Abstract
Monocytes are key players in the immune system. Crossing the blood barrier, they infiltrate tissues and differentiate into (i) macrophages that fight off pathogens and (ii) dendritic cells (DCs) that activate the immune response. A hallmark of monocyte/macrophage activation is the generation of reactive oxygen species (ROS) as a defense against invading microorganisms. How monocytes, macrophages, and DCs in particular respond to ROS is largely unknown. Here we studied the sensitivity of primary human monocytes isolated from peripheral blood and compared them with macrophages and DCs derived from them by cytokine maturation following DNA damage induced by ROS. We show that monocytes are hypersensitive to ROS, undergoing excessive apoptosis. These cells exhibited a high yield of ROS-induced DNA single- and double-strand breaks and activation of the ATR-Chk1-ATM-Chk2-p53 pathway that led to Fas and caspase-8, -3, and -7 activation, whereas macrophages and DCs derived from them were protected. Monocytes are also hypersensitive to ionizing radiation and oxidized low-density lipoprotein. The remarkable sensitivity of monocytes to oxidative stress is caused by a lack of expression of the DNA repair proteins XRCC1, ligase IIIα, poly(ADP-ribose) polymerase-1, and catalytic subunit of DNA-dependent protein kinase (DNA-PKcs), causing a severe DNA repair defect that impacts base excision repair and double-strand break repair by nonhomologous end-joining. During maturation of monocytes into macrophages and DCs triggered by the cytokines GM-CSF and IL-4, these proteins become up-regulated, making macrophages and DCs repair-competent and ROS-resistant. We propose that impaired DNA repair in monocytes plays a role in the regulation of the monocyte/macrophage/DC system following ROS exposure.
Keywords: DNA damage response, monocytes, macrophages, dendritic cells
Monocytes are key players in the immune system. They originate from bone marrow precursor cells and are released, upon maturation, into the bloodstream. Crossing the blood barrier, they enter the tissue and differentiate into macrophages and myeloid dendritic cells (DCs) (1). DCs activate the immune response by presenting antigenic peptides on their surface that stimulate the differentiation and expansion of T cells (2). They may also activate, via T cells, macrophages (3). A hallmark of macrophages is their ability to phagocytose invading microorganisms and to generate a burst of reactive oxygen species (ROS), which is part of the organism's defense against infections (4).
The monocyte/macrophage system also plays a central role in the pathogenesis of chronic inflammatory diseases, whereby neutrophils become permanently activated, leading to long-term generation of ROS (5). As ROS easily penetrate surrounding cells and damage DNA and other cellular components, a sustained high ROS level together with cytokines and chemokines produced by excessive and chronic monocyte activation may cause severe damage of the normal tissue, leading to tissue degeneration and severe disorders such as rheumatoid arthritis, psoriasis, inflammatory bowel disease, neurodegenerative disorders, and atherosclerosis (6). Some of the DNA lesions induced by ROS, such as 8-oxo-guanine (8oxoG) and thymine glycol, have mispairing and noninstructive properties, respectively, giving rise to genomic instability, mutations, and finally cancer (7). Therefore, the activation of the ROS- and cytokine-producing monocyte/macrophage/DC system following infection and inflammation must be subject to fine-tuned regulation to avoid an excessive and adverse immune response and ROS production that may damage the normal tissue. How this regulation occurs is largely unknown.
Here, we investigated how human monocytes obtained from healthy volunteers, and DCs and macrophages derived from them, respond to genotoxic stress that generates oxidative DNA damage. We demonstrate that human macrophages and DCs are protected against ROS-inducing exposures including chemical agents, ionizing radiation (IR), and oxidized low density lipoprotein (oxLDL), displaying a high level of resistance to DNA breakage, DNA damage response (DDR), and apoptosis induction. In contrast, their precursor cells, the monocytes, display a high sensitivity to the genotoxic and killing effects of all these ROS-producing treatments. This is a result of a multiple DNA repair defect caused by the lack of expression of four DNA repair proteins that play a key role in base excision repair (BER) and DNA double-strand break (DSB) repair via nonhomologous end joining (NHEJ). The severe DNA repair defect gives rise to a high level of DSBs in human monocytes and activation of DDR and apoptosis pathways following ROS-producing chemical agents, IR, and endogenous ROS triggered by the uptake of oxLDL. We propose the hypothesis that the DNA repair defect that causes preferential killing of monocytes following ROS-induced DNA damage represents a homeostatic self-regulatory mechanism in the monocyte/macrophage/DC system. It attenuates the immune response and ROS production by depleting the precursor of macrophages and myeloid DCs, thus maintaining an optimal level of ROS and cytokine-producing immunocompetent cells.
Results
Monocytes, but Not DCs and Macrophages Derived from Them, Are Hypersensitive to Oxidative Agents.
