Abstract
Gaucher disease (GD) is caused by a spectrum of genetic mutations within the gene encoding the lysosomal enzyme glucocerebrosidase (GCase). These mutations often lead to misfolded proteins that are recognized by the unfolded protein response system and are degraded through the ubiquitin–proteasome pathway. Modulating this pathway with histone deacetylase inhibitors (HDACis) has been shown to improve protein stability in other disease settings. To identify the mechanisms involved in the regulation of GCase and determine the effects of HDACis on protein stability, we investigated the most prevalent mutations for nonneuronopathic (N370S) and neuronopathic (L444P) GD in cultured fibroblasts derived from GD patients and HeLa cells transfected with these mutations. The half-lives of mutant GCase proteins correspond to decreases in protein levels and enzymatic activity. GCase was found to bind to Hsp70, which directed the protein to TCP1 for proper folding, and to Hsp90, which directed the protein to the ubiquitin–proteasome pathway. Using a known HDACi (SAHA) and a unique small-molecule HDACi (LB-205), GCase levels increased rescuing enzymatic activity in mutant cells. The increase in the quantity of protein can be attributed to increases in protein half-life that correspond primarily with a decrease in degradation rather than an increase in chaperoned folding. HDACis reduce binding to Hsp90 and prevent subsequent ubiquitination and proteasomal degradation without affecting binding to Hsp70 or TCP1. These findings provide insight into the pathogenesis of GD and indicate a potent therapeutic potential of HDAC inhibitors for the treatment of GD and other human protein misfolding disorders.
Gaucher disease (GD), the most prevalent human hereditary metabolic storage disorder, is caused by mutations in the gene (GBA) that codes for glucocerebrosidase (GCase), leading to accumulation of glucocerebroside in affected tissues (1, 2). GD is classified into three clinical types. Type I GD is nonneuronopathic and is characterized by hepatosplenomegaly, cytopenia, and bone disease. Types II and III GD are neuronopathic with either acute (type II) or chronic (type III) progression of CNS degeneration (3). Although any of the ≈300 mutations identified in GBA may lead to the disease, these mutations do not fully account for the phenotypic variation among patients with the same genotype. Even siblings with the same mutation often present with discordant phenotypes (4, 5), suggesting a more complex mechanism of disease involved in a single gene mutation.
Previous findings indicated that the inconsistent genotype–phenotype correlation in GD is partly the result of differences in sensitivity of mutant GCase to degradation by mediators of the protein quality control system (6). Proteins undergo significant posttranslational modification in the endoplasmic reticulum (ER). Nascent peptides form complexes with several chaperone proteins that facilitate proper folding and targeting. Misfolded proteins bind to other chaperones that direct them toward the ubiquitin–proteasome pathway for degradation. Missense mutations in GCase destabilize the protein, rendering it vulnerable to retention and degradation in the ER (7–9). In GD, this process causes loss of cellular GCase catalytic activity due to reduced localization to the lysosome and proteasomal degradation of the mutant enzyme, rather than a decrease in its intrinsic function (6). Thus, targeting mediators of protein homeostasis, or proteostasis, may prevent GCase degradation and restore function in affected cells.
Histone deacetylase inhibitors (HDACis) are a class of proteostasis regulators that may increase the quantity of functional GCase. HDAC inhibition has been demonstrated to be effective in correcting the cellular phenotype of other diseases of aberrant protein folding, including Niemann-Pick type C disease (10, 11), cystic fibrosis (12), and type II diabetes mellitus (13). HDACs regulate cellular function by posttranslational modification of histones, transcriptional factors, and chaperones including Hsp90 (14). By altering acetylation of these proteins, HDACis can modulate gene expression of proteins in the heat shock response, alter the sensitivity of the unfolded protein response, and decrease ubiquitination and proteasomal degradation to restore the function of misfolded proteins. We investigated the effect of suberoylanilide hydroxamic acid (SAHA, vorinostat), a clinically available HDACi, and a unique investigational HDACi, LB-205, on the stability of mutant GCase with two common mutations, N370S in type I GD and L444P in types II and III GD.
Results
LB-205 Inhibits HDAC Activity and Exhibits a Longer Half-Life than SAHA in Vivo.
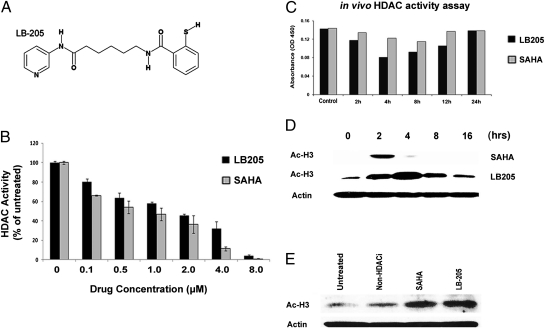
LB-205 contains a metal-binding functional group and, like SAHA, inhibits Zn2+ dependent class I and class II HDACs (Fig. 1A). HDAC activity assays were performed using the DAOY medulloblastoma cell line (15) and DAOY xenograft tumors to compare pharmacodynamic and pharmacokinetic properties of LB-205 to SAHA. LB-205 and SAHA demonstrated dose-dependent HDAC inhibition (Fig. 1B). To determine the biological half-life of LB-205, we measured HDAC activity in s.c. xenografts of DAOY from SCID mice treated with i.p. injections of SAHA and LB-205 after 2, 4, 8, and 12 h. HDACi function of SAHA decreased after 4–8 h, whereas that of LB-205 was sustained up to 12 h (Fig. 1C). Western blotting for acetylated histone 3 (Ac-H3) demonstrated a longer effectiveness of LB-205 in vitro (Fig. 1D). LB-205 and SAHA also inhibited HDACs in GD type 1 (N370S/N370S) fibroblasts. A non-HDACi small molecule, LB-100 (16), was used as a nonspecific control (Fig. 1E).
