Abstract
Research involving model organisms necessitates recording and archiving many types of animal maintenance and use data. We developed a comprehensive inventory system using FileMaker Pro® to incorporate, record, and archive data on zebrafish stocks, tank organization, husbandry, and fish usage. Our relational database is constructed of tables containing detailed information on fish identity, parents of origin, tank location, mutant phenotypes, caretakers, natural mating and in vitro fertilization experiments, and fish mortality. In addition to its basic annotation and reporting capabilities, the database allows barcode scan entry of several actions, for example, moving a tank of fish, mating or performing in vitro fertilization with specific fish, and recording dead fish. All data are input in real time using either barcode scanning or manual entry. The database provides several types of preformatted reports, as well as printed labels for tank location and stock identification. In summary, we have created a versatile, multipurpose inventory system that can be personalized and enhanced for any zebrafish facility and can be further adapted to organize data and archival information for other model systems or applications.
Introduction
For decades, electronic databases have provided accurate and detailed recording for animal facility management in the maintenance of nonhuman primates,1 mice,2–4 and dairy herds.5 Several electronic databases are suitable for use with multiple types of organisms.6–11 Specialized databases provide features such as storage of mutagenesis screening data (Mutabase3) or statistical and genetic analyses of phenotypes (MouseTRACS4). Inclusion of features that help manage Institutional Animal Care and Use Committee (IACUC) protocols, such as assignment of project numbers to new stocks4 and reminders and submission of animal study protocol updates,10 can further facilitate research studies.
The zebrafish (Danio rerio) has been well established as a model organism for vertebrate studies. Research laboratories and facilities must record and archive many types of data regarding the maintenance and use of zebrafish stocks and the generation and propagation of mutant and transgenic lines. Recording data on animal maintenance, use, and numbers of individuals can be valuable for reporting to institutional animal care and use committees and for inspections, as well as protect from overuse or misuse of fish lines. Often, laboratories will collect several types of data in multiple formats, sometimes in a mix of computer databases, logbooks, and calendars. The comprehensive inventory system we describe here provides one simple interface for recording, querying, and summarizing all relevant data.
At each research institution or laboratory, there may be several, perhaps dozens, of researchers and animal caretakers who will regularly interact with the database. Therefore, real-time capability is essential in maintaining the integrity of the data.3 Certain tasks, such as moving tanks within a facility or creating a new stock record for the purposes of putting larvae into the nursery, are best recorded at the time they are performed. Our database has been created in FileMaker Pro (FMP), a commonly used relational database software package. We configured our application such that the database file and FMP Server software are stored on a central server and FMP client software is used on all desktop computers and facility laptops. This arrangement allows multiple users to access the database concurrently. Thus, a technician may scan barcodes in the fish facility, such as to report a dead fish in a tank, at the same time that a graduate student is creating new stock records or reports from his/her desktop computer. Further, FMP allows for designation of different types of users with various privileges and restrictions. Using administrative privileges, the database file can be readily modified to serve the needs of different facilities. Examples of personalization may include adding new fields to data tables, creating report templates, and changing the room and tank locations to reflect the physical setup of a given aquatics facility.
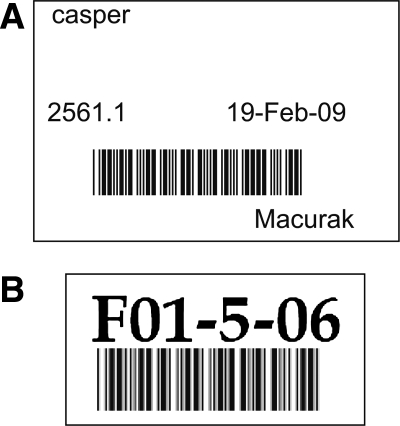
Always germane to the discussion of archival information is the introduction of human error during data entry. The use of barcoded labels can reduce much of this error and is currently used in many animal facilities.3,6,8 We sought to both minimize error and streamline data entry by barcoding not only stock and tank location labels, but also regularly performed tasks such as mating fish or changing the location of a tank of fish (e.g., moving fish to a smaller tank). Providing each facility space or room with the means to scan data and functions readily, such as a laptop and a dedicated water-resistant barcode scanner (Fig. 1), not only reduces human error but also ensures user compliance in updating information in a timely and consistent manner.