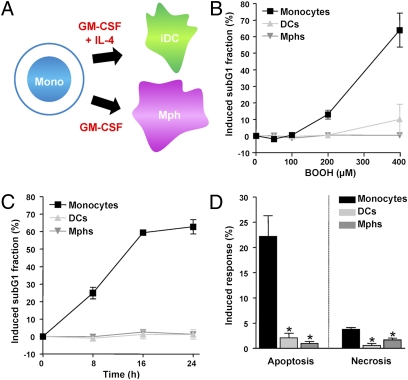
Human monocytes were obtained from peripheral blood of healthy donors as previously described (8) and differentiated into DCs and macrophages by treatment with the cytokines GM-CSF and IL-4 (Fig. 1A). A check for surface markers confirmed the maturation process in each experiment (Fig. S1A). Neither the isolated monocytes nor DCs and macrophages derived from them proliferate during the maturation process. To compare the sensitivity of monocytes with DCs and macrophages to oxidative stress, we determined the apoptotic response of the three cell populations upon treatment with tert-butyl hydroperoxide (BOOH), which is a potent oxidative agent (9). By using two different methods for quantifying apoptosis, we observed that monocytes are highly sensitive to BOOH whereas DCs and macrophages did not show significant toxicity (Fig. 1B for dose dependence and Fig. 1C for time dependence of sub-G1 fraction). Flow cytometry of annexin V/propidium iodide (PI) double-stained cells revealed that most of the cytotoxicity in monocytes is a result of apoptosis whereas necrosis amounts for only approximately 20% of the total cell kill fraction (Fig. 1D shows quantification and Fig. S1B shows a representative plot). Similar data were obtained with H2O2 (Fig. S2 A and B).
Fig. 1.
Death of monocytes, DCs, and macrophages following treatment with oxidizing agents. (A) Maturation of human monocytes into macrophages (Mphs) and DCs. (B) Dose dependence of cell death of monocytes, DCs, and macrophages following treatment with BOOH for 24 h. (C) Time dependence of the apoptotic response after treatment with 400 μM BOOH. Apoptosis was determined by quantifying the sub-G1 fraction by flow cytometry. (D) Frequency of induced apoptosis and necrosis of monocytes, DCs, and macrophages 24 h after treatment with 400 μM BOOH (determined by annexin V/PI double staining). *P < 0.001 for comparison of monocytes and DCs and macrophages (t test). All data are the mean of at least three independent experiments ± SD.
Hypersensitivity of monocytes to oxidative agents could theoretically be caused by a lack in radical scavenging. Therefore, we determined the ROS level in the cells upon treatment with BOOH. The ROS level increased significantly with treatment time (Fig. S3A), and monocytes did not display a higher ROS level than DCs and macrophages (Fig. S3B). Therefore, impaired radical scavenging cannot explain the hypersensitivity of monocytes to ROS-producing agents. This was confirmed by the cellular level of 8oxoG, the principal mutagenic oxidative DNA lesion, which was similar in monocytes, DCs, and macrophages (Fig. S3C).
Monocytes Display a High DNA Strand Break Level Following ROS Treatment.
ROS react with DNA, producing strand breaks and DNA base modifications that have the capacity to trigger cell death (10). Therefore, we assessed the possibility that ROS-induced DNA damage is not properly repaired in monocytes. To this end, we determined the ability of the cells to repair oxidative DNA damage by using alkaline single-cell gel electrophoresis (ie, comet assay). As shown in Fig. 2A, DNA single-strand breaks (SSBs) are formed at a high level already 10 min after the onset of treatment, and continue to accumulate over time. The level of SSBs was, however, lower in DCs and macrophages than in monocytes. After an exposure time of 240 min, the level of SSBs clearly declined in DCs and macrophages, whereas no significant decrease was observed in monocytes (Fig. 2A). Oxidative DNA damage is mainly repaired by BER, and SSBs are generated as intermediates of this repair process as a result of base removal and cleavage of apurinic/apyrimidinic (AP) sites by 8oxoG-DNA glycosylase (OGG1) and AP endonuclease (APE) (11). Therefore, the data suggests that SSBs produced during BER of oxidative DNA lesions cannot be sealed in monocytes, whereas they are sealed in DCs and macrophages, leading to a lower overall level of SSBs and a more rapid decrease with time.
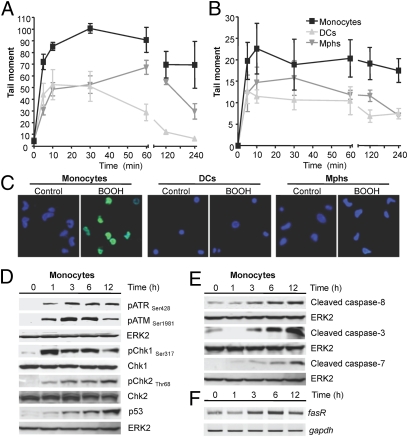
Fig. 2.
DNA breaks, DDR, and apoptosis in human monocytes. (A) Induced DNA SSBs following 400 μM BOOH, as determined by the alkaline comet assay. (B) Induced DSBs following 400 μM BOOH, as determined by the neutral comet assay. The level of DNA breakage was expressed by the tail moment (32). Data are the mean of at least three independent experiments ± SD. (C) γH2AX pan-staining in monocytes, DCs and macrophages (Mphs) after 1 h treatment with 400 μM BOOH. Blue, ToPro3 nuclear staining; green, γH2AX staining. (D) Western blot analysis of ATR, ATM, Chk1, and Chk2 phosphorylation and p53 stabilization in cell extracts of monocytes at different times after treatment with 400 μM BOOH. ERK2 served as loading control. (E) Time dependence of cleaved initiator caspase-8 and executor caspases 3 and 7 in cell extracts of monocytes following treatment with 400 μM BOOH. (F) Expression of fasR in monocytes, determined by semiquantitative RT-PCR. gapdh served as internal control.
Monocytes Display a High Level of DSB Following ROS Treatment.