Fig. 1.
LB-205 inhibits HDAC activity in vitro and in vivo. (A) Chemical structure of LB-205. LB-205 has a zinc binding moiety to inhibit zinc-dependent class I and class II HDACs. (B) Effect of 0–8 μM of LB-205 or SAHA on HDAC activity in DAOY cells. (C) HDAC activity in DAOY s.c. xenografts in SCID mice after i.p. injections of LB-205 and SAHA. (D) Western blot of Ac-H3 in DAOY xenografts confirm the stability of LB-205 for at least 4 h longer than that of SAHA in vivo. (E) Western blot for Ac-H3 demonstrating HDAC inhibition in type 1 GD fibroblasts treated with LB-205 (2.5 μM) and SAHA (2.5 μM).
HDAC Inhibitors Increase the Quantity of Functional GCase in GD Patient-Derived Fibroblasts.
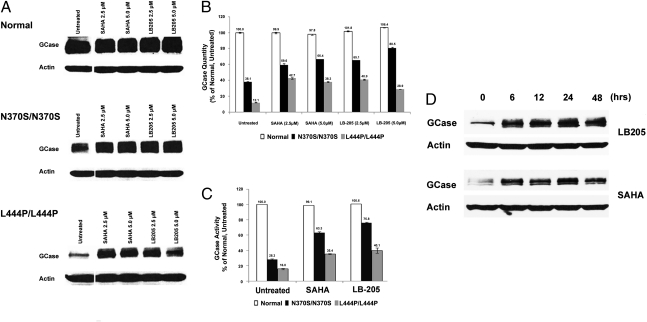
GCase protein levels were analyzed in patient-derived type I (N370S/N370S) and type II/III (L444P/L444P) GD fibroblasts treated with SAHA or LB-205 to determine whether HDACis increase the reduced levels of GCase in fibroblasts with N370S and L444P mutations. Western blotting demonstrated increased GCase levels, from 38% to 60% of untreated control in type I (N370S/N370S) fibroblasts treated with SAHA and up to 80% in those treated with LB-205. GCase levels also increased in type II/III (L444P/L444P) GD fibroblasts treated with LB-205, from 12% of untreated control to 40% and to 42% in those treated with SAHA (Fig. 2 A and B). Assays of enzymatic activity demonstrated a significant increase in functional GCase after treatment with HDACis, indicating that increases in the quantity of GCase protein correlated with increases in cellular enzyme activity. LB-205 increased catalytic activity of N370S GCase from 28% to 76% of untreated GD fibroblasts and L444P GCase from 16% to 40% of untreated GD fibroblasts. Similarly, SAHA increased catalytic activity of N370S GCase from 28% to 63% and of L444P GCase from 16% to 35% (Fig. 2C). Western blotting for GCase in L444P/L444P GD fibroblasts after 0, 6, 12, 24, and 48 h of LB-205 or SAHA treatment demonstrated that the increases in GCase after HDACi treatment are maintained up to 48 h (Fig. 2D).
Fig. 2.
HDAC inhibitors increase functional GCase levels in patient-derived Gaucher fibroblasts. (A) Western blot of analysis of GCase in normal and GD fibroblasts homozygous for N370S or L444P mutations after treatment with LB-205 and SAHA. (B) Quantification of GCase protein by densitometric analysis of Western blots. (C) GCase catalytic activity in normal fibroblasts, untreated GD fibroblasts, and GD fibroblasts treated with LB-205 (2.5 μM) and SAHA (2.5 μM) for 24 h. GCase activity is quantified as a percentage of that of normal fibroblasts cells. (D) Increase in GCase levels in L444P/L444P GD fibroblasts treated with LB-205 (2.5 μM) or SAHA (2.5 μM) was maintained for up to 48 h.
Decreased Stability of N370S and L444P Mutant GCases.
On the basis of the finding that loss of GCase activity in GD is caused by a quantitative reduction of GCase protein, but not a change in its intrinsic catalytic function (6), we hypothesized that the loss of GCase protein is due to a change in protein stability and rapid degradation after translation. To test this possibility, the stability of GCase protein with pathogenic mutations in type I and type III GD was investigated. Two mutations in GBA (ΔGBA-N370S, ΔGBA-L444P) were cloned into pCMV6-Entry vector and trans-fected into HeLa cells. Quantitative changes of GCase pro-tein were monitored through a [35S]-methionine–labeled pulse chase assay.
