The use of directing fields to control the assembly of colloidal particles, which range in size from tens of nanometers to several micrometers, is a promising route for bottom-up fabrication of new nano- and microstructured functional materials (1). Recent examples include the use of electric or magnetic fields to fabricate photonic crystals from colloidal fluids by controlling particle orientation, interactions, and spatial distribution (2, 3). Electromagnetic fields can be substituted by structured solvents, such as nematic liquid crystals, which define a directing field for larger particles through distortions of the average molecular orientation of the polymer (4, 5). In this spirit of directed assembly, a study in PNAS by Cavallaro et al. (6) extends these principles to control particles at 2D fluid interfaces. By using a combination of theory and experiment, they demonstrate an ability to define directing fields by controlling the interface curvature to generate forces and torques on particles that lead to their placement at exact locations on the surface.
The behavior of particles at fluid interfaces is scientifically and technologically important. Colloidal particles spontaneously adsorb to the interface between immiscible fluids, such as oil and water. By going to the interface, particles essentially reduce the unfavorable contact between the fluids, and thus lower the interfacial energy. For micrometer-diameter particles, the attachment energy is often many orders of magnitude stronger than the thermal energy kT, which means they are, in any practical sense, irreversibly bound to the interface. Ramsden first noted this adsorption in 1903, followed later by Pickering, both of whom observed several novel effects, including the resulting stiff interfacial mechanics when particles have been confined to this 2D world (7, 8). Pickering focused on the use of particles as efficient stabilizers for dispersing one immiscible fluid into another, an application that has reemerged as it becomes desirable to identify more environmentally benign emulsifiers than commonly used small-molecule or polymeric surfactants. A recent surge of interest has led to a range of other applications as well, such as particle capsules (9), anisotropic droplets (10), and bicontinuous interfacially jammed emulsion gels (or “bijels”) (11, 12). The interpenetrating fluid networks of bijels may be useful as fluid microreactors, bulk heterojunction photovoltaics, or precursors to structured materials like catalyst supports. Alternatively, the energy of the interface between immiscible fluids could be thought of as a directing field in its own right for templating micro- and nanoparticles into percolating structures (13).
What are the governing rules of particle assembly at interfaces, and how can it be controlled? To assemble particles, their interparticle interactions must be tailored. Typically, colloids have a significant amount of dissociable charged groups on their surface, which ensures their stability as aqueous dispersions when the resulting double-layer repulsion is in excess of the ubiquitous van der Waals attractions. The range and strength of electrostatic repulsions between particles at the interface are enhanced by the asymmetric charge dissociation between high (e.g., water) and low (e.g., oil or air) permittivity fluids (14, 15). Repulsion leads to beautiful, highly stable interfacial colloidal crystals of the kind first reported by Pieranski (16).
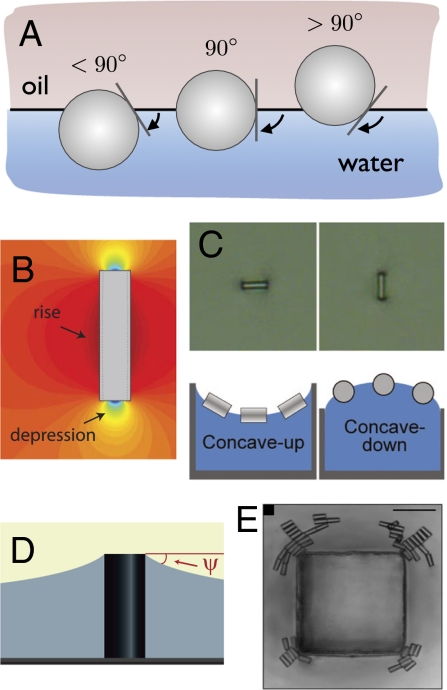
Alternately, strong attractive interactions between particles arise as a result of the local interface deformation. To understand this, first consider the case of an adsorbed homogeneous sphere. If the sphere is partially wetted by both fluids, it adopts an equilibrium position normal to the interface to match the three-phase equilibrium contact line, as shown in Fig. 1A. The contact angle is a function of the surface energies of the particle–fluid and fluid–fluid interfaces. Changes in these surface energies will favor higher or lower contact angles, and the sphere will adjust its height. However, the interface always remains flat.
Fig. 1.
(A) The three-phase contact line for spherical particles. (B) The quadrupolar interfacial deformation around a cylinder leads to (C) cylinder orientation that depends on the interface curvature. Adapted with permission from Lewandowski et al., Langmuir 2008, 24, 9302–9307. Copyright 2008 American Chemical Society. (D) Microfabricated posts define the interface curvature. (E) Cylinder particle assembly at the corners of a micropost with a square cross-section.
