Abstract
Transposon control is a critical process during reproduction. The PIWI family proteins can play a key role, using a piRNA-mediated slicing mechanism to suppress transposon activity posttranscriptionally. In Drosophila melanogaster, Piwi is predominantly localized in the nucleus and has been implicated in heterochromatin formation. Here, we use female germ-line–specific depletion to study Piwi function. This depletion of Piwi leads to infertility and to axis specification defects in the developing egg chambers; correspondingly, widespread loss of transposon silencing is observed. Germ-line Piwi does not appear to be required for piRNA production. Instead, Piwi requires Aubergine (and presumably secondary piRNA) for proper localization. A subset of transposons that show significant overexpression in germ-line Piwi-depleted ovaries exhibit a corresponding loss of HP1a and H3K9me2. Germ-line HP1a depletion also leads to a loss of transposon silencing, demonstrating the functional requirement for HP1a enrichment at these loci. Considering our results and those of others, we infer that germ-line Piwi functions downstream of piRNA production to promote silencing of some transposons via recruitment of HP1a. Thus, in addition to its better-known function in posttranscriptional silencing, piRNA also appears to function in a targeting mechanism for heterochromatin formation mediated by Piwi.
Transposons are molecular parasites known to play critical roles in the biology of their host in multiple ways, including being a major force shaping the evolutionary history of a lineage (1, 2). For individuals, germ-line defense against transposon invasion and mobilization is necessary to maintain the fidelity of genome transmission and general fitness of the offspring. Several different systems involving small RNAs have evolved in eukaryotes for transposon control (3). In many cases, a system involving an RNA-dependent RNA polymerase (RDRP) amplification and Dicer processing of precursor transcripts is used (4, 5). This endogenous small RNA defense mechanism shares many features with the RNAi mechanism first described in Caenorhabditis elegans (6). However, in some animals, a distinct small RNA defense mechanism has been described (7) using small RNAs that interact specifically with the PIWI clade of argonaute proteins (piRNA) (8–12). Production of piRNA is independent of Dicer enzymes (11) and, correspondingly, these small RNAs are slightly larger in size (24∼30 nt). Rather than use of an RDRP, the amplification of piRNAs has been reported to rely on reciprocal slicing of single-stranded precursor transcripts by PIWI proteins, a process referred to as ping-pong amplification (8, 9).
In the Drosophila melanogaster female gonad, two distinct piRNA pathways have been identified that drive transposon silencing in the germ line and the soma, respectively (13–15). In the germ line, piRNA biogenesis involves both primary processing and a secondary amplification pathway (ping-pong amplification) (8, 9, 16), whereas piRNAs in the soma are generated solely from primary transcripts (primary pathway) (14, 15, 17).
Despite recent progress, the mechanisms used by piRNA to promote silencing are not clear; evidence supporting both transcriptional and posttranscriptional silencing mechanisms has been reported (10, 18–21). The fly genome codes for three PIWI family argonaute proteins used in the piRNA pathways are Piwi, Aub, and AGO3 (22). Although Aub and AGO3 are restricted to the germ-line cytoplasm, Piwi localizes predominantly in the nucleus, while still present in the cytoplasm of both the germ line and the ovarian soma (8, 10, 23). Correspondingly, Piwi appears to be a key component of both germ line and somatic piRNA pathways (14, 15). Aub and AGO3 are the enzymes that generate the 5′ end of secondary piRNAs (8, 9). However, the exact role(s) of Piwi, potentially distinct in germ line and soma, remains to be elucidated.
Piwi was originally identified as a gene required for maintenance of germ-line stem cells in D. melanogaster (24). Its identification as an argonaute protein (22) led to the identification of piRNAs and their role in transposon silencing. In Drosophila, Piwi was first proposed to take part in the “ping-pong” amplification of secondary piRNAs, which drives a robust posttranscriptional transposon silencing mechanism (8, 9). However, recent high-throughput sequencing analysis (14) has revealed that Piwi is not required for ping-pong amplification; nonetheless, a role for Piwi in germ-line transposon silencing has been demonstrated (25). How germ-line Piwi functions in transposon silencing is thus an open question.
Although Piwi is likely not involved in the cytoplasmic ping-ping amplification, it could participate in other steps of piRNA biogenesis. Alternatively, Piwi could function directly in transposon silencing by using piRNAs. The majority of Drosophila piRNAs map to the pericentric or telomeric heterochromatin (8, 10). In Schizosaccharomyces pombe, the RITS complex uses both an argonaute protein, Ago1, and an HP1 protein, Chp1, in targeting heterochromatin assembly (26). Drosophila Piwi and HP1a interact directly in the yeast two-hybrid system and coimmunoprecipitate from embryo lysates (18). In vitro studies indicate that the Piwi N-terminal peptide binds to a dimer of the HP1a chromo shadow domain by using a PXVXL motif (27). These observations suggest a role for Piwi in targeting HP1a to silence transposons through a chromatin-based mechanism. However, Piwi is capable of slicing an RNA substrate in vitro (10), which argues for a posttranscriptional or cotranscriptional silencing function.
Most prior functional analyses of Piwi have used mutant lines deficient in Piwi in both germ line and soma (11, 12, 14). This strategy results in a mixture of germinal and somatic piwi phenotypes and could reflect the mixed features of Piwi in two (or more) independent pathways. Further, a lack of functional Piwi in the ovarian soma leads to a block in oogenesis, with pleiotropic consequences (23).
In this study, we specifically deplete Piwi in the germ line to gain a mechanistic understanding of its function there. We observe that germ-line Piwi apparently functions downstream of piRNA production to silence a subset of transposons; loss of transposon silencing generally correlates with loss of HP1a and H3K9me2 from the repetitious element. The results support a chromatin-based transcriptional silencing mechanism dependent on germ-line Piwi and suggest a possible mechanism for targeting heterochromatin formation.
