Abstract
Despite its advantages as a chronobiological technique, the ultra-short sleep/wake protocol remains underutilized in circadian rhythm research. The purpose of this study was to examine circadian rhythms of psychomotor vigilance (PVT), mood, and sleepiness in a sample (n = 25) of healthy young adults while they adhered to a 3-h ultra-short sleep/wake protocol. The protocol involved 1-h sleep intervals in darkness followed by 2-h wake intervals in dim light, repeated for 50–55 h. A 5-min PVT test was conducted every 9 h with the standard metrics of mean reaction time (RT; RTmean), median RT (RTmed), fastest 10% of responses (RT10fast), and the reciprocal of the 10% slowest responses (1/RT10slow). Subjective measures of mood and sleepiness were assessed every 3 h. A cosine fit of intra-aural temperature, assessed three times per wake period, established the time of the body temperature minimum (Tmin). Mood, sleepiness, and PVT performances were expressed relative to individual means and compared across eight times of day and across twelve 2-h intervals relative to Tmin. Significant time-of-day and circadian patterns were demonstrated for each of the PVT metrics, as well as for mood and sleepiness. Most mood subscales exhibited significant deterioration in day 2 of the protocol without an alteration of circadian pattern. However, neither sleepiness nor performance was worse on the second day of observation compared to the first day. These data provide further support for the use of the ultra-short sleep/wake protocol for measurement of circadian rhythms.
Keywords: ultra-short sleep/wake protocol, circadian, neurobehavioral performance, mood, sleepiness
INTRODUCTION
Reliable assessment of human circadian rhythms requires chronobiological protocols that minimize or control for the numerous environmental and behavioral “masking” factors that could confound their measurement. A limited number of such protocols have been developed, the most common being the constant routine and forced desynchrony protocols. Each of these techniques has advantages and limitations.
The constant routine protocol is useful for examining circadian rhythms over ~24 h in many physiologic variables (Duffy & Dijk, 2002), but it is less useful for variables that are affected by sleep deprivation (e.g., cognitive performance, mood) or for variables which require several hours of recovery between trials (e.g., athletic performance; Kline et al., 2007). The forced desynchrony protocol is superior for delineating circadian and homeostatic influences on a variable, but requires multiple cycles (e.g., six 20- or 28-h cycles for only one beat cycle), making it a time-intensive protocol (Wright et al., 2002; Wyatt et al., 1999).
A chronobiological technique that has received much less attention is the ultra-short sleep/wake protocol, which could be considered a variation of the forced desynchrony protocol. In this protocol, participants adhere to repeated ultradian “day” cycles, with each cycle being shorter than the normal sleep/wake cycle and containing a fixed period of wakefulness in dim light followed by a fixed period of attempted sleep in total darkness. Cycle lengths have varied between studies, ranging from 20 min (13 min wake, 7 min sleep; Lavie & Scherson, 1981) to 30 min (20/10; Kubota et al., 2002) to 90 min (60/30; Buysse et al., 2005; Kripke et al., 2007) to 180 min (120/60; Weitzman et al., 1974a), and the protocol can be sustained successfully for up to 10 days (Weitzman et al., 1974b). Instead of eliminating the influence of factors that might mask circadian measurement (e.g., physical activity, sleep/wake state, food ingestion), these effects are distributed evenly across all cycles. Circadian rhythms free-run in these protocols, delaying approximately 0.30 h per day (Kripke et al., 2007). Though sleep is fragmented into short bouts, sleep deprivation is not nearly as extreme as that induced by the constant routine protocol. Furthermore, many assessments can be accomplished in much shorter time in the ultra-short sleep/wake schedule compared to the forced desynchrony protocol.
The ultra-short sleep/wake protocol has been used to establish circadian rhythms in melatonin (Kripke et al., 2005), sleep propensity (Kudo et al., 1999; Lavie et al., 1981), sleep architecture (Carskadon & Dement, 1975; Stampi, 1992), and neuroendocrine function (Weitzman et al., 1974a; Weitzman et al., 1974b), and to examine phase response curves for bright light (Kripke et al., 2007). However, the ultra-short sleep/wake protocol has been sparsely used in assessing circadian patterns of neurobehavioral performance and mood (Lavie et al., 1987). Use of this protocol would appear especially well-suited for examining circadian fluctuation in these variables, which are sensitive to sleep loss.
The primary aim of this study was to examine circadian variation in psychomotor vigilance task (PVT) performance, mood, and sleepiness using the ultra-short sleep/wake protocol. A secondary aim was to examine associations of PVT performance with body temperature and mood to explore the mediating influence of these variables. It was hypothesized that significant circadian rhythms would be found for PVT performance, mood and sleepiness, and that the rhythms of PVT performance would be modestly correlated with body temperature, mood and sleepiness. The present study was ancillary to a study investigating circadian variation in swim performance, the results of which are presented elsewhere (Kline et al., 2007).
METHODS
Participants
Twenty-five healthy young adults (19.58 ± 0.95 yr, 1.76 ± 0.02 m, 73.92 ± 2.46 kg, 13 female) were recruited for the study. All participants were physically active and free of depression, health or sleep problems. In addition, no participants were of extreme chronotype [i.e., Horne-Ostberg Morningness-Eveningness Questionnaire (MEQ; Horne & Ostberg, 1976) score > 69 or < 31] or had reported shift-work or travel across multiple time zones in the month prior to the study. The study protocol was approved by the Institutional Review Board at the University of South Carolina and was conducted in accordance with the ethical standards of Chronobiology International for the conduct of human biological rhythm research (Portaluppi et al., 2008).
Experimental Procedure
Home observation
During the week prior to laboratory observation, participants maintained stable sleep-wake times (i.e., bed and wake times not varying by more than 1.5 h) that were consistent with their average sleep schedules. Adherence to the sleep schedule was verified by continuous wrist actigraphic recording (Octagonal Basic Motionlogger; Ambulatory Monitoring, Inc., Ardsley, NY, USA) and completion of a daily sleep diary. During this week, participants averaged 5:54 ± 00:13 h of actigraphically-assessed sleep each night.
Participants were asked to refrain from alcohol and caffeine during the two days prior to laboratory observation, though compliance was not objectively assessed. During this time, participants visited the laboratory to practice PVT performance and review the upcoming laboratory procedures.
