Summary
Lyn is a Src-family kinase (SFK) present in B-lymphocytes and myeloid cells. In these cell types Lyn establishes signaling thresholds by acting as both a positive and a negative modulator of a variety of signaling responses and effector functions. Lyn-deficiency in mice results in the development of myeloproliferation and autoimmunity. The latter has been attributed to the hyper-reactivity of Lyn-deficient B cells due to the unique role of Lyn in down-modulating B-cell receptor (BCR) activation, mainly through phosphorylation of inhibitory molecules and receptors. Myeloproliferation results, on the other hand, from the enhanced sensitivity of Lyn-deficient progenitors to a number Colony Stimulating Factors (CSFs). The hyper-sensitivity to myeloid growth factors may also be secondary to poor inhibitory receptor phosphorylation, leading to impaired recruitment/activation of tyrosine phosphatases and reduced down-modulation of CSF signaling responses. Despite these observations, the overall role of Lyn in the modulation of myeloid cell effector functions is much less well understood, as often both positive and negative roles of this kinase have been reported. In this review we discuss the current knowledge of the duplicitous nature of Lyn in the modulation of myeloid cell signaling and function.
Keywords: Lyn, kinase, myeloid cells, myeloproliferation, inflammation
Introduction
Members of the Src-family of protein tyrosine kinase (SFKs) have been implicated in multiple signaling pathways where they play critical roles in the regulation of immune system function and cellular homeostasis. There are nine SFKs, which are expressed in overlapping patterns in both immune cells and non-hematopoietic cells. The SFKs participate in a variety of signaling pathways through different receptor types in response to multiple environmental stimuli (reviewed in 1, 2–7). SFKs interact with a large repertoire of receptors in immune cells, including a number of different types of immunoreceptors (such as the T-cell/B-cell receptors or the Fc receptors), growth factor receptors, integrins, trimeric G protein-coupled receptors and selectin counter-receptors. As a result, SFKs are implicated in the modulation of key leukocyte functions, including cell migration, adhesion, phagocytosis, cell survival and proliferation. To date, the role SKFs has been most heavily studied in the immunoreceptor and integrin signaling pathways. Though structurally different, both of these receptor types utilize common protein domains (either within the receptor or on associated protein subunits) known as immunoreceptor tyrosine-based activation motifs (ITAM), to initiate signaling that leads to activation of the tyrosine kinase Syk/ZAP70 and downstream pathways and functional responses (reviewed in 8). In myeloid cells in particular, the similar phenotype of immune cells derived from SFK and Syk-deficient mice has clearly revealed the interrelationships between these two key kinases in the activation of different signaling pathways. In general, SFKs initiate the ITAM/Syk/ZAP70 pathway to affect a positive functional role in activation of leukocyte functional responses (such as phagocytosis and adhesion-dependent activation). SFKs have also been implicated as positive regulators of pathways that are independent of Syk/ZAP70, such as signaling downstream of chemoattractant G protein-coupled or cytokine receptors. On the other hand, it is now clear that SFKs, have also a negative or inhibitory role in the modulation of signaling responses (3, 9). In these inhibitory pathways, SFKs phosphorylate residues within receptor cytoplasmic domains referred to as immunoreceptor tyrosine-based inhibitory motifs (ITIMs) that are present on molecules such as PIR-B (paired Ig-like receptor) and/or SIRPα (signal regulatory protein-α). Phospho-ITIM domains serve as docking sites for several types of phosphatases, such as SHP-1 (Src homology 2 domain containing protein tyrosine phosphatase-1/2) or SHIP-1 (SH-2-containing inositol phosphatase), which then become active and function to downmodulate signaling responses. SFKs can also inhibit signaling responses via phosphorylation of p62 DOK family cytoplasmic proteins that in turn recruit the Ras GTPase-activating protein (rasGAP) and SHIP-1 to downmodulate further signaling reactions (3). Overall, it is now clear that different SFKs have a duplicitous nature that allows them to act as rheostats of positive or negative signaling in response to a number of stimulatory conditions.
Lyn is a SFK-member expressed in all blood cells, other than T lymphocytes, and is the first Src-family kinase member found to have a dual role acting both as a positive and a negative signaling molecule (Fig. 1) (3, 9). The evidence that Lyn is playing a crucial role in downmodulating signaling responses was revealed by the observation that lyn−/− mutant mice develop a lupus-like disease and immune complex nephritis (10–13). To date, the autoimmune phenotype of lyn−/− mice has been mainly attributed to the hyper-responsiveness of lyn−/− B cells to B cell receptor (BCR) ligation leading to abnormal B-cell selection and/or tolerance resulting in production of self-reactive antibodies (9, 14). In B cells, due to the redundant expression of multiple SFKs, the role of Lyn in the positive modulation of signaling responses seems to be dispensable, while this SFK member plays a unique role in down-modulating BCR signaling events. As extensively reviewed (3, 9, 14), Lyn is responsible for phosphorylating several inhibitory (ITIM-containing) receptors in B cells, such as CD22 and Fc receptor gamma IIb (FcγRIIb) and for the subsequent recruitment of tyrosine phosphatases such as SHP-1, SHP-2 or the inositol phosphatase SHIP-1 required to turn off activating responses in B cells.
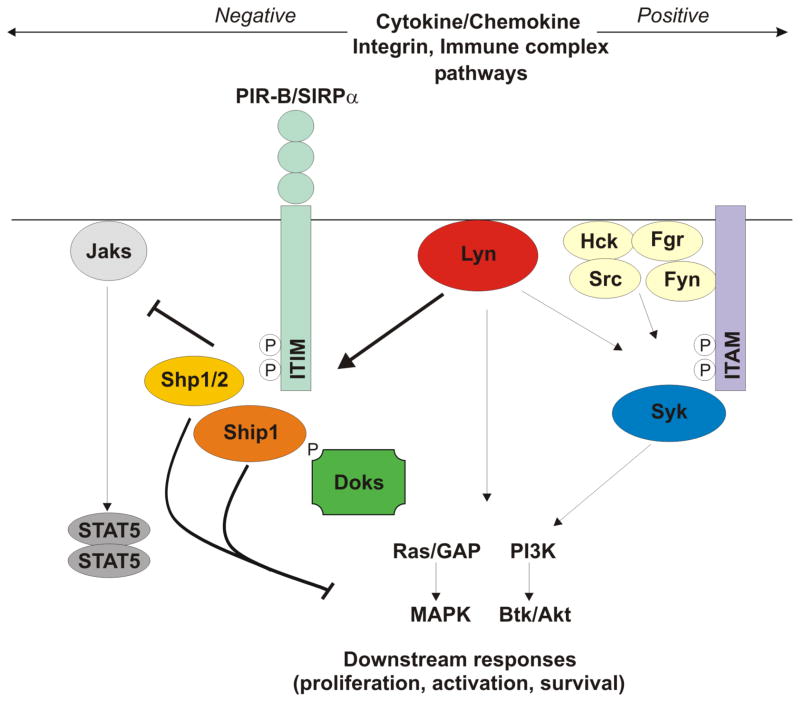
Figure 1. Proposed model of the dual role of Lyn in the modulation of signaling pathways in myeloid cells.
Lyn exerts its negative role in the modulation of signaling pathways in myeloid cells mainly by phosphorylating ITIM-containing inhibitory receptors, such as PIR-B and SIRPα thus recruiting inhibitory phosphatases, such as SHP1/2 or SHIP-1, which in turn downmodulate both proximal responses from Jak kinases and downstream responses at multiple sites distal to Ras. Additionally, Lyn can negatively modulate signaling responses in myeloid cells through the phosphorylation of the DOK family of cytoplasmic proteins that in turn recruit the Ras GTPase-activating protein (rasGAP) and SHIP-1 to down-modulate further signaling reactions. This inhibitory function of Lyn occurs especially in integrin, growth factor receptor and FcεRI signaling pathways. The mechanisms through which Lyn positively modulates signaling responses in myeloid cells are, on the other hand, less clear. Lyn can directly phosphorylate ITAM domains in various immunoreceptors associated signaling adapters (such as the FcRγ chain) leading to recruitment of Syk and activation of downstreamreactions. This positive regulatory function of Lyn occurs mainly in Fc signaling pathways. Lyn can also positively modulate signaling responses downstream to cytokine receptors, such as GM-CSF, IL-5, or chemokine receptors, but the mechanisms responsible for this phenomenon are not fully understood.
However, there are numerous other cell types where Lyn has been found to have both positive and negative regulatory functions, including hematopoietic progenitors, mature myeloid cells (neutrophils, macrophages and dendritic cells), platelets and erythrocytes (Table 1) (15–19). In these cell types, Lyn acts downstream not only from immunoreceptors (such as FcγRs) but from non-immunoreceptors, such as cytokine and integrin receptors, often both activating certain functional responses and inhibiting others. In this dual signaling mode, the loss of inhibitory function is usually dominant; as a result, lyn−/− mice display strong perturbations in myelopoiesis and have hyper-active mature innate immune cells. Interestingly, a positive role for Lyn in the modulation of signaling pathways in myeloid cells has also been reported (15, 20). Besides Lyn, the other main SFKs expressed in myeloid cells are Hck and Fgr in granulocytes, monocyte/macrophages and dendritic cells, and Hck, Src and Fyn in mast cells (3, 21). According to the cell type and the receptor/signaling pathways implicated, Lyn seems to exert its positive or negative role synergistically with, or in opposition to, the other SFKs expressed in the different myeloid cell subtypes.
TABLE 1.
Dual role of Lyn in the modulation of myeloid-cell signaling responses and functions
| RECEPTOR | Ligand | Cell type | Function |
|---|---|---|---|
| Negative role
| |||
| Type I cytokine receptors | GM-CSF, G-CSF,IL-3, IL-6 | MP, Mφ,DC,MC | proliferation, survival |
| Immunoglobuline superfamily receptors | M-CSF, SCF | MP, Mφ, MC | proliferation |
| FcεRI | IgE | MC | proliferation, degranulation, cytokine production |
| Integrin | extracellular matrix proteins, counter receptors | Mφ,PMN,MP | adhesion, activation, survival |
|
| |||
| Positive role
| |||
| Type I cytokine receptors 7 transmembrane α-helical receptors |
IL-5, G-CSF, GM-CSF chemokines |
PMN, E PMN, Mφ, MP, MC |
survival, proliferation activation, migration |
The table list specific receptors, ligands, cell types and functions that are either positively or negatively regulated by Lyn. MP, myeloid precursors; Mφ, macrophages; DC, dendritic cells; MC, mast cells; PMN, neutrophils; E, eosinophils.
In this review we will summarize the reported observations for the role of Lyn in modulating signaling in myeloid progenitors and different subtypes of mature myeloid cells, mainly focusing on data obtained with cells derived from Lyn-deficient mice. The possible implications of deregulated effector functions in Lyn-deficient myeloid cells during inflammatory and autoimmune diseases will also be discussed.