Results
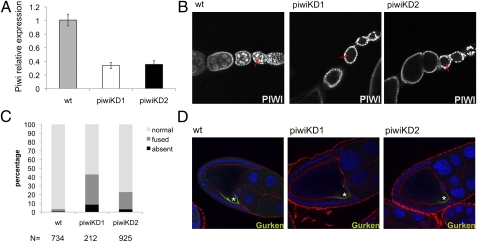
We depleted Piwi using a female germ-line–specific GAL4 driver, NGT40 (28), driving an RNAi knockdown construct (29) in conjunction with overexpression of DCR2 (SI Appendix, Fig. S1). To ensure target specificity, two RNAi knockdown hairpins with no overlapping 19-mers (examined by a sliding window analysis) were used (Materials and Methods). Both hairpin constructs have Piwi as their only target in the fly genome. Knockdown experiments using these hairpins result in a decrease in the level of piwi transcript in the ovaries to one-third that of the wild type (Fig. 1A). Because the transcript level was measured by using whole ovaries, the majority of the residual piwi transcripts likely come from the somatic follicle cells. Immunofluorescent staining of the knockdown ovaries with Piwi antibody shows that the signal in the germ cells is strongly depleted, whereas the signal in the surrounding somatic follicle cells is not affected (Fig. 1B), demonstrating that knockdown is significant and specific to the germ line.
Fig. 1.
Germ-line–specific Piwi depletion leads to axis specification defects in developing egg chambers. (A) Quantitative RT-PCR analysis of the piwi expression level in germ-line Piwi knockdown ovaries. Expression levels are given relative to the RPL32 locus. (Bars represent the mean ± SEM) (B) Piwi antibody staining of developing egg chambers. Piwi depletion specifically in the germ line (red arrows) is achieved with either of two independent knockdown constructs without affecting the surrounding somatic follicle cells. (Full genotypes of piwiKD1 and piwiKD2 are given in SI Appendix, Table S5.) (C) The cumulative percentage of dorsal appendage phenotype of embryos produced by germ-line Piwi knockdown females. (N represents the total number of embryos scored for each genotype.) (D) Gurken (green) immunofluorescent staining of stage 9 developing egg chambers. The oocyte nucleus is indicated (asterisk). DAPI staining (blue) marks the nuclei and the actin filament (red) marks the cell boundaries. Gurken localization is diminished in the Piwi knockdown lines.
As reported earlier using a mitotic recombination strategy (23), germ-line Piwi knockdown does not block oogenesis. However, the eggs laid show a high frequency of collapse and a very low rate of hatching (SI Appendix, Table S1). A significant portion of the embryos produced here from germ-line Piwi knockdown females show fused or absent dorsal appendages (Fig. 1C). Correspondingly, Gurken localization to the dorsal region of the developing oocyte (required for specification of the dorsal/ventral axis) is decreased (Fig. 1D). A similar shift in Gurken localization and a concomitant dorsal appendage defect have been observed with other mutations in the piRNA pathway (30).
It was suggested that the axis polarity specification defects resulting from mutations in piRNA pathway genes are likely a secondary effect because of loss of transposon control (30, 31). Transposon transposition creates DNA double-strand breaks; a DNA damage response can occur, leading to a checkpoint arrest and polarity specification defects. Indeed, Kalmykova et al. have shown that the progeny of piwi mutants can exhibit new insertion sites for the mdg1 transposon (32). This report of actual transposition events provides strong evidence linking transposon activity with the polarity specification defects commonly observed in mutants deficient in piRNA pathway components and seen here.
Examining transposon expression levels in these Piwi germ-line knockdown lines, we observe a loss of silencing for over one-half of the ≈30 transposons tested by quantitative PCR using total ovarian cDNA (Table 1). Telomeric retrotransposon HeT-A and LTR retrotransposon Burdock show the most dramatic effects. In general, transposons that show increased expression in germ-line Piwi knockdown lines were the same as those that showed increased expression in an ago3 mutant line as reported in Li et al. (13) (Table 1). This correlation argues that germ-line Piwi functions in the same pathway as Aub and AGO3 (8, 9). However, three cases clearly do not follow this pattern: Transposons Max, Idefix, and Invader1 are significantly up-regulated in Piwi germ-line–knockdown ovaries (Table 1), but are reported to show little to no response to an ago3 mutation (13). This discrepancy suggests an additional role for Piwi.
Table 1.
Transposon response to germ-line Piwi knockdown
| Element (type)* | Fold expression† | AGO3 response‡ | AGO3 grouping§ |
| Strong¶ | |||
| Bari1 (T) | 3.94 ± 1.48 | I | I |
| Blood (L) | 4.67 ± 0.03 | S | III |
| Burdock (L) | 7.81 ± 0.87 | S | I |
| Diver (L) | 4.24 ± 0.09 | S | I |
| HeT-A (N) | 8.70 ± 1.44 | S | I |
| Idefix (L) | 4.03 ± 0.95 | W | III |
| Invader1 (L) | 3.40 ± 1.66 | W | II |
| Invader4 (L) | 5.32 ± 1.44 | I | I |
| Max (L) | 3.19 ± 0.07 | W | I |
| Intermediate¶ | |||
| 1360 (T) | 1.91 ± 0.69 | I | I |
| 1731 (L) | 2.83 ± 1.15 | W | I |
| 412 (L) | 2.27 ± 0.15 | I | III |
| Accord (L) | 1.91 ± 1.60 | I | I |
| Diver2 (L) | 2.79 ± 0.87 | W | II |
| Jockey (N) | 2.85 ± 0.05 | I | I |
| R1A1 (N) | 2.10 ± 0.44 | W | I |
| Rt1a (N) | 1.91 ± 0.34 | W | I |
| Weak¶ | |||
| 297 (L) | 1.21 ± 0.95 | W | III |
| Aurora (L) | 1.28 ± 0.01 | W | I |
| Doc (N) | 1.56 ± 0.69 | W | I |
| F-element (N) | 0.80 ± 0.51 | W | I |
| Gypsy6 (L) | 1.43 ± 0.90 | W | III |
| Hopper (T) | 1.59 ± 0.53 | W | II |
| INE-1 (S) | 1.33 ± 0.98 | W | I |
| Mdg1 (L) | 0.76 ± 0.04 | W | III |
| NOF (T) | 0.56 ± 0.58 | W | I |
| Opus (L) | 0.82 ± 0.52 | W | I |
| Roo (L) | 0.28 ± 0.24 | W | I |
| S-element (T) | 1.36 ± 0.43 | W | I |
*Element type abbreviations used are: T, TIR element; L, LTR retrotransposon; N, non-LTR retrotransposon; S, SINE element.