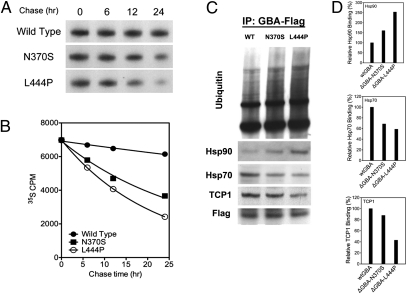
Autoradiography demonstrated normal protein synthesis in the pathogenic mutations. There were no differences in the quantities of radioactively labeled GCase protein between wild type and mutants, suggesting comparable gene transcription/translation efficiency. A quantitative loss of [35S]-labeled GCase protein in ΔGBA-N370S and ΔGBA-L444P mutations was observed over time (Fig. 3A). This finding is comparable to studies that demonstrated that nascent GCase mutant proteins are retained in the ER and efficiently degraded within 24 h (8, 17). [35S]-GCase protein was quantified through liquid scintillation on X-ray film (Fig. 3B). Changes in the quantity of proteins revealed an exponential decay over time. After a 24-h chase, 88% wild-type GCase protein remained in the cells, whereas N370S and L444P mutant proteins were reduced to 52% and 35%, respectively.
Fig. 3.
Mutated GCases exhibit reduced half-life and abnormal binding to chaperonins. (A) Metabolic [35S] pulse chase assay shows quantitative loss of [35S]-labeled GCase protein in HeLa cells with ΔGBA-N370S and ΔGBA-L444P mutant vectors with time. (B) Quantification of [35S]-GCase protein through liquid scintillation measurement confirmed the loss of stability of mutant GCase proteins. (C) Western blot of immunoprecipitated N370S and L444P GBA-Flag proteins suggests increased degradation of mutated GCases, as evidenced by reduced binding to chaperonins Hsp70 and TCP1 and increased ubiquitination and Hsp90 binding. (D) Densitometric quantification of Hsp70, Hsp90, and TCP1 binding to L444P GCase.
Abnormal Chaperonin Binding to Mutated GCase Proteins.
The rapid degradation of GCase after translation indicated the presence of dysfunction in protein folding and posttranslational modification of GCase mutants. To further elucidate the degradation pathways of N370S and L444P mutants, chaperonin binding to GCase was assessed using coimmunoprecipitation of Flag-tagged GCase with monoclonal antibodies against Flag (DYKDDDDK). We found decreased binding of Hsp70 and TCP1 to mutant GCases, suggesting that the mutated proteins were less likely to be recognized by chaperonins and may be unable to form mature GCase. In contrast, GCase was found to have elevated Hsp90 binding and protein ubiquitination in both N370S and L444P mutants, suggesting that the mutant protein was trapped by the Hsp90 system and directed toward ubiquitin–proteasome-mediated degradation (Fig. 3C).
Increased GCase Stability After Treatment with Proteostasis Regulators.
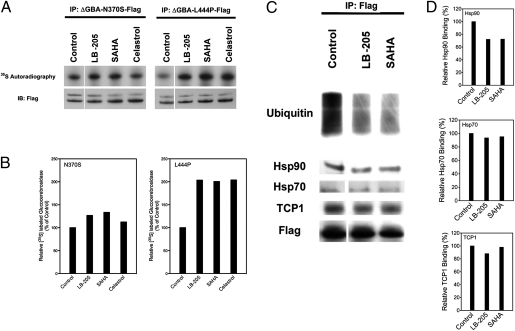
Mutant GCases produced by the above genotypes largely retain their intrinsic catalytic activity. The reduced activity of GCase in these patients with GD seems to be due to rapid posttranslational degradation rather than to impaired function. Modulation of proteostasis regulators that affect prefunctional deg-radation may be an effective therapeutic approach by increasing the amount of functional GCase. In the present study, HeLa cells with ΔGBA-N370S or the ΔGBA-L444P mutant vector were treated with SAHA, LB-205, and celastrol for 48 h, and residual protein levels were investigated by a pulse chase assay. Celastrol is a proteostasis regulator that induces heat shock response in human cells by activating the heat shock transcription factor (HSF-1) (18, 19). LB-205, SAHA, and celastrol increased protein stability in the N370S mutant (Fig. 4A). The amount of radioactively labeled protein increased by 27% after treatment with LB-205, by 12% after treatment with SAHA, and by 33% after treatment with celastrol. For the L444P mutant, [35S]-GCase was found to increase by 104% after LB-205 treatment, by 101% after SAHA treatment, and by 104% after treatment with celastrol (Fig. 4B).
Fig. 4.
Proteostasis regulators prevent degradation of N370S and L444P GCase and promote proper binding to chaperonins. (A) [35S] pulse chase assay after treatment of HeLa cells with ΔGBA-N370S and ΔGBA-L444P mutant vectors with proteostasis regulators LB-205 (10 μM), SAHA (1 μM), and celastrol (0.8 μM) for 48 h. All three proteostasis regulators increased GCase quantity in both N370S and L444P mutants. (B) Densitometric quantification of mutant GCase after treatment. (C) Western blot of coimmunoprecipitated GBA-Flag proteins in L444P mutants treated with LB-205 and SAHA. HDAC inhibition reduced ubiquitin and Hsp90 binding with no significant change in TCP1 and Hsp70 binding. (D) Densitometric quantification of Hsp70, Hsp90, and TCP1 binding to L444P GCase treated with LB-205 and SAHA.