The role of Lyn in myeloid progenitors and myelopoiesis
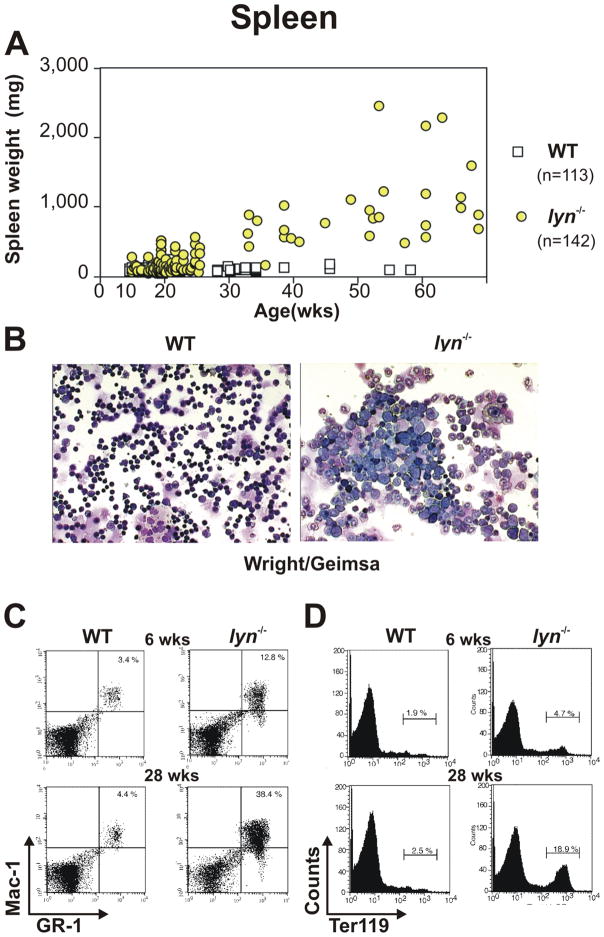
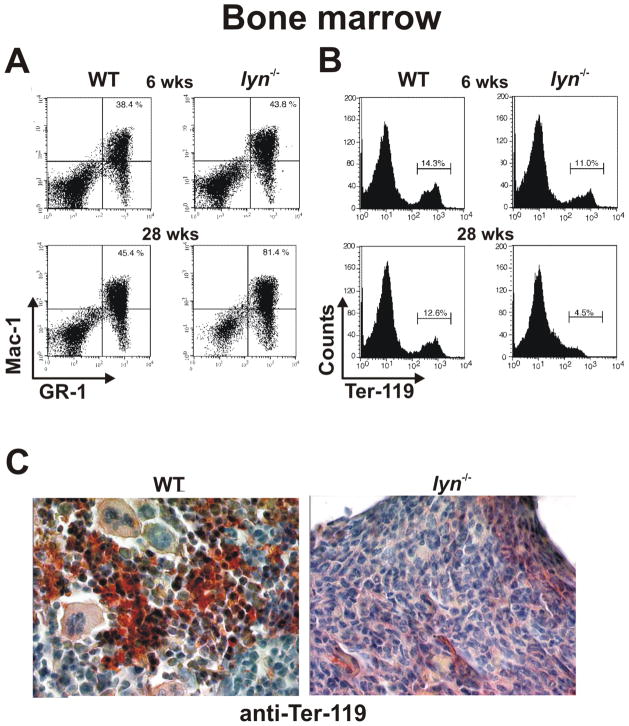
A number of studies have demonstrated that Lyn functions as a negative regulator of myelopoiesis (16, 22). Lyn−/− mice (on a mixed B6/129 genetic background) develop an age-dependent increase in spleen size (Fig. 2A) beginning at about 6 weeks of age, which progressively increases with time to a size of 10 – 20 fold that of wild-type (WT) animals by 50 weeks of age (on the inbred B6 genetic background the splenomegaly is roughly ½ of this). Microscopic examination of splenic cells from 28 week old lyn−/− mice reveals the presence of large numbers of cells with a blast-like phenotype, compared to mainly the mature lymphocytes found in WT spleens (Fig. 2B). Furthermore, flow cytometric analysis of splenic cells stained with anti-Gr-1 and anti-Mac-1 specific antibodies demonstrates a dramatic increase in myeloid cell percentage in lyn−/− mice beginning at 6 weeks of age, with over 38% of the splenic compartment being represented by cells of the myeloid lineage by 28 weeks (Fig. 2C). Additionally, as often observed in disease conditions such as leukemia (23, 24), the enhanced myeloproliferation correlates with perturbations in erythropoiesis and development of extramedullary hematopoiesis in the spleens of lyn−/− mice, as revealed by the progressive increase in splenic TER-119+ erythroid progenitors (Fig. 2D). The bone marrow (BM) of Lyn-deficient mice also fills with myeloid cells, mainly mature granulocytes, which in turn displace erythroid cells (Fig. 3A, B). The replacement of erythroid and other BM cell types by mature granulocytes is especially apparent in immunohistochemical staining for Ter-119 (Fig. 3C). Eventually the bones of lyn−/− mice appear completely white to the naked eye.
Figure 2. Lyn-deficient mice develop splenomegaly due to myeloproliferation and extra-medullary erythropoiesis.
(A) Spleen weight of WT and lyn−/− mice monitored over 60 weeks. (B) Splenic cells derived from WT and lyn−/− mice stained with Wright/Giemsa. (C, D) Flow cytometric analysis of WT and lyn−/− splenocytes from 6 and 28 week old mice. Percentages of Mac-1/Gr-1 double positive myeloid cells (C) or Ter-119 positive erythroid cells (D) are reported.
Figure 3. Myeloproliferation and loss of erythropoiesis in the bone marrow of Lyn-deficient mice.
(A, B) Flow cytometric analysis of WT and lyn−/− bone marrow from 6 and 28 week old mice. Percentages of Mac-1/Gr-1 double positive myeloid cells – mainly neutrophils – (A) or Ter-119 positive erythroid cells (B) are reported. (C) Paraffin embedded bone marrow sections from 28 week old WT and lyn−/− mice probed with anti-TER-119 Ab.
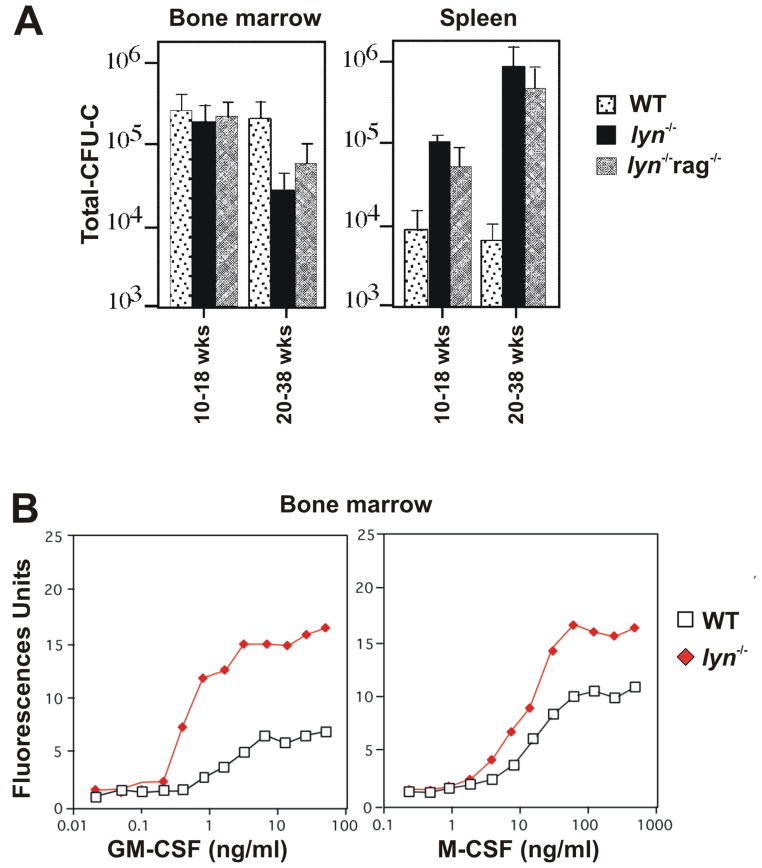
The assessment of hematopoietic progenitor numbers in colony-forming cell assays further confirms the myeloproliferative phenotype of lyn−/− mice. The number of hematopoietic progenitors responsive to myeloid specific growth factors such as GM-CSF, G-CSF, M-CSF (or CSF-1), interleukin-3 (IL-3), IL-6, stem cell factor (SCF) is dramatically increased in the spleen, peripheral blood and lymph nodes of lyn−/− mice (S.P. and C.L., unpublished observations, 16, 22). Splenic progenitor numbers steadily increase and reach nearly 100 fold over WT animals by 30+ weeks of age (Fig. 4A). However, with increasing age, myeloid progenitor numbers start to fall in the BM, concomitant with the replacement most of the BM by mature neutrophils and the loss of Ter-119+ cells (Fig. 4A). The shift of progenitors from the BM to the spleen in aging lyn−/− mice is reflected also in counts of SCF responsive cells: splenic SCF-responsive progenitors are higher while BM lineage negative (Lin−) progenitor cells produce lower number of colonies with reduced proliferative capacity to SCF (15, 22). It is possible that the dramatic expansion of mature myeloid cells observed in the BM of lyn−/− mice, and consequent potential limitation in stromal-cell support, might induce significant secondary changes in the Lin− progenitors (such as changes in c-Kit expression or alterations in downstream signaling molecules), that would limit interpretation of the colony-forming assay results in the BM. Nevertheless, the overall hematopoietic picture in Lyn deficiency is of relentless accumulation of myeloid cells (including monocytes/macrophages, neutrophils, eosinophils, mast cells and dendritic cells) in the spleen, lymph nodes and BM accompanied by the development of severe extramedullary hematopoiesis, which together strongly suggests that Lyn is playing a negative role in the modulation of myelopoiesis.
Figure 4. Hyper-responsiveness of Lyn-deficient bone marrow cells to cytokine stimulation.
(A) Cells derived from bone marrow and spleen obtained from WT, lyn−/− and lyn−/− rag−/− (lacking mature B and T cells) mice at the indicated ages, were cultured in methylcellulose media supplemented with IL-3, IL-6, SCF and erythropoietin for 7 days and the resultant number of colony forming units were counted. Total CFU-C were calculated based on the total cell yield from each site (bone marrow or spleen). The experiments were repeated a minimum of three times in triplicate and averaged results are reported. (B) Equal numbers of bone marrow derived cells from WT and lyn−/− mice were cultured with varying concentrations of GM-CSF or M-CSF for 7 days. Adherent cells number at the end of the culture period was determined using CyQuant fluorescent cell counting. The experiment was repeated a minimum of three times and the data shown are representative.
In relationship to human disease, this process most resembles a myelodysplastic syndrome seen in many pre-leukemic patients. However, there is no evidence that lyn−/− mice develop frank leukemia – there are no reports of overwhelming blast cell proliferation or accumulation of blasts in multiple tissue sites. Instead, this process mainly reflects expansion of mixed myeloid cells, ranging from mostly mature phenotypes to increased progenitors. Under some conditions, the extramedullary accumulation of myeloid cells can be extreme, to the point where older (> 1 year) lyn−/− mice sporadically develop large aggregates of myelomonocytic cells on the tail, ears, and legs (22). These abnormal monocyte/macrophage cells are tumorigenic when transferred to SCID hosts, indicating that rarely the loss of Lyn kinase can lead to transformation of myeloid lineage cells, though not frank myeloid leukemia. The potential tumor suppressive function of Lyn is undoubtedly affected by genetic background and other environmental factors (such as chronic infection or spectrum of commensal flora), since we have never observed monocyte/macrophage like tumors in lyn−/− mice within the UCSF animal facility.
Mice lacking both Lyn and Hck develop an even more exaggerated form of myelodysplasia (25). Lyn−/− hck−/− double mutants rapidly develop severe extramedullary hematopoiesis, with accumulation of abnormal progenitors that manifest increased proliferative responses and reduced apoptosis. These animals accumulate large numbers of mature myeloid cells, including M2 macrophages that invade the lungs, resulting in early (8 weeks or less) death. The myeloproliferative disease can be conferred to naïve recipient mice by transfer of Lin− hematopoietic progenitors. Despite the very aggressive nature of this disease, there is no evidence that loss of these SFKs results in transformation to frank leukemia or lymphoma. The accumulated cells appear morphologically heterogeneous, with mainly mature cells present, as eventually develops in lyn−/− single mutant mice. Single mutant hck−/− animals show none of these hematopoietic phenotypes, demonstrating that this SFK mainly facilitates the inhibitory function of Lyn. However, in vitro the hck−/− myeloid progenitors do show a modestly increased response to G-CSF stimulation thus helping explain the synergistic effect of loss of both of these SFKs (26).
As in many myelodysplastic syndromes, lyn−/− progenitor cells show obvious hyper-responsiveness to colony stimulating factors in liquid culture, producing a left shift in the dose-response curves to both GM-CSF and M-CSF (Fig. 4B). Lyn−/− progenitors also proliferate more rapidly, reaching saturation density one to two days in advance of WT cells, at fully activating CSF concentrations (S.P. and C.L., unpublished observations, 16). It is quite likely that this hyper-proliferative phenotype contributes to both the increased numbers of myeloid progenitor cells (mainly in the spleen) and the relentless development of myelodysplasia described above. However, at least in the lyn−/− hck−/− double mutant mice, impairments in the rates of spontaneous apoptosis (as determined by annexin V staining) may also be contributory (25). In contrast, we have found no changes in the rates of apoptosis of mature lyn−/− macrophages following cytokine/growth factor withdrawal, though Harder et al (22) did report a subtle resistance to cytokine withdrawal-induced apoptosis in lyn−/− mature macrophages. Thus, the myelodysplasia in lyn−/− mice seems to be mainly a consequence of hyper-responsiveness to cytokine stimulation and not impaired apoptosis.