†Average (±SD) of two Piwi knockdown lines relative to a wild-type control. Data presented using a log 2 scale.
‡Transposon expression in response to an ago3 mutation. The response is classified based on results from Li et al. (13). I, intermediate; S, strong; W, weak to none.
§Transposon groupings based on piRNA sequencing results (13).
¶The extent of response is categorized into three groups using cutoff values of 3.0-fold and 1.6-fold increased expression for each transposon (log 2 scale).
To confirm that the observed transposon overexpression phenotype is a direct consequence of Piwi depletion in the germ line, we used a DFS-FLP strategy to populate the entire germ line with homozygous piwi1 germ cells (SI Appendix, Fig. S2) (33) and assayed the effect on transposon expression with and without the presence of a wild-type Piwi rescue construct. Germ-line piwi flip-out ovaries exhibit strong up-regulation of expression from transposons HeT-A, Burdock, Blood, and Invader1 (SI Appendix, Fig. S3), similar to that seen for germ-line Piwi knockdown ovaries (Table 1). A wild-type Piwi transgene results in rescue, with all four tested transposons reverting to wild-type levels of expression (SI Appendix, Fig. S3). This result confirms the specificity of the knockdown effect and indicates that some transposons, e.g., Invader1, require Piwi but not AGO3 (and the secondary piRNA it helps produce) for proper regulation, suggesting an additional mechanism. In addition, in the cases tested, a Piwi transgene with a valine to alanine substitution at amino acid 30, PiwiV30A (18), also rescues the overexpression phenotype (SI Appendix, Fig. S3), suggesting that an intact PXVXL motif is not required for Piwi to silence transposons in the germ line.
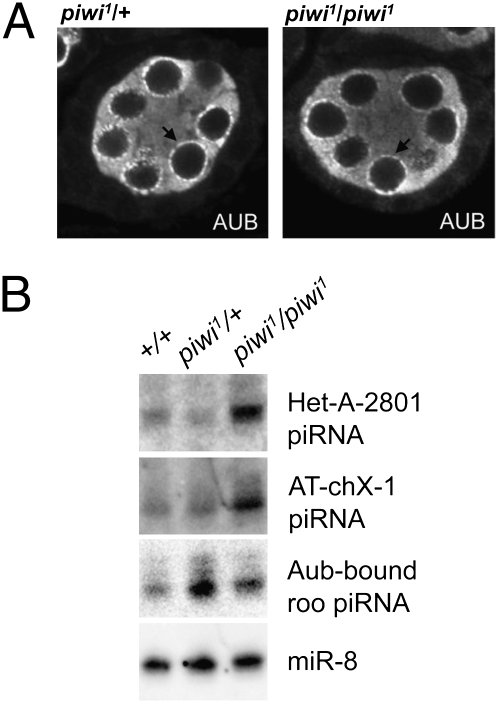
Using the DFS-FLP strategy to replace wild-type piwi with the piwi1 loss-of-function allele (SI Appendix, Fig. S2), we next looked at the impact of germ-line Piwi depletion on the localization pattern of Aub. Mutations disrupting the ping-pong amplification process can lead to mislocalization of Aub from the peri-nuclear structure nuage (13, 34), the proposed site of secondary piRNA production (34). Similar to earlier observations using mutants that disrupt Piwi in both germ line and soma (14), we found no impact on Aub localization to the nuage (Fig. 2A). The lack of change is in agreement with the earlier finding that germ-line Piwi is not required for the ping-pong amplification process (14).
Fig. 2.
Depletion of germ-line Piwi does not disrupt Aub function. (A) Aub immunofluorescent staining of stage 4/5 egg chambers bearing piwi1/+ or piwi1/piwi1 germ line. The peri-nuclear structure nuage (black arrow) and Aub localization are not perturbed in the piwi1/piwi1 germ line. (B) Small RNA Northern blot analysis using three different piRNA probes, HeT-A-2801, AT-chX-1, and Aub-bound roo, along with a microRNA probe, miR-8, as a loading control. (A and B) Genotypes indicated are germ-line genotype at the piwi locus.
To ask whether germ-line Piwi is involved in other steps of the piRNA biogenesis pathway, we assayed piRNAs originating from three independent loci, HeT-A (a telomeric non-LTR retrotransposon), Roo (an abundant LTR retrotransposon), and AT-chX-1 (a nontransposon repetitive DNA element) (35), by Northern blot. In contrast to findings using mutants that disrupt piwi in both germ line and soma (11), we did not observe a disruption of piRNA production in germ-line piwi1 mutant ovaries. Instead, we see an increase in piRNA from all three elements (Fig. 2B), suggesting that germ-line Piwi is not required for piRNA biogenesis. Results from germ-line Piwi knockdowns are similar (SI Appendix, Fig. S4).
