Abstract
Coupling of spindle orientation to cellular polarity is a prerequisite for epithelial asymmetric cell divisions. The current view posits that the adaptor Inscuteable (Insc) bridges between Par3 and the spindle tethering machinery assembled on NuMA∶LGN∶GαiGDP, thus triggering apico-basal spindle orientation. The crystal structure of the Drosophila ortholog of LGN (known as Pins) in complex with Insc reveals a modular interface contributed by evolutionary conserved residues. The structure also identifies a positively charged patch of LGN binding to an invariant EPE-motif present on both Insc and NuMA. In vitro competition assays indicate that Insc competes with NuMA for LGN binding, displaying a higher affinity, and that it is capable of opening the LGN conformational switch. The finding that Insc and NuMA are mutually exclusive interactors of LGN challenges the established model of force generators assembly, which we revise on the basis of the newly discovered biochemical properties of the intervening components.
Asymmetric cell divisions regulate the position and the fate choice of daughter cells, with impact on numerous phenotypes of multicellular organisms. During development, asymmetric divisions coordinate cell growth with cell specification to determine tissue morphogenesis, while in adult life they sustain tissue homeostasis and regeneration (1). In asymmetric divisions specific cortical landmarks instruct the orientation of the mitotic spindle to promote unequal partitioning of fate determinants in cellular systems as diverse as Caenorhabditis elegans zygotes, Drosophila neuroblasts, as well as vertebrate skin and neural progenitors (2). Spindle coupling to polarity cues involves the recruitment at cortical sites of molecular devices, known as force generators, whose main task is to capture astral microtubules emanating from the spindle poles and to establish pulling forces. Core components of force generators are the evolutionary conserved NuMA∶LGN∶Gαi complexes, termed Mud∶Pins∶Gαi in flies. Topologically, tetratricopeptide repeats (TPR) present in the N-terminal portion of LGN mediate the interactions with NuMA, while GoLoco motifs at the C terminus serve as a docking platform for four GαiGDP subunits anchored at the plasma membrane via a myristoyl group (3). LGN exclusively binds to GDP-loaded Gαi (4). The association of cortical NuMA with the microtubule motor Dynein/Dynactin (5) provides a sliding anchorage for depolymerizing microtubules, whose shrinkage pulls towards the cortex the connected spindle pole. FRET studies revealed that LGN behaves as a conformational switch held in a closed form in interphase by head-to-tail interactions (3).
An issue intimately related to how force generators are assembled is how they are recruited at sites of polarization. In polarized asymmetric divisions, the apico-basal polarity axis is established by the asymmetrical distribution of Par3∶Par6∶aPKC at the apical cortex, which are able to recruit NuMA∶LGN∶GαiGDP via an adaptor named Inscuteable (Insc) (6). Insc was first identified in larval Drosophila neuroblasts as a partner of Par3 (Bazooka in flies) colocalizing with polarity proteins right after delamination (7–9), and later shown to bind Pins (10). Mammalian Insc homologues endowed with similar properties have been discovered in rat retina (11) and in mouse developing skin (12). Insc overexpression is sufficient to induce apico-basal divisions in cells that normally divide in a planar fashion such as Drosophila embryonic ephitelial cells (7), vertebrate neuroepithelial progenitors (13), and mouse skin progenitors (6). The portion of fly Insc encompassing residues 252–615 recapitulates Insc functions in neuroblasts (hence termed “asymmetric domain”), and directly interacts with Pins (9). Based on its interaction with both Par3 and LGN, Insc has been considered the molecular link between cortical Par proteins and NuMA∶LGN∶GαiGDP complexes. This notion is mainly substantiated by imaging analyses conducted in fly neuroblasts and mouse skin progenitors showing that during asymmetric metaphases Par3, Insc, LGN, and NuMA colocalyze in a cortical region underlying one of the spindle poles (1, 6, 12). However, no proof has been provided for the simultaneous association of LGN with Insc and NuMA, which remains a key question to elucidate how force generators work. We thus set out to study how LGN interacts with Insc and NuMA.
Results
Determination of the Crystal Structure of Pins∶Insc.