The most critical lesions induced by genotoxins are DSBs, which are formed for most chemical agents in a DNA replication-dependent manner (10). Monocytes, DCs, and macrophages are, however, nonreplicating cells. Therefore, we wondered whether DSBs are formed upon BOOH treatment in these blood cell populations. By using the neutral comet assay, which is a reliable method for detecting DSB, we observed a higher DSB level in monocytes than in DCs and macrophages (Fig. 2B). Even after a recovery time of 4 h, the level remained high in monocytes, indicating impaired repair of DSBs upon oxidative stress. Furthermore, we determined the level of γH2AX foci formation as a biomarker for the presence of DSBs (12) upon BOOH treatment. In monocytes, γH2AX foci were induced at high level 20 min after treatment (Fig. S4) and were still present following 60 min of incubation. In contrast, only a low amount of γH2AX foci formation was observed in DCs and macrophages (Fig. 2C and Fig. S4), supporting the notion that monocytes are impaired in DSB repair.
DDR and Cell Death Pathways Are Strongly Activated in Monocytes.
DSBs induce the DDR pathway, which is driven by ATM, ATR, Chk1, Chk2, and p53 (13). These key players of DDR were assayed for in monocytes, DCs, and macrophages. Both ATR and ATM became activated in monocytes following BOOH treatment, which was accompanied by Chk1 and Chk2 activation and p53 stabilization (Fig. 2D). In DCs and macrophages treated with BOOH, the activation of the DDR pathway was very weak compared with monocytes (Fig. S5). We should note that both ATM and ATR already became activated 1 h after treatment (Fig. 2D), which is in line with the finding that SSBs and DSBs are produced at high levels in monocytes immediately after treatment.
Cell death induced in monocytes by oxidative stress is executed mainly by apoptosis (Fig. 1D). To determine which apoptosis pathway is activated in monocytes in response to oxidative DNA damage, we determined the level of expression of proteins involved. As shown in Fig. 2E, caspase-8, caspase-3, and caspase-7 already became activated 3 h after BOOH treatment. We also observed up-regulation of the Fas receptor (Fig. 2F). Inhibition of the Fas receptor by a neutralizing Fas antibody significantly atten-uated BOOH-induced apoptosis, similar to a caspase inhibitor (Fig. S6), indicating that the death receptor pathway triggers the genotoxic killing response in monocytes. In this process, p53 that precedes fas up-regulation (Fig. 2D shows p53 expression and Fig. 2F shows fasR expression) and regulates its transcription (14) is involved, as inhibition of the transactivating function of p53 by pifithrin-α reduced the level of apoptosis (Fig. S6).
Monocytes Are Impaired in BER.
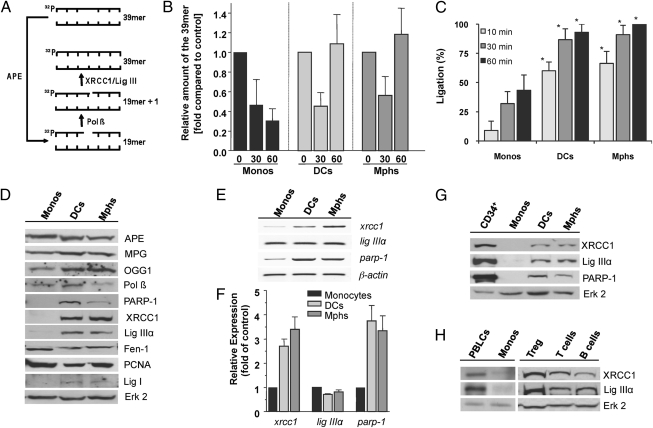
The finding of a high SSB level in monocytes following oxidative stress (Fig. 2A) prompted us to investigate the BER capacity of the cells. We used an in vitro assay (15), which is based on cleavage of a double-stranded oligonucleotide containing a single AP site and subsequent re-ligation of the resulting SSB catalyzed by DNA polymerase β (Pol β), XRCC1, and ligase (Lig) IIIα (Fig. 3A). This experiment (a representative example is shown in Fig. S7A) revealed that extracts of all cell types, i.e., monocytes, DCs, and macrophages, are able to cleave the oligonucleotide, but restitution to the full-length fragment did not occur in monocytes (Fig. 3B). We also applied a ligation assay by using a 32P-labeled oligonucleotide (a representative blot is shown in Fig. S7B), which shows that ligation is indeed hampered when extracts of monocytes were used, compared with DCs and macrophages (Fig. 3C). The data support the notion that monocytes are defective in BER.
Fig. 3.
BER and expression of repair proteins in monocytes, DCs, and macrophages (Mphs). (A) In vitro repair reactions were performed by using whole-cell extracts and a 32P-labeled oligonucleotide containing a single AP site. Schematic outline of the repair reaction. The 39-mer fragment (full length) represents molecules that contain the lesion or are fully repaired. (B) Relative amount of the 39-mer at increasing time points (0, 30, and 60 min). Data of three independent experiments are pooled. (C) DNA ligation assay. Relative amount of the 39-mer ligation product at increasing times of incubation (10, 30, 60 min). It was set to 100% in macrophages. Data of three independent experiments are pooled (*P < 0.001 comparing monocytes with DCs and macrophages). (D) Protein expression of APE, methylpurine-DNA glycosylase (MPG), OGG1, Pol β, Parp-1, XRCC1, Lig IIIα, Fen-1, proliferating cell nuclear antigen (PCNA), and Lig I in cell extracts of monocytes, DCs, and macrophages. Erk2 represents control. (E) mRNA level of xrcc1, lig IIIα, and parp-1 mRNA in monocytes, DCs, and macrophages, detected by semiquantitative RT-PCR. β-actin was used as internal control. (F) Expression of xrcc1, lig IIIα, and parp-1 in monocytes, DCs, and macrophages determined by quantitative RT-PCR. (G) XRCC1, Lig IIIα, and PARP-1 protein in CD34+ hematopoietic progenitor cells in comparison with monocytes, DCs, and macrophages. (H) Expression of XRCC1 and Lig IIIα in PBLCs, regulatory T cells, T cells, and B cells compared with monocytes.