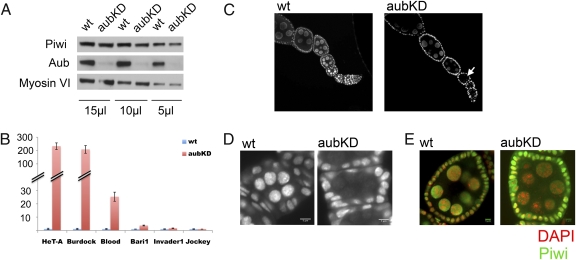
These results indicate a role for germ-line Piwi downstream of piRNA production, potentially downstream of Aub/Ago3 activity. Germ-line–specific knockdown of Aub (Fig. 3A) results in a strong depletion of the Roo element, AT-chX-1, and 3′-UTR HeT-A piRNAs in ovaries (SI Appendix, Fig. S4 A and B). All of these probes hybridize extensively with ping-pong–amplified piRNAs (SI Appendix, Table S2); their depletion confirms that germ-line Aub knockdown disrupts the ping-pong amplification cycle. Inspection of transposon expression levels shows significant up-regulation of multiple transposons on Aub knockdown (Fig. 3B). In particular, retrotransposons HeT-A and Burdock, which showed strong up-regulation upon germ-line Piwi depletion (Table 1 and SI Appendix, Fig. S3), show similar up-regulation here, supporting the idea that Piwi and Aub are functioning in the same pathway.
Fig. 3.
Germ-line Aub knockdown perturbs proper Piwi nuclear localization and leads to overexpression of some transposons. (A) Western blot analysis of Piwi or Aub protein levels in Aub knockdown ovaries shows no significant loss of Piwi. Myosin VI is used as the loading control; the volume of lysate loaded in each lane is indicated beneath. (B) Quantitative RT-PCR analysis of transposon expression levels in germ-line Aub knockdown ovaries. Expression levels are given relative to the RPL32 locus. (Bars represent the mean ± SEM.) (C) Piwi immunofluorescent staining of ovarioles. In the Aub knockdown germ line, Piwi is barely visible in the nuclei of early stage egg chambers (arrow) in contrast to wild type. (D) The diffuse pattern of Piwi staining in an Aub knockdown germ line is most apparent in stage 2/3 egg chambers. (E) Piwi immunofluorescent staining of stage 6/7 egg chambers. DNA staining is shown in red to delineate the nucleus. The overall Piwi signal in the Aub knockdown egg chambers is adjusted so that the signal strength in the germline nuclei matches the corresponding region in the wild-type egg chamber. (Scale bars: 5 μm.)
No significant impact on Piwi expression levels is observed in Aub knockdown ovaries (Fig. 3A). However, mutations in piRNA pathway components can lead to mislocalization of Piwi protein (14, 36); aubQC42/aubHN2 results in a strong decrease in the level of Piwi in the nucleus (13). Here, immunofluorescent staining experiments show a notable depletion of Piwi signal in the germ-line nuclei of Aub knockdown ovaries (Fig. 3C). Because the total Piwi protein level is not affected (Fig. 3A), we reason that Aub knockdown results in a dispersed Piwi localization pattern. The diffuse nuclear localization is most obvious in early stage egg chambers (Fig. 3D), whereas the reduced nuclear-to-cytoplasmic Piwi signal ratio is more obvious in latter stage egg chambers (Fig. 3E). This Piwi staining pattern in early stage egg chambers (Fig. 3D) is very similar to that reported in zucchini mutant ovaries (36). The evidence as a whole argues for a role for Aub in Piwi nuclear localization and indicates a function for germ-line Piwi in transposon silencing downstream of piRNA production.
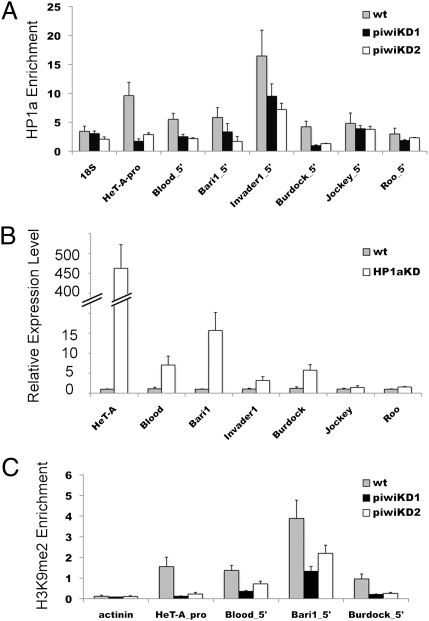
Previous studies have supported a role for the piRNA pathway in heterochromatin-dependent silencing (18, 20, 21), in particular implicating a direct interaction between Piwi and HP1a (18, 27). Chromatin immunoprecipitation experiments show a significant loss of HP1a after germ-line Piwi knockdown at five transposon sites of seven tested, looking at their promoter region or 5′ end (Fig. 4A). The Roo element is not regulated by germ-line Piwi (Table 1), and we observe little to no impact on its HP1a enrichment (Fig. 4A). Among the transposons tested, HeT-A and Burdock show the most dramatic depletion of HP1a, whereas Blood, Bari1, and Invader1 also show a significant decrease (Fig. 4A). Similar (but less potent in the case for HeT-A) results were observed when the internal regions of these transposons were examined (SI Appendix, Fig. S5). Transposon Jockey shows little to no HP1a depletion, suggesting additional mechanisms for Piwi silencing.
Fig. 4.
Germ-line Piwi functions in silencing some transposons through an HP1a-dependent chromatin-based mechanism. (A) ChIP-quantitative PCR analysis at 5′ ends or promoter regions (as indicated in the label) of a set of transposons using antibodies against HP1a in germ-line Piwi knockdown ovaries. The enrichment levels are relative to the α-actinin locus. (B) Quantitative RT-PCR analysis of expression levels for the same set of transposons in germ-line HP1a knockdown ovaries. Fold expression levels are relative to RPL32 expression. (C) ChIP-quantitative PCR analysis at 5′/promoter regions of a set of transposons using antibodies against H3K9me2 in germ-line Piwi knockdown ovaries. The enrichment levels are relative to the 18S ribosomal DNA locus. Bars represent mean ± SEM of three biological replicate experiments.