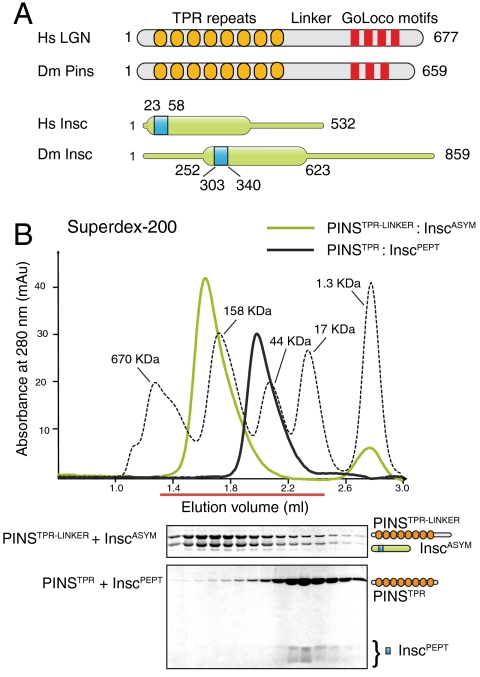
Human Insc∶LGN∶GαiGDP was refractory to crystallization. We thus took advantage of the functional information available on fruitfly proteins (Fig. 1A), and generated a dicistronic vector for coexpression in bacteria in which we cloned the TPR repeats and part of the linker region of Pins (PinsTPR-LINKER) fused with a GST moiety in the first cassette, and a fragment corresponding to the asymmetric domain of Insc (InscASYM) in the second cassette. This strategy yielded a soluble PinsTPR-LINKER∶InscASYM complex eluting from a size exclusion chromatography (SEC) as a monodisperse sample (Fig. 1B). In order to obtain diffraction quality crystals, we had to further shorten these Pins and Insc constructs by limited proteolysis. When subjected to trypsinization, both subunits of PinsTPR-LINKER∶InscASYM were trimmed to smaller fragments (Fig. S1), which were assigned by mass spectrometry to Pins25–406 and Insc303–340 respectively. SEC analysis confirmed that Pins25–406 stably associates with Insc303–340 with a 1∶1 stoichiometry (Fig. 1B). To gain an understanding of the organizational principles of the interaction, we determined the structure of the proteolitically defined Pins25–406∶Insc303–340 complex, referred to as PinsTPR∶dInscPEPT (where “d” stands for Drosophila).
Fig. 1.
Generation of the Pins:Insc complex. (A) Scheme of LGN and Insc. (B) SEC elution profiles of PinsTPR-LINKER∶InscASYM and PinsTPR∶dInscPEPT.
To determine the crystallographic structure, we used SAD phases from selenomethionine-substituted protein crystals. The structure has been refined to 2.1 Å resolution with an Rfree of 25.6% (Table S1). The final model includes residues 307 to 335 of dInsc peptide, and residues 39–386 of Pins.
General Architecture of the Pins∶Insc Assembly.
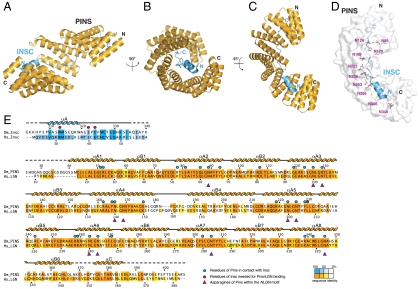
The PinsTPR domain folds into a cradle-shaped arch harboring the extended dInscPEPT into its inner concave surface (Fig. 2 A–C). In such arrangement, the Pins and dInsc polypeptide chains run with opposite direction, with the helical N terminus of dInscPEPT contacting the C-terminal portion of PinsTPR. Spiraling of the PinsTPR domain around dInscPEPT generates an extensive interaction surface of about 1,900 Å2, corresponding to approximately 50% of the dInscPEPT surface area.
Fig. 2.
Structure of the PinsTPR∶dInscPEPT dimer. (A–C) Ribbon models of the PinsTPR∶dInscPEPT complex viewed at the indicated orientations. (D) Surface representation of PinsTPR with the dInscPEPT lining along the conserved asparagines (purple). (E) Sequence and secondary structure of PinsTPR and dInscPEPT colored according to the conservation.
The PinsTPR domain consists of 17 antiparallel helices forming 8.5 TPR repeats (Fig. S2), with a pattern of hydrophobic amino acids consistent with the canonical TPR consensus (14). The sequence 39–76 adopts a TPR-like arrangement despite deviating from the consensus. In addition, the fourth TPR repeat presents an insertion between the first and the second helix (Fig. S2) resulting in longer α4A and α4B helices joined by a 8-residue loop. Consecutive TPR units stack in much the same way as reported for the TPR-containing domain of O-linked N-actylglucosamine transferase (15), giving rise to a right-handed superhelical twist of the molecule. The unusual sequence composition of the TPR4 repeat distorts the superhelix by an outward displacement of the subsequent TPR units with respect to the helical axis (see Fig. S3).
A peculiar aspect of the PinsTPR structure is the presence within the groove of a set of asparagines positioned on the third and fourth turn of the αA helix of each TPR repeat, which generate a ridge extending throughout the concave surface of the molecule (Fig. 2D). These asparagine residues occupy the positions 6 and 9 of the TPR consensus, within an invariant NLGN motif found in most of the Pins TPR repeats. Not surprisingly, in the binary complex dInscPEPT lines up with this asparagine ladder. This binding mode is reminiscent of the binding of similarly extended ligands to multiple ARM-repeat proteins such as the NLS peptides bound to karyopherin α (16), and the E-cadherin peptide bound to β-catenin (17), thus suggesting a general recognition mechanism common to all these topologically diverse helical scaffolds.