To better understand the molecular features of GCase degradation during treatment with HDACis, protein ubiquitination and Hsp90, Hsp70, and TCP1 binding were examined by coimmunoprecipitation of Flag-tagged GCase with monoclonal antibodies against Flag (Fig. 4C). Ubiquitination of mutant GCase protein and binding to Hsp90 were elevated in untreated transfected HeLa cells, which is consistent with the shortened half-life of the mutant protein (Fig. 4D). Treatment with LB-205 and SAHA decreased GCase ubiquitination without changing the level of TCP1 binding, indicating that a decrease in protein degradation through the ubiquitin–proteasome pathway mediates the increase in GCase after HDACi, rather than an up-regulation of the chaperones involved in the unfolded protein response.
Discussion
We report a potential approach for the treatment of GD by inhibiting HDAC activity that results in an increase in catalytic activity of mutant GCases in tissues. After translation at the rough ER, nascent GCase is cleaved and glycosylated at four asparagine residues for translocation through the ER (20). GCase undergoes further modifications in the Golgi apparatus, and the mature protein is trafficked to lysosomes to carry out its function (21). Alternatively, mutated GCase proteins are eliminated by ER-associated degradation before they reach the lysosome. Generally, proteins that are unable to fold properly are identified by ER chaperones and returned to the cytosol for ubiquitin-dependent proteasomal degradation. Although several mutations produce the various phenotypes of GD, improper folding and subsequent degradation of mutant GCase by the ubiquitin–proteasome pathway seems to be a common mechanism that ultimately leads to cellular accumulation of glucocerebroside in many of these mutations (22). Previously, we found that the catalytic activity of GCase was not impaired by common mutations found in GD types I, II, and III, but that enzymatic activity was correlated with the concentration of the protein in the cell (6). Therefore, the use of proteostasis regulators such as HDACis to prevent degradation of GCase seems to be a useful approach to rescue GCase function.
The preponderant treatment for GD uses enzyme replacement therapy with recombinant GCase (23). Although effective for reducing the accumulation of glucocerebrosides in the viscera, enzyme replacement cannot treat neuronopathic forms of GD because GCase does not cross the blood–brain barrier efficiently (24, 25). Recent insight into the misfolding of GCase has led to the development of pharmacological chaperones, such as isofagomine, that are designed to aid in the proper folding and delivery of GCase to the lysosome. These agents increase lysosomal delivery of several mutant variants of GCase in vitro (26, 27) and in preclinical animal models have also shown efficacy in restoring GCase activity, further suggesting that modulating protein stability can provide therapeutic benefits for GD (28). Currently, pharmacological chaperones are under investigation in phase II and phase III clinical trials for the treatment of type I GD and other lysosomal storage diseases (29, 30). However, these agents are also active-site inhibitors that require potentially complex on–off regimens to wash the drug out of the lysosome before restoring enzyme activity (31, 32). Other compounds can also modulate proteostasis and may increase the quantity of active GCase without the need for a washout period. Celastrol has also been shown to rescue mutant GCase from degradation (9). However, it is cytotoxic at concentrations higher than 1 μM, indicating the need for less toxic equivalents. HDACis have been shown to increase proper folding and trafficking in several other protein misfolding disorders (10–13). This strategy could be applied to GD as a means to improve endogenous GCase stability and increase delivery of active enzyme to the lysosome.
The effects of two broad-spectrum HDACis, SAHA and LB-205, were investigated to determine whether HDACis could modulate the quality control and degradation of mutant GCase. SAHA (vorinostat) was the first HDACi approved for clinical use as treatment for advanced cutaneous T-cell lymphoma (33). SAHA inhibits class I HDACs 1, 2, 3, and 8, as well as class II HDACs 6, 10, and 11 (34). LB-205 (Fig. 1A) has a similar HDAC inhibitory function as SAHA and a longer biological half-life than SAHA in vivo (Fig. 1C). The concentrations used in this study are consistent with serum concentrations of SAHA achieved in patients (35). Western blot analysis of patient-derived GD fibroblasts treated with LB-205 and SAHA revealed a significant increase in GCase quantity in both N370S and L444P fibroblasts. Similar increases in protein stability and enzymatic activity were observed in HeLa cells transfected with ΔGBA-N370S and ΔGBA-L444P after treatment with LB-205 and SAHA. Although the relative increase in GCase quantity was less than what was observed in patient-derived type I and type III GD fibroblasts, the effect of HDACis on increasing total GCase was substantiated. The discrepancy may be explained by an increase in transcription of mutant GCase in transfected cells resulting in elevated GCase protein compared with patient-derived fibroblasts with the same mutations. Treatment with HDACis increased the relative proportion of L444P mutant GCase protein more than N370S, similar to the trend observed in patient-derived fibroblasts. Another known proteostasis regulator, celastrol (18, 19, 36), yielded similar increases in the quantity of GCase as treatment with HDACis, providing further support that HDACis act through the protein quality control system.
