Abstract
BACKGROUND
The CD8 T cell response to prostate and other cancers is often functionally diminished or absent. This may occur via deletion of tumor-specific T cells, through acquisition of an anergic phenotype, or via active suppression mediated by another population of cells.
METHODS
We used a double transgenic model in which mice express CD8 T cells specific for a prostate/prostate cancer antigen to study the response of CD8 T cells to evolving autochronous prostate tumors in TRAMP mice. CD8 T cells were analyzed for functionality by measuring IFN-γ production via flow cytometry and via an in vivo CTL killing assay. In addition, pathological scoring of the prostates of the double transgenic mice was compared to scoring of tumor burden prostates of ProTRAMP mice.
RESULTS
Tumor-specific CD8 T cells were not grossly deleted in these animals, but evidenced a clearly non-functional phenotype. Interestingly, full lytic function was rapidly recovered upon removal from tumor-bearing mice.
CONCLUSIONS
These data indicate a role for continuous antigen exposure in the maintenance of tumor-specific CD8 T cell tolerance to prostate cancer.
Keywords: anergy, tolerance, TRAMP, Clone 4, hemagglutinin
Introduction
The mammalian immune system employs multiple redundant mechanisms to prevent the induction of autoimmunity. Not surprisingly, many of these mechanisms have been co-opted by tumors, which subvert or evade active cellular immunity as they progress in immune-competent hosts. These mechanisms may include central deletion, as has been demonstrated in a murine model of plasmacytoma[1, 2]. More commonly, though, tumor-specific T cells are rendered anergic, as occurs when engagement of the T Cell Receptor (TCR) by the antigen/MHC complex transpires in the absence of adequate costimulation[3]. Anergy as a mechanism of T cell tolerance to tumors has been demonstrated for both antigen-specific CD4[4, 5]and CD8 T cells[6], and may occur even when tumor-specific T cells are pre-activated prior to tumor antigen encounter[7]. Other mechanisms of CD8 T cell tolerance to tumors include myeloid-derived suppressor cells[8] and regulatory T cells[9, 10]. As recent data support the notion that tumors in patients most likely arise slowly over time[11], the role of ongoing T cell encounter with tumor antigens is of particular interest, as stably expressed antigen has been shown to mitigate T cell function in both the absence[12], and presence[13] of cancer.
To investigate the CD8 T cell response to tumors in a physiological setting, and to address issues surrounding persistent antigen exposure, several groups have studied double transgenic cancer models. These mice were created by crossing TCR transgenic animals with animals that develop spontaneous tumors expressing the TCR's cognate antigen. Such studies have revealed deletion as a mechanism of self tolerance[14], reversible tolerance[15], and even T cell activation without tolerance[16]. These double-transgenic models, however, have not specifically addressed prostate cancer, the second most common cause of cancer death in men. The prostate gland is of particular interest, as prior studies in humans[17] and animals[5] suggest that the prostate gland may be immunologically ignored in the absence of malignancy. We created a relevant double transgenic model by crossing animals that develop autochthonous prostate cancers which express hemagglutinin (ProHA × TRAMP)[5, 10, 18–20] with TCR transgenic mice that generate CD8 T cells specific for hemagglutinin (HA) (known as Clone 4)[21]. We found that tumor-specific CD8 T cells were not grossly deleted in Clone 4 × (ProHA × TRAMP) mice. Despite the observation that such cells were non-functional in several assays, the growth of prostate tumors was significantly attenuated in double-transgenic animals, suggesting that CD8 T cells can mediate some level of anti-tumor effector function during the process of in vivo activation.
Materials and Methods
Mice and vaccines
Non-transgenic control mice (B10.2 [Thy1.2+, H-2d] were obtained from the Jackson laboratory. ProHA × TRAMP mice on a B10.2 background were described previously[5]. Donor mice (Clone 4 TCR transgenic [Thy1.1+, Kd, HA-specific]) were a generous gift of L. Sherman (The Scripps Research Institute, La Jolla, California, USA), and were bred and maintained in the JHU vivarium. Double transgenic mice were bred by crossing Clone 4 TCR transgenic mice with ProHA × TRAMP mice and then subsequently crossing the progeny of the F1 population. Recombinant vaccinia virus expressing wild type HA protein (Vac-HA) was constructed as previously described[5].
