Abstract
Background
We retrospectively explored changes in immunological parameters in men with biochemically recurrent prostate cancer treated with either 5mg or 25mg of lenalidomide in a randomized phase 2 trial, and determined whether those changes correlated with disease progression.
Methods
Cytokine levels were compared for each patient at baseline and after 6 months of treatment with lenalidomide. Regression models for correlated data were used to assess associations of cytokine levels with lenalidomide treatment effect. Estimates were obtained using generalized estimating equations (GEE). Changes in circulating anti-prostate antibodies were evaluated using a high-throughput immunoblot technique.
Results
Treatment with lenalidomide was associated with global changes in immune-reactivity to a number of prostate-associated antigens, as well as with changes in circulating levels of the TH2 cytokines IL-4, IL-5, IL-10 and IL-13. Disease progression in treated patients was associated with an increase in circulating IL-8 levels, while IL-8 levels decreased significantly in non-progressors.
Conclusions
Lenalidomide demonstrates immunomodulatory properties in patients with biochemically recurrent prostate cancer. The induction of novel anti-prostate antibodies is a potential mechanism for lenalidomide response. Changes in serum IL-8 levels may serve as a potential biomarker in treated patients. These hypotheses require formal testing in future prospective trials.
Keywords: prostate cancer, antibody, cytokine, IL-8, lenalidomide
Introduction
Prostate cancer is the most common malignancy in men, with over 200,000 new cases in the United States alone each year[1]. While radical prostatectomy is curative for most patients with localized prostate cancer, approximately 20–40% of men will experience disease recurrence within 10 years of surgery[2]. Due to the near universal availability and high sensitivity of PSA testing, most patients with disease recurrence after prostatectomy present with a rising PSA in the absence of detectable local or distant recurrence. Once this state of ‘biochemical recurrence’ has occurred it is often incurable although survival may be prolonged [3]. Currently, there is no consensus on the optimal management of non-metastatic biochemically recurrent prostate cancer[4,5].
A recent study of sixty men with non-castrate, non-metastatic, biochemically recurrent prostate cancer showed that lenalidomide, a thalidomide analogue with proven efficacy in the treatment of patients with multiple myeloma, was associated with long-term disease stabilization and PSA declines[6]. Both in vitro and clinical studies provided evidence that lenalidomide may mediate its anti-tumor effect through a variety of mechanisms including inhibition of angiogenesis[7], direct induction of cell cycle arrest[8], and through immunological mechanisms such as enhancement of T-cell responses, inhibition of T regulatory cells[9] and/or an increase in NK cell activity leading to enhanced antibody-dependent cellular cytotoxicity (ADCC)[10]. Immune-enhancing aspects of lenalidomide have also been demonstrated in patients treated on a phase I study in patients with solid tumors. In these studies, increased circulating activated/memory CD45RO+ T cells were noted, in addition to increased serum levels of cytokines such as soluble interleukin-2 (sIL-2) receptor, granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-12, tumor necrosis factor-α (TNF-α) and IL-8[11].
Our study of lenalidomide in the treatment of biochemically recurrent prostate cancer patients provided a unique opportunity to examine the immunological effects of lenalidomide in this population. It is currently unknown how lenalidomide modulates the immune responses in these patients, particularly in patients who show evidence of a clinical response to treatment with lenalidomide. Here we aimed to determine the immunological effects of lenalidomide treatment in men with prostate cancer, and to determine whether specific immunological changes could be associated with clinical response.
Patients and Methods
Patients
Serum samples were prospectively collected from patients participating in a blinded, randomized controlled trial examining two dose-levels of lenalidomide in men with non-metastatic biochemically-recurrent prostate cancer. Detailed experimental methods from that trial have been published previously[6]. Briefly, this was a single-center 60-patient study conducted at the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center. Eligible patients met the following criteria: they were ≥18 years old, had histologically confirmed prostatic adenocarcinoma with a rising serum PSA level after local therapy (prostatectomy, radiotherapy, or both) and no evidence of loco-regional or distant metastasis as determined by radiographic studies, had adequate bone marrow function, adequate renal and hepatic function, and non-castrate serum testosterone levels (>150 ng/dL). Patients were required to have an Eastern Cooperative Oncology Group (ECOG) performance status ≤2. Prior systemic chemotherapy, androgen deprivation therapy (ADT), and biologic or vaccine therapy must have been discontinued for at least 6 months prior to study entry. All patients signed an IRB-approved consent form. Eligible patients were stratified according to PSA doubling time (PSADT) (<3 months vs. 3.0–8.9 months vs. ≥9.0 months), prior local treatment (surgery and/or radiation therapy), and prior ADT use, and were then randomly assigned to receive identically appearing oral lenalidomide capsules at doses of 5mg/day or 25 mg/day. Both the patients and the investigators were blinded to the dose of lenalidomide assigned. Treatment was administered on days 1 through 21 of a 28-day cycle, and continued until disease progression or dose-limiting toxicity was observed.
Outcomes: Definition of disease progression
Patients underwent monthly follow-up visits for toxicity, and received physical exams every two months. Serum PSA and testosterone levels were obtained on the first days of cycles 1, 2, 4, and 6; and were processed by a single core laboratory at the Johns Hopkins Hospital. Imaging studies (CT and bone scan) were performed every 6 months. Protocol treatment continued until biochemical or clinical disease progression or dose-limiting toxicity occurred. Biochemical progression was defined as a ≥25% increase in PSA level over baseline. Clinical progression was defined as new evidence of disease by physical exam (including new findings on digital rectal exam) or radiographic scans that were suggestive of local or distant disease recurrence. Patients without evidence of disease progression or dose-limiting toxicity after each 6 month period of treatment were treated for an additional 6 months, or until one of the above events occurred. For the present analysis, we dichotomized lenalidomide progressors from non-progressors using the following definition: patients who had evidence of biochemical, clinical, or radiographic progression during the first 6 months of treatment were defined as experiencing disease progression, while those who had not developed biochemical, clinical, or radiographic progression at the first 6-month evaluation were defined as not experiencing disease progression.