For anisotropic particles, such as colloidal ellipsoids, rods, or doublets, it is impossible to satisfy the three-phase contact angle over the entire particle perimeter without inducing a deformation in the interface. A cylinder is a simple case to envision: if the rod sits at a equilibrium position that satisfies the contact angle on the curved surface (similar to a sphere), then the contact line on the flat surfaces of the cylinder ends cannot be satisfied without inducing a strong interfacial deformation. Such interface distortion creates excess surface area and a higher energy. The equilibrium position of the cylinder reflects a minimization of the overall deformation, in which some areas rise up and others bend down (Fig. 1B); thus, the deformation takes on a quadrupolar form that is oriented with the anisotropic particle. The effect of this perturbation is a long-range “capillary force” that acts to bring particles together to minimize the local interfacial deformation, and hence, minimize the area of liquid–vapor or liquid–liquid interface (17). An often-cited effect of capillary attraction is the clustering of breakfast cereal in a bowl of milk. That case is somewhat different from that for colloids. The gravitational force acting on the cereal particles pulls them down and causes the interface to distort. In contrast, the gravitational force is negligible for colloids; the Bond number—the ratio of gravitational to surface tension forces—is much less than 1.
The interfacial deformation that gives rise to capillary interactions between particles provides the clues for manipulating their orientation and placement on an interface. Previous work showed that the quadrupolar deformation around particles couples to curved interfaces, again in a manner that minimizes the total interface distortion, and thus the total interfacial energy (18). Because the direction of the local interface deformation around the particle varies, the orientation of a rod changes by alternating the sign of the macroscopic interface curvature, as shown in Fig. 1C for a plane–parabolic interface—one with a single principal radius of curvature. Changing the interface curvature from concave up to concave down generates a torque on the cylinders that reorients them. The concave-up surface matches the local bend down near the cylinder ends to minimize the total interface deformation. Likewise, the concave-down interface matches the upward bend of the cylinder's round sides, leading to the particles’ orientation perpendicular to the curvature. Similarly, particles will move along gradients in the interface curvature to regions in which the total interfacial energy is minimized. This leads naturally to the advances reported by Cavallaro et al. (6), who demonstrate that interface curvature can be used to orient and position colloids, thus acting as a directing field for particle assembly.
In their study, Cavallaro et al. (6) precisely control the interface curvature by using microfabricated posts. The posts pin the interface locally at a greater height than its surroundings, and the shape of the post determines the local curvature and its gradients, as shown in Fig. 1D. A simple micropost with circular cross-section creates two principal axes of curvature—one in the angular direction and one that decays radially. The total curvature is a maximum at the post tip, so particles will spontaneously migrate toward the post, or “uphill” from the surrounding flat
Interface curvature can be used to orient and position colloids, thus acting as a directing field for particle assembly.
interface. As they do, the particles also orient tangentially to the post surface to match the radial and angular curvatures.
More sophisticated control over the position and orientation of particles is demonstrated by using posts with different cross-sections. Microposts with elliptical or square cross-sections create locations on the interface with a high degree of curvature. These points strongly attract particles. A stunning example is shown for a square post, which is reproduced in Fig. 1E. Cylinders arrange at the corners in an arcing pattern of both tip-to-tip and side-by-side assembly. Notably, the flat surfaces of the post create capillary repulsion that further directs the particles to the corners. All of this is in good agreement with theory, which means that design rules for particle orientation, placement, and assembly can be rigorously defined. Increasingly complex posts or arrays of posts should produce more complex assembly behavior and directed structures.
Finally, the particle interactions at interfaces will ultimately determine the form of the self-assembled structures as they are directed along the interface. These interactions are not merely limited to simple geometric shapes like rods or ellipsoids. Particles with complex cross-sections can be used to create “programmable” sites of interaction, which lead to highly specific capillary forces between them (19, 20). Moreover, even spheres may be subject to such directing fields. The attractive interactions between latex spheres suggest similar capillary forces occur as a result of surface roughness or chemical heterogeneity (21, 22). The far-field interface deformation around a sphere should be quadrupolar, and thus would couple to curvature gradients in much the same way as the cylinders discussed here. Moreover, capillary forces are strong even at nanoparticle dimensions, providing a new opportunity to direct their assembly.
From this impressive set of emerging design tools, including programmable interactions and directing fields, it is clear that engineering directed colloidal assembly by using interfaces has a strong potential for the bottom-up fabrication of novel structured materials.
Acknowledgments
Work on directed self-assembly in the author's laboratory is supported by the United States Department of Energy, Office of Basic Energy Sciences, Division of Materials Sciences and Engineering, under Award DE-FG02-09ER46626 for assembly from nanoparticle dispersions; and by National Science Foundation Chemical, Bioengineering, Environmental and Transport Systems Grant 0930549 for dicolloid assembly in electric fields.
Footnotes
The author declares no conflict of interest.
See companion article on page 20923.
