:867–873. doi: 10.1038/nm0703-867. [DOI] [PubMed] [Google Scholar]
- 2.Nicholson LK, et al. Flexibility and function in HIV-1 protease. Nat Struct Biol. 1995;2:274–280. doi: 10.1038/nsb0495-274. [DOI] [PubMed] [Google Scholar]
- 3.Ishima R, Louis JM. A diverse view of protein dynamics from NMR studies of HIV-1 protease flaps. Proteins. 2008;70:1408–1415. doi: 10.1002/prot.21632. [DOI] [PubMed] [Google Scholar]
- 4.Perryman AL, Lin J-H, McCammon JA. HIV-1 protease molecular dynamics of a wild-type and of the V82F/I84V mutant: Possible contributions to drug resistance and a potential new target site for drugs. Protein Sci. 2004;12:1108–1123. doi: 10.1110/ps.03468904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hornak V, Simmerling C. Targeting structural flexibility in HIV-1 protease inhibitor binding. Drug Discov Today. 2007;12:132–138. doi: 10.1016/j.drudis.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piana S, Carloni P, Rothlisberger U. Drug resistance in HIV-1 protease: Flexibility-assisted mechanism of compensatory mutations. Protein Sci. 2002;11:2393–2402. doi: 10.1110/ps.0206702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang C-E, Shen T, Trylska J, Tozzini V, McCammon JA. Gated binding of ligands to HIV-1 protease: Brownian dynamics simulations in a coarse-grained model. Biophys J. 2006;90:3880–3885. doi: 10.1529/biophysj.105.074575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller M, et al. Structure of a complex of synthetic HIV-1 protease with a substrate-based inhibitor at 2.3 Å resolution. Science. 1989;246:1149–1152. doi: 10.1126/science.2686029. [DOI] [PubMed] [Google Scholar]
- 9.Torbeev VY, Mandal K, Terechko VA, Kent SBH. Crystal structure of chemically synthesized HIV-1 protease and a ketomethylene isostere inhibitor based on the p2/NC cleavage site. Bioorg Med Chem Lett. 2008;18:4554–4557. doi: 10.1016/j.bmcl.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 10.Das A, et al. Crystal structure of HIV-1 protease in situ product complex and observation of a low-barrier hydrogen bond between catalytic aspartates. Proc Natl Acad Sci USA. 2006;103:18464–18469. doi: 10.1073/pnas.0605809103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das A, et al. X-ray snapshot of HIV-1 protease in action: Observation of tetrahedral intermediate and short ionic hydrogen bond SIHB with catalytic aspartate. J Am Chem Soc. 2010;132:6366–6373. doi: 10.1021/ja100002b. [DOI] [PubMed] [Google Scholar]
- 12.Dawson PE, Muir TW, Clark-Lewis I, Kent SBH. Synthesis of proteins by native chemical ligation. Science. 1994;266:776–779. doi: 10.1126/science.7973629. [DOI] [PubMed] [Google Scholar]
- 13.Bang D, Pentelute BL, Kent SBH. Kinetically controlled ligation for the convergent chemical synthesis of proteins. Angew Chem Int Ed Engl. 2006;45:3985–3988. doi: 10.1002/anie.200600702. [DOI] [PubMed] [Google Scholar]
- 14.Torbeev VY, Kent SBH. Convergent chemical synthesis and crystal structure of a 203 amino acid ‘covalent dimer’ HIV-1 protease enzyme molecule. Angew Chem Int Ed Engl. 2007;46:1667–1670. doi: 10.1002/anie.200604087. [DOI] [PubMed] [Google Scholar]
- 15.Yamazaki T, et al. NMR and X-ray evidence that the HIV protease catalytic aspartyl groups are protonated in the complex formed by the protease and a non-peptide cyclic urea-based inhibitor. J Am Chem Soc. 1994;116:10791–10792. [Google Scholar]
- 16.Lam PY, et al. Cyclic HIV protease inhibitors: Synthesis, conformational analysis, P2/P2′ structure-activity relationship, and molecular recognition of cyclic ureas. J Med Chem. 1996;39:3514–3525. doi: 10.1021/jm9602571. [DOI] [PubMed] [Google Scholar]
- 17.Pannier M, Veit S, Godt A, Jeschke G, Spiess HW. Dead-time free measurement of dipole-dipole interactions between electron spins. J Magn Reson. 2000;142:331–340. doi: 10.1006/jmre.1999.1944. [DOI] [PubMed] [Google Scholar]
- 18.Torbeev VY, et al. Dynamics of “flap” structures in three HIV-1 protease/inhibitor complexes probed by total chemical synthesis and pulse-EPR spectroscopy. J Am Chem Soc. 2009;131:884–885. doi: 10.1021/ja806526z. [DOI] [PubMed] [Google Scholar]
- 19.Wlodawer A, et al. Conserved folding in retroviral proteases: Crystal structure of a synthetic HIV-1 protease. Science. 1989;245:616–621. doi: 10.1126/science.2548279. [DOI] [PubMed] [Google Scholar]
- 20.Jarymowycz VA, Stone MJ. Fast time scale dynamics of protein backbones: NMR relaxation methods, applications, and functional consequences. Chem Rev. 2006;106:1624–1671. doi: 10.1021/cr040421p. [DOI] [PubMed] [Google Scholar]
- 21.Freedberg DI, et al. Rapid structural fluctuations of the free HIV protease flaps in solution: Relationship to crystal structures and comparison with predictions of dynamic calculations. Protein Sci. 2002;11:221–232. doi: 10.1110/ps.33202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmer AG, Kroenke CD, Loria JP. Nuclear magnetic resonance methods for quantifying microsecond-to-millisecond motions in biological macromolecules. Methods Enzymol. 2001;339:204–238. doi: 10.1016/s0076-6879(01)39315-1. [DOI] [PubMed] [Google Scholar]
- 23.Korzhnev DM, et al. Low-populated holding intermediates of Fyn SH3 characterized by relaxation dispersion NMR. Nature. 2004;430:586–590. doi: 10.1038/nature02655. [DOI] [PubMed] [Google Scholar]
- 24.Baca M, Kent SBH. Catalytic contribution of flap-substrate hydrogen bonds in HIV-1 protease explored by chemical synthesis. Proc Natl Acad Sci USA. 1993;90:11638–11642. doi: 10.1073/pnas.90.24.11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baca M, Kent SBH. Protein backbone engineering through total chemical synthesis: New insight into the mechanism of HIV-1 protease catalysis. Tetrahedron. 2000;56:9503–9513. [Google Scholar]
- 26.Suguna K, Padlan EA, Smith CW, Carlson WD, Davies DR. Binding of a reduced peptide inhibitor to the aspartic proteinase from Rhizopus chinensis: Implications for a mechanism of action. Proc Natl Acad Sci USA. 1987;84:7009–7013. doi: 10.1073/pnas.84.20.7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davies DR. The structure and function of the aspartic proteinases. Annu Rev Biophys Biophys Chem. 1990;19:189–215. doi: 10.1146/annurev.bb.19.060190.001201. [DOI] [PubMed] [Google Scholar]
- 28.Piana S, Bucher D, Carloni P, Rothlisberger U. Reaction mechanism of HIV-1 protease by hybrid Car-Parrinello/classical MD simulations. J Phys Chem B. 2004;108:11139–11149. [Google Scholar]
- 29.Dunn BM. Structure and mechanism of the pepsin-like family of aspartic peptidases. Chem Rev. 2002;102:4431–4458. doi: 10.1021/cr010167q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.