Although the exact mechanism of how HDACis facilitate the increase in catalytically active protein is unclear, we propose that at least two parallel processes shift the cellular machinery toward increased protein expression and decreased protein degradation. The first is a direct action of HDACis on the hyperacetylation and inactivation of Hsp90, a chaperone protein implicated in the degradation of misfolded GCase. HDACis could regulate proteostasis through several possible mechanisms involving chaperonins and the ubiquitin–proteasome degradation pathway. Protein ubiquitination and binding to three well-accepted mediators of protein folding and maturation, Hsp90, Hsp70, and TCP1 (27, 37), was investigated to elucidate which of these mechanisms played a predominant role in the proteostasis of GCase. Both N370S and L444P mutants demonstrated increased ubiquitination, increased binding to Hsp90, and decreased binding to Hsp70 and TCP1 (Fig. 4). These observations are consistent with previous findings with regard to the von Hippel-Lindau tumor suppressor protein (37) and merlin (38), in which two distinct pathways mediate folding and quality control. Hsp70 and TRiC are required for correct folding of nascent protein, whereas defective protein is transferred to the Hsp90 complex for degradation. Treatment with HDACis decreased ubiquitination in N370S and L444P mutants compared with the control, suggesting that HDAC inhibition curbed the rate of degradation by modulating the Hsp70–Hsp90 system. These findings are consistent with previous studies that have shown that HDAC6 inhibition leads to hyperacetylation of Hsp90, the disassociation of Hsp90 from its cochaperone, p23, and loss of chaperone activity (39). By reducing the rate of ubiquitination and degradation and maintaining the rate of transcription, translation, and folding, the half-life of synthesized GCase increases along with its steady-state concentration in the lysosome and restores sufficient enzymatic activity to potentially rescue cells from glucocerebroside toxicity.
A separate, indirect mechanism of HDACis may act in concert with the direct modulation of the Hsp70–90 pathway to nonspecifically saturate the protein quality control system. This mechanism may decrease selectivity and allow misfolded proteins to evade proteasomal degradation. HDACis alter the acetylation of histones modulating the transcription of multiple genes that act alone or in concert to promote proper folding of GCase and other proteins (12). This mechanism is consistent with studies on HDAC inhibitor function in multiple myeloma and other human solid and hematologic cancers. SAHA works synergistically with proteasome inhibitors to promote protein buildup and aggregation, inducing cell death in multiple myeloma cells with uniquely high protein synthetic load (40). Although cells affected by GD are unlike cancer cells in their rate of protein synthesis, a similar principle may apply due to the general increase in gene expression after treatment with HDACis. By increasing the synthetic load and decreasing the available number of chaperones, HDACis may limit the effective binding of newly synthesized GCase to Hsp90 and prevent further ubiquitination and degradation. Such a mechanism may also lead to an undesirable increase in the formation of toxic protein aggregates by triggering an ER stress response. Proteasome inhibitors have been associated with the overexpression of parkin, an E3 ubiquitin ligase responsible for the aggregation of mutant GCase, resulting in premature neuronal death and leading to Parkinson's disease even in heterozygous carriers of GBA mutations. However, this effect has yet to be demonstrated with HDACis, and further investigation must be conducted to determine the magnitude of this risk. We do not believe this risk precludes the potential benefit of HDACis in restoring GCase activity in GD.
Our findings suggest that HDACis may be valuable as a therapeutic option for neuronopathic and nonneuronopathic GD by decreasing the rate of degradation of mutant GCases. HDACis increased the catalytic activity of N370S and L444P in GD fibroblasts nearly twofold, which may be sufficient to ameliorate some or all of the manifestations of Gaucher disease (32). These findings underscore the hypothesis that misfolded proteins that lose conformational stability but otherwise retain intrinsic function can be rescued from degradation by HDAC inhibition. Although both direct and indirect inhibition of protein degradation are likely involved in the rescue of mutant GCase, clarifying the mechanism by which HDACis mediate proteostasis has significant implications for many other diseases caused by misfolded proteins, including other lysosomal storage disorders (9, 41), neurofibromatosis type 2 (38), cystic fibrosis (12), and type II diabetes mellitus (13). By further understanding and targeting the pathways involved in proteostasis, regulators such as HDACis may be able to restore adequate protein function in these common diseases.
Materials and Methods
Cell Culture and HDACi Treatment.
Fibroblasts from GD patients were maintained as previously described (6). Cell lines were obtained from the Development and Metabolic Neurology Branch of the National Institute of Neurological Disorders and Stroke. The type I GD fibroblasts are homozygous for the N370S glucocerebrosidase mutation. Type II and III GD fibroblasts are homozygous for L444P mutation. Cells were cultured in Eagle's minimum essential medium (Invitrogen) supplemented with 10% FBS. A total of 2.5 × 104 cells were seeded in 24-well plates, allowed to attach for at least 24 h, and then treated with SAHA (Cayman Chemical) and LB-205 dissolved in dimethyl sulfoxide (Sigma-Aldrich) at concentrations of 2.5 μM and 5.0 μM for 24 h. Transfected HeLa cells were treated with 10 μM of LB-205, 1 μM of SAHA, and 0.8 μM of celastrol (Sigma-Aldrich). LB-205 was provided by Lixte Biotechnology Holdings.