Laboratory observation
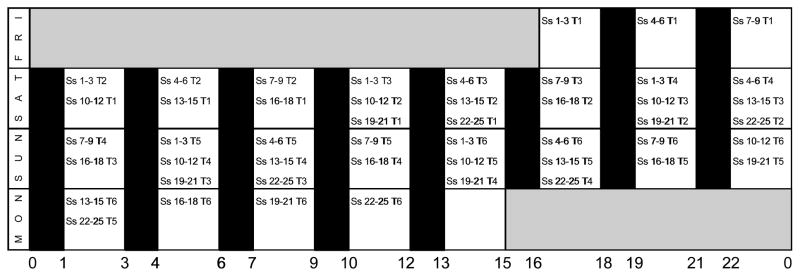
Immediately following home observation, each participant spent 50–55 h completing a weekend laboratory protocol. For most participants, arrival into the protocol occurred in blocks of n = 3 at one of eight times of day, approximately 1 h before PVT trial #1 (Figure 1). To avoid sleep interruption in participants scheduled to perform PVT trial #1 at 02:00 or 05:00 h, these participants (n = 6) were required to arrive in the laboratory 1 h before their usual bedtimes and to stay in the laboratory for 2–5 h longer than the other participants. Each participant performed a PVT trial every 9 h, with 6 trials per participant. The randomization process was structured to obtain 18 PVT assessments at each of 8 possible time-points (i.e., 02:00, 05:00, 08:00, 11:00, 14:00, 17:00, 20:00, or 23:00 h), with potential fatigue associated with multiple trials evenly dispersed across the 24-h day. Thus, for example, three PVT performances during each time-point were PVT trial #1 for some participants, three PVT performances during each time-point were PVT trial #2 for some participants, three PVT performances were PVT trial #3 for some participants, and so on (Figure 1).
Figure 1. Laboratory observation and performance trial schedule.
Key: “Ss” indicates participants (e.g., “Ss 1–3” indicates participants 1–3); “T” (followed by a number) indicates the trial number for that set of participants. Open bars are 120-min periods of wakefulness in dim light (< 30 lux); black bars indicate 60-min periods of sleep (< 1 lux). Days of experiment are listed on far-left column. Time of day is listed across the bottom. Participants entered the protocol in groups of n = 3, with each group performing a PVT trial every 9 h (6 trials per participant). The start times for participants were staggered so that ~18 performances would be obtained during each of the 8 wake periods, and that each set of performances was at a different point in the progression of trials. The intent of this scheduling was to evenly distribute any fatigue effects that may occur from multiple testing across the 24-h day.
Throughout the 50–55 h of laboratory observation, participants followed a 3-h ultra-short sleep/wake schedule, involving 2 h of out-of-bed wakefulness in dim light (< 30 lux) followed by 1 h of attempted sleep in darkness (< 1 lux). At min 90 of each 2-h wake period, participants ate a standardized meal, designed by a registered dietician (TAM), based upon each participant’s individual energy intake needs [i.e., body weight, age, gender, and activity level (Frankenfield et al., 2003)]. Water and non-caffeinated, calorie-free beverages were allowed ad libitum.
Participants were free to engage in sedentary activities of their choice during wake periods. Every 9 h (in the wake period following PVT performances), participants performed a maximal-effort 200-m swim trial (Kline et al., 2007). Besides the scheduled swim trials, exercise was not allowed. Participants left the laboratory only to perform the swim trial or use the restroom, and were required to wear dark sunglasses [Uvex Genesis (dark gray lenses; 10% available light transmittance), Smithfield, RI, USA] to keep light exposure relatively constant outside of the laboratory.
Body temperature measurements
Intra-aural temperature (Taur) was assessed at min 15, 60, and 105 of each 2-h wake period throughout laboratory observation. Staff measured each participant’s Taur using a hospital-grade infra-red ear thermometer (Braun ThermoScan 4000; Welch Allyn, Inc., San Diego, CA, USA). Three measurements were taken at each time-point and averaged.
Psychomotor vigilance assessments
PVT performance was assessed at min 60 of the first wake period and every 9 h thereafter using a pre-programmed portable monitor (PVT-192; Ambulatory Monitoring, Inc., Ardsley, NY, USA) (Dinges & Powell, 1985). These trials always preceded the swim trials by one wake period. The test was performed over 5 min in a room free of surrounding distractions. It was a fully electronic test for simple visual reaction time. Participants were presented with a visual stimulus (a display of the response time in ms) and were asked to react when they saw the stimulus by pressing a button. Various performance measures were computed by standard PVT software (PVTcommW version 2.10.1.1, REACT version 1.1.05; Ambulatory Monitoring, Inc., Ardsley, NY, USA), including mean reaction time (RTmean), median reaction time (RTmed), fastest 10% of responses (RT10fast) and the reciprocal of slowest 10% of responses (1/RT10slow). Lapses (i.e., responses > 500 ms) were not included in the analysis because, upon initial inspection of the data, they occurred very infrequently. Participants averaged only 0.65 ± 0.13 lapses per trial, with 12 of the 25 participants recording ≤ 2 lapses across all six trials combined.
Mood
At min 15 of each wake period, mood was assessed with the Profile of Mood States Questionnaire (POMS; McNair et al., 1971). Participants were prompted to indicate how they felt “right now”. The POMS assesses the present intensity of 65 different emotions/feelings on a 5-point scale: (0) not at all; (1) a little; (2) moderately; (3) quite a bit; (4) extremely. Subscale scores for Tension-Anxiety, Depression-Dejection, Anger-Hostility, Vigor-Activity, Fatigue-Inertia, and Confusion-Bewilderment, and a global Total Mood Disturbance (TMD) score were calculated.
Sleep and sleepiness
Sleep/wake state was recorded over the course of laboratory observation by wrist actigraphy. At min 15 of each wake period, sleepiness was assessed with the Stanford Sleepiness Scale (SSS; Hoddes et al., 1973). The SSS is a single-item scale on which participants rate their sleepiness “at that very moment” in categories ranging from 1 (“feeling active and vital, alert, wide awake”) to 7 (“almost in reverie, sleep onset soon, lost struggle to remain awake”).
Data Analysis
Data were analyzed with SAS (version 9.2, Cary, NC) and SPSS for Windows (version 16.0, Chicago, IL, USA) and are presented as mean ± standard error (SE). Statistical significance was set at p < 0.05.
Circadian rhythm of Taur
The circadian rhythm of body temperature was determined by least-squares estimation of the best-fitting cosine curve of temperature data using Action4 software (version 1.12, Ambulatory Monitoring, Ardsley, NY, USA). To eliminate the effect of swimming and water temperature on Taur, temperature data immediately following swim performances were excluded from analysis. The circadian nadir (Tmin) was calculated as the time of the lowest value of the cosine-fitted rhythm for each of the 25 participants. Average goodness of fit was R2 = 0.68 ± 0.03.