Myleoproliferative phenotypes are observed in a number of mouse models, most often in animals with associated autoimmunity. A good example is the disease spectrum of SHP-1 mutant mice (motheaten animals) or animals lacking the inositol phosphatase SHIP-1 (27, 28). Though much more severe, the general similarity of the myeloproliferative phenotype in motheaten, SHIP-1 and lyn−/− mice was the first evidence suggesting that Lyn’s negative regulatory function involves activation of these phosphatase pathways. However, myeloproliferation is also seen in other mouse models of autoimmunity, such as the NZB/NZW model (29), where myeloid cell expansion could be a consequence of the inflammatory effects of immune complexes produced by self-reactive B-lymphocytes. The latter observation raises the question whether the myeloproliferation in lyn−/− mice results from an intrinsic defect in stem cell or progenitor signaling or is more of a consequence of the inflammation and autoimmune phenotype developed by these animals. Harder and co-workers showed that myeloproliferation in lyn−/− mice is not dependent on B-cell mediated autoimmunity, as lyn−/−μMT/μMT mice (mice that lack mature B cells), still develop myeloproliferation indistinguishable from lyn−/− mice (22). Similarly, myeloproliferation and expansion of splenic progenitor numbers still occurs when lyn−/− mice are crossed to Rag mutant animals (lacking mature B and T cells; Fig. 4A). These observations suggest that the increased myeloproliferation in lyn−/− mice is due to intrinsic hyper-responsiveness of progenitors and not just a consequence of autoimmunity. Indeed, the myeloproliferative disease of SHP-1 mutant motheaten mice is also unaffected when these animals are rendered genetically deficient of lymphocytes (30). Of course, this raises the question of what the progenitors in lyn−/− or motheaten mice are hyper-responding to? We have observed that aged lyn−/− mice have elevated blood levels of a number of inflammatory cytokines (P.S. and C.L., unpublished observation), while lyn−/− hck−/− BM cells spontaneously secrete IL-3 and GM-CSF (25), which undoubtedly drives myeloproliferation. It is possible that increased responses to endogenous Toll-like receptor (TLR) ligands, present in normal bacterial flora, may drive these responses since disruption of TLR signaling in lyn−/− mice (by crossing to MyD88−/− animals) blocks development of autoimmunity and myeloproliferation (31). MyD88 deficiency also blocks autoimmunity and myeloproliferation in motheaten mice (32). The hypothesis that increased inflammatory cytokine release, and subsequent hyper-responsiveness to these cytokines could drive the disease in lyn−/− mice is supported by the observation that when lyn−/− mice are crossed to transgenic animals expressing a low level of Bruton’s tyrosine kinase (Btk), the bi-genic lyn−/− Btklow mice manifest a strong reduction of myeloproliferation and autoimmunity despite the fact that the intrinsic B-cell hyper-responsive phenotype is still present (33). In this study the authors suggest that Btk-dependent increased cytokine secretion, either directly by the expanded and hyper-activated myeloid cells or, indirectly, by T cells receiving antigenic stimulation from the hyper-activated myeloid cells, may sustain the inflammation and myeloproliferation in lyn−/− mice ultimately leading to autoimmunity. In support of the latter hypothesis we recently found that the secretion of interferon γ (IFNγ) by activated T cells strongly contributes to the development of both myeloproliferation and autoimmunity in lyn−/− mice (P.S. and C.L., unpublished observation). The mechanisms responsible for this phenomenon are currently under investigation, but they correlate with an IFNγ-dependent increased production of inflammatory cytokines and myeloid cell growth factors induced by Lyn-deficiency. It is therefore possible, that the intrinsic hyper-responsiveness of lyn−/− progenitors and myeloid cells leads to activation of T-cells, which in turn further activate myeloid cells (through production of INFγ) leading to a positive feedback loop that contributes to myeloproliferation and autoimmunity.
The signaling mechanisms in primary myeloid progenitors through which Lyn is down modulating responses to growth factors are poorly studied. To our knowledge, the only finding obtained in less differentiated myeloid cells is the observation of increased Erk activation in lyn−/− granulocyte precursors following G-CSF stimulation (26). More detailed studies have been performed in mature lyn−/− macrophages and granulocytes (see below). However, the overall hypothesis for Lyn’s signaling function in myeloid progenitors is the same as reported for mature B-cells – namely that Lyn must be phosphorylating ITIM containing receptors or intracellular molecules to recruit phosphatases such as SHP-1 and SHIP-1 (Fig. 1). However, the nature of the inhibitory ITIM containing receptors is unknown. Notably, deficiency of the major myeloid inhibitory molecules PIR-B and SIRPα, which Lyn phosphorylates in mature cells, does not produce a myeloproliferative phenotype (34, 35). Thus it is likely that other Lyn substrates in myeloid progenitors are involved in the activation of phosphatases to mediate the inhibitory function of Lyn kinase. It is possible that, downstream of the phosphatases, one of the pathways regulated by Lyn kinase involves STAT5 activation, as shown below for GM-CSF-stimulated macrophages (Fig. 1). In line with this hypothesis, SHIP-1−/− mice and lyn−/− hck−/− double mutant progenitors show hyper-activation of STAT5 (25). The substrates on which Lyn and/or Hck are operating in this model must involve recruitment of SHIP-1 to the membrane, since expression of a constitutively membrane localized version of SHIP-1 will reverse the STAT5 hyper-activation seen in lyn−/− hck−/− cells and blocks development of myeloproliferation. Hence, it is possible that some transmembrane receptor to which (at least) SHIP-1 associates is a primary target of Lyn and/or Hck in hematopoietic stem cells and progenitors.
The role of Lyn in myeloid leukemia
Given the clear role of Lyn kinase as an inhibitor of myeloid progenitor responses to CSFs, it is surprising that a number of studies have shown that various myeloid leukemias depend on Lyn signaling for survival and proliferation. Indeed, therapeutic targeting of Lyn kinase and other SFKs is now entering advanced stage trials in various forms of myeloid leukemia (36). The most convincing evidence comes from studies of cell lines established from patients with chronic myelogenous leukemia (CML) (37). In many of these cell lines, Lyn is hyper-activated (as judged by tyrosine phosphorylation) and this correlates with the resistance of these lines to inhibitors that target the BCR/ABL fusion protein that causes CML. Knock-down of Lyn expression, by siRNA targeting, results in increased apoptosis and a renewed susceptibility of the CML lines to BCR/ABL inhibitors. Therapeutic targeting of Lyn (and other SFKs present in the CML cells) using novel inhibitors (such as dasatinib) leads to remission of CML disease in patients not responding to the BCR/ABL inhibitors. In some studies, the development of Lyn kinase dependent signaling is associated with new mutations in the kinase, or at least the appearance of new phosphorylation sites and associated molecules (36). However, the specific pathways that Lyn is modulating in this positive signaling function are unknown, but may involve activation of Gab2 and downregulation of another inhibitory molecule, c-Cbl (38). Correlating with the involvement of STAT5 in the myeloproliferative disease in lyn−/− hck−/− mice, inhibitors such as dasatinib also reduce phospho-STAT5 levels in myeloid leukemia cell lines (39).
A similar result has been reported in acute myeloid leukemia (AML). Though a very different disease that CML, cell lines and primary progenitors from patients with AML also show high levels of phospho-Lyn kinase (40). Inhibition of SFKs with semi-selective inhibitor PP2 (7) markedly reduces AML cell proliferation and leads to induction of apoptosis of blasts in patient samples without affecting the normal myeloid progenitors. Similar effects are seen by siRNA mediated Lyn knockdown. In these cells, disruption of Lyn kinase signaling leads to diminished mTOR activation, suggesting that Lyn is functioning somewhere in this pathway as a positive regulator. In the well established myeloid 32D cell line, Lyn kinase associates with Fms-like tyrosine kinase receptor FLT3 to mediate constitutive activation of STAT5 phosphorylation; PP2 mediated inhibition of Lyn or siRNA knockdown blocks the FLT3/Lyn dependent STAT5 activation (41).
Thus, we are left with the very paradoxical result that Lyn kinase seems to be an inhibitor of normal myeloid cell development, yet is positive regulator of myeloid leukemic cell proliferation, and at least in the case of STAT5, through the very same signaling pathways. Even more confusing is the fact that mice with a gain-of-function mutation in Lyn, resulting in a constitutively active kinase, do not develop myeloproliferative disease and are not prone to myeloid tumors (22). Obviously, there can be many reasons why signaling responses in normal versus malignant myeloid progenitors show such opposite effects of Lyn inhibition. Nevertheless, these observations are prime examples of the duplicitous nature of Lyn in myeloid cell signaling.
Role of Lyn in macrophages
The role of Lyn in the modulation of macrophage function has been mainly investigated in response to cytokine stimulation or adhesion. As in myeloid progenitors, Lyn exerts mainly a negative role in the modulation of these responses in primary macrophages, primarily through phosphorylation of ITIM receptors such as PIR-B and SIRPα (Fig. 1 and Table 1). In contrast, most other SFKs have a positive regulatory role in these cells (reviewed in 7).
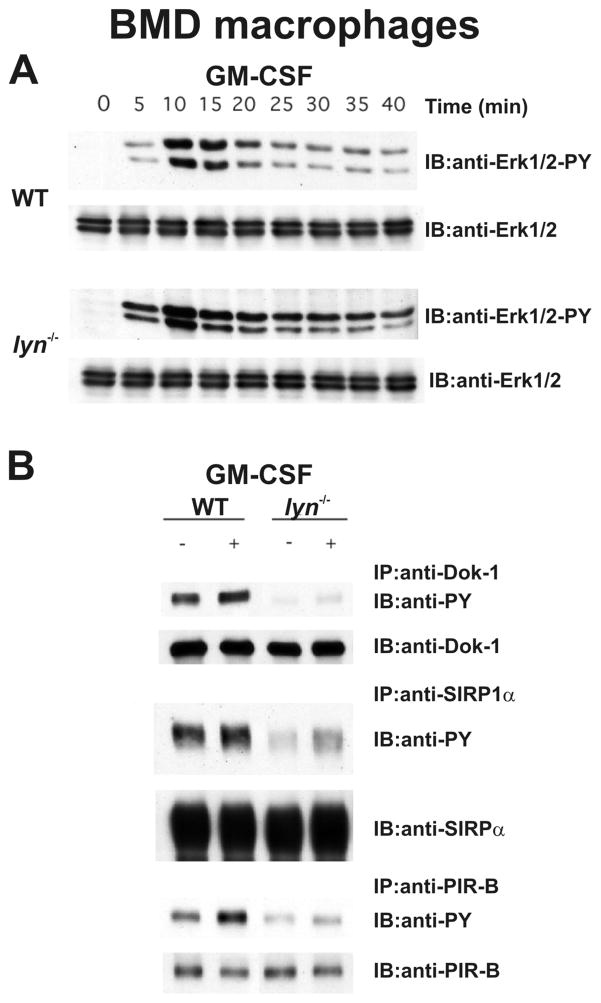
The primary cytokines to which macrophages respond include M-CSF and GM-CSF, which induce macrophage proliferation and survival. The inhibitory role of Lyn kinase in the CSF signaling has been clearly demonstrated using both cell line models and in primary bone marrow-derived (BMD) macrophages from WT and lyn−/− mice (16). Most convincingly, primary BMD macrophages treated with either M-CSF or GM-CSF show robust activation of the Erk1/2 pathway; in lyn−/− macrophages, Erk1/2 activation is higher and more prolonged (Fig. 5A). These cytokines also induce STAT5 activation in BMD macrophages. As seen in lyn−/− hck−/− myeloid progenitors, formation of phospho-STAT5 is much stronger in GM-CSF stimulated lyn−/− macrophages compared to WT cells (S.P. and C.L., unpublished observation). In the case of M-CSF stimulation, lyn−/− BMD macrophages also display much higher phosphorylation of the M-CSF receptor itself (S.P. and C.L., unpublished observation), which directly suggests that loss of the kinase results in impaired recruitment of a tyrosine phosphatase such as SHP-1.
Figure 5. Lyn-deficient bone marrow derived macrophages manifest increased Erk1/2 activation and reduced inhibitory receptor phosphorylation following GM-CSF stimulation.
(A) WT and lyn−/− BMD macrophages were stimulated with 100ng/ml GM-CSF for varying lengths of time and Erk1/2 activation was determined by immunoblot (IB) using phospho-specific mAbs Lower panels show IBs with total Erk/1/2 to demonstrate equal loading. (B) WT and lyn−/− BMD macrophages were stimulated with 100ng/ml GM-CSF for 15 minutes and cell lysates were immunoprecipitated (IP) with anti-Dok-1, anti-SIRPα and anti-PIR-B followed by anti-phosphotyrosine (anti-PY) immunoblotting (IB) to determine phosphorylation. Lower panels show IBs against the precipitated protein to demonstrate equal loading.
SHP-1 and SHIP-1 are well established negative regulators of growth factor signaling (27, 28). It was therefore likely that the potential mechanism of Lyn-deficient macrophages hyper-responsiveness to M-CSF and GM-CSF is related to an impaired recruitment and activation of these phosphatases (Fig. 1). This hypothesis has been supported by many experiments performed by several groups, including ours.
SIRP1α and PIR-B are the major macrophage inhibitory receptors that recruit SHP-1 and SHP-2 (42–44). We have found that phosphorylation of both SIRPα and PIR-B is reduced in both resting and GM-CSF stimulated lyn−/− BMD macrophages (Fig. 5B). In addition to these receptors, the intracellular ITIM containing molecule Dok-1, which is know to recruit the phosphatase SHIP-1 and RasGAP to downmodulate growth factor signaling (45–47), is also poorly phosphorylated in GM-CSF stimulated lyn−/− macrophages (Fig. 5B). Impaired phosphorylation of PIR-B, SIRPα and Dok-1 also occurs in M-CSF stimulated lyn−/− BMD macrophages (S.P. and C.L., unpublished observation). The lack of phosphorylation of these receptors leads to poor recruitment and activation of the phosphatases. Indeed, membrane fractions from lyn−/− BMD macrophages have reduced levels of both SHP-1 and SHP-2 as well as reduced overall phosphatase activity, suggesting poor recruitment of these enzymes to inhibitory receptors (22). Poor association of SIRPα and PIR-B with SHP-1 was also observed by co-immunoprecipitation experiments from lyn−/− macrophages (22). These data directly implicate Lyn as the primary SFK that phosphorylates these inhibitory receptors leading to phosphatase activation – lack of this activation explains the hyper-proliferative response of lyn−/− myeloid progenitors and mature cells to growth factors such as GM and M-CSF.