Determinants of Interactions Between Pins and Insc.
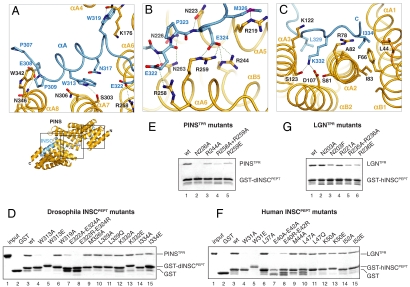
We next addressed the structural determinants of the PinsTPR∶dInscPEPT assembly. As expected for TPR repeat-containing proteins, the hetero-dimer interface is contributed by the amino acids of the αA helices, facing the inner side of the domain. To facilitate the description, we will divide the interactions into three major modules roughly matching the dInscPEPT N-terminal α-helix, the central E322-P323-E324Insc motif, and the elongated C-terminal stretch. The first module involves the helix αA of dInscPEPT that packs against the TPR repeats 6 and 7 of PinsTPR with an orientation almost perpendicular to the repeats’ axis (Fig. 3A). This helix contains the invariant Trp313Insc that is hydrogen-bonded to Ser303Pins and further stabilized by stacking interactions with Asn306Pins. The correct orientation of the αA helix is ensured on one end by a hydrogen bond between Glu308Insc and Asn346Pins, and on the other end by the hydrogen bond between the Trp319Insc and the carbonyl group of Lys176Pins located in the TPR4 insertion. At Trp319Insc the dInscPEPT chain turns sharply to progress parallel to the αA5 helix of PinsTPR until Met326Insc, crossing the whole surface of the TPR domain (Fig. 3B). Hydrogen bonds from the conserved asparagines Asn223Pins, Asn226Pins, and Asn263Pins anchor the dInscPEPT main chain to the TPR scaffold, and a number of salt bridges between Glu322Insc and Glu324Insc and Arg244Pins, Arg258Pins and Arg259Pins greatly contribute to strengthen the interaction. The electrostatic potential of the PinsTPR surface in this area confirms that the negatively charged EPEInsc triplet is accommodated into a positively charged patch of the central portion of the PinsTPR domain (Fig. S4), thus suggesting that charge complementarity might constitute the major factor dictating the ligand specificity here. The third area of interactions involves the C-terminal end of dInscPEPT and the first three TPR units of PinsTPR. This portion of dInscPEPT inserts the side chains of Lys332Insc and Ile334Insc into two adjacent pockets at opposite sites of the helix (Fig. 3C). Lys332Insc snugly fits into the negatively charged cavity formed by Ser81Pins, Ser123Pins, and Asp107Pins, whereas Ile334Insc sits into a predominantly hydrophobic environment contributed by Leu44Pins, Phe66Pins, and Ile83Pins.
Fig. 3.
Analysis and conservation of the PinsTPR∶dInscPEPT dimer interface. (A–C) Enlarged views of the dimer interface. (D) GST-dInscPEPT, mutated as indicated above, on GSH beads was incubated with PinsTPR. After washing species on beads were analyzed by SDS-PAGE. (E) Binding of PinsTPR mutants to wt-dInscPEPT. (F and G) Same assays as in boxes D and E performed with human LGNTPR and hInscPEPT.
To address the relevance of each interaction module for the dimer assembly, we substituted key residues identified by our structural analysis. The binding ability of the mutated proteins was tested in a pull-down assay performed with GST-dInscPEPT immobilized onto glutathione sepharose (GSH) beads and purified PinsTPR constructs in solution. We first asked if dInscPEPT mutants were able to bind wild-type PinsTPR (Fig. 3D). As expected, double replacement of E322-E324Insc with alanine or arginine fully abrogates the binding. The same effect is caused by substitution of the Trp313Insc with alanine or glutamic acid, underscoring a fundamental role of this triptophane in docking the dInscPEPT helical fragment onto PinsTPR. Mutations of Trp319InscAla and Met326InscAla, as well as substitution of the invariant residues Leu329Insc, Lys332Insc, and Ile334Insc on the dInscPEPT C terminus do not severely affect binding. We next tested the structural determinants of the binding on the PinsTPR side, focusing our mutational analysis on the central EPEInsc module. In keeping with the role predicted for the invariant asparagines of PinsTPR in anchoring the dInscPEPT backbone, removal of the Asn226Pins side chain is sufficient to abolish binding (Fig. 3E). Single mutations of Arg244Pins and Arg259Pins to alanine or glutamic acid strongly reduce binding, while double substitution of Arg258Pins and Arg259Pins with alanine results in a PinsTPR mutant unable to associate with dInscPEPT. In summary, our results indicate that; (i) all three dInscPEPT portions including the N-terminal α-helix, the central EPEInsc motif, and the extended C-terminal tail synergize in binding PinsTPR; (ii) the polar interactions between the negative charges of the EPEInsc motif and the positively charged inner surface of PinsTPR is a hallmark of dInscPEPT recognition; (iii) besides Glu322Insc and Glu324Insc, a key determinant of the dimeric assembly is the invariant Trp313Insc.