Cytokine analysis
Frozen serum samples collected during the trial were available for 51 out of the 60 trial participants. All samples were stored at −80°C until used for analysis. For cytokine analysis, a patient’s serum sample obtained during treatment cycle 6 was compared to the serum sample obtained prior to the initiation of lenalidomide (i.e. the baseline sample). If a patient experienced disease progression prior to cycle 6, a serum sample collected at the start of the last treatment cycle was used for analysis.
The Bioplex 200 (Bio-Rad, Hercules, CA) platform was used to determine the absolute concentration (in pg/ml) of 28 target proteins in banked sera samples. A multiplexed bead-based immunoassay was performed in duplicate for each serum sample following the manufacturer’s protocols and using the supplied cytokine standards. The concentration of each cytokine was determined using a 5-parameter log curve fit using the supplied software. The Human 27-plex panel includes validated assays for IL-1b, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12(p70), IL-13, IL-15, IL-17, eotaxin, basic FGF, G-CSF, GM-CSF, IFNγ, IP-10, MCP-1, MIP1a, MIP1b, PDGF-BB, VEGF and TNFα. Levels of TGFβ were assessed separately using the MAP TGFβ1 single-plex assay (Millipore, Bilerica, MA).
Statistical methods
The primary statistical endpoints considered in this study were treatment-related changes in the 28 cytokine levels from serum samples taken before and after treatment with two different doses of lenalidomide. Extensive univariate analyses stratified by dose showed that cytokine levels were altered similarly with the two doses used in the trial. Likewise, adjusting pre- and post-treatment changes by dose confirmed a lack of a dose-response relationship on cytokine levels. Therefore, data from the low (5 mg/day) and high (25 mg/day) lenalidomide doses were subsequently combined for further analyses. Cytokine levels that were out of the range of detection were either: treated as being equal to the lower limit of detection for levels that were below the lower limit of detection, or discarded for levels above the upper level of detection.
The primary comparisons included the pre- and post-treatment changes in cytokine levels by dose and progression status. Our hypothesis was that treatment with lenalidomide would modulate cytokine levels and that this change would be greater among patients responding to treatment (i.e. the non-progressors). A within-patient median normalization and log (base 2) transformation was applied before analysis. Because these analyses are exploratory and hypothesis-generating rather than confirmatory, no adjustment was made for multiple comparisons.
Comparisons were visualized using box-and-whisker plots of the median-normalized data and the differences obtained when subtracting pre-treatment values from post-treatment values. The length of the box is the interquartile range (IQR) of the data and depicts the spread of the middle 50 percent of the observations. The median is displayed with a horizontal line inside of this box. The lines projecting out from the box extend from the upper and lower quartiles to values defined as adjacent values. The adjacent values are the upper quartile plus 1.5 × IQR and the lower quartile minus 1.5 × IQR. Any value lying outside of this range is displayed with an open circle and can be considered an outlier.
Cytokine levels were compared for each patient at two time points: at baseline (before study entry) and after 6 months of treatment with lenalidomide. This resulted in two repeated measures per patient for each outcome. Regression models for correlated data were used to assess associations of cytokine levels with lenalidomide treatment. Estimates were obtained using the generalized estimating equations (GEE) approach of Liang and Zeger[12], as previously described in more detail [13,14]. A compound symmetric covariance structure was assumed for these regression models since there was no reason to believe that measures on the different specimens, pre- or post-treatment, would have different variances or that pairs of these measures would have different correlations. For comparisons by clinical response status (progressors versus non-progressors), the vector of pre- and post-treatment cytokine levels among patients was modeled as a function of time point, response, and the interaction of the two.
Boxplots were generated using R [15] and all other statistical computations were performed using SAS (version 8.0; SAS Institute, Cary, NC). The SAS procedure GENMOD was used for GEE regression analyses. All p values are two-sided and all confidence intervals (CI) are at the 95% level.
High Throughput Immunoblot (HTI)
Stored serum samples were also used to perform HTI analyses. Forty-nine of 51 patients treated on the lenalidomide trial with available sera were analyzed. A series of 34 patients with non-metastatic biochemically-recurrent prostate cancer who were treated with standard androgen deprivation therapy (ADT) were used as a comparison group for these studies. These patients had serum samples collected before and 3 months after ADT initiation. Phage immunoblot was performed as previously described [16,17]. In brief, lambda phage encoding 126 unique antigens were spotted in triplicate in a 16×24 array onto E. coli bacterial lawns using a Biomek FX liquid handling robot. These individual antigens included 29 cancer-testis antigens [18], 40 antigens identified in patients with chronic prostatitis [3], and 57 antigens identified in individual patients, some of whom were treated with androgen deprivation or other immunomodulatory therapies [19,20,21]. Encoded proteins were transferred to nitrocellulose membranes, washed, blocked, and probed with sera from patients pre- or post-treatment, diluted 1:100 in isotonic buffer. Human IgG was then detected with an IgG-specific secondary antibody conjugated to alkaline phosphatase and immunoreactivity was detected by development with 0.3 mg/mL nitro blue tertazolium chloride (NBT) (Fisher Biotech, Hampton, NH) and 0.15 mg/mL 5-bromo 4-chloro 3-indoylphosphate (BCIP) (Fisher Biotech). Membranes were digitally scanned and then assessed visually, with individual plaques scored positive or not positive by four independent observers, blinded to the treatment, timing of sample acquisition and membrane layout, as previously reported [22,16]. All of the membranes for the entire study were evaluated by the same observers in a single session. Triplicate samples were evaluated for each antigen, and immunoreactivity to individual antigens was scored positive if there was concordance among 3 of 4 observers, and if immunoreactivity was scored positive in at least two of the three replicates. Heatmap Builder software (version 1.1; Stanford University, Stanford, CA) was used to generate heatmaps displaying changes (gain, loss, or no change) in IgG immunoreactivity to individual antigens following treatment with lenalidomide or ADT.