To ask whether this Piwi-dependent enrichment of HP1a at transposon sites is established through a mechanism downstream of secondary piRNA production, we examined the impact of Aub knockdown on HP1a enrichment. Transposons HeT-A, Blood and Burdock all show loss of HP1a enrichment similar to that seen in germ-line Piwi knockdown (SI Appendix, Fig. S6). A similar lack of impact on the Roo element is also observed. These findings suggest that for transposons HeT-A, Blood, and Burdock, Piwi recruits HP1a to transposon sites through a mechanism downstream of secondary piRNA production.
For six of seven transposons tested in germ-line Piwi knockdown ovaries, we observe a strong correlation between depletion of HP1a occupancy and increase in transcript levels (Fig. 4A and Table 1). To directly test this relationship, we examined transposon expression levels in germ-line HP1a-depleted ovaries. Germ-line HP1a knockdown blocks oogenesis and results in abnormal ovaries (SI Appendix, Fig. S7), but the incomplete penetrance and variable expressivity of this phenotype in our system allowed us to prepare ovarian cDNA (primed with random hexamers) to profile expression of these transposons. We observe a high degree of correlation between the two datasets (Fig. 4 A and B). Significant up-regulation of expression in the absence of germ-line HP1a (Fig. 4B) is seen for all five transposons that show significant HP1a depletion at their 5′ end/promoter region in germ-line Piwi knockdown ovaries (Fig. 4A). Transposons Jockey and Roo show no significant change in expression (Fig. 4B), correlating with the lack of impact on HP1a enrichment levels in germ-line Piwi knockdown ovaries (Fig. 4A). These results demonstrate that enrichment of HP1a is critical for maintaining proper control of expression for a subset of transposons.
HP1a functions as a structural component of pericentric heterochromatin (37), binding di- and trimethylated histone H3 lysine 9 (H3K9me2/3) through its chromo domain (38, 39) and interacting with SU(VAR)3–9, a histone 3 lysine 9 methyltransferase (40). To look for evidence of a similar mechanism here, we examined the impact of germ-line Piwi knockdown on the enrichment level of H3K9me2 at those transposons. Strong reductions in H3K9me2 levels are seen at the HeT-A promoter region and at the 5′ end of Burdock, with significant but less potent depletion at the 5′ ends of Blood and Bari1 (Fig. 4C). Taken together with the observed loss of HP1a occupancy at these same sites (Fig. 4A), the results suggest that the Piwi-dependent, HP1a-dependent germ-line transposon silencing is likely functioning through such heterochromatin formation.
Discussion
The results above lead us to conclude that germ-line Piwi functions in silencing a subset of transposons either through recruiting HP1a to the transposon sites, likely directed by piRNAs, or through an unknown mechanism(s) to maintain HP1a enrichment at transposon sites. The former interpretation is supported by the ChIP results obtained from Aub knockdown ovaries (SI Appendix, Fig. S6) and fits well with the small RNA targeting model for heterochromatin formation first described in fission yeast, S. pombe (26, 41). However, as in many previous studies, we find that not all transposable elements behave in the same way, and that it is necessary to invoke more than one mechanism to achieve silencing of all transposons. For example, we observed three cases that exhibit obvious up-regulation in germ-line Piwi knockdown ovaries that did not respond to mutations in ago3 (Table 1). One possibility is that Piwi functions in these cases through a primary piRNA mediated mechanism (16).
We find that germ-line Piwi is not required to maintain wild-type levels of piRNA (Fig. 2), which is in contrast to an earlier study (11) describing a significant decrease in Roo piRNA levels in piwi homozygous mutant ovaries. The major difference between the two studies likely comes from the difference in tissue type. Depletion of Piwi specifically in the germ line, as done here, allows oogenesis to occur normally (23), whereas depletion in the ovarian soma also leads to blockage of oogenesis, resulting in ovariole bundles composed mostly of somatic cells (24). Thus, the signals detected in the latter experiments probably reflect functions of somatic Piwi. Using an ovarian somatic cell line (OSC), Saito et al. have shown that Piwi is required to maintain normal piRNA levels in these cells (17). Although the mechanism remains unclear, their results in soma taken together with our observations in germ line highlight a distinction in Piwi function between the two tissues.
The significance of the observed increase for HeT-A and AT-chX-1 piRNA levels in germ-line Piwi depleted ovaries (Fig. 2B) remains unclear. One attractive interpretation would be that the increase in transcript levels in the absence of germ-line Piwi could result in an increase in substrate available for the ping-pong amplification cycle (8, 9). The strong depletion in AT-chX-1 piRNA level in Piwi-Aub double knockdown ovaries (SI Appendix, Fig. S4A) supports this idea.
In Aub knockdown germ line, we observe a more diffuse Piwi localization pattern (Fig. 3). One attractive interpretation would be that germ-line Piwi requires loading of piRNAs to be licensed for its nuclear entrance (13). Depletion of Aub leads to disruption of the ping-pong amplification cycle and would therefore disrupt any nuclear localization of Piwi dependent on secondary piRNA interaction. The remaining nuclear Piwi signal could come from Piwi proteins loaded with primary piRNAs, or alternatively result from incomplete Aub depletion in the knockdown ovaries.
Jockey appears to be a singular case among transposons tested here. Although it requires both Piwi and AGO3 for proper control of expression (Table 1), it does not seem to respond to germ-line Aub knockdown (Fig. 3B). Moreover, the chromatin immunoprecipitation results and HP1a knockdown results indicate that Jockey expression is regulated via a mechanism that is independent of HP1a. As Aub knockdown leads to an increase in cytoplasmic Piwi relative to the nuclear fraction (Fig. 3E), Piwi could execute Jockey silencing in the cytoplasm. Further studies on how germ-line Piwi silences Jockey could be very informative in understanding how Piwi functions in general.