The Pins∶Insc Interface Is Evolutionary Conserved.
One of the open questions in the stem cell field is to which extent the molecular mechanisms underlying Drosophila neuroblast divisions are conserved in vertebrates. To gain insight into this issue, we generated constructs of human LGN and Insc corresponding to the fruitfly domains we had used for the structure determination, and repeated the GST pull-down assays with an analogous battery of mutants. Sequence analysis revealed that the counterparts of the dInscPEPT spans residues 23 to 58 of the human Insc, to which we refer as hInscPEPT, where “h” stands for human. As predicted by the strong sequence conservation of the ortholog proteins in these regions (Fig. 2E, Fig. S5), equivalent mutations on hInscPEPT and dInscPEPT impair the association with the cognate LGNTPR and PinsTPR domains (Fig. 3 F and G). In particular, replacement of Trp31hInsc or of the glutamic acid couple E40-E42hInsc fully abrogates the interaction with LGNTPR. We next assessed the contribution of the conserved Asn203LGN, Arg221LGN, Arg235LGN, and Arg236LGN toward hInsc binding. Simultaneous substitution of Arg235LGN and Arg236LGN with alanine, as well as charge reversal of Arg236LGN to Glu severely perturbed the interaction, mirroring the effect observed for the corresponding PinsTPR mutations (Fig. 3G). Only upon replacement of Asn203LGN with a bulky phenylalanine we could score a binding reduction recapitulating the impairment observed with Asn226Pins. Finally, to evaluate whether equivalent chemical interactions sum up to similar overall binding affinities, we measured the strength of the binary interaction in both species by Isothermal Titration Calorimetry. The dissociation constants were 5 nM for the PinsTPR∶dInscPEPT interaction and 13 nM for the LGNTPR∶hInscPEPT one (Fig. S6). Collectively these results indicate that the LGN∶Insc interface is evolutionary conserved.
NuMA Binds to LGN with an EPE-Motif.
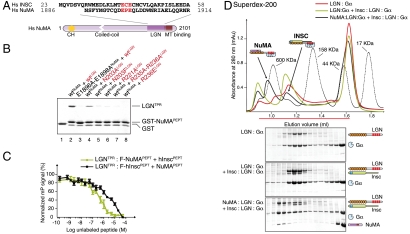
In mitosis the association of LGN with NuMA is fundamental to sustain the spindle orientation process. Previous studies have mapped the LGN-binding site of NuMA within a C-terminal portion of about 20 KDa encompassing residues 1,878–1,910 (18). This fragment of NuMA has been shown to enter a complex with LGNTPR, raising the question as to whether it is compatible with the concomitant presence of Insc on the same domain. Primary sequence analysis of NuMA revealed the existence of a conserved EPE motif at position 1,896–1,898, strongly suggesting that NuMA could interact with LGN with the same binding mode displayed by Insc (Fig. 4A). To test this hypothesis, we expressed NuMA1,886–1,914 (referred to as NuMAPEPT hereon) fused to a GST moiety, and checked its ability to interact with LGNTPR by GST pull-down assay. Indeed, NuMAPEPT binds LGNTPR in an EPE-dependent manner (Fig. 4B). Furthermore, all asparagine and arginine residues of LGNTPR found to be in contact with EPE motif of hInscPEPT are also important for NuMAPEPT recognition. Single substitutions on LGNTPR abrogate binding to NuMAPEPT due to the lower binding affinity displayed by NuMAPEPT for LGNTPR with respect to the one between hInscPEPT and LGNTPR, with dissociation constants of about of 50 nM and 13 nM respectively (Fig. S6). We conclude that NuMAPEPT and hInscPEPT associate with LGNTPR via a conserved EPE motif.
Fig. 4.
NuMA competes with Insc for the binding to LGN via the invariant EPE motif. (A) Alignment of hInscPEPT and NuMAPEPT around the conserved ECE/EPE motif. (B) Validation of the NuMAPEPT:LGNTPR interface. (C) Fluorescence polarization-based competition assays showing the decrease in polarization signal upon titration of unlabeled hInscPEPT into a mixture of LGNTPR and fluorescein-NuMAPEPT, or titration of unlabeled NuMAPEPT into a mixture of LGNTPR and fluorescein-hInscPEPT. (D) SEC analysis of the competitive interactions of Insc and NuMA1,807–1,987 with LGN∶GαiGDP.