Monocytes Lack the Expression of XRCC1, Lig IIIα, and Poly(ADP Ribose) Polymerase 1.
To determine the factor(s) of BER for which monocytes are defective, we studied the expression of BER proteins in monocytes, DCs, and macrophages from the same donor. As shown in Fig. 3D, the three cell populations expressed similar levels of APE, N-methylpurine-DNA glycosylase (MPG), OGG1, Pol β, Fen-1, and proliferating cell nuclear antigen. However, monocytes did not express poly(ADP-ribose) polymerase-1 (PARP-1), XRCC1, and Lig IIIα at a detectable level; all of them were clearly expressed in DCs and macrophages. Lig I was also not expressed in monocytes and expressed, albeit at very low level, in DCs and macrophages following maturation (Fig. 3D). XRCC1 and PARP-1 were also hardly detectable in monocytes on RNA level; its amount, however, clearly increased following their maturation in DCs and macrophages, whereas Lig IIIα mRNA was not enhanced during DC and macrophage maturation (Fig. 3E shows a representative blot and Fig. 3F shows quantitative RT-PCR). The data indicate that the low XRCC1 and PARP-1 protein levels are a result of transcriptional down-regulation. As Lig IIIα protein is stabilized by XRCC1 (16), its lack in monocytes is likely a secondary effect of XRCC1 down-regulation. The expression of XRCC1, Lig IIIα, and PARP-1 protein was also determined in several other blood cell populations, such as CD34+ hematopoietic progenitor cells (Fig. 3G), peripheral blood lymphocytes (PBLCs), T cells, B cells, and regulatory T cells (Fig. 3H), and found to be present in all these cell populations. It thus appears that monocytes represent a peculiar cell type that is characterized by simultaneous down-regulation of several DNA repair proteins that occupy key positions in the BER pathway of oxidative DNA adducts.
Monocytes Are Radiation-Hypersensitive and Impaired in DSB Repair.
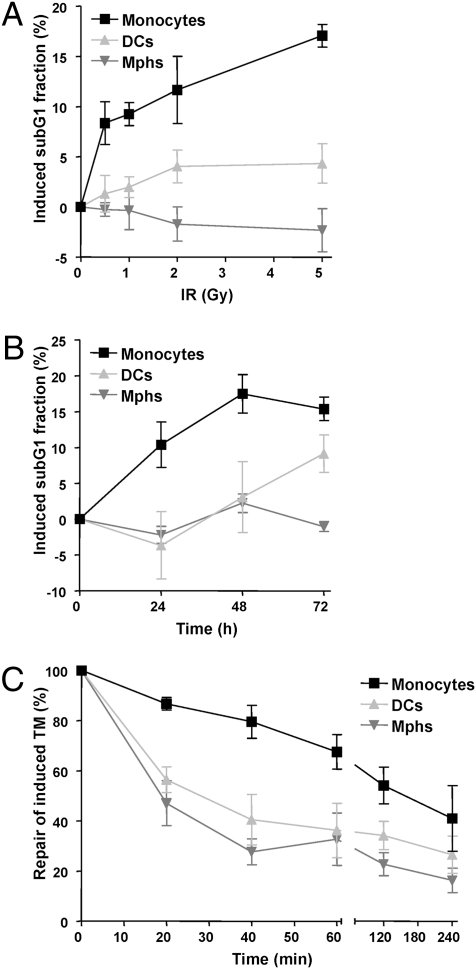
XRCC1, Lig IIIα, and PARP-1 are not only indispensable for BER (17), but also required for a subpathway of DSB repair, the backup pathway of NHEJ (18). Therefore, we posited that the BER defect leads to the formation of DSBs in overlapping BER repair patches that are not repaired in monocytes. We should note that, in monocytes, DCs, and macrophages, DSB repair cannot be executed by homologous recombination, which occurs in S and G2 phase (19), as the cells do not proliferate. Therefore, NHEJ is likely the main route of DSB repair in these cells. To substantiate this, we determined the sensitivity and capacity of the cells for repairing DSBs following exposure to IR. Although DCs and macrophages showed only very weak apoptosis at low dose IR (0.5 and 1 Gy), apoptosis was induced at a high level in monocytes in this therapy-relevant dose range (Fig. 4A shows dose response and Fig. 4B shows time response). The hypersensitivity of monocytes to IR was accompanied by attenuated DSB repair, which was slower than in DCs and macrophages, as shown in the neutral comet assay (Fig. 4C) and by γH2AX immunocytochemistry (Fig. S8). The data confirm that monocytes are impaired not only in BER, but also in DSB repair. The data also demonstrate that the capacity of DSB repair is elevated when monocytes differentiate into DCs and macrophages.
Fig. 4.