Rhythms of PVT performance, mood and SSS
PVT performance, mood and SSS data were transformed into deviation from the individual mean so that variations in performance and mood could be made relative to each participant. For each day of the protocol, data were expressed relative to time of day (i.e., 8 times) and also organized into twelve 2-h bins relative to Tmin, with Tmin (i.e., 1 h before to 1 h after the Tmin) assigned a phase of 0°. SAS PROC MIXED, with day (1 or 2) and time (8 times of day or 12 circadian bins) as within-subjects factors with time specified as a repeated factor, was used to evaluate each PVT, mood and SSS outcome separately. Possible effects of the length of observation or accumulated sleep loss on PVT performance were further analyzed using SAS PROC MIXED, with trial as a repeated factor, for each of the PVT metrics. Analyses indicated that we had 96% power to detect a 0.50 standard deviation decrement in performance from day 1 to day 2 of the protocol, and we had 80% power to detect a 0.37 standard deviation decrement (SamplePower; SPSS, Inc.).
All analyses utilized Kenward-Roger’s adjusted degrees of freedom, but the original degrees of freedom are reported. Bonferroni adjustments were made for multiple comparisons between individual time points and circadian bins for each of the PVT metrics, POMS subscales, and SSS data.
Sleep
Actigraphic sleep/wake status during laboratory observation was estimated by an algorithm associated with Action4 software. Cosinor analysis of sleep/wake status was performed separately for days 1 and 2 of the protocol, and values of acrophase, mesor and amplitude were compared with a paired samples t-test. For laboratory observation, sleep efficiency and total sleep time were calculated and compared between days 1 and 2 of the protocol with a paired samples t-test.
Correlations of performance with mood and temperature
Associations of PVT performance with temperature, POMS and SSS values were evaluated with cross-correlation analysis. For each participant, cross-correlations over the entire course of observation were performed using 3-h time lags. For each time lag interval, the individual correlation coefficients initially went through Fisher’s z-transformations before being averaged across all participants and being converted back to r values. Correlations were assessed for significance against the null hypothesis of ρ = 0 via t-tests against the transformed values.
RESULTS
Body Temperature
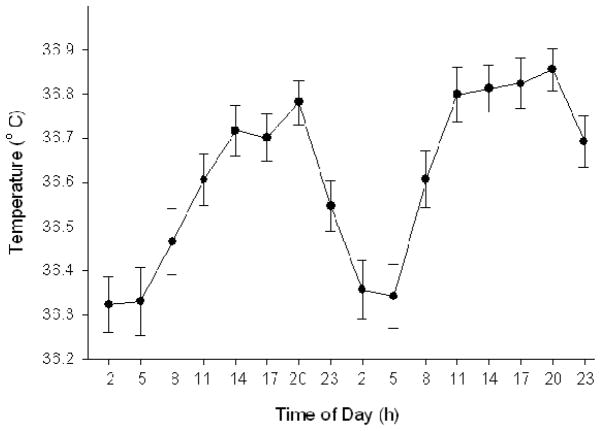
On average, 38.8 ± 0.3 body temperature data points were obtained for each participant during laboratory observation, with an average coefficient of variation for the three measurements at each time-point of 0.16 ± 0.01 %. Group Tmin was 04:16 ± 00:23 h, with a mesor of 36.60 ± 0.05° C and an amplitude of 0.25 ± 0.02° C (Figure 2). Individual assessments of Tmin were significantly associated with self-reported circadian type (r = −0.58, p < 0.001), with an earlier Tmin being associated with a higher MEQ score (i.e., higher “morningness”).
Figure 2. Pattern of intra-aural temperature during the laboratory protocol, averaged across the 25 participants.
Data are expressed as mean ± SE. Average Tmin was 04:16 ± 00:23 h.
Psychomotor Vigilance
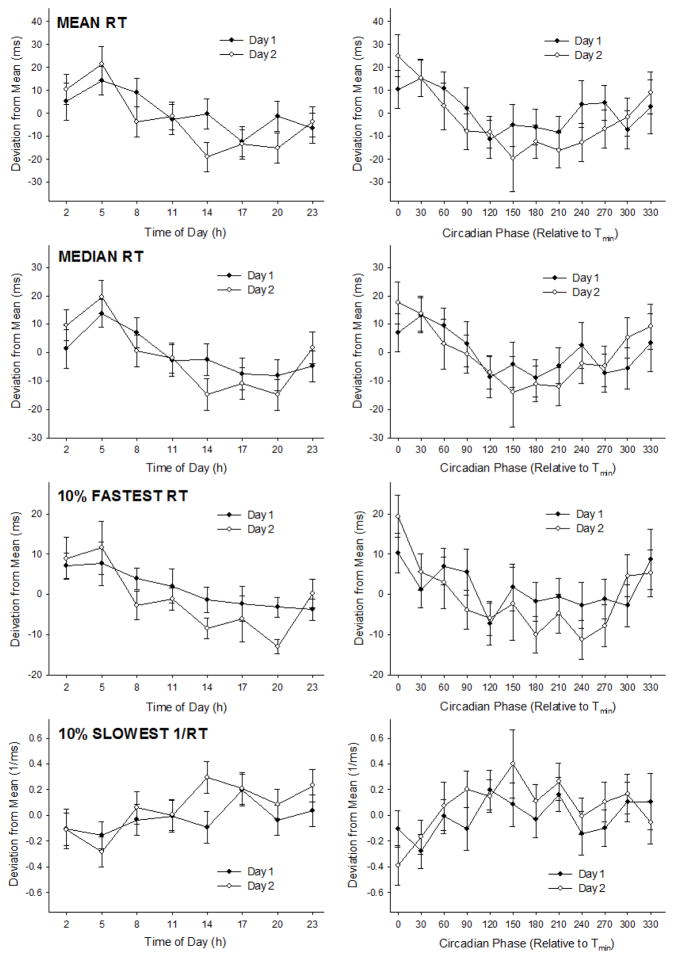
Each of the four PVT performance metrics differed significantly by time of day (Table 1; Figure 3). Although differing slightly between metrics, performance was generally significantly worse at 02:00–05:00 h compared to 14:00–20:00 h (Table 1). Furthermore, each of the four PVT metrics differed significantly when expressed relative to Tmin (Table 1; Figure 3). Again, although differing slightly between measures, performance was typically worse at 0–30° circadian phase compared to 180–210° (Table 1).
Table 1. Metrics of PVT performance relative to time of day and circadian phase.