Results
Treatment with lenalidomide induces changes in immunoreactivity to prostate-associated antigens
We examined changes (gain, loss, or no change) in IgG immunoreactivity to 126 individual prostate-associated antigens following lenalidomide treatment in 49 of the 51 available patient samples using the HTI method. A heatmap depicting changes in IgG response to these antigens during lenalidomide treatment compared to before initiation of lenalidomide is shown in Figure 2B. For comparison, a heatmap depicting IgG reactivity changes in 34 separate patients before and after initiation of ADT is shown in Figure 2A. As demonstrated, treatment with lenalidomide is associated with global changes in immunoreactivity to the prostate-associated antigens that we interrogated. In the 49 patients studied, there were 87 instances where immunoreactivity to an antigen was gained and 47 instances where immunoreactivity was lost following lenalidomide treatment. This equates to an average change in immunoreactivity of 2.73 antigens per patient. In contrast, among the 34 patients who underwent ADT, there were 28 instances of gained immunoreactivity and 18 instances of lost immunoreactivity to the same set of antigens, an average of 1.35 changes in immunoreactivity per patient. More specifically, immunoreactivity changes that were differentially observed in lenalidomide-treated patients but not in ADT-treated patients included those involving PSA, RP11-738B7, O-fucosyltransferase, adducin 1, chitobiase, cytovillin 2, SSX1, SSX2, LAGE-1, SPA 17, MAGE-B1, cancer/testis antigen 2, prolactin-induced protein, plexin B2, acetyl-coenzyme A acyltransferase 1, neuronal PAS domain protein 2, FLT3 ligand, and chromosome 4 gene contig. A summary table of the most frequent immunoreactivity changes by antigen for the lenalidomide-treated patients is shown in Table 2. Interestingly, 7 of the lenalidomide-treated patients (14%) gained immunoreactivity to prostate specific antigen (PSA) while this did not occur in any of the men treated with ADT. In Kaplan-Meier analysis, progression-free survival was greater among lenalidomide-treated men who gained immunoreactivity to PSA after initiation of lenalidomide treatment compared to those who did not (log-rank test; p = 0.046) (Figure 2C).
Figure 2. High Throughput Immunoblot Analysis.
Columns represent individual patients, rows represent individual antigens assayed. Background green = no change, light green = new reactivity, black = loss of reactivity. All comparisons are pre versus post treatment.
A. Patients treated with androgen suppression (N = 34)
B. Patients treated with lenalidomide (N = 49)
C. Kaplan-Meier analysis of progression-free survival in lenalidomide-treated men who gained immunoreactivity to PSA after initiation of lenalidomide treatment compared to those who did not
Table 2.
| Antigen | # of patients (out of 49) gaining immunoreactivity | # of patients (out of 49) losing immunoreactivity |
|---|---|---|
| Chromosome 1 gene contig3 | 7 | 7 |
| Lage 1 | 9 | 3 |
| Prolactin-induced protein | 8 | 4 |
| Recombination signal binding protein (RBPJK) | 6 | 3 |
| SSX-2 | 5 | 4 |
| Adducin 1 | 6 | 2 |
| Prostate specific antigen | 7 | 0 |
| Chitobiase | 5 | 1 |
| SSX-1 | 3 | 1 |
| Mage B1 | 2 | 0 |
| Mage A4 | 1 | 1 |
| Mage A3 | 0 | 1 |
| Total events for 49 patients* | 87 | 47 |
Changes in cytokine concentrations are associated with lenalidomide treatment
Since it has been hypothesized that lenalidomide treatment results in immunologic effects, we examined selected cytokine levels in sera from prostate cancer patients before and 6 months after initiation of lenalidomide to determine whether treatment was associated with changes in systemic cytokine concentrations (Figure 3). Table 3 shows the results of our analysis for 12 biologically-relevant cytokines that were assayed. Significant changes in the levels of IL-2, IL-4, IL-5, IL-10, IL-13, and G-CSF were observed in patients after treatment with lenalidomide was initiated. In all cases, lenalidomide treatment was associated with increased serum levels of these six cytokines. This pattern is especially interesting, in that it corresponds generally to a TH2 signature [23], as would be expected, given the changes in IgG antibody production noted above.
Figure 3. Lenalidomide treatment is associated with changes in cytokine levels.
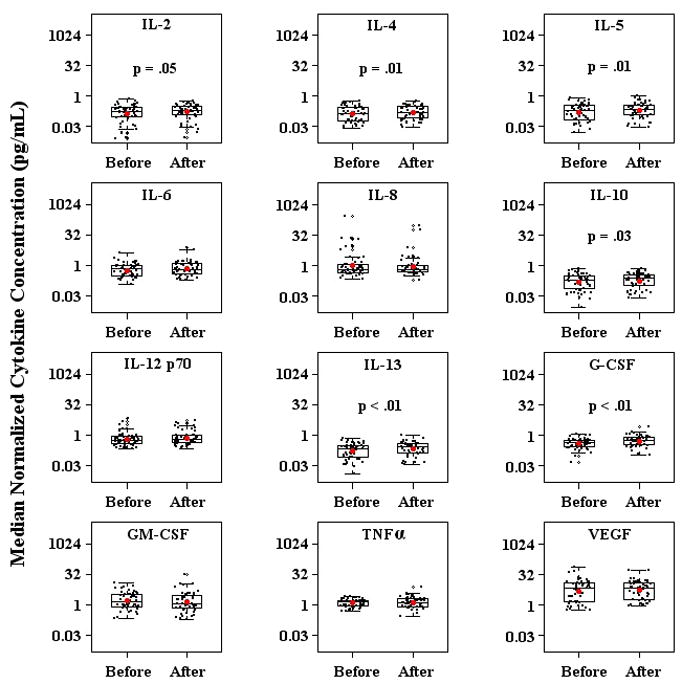
Median normalized cytokine concentrations before and after initiation of lenalidomide treatment in all 51 analyzed patient samples. The height of each box is the interquartile range (IQR), the horizontal line inside the box is the median, and the whiskers extend beyond the upper and lower quartiles by 1.5× IQR. Values lying outside of this range are considered outliers and are denoted by open circles. P values compare the change in cytokine concentration before and after initiation of lenalidomide.