Sensitivity of monocytes, DCs and macrophages to IR. (A) Apoptotic response of cells 24 h after irradiation with different doses of γ-rays. (B) Time dependence of the apoptotic response of monocytes, DCs, and macrophages after irradiation with 5 Gy. Apoptosis was determined by flow cytometry (sub-G1 fraction). (C) Kinetics of repair of induced DSBs, determined by the neutral comet assay [tail moment (TM)] after irradiation with 5 Gy. Data are the mean of at least three independent experiments ± SD.
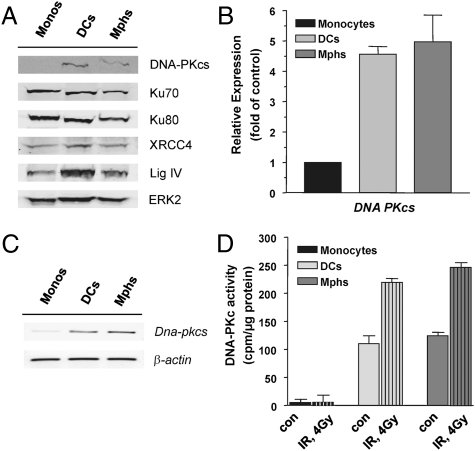
Monocytes Lack Catalytic Subunit of DNA-Dependent Protein Kinase.
The main pathway of DSB repair in nonproliferating cells is NHEJ, in which the key player is DNA-dependent protein kinase (PK) composed of Ku70, Ku80, and the catalytic subunit of DNA-PK (DNA-PKcs). Further, XRCC4 and Lig IV are involved. We determined the expression level of these NHEJ proteins and observed that all of them are expressed in monocytes and did not change in the level during DC and macrophage maturation, with the exception of DNA-PKcs, which was surprisingly not detectable in monocytes (Fig. 5A). RT-PCR analysis revealed that the DNA-PKcs mRNA level was very low in monocytes and became up-regulated during their maturation into DCs and macrophages (Fig. 5B shows quantitative PCR and Fig. 5C shows a representative blot), indicating down-regulation of DNA-PKcs on a transcriptional level in monocytes. We also determined the DNA-PKcs enzyme activity following treatment with IR and found nearly no detectable basal and induced activity in monocytes, whereas in DCs and macrophages, derived from them, DNA-PKcs activity was present and significantly enhanced following radiation (Fig. 5D). The data support the conclusion that monocytes are defective in NHEJ.
Fig. 5.
Expression of NHEJ proteins in monocytes, DCs, and macrophages (Mphs). (A) Western blot analysis of DNA-PKcs, Ku70, Ku80, XRCC4, and Lig IV. ERK2 served as loading control. (B) Determination of mRNA expression of DNA-PKcs by quantitative PCR, demonstrating up-regulation of the mRNA level during maturation of monocytes into DCs and macrophages. (C) DNA-PKcs mRNA level determined by semiquantitative RT-PCR to demonstrate the specificity of the reaction. (D) DNA-PK activity in cell extracts of monocytes, DCs, and macrophages not treated (con) or treated with IR (4 Gy). Data are the mean of at least three independent determinations.
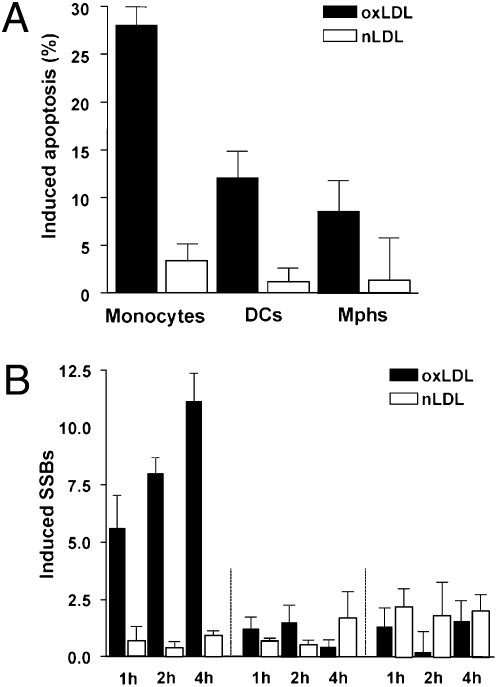
Repair Defect Sensitizes Monocytes to Oxidized Lipoprotein.
An important pathophysiological inducer of ROS is oxLDL, which is formed during oxidation of LDL. It is taken up by monocytes, macrophages, and DCs via scavenger receptor/LDL receptor complexes (20). Following uptake, oxLDL provokes intracellular ROS formation (21). As shown in Fig. 6A, oxLDL is clearly toxic, which is in contrast to nonoxidized LDL (nLDL), which is not toxic. Importantly, the experiments revealed that monocytes display a significantly higher apoptotic response than DCs and macrophages following oxLDL (Fig. 6A). This prompted us to measure the DNA damage following oxLDL treatment, and we found that, under the same treatment conditions, monocytes displayed a high level of DNA strand breaks that increased with time of oxLDL exposure, whereas nLDL was ineffective in inducing DNA strand breaks. In contrast to monocytes, oxLDL induced nearly no strand breaks in DCs and macrophages (Fig. 6B). The data revealed that monocytes are hypersensitive to oxLDL-induced cell death and are, in contrast to DCs and macrophages, not protected against oxLDL-induced DNA breakage.
Fig. 6.