FIG. 1.
The Aquatics Facility FileMaker Pro (FMP) database allows for easy and accurate barcode scan entry. (A) Actions, such as reporting that a tank of fish has been used for in vitro fertilization, can easily be entered by barcode scan. This demonstration illustrates the use of a handheld scanner, the scan guide, and the tank label. Also shown is the tank location label (on the rack). (B) Each station is composed of a laptop and handheld Bluetooth scanner, paired by serial number. This pairing allows for multiple stations and database users in one room.
Currently, there are several commercially available animal inventory systems. In 2001, eight such software packages were compared.12 The programs ranged in cost: LAMS™, a simple system for colony inventory, was reported as free for academic, educational, and nonprofit institutions, whereas Topaz™, a system that incorporates animal ordering and IACUC protocol data, has an upper end of $100,000. Other software found through searching the web include an application employing barcodes in agrobusiness management (Argos software) and the Dairybase system, which couples animal sensors and a relational database for dairy cattle management.5 Nonbarcoding systems include one geared toward businesses such as ranches and kennels managing animal inventory and financial data (Animal Trac; Starshine Enterprizes) and web-based systems such as MouseTRACS, a system for husbandry and screen management,4 and mLIMS Online Mouse Info System for mouse colony management (BIOINFORX). At the time we embarked on this project, commercial software choices were limited and none were specifically tailored for the needs of zebrafish researchers. We, therefore, set out to design a program that incorporates the benefits of a relational database and adds specialized features for management of genetic research in a large-scale aquatics facility.
Data Tables
The comprehensive inventory system we developed is based on a relational database model. Relational databases are composed of tables that relate to each other through shared characteristics (e.g., stock number or caretaker). Each entry in a data table is referred to as a record. In FMP, records can be sorted, queried, and viewed independently or in a list format. Our relational database is composed of tables storing stock information, tank location, assigned caretaker, and mutant/transgenic properties.
The records in the stock table contain all the information that was traditionally archived in logbooks in zebrafish research labs, such as parental identity, date of birth, and allele designations (Fig. 2). New records are automatically assigned a unique stock number. Records display additional relational data such as associated tank locations, history of deaths, and a record of fish usage (namely, mating and in vitro fertilization [IVF]). A stock management feature of the database enables the use of given fish lines to be limited to certain individuals. For example, although all individuals may record mortalities and health problems for any tank, only an approved individual may work with the fish in a restricted use stock.
FIG. 2.
Storage of stock-related data is much improved over previous methods. Screen-shot of a sample stock record from the Aquatics Facility database.
Tank location tables contain information specific to a given facility, such as room identifiers, rack numbers, row number on a given rack, and tank position within a row. As such, the tank location is composed of practical positional information and guides the user to locate any tank efficiently. Further, the tank location aspect of the database supports a dynamic facility in that rooms, racks, rows, and positions can be changed to reflect changes in the physical space. A simple example involves swapping a 12-position, 3-L tank manifold with a 20-position, 1-L tank manifold: eight additional tank positions are “activated” in the database and new barcode labels for these positions are automatically queued for printing. Relational data that are displayed in the tank record view include the associated stocks, any recorded dead fish or problems, and usage information.
The mutation table contains detailed information regarding mutant and transgenic lines isolated or established and bred in the facility (Fig. 3). The record view contains fields that provide ample space for assigning allele numbers and detailing morphological and gene expression phenotypes. An image container field allows for placement of digital images. The related stock numbers are displayed and provide a link to the stock records.
FIG. 3.
Descriptive data, both written and imaged, may be stored in our Aquatics Facility database. (A) Screen-shots showing sample mutant data and (B) sample transgenic data from the mutants and transgenics section of the Aquatics Facility FMP database.
Employee tables include contact information, lab association, and e-mail preferences. As lab members are often responsible for raising and maintaining multiple lines of stock, the database allows for assignment of ownership (“caretaker”) to each stock. The employee record view displays the stocks and tank locations that a caretaker manages.
