Abstract
Objective
To determine the effect of cryotherapy on HIV-1 cervical shedding.
Design
Prospective cohort study.
Methods
Five hundred HIV-positive women enrolled at an HIV treatment clinic in Nairobi, Kenya were screened for cervical cancer. Women diagnosed with cervical intraepithelial neoplasia stage 2 or 3 (CIN 2/3) by histology were offered cryotherapy treatment. The first 50 women had cervical swabs taken at baseline and at 2 and 4 weeks following treatment. Swabs were analyzed for HIV-1 RNA and compared using General Estimating Equation (GEE) with binomial or Gaussian links.
Results
Of the 50 women enrolled, 40 were receiving antiretroviral therapy (ART) and 10 were not receiving ART at the time of cryotherapy and during study follow-up. Among all women, the odds of detectable cervical HIV-1 RNA did not increase at 2 weeks [odds ratio (OR) 1.18; 95% confidence interval (CI) 0.65–2.13] or 4 weeks (OR 1.29; 95% CI 0.71–2.33) following cryotherapy. Among 10 women not receiving ART, the OR of detectable shedding at 2 weeks was higher, but not statistically significant (OR 4.02; 95% CI 0.53–30.79; P = 0.2), and at 4 weeks remained unchanged (OR 1.00; 95% CI 0.27–3.74).
Conclusion
There was no increase in detectable cervical HIV-1 RNA among HIV-positive women after cryotherapy. The risk of HIV-1 transmission after cryotherapy may not be significant, particularly among women already on ART at the time of cervical treatment. However, further investigation is needed among women not receiving ART.
Keywords: Africa, antiretroviral therapy, cervical intraepithelial neoplasia, cryotherapy, HIV-1, shedding
Introduction
In resource-limited settings, `see and treat' has been promoted as an economical and effective method to detect and treat cervical intraepithelial neoplasia (CIN) [1]. In such a program, women are examined for cervical neoplasia using visual inspection with acetic acid (VIA) and are treated on the same day with cryotherapy to remove any cervical lesions [2]. This method is inexpensive to administer and decreases loss to follow-up by combining diagnosis and treatment on the same day [3]. As a result, a `see and treat' program can handle a large number of patients and address the immense backlog of women needing cervical cancer screening in resource-limited settings.
Many of the same areas where `see and treat' programs are implemented also have high prevalence of HIV-1 [4], yet the impact of cryotherapy on cervical shedding of HIV-1 among HIV-positive women is unclear. Cervical treatments for CIN 2/3 disease, such as cryotherapy, may inflame the cervix and cause ulcerations and watery discharge that increase HIV-1 shedding [5–7]. High levels of HIV-1 cervical shedding may in turn increase an HIV-1-positive woman's infectivity and risk of HIV-1 transmission to HIV-uninfected sexual partners [8].
In order to understand the effect cryotherapy may have on cervical HIV-1 shedding, we examined HIV-1 RNA cervical levels over 4 weeks among HIV-positive women receiving cryotherapy treatment at an HIV treatment clinic in Kenya.
Methods
Five hundred HIV-1-positive women who were enrolled at the Coptic Hope Center for Infectious Diseases in Nairobi, Kenya were invited to participate in a trial comparing cervical cancer screening methods [9]. Enrollment and clinic procedures for HIV care at the Coptic Hope Center have been described previously [10]. Women were eligible to participate in the cervical screening study if they were at least 18 years of age, had an intact cervix, were HIV-positive, and had never had cervical treatment for cancerous or precancerous lesions. The study protocol was reviewed and approved by the institutional review boards at the University of Washington (Seattle, Washington, USA) and Kenyatta National Hospital (Nairobi, Kenya). After signing an informed consent, all women underwent Papanicolaou (Pap) smear, VIA, human papillomavirus (HPV) testing, and colposcopy-directed biopsy. Blood was also drawn for CD4 cell count and urine pregnancy test was performed.