A role of Lyn in the recruitment of SHIP-1, and consequent negative regulation of M-CSF-induced Akt activation, has been suggested by Baran C.P. and co-workers (48). These authors showed that Lyn and SHIP-1 co-associate, via an SH2 interaction, when co-transfected into the human monocyte cell line THP-1. Furthermore, in 3T3-Fms cells, which express the human M-CSF-R, Lyn enhanced the ability of SHIP-1 to regulate Akt activation by stabilizing SHIP-1 at the cellular membrane. In line with these observations, BMD macrophages isolated from both SHIP-1 and Lyn-deficient mice exhibited enhanced Akt phosphorylation following M-CSF stimulation (48). Impaired recruitment of SHIP-1 to phospho-Dok-1 may also contribute to poor regulation of Akt phosphorylation in lyn−/− macrophages.
Lyn also operates as a negative regulator of integrin signaling during adhesion-dependent macrophage activation (49). Lyn−/− BMD macrophages display an increased rate of macrophage spreading following plating on non-tissue culture plastic (VALMARK) plates, which have been shown to engage β2 integrins leading to firm adhesion and integrin-dependent signaling (49). Similar to M-CSF and GM-CSF signaling responses, the hyper-adhesive phenotype of Lyn-deficient macrophages is due to an impaired PIR-B and SIRPα mediated recruitment of SHP-1 and consequent lack of down-regulation of signaling events. Following adhesion to VALMARK plates WT BMD macrophages display increased phosphorylation of both PIR-B and SIRPα. However, adhesion-dependent phosphorylation of these two proteins is decreased in lyn−/− BMD macrophages (49). Furthermore, in line with the reduced phosphorylation of both PIR-B and SIRPα, co-immunoprecipitation of these molecules with SHP-1 is strongly reduced in lyn−/− BMD-macrophages compared to WT cells. Consistent with these findings, BMD macrophages from SHP-1 mutant motheaten mice display a hyper-adhesive phenotype (50). Lyn has also been implicated as a negative regulator of LFA-1 mediated adhesion of monocytes to activated endothelial cells – siRNA knockdown of Lyn in monocytes induced increased adhesion, as observed in lyn−/− macrophages (51). Interestingly, Hck- and Fgr-double deficiency induces an opposite phenotype to Lyn-deficiency, as hck−/− fgr−/− macrophages display an impaired integrin-mediated signaling transduction and functions (52), suggesting a positive role of Hck and Fgr in the modulation of these responses.
In contrast to the inhibitory role in integrin signaling, Lyn seems to play a positive role in macrophage adhesion through the class A scavenger receptor (SR-A) (53). During SR-A mediated spreading of peritoneal macrophages Lyn kinase displays prolonged (up to 2h) activation while other SFKs present in these cells are not activated. Treatment with PP2 blocks SR-A-dependent Lyn phosphorylation, phosphatidylinositol-3 kinase (PI-3K) activation, paxillin phosphorylation and consequent SR-A-induced cell adhesion.
Lyn kinase has also been implicated as a positive modulator in chemokine/chemoattractant signaling events in macrophages. Signaling through CCR5 in primary human macrophages depends on Lyn kinase, since peptide mediated inhibition of Lyn results in reduced Erk activation following MIP-1β stimulation of macrophages (54). Similarly, knockdown of Lyn in monocytes using siRNA decreases SDF-1α-mediated migration through monolayers of endothelial cells (51). CXCR4 signaling in hematopoietic progenitors is also dependent on Lyn kinase, since siRNA knockdown of Lyn in these cells also reduces responses to CXCR4 agonists (SDF-1α) (55). However, assays assessing migration of Lyn-deficient cells in response to chemokine stimulation are complicated by the hyper-adhesive phenotype observed in these cells. Decreased migratory responses may reflect the increased integrin mediated adhesion seen in lyn−/−cells and not defects in chemokine/chemoattractant signaling per se. In contrast, it is well established that macrophage migration depends on signaling from other SFKs. In both in vitro wound healing and transwell assays, the migration of hck−/− fgr−/− macrophages is significantly decreased. Furthermore, hck−/− fgr−/− lyn−/− macrophages display reduced migration in the thioglycollate-induced peritonitis model in vivo (4, 6, 56). To our knowledge, no similar studies have been performed with Lyn single mutant macrophages, although such experiments might help to better understand the increased macrophage infiltration in different tissues displayed by lyn−/− mice (22).
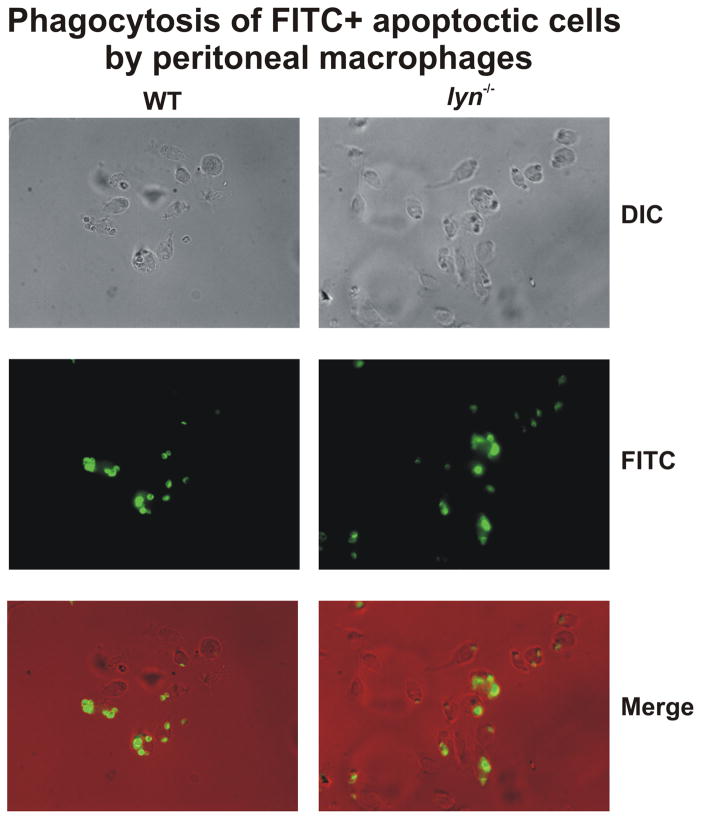
Lyn seems to play a redundant role in the modulation of macrophage phagocytosis. This kinase is activated during Fc receptor γ (FcγR)- mediated phagocytosis (57, 58) but defects in IgG-dependent phagocytosis are only evident in macrophages lacking all the three SFKs (lyn−/− hck−/− fgr−/− macrophages) and not in hck−/− fgr−/− double mutant or lyn−/− single mutant cells (58). On the other hand, Lyn-deficient macrophages do show a modest signaling defect (reduced overall levels of tyrosine phosphorylation) following engagement of FcγRs while hck−/− fgr−/− cells do not, but this mild signaling impairment has no consequences in functional uptake of IgG coated particles. Macrophages lacking the membrane tyrosine kinase c-mer have defective phagocytosis of apoptotic cells, despite normal phagocytosis of other particles (59). Deficiency of this kinase and other members of the TAM family of receptor kinases results in a number of signaling abnormalities and development of autoimmune disease similar to lyn−/− mice (60). We therefore investigated whether a similar defect was present in lyn−/− macrophages, but as shown in Fig. 6 there were no differences in the phagocytosis of apoptotic cells between lyn−/− macrophages and WT cells, further excluding an important role of Lyn in the modulation of phagocytosis in macrophages.
Figure 6. Lyn-deficient macrophages phagocytose apoptotic thymocytes normally.
WT and lyn−/− peritoneal macrophages were collected 5 days after IP injection with aged 3% thioglycollate and cultured overnight. Macrophages were exposed to 20-fold excess of apoptotic thymocytes, labeled with CellTracker green, for 30 min an examined by fluorescence microscopy, as described (108).
No conclusive data has been reported on the role of Lyn in the modulation of cytokine production by macrophages. Macrophages lacking Hck, Fgr and Lyn display a mild enhancement in cytokine secretion (IL-1, IL-6 and TNFα) following stimulation with the TLR ligand, lipopolysaccharide (LPS) (61). Similar results have been reported for syk−/− cells and map to a role of SFKs and Syk acting on the TREM-2 receptor, and its ITAM containing adapter protein DAP-12, to downmodulate TLR responses (62). These results reflect the growing recognition of interactions between ITAM pathways and TLR pathways (63). On the other hand it has been reported that PP2, inhibits cytokine production in response to LPS and a number of other TLR agonists (64). This effect of PP2 seems to be due to the ability of this molecule to block AP-1 nuclear accumulation in response to LPS (64), although possible off-target effects of PP2 may explain its ability to block TLR signaling. Further studies are needed to better clarify the specific role of Lyn in the modulation of cytokine production by macrophages in response to different agonists.
No studies have so far investigated the role of Lyn-deficient macrophages in inflammatory or host-defense responses in vivo. Given the general enhancement of macrophage responses to growth factors and integrin signaling seen in Lyn-deficient mice, it is quite possible that these animals may manifest increased resistance to bacterial (or other pathogen) challenge. This remains to be tested.
Role of Lyn in neutrophils
Lyn seems to exert both a positive and a negative role in the modulation of neutrophil function. To date, a role of Lyn in the modulation of neutrophil granulopoiesis, apoptosis and adhesion are the main responses that have been investigated.
With age, lyn−/− mice develop increased peripheral blood (and splenic) neutrophil counts (26). G-CSF is one of the main regulators of granulopoiesis (65) and the progressive neutrophilia observed in lyn−/− mice suggests that this kinase functions as a negative regulator of G-CSF signaling. Unfortunately, this is not well established. In vivo lyn−/− mice display an exaggerated granulopoiesis in response to low-dose G-CSF injection, while Lyn-deficient c-kit+Lin− progenitors display decreased proliferative responses to G-CSF in culture (26). This apparently confusing result may be explained by the fact that Lyn-deficiency results in development of higher numbers of myeloid committed progenitors, suggesting that the real effect of Lyn is on signaling pathways earlier in myelopoiesis. As mentioned in the myeloid progenitor section, an inhibitory role for Lyn in the G-CSF pathway is also suggested by the observation that primary lyn−/− c-kit+Lin− progenitors manifest increased and prolonged activation of Erk kinase following G-CSF stimulation. Nevertheless, many other studies in both primary cells and cell lines suggest just the opposite – concluding that Lyn is an important positive transducer of G-CSF responses (reviewed in 66). Stimulation of mature neutrophils with G-CSF induces the activation of Lyn, which co-precipitates with the G-CSF receptor (67). In Ba/F3 cells, G-CSF stimulation leads to Lyn dependent phosphorylation of the Gab2 adapter protein, which helps link G-CSF receptor signaling to downstream PI-3K and Erk pathways, to regulate differentiation responses (68). Furthermore, Lyn has been shown to act as a positive modulator of G-CSF induced reactive oxygen species (ROS) production (69). Therefore, it is possible that Lyn is a negative modulator of G-CSF induced responses in neutrophil precursors, while it acts as a positive modulator of G-CSF induced responses in more differentiated cells (Table 1), but clearly more studies are required to provide a molecular understanding of these observations.
Apoptosis is the major mechanism for limiting neutrophil numbers in vivo. Extracellular stimuli, such as proinflammatory cytokines, cell adhesion, phagocytosis, can modulate neutrophil apoptotic death (70). Controversial data for both a positive and a negative role of Lyn in the regulation of neutrophil apoptosis have been reported. GM-CSF is a well known inhibitor of neutrophil apoptosis (71). Lyn is physically associated with GM-CSF receptor in neutrophils and rapidly activated after GM-CSF stimulation of these cells (20). GM-CSF inhibition of neutrophil apoptosis seems to be completely dependent on Lyn as antisense knockdown of Lyn completely reverses the cell survival advantage provided by GM-CSF in human neutrophils (20). In line with this evidence, we also found that GM-CSF as well as IFNγ-induced survival is impaired in lyn−/− neutrophils, although G-CSF-induced survival is not affected by Lyn-deficiency (P.S. and C.L., unpublished observation). In addition, the mechanism through which death domain-containing receptors of the TNF family disrupt anti-apoptosis pathways initiated by survival factors, such as GM-CSF, in neutrophils has been shown to be dependent on a SHP-1-induced inhibition of Lyn activation (72). Daigle and co-workers found that death-receptor activation blocks GM-CSF-mediated pro-survival signal by increasing SHP-1 binding to Lyn, thus decreasing Lyn activity (72). Furthermore, a role for Lyn in the inhibition of constitutive neutrophil apoptosis, as well as in the mediation of the pro-survival effect of LPS, has also been reported (73). In this study, the authors show that Lyn-mediated phosphorylation of caspase-8, both under resting conditions and after LPS stimulation, renders it resistant to activation cleavage and inhibits progression of apoptosis. Lyn exerts this effect in antagonism with SHP-1, which is responsible of caspase-8 dephosphorylation and activation of the apoptosis cascade (73). These observations suggest that Lyn may be a negative regulator of neutrophil apoptosis under specific stimulatory conditions. On the other hand, a positive role of Lyn in modulation of neutrophil apoptosis has also been suggested. Gardai and co-workers reported that Lyn is a key activator of accelerated apoptosis induced by combined anti- and pro-apoptotic stimuli (74). The authors report that in the combined presence of integrin engagement (pro-survival stimulus) and pro-apoptotic stress stimuli, such as TNFα and Fas ligand stimulation, Lyn is involved in the recruitment of SHIP-1 to the plasma membrane which in turn blocks the pro-survival effect of integrin-induced Akt activation by reducing phosphoinositide 3,4,5-trisphosphate (PIP3) levels. As a result, lyn−/− neutrophils and SHIP-1−/− neutrophils do not manifest enhanced apoptosis with this stimulus combination (74). The inhibitory function of Lyn in regulation of integrin signaling (49) may confound these results by differentially affecting the pro-survival cascade induced by integrin engagement versus the pro-survival effect induced by other stimuli such as GM-CSF, where the kinase may have a more positive role (Table 1). Considering the relevant role of integrin engagement during the inflammatory response, further study of Lyn function in the modulation of neutrophil apoptosis in vivo should be performed.