NuMA and Insc Are Competitive Interactors of LGN.
To ascertain whether hInscPEPT and NuMAPEPT are mutually exclusive interactors of LGNTPR, we developed a fluorescence polarization-based competition assay. Fluorescein-labeled NuMAPEPT at a concentration of 15 nM was mixed with 250 nM of LGNTPR. Based on their reciprocal affinity, under these conditions about 85% of the fluorescein-NuMAPEPT are in complex with LGNTPR (Fig. S7). We then titrated into the reaction increasing amounts of unlabeled hInscPEPT, and measured the residual fluorescein-NuMAPEPT polarization at steady-state. The monotonic decrease of the polarization signal upon hInscPEPT addition indicates that hInscPEPT effectively competes with NuMAPEPT, and implies that the simultaneous binding of the two peptides on LGNTPR is precluded (Fig. 4C). We confirmed this result by performing an analogous competition experiment in which we titrated unlabeled NuMAPEPT to compete the binding of fluorescein-labeled hInscPEPT to LGNTPR. The fourfold lower affinity displayed by NuMAPEPT towards LGNTPR with respect to the one of hInscPEPT predicts that higher doses of NuMAPEPT are required to fully displace hInscPEPT from LGNTPR. The shift observed in the NuMA-inhibited curve as compared to the Insc-inhibited one fully satisfies this prediction.
We next asked if the competition observed for the short NuMAPEPT and hInscPEPT peptides reflects a genuine inability of full-length Inscutable and NuMA to concomitantly enter a complex with LGN. We purified a homogeneous form of human LGN∶GαiGDP from insect cells following a previously described colysis method (4). When injected on a SEC column, the LGN∶GαiGDP complex elutes at an apparent molecular weight compatible with the expected 1∶4 stoichiometry (Fig. 4D). We also adapted the purification protocol to generate an Insc∶LGN∶GαiGDP complex by coexpression of Insc and LGN in insect cells followed by colysis with Gαi. We then mixed LGN∶GαiGDP and Insc∶LGN∶GαiGDP in roughly equimolar amounts and analyzed the sample by SEC. Despite the significant difference in the theoretical molecular weights caused by the presence of Insc, the two complexes coelutes in about the same fractions. To test the effect of NuMA, we additioned the LGN∶GαiGDP plus Insc∶LGN∶GαiGDP mixture with a 20-fold molar excess of NuMA1,807–1,987, and separated the resulting species by SEC. NuMA1,807–1,987 readily bound to free LGN∶GαiGDP and eluted in a well separated peak distinct from the Insc∶LGN∶GαiGDP one. In agreement with the competition model, the NuMA1,807–1,987 excess was unable to dissociate Insc from LGN∶GαiGDP due to its lower binding affinity. Of note, NuMA∶LGN∶GαiGDP elutes earlier than the Insc containing complex in spite of its smaller theoretical mass. We suspect that such behavior might be ascribed to specific hydrodynamical properties of the NuMA assembly, although we cannot rule out a dimerization of the NuMA-containing hetero-trimer. Collectively these findings demonstrate that Insc and NuMA utilize the ECE/EPE recognition motif to competitively associate with LGN.
Both NuMA and Insc Open the LGN Conformational Switch.
One of the key events in the cortical force generators assembly is the release of the head-to-tail interactions holding LGN in an inhibited state. Previous studies have shown that the association of NuMA to LGNTPR is capable of opening the conformational switch (3). Given the similarity in the binding mode of NuMA and Insc toward LGNTPR, we enquired if Insc would also induce a similar conformational transition. To this end, we first reproduced the displacement the C-terminal LGNGoLoco region from LGNTPR observed upon NuMA addition (Fig. S8). We next took advantage of the stable LGNTPR∶LGNGoLoco assembly mimicking the closed conformation of LGN to investigate the effect of Insc binding on the LGN conformational state. Our previous results proved that hInscPEPT forms a tight complex with LGNTPR. When we repeated a similar GST pull-down experiment using LGNTPR∶LGNGoLoco in solution, only the LGNTPR half of the sandwich remained associated with the beads (Fig. 5A), providing compelling evidence that Insc opens the LGN switch. An analogous behavior is observed upon incubation of LGNTPR∶LGNGoLoco with NuMA1,807–1,987, that we included as a positive control.
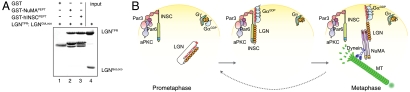
Fig. 5.