Although our results clearly indicate that germ-line Piwi functions through recruiting HP1a to some transposon sites to induce local heterochromatin formation and silence transposons, the actual mechanism of HP1a recruitment by Piwi remains to be determined. The direct interaction between Piwi and HP1a by using a PXVXL motif, observed both in vitro (27) and in a yeast two-hybrid assay (18), provides a possible means to mediate this process. However, a direct test using a V30A mutant piwi transgene showed rescue of transposon silencing in the piwi1 germ line, indicating that germ-line Piwi does not require an intact PXVXL motif for this silencing function (SI Appendix, Fig. S3). Nonetheless, the results above showing loss of HP1a deposition in response to Piwi depletion, taken together with the reported coimmunoprecipitation of Piwi and HP1a (18), argue for a link; we suggest that there are likely additional interactions bridging between Piwi and HP1a. Alternatively, Piwi could recruit HP1a through an indirect mechanism, yet to be elucidated. Further exploration will be needed to determine the mechanisms for Piwi dependent recruitment of HP1a.
In summary, our results using a system that can deplete Piwi specifically in the female germ line provide unique findings that correspond well with the current literature and support the hypothesis of a chromatin-based transposon silencing mechanism for germ-line Piwi in Drosophila (see model, SI Appendix, Fig. S8). Our observations are in agreement with an earlier study from Gvozdev and colleagues, who used spn-E mutants to look at the impact of piRNA pathway mutations on chromatin structure at transposon sites (20). In addition, our study further positions Piwi downstream of piRNA production to function in directing assembly of a proper chromatin structure at transposon sites to achieve silencing.
Materials and Methods
Fly Stocks, Husbandry, and Genetics.
All crosses were performed at 25 °C, 70% humidity by using regular cornmeal sucrose-based medium. Full genotypes of the fly lines used are listed in SI Appendix, Table S3. For female germ-line knockdown experiments, male flies from the driver line were crossed with female virgins from the respective hairpin target lines (SI Appendix, Fig. S1). Hairpin lines used in this study were yw;+/+;P{my+=UAS-PIWIhp8} (piwiKD2), w1118;P{GD11827}22235 (piwiKD1), w1118;P{GD12524}v31995 (HP1aKD), and w1118; P{GD11831}v30125 (aubKD) (29) (abbreviations given in SI Appendix, Table S5). The DFS-FLP experiment was carried out as described (23) (SI Appendix, Fig. S2). Ovaries were dissected from 3- to 5-d-old females provided with fresh yeast overnight.
Hairpin Transgenic Line Construction.
Hairpin line construction was carried out as described (42) except that the piwi fragment was amplified from a cDNA clone, GM05853 (43), using the following primer pair: forward 5′-GCT CTA GAT CCG GTT GAG CTG GTA TCC AAG AA-3′ and reverse 5′-GCT CTA GAA GAT CGT CTC GGT GCG CAT AAC TT-3′. Seven transgenic lines with different insertion sites were recovered (SI Appendix, Table S4).
Immunostaining and Confocal Imaging.
Flies were dissected in EBR (an iso-osmotic buffer) and dissected ovaries were fixed in 6% formaldehyde saturated with heptane (44). Antibodies used for immunostaining are P4D2 anti-Piwi (1:2) (10), 1D12 anti-Gurken (1:20) (Developmental Studies Hybridoma Bank), 4D10 anti-Aub (1:200) (35), and C1A9 anti-HP1a (1:10) (45). Phaloidine-Alex568 (Invitrogen) (1:100) was used to stain actin. Secondary antibodies were Alexa Fluor-conjugated antibodies from Invitrogen. Images were collected on a Nikon A1 confocol microscope. Each image was averaged over 16 scans of a single focal plane and processed by using Image J software and Adobe Photoshop.
Western Blot Analysis.
Ovarian lysate was prepared as described (46). Electrophoresis was carried out with a 4–20% polyacrylamide gradient gel (Bio-Rad) in SDS running buffer. Proteins were wet-transferred to a 0.45-μm nylon membrane, and the membrane was probed with the respective antibodies in 5% milk TBST using the following dilutions: P4D2 anti-Piwi (1:66) (10), 4D10 anti-Aub (1:1,000) (35), and 3C7 anti-myosin VI (1:20) (47). HRP (horseradish peroxidase) conjugated secondary antibodies (KPL) and substrates (Millipore) were used according to vendors’ instructions to visualize the results.
Quantitative RT-PCR and Northern Blot.
RNA was isolated with TRIzol by following vendor's instructions. For each biological sample, 20 pairs of ovaries were hand-homogenized in 1 mL of TRIzol reagent by using a small pestle. RNA templates for RT reactions were treated with DNase I (Fermantas). cDNA was generated using random hexamers (Invitrogen) and SuperScript III (Invitrogen) by following vendor's instructions. Quantitative PCR was performed by using iQ SYBR Green Supermix (Bio-Rad) on an ABI 7500 or a Cepheid Smart Cycler. Primers used are listed in SI Appendix, Table S6. Results were analyzed by using the ΔΔCT method (48). Small RNA Northern blots were done as described (49) but omitting the size selection step. Probes used are listed in the SI Appendix, Table S7.
Chromatin Immunoprecipitation.