Upper panel: Statistical analysis results with the factors day, time of day/circadian phase, and interaction. Lower panel: Individual time-point differences for each PVT metric. Refer to Figure 3 for a graphical display of the results. PVT: psychomotor vigilance task; RT: reaction time. Degrees of freedom for time-of-day analyses: day 1, 134; time of day 7, 134; day × time of day 7, 134; for circadian analyses: day 1, 126; circadian phase 11, 126; day × circadian phase 11, 126.
| Metric & Factor: | Time of Day:
|
Circadian Phase:
|
||
|---|---|---|---|---|
| F-value: | P-value: | F-value: | P-value: | |
| Mean RT | ||||
| Day | 0.49 | 0.486 | 0.45 | 0.502 |
| Time/Phase | 6.10 | <0.001 | 3.93 | <0.001 |
| Day × Time/Phase | 1.48 | 0.182 | 0.96 | 0.491 |
| Median RT | ||||
| Day | 0.00 | 0.953 | 0.00 | 0.977 |
| Time/Phase | 7.43 | <0.001 | 3.63 | <0.001 |
| Day × Time/Phase | 1.29 | 0.262 | 1.07 | 0.395 |
| 10% Fastest RT | ||||
| Day | 1.40 | 0.243 | 0.90 | 0.349 |
| Time/Phase | 4.79 | <0.001 | 3.36 | <0.001 |
| Day × Time/Phase | 0.87 | 0.532 | 0.80 | 0.645 |
| 10% Slowest 1/RT | ||||
| Day | 1.00 | 0.319 | 1.04 | 0.311 |
| Time/Phase | 3.80 | <0.001 | 2.56 | 0.006 |
| Day × Time/Phase | 1.46 | 0.187 | 1.10 | 0.370 |
| Metric: | Time of Day: | Circadian Phase: |
|---|---|---|
| Mean RT | 05:00 < 11:00–23:00 h | 0° < 120–240°, 300°; 30° < 210° |
| Median RT | 05:00 < 11:00–23:00 h | 0° < 120, 180°, 210°, 270°; 30° < 180° |
| 10% Fastest RT | 2:00 < 20:00 h; 5:00 < 14:00–20:00 h | 0° < 120°, 180–270° |
| 10% Slowest 1/RT | 5:00 < 14:00, 17:00, 23:00 h | 0° < 120°, 210° |
Figure 3. PVT performance metrics plotted relative to time of day and circadian phase.
Data are separated by day and presented as mean ± SE. See Table 1 for statistical results and significant differences between time-points.
PVT performance was not significantly different between days 1 and 2 of the protocol, and neither time of day × day nor circadian phase × day interactions were significant for any of the four PVT metrics (Table 1). Furthermore, no significant trial effect on performance was revealed for any of the PVT metrics (each p > 0.10).
Mood
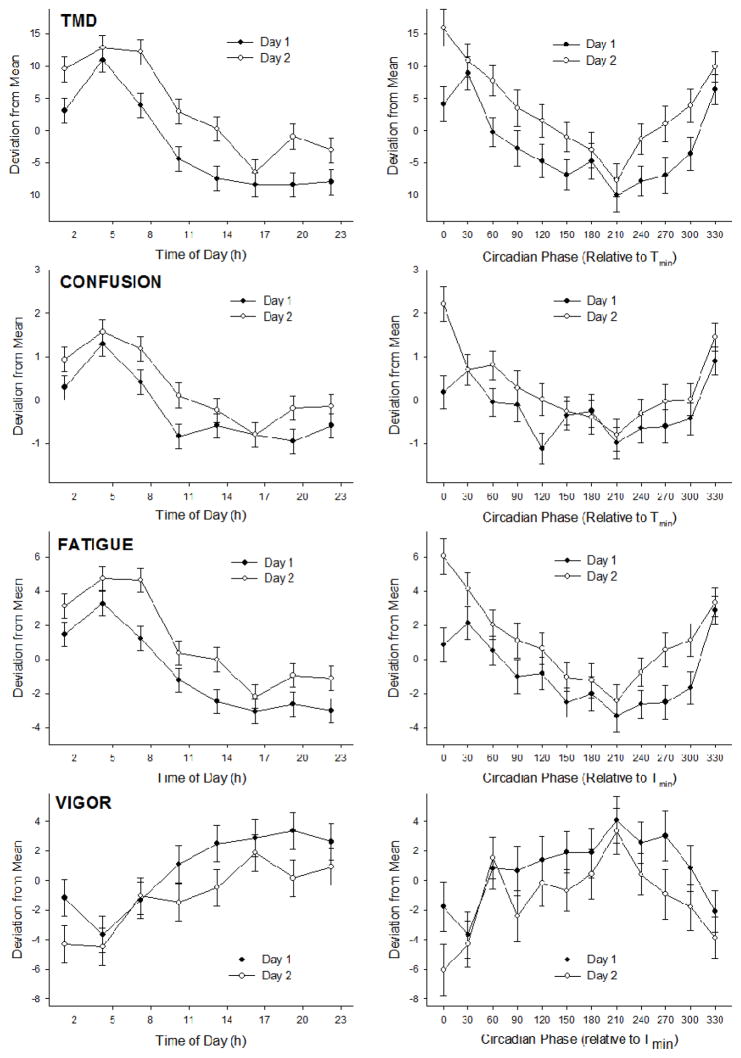
Mood, as assessed by each POMS subscale, demonstrated significant time-of-day and circadian variation (Table 2). Mood was generally most impaired at 04:15 h and highest from 10:15 h to 23:15 h. Relative to circadian phase, mood was most impaired at 30° and highest from 90° to 300° relative to Tmin. Mood was significantly worse on the second day of the protocol for the POMS measures of TMD, confusion, fatigue and vigor (Table 2, Figure 4). The time-of-day or circadian variation was not significantly different between days for any of the measures.
Table 2. Variation of mood and sleepiness relative to time of day and circadian phase.