In a subset analysis, the first 50 women in the trial who were diagnosed with CIN 2/3 by histology and received cryotherapy treatment had cervical swabs and blood samples obtained at baseline before cryotherapy and then at 2 and 4 weeks after the intervention. To obtain the cervical specimens, Dacron swabs (Puritan, Guilford, Maine, USA) were inserted 1 cm into the cervical os, the site of cryotherapy treatment, and rotated one full turn [11]. One swab was kept dry in a 2-ml vial and one swab was placed in a 2-ml vial containing 1 ml of solution (70% RPMI, 20% fetal bovine serum, and 10% dimethyl sulfoxide). Cervical and plasma samples were frozen at −80°C and shipped to Seattle on dry ice for HIV-1 RNA quantitation.
In Seattle, dry swab samples were thawed into 500 μl of guanadinium lysis buffer [4 mol guanadinium thiocyanate/l, 25 mmol sodium citrate/l (pH 7), 0.5% N-lauroylsarcosine, and 0.1 mol 2-mercaptoethanol/l] for 15 min at ambient temperatures and vortexed briefly before HIV-1 RNA was extracted with silica gel to remove inhibitory factors [12]. The swabs placed in RPMI solution remain in storage for future viral analyses. HIV-1 RNA was quantified using an independently validated TaqMan real-time PCR assay as previously described [13]. The lower limit of HIV-1 quantification for plasma was 30 (1.48 log10) HIV-1 RNA copies/ml and for cervical swabs was 210 (2.32 log10) HIV-1 RNA copies/ml of swab fluid. Viral levels that were measured below the lower limit of detection were assigned a value at the midpoint between the lower limit of detection and zero. CD4 cell counts were determined using flow cytometry (FACScan; Becton Dickinson, Franklin Lakes, New Jersey, USA).
Viral levels were compared at different time points using General Estimating Equations (GEE) with binomial (for binary outcome) or Gaussian (for continuous outcome) links, exchangeable correlation structures, and robust variance estimates.
Results
Between June and November 2009, 500 women were enrolled in the cervical screening study, 498 completed testing, and 101 received cryotherapy after being diagnosed with CIN 2 (n=40) or 3 (n=61). Of these women, 50 were examined in this subset analysis and had blood and cervical specimens collected at baseline, 2, and 4 weeks after treatment.
The median age of the 50 study participants was 38 years [interquartile range (IQR) 34–45], the median CD4 cell count was 296 cells/μl (IQR 178–403), and 23 women (64%) had only one sexual partner in their lifetime (Table 1). Of the 50 women, 40 were receiving antiretroviral therapy (ART) (median duration, 529 days; IQR 108–1048) at the time of cryotherapy, whereas 10 had never received ART and were not receiving ART at the time of cryotherapy or during the subsequent 4-week follow-up period. Of the women receiving ART, 34 were on a nonnucleoside reverse transcriptase inhibitor (NNRTI)-based regimen of either nevirapine or efavirenz, and six were on a ritonavir-boosted protease inhibitor-based regimen of either lopinavir or saquinavir. None were pregnant.
Table 1.
Baseline clinical and socio-demographic characteristics.
| Characteristics | N | Median or N (IQR or %) |
|---|---|---|
| Age (years) | 50 | 38 (34–45) |
| CD4 cell count (cells/μl) | 50 | 296 (178–403) |
| On ART | 40 | 289 (177–414) |
| Off ART | 10 | 319 (213–375) |
| CD4 cell count (cells/μl) | 50 | |
| 0–199 | 13 (26%) | |
| 201–499 | 29 (58%) | |
| ≥500 | 8 (16%) | |
| Plasma HIV-1 RNA (log10 copies/ml) | 50 | 1.18 (1.18–3.25) |
| On ART | 40 | 1.18 (1.18–1.68) |
| Off ART | 10 | 4.09 (3.74–4.81) |
| Weight (kg) | 50 | 59 (52–69) |
| WHO stage | 49 | |
| I | 15 (31%) | |
| II | 10 (20%) | |
| III | 21 (43%) | |
| IV | 3 (6%) | |
| Marital status | 50 | |
| Married | 22 (44%) | |
| Single | 12 (24%) | |
| Divorced/separated | 12 (24%) | |
| Widowed | 4 (8%) | |
| Education level | ||
| None | 2 (4%) | |
| Primary | 50 | 10 (20%) |
| Secondary | 24 (48%) | |
| College | 14 (28%) | |
| Employment | 50 | |
| Employed | 40 (80%) | |
| Unemployed | 10 (20%) | |
| No. of lifetime sexual partners | ||
| 1 | 36 | 23 (64%) |
| 2 | 8 (22%) | |
| ≥3 | 5 (14%) | |
| On ART | 50 | |
| Yes | 40 (80%) | |
| No | 10 (20%) | |
| Duration on ART (days) | 40 | 529 (108–1048) |
ART, antiretroviral therapy; IQR, interquartile range.