Chromatin preparation was carried out as described (19). Sonication used a Branson sonifier with a microprobe at 100% duty cycle and output setting 2. Four 12-s bursts with 2-min intervals on ice gave a sample with fragment sizes between 100–1,000 bp. Immunoprecipitation was carried out by following the modENCODE protocol (http://www.modencode.org) using antibodies WA191 (121701) anti-HP1a (50) (1:50) and Ab1220 anti-H3K9me2 (lot 765092; Abcam) (1:100). The relative enrichment of each mark at the designated region was quantified by quantitative PCR. Primers used are listed in SI Appendix, Table S6, Lower. The 5′ primers were designed to amplify the junction between LTR and internal TE sequence. For consistent amplicon size, primer pairs are tested by in silico PCR on the University of California, Santa Cruz genome browser Web site. The percent input of each IP at each locus was determined by using input sample dilutions. Relative enrichment at a given locus was then determined by normalizing the locus percent input over α-actinin or 18S ribosomal DNA percent input. The mean of the normalized value from three biological replicates is reported.
Supplementary Material
Acknowledgments
We thank Christopher Zugates, Elena Gracheva, and Brent Brower-Toland for help in constructing our piwi hairpin lines; the Bloomington Drosophila Stock Center, Vienna Drosophila RNAi Center, and Haifan Lin for fly stocks; the Developmental Studies Hybridoma Bank, Mamiko Isaji, and Mikiko and Haruhiko Siomi for antibodies; Mary Lou Pardue for clones; Wilson Leung and Ruth Nan for technical support; and Annie Shieh and Elizabeth Tempel for help in manuscript preparation. This work was supported by National Institutes of Health Grants GM073190 and GM068388 (to S.C.R.E.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.M. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1107892109/-/DCSupplemental.
References
- 1.Bannert N, Kurth R. The evolutionary dynamics of human endogenous retroviral families. Annu Rev Genomics Hum Genet. 2006;7:149–173. doi: 10.1146/annurev.genom.7.080505.115700. [DOI] [PubMed] [Google Scholar]
- 2.Wang T, et al. Species-specific endogenous retroviruses shape the transcriptional network of the human tumor suppressor protein p53. Proc Natl Acad Sci USA. 2007;104:18613–18618. doi: 10.1073/pnas.0703637104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Girard A, Hannon GJ. Conserved themes in small-RNA-mediated transposon control. Trends Cell Biol. 2008;18:136–148. doi: 10.1016/j.tcb.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gu W, et al. Distinct argonaute-mediated 22G-RNA pathways direct genome surveillance in the C. elegans germline. Mol Cell. 2009;36:231–244. doi: 10.1016/j.molcel.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Law JA, Jacobsen SE. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat Rev Genet. 2010;11:204–220. doi: 10.1038/nrg2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fire A, et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 7.Saito K, Siomi MC. Small RNA-mediated quiescence of transposable elements in animals. Dev Cell. 2010;19:687–697. doi: 10.1016/j.devcel.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Brennecke J, et al. Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell. 2007;128:1089–1103. doi: 10.1016/j.cell.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 9.Gunawardane LS, et al. A slicer-mediated mechanism for repeat-associated siRNA 5′ end formation in Drosophila. Science. 2007;315:1587–1590. doi: 10.1126/science.1140494. [DOI] [PubMed] [Google Scholar]
- 10.Saito K, et al. Specific association of Piwi with rasiRNAs derived from retrotransposon and heterochromatic regions in the Drosophila genome. Genes Dev. 2006;20:2214–2222. doi: 10.1101/gad.1454806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vagin VV, et al. A distinct small RNA pathway silences selfish genetic elements in the germline. Science. 2006;313:320–324. doi: 10.1126/science.1129333. [DOI] [PubMed] [Google Scholar]
- 12.Yin H, Lin H. An epigenetic activation role of Piwi and a Piwi-associated piRNA in Drosophila melanogaster. Nature. 2007;450:304–308. doi: 10.1038/nature06263. [DOI] [PubMed] [Google Scholar]
- 13.Li C, et al. Collapse of germline piRNAs in the absence of Argonaute3 reveals somatic piRNAs in flies. Cell. 2009;137:509–521. doi: 10.1016/j.cell.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malone CD, et al. Specialized piRNA pathways act in germline and somatic tissues of the Drosophila ovary. Cell. 2009;137:522–535. doi: 10.1016/j.cell.2009.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saito K, et al. A regulatory circuit for piwi by the large Maf gene traffic jam in Drosophila. Nature. 2009;461:1296–1299. doi: 10.1038/nature08501. [DOI] [PubMed] [Google Scholar]
- 16.Siomi MC, Miyoshi T, Siomi H. piRNA-mediated silencing in Drosophila germlines. Semin Cell Dev Biol. 2010;21:751–759. doi: 10.1016/j.semcdb.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Saito K, et al. Roles for the Yb body components Armitage and Yb in primary piRNA biogenesis in Drosophila. Genes Dev. 2010;24:2493–2498. doi: 10.1101/gad.1989510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brower-Toland B, et al. Drosophila PIWI associates with chromatin and interacts directly with HP1a. Genes Dev. 2007;21:2300–2311. doi: 10.1101/gad.1564307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klattenhoff C, et al. The Drosophila HP1 homolog Rhino is required for transposon silencing and piRNA production by dual-strand clusters. Cell. 2009;138:1137–1149. doi: 10.1016/j.cell.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klenov MS, et al. Repeat-associated siRNAs cause chromatin silencing of retrotransposons in the Drosophila melanogaster germline. Nucleic Acids Res. 2007;35:5430–5438. doi: 10.1093/nar/gkm576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pal-Bhadra M, et al. Heterochromatic silencing and HP1 localization in Drosophila are dependent on the RNAi machinery. Science. 2004;303:669–672. doi: 10.1126/science.1092653. [DOI] [PubMed] [Google Scholar]