Refer to Figure 4 for a graphical display of POMS TMD, Confusion, Fatigue, and Vigor results. Refer to Figure 5 for a graphical display of SSS results. POMS: Profile of Mood States; TMD: Total Mood Disturbance; SSS: Stanford Sleepiness Scale. Degrees of freedom for time-of-day analyses: day 1, 382; time of day 7, 382; day × time of day 7, 382; for circadian phase analyses: day 1, 374; circadian phase 11, 374; day × circadian phase 11, 374.
| Subscale & Effect: | Time of Day: | Circadian Phase: | ||
|---|---|---|---|---|
| F-value: | P-value | F-value: | P-value: | |
| POMS TMD | ||||
| Day | 39.58 | <0.001 | 38.99 | <0.001 |
| Time/Phase | 26.07 | <0.001 | 9.85 | <0.001 |
| Day × Time/Phase | 0.98 | 0.449 | 0.79 | 0.647 |
| POMS Anger | ||||
| Day | 4.04 | 0.051 | 3.13 | 0.079 |
| Time/Phase | 5.02 | <0.001 | 2.65 | 0.004 |
| Day × Time/Phase | 1.35 | 0.251 | 1.20 | 0.292 |
| POMS Confusion | ||||
| Day | 13.82 | <0.001 | 14.13 | <0.001 |
| Time/Phase | 15.89 | <0.001 | 7.09 | <0.001 |
| Day × Time/Phase | 0.60 | 0.753 | 1.27 | 0.243 |
| POMS Depression | ||||
| Day | 3.53 | 0.226 | 3.08 | 0.086 |
| Time/Phase | 4.89 | <0.001 | 2.25 | 0.014 |
| Day × Time/Phase | 1.26 | 0.275 | 0.91 | 0.536 |
| POMS Fatigue | ||||
| Day | 28.48 | <0.001 | 29.68 | <0.001 |
| Time/Phase | 26.13 | <0.001 | 9.99 | <0.001 |
| Day × Time/Phase | 0.58 | 0.773 | 0.94 | 0.504 |
| POMS Tension | ||||
| Day | 3.87 | 0.056 | 3.02 | 0.090 |
| Time/Phase | 4.31 | <0.001 | 2.02 | 0.030 |
| Day × Time/Phase | 1.29 | 0.263 | 1.30 | 0.227 |
| POMS Vigor | ||||
| Day | 10.11 | 0.002 | 10.79 | 0.001 |
| Time/Phase | 6.55 | <0.001 | 4.29 | <0.001 |
| Day × Time/Phase | 0.59 | 0.767 | 0.45 | 0.930 |
| SSS Sleepiness | ||||
| Day | 1.63 | 0.202 | 1.51 | 0.220 |
| Time/Phase | 23.18 | <0.001 | 9.54 | <0.001 |
| Day × Time/Phase | 0.97 | 0.452 | 0.53 | 0.883 |
Figure 4. Selected POMS mood variables plotted relative to time of day and circadian phase.
Data are presented as mean ± SE and separated by day. The four POMS subscales with the most robust worsening of mood on day 2 of the protocol are shown. See Table 2 for statistical test results.
Sleep and Sleepiness
The average amount of sleep obtained per 24 h of laboratory observation was 5:06 ± 0:12 h. Average sleep efficiency during the scheduled naps was 65.06 ± 1.90%, and did not differ between days 1 and 2 of the protocol (65.30 ± 2.86% vs. 64.81 ± 2.56%, respectively; t23 = 0.290, p = 0.774). Average sleep acrophase (i.e., time of highest sleep propensity) was 06:30 ± 00:24 h. Sleep acrophase, mesor, and amplitude values did not differ between days 1 and 2.
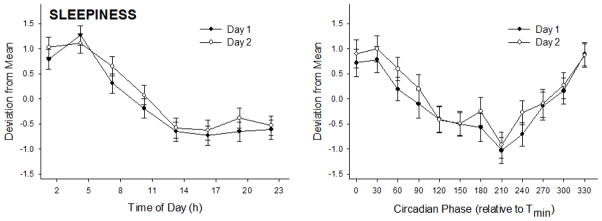
Subjective sleepiness, assessed by the SSS, differed significantly when expressed relative to time of day and circadian phase (Table 2; Figure 5). Sleepiness was greatest from 01:15–07:15 h compared to all other times of day, with a nadir from 13:15–16:15 h (albeit nonsignificant from surrounding time-points). Relative to circadian phase, sleepiness was greatest near Tmin (i.e., 330° to 60°) compared to all other phases, and reduced at 210° compared to 300°. Sleepiness did not differ between days, and the time-of-day and circadian variation was similar between days.
Figure 5. Subjective sleepiness plotted relative to time of day and circadian phase.
Data are presented as mean ± SE and separated by day to display the lack of increased sleepiness on day 2 of the protocol. See Table 2 for statistical test results.
Associations of Mood and Temperature with Performance
With few exceptions, the strongest correlations with performance came at 0 h time lag (i.e., at same time-point; Table 3). Furthermore, across most metrics, body temperature exhibited the strongest association with PVT performance, though these correlations were relatively modest (r < 0.50; Table 3).
Table 3.
Cross-correlations between mood/sleepiness and metrics of PVT performance.
| Variable: | Mean RT:
|
Median RT:
|
||
|---|---|---|---|---|
| r-value: | Lag: | r-value: | Lag: | |
| Intra-aural Temperature | −0.36 ± 0.11* | 0 h | −0.45 ± 0.11* | 0 h |
| POMS Anger | 0.30 ± 0.16 | 0 h | 0.27 ± 0.14 | 0 h |
| POMS Confusion | 0.30 ± 0.16 | 0 h | 0.42 ± 0.14* | 0 h |
| POMS Depression | 0.31 ± 0.08* | +3 h | 0.32 ± 0.14* | 0 h |
| POMS Fatigue | 0.44 ± 0.19* | 0 h | 0.44 ± 0.14* | 0 h |
| POMS Tension | 0.26 ± 0.15 | 0 h | 0.24 ± 0.14 | 0 h |
| POMS Vigor | −0.35 ± 0.11* | 0 h | −0.39 ± 0.13* | 0 h |
| POMS TMD | 0.38 ± 0.14* | 0 h | 0.43 ± 0.14* | 0 h |
| SSS Sleepiness | 0.39 ± 0.14* | 0 h | 0.40 ± 0.13* | 0 h |
| Variable: | 10% Fastest RT:
|
10% Slowest 1/RT:
|
||
|---|---|---|---|---|
| r-value: | Lag: | r-value: | Lag: | |
| Intra-aural Temperature | −0.43 ± 0.10* | 0 h | 0.19 ± 0.12 | 0 h |
| POMS Anger | 0.25 ± 0.09* | +3 h | −0.26 ± 0.14 | 0 h |
| POMS Confusion | 0.34 ± 0.13* | 0 h | −0.25 ± 0.16 | 0 h |
| POMS Depression | 0.30 ± 0.12* | 0 h | −0.26 ± 0.06* | +3 h |
| POMS Fatigue | 0.28 ± 0.13* | 0 h | −0.25 ± 0.17 | 0 h |
| POMS Tension | 0.26 ± 0.13 | 0 h | −0.21 ± 0.14 | 0 h |
| POMS Vigor | −0.33 ± 0.12* | 0 h | 0.16 ± 0.11 | 0 h |
| POMS TMD | 0.33 ± 0.14* | 0 h | −0.20 ± 0.15 | 0 h |
| SSS Sleepiness | 0.32 ± 0.12* | 0 h | −0.24 ± 0.14 | 0 h |
Data are expressed as mean ± SE.
indicates that the correlation is significantly different from 0 (p < 0.05). POMS: Profile of Mood States; TMD: Total Mood Disturbance; SSS: Stanford Sleepiness Scale.