Among all 50 participants, cervical HIV-1 RNA was detected in 18 of 50 (36%) women at baseline, 19 of 48 (40%) at 2 weeks, and 21 of 50 (42%) at 4 weeks after cryotherapy. The odds of detectable cervical shedding did not increase at either 2 weeks [odds ratio (OR) 1.18; 95% confidence interval (CI) 0.65–2.13] or 4 weeks (OR 1.29; 95% CI 0.71–2.33) following treatment. Cervical mean log10 HIV-1 RNA at baseline was 2.64 copies/ml (95% CI 2.39–2.90) and did not differ significantly at 2 weeks (2.58 copies/ml; 95% CI 2.33–2.84; P=0.7) or 4 weeks (2.64 copies/ml; 95% CI 2.38–2.90; P=1.0) following cryotherapy.
Among the 40 women receiving ART, cervical HIV-1 RNA was detected in 11 of 40 (28%) at baseline, 11 of 39 (28%) at 2 weeks, and 14 of 40 (35%) at 4 weeks after cryotherapy. The OR of cervical HIV-1 RNA shedding did not increase at either 2 weeks (OR 1.01; 95% CI 0.46–2.23) or 4 weeks (OR 1.42; 95% CI 0.67–3.02) following treatment. Cervical mean log10 HIV-1 RNA at baseline was 2.43 copies/ml (95% CI 2.20–2.66) and did not differ significantly at 2 weeks (2.30 copies/ml; 95% CI 2.14–2.45; P=0.2) or 4 weeks (2.43 copies/ml; 95% CI 2.21–2.64; P=1.0) following cryotherapy (Fig. 1).
Fig. 1. Mean and 95% confidence interval of log10 cervical HIV-1 RNA viral load by weeks since cryotherapy.
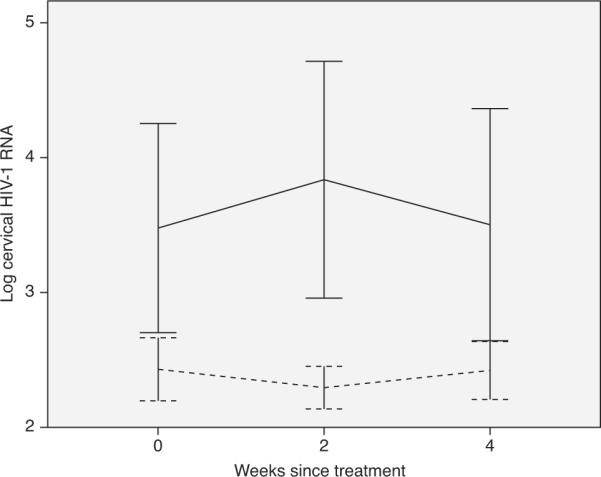
Vertical lines are 95% confidence intervals (CIs) and horizontal lines are interpolation lines between the mean values. Dashed line = on antiretroviral therapy (ART); solid line = off ART.
Among the 10 ART-naive women, cervical HIV-1 RNA was detected in seven of 10 (70%) at baseline, nine of 10 (90%) at 2 weeks, and seven of 10 (70%) at 4 weeks after cryotherapy. The OR of detectable shedding at 2 weeks after treatment was higher than that at baseline, but this was not statistically significant (OR 4.02; 95% CI 0.53–30.79), and at 4 weeks, the OR remained unchanged (OR 1.00; 95% CI 0.27–3.74). Cervical mean log10 HIV-1 RNA at baseline was 3.48 copies/ml (95% CI 2.71–4.25) and did not differ significantly at 2 weeks (3.83 copies/ml; 95% CI 2.95–4.71; P=0.5) or 4 weeks (3.50 copies/ml; 95% CI 2.65–4.36; P=1.0) following cryotherapy (Fig. 1).