Table 3.
| Cytokine | Fold Change (FC) | 95% Confidence Interval of FC | p value* |
|---|---|---|---|
| IL-2 | 1.22 | 1.01–1.47 | 0.05 |
| IL-4 | 1.14 | 1.04–1.26 | 0.01 |
| IL-5 | 1.27 | 1.07–1.50 | 0.01 |
| IL-6 | 1.20 | 1.00–1.44 | 0.06 |
| IL-8 | 0.89 | 0.53–1.47 | 0.65 |
| IL-10 | 1.18 | 1.02–1.36 | 0.03 |
| IL-12 p70 | 1.16 | 0.93–1.45 | 0.19 |
| IL-13 | 1.29 | 1.11–1.49 | <0.01 |
| G-CSF | 1.34 | 1.15–1.57 | <0.01 |
| GM-CSF | 0.86 | 0.64–1.15 | 0.31 |
| TNFα | 1.08 | 0.96–1.22 | 0.20 |
| VEGF | 1.07 | 0.90–1.27 | 0.10 |
Changes in IL-8, IL-12 p70, and IL-13 concentrations after lenalidomide treatment are associated with clinical response
We next investigated whether changes in cytokine levels after initiation of lenalidomide correlated with disease progression in these patients. To perform these analyses, we calculated fold changes in the 12 relevant cytokines delineated above (Figure 3) after stratifying patients by their disease progression status: those in whom progression occurred prior to the 6-month evaluation, versus those without progression after 6 months of treatment. As shown in Figure 4 and Table 4, changes in the concentrations of IL-8, IL-12 p70, and IL-13 differed pre-and post-lenalidomide treatment among patients with versus without disease progression. Most significantly, patients who did not experience disease progression showed a mean 51% decrease in IL-8 levels after treatment with lenalidomide as opposed to a 58% increase in IL-8 levels in patients who did experience progression. IL-12 p70 showed a different pattern than IL-8, with a relative increase in patients without progression, while remaining relatively unchanged in patients with progression. This analysis confirmed the changes in IL-13 noted earlier, but suggested that this was a lenalidomide (rather than disease-associated) process, as similar changes were noted in both progressors and non-progressors. In summary, only a few of the cytokine changes noted previously were different between progressors and non-progressors, but among these, the changes in IL-8 are of particular interest as the relative increase in progressors corresponded to a relative decrement in non-progressors; suggesting that IL-8 levels could potentially be re-evaluated as a biomarker in future studies of prostate cancer patients treated with lenalidomide.
Figure 4. Cytokine changes in lenalidomide treated patients stratified by progression status.
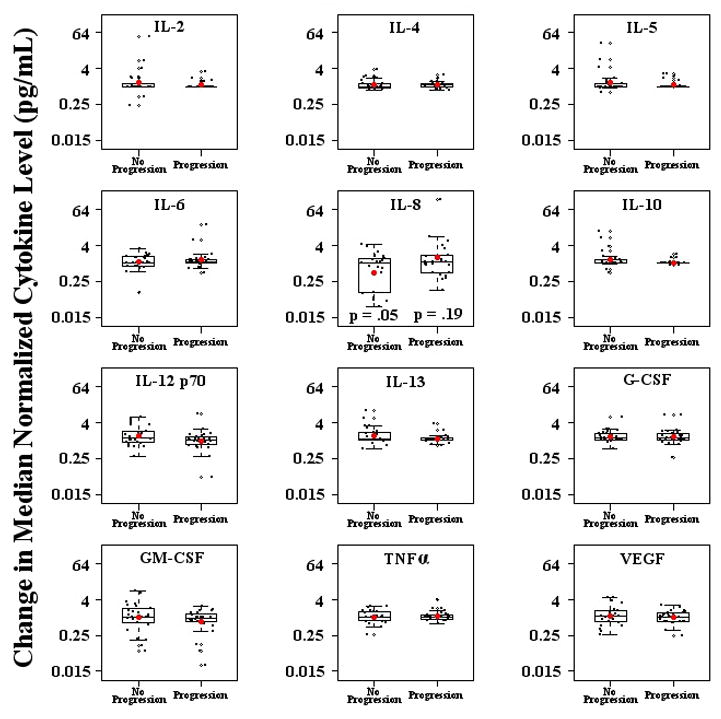
Change in the median normalized cytokine concentrations before and after treatment with lenalidomide, comparing patients that did and did not develop disease progression. P values denote the change in cytokine concentrations before and after initiation of lenalidomide for patients who experienced disease progression (progressors) versus those who did not (non-progressors). On the y-axis, a value of 1 is equivalent to no change in cytokine level after lenalidomide treatment.
Table 4.
| Cytokine | Fold Change (FC) in Progressors (95% CI; p-value) | FC in Non-Progressors (95% CI; p-value) |
|---|---|---|
| IL-2 | 1.11 | 1.34 |
| IL-4 | 1.11 | 1.18 |
| IL-5 | 1.16 | 1.36 |
| IL-6 | 1.30 | 1.11 |
| IL-8 | 1.58 (0.81–3.09; 0.19) | 0.49 (0.24–0.97; 0.05) |
| IL-10 | 1.06 | 1.32 |
| IL-12 p70 | 0.97(0.71–1.31; 0.84) | 1.40 (1.04–1.89; 0.04) |
| IL-13 | 1.12 (0.99–1.27; 0.09) | 1.48 (1.14–1.91; 0.01) |
| G-CSF | 1.33 | 1.35 |
| GM-CSF | 0.73 | 1.01 |
| TNFα | 1.12 | 1.05 |
| VEGF | 1.02 | 1.13 |
Discussion
Several groups, including our own[23], have suggested that prostate cancer may arise in a setting of chronic inflammation, with early pre-malignant lesions detectable adjacent to areas marked by an inflammatory infiltrate[24]. This observation suggests that prostate cancer may be recognized by the adaptive immune system, a finding supported by the detection of prostate-associated antibodies in the sera of prostate cancer patients in multiple studies[25,16,26,27,21]. Several of these studies focused on the use of serum antibody levels (or serum antibody profile) as a potential tool for prostate cancer diagnosis[25,26,27], while other groups suggested that antibody induction may be associated with treatment. In that regard, antigen-specific antibody responses have been noted in patients treated with either hormonal or radiation therapy[28]. Interestingly, one group has described antibodies to the ligand-binding domain of the androgen-receptor. As this region is normally intracellular, these data suggest that antibody induction could be driven by processes associated with tissue destruction and immunogenic recognition of associated proteins[29]. In the present study, we show that treatment of men with biochemically recurrent prostate cancer with lenalidomide resulted in the induction of new prostate–associated antibodies. This induction was greater than that observed in men treated with ADT alone. These data are novel in that we were unable to find prior reports showing the induction (or augmentation) of cancer tissue-related antibodies in response to lenalidomide treatment in the current literature. Moreover, these results are consistent with recent data showing that patients with multiple myeloma mounted an augmented response to a polyvalent pneumococcal vaccine if under treatment with lenalidomide at the time of vaccination (Borrello, I, unpublished). It is thus tempting to speculate that these data suggest that a novel mechanism of action for lenalidomide might be the induction of anti-tumor antibodies, but this hypothesis requires further evaluation in prospective clinical trials.