References
- 1.Grzelczak M, Vermant J, Furst EM, Liz-Marzán LM. Directed self-assembly of nanoparticles. ACS Nano. 2010;4:3591–3605. doi: 10.1021/nn100869j. [DOI] [PubMed] [Google Scholar]
- 2.Forster JD, et al. Assembly of optical-scale dumbbells into dense photonic crystals. ACS Nano. 2011;5:6695–6700. doi: 10.1021/nn202227f. [DOI] [PubMed] [Google Scholar]
- 3.Ding T, Song K, Clays K, Tung C-H. Fabrication of 3D photonic crystals of ellipsoids: Convective self-assembly in magnetic field. Adv Mater. 2009;21:1–5. [Google Scholar]
- 4.Poulin P, Stark H, Lubensky TC, Weitz DA. Novel colloidal interactions in anisotropic fluids. Science. 1997;275:1770–1773. doi: 10.1126/science.275.5307.1770. [DOI] [PubMed] [Google Scholar]
- 5.Loudet JC, Barois P, Poulin P. Colloidal ordering from phase separation in a liquid-crystalline continuous phase. Nature. 2000;407:611–613. doi: 10.1038/35036539. [DOI] [PubMed] [Google Scholar]
- 6.Cavallaro M, Jr., Botto L, Lewandowski EP, Wang M, Stebe KJ. Curvature-driven capillary migration and assembly of rod-like particles. Proc Natl Acad Sci USA. 2011;108:20923–20928. doi: 10.1073/pnas.1116344108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramsden W. Separation of solids in the surface-layers of solutions and suspensions. Proc R Soc Lond. 1903;72:156–164. [Google Scholar]
- 8.Pickering SU. Emulsions. J Chem Soc. 1907;91:2001–2021. [Google Scholar]
- 9.Velev O, Furusawa K, Nagayama K. Assembly of latex particles by using emulsion droplets as templates. 1. Microstructured hollow spheres. Langmuir. 1996;12:2374–2384. [Google Scholar]
- 10.Pawar AB, Caggioni M, Ergun R, Hartel RW, Spicer PT. Arrested coalescence in Pickering emulsions. Soft Matter. 2011;7:7710–7716. [Google Scholar]
- 11.Stratford K, Adhikari R, Pagonabarraga I, Desplat JC, Cates ME. Colloidal jamming at interfaces: A route to fluid-bicontinuous gels. Science. 2005;309:2198–2201. doi: 10.1126/science.1116589. [DOI] [PubMed] [Google Scholar]
- 12.Cates ME, Clegg PS. Bijels: A new class of soft materials. Soft Matter. 2008;4:2132–2138. [Google Scholar]
- 13.Bose S, et al. Phase separation as a tool to control dispersion of multiwall carbon nanotubes in polymeric blends. ACS Appl Mater Interfaces. 2010;2:800–807. doi: 10.1021/am9008067. [DOI] [PubMed] [Google Scholar]
- 14.Hurd AJ. The electrostatic interaction between interfacial colloidal particles. J Phys A. 1985;18:1055–1060. [Google Scholar]
- 15.Masschaele K, Park BJ, Furst EM, Fransaer J, Vermant J. Finite ion-size effects dominate the interaction between charged colloidal particles at an oil-water interface. Phys Rev Lett. 2010;105:048303. doi: 10.1103/PhysRevLett.105.048303. [DOI] [PubMed] [Google Scholar]
- 16.Pieranski P. Two-dimensional interfacial colloidal crystals. Phys Rev Lett. 1980;45:569–572. [Google Scholar]
- 17.Loudet JC, Alsayed AM, Zhang J, Yodh AG. Capillary interactions between anisotropic colloidal particles. Phys Rev Lett. 2005;94:018301. doi: 10.1103/PhysRevLett.94.018301. [DOI] [PubMed] [Google Scholar]
- 18.Lewandowski EP, Bernate JA, Searson PC, Stebe KJ. Rotation and alignment of anisotropic particles on nonplanar interfaces. Langmuir. 2008;24:9302–9307. doi: 10.1021/la801167h. [DOI] [PubMed] [Google Scholar]
- 19.Lucassen J. Capillary forces between solid particles in fluid interfaces. Colloids Surf. 1992;65:131–137. [Google Scholar]
- 20.Lewandowski EP, Bernate JA, Tseng A, Searson PC, Stebe KJ. Oriented assembly of anisotropic particles by capillary interactions. Soft Matter. 2009;5:886–890. [Google Scholar]
- 21.Stamou D, Duschl C, Johannsmann D. Long-range attraction between colloidal spheres at the air-water interface: the consequence of an irregular meniscus. Phys Rev E. 2000;62(4 Pt B):5263–5272. doi: 10.1103/physreve.62.5263. [DOI] [PubMed] [Google Scholar]
- 22.Park BJ, Furst EM. Attractive interactions between colloids at the oil–water interface. Soft Matter. 2011;7:7676–7682. doi: 10.1039/c5sm02001h. [DOI] [PubMed] [Google Scholar]