Both NuMA and Insc open the LGN conformational switch. (A) Upon incubation of 2 μM GST-hInscPEPT with 4 μM of preformed LGNTPR∶LGNGoLoco, only LGNTPR is retained on beads, demonstrating that the simultaneous presence of LGNGoLoco and Insc on the TPR domain is excluded. (B) Revised model of force generators functioning during asymmetric divisions.
Discussion
In this study we report the characterization of the PinsTPR∶dInscPEPT complex, and provide a molecular explanation for the mutual exclusive interaction of Insc and NuMA to LGN. While this manuscript was in preparation, Zhu and coworkers arrived to similar conclusions analyzing the structure of the LGN∶NuMA complex (19).
A 38-residue fragment of Drosophila Insc encompasses the PinsTPR binding region. This fragment of Insc shares a high sequence similarity to functional homologues recently discovered in mammals, fully supporting the notion that the basic mechanism responsible for the recruitment of force generators at polarity sites is evolutionary conserved. With the exception of a short N-terminal α-helix, the InscPEPT is intrinsically unstructured, and lines on the scaffold provided by the superhelical TPR arrangement of Pins with an extended conformation. The interaction surface is organized around a core module involving the EPE motif of InscPEPT and the central TPR5-6 of Pins, whose specificity is primarily dictated by charge complementarity. The binding is further stabilized by polar and hydrophobic interactions contributed by the αA helix of InscPEPT. Not surprisingly, the large interaction surface characterizing the topology of the PinsTPR∶InscPEPT heterotypic dimer accounts for an elevated binding affinity (of about 5 nM for the fly proteins and 13 nM for the human counterparts). The structure of mouse LGN191–350, corresponding to what we name TPR5-8, with Insc19–40 suggests that vertebrate proteins assemble with organizational principles similar to the fly ones (19). However, the short mouse constructs only depict the interaction of LGNTPR with the αA helix of InscPEPT, up to the first Glu of the EPE motif. Intriguingly, the mouse LGN∶Insc interaction seems to be characterized by lower affinity compared to human and fly ones (with KD of 63 nM for LGNTPR5–8∶Insc19–40, and of 47 nM for LGNTPR1–8∶Insc20–57).
The evidence that NuMA forms a complex with the same LGNTPR domain associating to Insc raised the question of whether it binds in a similar manner. Indeed, comparison of the primary sequence of InscPEPT with the known LGN-binding portion of NuMA revealed the presence of an EPE triplet that turned out to be essential for the LGN recognition, with a similar molecular signature of the EPE motif of the InscPEPT. Notably, the NuMA ortholog in fly (Mud) codes for two consecutive EDE-EGE motifs in the Pins-binding region, whose interplay remains to be clarified (Fig. S9). The structure of LGN in complex with NuMAPEPT fully supports the notion that the EPE-interaction module represents a common region required for docking unstructured ligands on the LGNTPR scaffold (19). In the case of NuMA, the interface is further contributed by a helical fragment forming a bundle with helices αA2 and αA3 of LGNTPR. The consequence of the partial overlap between the Insc and NuMA binding sites is that their concomitant loading on LGN is excluded.
A key step during the assembly of the force generators is the opening of the LGN conformational switch that keeps the molecule in an inactive state (3). Binding of NuMA to LGNTPR induces the release of the intramolecular interactions holding the molecule in a closed form. In agreement with the similarity in the binding modes, we demonstrated that also Insc disengages the LGN GoLoco motifs from the TPR domain. Together these findings imply that the GoLoco motifs contact the TPR repeats in the same region occupied by Insc and NuMA. Primary sequence inspection revealed that the GoLocos of both Pins and LGN do not contain EPE triplets, suggesting that either the head-to-tail interaction involves alternative TPR patches sterically occluded by the presence of Insc and NuMA, or that less conserved negatively charged triplets are accommodated on the same TPR5-6 of LGN.