- 22.Williams RW, Rubin GM. ARGONAUTE1 is required for efficient RNA interference in Drosophila embryos. Proc Natl Acad Sci USA. 2002;99:6889–6894. doi: 10.1073/pnas.072190799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cox DN, et al. A novel class of evolutionarily conserved genes defined by piwi are essential for stem cell self-renewal. Genes Dev. 1998;12:3715–3727. doi: 10.1101/gad.12.23.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin H, Spradling AC. A novel group of pumilio mutations affects the asymmetric division of germline stem cells in the Drosophila ovary. Development. 1997;124:2463–2476. doi: 10.1242/dev.124.12.2463. [DOI] [PubMed] [Google Scholar]
- 25.Chambeyron S, et al. piRNA-mediated nuclear accumulation of retrotransposon transcripts in the Drosophila female germline. Proc Natl Acad Sci USA. 2008;105:14964–14969. doi: 10.1073/pnas.0805943105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verdel A, et al. RNAi-mediated targeting of heterochromatin by the RITS complex. Science. 2004;303:672–676. doi: 10.1126/science.1093686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mendez DL, et al. The HP1a disordered C terminus and chromo shadow domain cooperate to select target peptide partners. ChemBioChem. 2011;12:1084–1096. doi: 10.1002/cbic.201000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tracey WD, Jr, Ning X, Klingler M, Kramer SG, Gergen JP. Quantitative analysis of gene function in the Drosophila embryo. Genetics. 2000;154:273–284. doi: 10.1093/genetics/154.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dietzl G, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- 30.Klattenhoff C, et al. Drosophila rasiRNA pathway mutations disrupt embryonic axis specification through activation of an ATR/Chk2 DNA damage response. Dev Cell. 2007;12:45–55. doi: 10.1016/j.devcel.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Ghildiyal M, Zamore PD. Small silencing RNAs: An expanding universe. Nat Rev Genet. 2009;10:94–108. doi: 10.1038/nrg2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalmykova AI, Klenov MS, Gvozdev VA. Argonaute protein PIWI controls mobilization of retrotransposons in the Drosophila male germline. Nucleic Acids Res. 2005;33:2052–2059. doi: 10.1093/nar/gki323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chou TB, Perrimon N. The autosomal FLP-DFS technique for generating germline mosaics in Drosophila melanogaster. Genetics. 1996;144:1673–1679. doi: 10.1093/genetics/144.4.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim AK, Kai T. Unique germ-line organelle, nuage, functions to repress selfish genetic elements in Drosophila melanogaster. Proc Natl Acad Sci USA. 2007;104:6714–6719. doi: 10.1073/pnas.0701920104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishida KM, et al. Gene silencing mechanisms mediated by Aubergine piRNA complexes in Drosophila male gonad. RNA. 2007;13:1911–1922. doi: 10.1261/rna.744307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olivieri D, Sykora MM, Sachidanandam R, Mechtler K, Brennecke J. An in vivo RNAi assay identifies major genetic and cellular requirements for primary piRNA biogenesis in Drosophila. EMBO J. 2010;29:3301–3317. doi: 10.1038/emboj.2010.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eissenberg JC, Elgin SC. The HP1 protein family: Getting a grip on chromatin. Curr Opin Genet Dev. 2000;10:204–210. doi: 10.1016/s0959-437x(00)00058-7. [DOI] [PubMed] [Google Scholar]
- 38.Lachner M, O'Carroll D, Rea S, Mechtler K, Jenuwein T. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature. 2001;410:116–120. doi: 10.1038/35065132. [DOI] [PubMed] [Google Scholar]
- 39.Eskeland R, Eberharter A, Imhof A. HP1 binding to chromatin methylated at H3K9 is enhanced by auxiliary factors. Mol Cell Biol. 2007;27:453–465. doi: 10.1128/MCB.01576-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schotta G, et al. Central role of Drosophila SU(VAR)3-9 in histone H3-K9 methylation and heterochromatic gene silencing. EMBO J. 2002;21:1121–1131. doi: 10.1093/emboj/21.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Volpe TA, et al. Regulation of heterochromatic silencing and histone H3 lysine-9 methylation by RNAi. Science. 2002;297:1833–1837. doi: 10.1126/science.1074973. [DOI] [PubMed] [Google Scholar]
- 42.Brower-Toland B, Riddle NC, Jiang H, Huisinga KL, Elgin SC. Multiple SET methyltransferases are required to maintain normal heterochromatin domains in the genome of Drosophila melanogaster. Genetics. 2009;181:1303–1319. doi: 10.1534/genetics.108.100271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rubin GM, et al. A Drosophila complementary DNA resource. Science. 2000;287:2222–2224. doi: 10.1126/science.287.5461.2222. [DOI] [PubMed] [Google Scholar]
- 44.Cooley L, Verheyen E, Ayers K. chickadee encodes a profilin required for intercellular cytoplasm transport during Drosophila oogenesis. Cell. 1992;69:173–184. doi: 10.1016/0092-8674(92)90128-y. [DOI] [PubMed] [Google Scholar]
- 45.James TC, et al. Distribution patterns of HP1, a heterochromatin-associated nonhistone chromosomal protein of Drosophila. Eur J Cell Biol. 1989;50:170–180. [PubMed] [Google Scholar]
- 46.Tomari Y, et al. RISC assembly defects in the Drosophila RNAi mutant armitage. Cell. 2004;116:831–841. doi: 10.1016/s0092-8674(04)00218-1. [DOI] [PubMed] [Google Scholar]
- 47.Miller KG, Field CM, Alberts BM. Actin-binding proteins from Drosophila embryos: A complex network of interacting proteins detected by F-actin affinity chromatography. J Cell Biol. 1989;109:2963–2975. doi: 10.1083/jcb.109.6.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 49.Blevins T. Northern blotting techniques for small RNAs. Methods Mol Biol. 2010;631:87–107. doi: 10.1007/978-1-60761-646-7_9. [DOI] [PubMed] [Google Scholar]
- 50.Stephens GE, Slawson EE, Craig CA, Elgin SC. Interaction of heterochromatin protein 2 with HP1 defines a novel HP1-binding domain. Biochemistry. 2005;44:13394–13403. doi: 10.1021/bi051006+. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.