Assay of HDAC Activity.
Cultured DAOY cells were plated in 175-cm3 flasks. When the cells were 80% confluent, the media was replaced with media that contained SAHA (0–8 μM) or LB-205 (0–8 μM). After 1 h, the cells were washed three times with a 0.9% normal saline solution. Cells were lysed in tissue protein extraction reagent (T-PER) (Pierce Biotechnology), sonicated, and centrifuged. Nuclear extracts (40 μg per well) were assayed using the HDAC Assay Kit (Active Motif).
HDAC activity in DAOY s.c. xenografts was assayed in 6- to 8-wk-old SCID mice (Taconic) using the above conditions. Each mouse weighed ≈20 g. Animals were housed under barrier conditions and maintained on a 12-h light/12-h dark cycle with adequate food and water. After being observed for 7 d, mice were injected s.c. in both flanks with 5 × 106 DAOY tumor cells suspended in PBS. After the xenografts reached 1.0 ± 0.5 cm, animals received i.p. injections of PBS vehicle, 25 mg/kg of LB-205, or 25 mg/kg of SAHA. Tumor tissues were harvested after 2, 4, 8, 12, and 24 h and washed three times with a 0.9% normal saline solution for HDAC activity assays. All animal experiments were approved for use and care of animals under the protocol guidelines of the National Institutes of Health Animal Care and Use Committee.
GCase Enzyme Activity Assay.
GCase activity was determined as previously described (42). Samples were loaded into a 96-well plate, and fluorescence was measured with a VICTOR3 multilabel counter (Perkin-Elmer) at an excitation/emission setting of 355 nm/460 nm. One unit of GCase activity was designated as 1 nmol of 4-methylumbelliferone released per hour.
Western Blotting.
Cell pellets were lysed in T-PER solution (Thermo Scientific), sonicated, and centrifuged. Protein was determined in the supernatant solution using the Bio-Rad Protein Assay kit. Proteins were separated by SDS/PAGE on 4–15% acrylamide gels (Invitrogen) and transferred to nitrocellulose membranes (Invitrogen). Blocking buffer solution was used before immunoblotting with primary antibody. Expression of GCase was determined by Western blotting using monoclonal antibody (Abcam) at a dilution of 1:1,000. Ac-H3 rabbit antibody (Cell Signaling Technology) was used to detect HDACi function. A goat antibody directed against actin (sc-1616; Santa Cruz Biotechnology) at a dilution of 1:1,000 was used as a loading control. Detection of antibodies was performed with a horseradish peroxidase-conjugated species-specific secondary antibody and an enhanced chemiluminescence system. Densitometric analysis using ImageJ software (National Institutes of Health) was used to quantify the expression of GCase.
Immunoprecipitation.
Immunoprecipitation was performed using a DynaBeads Protein G immunoprecipitation kit (Invitrogen). Cells were harvested and extracted for protein using IP lysis buffer (Thermo Scientific). Two hundred micrograms of whole-cell protein were precipitated at 4 °C with anti-Flag monoclonal antibody (1:200, Origene). Precipitated protein was eluted and analyzed by Western blotting.
DNA Cloning and Site-Directed Mutagenesis.
The human GBA gene was cloned into pCMV6-Entry vector (Origene). Mutagenesis was performed using a QuikChange Lightning Site-directed mutagenesis kit (Agilent). Mutagenesis was conducted using primers with N370S and L444P mutation sites. The sequence of GBA genes was verified by sequencing the entire coding regions for both mutants.
Metabolic [35S] Labeling and Pulse Chase Assay.
Metabolic [35S] pulse chase assay was performed as previously described with minor modifications (38). A total of 5 × 105 HeLa cells were transfected with GBA vectors through FuGene 6 transfection reagent (Roche). Cells were used for pulse chase assay 24 h after transfection. Transfected cells treated with LB-205, SAHA, and celastrol for 48 h before pulse chase assay. Cells were starved in methionine-depleted medium for 15 min, followed by 15 min labeling in methionine-free Delbecco's modified Eagle medium (DMEM) supplemented with 10% FBS and 0.2 mCi/mL [35S]-methionine (>1,000 Ci/mL specific activity; Perkin-Elmer). The cells were then chased in DMEM with 10% FBS and 3 mg/mL methionine. Cells were extracted for protein at varying times after radioactive labeling. Cells were lysed in radioimmunoprecipitation assay lysis buffer containing proteinase inhibitor mixture (Thermo Scientific). GCase proteins were purified by immunoprecipitation using 200 μg total lysate with Flag antibody (Origene). Precipitated proteins were resolved on NuPAGE Novex 4–12% Bis-Tris Gel (Invitrogen) and measured by liquid scintillation counting. The gel was fixed and incubated in EN3HANCE (Perkin-Elmer) for 45 min and dried before exposure to X-ray film.
Acknowledgments
This research was supported (in part) by the Intramural Research Program of the National Institutes of Health (NIH), National Institute of Neurological Disorders and Stroke (NINDS).
Footnotes
The authors declare no conflict of interest.