DISCUSSION
In this ultra-short sleep/wake protocol with healthy young adults, PVT performance, mood and sleepiness showed robust circadian variation. The PVT performance patterns were modestly associated with body temperature, and slightly less correlated with mood and sleepiness.
PVT Performance
Reaction time on four metrics of the PVT showed a clear time-of-day and circadian pattern. When expressed relative to time of day, reaction time was generally slowest at 2:00–05:00 h and optimal between 14:00 and 20:00 h (Figure 3). When expressed relative to circadian phase, reaction time was generally worst from 0–30° and best from 180–210° (Figure 3). Of the four metrics, RTmed demonstrated the most robust variation relative to diurnal and circadian time. Although 1/RT10slow exhibited the weakest variation of the four PVT metrics, performance was still significantly different across different times and circadian phases (Table 1).
These data are consistent with other studies that have assessed PVT performance with circadian protocols. Wright et al. (2002), using a forced desynchrony protocol with 28-h cycles, found 10-min PVT performance to differ by circadian phase for the measures of RT10fast, 1/RT10slow, and lapses (i.e., responses > 500 ms), but not for RTmed. Wyatt et al. (1999), using a forced desynchrony protocol with 20-h cycles, found similar results, with lapses differing significantly by circadian phase and RTmed exhibiting a circadian trend (p = 0.0545). In both of these studies, performance on each PVT metric was generally optimal at approximately 240° circadian phase and worst at or just after 0°.
Blatter et al. (2006) and Graw et al. (2004) utilized the ultra-short sleep/wake protocol to identify significant time-of-day effects in 5-min PVT performance for RTmed, the 10th and 90th percentile of RT, RT variability, and lapses. Although the times of peak and worst performance were not reported in these studies and performance was not tracked relative to circadian time, the results are consistent with the data reported herein.
Mood and Sleepiness
Mood also demonstrated significant time-of-day and circadian patterns. The most robust mood rhythms were found for TMD, confusion, fatigue, and vigor, but all subscales demonstrated significant circadian rhythms. In general, mood was worst at the body temperature minimum (330–30° circadian phase), corresponding to 01:00–07:00 h, and was highest from 90°–300°, corresponding with 11:00–23:00 h.
Our results extend the finding of circadian variation in mood state, which has previously been documented in other chronobiological studies (Boivin et al., 1997; Koorengevel et al., 2003; Monk et al., 1992; Monk et al., 1997; Murray et al., 2002). However, to our knowledge, this is the first study to examine the circadian variation of mood in the ultra-short sleep/wake protocol.
The protocol resulted in significant mood deterioration in the majority of mood subscales, across all time points on day 2 relative to day 1 (Figure 4). However, it is noteworthy that the mood impairment was likely of minimal clinical relevance, as values on day 2 of the protocol were still within the range of normal functioning (Nyenhuis et al., 1999). For example, aggregate TMD values changed from 3.6 ± 15.6 to 9.1 ± 17.9 between day 1 and day 2. Nevertheless, the good overall mood of the participants was likely due to the inclusion criteria, namely young age, high level of physical activity, and absence of significant mood and/or sleep disturbances.
To our knowledge, this is the first report of mood deterioration with the ultra-short sleep/wake protocol. These results are not consistent with the results of Kripke et al. (2007), who found no mood impairment from day 1 to day 4 of a ~5-day ultra-short sleep/wake protocol. A slight deterioration of mood has also been documented in a previous study using the forced desynchrony protocol (Koorengevel et al., 2003). Most chronobiological studies have not reported whether there were mood decrements associated with the protocols. However, the potential for such decrements seems apparent, considering the sleep loss (Dinges et al., 1997), dimly lit environment (Park et al., 2007), relative isolation (Keller & Nesse, 2005), etc., which are associated with these protocols. Future studies should carefully assess these effects, particularly in examining participants with pre-existing mood disturbances.
Both objective sleep and subjective sleepiness exhibited circadian variation, which is in agreement with previous studies that have utilized the ultra-short sleep/wake protocol (Buysse et al., 2005). Neither objective sleep nor subjective sleepiness were different between days 1 and 2 of the protocol, suggesting that an accumulation of homeostatic sleep drive did not occur, although participants entered the study with differing homeostatic sleep drives due to staggered start times. Whether the ultra-short sleep/wake protocol would be effective at counteracting an accumulation of homeostatic sleep drive over a longer period of observation remains to be studied.
Correlations of Mood and Temperature with Performance
Core body temperature (or, inversely, skin temperature) has been hypothesized to modulate neurobehavioral performance (Wright et al., 2002), possibly via altered central or peripheral synaptic conduction velocity (Rutkove et al., 1997) and/or effects at the preoptic area/anterior hypothalamus, a brain region involved with both thermoregulation and control of vigilance (Raymann & van Someren, 2007). In this study, the association between PVT performance and body temperature was significant but rather modest (r = 0.19–0.45; Table 3). These data are in agreement with those of Wright et al. (2002), who reported correlations of r = −0.24–0.31 between body temperature and PVT performance. Moreover, in the Wright et al. (2002) study, higher versus lower body temperatures did not affect PVT performance when controlling for circadian phase and duration of wakefulness.
We found that PVT performance was slightly less strongly correlated with mood than with body temperature (Table 3), which is consistent with previous findings (Monk et al., 1997). Interestingly, in the same set of participants, mood state was more strongly associated with 200-m swim performance than body temperature (Kline et al., 2007). Thus, at least in our sample of healthy young adults, body temperature seems to be more closely linked to neurobehavioral performance than to anaerobic athletic performance.
There was no apparent homeostatic effect of sleep loss on PVT performance, as no significant trial effects on performance were found and performance was not significantly different between the two days of observation. This is in agreement with our findings that objective sleep and subjective sleepiness were not increased during the second day of observation (Figure 5). However, the associations between subjective sleepiness and PVT performance were similar to those of mood and performance. These results agree with prior studies conducted under conditions of sleep deprivation or chronic sleep restriction (Phipps-Nelson et al., 2003), and extends those findings by indicating that subjective sleepiness remains associated with performance independent of significant homeostatic sleep pressure accumulation.