Data Entry by Barcode Scan or Manual Entry
Nine separate actions may be recorded in real time by barcode scan (Fig. 4) or by manual entry of data. The nine actions, or functions, fall into four categories (Fig. 5). The first category, moving a tank of fish, is composed of three actions: moving a tank onto the system, off the system, or from one location to another within the system. The second category involves fish usage, namely, performing IVF on or mating a tank of fish. The third category contains two actions pertinent to mortality and health data: reporting dead fish or problems within a tank. Last, two actions create new stock records that are appended with a decimal plus positive integers to distinguish identified founders or carriers of a gene or transgene of interest from their stock of origin. The new stock records are derived from the source stock record and thus share the base stock number (e.g., stocks 1234 and 1234.1). Modifying stock numbers by the addition of an informational suffix allows for efficient recognition of carriers, reducing confusion between unidentified fish in stocks and the corresponding identified heterozygotes, homozygous mutants, or transgenic founders.
FIG. 4.
Sample stock barcode output from the Aquatics Facility database. (A) Stock labels are one of several barcoded items in the database. They may be printed on static cling, vinyl laser labels and placed on the tank as an adhesive-free and waterproof solution. (B) Tank location labels may be printed on permanent, polyester laser labels and affixed to tank racks.
FIG. 5.
Nine actions may be recorded by barcode scan or manual entry. (A) Screen-shot of the manual data entry section of the Aquatics Facility database. Upper left box records actions relating to fish use, lower left box relates to creating new stock records upon identification of carriers or founders of interest, middle box relates to moving tanks of fish, and right box relates to reporting dead fish or problems within a tank. (B) Sample flowchart from Scan Instruction Sheet. Barcode scan entry is performed in three or four steps, depending on the action. Recording fish crosses involves three scans: employee barcode, the cross fish function barcode, and the relevant tank location barcode.
An important aspect of the nine functions is that they quickly test whether the scanned entry is acceptable and output either a positive or negative feedback sound. For example, if a user scans the barcode for the function to “cross fish” and then scan enters a tank label barcode for fish that have been already used for mating within the same week, a feedback sound will first indicate that the barcodes were successfully scanned. A distinct sound will then signal that the function is unacceptable. Other tests performed by the database include whether a given tank may be used for IVF based on the timing of their prior use, whether a tank position does not correspond with an active fish record, or whether the user has permission to work with a given tank. Further, the feedback sounds correspond to sound files that can be personalized for each system (e.g., Homer Simpson's catch phrase “D'oh!” serves as our negative feedback sound). The selection of different feedback sounds and the prerequisite pairing of each scanner and laptop permit multiple, independent stations (see hardware discussion below and Fig. 1) to be used concurrently within the same room.
Having the option to introduce data through either manual or barcode scan entry provides users with flexibility on where and when to update their database information. If all of the barcode scanners are in use, a lab member may choose to enter data from his or her personal computer or at another station in the facility rather than wait for an available scanner. Manual data entry is performed using a computer keyboard to enter the relevant data when prompted, such as stock and tank location numbers. Instead of playing sound files after each step, feedback is received only when there is an error, and in those instances, descriptive error boxes are displayed on the screen.
An important component for implementation of the database is the research and selection of a suitable hardware. We prioritized three characteristics: simplicity, reliability, and budget. We chose Bluetooth (BT) technology for data transmission, because it is wireless, inexpensive, and easy to use and there are a multitude of handheld scanners employing BT technology on the market. We selected the Wasp Freedom handheld barcode scanner for its impact- and water-resistant properties, 160-foot BT range, and reasonable price ($400–$500 USD, WWS800; Wasp Barcode Technologies). For the aquatics facility, we selected laptops meeting minimal requirements: network connectivity, a USB communications port for BT adapters, a sound card for audio notifications, and the ability to run FMP software. Each scanning station is composed of a laptop and BT scanner, paired by serial number. We placed two scanning stations in a 52-rack facility and one in each of two smaller, alternate light cycle rooms. Although most computers have innate BT capability, we chose Linksys Bluetooth USB adapters (USBBT100; Cisco) to boost the range of wireless connectivity for inexpensive PC laptops.