Plasma HIV-1 RNA levels were significantly associated with cervical HIV-1 RNA detection. For every 1-log10 increase in plasma HIV-1 RNA viral load, there was a 1.97-fold increased OR of detecting cervical HIV-1 RNA (95% CI 1.40–2.78; P < 0.001).
Discussion
In this study, there was no significant increase in detectable cervical HIV-1 RNA shedding among 50 HIV-positive women who were examined at 2 and 4 weeks following cervical cryotherapy for CIN 2/3. A majority of these women (80%) had been receiving ART at the time of cervical treatment and during study follow-up. Among the small subgroup of 10 women who had not received ART, there was an increase from baseline in detectable cervical viral shedding at 2 weeks following cryotherapy, but this was not statistically significant. A strong association between increased plasma HIV-1 RNA level and detection of cervical HIV-1 RNA was also demonstrated.
In a study by Wright et al. [5], increased cervical shedding of HIV-1 RNA was found among HIV-positive women treated with cryotherapy, loop electrosurgical excision procedure (LEEP), and cold-knife cone biopsy; however, the study was limited to 14 women who were not on ART and no statistical comparisons between time points were presented. Our study examined a comparatively larger number of women and evaluated a specific homogeneous cervical treatment intervention (cryotherapy alone) instead of a number of different interventions (e.g., cryotherapy, LEEP, and biopsy). Most women in our study were also on ART during cryotherapy, reflecting the composition of a typical cervical cancer screening program based at an HIV treatment clinic in Africa [4].
ART has been demonstrated to rapidly decrease genital shedding of HIV-1 [14]. These effects may be due, in part, to a reduction in plasma HIV-1 RNA [15], which was strongly associated with cervical HIV-1 levels in this study. Over two-thirds of women (70%) not receiving ART had detectable cervical HIV-1 RNA compared with only 28% of women who were on ART at the time of cryotherapy. Women on ART in this study also had lower baseline cervical HIV-1 levels compared to those not receiving ART (mean log10 HIV-1 RNA, 2.43 vs. 3.48 copies/ml), and this more than 1 log10 difference in cervical HIV-1 RNA was maintained for 4 weeks after cryotherapy. As such, ART may limit cervical HIV-1 RNA shedding associated with tissue damage and inflammation caused by cryotherapy [16] and thereby decrease the risk of HIV-1 transmission to uninfected sexual partners by more than half [8].
These results suggest that implementation of `see and treat' programs in Africa may be safely scaled up in HIV treatment clinics without significant risk of HIV-1 transmission due to increased cervical HIV-1 shedding after cryotherapy [17]. However, patients should still be cautioned against engaging in unprotected sexual activity in the first 2 weeks after treatment. Among those not taking ART, one strategy could be to prescribe antiretroviral medications around the time of cryotherapy, similar to how these drugs are administered during pregnancy to decrease mother-to-child transmission of HIV-1 [18,19].
A limitation of the study is the relatively small numbers of women tested who were not on ART. There appeared to be a trend for increased shedding at 2 weeks among the 10 women who were not receiving ART, but this was not statistically significant. This may be due to a lack of statistical power given the small numbers in this subgroup. Cervical samples were also not tested separately for HIV-1 DNA, a measurement of HIV-1-infected cells, which could persist in inflamed cervical secretions despite ART [14,20].
In conclusion, no significant increase in HIV-1 RNA cervical shedding was found at 2 and 4 weeks after cryotherapy among HIV-positive women diagnosed with CIN 2/3 disease. The risk of shedding and HIV-1 transmission after cryotherapy may, therefore, not be significant, particularly among women already on ARTat the time of cervical treatment. This is generally reassuring and supports the scale-up of `see and treat' programs at HIV treatment clinics across Africa, but further studies are needed in order to understand more clearly the risk of HIV-1 cervical shedding among women not receiving ART at the time of cryotherapy.
Acknowledgements
The authors would like to thank the research personnel, clinic and laboratory staff, and data management teams in Nairobi, Kenya and Seattle, Washington for their work. They recognize the Coptic Hope Center for Infectious Diseases for their cooperation and their patients for participation and support.