Genotoxic response of monocytes, DCs, and macrophages following oxLDL and cytokine production following stimulation with LPS. (A) Frequency of induced apoptosis 16 h after treatment with 200 μg/mL oxLDL and nLDL, determined by annexin V/PI double staining, in monocytes, DCs, and macrophages. (B) Formation of induced SSBs (alkaline comet assay) following treatment of cells with 200 μg/mL oxLDL and nLDL.
Discussion
Here we compared the genotoxic response of human monocytes, DCs and macrophages following ROS exposure. We demonstrate that human monocytes isolated from the blood of healthy volunteers are highly sensitive to oxidative stress, which results from defects in DNA repair. The severe impairment of repair in monocytes is caused by a lack of expression of four proteins, XRCC1, Lig IIIα, PARP-1, and DNA-PKcs, which are key players in the last step of BER (22) and DSB repair via the canonical and backup pathway of NHEJ (18, 23). XRCC1, Lig IIIα, and PARP-1 are also involved in SSB repair (24). Therefore, down-regulation of these DNA repair proteins simultaneously affects several DNA repair pathways. All other BER and NHEJ proteins for which we assayed were clearly expressed in monocytes. The expression of MPG, OGG1, APE1, and Pol β explains the finding that monocytes are able to perform the first steps of BER, i.e., base removal, DNA incision, and gap formation. It is reasonable to conclude that the inability of executing the last step in BER leads to an accumulation of SSB, which was observed in our assays. If this faulty BER process occurs in overlapping repair patches, DSBs will be formed (25) which may happen even in nonreplicating cells. This is indeed the case; monocytes exhibited a higher amount of DSBs, as determined by the neutral comet and γH2AX assays, compared with DCs and macrophages following chemical ROS or IR treatment. The additional lack of DNA-PKcs exacerbates the repair defect, strongly reducing the monocyte's ability to repair DSBs formed indirectly, as a consequence of BER, or directly, following IR or high-level ROS exposure. With the down-regulation of these four repair proteins, monocytes seem to follow a strategy of exacerbating the effect of oxidative DNA lesions, which ultimately leads to cell death. It can therefore be considered as a suicide strategy for monocytes following genotoxic stress that can be tolerated by normal, repair-competent cells.
It is anticipated that, in monocytes, nonrepaired DSBs provide a strong signal for the DDR. This was indeed the case. We observed a strong activation of ATM, ATR, Chk1, Chk2, and p53 that triggered the apoptotic pathway involving FasR up-regulation and caspase-8, -3, and -7 activation. It is of interest that ATM and ATR became activated even though the cells were not proliferating. This is explained by a large amount of SSBs and DSBs produced by the faulty BER process that obviously activates several DDR pathways, including p53 that triggers the Fas/CD95/Apo-1–controlled cell death mechanism (10). Importantly, although DNA-PKcs is not active in monocytes, H2AX phosphorylation occurred at a high level because of the activation of ATM and ATR.
We should note that monocytes express even a higher level of O6-methylguanine-DNA methyltransferase than macrophages and DCs (Fig. S9A), indicating that only a small but important set of DNA repair genes is silenced in monocytes. As the cells are not proliferating, the differences in killing responses among monocytes, DCs, and macrophages cannot be attributed to replication-mediated conversion of critical DNA adducts into killing lesions. This makes the system different from proliferating myoblasts and nonproliferating myotubes of mice that were shown to differ in the expression of XRCC1 and Lig IIIα (26). We should also note that monocytes isolated by the method of adherence (27) or by CD14+ magnetic bead separation (Miltenyi) gave essentially the same result in terms of XRCC1 and Lig IIIα expression (Fig. S9 B and C), demonstrating that the phenotype is not related to the monocyte isolation procedure. The up-regulation of the repair genes studied here following monocyte maturation is clearly driven by GM-CSF/IL-4, as primary monocytes do not survive long-term cultivation in the absence of cytokines.
It is striking that not only DCs and macrophages, but also PBLCs, CD34+ progenitor cells, B cells, T cells, and regulatory T cells, display XRCC1 and Lig IIIα expression. Therefore, the observed down-regulation of repair proteins seems to be a specific peculiarity of monocytes. XRCC1, PARP-1, and DNA-PKcs regulation appears to occur on a gene level because corresponding transcripts were not (or borderline) detected in monocytes. Lig IIIα transcripts were observed; therefore, it appears that lack of the protein is a secondary effect resulting from the absence of XRCC1, which stabilizes the ligase. Our data further demonstrate that XRCC1, PARP-1, and DNA-PKcs are subject of coregulation by the proinflammatory cytokines GM-CSF and IL-4. Thus, cultivation of monocytes in medium containing either GM-CSF or IL-4, or both of them, provokes prompt up-regulation of these repair proteins, making the cells competent for the repair of ROS-induced DNA damage. Thus, the data at the same time provide strong evidence that DNA repair is subject to regulation by cytokines.
The BER defect in monocytes renders them highly sensitive to oxLDL, which is known to induce intracellular ROS upon uptake (21) and plays a key role in atherosclerosis (28). It has previously been shown that oxLDL induces a higher apoptotic response in monocytes than macrophages (29). Here, we extend this finding by demonstrating that monocyte-derived DCs are also protected from oxLDL and that the strong killing response of monocytes following oxLDL treatment is a result of an accumulation of DNA breaks, resulting from a defect in the BER ligation function and DSB rejoining. It is tempting to speculate that the high sensitivity of monocytes to oxLDL may lead to a depletion of monocytes and monocyte-derived cell types in individuals exhibiting a high oxLDL blood level, which might have an impact on the immune response. It is obvious that the study has important implications that could be the subject of future investigations.