The well established model for force generators recruitment at polarity sites rests on the assumption that Insc and NuMA can be part of the same apically localized multisubunit complexes containing Par proteins. This model stems from colocalization experiments showing that in asymmetric mitoses Par3, Insc, LGN, and NuMA cluster together in apical crescents (6), complemented by coimmunoprecipitation assays in which LGN∶Gαi were found in association with Par3∶Insc (12) and NuMA (18). The finding that Insc and NuMA are mutually exclusive partners of LGN is both unexpected and puzzling. In particular, the higher affinity characterizing the Insc binding to LGN shifts the balance of the unmodified proteins towards the Insc∶LGN complex formation, which is instrumental in recruiting LGN with Par proteins at the onset of mitosis but cannot account for microtubule-pulling forces. What is the possible mechanism for transferring LGN from Insc to NuMA? The architecture of the InscPEPT∶PinsTPR structure whereby an extended ligand is accommodated on a large domain allows a high degree of regulation of the interaction strength. Posttranslational modifications on either side of the dimer might locally alter the contacts without affecting the rest of the interface, as it has been demonstrated for the similarly organized complex between the cytoplasmic domain of E-cadherin and β-catenin (17). Such modulating modifications can in principle occur on Insc, NuMA, or on LGN. To date, no experimental information is available regarding putative Insc or NuMA modifications. More controversial is the literature relative to LGN phosphorylations. In mitotic Drosophila neuroblasts, Pins has been found phosphorylated by Aurora-A on Ser436 at about half of the linker connecting the TPR domain with the GoLoco motifs (20). Using an “induced polarity” assay in S2 cells, phospho-Ser436Pins was shown to trigger a redundant NuMA-independent spindle orientation pathway engaging the membrane associated Dlg protein. It is to date unclear if such pathway is conserved in vertebrates. Notably, during oriented symmetric cell divisions of MDCK cells, phosphorylation on a similarly positioned Ser401 of LGN functions in excluding force generators from the apical cortex in order to prevent apico-basal spindle orientation (21). In this context, phospho-Ser401LGN would selectively prevent binding of LGN to apical Gαi. Based on our structural and biochemical results, it is difficult to provide a molecular explanation as to whether these LGN phosphorylations could also impact on the Insc and NuMA binding. Recent observations support the notion that the pool of NuMA∶LGN∶Gαi colocalizing with Par3∶Insc in embryonic mouse skin progenitors is tightly regulated to set the balance between symmetric and asymmetric divisions, though no mechanism for this has been put forward (22). In summary, we reckon more has to be learnt to understand what brings LGN from Insc to NuMA.
An additional question relates to the mechanism maintaining effective NuMA∶LGN∶GαiGDP species at the correct cortical sites in the absence of Insc. Based on the knowledge we acquired, we propose a step-wise model that can be schematized as follows (Fig. 5B): (i) in the early phases of mitosis LGN is brought to the apical membrane in conjunction with Par proteins by the high-affinity interaction with the preformed Par3∶Insc complex. Binding of LGN to Insc triggers the conformational switch transition enabling the relocation of GαiGDP moieties previously distributed all around the plasma-membrane with Gβγ; (ii) upon mitotic progression, when LGN is already at the correct sites, a yet unidentified molecular event alters the relative affinities of Insc and NuMA for LGN to shift the balance between the Insc-bound and the NuMA-bound LGN pools. We hypothesize that the four Gαi subunits present on LGN at this stage are sufficient to transiently hold cortical NuMA∶LGN∶GαiGDP in proximity of Par proteins to allow directional microtubule pulling. We speculate that NuMA∶LGN∶GαiGDP is a short-lived complex and disassemble, possibly assisted by a specialized GEF for Gαi such as as Ric-8A (23), releasing apo-LGN in the cytoplasm to start a new cycle. Such a dynamical interaction network would allow for a continuous regulation of the force exerted on astral microtubules throughout mitosis. Future attempts to validate the model in vivo will greatly benefit from the biochemical tools presented in this study.
Experimental Procedures
Detailed descriptions of crystallization, structure determination, and other experimental procedures are available in the SI Materials and Methods. Atomic coordinates and structure factors for the Pins∶Insc structure have been deposited to the Protein Data Bank under accession code 4A1S.
Supplementary Material
Acknowledgments.
M.M. is funded by the Italian Association for Cancer Research (AIRC), and the Italian Ministry of Health. S.C. and A.A. are FIRC postdoctoral fellows.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates have been deposited to the Protein Data Bank www.pdb.org (PDB ID code 4A1S).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1113077108/-/DCSupplemental.