As in macrophages, it is clear that Lyn is a major negative regulator of integrin-dependent functional responses in neutrophils (Table 1), while Hck and Fgr and Syk are positive modulators of these responses (49, 75, 76). While hck−/− fgr−/− and syk−/− neutrophils manifest a strong impairment of adhesion-induced functions (75–77), lyn−/− neutrophils display hyper-adhesive phenotype resulting in enhanced respiratory burst and secondary granule release when adherent to surfaces coated with either cellular counter-receptors (ICAM-1) or extracellular matrix proteins (fibrinogen or fibronectin) that engage integrins (49). As in macrophages, it is likely that Lyn functions to phosphorylate neutrophil inhibitory receptors, which in turn recruit SHP-1 (and other phosphatases) to downmodulate signaling (Fig. 1). Indeed, lyn−/− neutrophils display an exaggerated tyrosine phosphorylation response after integrin engagement (49). In line with this hypothesis, neutrophils isolated from SHP-1 mutant motheaten mice are also hyper-responsive following integrin engagement (49). Curiously, a study performed with human neutrophils treated with PP1 (the precursor SFK inhibitor to PP2) suggests a positive role of Lyn in TNFα-stimulated superoxide production by neutrophils adherent to fibrinogen (78). The fact that PP1 is a general SFK inhibitor and that hck−/− fgr−/− neutrophils display a defective superoxide production in response to integrin engagement, strongly suggests that the data obtained with lyn−/− neutrophils are more reliable for the understanding of the effective role of Lyn in the modulation of integrin signaling.
In contrast to the negative role that Lyn exerts in integrin-induced adhesion, this kinase seems to exert a positive role in the modulation of chemokine/chemoattractant-induced signaling (Table 1). Indeed Lyn plays a critical role in the activation of PI-3K after chemoattractant stimulation of human neutrophils (79, 80), similar to the role of Lyn in monocyte SDF-1α signaling (51). In line with these findings, we observed a reduced Ca2+ flux, MAP kinase activation and actin polymerization in lyn−/− neutrophils after stimulation with different chemokine/chemoattractant agonists (H.Z. and C.L., unpublished observation). Unfortunately, in vivo the situation seems to be more complicated and frankly understudied. In one clear example, lyn−/− neutrophils enter the skin normally and produce an equivalent amount of inflammatory tissue injury following induction of cutaneous inflammation in the LPS/TNF-induced local Schwartzman reaction (81). Interestingly, the other major SFKs in neutrophils, Hck and Fgr, function in the opposite fashion in the chemokine pathway. Neutrophils isolated from hck−/− fgr−/− mice display exaggerated signaling and functional responses following MIP-1α or MIP-2 stimulation (82). On the other hand, hck−/− fgr−/− neutrophils manifest defective responses to the chemoattractant fMLP (83). Hence, it is hard to make a general conclusion for the role of Lyn versus other SFKs in neutrophil chemoattractant signaling during inflammatory responses in different tissues. It is quite likely that signaling reactions elicited by different chemokines/chemoattractants utilize different pathways that are influenced or regulated by SFKs in different fashions. Clearly, this is an area needing further study.
Role of Lyn in dendritic cells (DCs)
As in other cells, Lyn plays both positive and negative regulatory roles in DC differentiation and maturation. The role SFKs, including Lyn, insignaling events in DCs has been studied mainly downstream of TLRs (84–87). Collectively, these studies suggest the importance of SFKs, in particular Lyn, in immune signaling in DCs. However, the majority of these studies have relied on the use of tyrosine kinase inhibitors with broad specificity thus complicating interpretation of the results.
Studies using lyn−/− mice clearly demonstrate that this kinase is involved in DC generation and maturation (88, 89). Loss of Lyn promotes DC expansion in vitro from BM precursors due to enhanced GM-CSF-induced cell division and differentiation from DC progenitors as well as higher survival rate of differentiated Lyn-deficient DCs (Table 1). In line with this in vitro evidence, the DC-population is increased in aged Lyn-deficient mice. The alterations in GM-CSF signaling in lyn−/− DCs are likely due to reduced phosphorylation of inhibitory receptors, as in macrophages. Indeed, antigen-pulsed BMD DCs from lyn−/− mice display less phosphorylation of PIR-B and diminished SHP-1 association, suggesting a synergistic activity of PIR-B and Lyn in modulating DC maturation (89). The high degree of similarity between lyn−/− and PIR-B−/− DC phenotypes strongly supports this hypothesis (34). Interestingly, in contrast to their enhanced generation, lyn−/− BMD DCs display, both in vitro and in vivo, a more immature phenotype as compare to WT cells. Lyn−/− BMD DCs fail to mature appropriately and display lower levels of MHC class II and co-stimulatory molecules even after stimulation with maturation stimuli such as TLR-ligands. In line with their more immature phenotype, IL-12 production and Ag-specific T-cell activation is reduced in lyn−/− DCs, resulting in impaired T helper type 1 (Th1) responses (88). The poor IL-12 production following TLR stimulation may be due to reduced activation of SHIP-1. SHIP-1 shows minimal tyrosine phosphorylation in lyn−/− DCs compared to WT cells, suggesting that it is less active and hence poorly regulates the PI-3K pathway. Hyper-activation of the PI-3K pathway leads to diminished IL-12 synthesis in DCs (89).
The immature, Th2 like phenotype of lyn−/− DCs may contribute an exaggerated development of Th2-dependent asthma following airway challenge with model Ags (89). Following airway challenge, lyn−/− mice display a dramatic increase in lung eosinophilia, mast cell hyper-degranulation, and hyper-IgE-production, which leads to prolonged bronchospasm. Adoptive transfer of Lyn-deficient DCs to naïve mice is sufficient to transfer the exaggerated allergic response, suggesting the asthma prone phenotype of these animals is caused by the immature/Th2 like DC phenotype versus B-cell dependent over production of IgE. The fact that lyn−/−μMT/μMT mice develop a similar disease severity as lyn−/− mice also rules out B-cell contributions to the allergic response. On the other hand, as reviewed below, Lyn is also expressed in eosinophils and mast cells where, at least for the latter cells, it has been clearly shown to play a negative role in modulating degranulation. Therefore it is possible that, besides the Th2-polarizing phenotype of lyn−/− DCs, the hyper-activated lyn−/− mast cells and eosinophils could be contributing to the severity of the immune response, inflammation and cytokine production during allergic airway challenge.
While the immature lyn−/− DCs may skew towards Th2 T-cell responses during lung inflammation/allergy models, they certainly do not do so in the experimental allergic encephalomyelitis (EAE) model (90). Following immunization with the myelin oligodendrocyte protein (MOG) p35-55, lyn−/− mice develop exaggerated CNS inflammation, cytokine production and more clinical severe disease symptoms than do WT mice. Transfer of serum from MOG-immunized lyn−/− mice worsened EAE in WT animals, suggesting that part of the increased response in the Lyn-deficient animals is due to enhanced B-cell production of anti-MOG IgM. In general, the hyper-responsiveness of Lyn-deficient myeloid effector cells in these models seems to overwhelm the poor ability of lyn−/− DCs to prime T-cell responses, resulting in just exaggerated inflammation, no matter what the stimulus.
The role of DCs in driving the autoimmunity in Lyn-deficient mice has been only peripherally examined. As in other models of murine autoimmunity, it is clear that signals from TLRs are required for disease development in Lyn-deficient animals, since genetic deletion of MyD88 strongly suppresses development of autoimmunity (as determined by autoAb production and development of glomerulonephritis) (31). Whether the loss of TLR signaling is affecting primarily DC maturation and presentation of self-Ags to T-cells remains to be determined. The strong reduction of B-cell hyper-activation and autoAb production by lyn−/− B cells observed in MyD88−/− lyn−/− double mutant mice, suggests that MyD88 deficiency is affecting B-cell responses, presumably through loss of TLR9 signals.
Finally, similar to hck−/− fgr−/− neutrophils, DCs from hck−/− fgr−/− double mutant or fgr−/− single mutant mice, display enhanced intracellular signaling (Ca 2+ flux, MAP kinase activation and actin polarization) and enhanced migration in vitro and in vivo in response to a number of different chemokines (82). This phenotype reminds the one of PIR-B-deficient DCs, suggesting once more the important signaling axis between SFKs and this inhibitory receptor. The role of Lyn in the modulation of chemokine responses in DCs has not been investigated so far.
Role of Lyn in mast cells
The role of Lyn in the regulation of mast-cell functions has been studied extensively, but many conflicting observations have been reported. Overall, Lyn seems to play mainly a negative role in the regulation of mast-cell functions such as degranulation, proliferation and cytokine production (Table 1). More complete reviews on the role of SFKs in mast cell function and signaling have been recently published (91, 92).
Mast cells proliferate mainly in response to SCF and IL-3. The data on the effect of Lyn in the regulation of SCF-and IL-3-induced mast cells proliferation are controversial. Indeed, evidence that Lyn-deficiency does not affect, or inhibits or enhances these responses have all been reported (47). In line with the observation that lyn−/− mice have more peritoneal mast cells that WT mice, Hernandez-Hansen and colleagues showed that lyn−/− BMD mast cells expand faster and proliferate more rapidly than WT cells, possibly due to enhanced IL-3-induced phosphorylation of Akt and ERK1/2 (93). The authors propose that the contradiction between their results and others could potentially be explained by differences in the culture conditions of the BMD mast-cell preparations or in the age or health of the donor mice. It is also unclear whether the peritoneal mast-cell expansion observed in lyn−/− mice is due to a direct effect of increased proliferation to mast-cell growth factors or to an enhanced survival response to the higher IgE serum levels present in these mice.
The role of Lyn in the modulation of mast cell migratory responses has been investigated only in one study where a positive role for Lyn in the modulation of SCF-induced chemotaxis has been suggested (15).
Several studies have been performed to investigate the role of Lyn in FcεRI-induced signaling and functional responses in mast cells. Old studies had initially proposed Lyn as a positive modulator of the ITAM-Syk signaling cascade down-stream to FcεRIβ and γ chain, similar to the FcγR in macrophages (3). However, more recent studies, mainly performed by utilizing lyn−/− BMD mast cells, generally demonstrate that Lyn-deficiency induces enhanced FcεRI mediated responses such as degranulation, cytokine and chemokine production. A partial explanation for these conflicting findings has been suggested by Xiao and co-workers, who propose that the intensity of the activating signal controls the positive versus negative nature of Lyn in FcεRI-induced responses (94). The authors found that Lyn is required for degranulation, cytokine production and survival in mast cells following “low intensity” stimulation (monomeric IgE, IgG plus anti-IgE Abs or IgE plus low antigen) but that during “high intensity” stimulation (IgE plus high antigen) of FcεRI, lyn−/− cells show an exaggerated response (94). Downstream signaling molecules, such as Akt and p38, are positively or negatively regulated by Lyn during the “low” versus “high intensity” stimulation, respectively. This dual function of Lyn may be mediated by its association/dissociation with FcεRIβ-ITAM chain. Low-intensity stimulation of the FcεRI leads to dissociation of Lyn from the FcεRIβ chain and phosphorylation of the canonical tyrosine residue of the FcεRIβ–ITAM, while high-intensity stimulation leads to an increased association of Lyn with the FcεRIβ–IITA and phosphorylation of non-canonical tyrosine residues (21, 94). The latter event leads to the recruitment and activation of SHIP-1 and SHP-1 and consequent down-modulation of FcεRI-induced responses. Interestingly, Hck may function as a crucial positive regulator of mast cell-induced activation by high-intensity FcεRI stimulation by phosphorylating the canonical tyrosine residue in FcεRIβ-ITAM and suppressing Lyn kinase activity (21).