Quantification of HA specific CD8 T cell responses
Mice were sacrificed via CO2 asphyxiation. The spleen and prostate draining lymph nodes were dissected, dissociated, and washed. To obtain prostate infiltrating lymphocytes, the prostate glands were first micro-dissected, then mechanically disrupted and incubated for 1 hour at 37° C with CTL media containing the enzyme liberase (Roche Applied Science) at a final concentration of 35 μg/mL. For FACS analysis, 106 lymphocytes were stained using anti-Vβ 8.1/8.2 antibody (directed against the Clone 4 TCR) and anti-CD8 antibody (BD Biosciences--Pharmigen). Cells were analyzed using a FACSCalibur system (BD). Intracellular staining for IFN-γ and TNF-α was performed as previously described[33]. Briefly, 106 lymphocytes were incubated with the immunodominant HA class I peptide (IYSTVASSL) at a concentration of 1 μg/mL and Golgi stop (BD) at 1:1000. Cells were stained with surface antibodies, fixed, and permeabilized using Cytofix/Cytoperm Plus Kit (BD Biosciences--Pharmigen). CD107a/b staining was performed during the 5 hour peptide stimulation. Anti-CD107/ab, anti-IFN-γ, and anti-TNF-α were all from BD Biosciences--Pharmigen.
Adoptive Transfers
Donor Clone 4 mice were sacrificed via CO2 asphyxiation. Spleens and axillary lymph nodes were collected and homogenized, and red blood cells were lysed. CD8+ T cells were purified using Miltenyi magnetically labeled beads (Miltenyi Biotec) according to the manufacturer's protocol. After separation, cells were washed twice and resuspended with HBSS for injections. 106 cells were injected per mouse in 0.2 mL of HBSS by tail-vein injection.
In vivo CTL assays
The assay was performed as previously described[28]; splenocytes from non transgenic B10.D2 mice were isolated and split into two groups. Group 1 was left untreated, while group 2 was loaded with synthetic immunodominant HA class I peptide (IYSTVASSL) at a concentration of 10 μg/mL, washed 3 times, counted and resuspended. Group 1 cells were labeled as CFSEhi and group 2 as CFSElo with 2.5 mM or 0.25 mM CFSE, respectively. After labeling, cells were washed with HBSS and counted. An equal number of HA-loaded CFSElo and unloaded CFSEhi target cells were combined and administered by tail vain injection. Twenty four hours after target transfer, splenocytes of recipient mice were analyzed by FACS, and the relative number of CFSEhi versus CFSElo peaks were determined to quantify percentage of specific lysis. Percent lysis was calculated as previously described.
Pathology
Ventral and dorsal lobes of the prostate glands were micro-dissected and fixed with 10% neutral buffered formalin solution (Richard Allen Scientific). Two surgical pathologists evaluated H&E sections of the lobes for tumor development in a double-blinded manner. Prostate histology was scored on a scale of 0–5 as previously described[19] : 0, no benign tissue; 1, PIN without cribriform formation; 2, PIN plus cribriform formation; 3, intraductal carcinoma; 4, moderately differentiated carcinoma; 5, poorly differentiated carcinoma/small cell carcinoma.
Statistics
P values were calculated using 2-tailed Student's t test as implemented in the Prism 4.0 package (GraphPad Software).