This research was supported by the Washington Global Health Alliance. M.H.C. was supported by a K23 grant, National Institutes of Health (5K23AI065222–04). G.C.J.-S. received support from an NICHD K24 Award (1K24HD054314–04). The Coptic Hope Center for Infectious Diseases is supported by the President's Emergency Plan for AIDS Relief (PEPFAR) through a cooperative agreement (U62/CCU024512–04) from the US Centers for Disease Control and Prevention (CDC). Laboratory support was provided by the University of Washington Center for AIDS Research (AI27757).
Footnotes
M.H.C. designed and implemented the study, supervised the on-site data management, interpreted the data, and wrote the paper. N.R.M. helped to implement the study and contributed to the study's design, analysis and writing. K.P.M. implemented the study and helped interpret the data. B.A.R. performed the statistical analysis and helped design the study and write the paper. G.C.J.-S. helped design the study, interpret the data, and write the paper. R.W.C. conducted the laboratory testing and helped interpret the data and write the paper. H.D.V. helped interpret the data and write the paper. J.W.N. helped implement the study, collected data, and conducted statistical analyses. E.N-M. implemented the study. S.R.S. helped implement and design the study.
Conflicts of interest None of the authors has a major conflict of interest in this study.
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or in the writing of the report.
The data were presented at the 18th Conference on Retroviruses and Opportunistic Infections (CROI 2011) held 27 February to 2 March 2011 in Boston, MA, USA (abstract # 768).
References
- 1.Visual inspection with acetic acid for cervical-cancer screening: test qualities in a primary-care setting. University of Zimbabwe/JHPIEGO Cervical Cancer Project. Lancet. 1999;353:869–873. [PubMed] [Google Scholar]
- 2.Denny L, Kuhn L, De Souza M, Pollack AE, Dupree W, Wright TC., Jr. Screen-and-treat approaches for cervical cancer prevention in low-resource settings: a randomized controlled trial. JAMA. 2005;294:2173–2181. doi: 10.1001/jama.294.17.2173. [DOI] [PubMed] [Google Scholar]
- 3.Gaffikin L, Blumenthal PD, Emerson M, Limpaphayom K. Safety, acceptability, and feasibility of a single-visit approach to cervical-cancer prevention in rural Thailand: a demonstration project. Lancet. 2003;361:814–820. doi: 10.1016/s0140-6736(03)12707-9. [DOI] [PubMed] [Google Scholar]
- 4.Franceschi S, Jaffe H. Cervical cancer screening of women living with HIV infection: a must in the era of antiretroviral therapy. Clin Infect Dis. 2007;45:510–513. doi: 10.1086/520022. [DOI] [PubMed] [Google Scholar]
- 5.Wright TC, Jr, Subbarao S, Ellerbrock TV, Lennox JL, Evans-Strickfaden T, Smith DG, et al. Human immunodeficiency virus 1 expression in the female genital tract in association with cervical inflammation and ulceration. Am J Obstet Gynecol. 2001;184:279–285. doi: 10.1067/mob.2001.108999. [DOI] [PubMed] [Google Scholar]
- 6.Kreiss J, Willerford DM, Hensel M, Emonyi W, Plummer F, Ndinya-Achola J, et al. Association between cervical inflammation and cervical shedding of human immunodeficiency virus DNA. J Infect Dis. 1994;170:1597–1601. doi: 10.1093/infdis/170.6.1597. [DOI] [PubMed] [Google Scholar]
- 7.McClelland RS, Wang CC, Mandaliya K, Overbaugh J, Reiner MT, Panteleeff DD, et al. Treatment of cervicitis is associated with decreased cervical shedding of HIV-1. AIDS. 2001;15:105–110. doi: 10.1097/00002030-200101050-00015. [DOI] [PubMed] [Google Scholar]
- 8.Baeten JM, Kahle E, Lingappa JR, Coombs RW, Delany-Moretlwe S, Nakku-Joloba E, et al. Genital HIV-1 RNA levels predict risk of heterosexual HIV-1 transmission. 18th Conference on Retroviruses and Opportunistic Infections; Boston. 2011. [Google Scholar]