References
- 1.Brady RO, Kanfer JN, Shapiro D. Metabolism of glucocerebrosides II. Evidence of an enzymatic deficiency in Gaucher's disease. Biochem Biophys Res Commun. 1965;18:221–225. doi: 10.1016/0006-291x(65)90743-6. [DOI] [PubMed] [Google Scholar]
- 2.Brady RO, Kanfer JN, Bradley RM, Shapiro D. Demonstration of a deficiency of glucocerebroside-cleaving enzyme in Gaucher's disease. J Clin Invest. 1966;45:1112–1115. doi: 10.1172/JCI105417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beutler E, Grabowski G. The Metabolic and Molecular Bases of Inherited Disease. 8th Ed. New York: McGraw-Hill; 2001. pp. 3635–3668. [Google Scholar]
- 4.Lachmann RH, Grant IR, Halsall D, Cox TM. Twin pairs showing discordance of phenotype in adult Gaucher's disease. QJM. 2004;97:199–204. doi: 10.1093/qjmed/hch036. [DOI] [PubMed] [Google Scholar]
- 5.Amato D, Stachiw T, Clarke JT, Rivard GE. Gaucher disease: Variability in phenotype among siblings. J Inherit Metab Dis. 2004;27:659–669. doi: 10.1023/b:boli.0000042983.60840.f3. [DOI] [PubMed] [Google Scholar]
- 6.Lu J, et al. Decreased glucocerebrosidase activity in Gaucher disease parallels quantitative enzyme loss due to abnormal interaction with TCP1 and c-Cbl. Proc Natl Acad Sci USA. 2010;107:21665–21670. doi: 10.1073/pnas.1014376107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ron I, Horowitz M. ER retention and degradation as the molecular basis underlying Gaucher disease heterogeneity. Hum Mol Genet. 2005;14:2387–2398. doi: 10.1093/hmg/ddi240. [DOI] [PubMed] [Google Scholar]
- 8.Schmitz M, Alfalah M, Aerts JM, Naim HY, Zimmer KP. Impaired trafficking of mutants of lysosomal glucocerebrosidase in Gaucher's disease. Int J Biochem Cell Biol. 2005;37:2310–2320. doi: 10.1016/j.biocel.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Mu TW, et al. Chemical and biological approaches synergize to ameliorate protein-folding diseases. Cell. 2008;134:769–781. doi: 10.1016/j.cell.2008.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pipalia NH, et al. Histone deacetylase inhibitor treatment dramatically reduces cholesterol accumulation in Niemann-Pick type C1 mutant human fibroblasts. Proc Natl Acad Sci USA. 2011;108:5620–5625. doi: 10.1073/pnas.1014890108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munkacsi AB, et al. An “exacerbate-reverse” strategy in yeast identifies histone deacetylase inhibition as a correction for cholesterol and sphingolipid transport defects in human Niemann-Pick type C disease. J Biol Chem. 2011;286:23842–23851. doi: 10.1074/jbc.M111.227645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hutt DM, et al. Reduced histone deacetylase 7 activity restores function to misfolded CFTR in cystic fibrosis. Nat Chem Biol. 2010;6:25–33. doi: 10.1038/nchembio.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ozcan U, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scroggins BT, et al. An acetylation site in the middle domain of Hsp90 regulates chaperone function. Mol Cell. 2007;25:151–159. doi: 10.1016/j.molcel.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobsen PF, Jenkyn DJ, Papadimitriou JM. Establishment of a human medulloblastoma cell line and its heterotransplantation into nude mice. J Neuropathol Exp Neurol. 1985;44:472–485. doi: 10.1097/00005072-198509000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Lu J, et al. The effect of a PP2A inhibitor on the nuclear receptor corepressor pathway in glioma. J Neurosurg. 2010;113:225–233. doi: 10.3171/2009.11.JNS091272. [DOI] [PubMed] [Google Scholar]
- 17.Bergmann JE, Grabowski GA. Posttranslational processing of human lysosomal acid beta-glucosidase: A continuum of defects in Gaucher disease type 1 and type 2 fibroblasts. Am J Hum Genet. 1989;44:741–750. [PMC free article] [PubMed] [Google Scholar]
- 18.Westerheide SD, et al. Celastrols as inducers of the heat shock response and cytoprotection. J Biol Chem. 2004;279:56053–56060. doi: 10.1074/jbc.M409267200. [DOI] [PubMed] [Google Scholar]
- 19.Westerheide SD, Morimoto RI. Heat shock response modulators as therapeutic tools for diseases of protein conformation. J Biol Chem. 2005;280:33097–33100. doi: 10.1074/jbc.R500010200. [DOI] [PubMed] [Google Scholar]
- 20.Erickson AH, Ginns EI, Barranger JA. Biosynthesis of the lysosomal enzyme glucocerebrosidase. J Biol Chem. 1985;260:14319–14324. [PubMed] [Google Scholar]
- 21.Takasaki S, et al. Structure of the N-asparagine-linked oligosaccharide units of human placental beta-glucocerebrosidase. J Biol Chem. 1984;259:10112–10117. [PubMed] [Google Scholar]