Results
Tumor specific CD8 T lymphocytes in double transgenic mice are present but non-functional
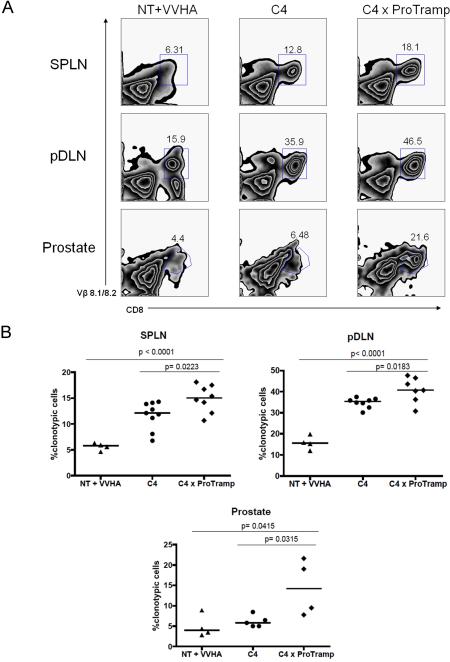
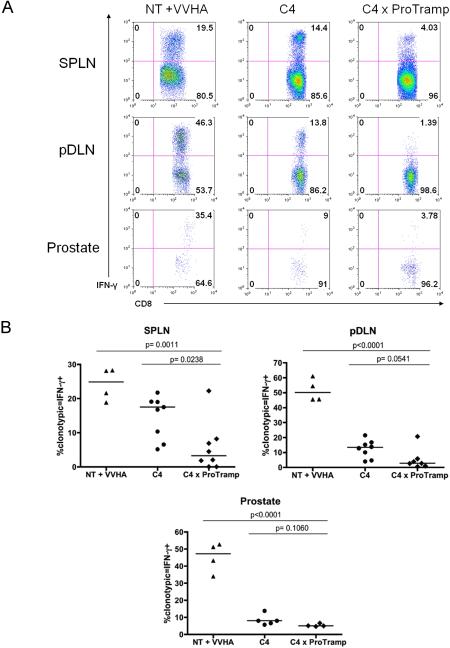
We first tested whether prostate-tissue/tumor specific CD8 T cells would be deleted as tumors developed in C4 × ProTRAMP (Clone 4 × (ProHA × TRAMP)) double transgenic mice, by comparing the relative percentage of clonotypic, HA-specific cells in parental Clone 4 mice to C4 × ProTRAMP mice using flow cytometry. As a positive control, CD8 T cells from C4 mice were adoptively transferred to nontransgenic (B10.D2) recipients, and activated by vaccinating animals with a vaccinia virus expressing hemagglutinin (Vac-HA). As shown in Figure 1A, HA-specific CD8 T cells were not deleted in C4 × ProTRAMP double transgenic animals. In fact, comparison of multiple litters of double transgenic animals with single transgenic littermates suggested that the frequency of tumor-reactive T cells was slightly increased in double-trangenic animals (Figure 1B). These data argue strongly against central deletion as a dominant mechanism of tolerance to this prostate/prostate-cancer restricted antigen. In order to examine the relative responsiveness of these antigen-specific CD8 T cells, we quantified cytokine production via intracellular staining. As shown in Figure 2C and 2D, IFN-γ secretion was significantly diminished in C4 × ProTRAMP animals as compared to either C4 or C4-> NT + Vac-HA animals. Interestingly, a significant difference in the secretion of TNF-α was not noted between these three groups (data not shown), consistent with the notion that IFN-γ secretion and TNF-α secretion may be differentially regulated.
Figure 1. Prostate cancer specific CD8 T cells are not deleted in double transgenic mice.
(1A) HA-specific CD8 T lymphocytes identified in the periphery and prostate by surface staining for CD8 and Vβ 8.1/8.2. One of three experiments shown.
(1B) Quantification of HA-specific CD8 T cells from spleen, prostate draining lymph nodes, and prostates. N = 3–5 / group, repeated × 2.
Figure 2. Prostate cancer specific CD8 T cells are non functional in double transgenic mice.
(2A) Intracellular cytokine staining for IFN-γ producing, antigen-specific CD8 T cells. One of three experiments shown.
(2B) Quantification of cytokine secretion by prostate-specific CD8 T cells. N = 3–5 / group, repeated × 2.