Study Limitations
One limitation of the study was that it was restricted to healthy young adults, so it is unknown whether these results generalize to other populations. For instance, Blatter et al. (2006) showed some evidence of blunted circadian regulation of PVT performance in older relative to younger adults in the ultra-short sleep/wake protocol. Moreover, research has suggested abnormal circadian function in individuals with affective disorders (Wirz-Justice, 2006).
The possible confounding influence of exercise on PVT performance and mood is another limitation of the study. While in the study, participants also performed six maximal 200-m freestyle swims at regularly scheduled times (Kline et al., 2007). However, since swim trials were always performed in the wake period following PVT performances, all PVT performances occurred 6 h after the swim trials. In addition, the swim trials were only 2–4 min in duration and were always followed by a 1-h bout of attempted sleep, so ~195 min elapsed between a swim performance and a subsequent mood assessment. Furthermore, mood was always assessed prior to the swim performances. Thus, exercise was unlikely to have contributed to changes in PVT performance (e.g., by increased arousal) or mood (e.g., by improving one’s mood state). It is also noteworthy that the participants were competitive swimmers well-accustomed to maximal swim efforts.
Another limitation of the study was that PVT performance was not assessed every wake period. The timing of PVT trials, every 9 h, was initially chosen so that assessments of neurobehavioral performance could be conducted in wake periods separate from swim performance trials. Although significant circadian rhythms were demonstrated for each of the PVT metrics, more frequent assessment of PVT performance would have provided even greater resolution of its circadian rhythmicity.
The lack of assessment of motivation was a notable limitation of the study. Motivation varies with circadian phase, and high levels of motivation are associated with improved cognitive performance independent of circadian phase or prior wakefulness (Hull et al., 2003). Inclusion of a motivation measure could have helped explain PVT performance variation or mood deterioration in the study. Future research should include measurement of subjective motivation, which could help in the interpretation of data.
Two days of recording were insufficient for estimating circadian period, though other studies have shown that period can be estimated with longer duration in the ultra-short sleep/wake cycle (Kripke et al., 2005; Kripke et al., 2007). Another limitation, related to the ultra-short sleep/wake protocol, was that the lack of homeostatic sleep pressure may limit generalizability of the results to normally entrained conditions, in which circadian variation of performance and mood interacts with up to 16–20 h of continuous wakefulness during the daytime. However, the study demonstrated the utility of the protocol for isolating circadian influences, and may be especially useful for the assessment of clinical disorders with a suspected circadian cause, such as certain sleep and affective disorders (Wirz-Justice, 2006). The ultra short sleep/wake protocol also might be good alternative to constant routine protocols, which seem more difficult for participants, and to forced desynchrony protocols, which are far more costly and time-consuming.
Summary
In summary, these results provide additional evidence for circadian variation in neurobehavioral performance, mood and sleepiness using the ultra-short sleep/wake protocol. Using a protocol that spanned slightly longer than 2 days, significant circadian variation was observed in four PVT metrics, SSS, and in each of the subscales of the POMS. These data provide support for further use of the ultra-short sleep/wake protocol to study circadian rhythms.
Acknowledgments
This study was supported by a Gatorade Sports Science Institute Grant, NIH grant HL71560, and a VA Merit Award. We are grateful for the help that Stephen C. Chen, Carrie E. Kline, Annie Y. Lee and Mark R. Zielinski provided with data collection. We are also indebted to Dr. Marsha Dowda for assistance with data analysis.
Footnotes
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Blatter K, Graw P, Munch M, Knoblauch V, Wirz-Justice A, Cajochen C. Gender and age differences in psychomotor vigilance performance under differential sleep pressure conditions. Behav Brain Res. 2006;168:312–317. doi: 10.1016/j.bbr.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Boivin DB, Czeisler CA, Dijk DJ, Duffy JF, Folkard S, Minors DS, Totterdell P, Waterhouse JM. Complex interaction of the sleep-wake cycle and circadian phase modulates mood in healthy subjects. Arch Gen Psychiatry. 1997;54:145–152. doi: 10.1001/archpsyc.1997.01830140055010. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Monk TH, Carrier J, Begley A. Circadian patterns of sleep, sleepiness, and performance in older and younger adults. Sleep. 2005;28:1365–1376. doi: 10.1093/sleep/28.11.1365. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Dement WC. Sleep studies on a 90-minute day. Electroencephalogr Clin Neurophysiol. 1975;39:145–155. doi: 10.1016/0013-4694(75)90004-8. [DOI] [PubMed] [Google Scholar]
- Dinges DF, Powell JW. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Beh Res Meth Inst Comp. 1985;17:652–655. [Google Scholar]
- Dinges DF, Pack F, Williams K, Gillen KA, Powell JW, Ott GE, Aptowicz C, Pack AI. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep. 1997;20:267–277. [PubMed] [Google Scholar]
- Duffy JF, Dijk DJ. Getting through to circadian oscillators: why use constant routines? J Biol Rhythms. 2002;17:4–13. doi: 10.1177/074873002129002294. [DOI] [PubMed] [Google Scholar]
- Frankenfield DC, Rowe WA, Smith JS, Cooney RN. Validation of several established equations for resting metabolic rate in obese and nonobese people. J Am Diet Assoc. 2003;103:1152–1159. doi: 10.1016/s0002-8223(03)00982-9. [DOI] [PubMed] [Google Scholar]
- Graw P, Krauchi K, Knoblauch V, Wirz-Justice A, Cajochen C. Circadian and wake-dependent modulation of fastest and slowest reaction times during the psychomotor vigilance task. Physiol Behav. 2004;80:695–701. doi: 10.1016/j.physbeh.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10:431–436. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- Hull JT, Wright KP, Jr, Czeisler CA. The influence of subjective alertness and motivation on human performance independent of circadian and homeostatic regulation. J Biol Rhythms. 2003;18:329–338. doi: 10.1177/0748730403253584. [DOI] [PubMed] [Google Scholar]