The SFK Fyn is also an important positive mediator of FcεRI-induced responses, in pathways independent of Lyn, involving activation of the Gab2/PI-3K complex leading to PIP3 production (95). By activating this pathway, Fyn crucially controls degranulation, as revealed by the complete block of this response in FcεR-stimulated BMD fyn−/− mast cells. On the other hand, other more Lyn-dependent functions, such as Ca2+ flux are unaffected by Fyn-deficiency. The fact that Fyn kinase activity, as well as PI-3K and PIP3 production, are enhanced in lyn−/− mast cells may explain their hyper-degranulation phenotype (95). In addition to increased signaling through the Fyn/Gab2/PI-3K pathway, Lyn-deficient mast cells also manifest reduced SHIP-1 phosphorylation. This may be due, in part, to an imbalance of expression of activating versus inhibitory FcRs in lyn−/− mast cells – Lyn-deficient BMD mast cells express lower levels of the inhibitory FcγRIIb and hence have reduced recruitment of SHIP-1 to the membrane than do WT cells (96). This could lead to the prolonged activation of the ERK1/2 pathway observed in FcεRI stimulated lyn−/− mast cells, which would contribute to increased TNFα and IL-2 release by these cells (97). Finally, Lyn-dependent phosphorylation of the inhibitory receptor PIR-B, which recruits SHP-1, has also been proposed as one of the mechanisms able to antagonize FcεRI-dependent signals (98). Overall these findings, suggest that the positive role of Lyn in the modulation of FcεRI-stimulated responses is probably non-crucial, due to the redundant positive role of Hck and Fyn in these functions. On the other hand, similar to B cells, macrophages and neutrophils, Lyn is playing a unique negative role in the modulation of FcεRI-induced responses.
The concept that Lyn is a negative modulator of mast-cell functions is further evidenced by the hyper-responsiveness of these cells during anaphylactic or allergic responses in vivo in lyn−/− mice (89, 99). Not only are mast cell-mediated inflammatory responses enhanced in Lyn-deficient mice, but the animals also spontaneously develop allergic-associated traits such as increased IgE serum levels, mast-cell expansion, increased circulating histamine and eosinophilia (99).
Role of Lyn in eosinophils
The role of Lyn in eosinophils has been less investigated than in other cells types, with most studies focused on IL-5 signaling.
IL-5 is the main cytokine regulating eosinophil differentiation, proliferation, survival and activation (100). Surprisingly, despite the fact that Lyn exerts mainly a negative role in the modulation of other myeloid cell-type responses to their specific growth factors, Lyn functions as a positive regulator of IL-5-induced responses in eosinophils (Table 1). IL-5R has two subunits, the ligand-specific α subunit and the β subunit which is in common with the IL-3R and GM-CSFR (101). Lyn associates with IL-5Rα subunit under basal conditions and phosphorylates both the IL-5Rα and β subunits in vitro (102). Lyn-deficiency, or the suppression of its activity with antisense oligodeoxynucleotides or through use of a specific peptide inhibitor, blocks eosinophil differentiation from BM stem cells (102, 103). Also, the number of IL-5-responsive eosinophil progenitors is strongly reduced in the BM of lyn−/− mice (89). Despite these observations, however, Lyn-deficient animals have significantly increased number of peritoneal eosinophils and develop progressive eosinophilia following asthma challenge, although this phenomenon might be secondary to altered mast cell activation and not a direct effect of IL-5 hyper-responsiveness (89, 99). This issue needs to be further investigated.
The role of Lyn in the regulation of eosinophil apoptosis is also unclear. Indeed, Lyn has been reported to play a positive role in IL-5/IL-3/GM-CSF-induced survival (102, 104), but also in Fas Receptor-mediated cell death (105).
Conclusion and future directions
Lyn is a SFK playing a fundamental role in the modulation of immune cell responses. In contrast to the majority of the different SFK members Lyn seems to exert a unique (i.e non-redundant) role in the modulation of effector functions in many different immune cell types. The profound effects that Lyn is exerting in the immune system are clearly proved by the dramatic phenotype induced by the genetic deletion of this kinase in mice which causes loss of inhibitory signaling in myeloid cells and B cells, ultimately resulting in myeloproliferation and autoimmunity (9, 16, 22). Whereas, the mechanisms through which Lyn is regulating B cell-responses have been well characterized (9, 106), the mechanisms involved in the modulation of myeloid cell-functions by Lyn are less clear. Indeed, as discussed throughout this review, many studies have investigated the role of Lyn in the modulation of multiple signaling pathways and effector responses in different subtypes of myeloid cells, but many conflicting observations on the possible positive and/or negative role of Lyn have been reported. Lyn can switch from being a positive to a negative modifier downstream to the same agonist in different cell types (i.e Lyn is a negative regulator of GM-CSF-induced proliferation in macrophages, but a positive modulator of GM-CSF-induced survival in neutrophils). Furthermore, in the same cell type, Lyn can not only positively and negatively modulate the responses to different stimulatory conditions, but also responses to the same agonists (i.e FcεR-induced responses in mast cells). What are the mechanisms regulating this switch? Is this dual action of Lyn regulated by the intrinsic activity of this kinase or due to complex interaction with other SFKs? Clearly, understanding how one kinase can have such dual functions is an area of active future research. In this context it is interesting that more recently a duplicitous nature of other SFKs, such as Fgr and Hck, have also been reported (82).
A key to finding these answers must involve an understanding of the real physiological implications of Lyn’s dual function. Too many separate observations in vitro have indeed been performed, resulting in a compartmentalization of a positive or a negative role of Lyn according to the cell type/function analyzed. But in the end, it is the in vivo picture for the overall function of the kinase that really matters. So this is where the real investigations should take place. For instance, in vitro Lyn plays both an inhibitory role in integrin-induced adhesion and activation but a positive role in some chemokine/chemoattractant-induced function. Yet how hyper-adhesion plus reduced attractant signaling adds up to altered in vivo migration in lyn−/− mice to different pathogenic or inflammatory stimuli remains to be determined. Similarly, how the dual functions of the kinase in multiple cell types affects host immune responses is also unclear – does the functional roles of Lyn in mast cells outweigh the inhibitory signaling in neutrophils? In this regard, we have recently found that the autoimmunity in lyn−/− mice results from the compound effect of this mutation on both B-cell signaling thresholds and loss of myeloid cell regulation, resulting in reciprocal cytokine production that establishes an amplification loop leading to immune complex formation and glomerulonephritis (P.S. and C.L., unpublished observations). Obviously, sorting out the interactive nature of Lyn mediated signaling in different cells types can only be done using conditional lineage-specific Lyn mutant mice.
Why is it important to address these compounding (and frankly confusing) functions of Lyn kinase in different immune cells types? Because we are giving patients Lyn kinase inhibitors to treat myeloid leukemia - with surprising success despite the apparent inhibitory role of the kinase in myelopoiesis. Perhaps we are missing the real therapeutic indication for these drugs? Would inhibition of Lyn help prime neutrophils/macrophages for host-defense in acute/chronic infection (for example in bacterial infection or AIDS)? How about using Lyn inhibitors to activate anti-tumor immunity by enhancing or modifying DC and/or macrophage antigen presentation/function? Although we have focused this review almost completely on myeloid cell functions for Lyn, these same issues arise when examining Lyn in other, non-hematopoietic cells. There is a growing body of literature suggesting an important growth regulatory function for Lyn in prostate cancer cells, for example (107). At this point, it is hard to make these therapeutic leaps without a more complete understanding of this important tyrosine kinase.
References
- 1.Korade-Mirnics Z, Corey SJ. Src kinase-mediated signaling in leukocytes. J Leukoc Biol. 2000;68:603–613. [PubMed] [Google Scholar]
- 2.Latour S, Veillette A. Proximal protein tyrosine kinases in immunoreceptor signaling. Curr Opin Immunol. 2001;13:299–306. doi: 10.1016/s0952-7915(00)00219-3. [DOI] [PubMed] [Google Scholar]
- 3.Lowell CA. Src-family kinases: rheostats of immune cell signaling. Mol Immunol. 2004;41:631–643. doi: 10.1016/j.molimm.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 4.Berton G, Mocsai A, Lowell CA. Src and Syk kinases: key regulators of phagocytic cell activation. Trends Immunol. 2005;26:208–214. doi: 10.1016/j.it.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Abram CL, Lowell CA. Convergence of immunoreceptor and integrin signaling. Immunol Rev. 2007;218:29–44. doi: 10.1111/j.1600-065X.2007.00531.x. [DOI] [PubMed] [Google Scholar]
- 6.Baruzzi A, Caveggion E, Berton G. Regulation of phagocyte migration and recruitment by Src-family kinases. Cell Mol Life Sci. 2008;65:2175–2190. doi: 10.1007/s00018-008-8005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abram CL, Lowell CA. The diverse functions of Src family kinases in macrophages. Front Biosci. 2008;13:4426–4450. doi: 10.2741/3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abram CL, Lowell CA. The expanding role for ITAM-based signaling pathways in immune cells. Sci STKE. 2007;2007:re2. doi: 10.1126/stke.3772007re2. [DOI] [PubMed] [Google Scholar]
- 9.Xu Y, Harder KW, Huntington ND, Hibbs ML, Tarlinton DM. Lyn tyrosine kinase: accentuating the positive and the negative. Immunity. 2005;22:9–18. doi: 10.1016/j.immuni.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Chan VW, Meng F, Soriano P, DeFranco AL, Lowell CA. Characterization of the B lymphocyte populations in Lyn-deficient mice and the role of Lyn in signal initiation and down-regulation. Immunity. 1997;7:69–81. doi: 10.1016/s1074-7613(00)80511-7. [DOI] [PubMed] [Google Scholar]
- 11.Hibbs ML, Tarlinton DM, Armes J, Grail D, Hodgson G, Maglitto R, Stacker SA, et al. Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell. 1995;83:301–311. doi: 10.1016/0092-8674(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 12.Nishizumi H, Taniuchi I, Yamanashi Y, Kitamura D, Ilic D, Mori S, Watanabe T, et al. Impaired proliferation of peripheral B cells and indication of autoimmune disease in lyn-deficient mice. Immunity. 1995;3:549–560. doi: 10.1016/1074-7613(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 13.Yu CC, Yen TS, Lowell CA, DeFranco AL. Lupus-like kidney disease in mice deficient in the Src family tyrosine kinases Lyn and Fyn. Curr Biol. 2001;11:34–38. doi: 10.1016/s0960-9822(00)00024-5. [DOI] [PubMed] [Google Scholar]
- 14.DeFranco AL, Chan VW, Lowell CA. Positive and negative roles of the tyrosine kinase Lyn in B cell function. Semin Immunol. 1998;10:299–307. doi: 10.1006/smim.1998.0122. [DOI] [PubMed] [Google Scholar]
- 15.O’Laughlin-Bunner B, Radosevic N, Taylor ML, Shivakrupa, DeBerry C, Metcalfe DD, Zhou M, et al. Lyn is required for normal stem cell factor-induced proliferation and chemotaxis of primary hematopoietic cells. Blood. 2001;98:343–350. doi: 10.1182/blood.v98.2.343. [DOI] [PubMed] [Google Scholar]