The proximal mechanism for the augmented antibody responses we noted here appears to be related to the cytokine changes we observed in patients after lenalidomide treatment. As previously reported, we found augmented IL-2 levels in treated patients[8]. This effect may be mediated by increased PKC-θ activation in T cells[30] as well as by increased AP-1 transcriptional activity[31]. We also noted a relative increase in the cytokines IL-4, IL-5, IL-10 and IL-13 in patients treated with lenalidomide. This represents a canonical TH2 cytokine profile[32], classically associated with a humoral immune response and antibody production[33]. This finding is perhaps not entirely consistent with previous work on the effects of lenalidomide-related agents on human T cells; in one of these studies a relative TH1 skewing was noted[34]. However, the relative TH1 versus TH2 skewing properties of the imide family of drugs is not completely clear; in another study, augmentation of cytokine production from both TH1 and TH2 cells was noted[31]. From that perspective, our data are thus consistent with the notion that lenalidomide treatment of patients with biochemically recurrent prostate cancer may boost an existing TH2 skewed response, rather than induce a response from naïve CD4 T cells or reprogram a pre-existing TH1 response[35]. Regardless of the precise mechanism involved, the induction of new anti-prostate antibodies after lenalidomide treatment is certainly consistent with the cytokine profile we observe here.
Perhaps the most interesting aspect of our data is the observation of a dichotomous trend in IL-8 levels in patients classified as lenalidomide “responders” versus “non-responders”: with IL-8 levels decreasing in patients who appeared to derive potential benefit from treatment, and increasing in patients with “progressive” disease. IL-8 is a pleotropic cytokine, produced by several cell types and affecting multiple cellular processes. Immunologically, IL-8 has a well-documented role in neutrophil recruitment[36]. In that light, it is interesting to note recent data showing that the corpora amylacea in men with prostate cancer contain lactoferrin and several other neutrophil-derived proteins, suggesting a potential role for neutrophil-mediated inflammation in prostate carcinogenesis[37]. In addition to a potential role in inflammation-associated tumor promotion, a second mechanism by which IL-8 might promote tumor progression involves angiogenesis. Expression of the receptors for IL-8 (CXR-1 and CXR-2) has been documented on tumor-associated endothelial cells, where binding of IL-8 may serve to promote angiogenesis[38]. Accordingly, IL-8 expression is enhanced by vascular endothelial growth vactor (VEGF), as well as hypoxia[39], and thus might serve as a surrogate marker for ongoing angiogenesis.
IL-8 might also serve to promote tumor cell growth and proliferation in an autocrine and/or paracrine fashion, as IL-8 expression has been documented in a number of tumor cell types, including lung[40], cervical[41], ovarian[42], breast[43], colon[44], and prostate cancer[45]. In prostate cancer, recent studies showed that IL-8 expression was undetectable in androgen-sensitive cancer cell lines such as LNCaP and LAPC-4, but that IL-8 is highly expressed in the castration-resistant cell line PC-3[46]. Transfection of prostate cancer cells with IL-8 increased both their motility and VEGF-production, correlating with increased microvessel density when transfectants were grown in immunocompromised hosts. Thus, our observation of a dichotomous IL-8 response in lenalidomide-treated patients may represent a downstream manifestation of numerous potential processes associated with prostate cancer progression.
Additionally, IL-8 levels have been shown to have prognostic value in several solid tumor types. A recent, well-powered study of patients with primary myelofibrosis showed that IL-8 levels were independently associated with survival in a multivariate analysis[47]. These recent data support earlier data in breast cancer[48], in which serum IL-8 levels correlated with bone marrow and lymph node involvement. Similar observations have been reported for hepatocellular carcinoma as well[49]. An intriguing and novel mechanistic explanation for these data may be the recent observation that IL-8 drives cancer cell expression of CCR7, a chemokine receptor strongly associated with lymph node homing, and more recently associated with the stimulation of prostate cancer growth[42].
In summary, the findings presented here suggest a novel mechanism for the clinical activity of lenalidomide; the induction of novel antibody specificities driven by TH2 cytokine polarization. These findings are clearly limited by the retrospective nature of the analyses performed and the small sample size, and require validation in a prospective manner. The recent development of several high-throughput antibody screening technologies for cancer patients should facilitate such investigations, both in prostate cancer and in other disease states. These data also raise the interesting possibility that circulating IL-8 levels could serve as a predictive biomarker in prostate cancer patients treated with lenalidomide. Although this hypothesis clearly requires further exploration in larger prospective trials, it is intriguing because identification of a predictive biomarker could potentially serve as an early indication of disease response to lenalidomide in prostate cancer patients, aiding clinical decision making, and possibly sparing patients from protracted treatment with agents that are not likely to alter their clinical outcome.
Supplementary Material
Figure 1.
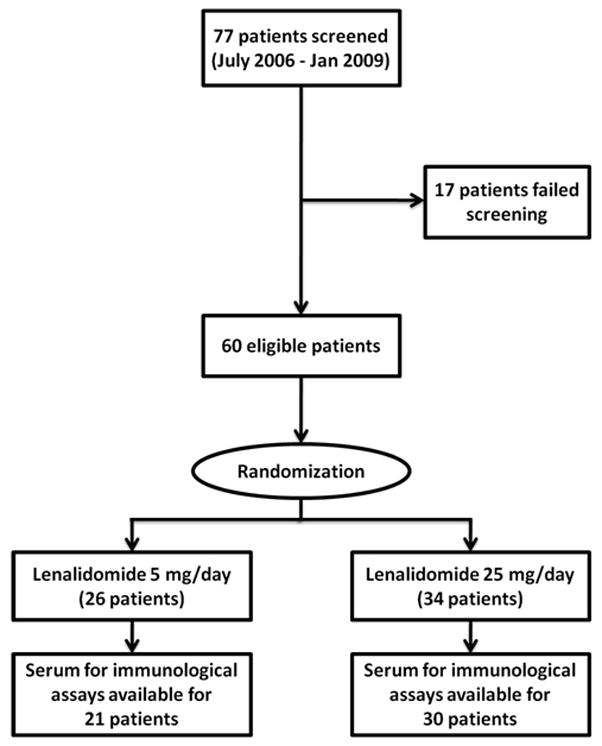
Study Schema
Table 1.