- Keller MC, Nesse RM. Is low mood an adaptation? Evidence for subtypes with symptoms that match precipitants. J Affect Disord. 2005;86:27–35. doi: 10.1016/j.jad.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Kline CE, Durstine JL, Davis JM, Moore TA, Devlin TM, Zielinski MR, Youngstedt SD. Circadian variation in swim performance. J Appl Physiol. 2007;102:641–649. doi: 10.1152/japplphysiol.00910.2006. [DOI] [PubMed] [Google Scholar]
- Koorengevel KM, Beersma DG, den Boer JA, van den Hoofdakker RH. Mood regulation in seasonal affective disorder patients and healthy controls studied in forced desynchrony. Psychiatry Res. 2003;117:57–74. doi: 10.1016/s0165-1781(02)00305-0. [DOI] [PubMed] [Google Scholar]
- Kripke DF, Elliott JA, Youngstedt SD, Rex KM. Circadian phase response curves to light in older and young women and men. J Circadian Rhythms. 2007;5:4. doi: 10.1186/1740-3391-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kripke DF, Youngstedt SD, Elliott JA, Tuunainen A, Rex KM, Hauger RL, Marler MR. Circadian phase in adults of contrasting ages. Chronobiol Int. 2005;22:695–709. doi: 10.1080/07420520500180439. [DOI] [PubMed] [Google Scholar]
- Kubota T, Uchiyama M, Suzuki H, Shibui K, Kim K, Tan X, Tagaya H, Okawa M, Inoue S. Effects of nocturnal bright light on saliva melatonin, core body temperature and sleep-propensity rhythms in human subjects. Neurosci Res. 2002;42:115–122. doi: 10.1016/s0168-0102(01)00310-8. [DOI] [PubMed] [Google Scholar]
- Kudo Y, Uchiyama M, Okawa M, Shibui K, Kamel Y, Hayakawa T, Kim K, Ishibashi K. Correlation between the circadian sleep propensity rhythm and hormonal rhythms under ultra-short sleep-wake cycle. Psychiatry Clin Neurosci. 1999;53:253–255. doi: 10.1046/j.1440-1819.1999.00536.x. [DOI] [PubMed] [Google Scholar]
- Lavie P, Gopher D, Wollman M. Thirty-six hour correspondence between performance and sleepiness cycles. Psychophysiology. 1987;24:430–438. doi: 10.1111/j.1469-8986.1987.tb00313.x. [DOI] [PubMed] [Google Scholar]
- Lavie P, Scherson A. Ultrashort sleep-waking schedule. I Evidence of ultradian rhythmicity in ‘sleepability’. Electroencephalogr Clin Neurophysiol. 1981;52:163–174. doi: 10.1016/0013-4694(81)90164-4. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Profile of Mood States Manual. San Diego: Educational and Industrial Testing Services; 1981. p. 40. [Google Scholar]
- Monk TH, Buysse DJ, Reynolds CF, III, Berga SL, Jarrett DB, Begley AE, Kupfer DJ. Circadian rhythms in human performance and mood under constant conditions. J Sleep Res. 1997;6:9–18. doi: 10.1046/j.1365-2869.1997.00023.x. [DOI] [PubMed] [Google Scholar]
- Monk TH, Buysse DJ, Reynolds CF, III, Jarrett DB, Kupfer DJ. Rhythmic vs homeostatic influences on mood, activation, and performance in young and old men. J Gerontol. 1992;47:221–227. doi: 10.1093/geronj/47.4.p221. [DOI] [PubMed] [Google Scholar]
- Murray G, Allen NB, Trinder J. Mood and the circadian system: investigation of a circadian component in positive affect. Chronobiol Int. 2002;19:1151–1169. doi: 10.1081/cbi-120015956. [DOI] [PubMed] [Google Scholar]
- Nyenhuis DL, Yamamoto C, Luchetta T, Terrien A, Parmentier A. Adult and geriatric normative data and validation of the profile of mood states. J Clin Psychol. 1999;55:79–86. doi: 10.1002/(sici)1097-4679(199901)55:1<79::aid-jclp8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Park DH, Kripke DF, Cole RJ. More prominent reactivity in mood than activity and sleep induced by differential light exposure due to seasonal and local differences. Chronobiol Int. 2007;24:905–920. doi: 10.1080/07420520701669677. [DOI] [PubMed] [Google Scholar]
- Phipps-Nelson J, Redman JR, Dijk DJ, Rajaratnam SM. Daytime exposure to bright light, as compared to dim light, decreases sleepiness and improves psychomotor vigilance performance. Sleep. 2003;26:695–700. doi: 10.1093/sleep/26.6.695. [DOI] [PubMed] [Google Scholar]
- Portaluppi F, Touitou Y, Smolensky MH. Ethical and methodological standards for laboratory and medical biological rhythm research. Chronobiol Int. 2008;25:999–1016. doi: 10.1080/07420520802544530. [DOI] [PubMed] [Google Scholar]
- Raymann RJ, van Someren EJ. Time-on-task impairment of psychomotor vigilance is affected by mild skin warming and changes with aging and insomnia. Sleep. 2007;30:96–103. doi: 10.1093/sleep/30.1.96. [DOI] [PubMed] [Google Scholar]
- Rutkove SB, Kothari MJ, Shefner JM. Nerve, muscle, and neuromuscular junction electrophysiology at high temperature. Muscle Nerve. 1997;20:431–436. doi: 10.1002/(sici)1097-4598(199704)20:4<431::aid-mus5>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Stampi C. The effects of polyphasic and ultrashort sleep schedules. In: Stampi C, editor. Why We Nap: Evolution, Chronobiology, and Functions of Polyphasic and Ultrashort Sleep. Boston: Birkhauser; 1992. pp. 137–179. [Google Scholar]
- Weitzman ED, Fukushima D, Nogeire C, Hellman L, Sassin J, Perlow M, Gallagher TF. Studies on ultradian rhythmicity in human sleep and associated neuroendocrine rhythms. In: Scheving LE, Halberg F, Pauly JE, editors. Chronobiology. Tokyo: Igaku Shoin; 1974a. pp. 503–505. [Google Scholar]
- Weitzman ED, Nogeire C, Perlow M, Fukushima D, Sassin J, McGregor P, Gallagher TF, Hellman L. Effects of a prolonged 3-hour sleep-wake cycle on sleep stages, plasma cortisol, growth hormone and body temperature in man. J Clin Endocrinol Metab. 1974b;38:1018–1030. doi: 10.1210/jcem-38-6-1018. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A. Biological rhythm disturbances in mood disorders. Int Clin Psychopharmacol. 2006;21:S11–S15. doi: 10.1097/01.yic.0000195660.37267.cf. [DOI] [PubMed] [Google Scholar]
- Wright KP, Jr, Hull JT, Czeisler CA. Relationship between alertness, performance, and body temperature in humans. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1370–R1377. doi: 10.1152/ajpregu.00205.2002. [DOI] [PubMed] [Google Scholar]
- Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk DJ. Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. Am J Physiol Regul Integr Comp Physiol. 1999;277:R1152–R1163. doi: 10.1152/ajpregu.1999.277.4.r1152. [DOI] [PubMed] [Google Scholar]