The findings raise the question of why monocytes are repair-defective and highly vulnerable to ROS. The simultaneous lack of four key repair proteins in human monocytes is likely not an incidental event. It is reasonable to suppose that it plays a biological role in the homeostatic regulation of the immune response. It is conceivable that overactivation of the immune response after infection or during inflammation is regulated by selective killing of monocytes as a result of a high ROS level in the infected/inflamed area. The selective killing of monocytes by ROS in the inflamed tissue may lead to a reduced maturation of macrophages, the main ROS producer, and DCs that otherwise might overstimulate the immune system by triggering the T-cell response (2). This mechanism, which is governed by a severe DNA repair defect in monocytes, would create a negative regulatory feedback that prevents excessive ROS production during inflammation. The data bear therapeutic implications: thus, during anti-inflammatory and tumor therapy, selective killing of monocytes may cause a depletion of DCs, and therefore a lack of T-cell activation following treatment of patients with genotoxic anticancer drugs.
Materials and Methods
Materials and Cells.
Monocytes were isolated from peripheral blood by Ficoll gradient centrifugation and harvested following 1 h substrate adherence. A part of the population was differentiated into immature DCs and macrophages on Petri dishes in X-VIVO-15 medium (Bio Whittaker) supplemented with 1.5% autologous plasma as described (27). The final concentrations of human recombinant cytokines were 800 U/mL GM-CSF (Bayer Healthcare) and 50 U/mL IL-4 (Miltenyi Biotec; SI Materials and Methods provides detailed information). BOOH was from Sigma and H2O2 from Roth. Pifithrin-α was obtained from Calbiochem and is a specific inhibitor of p53 (30). LDL was also purchased from Calbiochem. Before oxidation, LDL was dialyzed overnight against isotonic PBS solution to remove EDTA. LDL oxidation was provoked by 25 μM CuSO4 at 37 °C overnight. For removal of CuSO4, untreated and oxidized LDL were dialyzed overnight against PBS solution. The lipid peroxide content of native and oxidized LDL was determined by analyzing thiobarbituric acid-reactive substances (31).
Apoptosis, Necrosis, Comet, and Repair Assays.
Detailed information for apoptosis, necrosis, comet (32), and repair assays and all other assays such as BER (15) and DNA-PK assay (33), as well as Western blotting, is provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
The authors thank Simon Ernst and Teodora Nikolova for immunohistochemistry and help with LSM, Wynand P. Roos for a Western blot, Wolfgang Herr for samples of CD34+ cells, and Nicole Bacher and Kerstin Steinbrink for introducing the method of T-cell separation. This work was supported by Deutsche Forschungsgemeinschaft Grant KA724/14.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1111919109/-/DCSupplemental.
References
- 1.Hume DA. The mononuclear phagocyte system. Curr Opin Immunol. 2006;18:49–53. doi: 10.1016/j.coi.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Ueno H, Schmitt N, Palucka AK, Banchereau J. Dendritic cells and humoral immunity in humans. Immunol Cell Biol. 2010;88:376–380. doi: 10.1038/icb.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Enk AH, Jonuleit H, Saloga J, Knop J. Dendritic cells as mediators of tumor-induced tolerance in metastatic melanoma. Int J Cancer. 1997;73:309–316. doi: 10.1002/(sici)1097-0215(19971104)73:3<309::aid-ijc1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 4.Durackova Z. Some current insights into oxidative stress. Physiol Res. 2010;59:459–469. doi: 10.33549/physiolres.931844. [DOI] [PubMed] [Google Scholar]
- 5.Fialkow L, Wang Y, Downey GP. Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function. Free Radic Biol Med. 2007;42:153–164. doi: 10.1016/j.freeradbiomed.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 6.Valledor AF, Comalada M, Santamaría-Babi LF, Lloberas J, Celada A. Macrophage proinflammatory activation and deactivation: a question of balance. Adv Immunol. 2010;108:1–20. doi: 10.1016/B978-0-12-380995-7.00001-X. [DOI] [PubMed] [Google Scholar]
- 7.Tudek B, et al. Involvement of oxidatively damaged DNA and repair in cancer development and aging. Am J Transl Res. 2010;2:254–284. [PMC free article] [PubMed] [Google Scholar]
- 8.Briegert M, Kaina B. Human monocytes, but not dendritic cells derived from them, are defective in base excision repair and hypersensitive to methylating agents. Cancer Res. 2007;67:26–31. doi: 10.1158/0008-5472.CAN-06-3712. [DOI] [PubMed] [Google Scholar]