- 16.Harder KW, Quilici C, Naik E, Inglese M, Kountouri N, Turner A, Zlatic K, et al. Perturbed myelo/erythropoiesis in Lyn-deficient mice is similar to that in mice lacking the inhibitory phosphatases SHP-1 and SHIP-1. Blood. 2004;104:3901–3910. doi: 10.1182/blood-2003-12-4396. [DOI] [PubMed] [Google Scholar]
- 17.Karur VG, Lowell CA, Besmer P, Agosti V, Wojchowski DM. Lyn kinase promotes erythroblast expansion and late-stage development. Blood. 2006;108:1524–1532. doi: 10.1182/blood-2005-09-008243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yin H, Liu J, Li Z, Berndt MC, Lowell CA, Du X. Src family tyrosine kinase Lyn mediates VWF/GPIb-IX-induced platelet activation via the cGMP signaling pathway. Blood. 2008;112:1139–1146. doi: 10.1182/blood-2008-02-140970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maxwell MJ, Yuan Y, Anderson KE, Hibbs ML, Salem HH, Jackson SP. SHIP1 and Lyn Kinase Negatively Regulate Integrin alpha IIb beta 3 signaling in platelets. J Biol Chem. 2004;279:32196–32204. doi: 10.1074/jbc.M400746200. [DOI] [PubMed] [Google Scholar]
- 20.Wei S, Liu JH, Epling-Burnette PK, Gamero AM, Ussery D, Pearson EW, Elkabani ME, et al. Critical role of Lyn kinase in inhibition of neutrophil apoptosis by granulocyte-macrophage colony-stimulating factor. J Immunol. 1996;157:5155–5162. [PubMed] [Google Scholar]
- 21.Hong H, Kitaura J, Xiao W, Horejsi V, Ra C, Lowell CA, Kawakami Y, et al. The Src family kinase Hck regulates mast cell activation by suppressing an inhibitory Src family kinase Lyn. Blood. 2007;110:2511–2519. doi: 10.1182/blood-2007-01-066092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harder KW, Parsons LM, Armes J, Evans N, Kountouri N, Clark R, Quilici C, et al. Gain- and loss-of-function Lyn mutant mice define a critical inhibitory role for Lyn in the myeloid lineage. Immunity. 2001;15:603–615. doi: 10.1016/s1074-7613(01)00208-4. [DOI] [PubMed] [Google Scholar]
- 23.Beguin Y, Fillet G, Bury J, Fairon Y. Ferrokinetic study of splenic erythropoiesis: relationships among clinical diagnosis, myelofibrosis, splenomegaly, and extramedullary erythropoiesis. Am J Hematol. 1989;32:123–128. doi: 10.1002/ajh.2830320209. [DOI] [PubMed] [Google Scholar]
- 24.Hellebostad M, Ostbye KM, Halvorsen S. Leukaemia and anaemia in AKR/O mice. II. Role of the spleen as an erythropoietic organ during leukaemia development. Apmis. 1992;100:181–187. [PubMed] [Google Scholar]
- 25.Xiao W, Hong H, Kawakami Y, Lowell CA, Kawakami T. Regulation of myeloproliferation and M2 macrophage programming in mice by Lyn/Hck, SHIP, and Stat5. J Clin Invest. 2008;118:924–934. doi: 10.1172/JCI34013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mermel CH, McLemore ML, Liu F, Pereira S, Woloszynek J, Lowell CA, Link DC. Src family kinases are important negative regulators of G-CSF-dependent granulopoiesis. Blood. 2006;108:2562–2568. doi: 10.1182/blood-2006-05-024307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalesnikoff J, Sly LM, Hughes MR, Buchse T, Rauh MJ, Cao LP, Lam V, et al. The role of SHIP in cytokine-induced signaling. Rev Physiol Biochem Pharmacol. 2003;149:87–103. doi: 10.1007/s10254-003-0016-y. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J, Somani AK, Siminovitch KA. Roles of the SHP-1 tyrosine phosphatase in the negative regulation of cell signalling. Semin Immunol. 2000;12:361–378. doi: 10.1006/smim.2000.0223. [DOI] [PubMed] [Google Scholar]
- 29.Liu K, Li QZ, Yu Y, Liang C, Subramanian S, Zeng Z, Wang HW, et al. Sle3 and Sle5 can independently couple with Sle1 to mediate severe lupus nephritis. Genes Immun. 2007;8:634–645. doi: 10.1038/sj.gene.6364426. [DOI] [PubMed] [Google Scholar]
- 30.Yu CC, Tsui HW, Ngan BY, Shulman MJ, Wu GE, Tsui FW. B and T cells are not required for the viable motheaten phenotype. J Exp Med. 1996;183:371–380. doi: 10.1084/jem.183.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silver KL, Crockford TL, Bouriez-Jones T, Milling S, Lambe T, Cornall RJ. MyD88-dependent autoimmune disease in Lyn-deficient mice. Eur J Immunol. 2007;37:2734–2743. doi: 10.1002/eji.200737293. [DOI] [PubMed] [Google Scholar]
- 32.Croker BA, Lawson BR, Berger M, Eidenschenk C, Blasius AL, Moresco EM, Sovath S, et al. Inflammation and autoimmunity caused by a SHP1 mutation depend on IL-1, MyD88, and a microbial trigger. Proc Natl Acad Sci U S A. 2008;105:15028–15033. doi: 10.1073/pnas.0806619105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whyburn LR, Halcomb KE, Contreras CM, Lowell CA, Witte ON, Satterthwaite AB. Reduced dosage of Bruton’s tyrosine kinase uncouples B cell hyperresponsiveness from autoimmunity in lyn−/− mice. J Immunol. 2003;171:1850–1858. doi: 10.4049/jimmunol.171.4.1850. [DOI] [PubMed] [Google Scholar]
- 34.Ujike A, Takeda K, Nakamura A, Ebihara S, Akiyama K, Takai T. Impaired dendritic cell maturation and increased T(H)2 responses in PIR-B(−/−) mice. Nat Immunol. 2002;3:542–548. doi: 10.1038/ni801. [DOI] [PubMed] [Google Scholar]
- 35.Yamao T, Noguchi T, Takeuchi O, Nishiyama U, Morita H, Hagiwara T, Akahori H, et al. Negative regulation of platelet clearance and of the macrophage phagocytic response by the transmembrane glycoprotein SHPS-1. J Biol Chem. 2002;277:39833–39839. doi: 10.1074/jbc.M203287200. [DOI] [PubMed] [Google Scholar]
- 36.Lee F, Fandi A, Voi M. Overcoming kinase resistance in chronic myeloid leukemia. Int J Biochem Cell Biol. 2008;40:334–343. doi: 10.1016/j.biocel.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 37.Wu J, Meng F, Kong LY, Peng Z, Ying Y, Bornmann WG, Darnay BG, et al. Association between imatinib-resistant BCR-ABL mutation-negative leukemia and persistent activation of LYN kinase. J Natl Cancer Inst. 2008;100:926–939. doi: 10.1093/jnci/djn188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu J, Meng F, Lu H, Kong L, Bornmann W, Peng Z, Talpaz M, et al. Lyn regulates BCR-ABL and Gab2 tyrosine phosphorylation and c-Cbl protein stability in imatinib-resistant chronic myelogenous leukemia cells. Blood. 2008;111:3821–3829. doi: 10.1182/blood-2007-08-109330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nam S, Williams A, Vultur A, List A, Bhalla K, Smith D, Lee FY, et al. Dasatinib (BMS-354825) inhibits Stat5 signaling associated with apoptosis in chronic myelogenous leukemia cells. Mol Cancer Ther. 2007;6:1400–1405. doi: 10.1158/1535-7163.MCT-06-0446. [DOI] [PubMed] [Google Scholar]
- 40.Dos Santos C, Demur C, Bardet V, Prade-Houdellier N, Payrastre B, Recher C. A critical role for Lyn in acute myeloid leukemia. Blood. 2008;111:2269–2279. doi: 10.1182/blood-2007-04-082099. [DOI] [PubMed] [Google Scholar]
- 41.Okamoto M, Hayakawa F, Miyata Y, Watamoto K, Emi N, Abe A, Kiyoi H, et al. Lyn is an important component of the signal transduction pathway specific to FLT3/ITD and can be a therapeutic target in the treatment of AML with FLT3/ITD. Leukemia. 2007;21:403–410. doi: 10.1038/sj.leu.2404547. [DOI] [PubMed] [Google Scholar]
- 42.Barclay AN, Brown MH. The SIRP family of receptors and immune regulation. Nat Rev Immunol. 2006;6:457–464. doi: 10.1038/nri1859. [DOI] [PubMed] [Google Scholar]
- 43.Cant CA, Ullrich A. Signal regulation by family conspiracy. Cell Mol Life Sci. 2001;58:117–124. doi: 10.1007/PL00000771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takai T, Ono M. Activating and inhibitory nature of the murine paired immunoglobulin-like receptor family. Immunol Rev. 2001;181:215–222. doi: 10.1034/j.1600-065x.2001.1810118.x. [DOI] [PubMed] [Google Scholar]
- 45.Suzu S, Tanaka-Douzono M, Nomaguchi K, Yamada M, Hayasawa H, Kimura F, Motoyoshi K. p56(dok-2) as a cytokine-inducible inhibitor of cell proliferation and signal transduction. Embo J. 2000;19:5114–5122. doi: 10.1093/emboj/19.19.5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berg KL, Siminovitch KA, Stanley ER. SHP-1 regulation of p62(DOK) tyrosine phosphorylation in macrophages. J Biol Chem. 1999;274:35855–35865. doi: 10.1074/jbc.274.50.35855. [DOI] [PubMed] [Google Scholar]
- 47.Hibbs ML, Harder KW. The duplicitous nature of the Lyn tyrosine kinase in growth factor signaling. Growth Factors. 2006;24:137–149. doi: 10.1080/08977190600581327. [DOI] [PubMed] [Google Scholar]
- 48.Baran CP, Tridandapani S, Helgason CD, Humphries RK, Krystal G, Marsh CB. The inositol 5′-phosphatase SHIP-1 and the Src kinase Lyn negatively regulate macrophage colony-stimulating factor-induced Akt activity. J Biol Chem. 2003;278:38628–38636. doi: 10.1074/jbc.M305021200. [DOI] [PubMed] [Google Scholar]
- 49.Pereira S, Lowell C. The Lyn tyrosine kinase negatively regulates neutrophil integrin signaling. J Immunol. 2003;171:1319–1327. doi: 10.4049/jimmunol.171.3.1319. [DOI] [PubMed] [Google Scholar]
- 50.Roach TI, Slater SE, White LS, Zhang X, Majerus PW, Brown EJ, Thomas ML. The protein tyrosine phosphatase SHP-1 regulates integrin-mediated adhesion of macrophages. Curr Biol. 1998;8:1035–1038. doi: 10.1016/s0960-9822(07)00426-5. [DOI] [PubMed] [Google Scholar]
- 51.Malik M, Chen YY, Kienzle MF, Tomkowicz BE, Collman RG, Ptasznik A. Monocyte migration and LFA-1-mediated attachment to brain microvascular endothelia is regulated by SDF-1alpha through Lyn kinase. J Immunol. 2008;181:4632–4637. doi: 10.4049/jimmunol.181.7.4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suen PW, Ilic D, Caveggion E, Berton G, Damsky CH, Lowell CA. Impaired integrin-mediated signal transduction, altered cytoskeletal structure and reduced motility in Hck/Fgr deficient macrophages. J Cell Sci. 1999;112 (Pt 22):4067–4078. doi: 10.1242/jcs.112.22.4067. [DOI] [PubMed] [Google Scholar]
- 53.Nikolic DM, Cholewa J, Gass C, Gong MC, Post SR. Class A scavenger receptor-mediated cell adhesion requires the sequential activation of Lyn and PI3-kinase. Am J Physiol Cell Physiol. 2007;292:C1450–1458. doi: 10.1152/ajpcell.00401.2006. [DOI] [PubMed] [Google Scholar]
- 54.Tomkowicz B, Lee C, Ravyn V, Cheung R, Ptasznik A, Collman RG. The Src kinase Lyn is required for CCR5 signaling in response to MIP-1beta and R5 HIV-1 gp120 in human macrophages. Blood. 2006;108:1145–1150. doi: 10.1182/blood-2005-12-012815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakata Y, Tomkowicz B, Gewirtz AM, Ptasznik A. Integrin inhibition through Lyn-dependent cross talk from CXCR4 chemokine receptors in normal human CD34+ marrow cells. Blood. 2006;107:4234–4239. doi: 10.1182/blood-2005-08-3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meng F, Lowell CA. A beta 1 integrin signaling pathway involving Src-family kinases, Cbl and PI-3 kinase is required for macrophage spreading and migration. EMBO J. 1998;17:4391–4403. doi: 10.1093/emboj/17.15.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Strzelecka-Kiliszek A, Kwiatkowska K, Sobota A. Lyn and Syk kinases are sequentially engaged in phagocytosis mediated by Fc gamma R. J Immunol. 2002;169:6787–6794. doi: 10.4049/jimmunol.169.12.6787. [DOI] [PubMed] [Google Scholar]
- 58.Fitzer-Attas CJ, Lowry M, Crowley MT, Finn AJ, Meng F, DeFranco AL, Lowell CA. Fcγ receptor-mediated phagocytosis in macrophages lacking the Src family tyrosine kinases Hck, Fgr, and Lyn. J Exp Med. 2000;191:669–682. doi: 10.1084/jem.191.4.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cohen PL, Caricchio R, Abraham V, Camenisch TD, Jennette JC, Roubey RA, Earp HS, et al. Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. J Exp Med. 2002;196:135–140. doi: 10.1084/jem.20012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lemke G, Rothlin CV. Immunobiology of the TAM receptors. Nat Rev Immunol. 2008;8:327–336. doi: 10.1038/nri2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meng F, Lowell CA. Lipopolysaccharide (LPS)-induced macrophage activation and signal transduction in the absence of Src-family kinases Hck, Fgr, and Lyn. J Exp Med. 1997;185:1661–1670. doi: 10.1084/jem.185.9.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hamerman JA, Jarjoura JR, Humphrey MB, Nakamura MC, Seaman WE, Lanier LL. Cutting edge: inhibition of TLR and FcR responses in macrophages by triggering receptor expressed on myeloid cells (TREM)-2 and DAP12. J Immunol. 2006;177:2051–2055. doi: 10.4049/jimmunol.177.4.2051. [DOI] [PubMed] [Google Scholar]