Cytolytic activity of tumor-specific CD8 T cells is restored after removal from tumor-bearing hosts
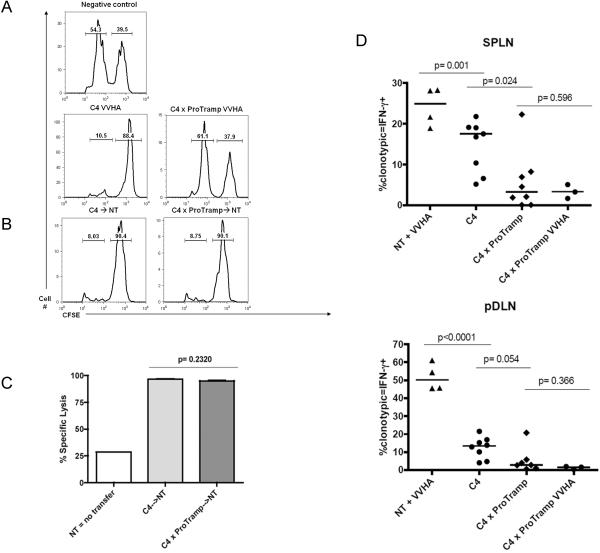
Because specific prostate/prostate cancer directed CD8 T cells were not deleted in double transgenic mice, we tested whether they could mediate lytic function in vivo. To quantify specific CD8 function in a physiologically relevant context, we used a standard in vivo CTL assay, in which fluorescently labeled antigen-loaded control or control cells were transferred to intact animals; 24 hours after transfer, in vivo lysis was quantified by flow cytometry. As shown in Figure 3A, no lytic function was observed in unvaccinated non-transgenic (NT) mice (negative control), while TCR-transgenic (C4) mice without prostate tumors showed the induction of robust lytic function after vaccination (Figure 3A). Correspondingly, almost no lytic function was observed in vaccinated double transgenic mice. These data suggest that vaccination alone is insufficient to reverse specific CD8 T cell dysfunction in tumor-bearing double transgenic mice (C4 × ProTRAMP). We next tested whether removing non-functional prostate cancer specific CD8 T cells from their tolerogenic host environment would be sufficient to allow for functional recovery. Specific CD8 T cells were harvested from double-transgenic C4 × ProTRAMP mice and transferred to tumor-free nontransgenic hosts. Surprisingly, these cells recovered full functionality after only 24 hours in tumor-free hosts, displaying in vivo lytic function comparable to cells obtained from control donors (Figure 3B, 3C). Returning to intact mice, we investigated the cellular mechanisms involved in the inability of tumor bearing C4 × ProTRAMP mice to respond to specific vaccination. As shown in Figure 3D, the ability of these mice to mount a lytic response to vaccination corresponded closely to the ability of HA-specific CD8 cells to secrete IFN-g, both in the spleen and in the prostate draining lymph nodes. In particular, antigen-specific CD8 cells in the prostate draining lymph nodes of double-transgenic mice were markedly non-functional, neither secreting IFN-γ at baseline, nor in response to specific vaccination (Figure 3D, right panel). Taken together these show that tumor-specific CD8 T cells may be profoundly non-functional, but that they can rapidly regain effector function upon removal from an antigen-containing environment.
Figure 3. Recovery of prostate-specific CD8 T cell function in an antigen-free environment.
(3A) CD8 T cell function assayed via in vivo CTL assay. Top Row: negative control, unvaccinated, nontransgenic hosts. Second Row: Vaccination of TCR transgenic (C4) or double transgenic (C4 x ProTRAMP) hosts.
(3B) Adoptive transfer of naïve Clone 4 (C4) or C4 from tumor bearing (C4 × ProTRAMP) donors into nontransgenic (NT) hosts, in both cases followed by vaccination. In all studies left peak represents antigen loaded targets, right peak unloaded control. N = 3–5 animals / group, representative of 3 independent experiments.
(3C) Quantification of CTL function post-transfer. Leftmost bar represents unvaccinated NT hosts (negative control). N = 3–5 / group, repeated × 3.
(3D) Quantification of cytokine production after vaccination of C4 or C4 × ProTRAMP (tumor bearing hosts. Leftmost bar (positive control) represents Clone 4 cells adoptively transferred into nontransgenic (NT) hosts. Remaining bars from in situ vaccination of animals indicated. Pooled data from 2 independent studies with a minimum of 3 animals / group.