| Group
|
Lenalidomide 5 mg | Lenalidomide 25 mg | p value |
|---|---|---|---|
| Parameter | n=26 | n=34 | |
|
| |||
| Age (years) | 63.7 (7.1) | 63.1 (7.2) | 0.76 |
| Mean (SD); range | (50 – 75) | (52 – 81) | |
|
| |||
| Gleason: | 7.1 (1.0) | 7.3 (1.1) | 0.51 |
| Mean (SD); range | (6 – 9) | (5 – 9) | |
|
| |||
| Local therapy: | |||
| Radical prostatectomy | 12 | 12 | 0.62 |
|
| |||
| Radiation therapy | 4 | 8 | |
|
| |||
| Surgery + Radiation therapy | 10 | 14 | |
|
| |||
| Pre-study PSA (ng/mL): | 11.9 (11.9) | 12.4 (13.6) | 0.88 |
| Mean (SD); range | (1.4 – 43.6) | (1.2 – 66.2) | |
|
| |||
| Pre-study PSADT (months) | |||
| <3 | 6 | 10 | 0.85 |
|
| |||
| 3 – 8.9 | 13 | 16 | |
|
| |||
| ≥9 | 7 | 8 | |
|
| |||
| Pre-treatment PSA slope | 0.16 (0.11–0.21) | 0.18 (0.13–0.23) | 0.55 |
| Mean; (range); (95% CI) | (0.0 – 0.45) | (0.03, 0.72) | |
Acknowledgments
CGD is a Damon Runyon-Lilly Clinical Investigator. These studies were also supported by National Institutes of Health R01 CA127153, 1P50CA58236-15, the Patrick C. Walsh Fund, the One-In-Six Foundation, the US Army Medical Research and Materiel Command Prostate Cancer Research Program (W81XWH-10-1-0495), and the Prostate Cancer Foundation.
Footnotes
DISCLOSURES
Research funding was received from Celgene, Inc.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA: a cancer journal for clinicians. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Roehl KA, Han M, Ramos CG, Antenor JAV, Catalona WJ. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. The Journal of urology. 2004;172(3):910–4. doi: 10.1097/01.ju.0000134888.22332.bb. [DOI] [PubMed] [Google Scholar]
- 3.Antonarakis ES, Chen Y, Elsamanoudi SI, Brassell SA, Da Rocha MV, Eisenberger MA, McLeod DG. Long-term overall survival and metastasis-free survival for men with prostate-specific antigen-recurrent prostate cancer after prostatectomy: analysis of the Center for Prostate Disease Research National Database. BJU international. 2010;107:1–8. doi: 10.1111/j.1464-410X.2010.09878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boccon-Gibod L, Djavan WB, Hammerer P, Hoeltl W, Kattan MW, Prayer-Galetti T, Teillac P, Tunn UW. Management of prostate-specific antigen relapse in prostate cancer: a European Consensus. International journal of clinical practice. 2004;58(4):382–90. doi: 10.1111/j.1368-5031.2004.00184.x. [DOI] [PubMed] [Google Scholar]
- 5.Sandler HM, Eisenberger MA. Assessing and treating patients with increasing prostate specific antigen following radical prostatectomy. The Journal of urology. 2007;178:S20–4. doi: 10.1016/j.juro.2007.04.034. [DOI] [PubMed] [Google Scholar]
- 6.Keizman D, Zahurak M, Sinibaldi V, Carducci M, Denmeade S, Drake C, Pili R, Antonarakis ES, Hudock S, Eisenberger M. Lenalidomide in nonmetastatic biochemically relapsed prostate cancer: results of a phase I/II double-blinded, randomized study. Clinical cancer research: an official journal of the American Association for Cancer Research. 2010;16(21):5269–76. doi: 10.1158/1078-0432.CCR-10-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu L, Payvandi F, Wu L, Zhang LH, Hariri RJ, Man HW, Chen RS, Muller GW, Hughes CCW, Stirling DI, Schafer PH, Bartlett JB. The anti-cancer drug lenalidomide inhibits angiogenesis and metastasis via multiple inhibitory effects on endothelial cell function in normoxic and hypoxic conditions. Microvascular research. 2009;77(2):78–86. doi: 10.1016/j.mvr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Bartlett JB, Dredge K, Dalgleish AG. The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nature reviews: Cancer. 2004;4(4):314–22. doi: 10.1038/nrc1323. [DOI] [PubMed] [Google Scholar]
- 9.Galustian C, Meyer B, Labarthe MC, Dredge K, Klaschka D, Henry J, Todryk S, Chen R, Muller G, Stirling D, Schafer P, Bartlett JB, Dalgleish AG. The anti-cancer agents lenalidomide and pomalidomide inhibit the proliferation and function of T regulatory cells. Cancer immunology, immunotherapy: CII. 2009;58(7):1033–45. doi: 10.1007/s00262-008-0620-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu L, Adams M, Carter T, Chen R, Muller G, Stirling D, Schafer P, Bartlett JB. Lenalidomide Enhances Natural Killer Cell and Monocyte-Mediated Antibody-Dependent Cellular Cytotoxicity of Rituximab-Treated CD20+ Tumor Cells. Clinical Cancer Research. 2008;14(4650) doi: 10.1158/1078-0432.CCR-07-4405. [DOI] [PubMed] [Google Scholar]
- 11.Bartlett JB, Michael A, Clarke IA, Dredge K, Nicholson S, Kristeleit H, Polychronis A, Pandha H, Muller GW, Stirling DI, Zeldis J, Dalgleish AG. Phase I study to determine the safety, tolerability and immunostimulatory activity of thalidomide analogue CC-5013 in patients with metastatic malignant melanoma and other advanced cancers. British journal of cancer. 2004;90(5):955–61. doi: 10.1038/sj.bjc.6601579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 13.Antonarakis ES, Heath EI, Walczak JR, Nelson WG, Fedor H, De Marzo AM, Zahurak ML, Piantadosi S, Dannenberg AJ, Gurganus RT, Baker SD, Parnes HL, DeWeese TL, Partin AW, Carducci MA. Phase II, randomized, placebo-controlled trial of neoadjuvant celecoxib in men with clinically localized prostate cancer: evaluation of drug-specific biomarkers. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27(30):4986–93. doi: 10.1200/JCO.2009.21.9410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antonarakis ES, Feng Z, Trock BJ, Humphreys EB, Carducci MA, Partin AW, Walsh PC, EM The natural history of metastatic progression in men with PSA-recurrent prostate cancer after radical prostatectomy: long-term follow-up. BJU international. 2011 doi: 10.1111/j.1464-410X.2011.10422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna Austria: R Foundation for Statistical Computing; 2008. [Google Scholar]