References
- 1.Morin X, Bellaiche Y. Mitotic spindle orientation in asymmetric and symmetric cell divisions during animal development. Dev Cell. 2011;21:102–119. doi: 10.1016/j.devcel.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 2.Knoblich JA. Asymmetric cell division: recent developments and their implications for tumour biology. Nat Rev Mol Cell Biol. 2010;11:849–860. doi: 10.1038/nrm3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Du Q, Macara IG. Mammalian Pins is a conformational switch that links NuMA to heterotrimeric G proteins. Cell. 2004;119:503–516. doi: 10.1016/j.cell.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 4.Tall GG, Gilman AG. Resistance to inhibitors of cholinesterase 8A catalyzes release of Galphai-GTP and nuclear mitotic apparatus protein (NuMA) from NuMA/LGN/Galphai-GDP complexes. Proc Natl Acad Sci USA. 2005;102:16584–16589. doi: 10.1073/pnas.0508306102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merdes A, Heald R, Samejima K, Earnshaw WC, Cleveland DW. Formation of spindle poles by dynein/dynactin-dependent transport of NuMA. J Cell Biol. 2000;149:851–862. doi: 10.1083/jcb.149.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams SE, Beronja S, Pasolli HA, Fuchs E. Asymmetric cell divisions promote Notch-dependent epidermal differentiation. Nature. 2011;470:353–358. doi: 10.1038/nature09793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kraut R, Chia W, Jan LY, Jan YN, Knoblich JA. Role of inscuteable in orienting asymmetric cell divisions in Drosophila. Nature. 1996;383:50–55. doi: 10.1038/383050a0. [DOI] [PubMed] [Google Scholar]
- 8.Kraut R, Campos-Ortega JA. Inscuteable, a neural precursor gene of Drosophila, encodes a candidate for a cytoskeleton adaptor protein. Dev Biol. 1996;174:65–81. doi: 10.1006/dbio.1996.0052. [DOI] [PubMed] [Google Scholar]
- 9.Yu F, Morin X, Cai Y, Yang X, Chia W. Analysis of partner of inscuteable, a novel player of Drosophila asymmetric divisions, reveals two distinct steps in inscuteable apical localization. Cell. 2000;100:399–409. doi: 10.1016/s0092-8674(00)80676-5. [DOI] [PubMed] [Google Scholar]
- 10.Schaefer M, Shevchenko A, Shevchenko A, Knoblich JA. A protein complex containing Inscuteable and the Galpha-binding protein Pins orients asymmetric cell divisions in Drosophila. Curr Biol. 2000;10:353–362. doi: 10.1016/s0960-9822(00)00401-2. [DOI] [PubMed] [Google Scholar]
- 11.Zigman M, et al. Mammalian inscuteable regulates spindle orientation and cell fate in the developing retina. Neuron. 2005;48:539–545. doi: 10.1016/j.neuron.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 12.Lechler T, Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature. 2005;437:275–280. doi: 10.1038/nature03922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konno D, et al. Neuroepithelial progenitors undergo LGN-dependent planar divisions to maintain self-renewability during mammalian neurogenesis. Nat Cell Biol. 2008;10:93–101. doi: 10.1038/ncb1673. [DOI] [PubMed] [Google Scholar]
- 14.Das AK, Cohen PW, Barford D. The structure of the tetratricopeptide repeats of protein phosphatase 5: implications for TPR-mediated protein-protein interactions. EMBO J. 1998;17:1192–1199. doi: 10.1093/emboj/17.5.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jinek M, et al. The superhelical TPR-repeat domain of O-linked GlcNAc transferase exhibits structural similarities to importin alpha. Nat Struct Mol Biol. 2004;11:1001–1007. doi: 10.1038/nsmb833. [DOI] [PubMed] [Google Scholar]
- 16.Conti E, Uy M, Leighton L, Blobel G, Kuriyan J. Crystallographic analysis of the recognition of a nuclear localization signal by the nuclear import factor karyopherin alpha. Cell. 1998;94:193–204. doi: 10.1016/s0092-8674(00)81419-1. [DOI] [PubMed] [Google Scholar]
- 17.Huber AH, Weis WI. The structure of the beta-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by beta-catenin. Cell. 2001;105:391–402. doi: 10.1016/s0092-8674(01)00330-0. [DOI] [PubMed] [Google Scholar]
- 18.Du Q, Stukenberg PT, Macara IG. A mammalian partner of inscuteable binds NuMA and regulates mitotic spindle organization. Nat Cell Biol. 2001;3:1069–1075. doi: 10.1038/ncb1201-1069. [DOI] [PubMed] [Google Scholar]
- 19.Zhu J, et al. LGN/mInsc and LGN/NuMA complex structures suggest distinct functions in asymmetric cell division for the Par3/mInsc/LGN and Galphai/LGN/NuMA pathways. Mol Cell. 2011;43:418–431. doi: 10.1016/j.molcel.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnston CA, Hirono K, Prehoda KE, Doe CQ. Identification of an Aurora-A/PinsLINKER/Dlg spindle orientation pathway using induced cell polarity in S2 cells. Cell. 2009;138:1150–1163. doi: 10.1016/j.cell.2009.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hao Y, et al. Par3 controls epithelial spindle orientation by aPKC-mediated phosphorylation of apical Pins. Curr Biol. 2010;20:1809–1818. doi: 10.1016/j.cub.2010.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poulson ND, Lechler T. Robust control of mitotic spindle orientation in the developing epidermis. J Cell Biol. 2010;191:915–922. doi: 10.1083/jcb.201008001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woodard GE, et al. Ric-8A and Gi alpha recruit LGN, NuMA, and dynein to the cell cortex to help orient the mitotic spindle. Mol Cell Biol. 2010;30:3519–3530. doi: 10.1128/MCB.00394-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.