- 22.Offman MN, Krol M, Silman I, Sussman JL, Futerman AH. Molecular basis of reduced glucosylceramidase activity in the most common Gaucher disease mutant, N370S. J Biol Chem. 2010;285:42105–42114. doi: 10.1074/jbc.M110.172098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brady RO. Enzyme replacement for lysosomal diseases. Annu Rev Med. 2006;57:283–296. doi: 10.1146/annurev.med.57.110104.115650. [DOI] [PubMed] [Google Scholar]
- 24.Sawkar AR, et al. Chemical chaperones and permissive temperatures alter localization of Gaucher disease associated glucocerebrosidase variants. ACS Chem Biol. 2006;1:235–251. doi: 10.1021/cb600187q. [DOI] [PubMed] [Google Scholar]
- 25.Sawkar AR, D'Haeze W, Kelly JW. Therapeutic strategies to ameliorate lysosomal storage disorders—a focus on Gaucher disease. Cell Mol Life Sci. 2006;63:1179–1192. doi: 10.1007/s00018-005-5437-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steet RA, et al. The iminosugar isofagomine increases the activity of N370S mutant acid beta-glucosidase in Gaucher fibroblasts by several mechanisms. Proc Natl Acad Sci USA. 2006;103:13813–13818. doi: 10.1073/pnas.0605928103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khanna R, et al. The pharmacological chaperone isofagomine increases the activity of the Gaucher disease L444P mutant form of β-glucosidase. FEBS J. 2010;277:1618–1638. doi: 10.1111/j.1742-4658.2010.07588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun Y, et al. Isofagomine in vivo effects in a neuronopathic Gaucher disease mouse. PLoS ONE. 2011;6:e19037. doi: 10.1371/journal.pone.0019037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amicus Therapeutics Study to compare the efficacy and safety of oral AT1001 and enzyme replacement therapy in patients with Fabry disease. ClinicalTrials.gov ID: NCT01218659. 2011. Available at: http://clinicaltrials.gov/ct2/show/NCT01218659. Accessed November 17, 2011.
- 30.Amicus Therapeutics A long-term extension study of AT2101 in type 1 Gaucher patients. ClinicalTrials.gov ID: NCT00813865. 2011. Available at: http://clinicaltrials.gov/ct2/show/NCT00813865. Accessed November 17, 2011.
- 31.Lieberman RL, et al. Structure of acid beta-glucosidase with pharmacological chaperone provides insight into Gaucher disease. Nat Chem Biol. 2007;3:101–107. doi: 10.1038/nchembio850. [DOI] [PubMed] [Google Scholar]
- 32.Yu Z, Sawkar AR, Whalen LJ, Wong CH, Kelly JW. Isofagomine- and 2,5-anhydro-2,5-imino-D-glucitol-based glucocerebrosidase pharmacological chaperones for Gaucher disease intervention. J Med Chem. 2007;50:94–100. doi: 10.1021/jm060677i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marks PA, Breslow R. Dimethyl sulfoxide to vorinostat: Development of this histone deacetylase inhibitor as an anticancer drug. Nat Biotechnol. 2007;25:84–90. doi: 10.1038/nbt1272. [DOI] [PubMed] [Google Scholar]
- 34.Marks PA, Xu WS. Histone deacetylase inhibitors: Potential in cancer therapy. J Cell Biochem. 2009;107:600–608. doi: 10.1002/jcb.22185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramalingam SS, et al. Phase I and pharmacokinetic study of vorinostat, a histone deacetylase inhibitor, in combination with carboplatin and paclitaxel for advanced solid malignancies. Clin Cancer Res. 2007;13:3605–3610. doi: 10.1158/1078-0432.CCR-07-0162. [DOI] [PubMed] [Google Scholar]
- 36.Nagai N, Nakai A, Nagata K. Quercetin suppresses heat shock response by down regulation of HSF1. Biochem Biophys Res Commun. 1995;208:1099–1105. doi: 10.1006/bbrc.1995.1447. [DOI] [PubMed] [Google Scholar]
- 37.McClellan AJ, Scott MD, Frydman J. Folding and quality control of the VHL tumor suppressor proceed through distinct chaperone pathways. Cell. 2005;121:739–748. doi: 10.1016/j.cell.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 38.Yang C, et al. Missense mutations in the NF2 gene result in the quantitative loss of merlin protein and minimally affect protein intrinsic function. Proc Natl Acad Sci USA. 2011;108:4980–4985. doi: 10.1073/pnas.1102198108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kovacs JJ, et al. HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol Cell. 2005;18:601–607. doi: 10.1016/j.molcel.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 40.Kikuchi J, et al. Histone deacetylases are critical targets of bortezomib-induced cytotoxicity in multiple myeloma. Blood. 2010;116:406–417. doi: 10.1182/blood-2009-07-235663. [DOI] [PubMed] [Google Scholar]
- 41.Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE. Biological and chemical approaches to diseases of proteostasis deficiency. Annu Rev Biochem. 2009;78:959–991. doi: 10.1146/annurev.biochem.052308.114844. [DOI] [PubMed] [Google Scholar]
- 42.Lee KO, et al. Improved intracellular delivery of glucocerebrosidase mediated by the HIV-1 TAT protein transduction domain. Biochem Biophys Res Commun. 2005;337:701–707. doi: 10.1016/j.bbrc.2005.05.207. [DOI] [PubMed] [Google Scholar]