- 16.Maricque BB, Eickhoff JC, McNeel DG. Antibody responses to prostate-associated antigens in patients with prostatitis and prostate cancer. The Prostate. 2011;71(2):134–46. doi: 10.1002/pros.21229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith HA, Maricque BB, Eberhardt J, Petersen B, Gulley JL, Schlom J, McNeel DG. IgG responses to tissue-associated antigens as biomarkers of immunological treatment efficacy. Journal of biomedicine & biotechnology. 2011 doi: 10.1155/2011/454861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dubovsky JA, Albertini MR, McNeel DG. MAD-CT-2 identified as a novel melanoma cancer-testis antigen using phage immunoblot analysis. Journal of immunotherapy. 2007;30(7):675–83. doi: 10.1097/CJI.0b013e3180de4d19. [DOI] [PubMed] [Google Scholar]
- 19.Mooney CJ, Dunphy EJ, Stone B, McNeel DG. Identification of autoantibodies elicited in a patient with prostate cancer presenting as dermatomyositis. International journal of urology: official journal of the Japanese Urological Association. 2006;13(3):211–7. doi: 10.1111/j.1442-2042.2006.01263.x. [DOI] [PubMed] [Google Scholar]
- 20.Dunphy EJ, McNeel DG. Antigen-specific IgG elicited in subjects with prostate cancer treated with flt3 ligand. Journal of immunotherapy. 2005;28(3):268–75. doi: 10.1097/01.cji.0000158853.15664.0c. [DOI] [PubMed] [Google Scholar]
- 21.Morse MD, McNeel DG. Prostate cancer patients on androgen deprivation therapy develop persistent changes in adaptive immune responses. Human immunology. 2010;71(5):496–504. doi: 10.1016/j.humimm.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunphy EJ, Eickhoff JC, Muller CH, Berger RE, McNeel DG. Identification of antigen-specific IgG in sera from patients with chronic prostatitis. Journal of clinical immunology. 2004;24(5):492–502. doi: 10.1023/B:JOCI.0000040920.96065.5a. [DOI] [PubMed] [Google Scholar]
- 23.DeMarzo AM, Platz EA, Sutcliffe S, Xu J, Grönberg H, Drake CG, Nakai Y, Isaacs WB, Nelson WG. Inflammation in prostate carcinogenesis. Nature Reviews: Cancer. 2007;7(4):256–269. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Marzo AM, Marchi VL, Epstein JI, Nelson WG. Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis. The American journal of pathology. 1999;155(6):1985–92. doi: 10.1016/S0002-9440(10)65517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Yu J, Sreekumar A, Varambally S, Shen R, Giacherio D, Mehra R, Montie JE, Pienta KJ, Sanda MG, Kantoff PW, Rubin MA, Wei JT, Ghosh D, Chinnaiyan AM. Autoantibody signatures in prostate cancer. The New England journal of medicine. 2005;353(12):1224–35. doi: 10.1056/NEJMoa051931. [DOI] [PubMed] [Google Scholar]
- 26.Bradley SV, Oravecz-Wilson KI, Bougeard G, Mizukami I, Li L, Munaco AJ, Sreekumar A, Corradetti MN, Chinnaiyan AM, Sanda MG, Ross TS. Serum antibodies to huntingtin interacting protein-1: a new blood test for prostate cancer. Cancer research. 2005;65(10):4126–33. doi: 10.1158/0008-5472.CAN-04-4658. [DOI] [PubMed] [Google Scholar]
- 27.Sreekumar A, Laxman B, Rhodes DR, Bhagavathula S, Harwood J, Giacherio D, Ghosh D, Sanda MG, Rubin MA, Chinnaiyan AM. Humoral immune response to alpha-methylacyl-CoA racemase and prostate cancer. Journal of the National Cancer Institute. 2004;96(11):834–43. doi: 10.1093/jnci/djh145. [DOI] [PubMed] [Google Scholar]
- 28.Nesslinger NJ, Sahota RA, Stone B, Johnson K, Chima N, King C, Rasmussen D, Bishop D, Rennie PS, Gleave M, Blood P, Pai H, Ludgate C, Nelson BH. Standard treatments induce antigen-specific immune responses in prostate cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2007;13(5):1493–502. doi: 10.1158/1078-0432.CCR-06-1772. [DOI] [PubMed] [Google Scholar]
- 29.Olson BM, McNeel DG. Antibody and T-cell responses specific for the androgen receptor in patients with prostate cancer. The Prostate. 2007;67(16):1729–39. doi: 10.1002/pros.20652. [DOI] [PubMed] [Google Scholar]
- 30.Payvandi F, Wu L, Naziruddin SD, Haley M, Parton A, Schafer PH, Chen RS, Muller GW, Hughes CCW, Stirling DI. Immunomodulatory drugs (IMiDs) increase the production of IL-2 from stimulated T cells by increasing PKC-theta activation and enhancing the DNA-binding activity of AP-1 but not NF-kappaB, OCT-1, or NF-AT. Journal of interferon & cytokine research: the official journal of the International Society for Interferon and Cytokine Research. 2005;25(10):604–16. doi: 10.1089/jir.2005.25.604. [DOI] [PubMed] [Google Scholar]
- 31.Schafer PH, Gandhi AK, Loveland MA, Chen RS, Man HW, Schnetkamp PPM, Wolbring G, Govinda S, Corral LG, Payvandi F, Muller GW, Stirling DI. Enhancement of cytokine production and AP-1 transcriptional activity in T cells by thalidomide-related immunomodulatory drugs. The Journal of pharmacology and experimental therapeutics. 2003;305(3):1222–32. doi: 10.1124/jpet.102.048496. [DOI] [PubMed] [Google Scholar]
- 32.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. Journal of immunology. 1986;136(7):2348–57. [PubMed] [Google Scholar]