- 9.Chung MH, McKenzie K, De Vuyst H, Pamnani R, Rana F, Njoroge J, et al. Comparing visual inspection with acetic acid, high-risk HPV testing, and PAP smear to colposcopic biopsy among HIVR women. 18th Conference on Retroviruses and Opportunistic Infections; Boston. 2011. [Google Scholar]
- 10.Chung MH, Drake AL, Richardson BA, Reddy A, Thiga J, Sakr SR, et al. Impact of prior HAART use on clinical outcomes in a large Kenyan HIV treatment program. Curr HIV Res. 2009;7:441–446. doi: 10.2174/157016209788680552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.John GC, Sheppard H, Mbori-Ngacha D, Nduati R, Maron D, Reiner M, et al. Comparison of techniques for HIV-1 RNA detection and quantitation in cervicovaginal secretions. J Acquir Immune Defic Syndr. 2001;26:170–175. doi: 10.1097/00042560-200102010-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baeten JM, Strick LB, Lucchetti A, Whittington WL, Sanchez J, Coombs RW, et al. Herpes simplex virus (HSV)-suppressive therapy decreases plasma and genital HIV-1 levels in HSV-2/HIV-1 coinfected women: a randomized, placebo-controlled, cross-over trial. J Infect Dis. 2008;198:1804–1808. doi: 10.1086/593214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zuckerman RA, Lucchetti A, Whittington WL, Sanchez J, Coombs RW, Zuniga R, et al. Herpes simplex virus (HSV) suppression with valacyclovir reduces rectal and blood plasma HIV-1 levels in HIV-1/HSV-2-seropositive men: a randomized, double-blind, placebo-controlled crossover trial. J Infect Dis. 2007;196:1500–1508. doi: 10.1086/522523. [DOI] [PubMed] [Google Scholar]
- 14.Graham SM, Holte SE, Peshu NM, Richardson BA, Panteleeff DD, Jaoko WG, et al. Initiation of antiretroviral therapy leads to a rapid decline in cervical and vaginal HIV-1 shedding. AIDS. 2007;21:501–507. doi: 10.1097/QAD.0b013e32801424bd. [DOI] [PubMed] [Google Scholar]
- 15.Cu-Uvin S, Caliendo AM, Reinert S, Chang A, Juliano-Remollino C, Flanigan TP, et al. Effect of highly active antiretroviral therapy on cervicovaginal HIV-1 RNA. AIDS. 2000;14:415–421. doi: 10.1097/00002030-200003100-00015. [DOI] [PubMed] [Google Scholar]
- 16.Gitau RW, Graham SM, Masese LN, Overbaugh J, Chohan V, Peshu N, et al. Effect of acquisition and treatment of cervical infections on HIV-1 shedding in women on antiretroviral therapy. AIDS. 2010;24:2733–2737. doi: 10.1097/QAD.0b013e32833f9f43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mwanahamuntu MH, Sahasrabuddhe VV, Pfaendler KS, Mudenda V, Hicks ML, Vermund SH, et al. Implementation of 'see-and-treat' cervical cancer prevention services linked to HIV care in Zambia. AIDS. 2009;23:N1–5. doi: 10.1097/QAD.0b013e3283236e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Connor EM, Sperling RS, Gelber R, Kiselev P, Scott G, O'Sullivan MJ, et al. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. N Engl J Med. 1994;331:1173–1180. doi: 10.1056/NEJM199411033311801. [DOI] [PubMed] [Google Scholar]
- 19.John GC, Nduati RW, Mbori-Ngacha DA, Richardson BA, Panteleeff D, Mwatha A, et al. Correlates of mother-to-child human immunodeficiency virus type 1 (HIV-1) transmission: association with maternal plasma HIV-1 RNA load, genital HIV-1 DNA shedding, and breast infections. 2001. J Infect Dis. 183:206–212. doi: 10.1086/317918. [DOI] [PubMed] [Google Scholar]
- 20.Lehman DA, Chung MH, John-Stewart GC, Richardson BA, Kiarie JN, Kinuthia J, et al. HIV-1 persists in breast milk cells despite antiretroviral treatment to prevent mother-to-child transmission. AIDS. 2008;22:1475–1485. doi: 10.1097/QAD.0b013e328302cc11. [DOI] [PMC free article] [PubMed] [Google Scholar]