- 63.Hamerman JA, Lanier LL. Inhibition of immune responses by ITAM-bearing receptors. Sci STKE. 2006;2006:re1. doi: 10.1126/stke.3202006re1. [DOI] [PubMed] [Google Scholar]
- 64.Smolinska MJ, Horwood NJ, Page TH, Smallie T, Foxwell BM. Chemical inhibition of Src family kinases affects major LPS-activated pathways in primary human macrophages. Mol Immunol. 2008;45:990–1000. doi: 10.1016/j.molimm.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 65.van de Geijn GJ, Aarts LH, Erkeland SJ, Prasher JM, Touw IP. Granulocyte colony-stimulating factor and its receptor in normal hematopoietic cell development and myeloid disease. Rev Physiol Biochem Pharmacol. 2003;149:53–71. doi: 10.1007/s10254-003-0014-0. [DOI] [PubMed] [Google Scholar]
- 66.Sampson M, Zhu QS, Corey SJ. Src kinases in G-CSF receptor signaling. Front Biosci. 2007;12:1463–1474. doi: 10.2741/2160. [DOI] [PubMed] [Google Scholar]
- 67.Corey SJ, Burkhardt AL, Bolen JB, Geahlen RL, Tkatch LS, Tweardy DJ. Granulocyte colony-stimulating factor receptor signaling involves the formation of a three-component complex with Lyn and Syk protein-tyrosine kinases. Proc Natl Acad Sci U S A. 1994;91:4683–4687. doi: 10.1073/pnas.91.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu QS, Robinson LJ, Roginskaya V, Corey SJ. G-CSF-induced tyrosine phosphorylation of Gab2 is Lyn kinase dependent and associated with enhanced Akt and differentiative, not proliferative, responses. Blood. 2004;103:3305–3312. doi: 10.1182/blood-2003-06-1861. [DOI] [PubMed] [Google Scholar]
- 69.Zhu QS, Xia L, Mills GB, Lowell CA, Touw IP, Corey SJ. G-CSF induced reactive oxygen species involves Lyn-PI3-kinase-Akt and contributes to myeloid cell growth. Blood. 2006;107:1847–1856. doi: 10.1182/blood-2005-04-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Luo HR, Loison F. Constitutive neutrophil apoptosis: mechanisms and regulation. Am J Hematol. 2008;83:288–295. doi: 10.1002/ajh.21078. [DOI] [PubMed] [Google Scholar]
- 71.Simon HU. Neutrophil apoptosis pathways and their modifications in inflammation. Immunol Rev. 2003;193:101–110. doi: 10.1034/j.1600-065x.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- 72.Daigle I, Yousefi S, Colonna M, Green DR, Simon HU. Death receptors bind SHP-1 and block cytokine-induced anti-apoptotic signaling in neutrophils. Nat Med. 2002;8:61–67. doi: 10.1038/nm0102-61. [DOI] [PubMed] [Google Scholar]
- 73.Jia SH, Parodo J, Kapus A, Rotstein OD, Marshall JC. Dynamic regulation of neutrophil survival through tyrosine phosphorylation or dephosphorylation of caspase-8. J Biol Chem. 2008;283:5402–5413. doi: 10.1074/jbc.M706462200. [DOI] [PubMed] [Google Scholar]
- 74.Gardai S, Whitlock BB, Helgason C, Ambruso D, Fadok V, Bratton D, Henson PM. Activation of SHIP by NADPH oxidase-stimulated Lyn leads to enhanced apoptosis in neutrophils. J Biol Chem. 2002;277:5236–5246. doi: 10.1074/jbc.M110005200. [DOI] [PubMed] [Google Scholar]
- 75.Lowell CA, Fumagalli L, Berton G. Deficiency of Src family kinases p59/61hck and p58c-fgr results in defective adhesion-dependent neutrophil functions. J Cell Biol. 1996;133:895–910. doi: 10.1083/jcb.133.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mocsai A, Ligeti E, Lowell CA, Berton G. Adhesion-dependent degranulation of neutrophils requires the Src family kinases Fgr and Hck. J Immunol. 1999;162:1120–1126. [PubMed] [Google Scholar]
- 77.Mocsai A, Zhou M, Meng F, Tybulewicz VL, Lowell CA. Syk is required for integrin signaling in neutrophils. Immunity. 2002;16:547–558. doi: 10.1016/s1074-7613(02)00303-5. [DOI] [PubMed] [Google Scholar]
- 78.Yan SR, Novak MJ. Src-family kinase-p53/Lyn p56 plays an important role in TNF-alpha-stimulated production of O2- by human neutrophils adherent to fibrinogen. Inflammation. 1999;23:167–178. doi: 10.1023/a:1020245129632. [DOI] [PubMed] [Google Scholar]
- 79.Ptasznik A, Prossnitz ER, Yoshikawa D, Smrcka A, Traynor-Kaplan AE, Bokoch GM. A tyrosine kinase signaling pathway accounts for the majority of phosphatidylinositol 3,4,5-trisphosphate formation in chemoattractant-stimulated human neutrophils. J Biol Chem. 1996;271:25204–25207. doi: 10.1074/jbc.271.41.25204. [DOI] [PubMed] [Google Scholar]
- 80.Ptasznik A, Traynor-Kaplan A, Bokoch GM. G protein-coupled chemoattractant receptors regulate Lyn tyrosine kinase. Shc adapter protein signaling complexes. J Biol Chem. 1995;270:19969–19973. doi: 10.1074/jbc.270.34.19969. [DOI] [PubMed] [Google Scholar]
- 81.Hirahashi J, Mekala D, Van Ziffle J, Xiao L, Saffaripour S, Wagner DD, Shapiro SD, et al. Mac-1 signaling via Src-family and Syk kinases results in elastase-dependent thrombohemorrhagic vasculopathy. Immunity. 2006;25:271–283. doi: 10.1016/j.immuni.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 82.Zhang H, Meng F, Chu CL, Takai T, Lowell CA. The Src family kinases Hck and Fgr negatively regulate neutrophil and dendritic cell chemokine signaling via PIR-B. Immunity. 2005;22:235–246. doi: 10.1016/j.immuni.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 83.Fumagalli L, Zhang H, Baruzzi A, Lowell CA, Berton G. The Src family kinases Hck and Fgr regulate neutrophil responses to N-formyl-methionyl-leucyl-phenylalanine. J Immunol. 2007;178:3874–3885. doi: 10.4049/jimmunol.178.6.3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Napolitani G, Bortoletto N, Racioppi L, Lanzavecchia A, D’Oro U. Activation of src-family tyrosine kinases by LPS regulates cytokine production in dendritic cells by controlling AP-1 formation. Eur J Immunol. 2003;33:2832–2841. doi: 10.1002/eji.200324073. [DOI] [PubMed] [Google Scholar]
- 85.Galgani M, De Rosa V, De Simone S, Leonardi A, D’Oro U, Napolitani G, Masci AM, et al. Cyclic AMP modulates the functional plasticity of immature dendritic cells by inhibiting Src-like kinases through protein kinase A-mediated signaling. J Biol Chem. 2004;279:32507–32514. doi: 10.1074/jbc.M403355200. [DOI] [PubMed] [Google Scholar]
- 86.Johnsen IB, Nguyen TT, Ringdal M, Tryggestad AM, Bakke O, Lien E, Espevik T, et al. Toll-like receptor 3 associates with c-Src tyrosine kinase on endosomes to initiate antiviral signaling. Embo J. 2006;25:3335–3346. doi: 10.1038/sj.emboj.7601222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sanjuan MA, Rao N, Lai KT, Gu Y, Sun S, Fuchs A, Fung-Leung WP, et al. CpG-induced tyrosine phosphorylation occurs via a TLR9-independent mechanism and is required for cytokine secretion. J Cell Biol. 2006;172:1057–1068. doi: 10.1083/jcb.200508058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chu CL, Lowell CA. The Lyn tyrosine kinase differentially regulates dendritic cell generation and maturation. J Immunol. 2005;175:2880–2889. doi: 10.4049/jimmunol.175.5.2880. [DOI] [PubMed] [Google Scholar]
- 89.Beavitt SJ, Harder KW, Kemp JM, Jones J, Quilici C, Casagranda F, Lam E, et al. Lyn-deficient mice develop severe, persistent asthma: Lyn is a critical negative regulator of Th2 immunity. J Immunol. 2005;175:1867–1875. doi: 10.4049/jimmunol.175.3.1867. [DOI] [PubMed] [Google Scholar]
- 90.Du C, Sriram S. Increased severity of experimental allergic encephalomyelitis in lyn−/− mice in the absence of elevated proinflammatory cytokine response in the central nervous system. J Immunol. 2002;168:3105–3112. doi: 10.4049/jimmunol.168.6.3105. [DOI] [PubMed] [Google Scholar]
- 91.Rivera J, Olivera A. A current understanding of Fc epsilon RI-dependent mast cell activation. Curr Allergy Asthma Rep. 2008;8:14–20. doi: 10.1007/s11882-008-0004-z. [DOI] [PubMed] [Google Scholar]
- 92.Furumoto Y, Gomez G, Gonzalez-Espinosa C, Kovarova M, Odom S, Ryan JJ, Rivera J. The role of Src family kinases in mast cell effector function. Novartis Found Symp. 2005;271:39–47. discussion 47–53, 95–39. [PubMed] [Google Scholar]
- 93.Hernandez-Hansen V, Mackay GA, Lowell CA, Wilson BS, Oliver JM. The Src kinase Lyn is a negative regulator of mast cell proliferation. J Leukoc Biol. 2004;75:143–151. doi: 10.1189/jlb.0503224. [DOI] [PubMed] [Google Scholar]
- 94.Xiao W, Nishimoto H, Hong H, Kitaura J, Nunomura S, Maeda-Yamamoto M, Kawakami Y, et al. Positive and negative regulation of mast cell activation by Lyn via the FcepsilonRI. J Immunol. 2005;175:6885–6892. doi: 10.4049/jimmunol.175.10.6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Parravicini V, Gadina M, Kovarova M, Odom S, Gonzalez-Espinosa C, Furumoto Y, Saitoh S, et al. Fyn kinase initiates complementary signals required for IgE-dependent mast cell degranulation. Nat Immunol. 2002;3:741–748. doi: 10.1038/ni817. [DOI] [PubMed] [Google Scholar]
- 96.Hernandez-Hansen V, Smith AJ, Surviladze Z, Chigaev A, Mazel T, Kalesnikoff J, Lowell CA, et al. Dysregulated FcεRI signaling and altered Fyn and SHIP activities in Lyn-deficient mast cells. J Immunol. 2004;173:100–112. doi: 10.4049/jimmunol.173.1.100. [DOI] [PubMed] [Google Scholar]
- 97.Hernandez-Hansen V, Bard JD, Tarleton CA, Wilder JA, Lowell CA, Wilson BS, Oliver JM. Increased expression of genes linked to FcεRI Signaling and to cytokine and chemokine production in Lyn-deficient mast cells. J Immunol. 2005;175:7880–7888. doi: 10.4049/jimmunol.175.12.7880. [DOI] [PubMed] [Google Scholar]
- 98.Uehara T, Blery M, Kang DW, Chen CC, Ho LH, Gartland GL, Liu FT, et al. Inhibition of IgE-mediated mast cell activation by the paired Ig-like receptor PIR-B. J Clin Invest. 2001;108:1041–1050. doi: 10.1172/JCI12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Odom S, Gomez G, Kovarova M, Furumoto Y, Ryan JJ, Wright HV, Gonzalez-Espinosa C, et al. Negative regulation of immunoglobulin E-dependent allergic responses by Lyn kinase. J Exp Med. 2004;199:1491–1502. doi: 10.1084/jem.20040382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Takatsu K, Nakajima H. IL-5 and eosinophilia. Curr Opin Immunol. 2008;20:288–294. doi: 10.1016/j.coi.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 101.Takatsu K. Interleukin 5 (IL-5) and its receptor. Microbiol Immunol. 1991;35:593–606. doi: 10.1111/j.1348-0421.1991.tb01591.x. [DOI] [PubMed] [Google Scholar]
- 102.Stafford S, Lowell C, Sur S, Alam R. Lyn tyrosine kinase is important for IL-5-stimulated eosinophil differentiation. J Immunol. 2002;168:1978–1983. doi: 10.4049/jimmunol.168.4.1978. [DOI] [PubMed] [Google Scholar]
- 103.Adachi T, Stafford S, Sur S, Alam R. A novel Lyn-binding peptide inhibitor blocks eosinophil differentiation, survival, and airway eosinophilic inflammation. J Immunol. 1999;163:939–946. [PubMed] [Google Scholar]
- 104.Yousefi S, Hoessli DC, Blaser K, Mills GB, Simon HU. Requirement of Lyn and Syk tyrosine kinases for the prevention of apoptosis by cytokines in human eosinophils. J Exp Med. 1996;183:1407–1414. doi: 10.1084/jem.183.4.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Simon HU, Yousefi S, Dibbert B, Hebestreit H, Weber M, Branch DR, Blaser K, et al. Role for tyrosine phosphorylation and Lyn tyrosine kinase in fas receptor-mediated apoptosis in eosinophils. Blood. 1998;92:547–557. [PubMed] [Google Scholar]
- 106.Chan VW, Lowell CA, DeFranco AL. Defective negative regulation of antigen receptor signaling in Lyn-deficient B lymphocytes. Curr Biol. 1998;8:545–553. doi: 10.1016/s0960-9822(98)70223-4. [DOI] [PubMed] [Google Scholar]
- 107.Park SI, Zhang J, Phillips KA, Araujo JC, Najjar AM, Volgin AY, Gelovani JG, et al. Targeting SRC family kinases inhibits growth and lymph node metastases of prostate cancer in an orthotopic nude mouse model. Cancer Res. 2008;68:3323–3333. doi: 10.1158/0008-5472.CAN-07-2997. [DOI] [PubMed] [Google Scholar]
- 108.Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, Earp HS, et al. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411:207–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]