- 33.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112(5):1557–69. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu W, Celeridad M, Sankar S, Webb DR, Bennett BL. CC-4047 promotes Th1 cell differentiation and reprograms polarized human Th2 cells by enhancing transcription factor T-bet. Clinical immunology. 2008;128(3):392–9. doi: 10.1016/j.clim.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 35.O’Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327(5969):1098–102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshimura T, Matsushima K, Tanaka S, Robinson EA, Appella E, Oppenheim JJ, Leonard EJ. Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proceedings of the National Academy of Sciences of the United States of America. 1987;84(24):9233–7. doi: 10.1073/pnas.84.24.9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sfanos KS, Wilson BA, De Marzo AM, Isaacs WB. Acute inflammatory proteins constitute the organic matrix of prostatic corpora amylacea and calculi in men with prostate cancer. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(9):3443–8. doi: 10.1073/pnas.0810473106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang S, Mills L, Mian B, Tellez C, McCarty M, Yang XD, Gudas JM, Bar-Eli M. Fully humanized neutralizing antibodies to interleukin-8 (ABX-IL8) inhibit angiogenesis, tumor growth, and metastasis of human melanoma. The American journal of pathology. 2002;161(1):125–34. doi: 10.1016/S0002-9440(10)64164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rofstad EK, Halsør EF. Hypoxia-associated spontaneous pulmonary metastasis in human melanoma xenografts: involvement of microvascular hot spots induced in hypoxic foci by interleukin 8. British journal of cancer. 2002;86(2):301–8. doi: 10.1038/sj.bjc.6600052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan A, Yu CJ, Luh KT, Kuo SH, Lee YC, Yang PC. Aberrant p53 expression correlates with expression of vascular endothelial growth factor mRNA and interleukin-8 mRNA and neoangiogenesis in non-small-cell lung cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2002;20(4):900–10. doi: 10.1200/JCO.2002.20.4.900. [DOI] [PubMed] [Google Scholar]
- 41.Fujimoto J, Sakaguchi H, Aoki I, Tamaya T. Clinical implications of expression of interleukin 8 related to angiogenesis in uterine cervical cancers. Cancer research. 2000;60(10):2632–5. [PubMed] [Google Scholar]
- 42.Davidson B, Goldberg I, Kopolovic J, Gotlieb WH, Givant-Horwitz V, Nesland JM, Berner A, Ben-Baruch G, Bryne M, Reich R. Expression of angiogenesis-related genes in ovarian carcinoma - a clinicopathologic study. Clinical & experimental metastasis. 2000;18(6):501–7. doi: 10.1023/a:1011858225144. [DOI] [PubMed] [Google Scholar]
- 43.Green AR, Green VL, White MC, Speirs V. Expression of cytokine messenger RNA in normal and neoplastic human breast tissue: identification of interleukin-8 as a potential regulatory factor in breast tumours. International journal of cancer Journal international du cancer. 1997;72(6):937–41. doi: 10.1002/(sici)1097-0215(19970917)72:6<937::aid-ijc3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 44.Haraguchi M, Komuta K, Akashi A, Matsuzaki S, Furui J, Kanematsu T. Elevated IL-8 levels in the drainage vein of resectable Dukes’ C colorectal cancer indicate high risk for developing hepatic metastasis. Oncology reports. 2002;9(1):159–65. [PubMed] [Google Scholar]
- 45.Inoue K, Slaton JW, Eve BY, Kim SJ, Perrotte P, Balbay MD, Yano S, Bar-Eli M, Radinsky R, Pettaway CA, Dinney CP. Interleukin 8 expression regulates tumorigenicity and metastases in androgen-independent prostate cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2000;6(5):2104–19. [PubMed] [Google Scholar]
- 46.Araki S, Omori Y, Lyn D, Singh RK, Meinbach DM, Sandman Y, Lokeshwar VB, Lokeshwar BL. Interleukin-8 is a molecular determinant of androgen independence and progression in prostate cancer. Cancer research. 2007;67(14):6854–62. doi: 10.1158/0008-5472.CAN-07-1162. [DOI] [PubMed] [Google Scholar]
- 47.Tefferi A, Vaidya R, Caramazza D, Finke C, Lasho T, Pardanani A. Circulating Interleukin (IL)-8, IL-2R, IL-12, and IL-15 Levels Are Independently Prognostic in Primary Myelofibrosis: A Comprehensive Cytokine Profiling Study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011;29(10):1356–63. doi: 10.1200/JCO.2010.32.9490. [DOI] [PubMed] [Google Scholar]
- 48.Benoy IH, Salgado R, Van Dam P, Geboers K, Van Marck E, Scharpé S, Vermeulen PB, Dirix LY. Increased serum interleukin-8 in patients with early and metastatic breast cancer correlates with early dissemination and survival. Clinical cancer research: an official journal of the American Association for Cancer Research. 2004;10(21):7157–62. doi: 10.1158/1078-0432.CCR-04-0812. [DOI] [PubMed] [Google Scholar]
- 49.Ren Y, Poon RTP, Tsui HT, Chen WH, Li Z, Lau C, Yu WC, Fan ST. Interleukin-8 serum levels in patients with hepatocellular carcinoma: correlations with clinicopathological features and prognosis. Clinical cancer research: an official journal of the American Association for Cancer Research. 2003;9(16):5996–6001. [PubMed] [Google Scholar]
- 50.Singh RK, Lokeshwar BL. The IL-8 regulated Chemokine Receptor CXCR7 stimulates EGFR signaling to promote prostate cancer growth. Cancer research. 2011;71(9):3268–77. doi: 10.1158/0008-5472.CAN-10-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