- 9.Plazar J, Zegura B, Lah TT, Filipic M. Protective effects of xanthohumol against the genotoxicity of benzo(a)pyrene (BaP), 2-amino-3-methylimidazo[4,5-f]quinoline (IQ) and tert-butyl hydroperoxide (t-BOOH) in HepG2 human hepatoma cells. Mutat Res. 2007;632:1–8. doi: 10.1016/j.mrgentox.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Roos WP, Kaina B. DNA damage-induced cell death by apoptosis. Trends Mol Med. 2006;12:440–450. doi: 10.1016/j.molmed.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Parsons JL, Dianova II, Dianov GL. APE1-dependent repair of DNA single-strand breaks containing 3′-end 8-oxoguanine. Nucleic Acids Res. 2005;33:2204–2209. doi: 10.1093/nar/gki518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 13.Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muller M, et al. p53 activates the CD95 (Apo-1/Fas) gene in response to DNA damage by anticancer drugs. J Exp Med. 1998;188:2033–2045. doi: 10.1084/jem.188.11.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parsons JL, Dianova II, Allinson SL, Dianov GL. Poly(ADP-ribose) polymerase-1 protects excessive DNA strand breaks from deterioration during repair in human cell extracts. FEBS J. 2005;272:2012–2021. doi: 10.1111/j.1742-4658.2005.04628.x. [DOI] [PubMed] [Google Scholar]
- 16.Caldecott KW, Tucker JD, Stanker LH, Thompson LH. Characterization of the XRCC1-DNA ligase III complex in vitro and its absence from mutant hamster cells. Nucleic Acids Res. 1995;23:4836–4843. doi: 10.1093/nar/23.23.4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dianov GL, et al. Base excision repair in nuclear and mitochondrial DNA. Prog Nucleic Acid Res Mol Biol. 2001;68:285–297. doi: 10.1016/s0079-6603(01)68107-8. [DOI] [PubMed] [Google Scholar]
- 18.Audebert M, Salles B, Calsou P. Involvement of poly(ADP-ribose) polymerase-1 and XRCC1/DNA ligase III in an alternative route for DNA double-strand breaks rejoining. J Biol Chem. 2004;279:55117–55126. doi: 10.1074/jbc.M404524200. [DOI] [PubMed] [Google Scholar]
- 19.Takata M, et al. Homologous recombination and non-homologous end-joining pathways of DNA double-strand break repair have overlapping roles in the maintenance of chromosomal integrity in vertebrate cells. EMBO J. 1998;17:5497–5508. doi: 10.1093/emboj/17.18.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zingg JM, Ricciarelli R, Azzi A. Scavenger receptor regulation and atherosclerosis. Biofactors. 2000;11:189–200. doi: 10.1002/biof.5520110305. [DOI] [PubMed] [Google Scholar]
- 21.Hsieh CC, Yen MH, Yen CH, Lau YT. Oxidized low density lipoprotein induces apoptosis via generation of reactive oxygen species in vascular smooth muscle cells. Cardiovasc Res. 2001;49:135–145. doi: 10.1016/s0008-6363(00)00218-2. [DOI] [PubMed] [Google Scholar]
- 22.Schreiber V, et al. Poly(ADP-ribose) polymerase-2 (PARP-2) is required for efficient base excision DNA repair in association with PARP-1 and XRCC1. J Biol Chem. 2002;277:23028–23036. doi: 10.1074/jbc.M202390200. [DOI] [PubMed] [Google Scholar]
- 23.Wang H, et al. DNA ligase III as a candidate component of backup pathways of nonhomologous end joining. Cancer Res. 2005;65:4020–4030. doi: 10.1158/0008-5472.CAN-04-3055. [DOI] [PubMed] [Google Scholar]
- 24.Caldecott KW, Aoufouchi S, Johnson P, Shall S. XRCC1 polypeptide interacts with DNA polymerase beta and possibly poly (ADP-ribose) polymerase, and DNA ligase III is a novel molecular ‘nick-sensor’ in vitro. Nucleic Acids Res. 1996;24:4387–4394. doi: 10.1093/nar/24.22.4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coquerelle T, Dosch J, Kaina B. Overexpression of N-methylpurine-DNA glycosylase in Chinese hamster ovary cells renders them more sensitive to the production of chromosomal aberrations by methylating agents—a case of imbalanced DNA repair. Mutat Res. 1995;336:9–17. doi: 10.1016/0921-8777(94)00035-5. [DOI] [PubMed] [Google Scholar]
- 26.Narciso L, et al. Terminally differentiated muscle cells are defective in base excision DNA repair and hypersensitive to oxygen injury. Proc Natl Acad Sci USA. 2007;104:17010–17015. doi: 10.1073/pnas.0701743104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jonuleit H, et al. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27:3135–3142. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 28.Victor VM, et al. Oxidative stress, endothelial dysfunction and atherosclerosis. Curr Pharm Des. 2009;15:2988–3002. doi: 10.2174/138161209789058093. [DOI] [PubMed] [Google Scholar]
- 29.Ermak N, Lacour B, Drüeke TB, Vicca S. Role of reactive oxygen species and Bax in oxidized low density lipoprotein-induced apoptosis of human monocytes. Atherosclerosis. 2008;200:247–256. doi: 10.1016/j.atherosclerosis.2007.12.052. [DOI] [PubMed] [Google Scholar]
- 30.Komarov PG, et al. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science. 1999;285:1733–1737. doi: 10.1126/science.285.5434.1733. [DOI] [PubMed] [Google Scholar]
- 31.Hessler JR, Morel DW, Lewis LJ, Chisolm GM. Lipoprotein oxidation and lipoprotein-induced cytotoxicity. Arteriosclerosis. 1983;3:215–222. doi: 10.1161/01.atv.3.3.215. [DOI] [PubMed] [Google Scholar]
- 32.Olive PL, Wlodek D, Banáth JP. DNA double-strand breaks measured in individual cells subjected to gel electrophoresis. Cancer Res. 1991;51:4671–4676. [PubMed] [Google Scholar]
- 33.Shao CJ, Fu J, Shi HL, Mu YG, Chen ZP. Activities of DNA-PK and Ku86, but not Ku70, may predict sensitivity to cisplatin in human gliomas. J Neurooncol. 2008;89:27–35. doi: 10.1007/s11060-008-9592-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.