The much-anticipated advent of electrostatic, vinyl label sheets that are compatible with laser printers has provided us with a solution for adhesive build-up on tanks and an alternative to expensive thermal printers. We print labels using Papilio Classic Color Laser Static Cling sheets (HPSCW8511KH; Papilio), trim the sheets to size, and place individual fish stock labels on the appropriate tanks (Fig. 3A). We use another permanent, bar-coded polyester laser label (44A-UN1267; Cils International) for each tank location (Fig. 3B), affixed to the steel racks. These water-resistant labels have been in place for 5 years.
A Versatile Tool for Facility Management
Aquatics facilities are typically located in rooms separate from the laboratory. To keep laboratory members apprised of the health of their stocks, the database provides each user an option to receive e-mail notifications regarding the fish that they are responsible for maintaining. The database will automatically e-mail the caretaker when dead fish and problems are reported.
The database includes several preformatted reports that provide the user with additional benefits. Two of the reports assist in arranging space for juvenile fish: one report lists all fish on the system that are younger than 1 month old and the other report lists all available tank locations within the facility. For facilities with nursery space, these reports can assist with planning how to relocate fish from the nursery to the area with adult tanks. Another report summarizes all tank contents in physical order, supplying a printout that may be double-checked for accuracy by performing a facility “walk-through.” To conserve electrostatic label sheets and streamline label production, the creation of new stock records automatically adds stock label entries to a queue for bulk printout. The “new stock labels” report queries the database for recently updated entries and provides a printable compilation of barcode labels that can be readily distributed to the correct tanks.
The database may be modified to accommodate the needs of a particular facility or institution. Personalization could range from changing the appearance of the interface to modifying the tank label format to adding or removing printed fields. Additional tables could be added, for example, to keep track of sperm freezing, shipping and receipt of fish, or for managing a mutagenesis screen.
Discussion
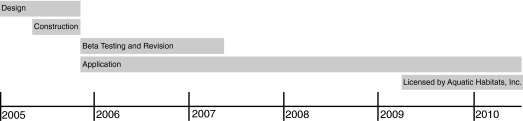
With the assistance of a database developer, we produced a real-time, network-accessible database to manage an ∼4000-tank aquatics facility and to store descriptive and archival information (Fig. 6). The database provides a facile and comprehensive way to preserve and access all data associated with maintaining a thriving zebrafish research facility. In 2009, we provided licensing rights to Aquatic Habitats to modify, support, and distribute the database, with the goal of making it accessible to the greater zebrafish research community. Upgraded versions along with the integration of improved computer hardware technology ensure that the inventory system will continue to evolve to fit the needs of its clients.
FIG. 6.
Timeline for design and implementation of the Aquatics Facility FMP database. The authors developed the database organization during the design phase. The program file was created during the construction phase and subsequently beta-tested and revised.
Using FMP software, the database can be readily manipulated and enhanced to suit the needs of any research group. One enhancement could involve adding a script that outputs the dead fish and health problems data into a timeline to determine trends and solve global facility issues. A unique strength of the database is that it contains not only active records but also archived records. A novel report could track the ancestral lineage for a current or recent stock. Because parental data are recorded for each stock, a multigenerational trace is possible. One valuable application is to follow mutations identified in mutagenized backgrounds; isolating a desired phenotype is often complicated by the presence of multiple mutations. The database provides an organized format to record the breeding scheme and details associated with mutant recovery. Genetic screen organization, transgenic line maintenance, and facility management are powerful features of the database. This comprehensive and versatile tool eliminates the need for multiple databases. With specialized modifications, we expect that it will serve as a valuable template for research facilities using other model organisms or for other applications.
Acknowledgments
The authors thank the Carnegie IT group Bill Kupiec, Mahmud Siddiqi, Rafael Villagaray, and Connie Jewell for supporting the project during the development phase and for helping to fine-tune and maintain the current database. The authors thank members of the Farber and Halpern laboratories for their suggestions for improvement, by assisting with beta testing and providing ongoing feedback, and, specifically, Courtney Akitake and Tim Mulligan for critical reading of the manuscript. The authors also gratefully acknowledge the zebrafish research groups at the University of Oregon in Eugene, who developed and generously shared the initial Filemaker database for recording zebrafish lines and established a valuable template for future modifications. The authors thank Jay Shepard of Ease Technologies for database implementation and Allan Spradling and the Carnegie Institution for Science for support of this project.
Disclosure Statement
In 2005, the authors designed a zebrafish facility database for the sole purpose of meeting the organizational and archival needs of our two research laboratories. The authors contracted a third-party software development company to help construct the database and to incorporate bar-coded scanning features. Although researchers at other institutions showed an interest in the software, the authors did not have the expertise or manpower to modify and support the database for the specific needs of outside users. The authors' regular communications with Aquatic Habitats, Inc. (AHAB), led to discussions regarding the database and its usefulness to the greater community of zebrafish researchers, with the outcome that, in 2009, AHAB was granted licensing rights to maintain, upgrade, and market it. The Carnegie Institution and the authors benefit from this arrangement through financial compensation for the commercialized product (FinCensus). The database is currently being improved upon by AHAB, who is incorporating feedback from users and plans to update the software regularly to serve the changing needs of researchers. The original FMP template is also available upon request from the authors for noncommercial use.
Note Added in Proof
We recently learned that Ted Moens (http://www.tswebwork.com) has developed a web-based application for managing zebrafish facilities and for easy tracking of genetic markers and stock lineages.
References
- 1.Sciabbarrasi SA. London WT. Computerized primate information retrieval system (PIRS) Lab Anim Sci. 1974;24:773–782. [PubMed] [Google Scholar]
- 2.Kalbach H. McCallum WF. Konvicka AJ. Holland MT. Computer record keeping in a large rodent colony. Lab Anim Sci. 1977;27:660–666. [PubMed] [Google Scholar]
- 3.Strivens MA. Selley RL. Greenaway SJ. Hewitt M. Liu X. Battershill K, et al. Informatics for mutagenesis: the design of mutabase—a distributed data recording system for animal husbandry, mutagenesis, and phenotypic analysis. Mamm Genome. 2000;11:577–583. doi: 10.1007/s003350010110. [DOI] [PubMed] [Google Scholar]
- 4.Ching KA. Cooke MP. Tarantino LM. Lapp H. Data and animal management software for large-scale phenotype screening. Mamm Genome. 2006;17:288–297. doi: 10.1007/s00335-005-0145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spahr SL. Dill DE. Leverich JB. McCoy GC. Dairybase: an electronic individual animal inventory herd management system. J Dairy Sci. 1993;76:1914–1927. [Google Scholar]
- 6.Wootton R. Animal house stock control based on bar-coded cage labels. Lab Anim. 1985;19:359–367. doi: 10.1258/002367785780887482. [DOI] [PubMed] [Google Scholar]
- 7.Wootton R. Flecknell PA. Towards the computerized animal house: the Newcastle University Animal House Management System. Lab Anim. 1986;20:165–172. doi: 10.1258/002367786780865115. [DOI] [PubMed] [Google Scholar]
- 8.Pryor C. Frankenfield D. Klein H. Terpeluk W. Washington SA. Mourad NT. A comprehensive, barcoded system for the management of animal information in a research facility. Lab Anim (NY) 2001;30:36–38. [PubMed] [Google Scholar]
- 9.Wasserman RS. Blumrick K. Liddell RW., 3rd An automated interactive animal colony management system. Lab Anim Sci. 1982;32:550–552. [PubMed] [Google Scholar]
- 10.Calzone T. Montijo KS. St. Claire MB. Lamoreaux E. An integrated database for managing animal study proposals and animal inventory for the small animal facility. Lab Anim (NY) 2001;30:28–31. [PubMed] [Google Scholar]
- 11.Rieger D. Beriault R. A microcomputer based program for the storage and retrieval of experimental animal information. Lab Anim Sci. 1983;33:390–394. [PubMed] [Google Scholar]
- 12.Novak G. Schub T. Software for lab animal facilities. Lab Anim (NY) 2001;30:39–43. [PubMed] [Google Scholar]