Attenuated tumorigeneis in C4 × ProTRAMP double transgenic mice
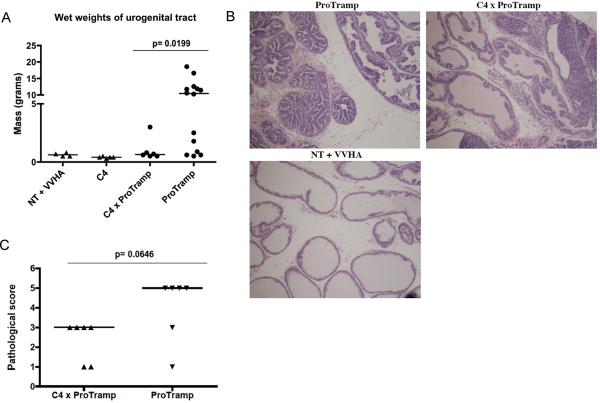
Based on the relative lack of function of HA-specific CD8 T cells in C4 × ProTRAMP double transgenic animals (Figures 1–3), we hypothesized that tumorigenesis in these animals would not be significantly different from age-matched, ProTRAMP mice. Comparing age-matched C4 × ProTRAMP mice to ProTRAMP controls showed this hypothesis to be incorrect. The wet weights of the urogenital tract (a gross index of tumor burden in TRAMP mice[22], were significantly decreased in C4 × ProTRAMP double transgenic mice (Figure 4A). These results were supported by a double blinded pathological analysis of the animals' prostate glands, which showed a significant decrease in pathological score in the C4 × ProTRAMP as compared to ProTRAMP controls (Figure 4B, 4C). Taken together, these data support the notion that antigen-specific CD8 T cells may mediate some level of anti-tumor efficacy, perhaps during antigen-specific expansion, which has been shown to be associated with a transient effector phase even when eventual functional tolerance is the final outcome[23].
Figure 4. Prostate cancer development is attenuated in C4 × ProTRAMP mice.
(4A) Wet weights of urogenital tracts of nontransgenic (+Vac-HA), C4, C4 × ProTramp, and control N = 4 – 14 animals / group.
(4B) Representative histology C4 × ProTramp versus ProTramp mice. (H&E staining, 10× magnification)
(4C) Pathological Scores (see methods for details) of double transgenic versus ProTRAMP mice. N = 6 / group.
Discussion
The results presented here are not entirely consistent with other “double transgenic” models of in vivo tumor tolerance. For example, the Engelhard group found that melanocyte-specific CD8 T cells were rapidly deleted in antigen-bearing mice, even in the absence of tumor expression [14]. Similar results were previously reported in plasmacytoma model[1]. In a pancreatic cancer model broadly analogous to ours, the Ohashi group bred LCMV-specific TCR transgenic mice with animals in which LCMV protein is expressed in evolving pancreatic tumors, and found neither deletion, nor evidence of functional tolerance[24]. In contrast, our data are consistent with multiple adoptive T cell transfer studies in TRAMP animals and their derivatives [7, 19, 25–28], corroborating functional T cell tolerance. These results recapitulate earlier studies in spontaneous murine cancer models [24, 29], again providing broad support to the notion that evolving tumors are associated with a non-functional CD8 T cell phenotype. The lack of central deletion in this model may reflect the observation that the target antigen (hemagglutinin) is not expressed in the thymus in these mice[5], but it is more difficult to understand the reasons for a lack of peripheral deletion, which may revolve around issues of antigen level[30] or the tumor microenvironment itself. The strengths of this particular model include the slowly evolving nature of prostate tumors on the TRAMP background, and the well-defined tumor-specific antigen provided by prostate-restricted expression hemagglutinin. It should further be noted that similar results – transient immunological repression of tumor growth followed by eventual CD8 T cell tolerance were recently observed in an elegant induced lung cancer model by the Jacks group [34].
Classical early studies by Ramsdell and Fowlkes demonstrated that the maintenance of the nonresponsive state of T cells to a self antigen required continuous exposure to antigen[12]. Those data were elegantly extended to a tumor model by the Offringa group[13], who showed that E1A specific CD8 T cells rendered tolerant by persistent antigen exposure recovered full function after transfer to naïve animals. Our functional studies are consistent with these previous studies, and suggest that one approach to overcome tumor-specific tolerance is the minimization or elimination of a tumor burden by standard therapy such as radiation or chemotherapy. Interestingly, complete elimination of a tumor burden may not be required, as studies in a breast cancer model showed that even partial resection of an evolving tumor was sufficient to meaningfully reverse immunological non-reactivity [31].
Perhaps the most interesting aspect of these results was the unexpected finding that double-transgenic animals showed a decrease in tumor burden, despite functional tolerance of antigen-specific CD8 T cells. One possible explanation for these findings would be the temporary acquisition of effector function by naïve T cells during the process of in vivo tolerance. This phenomena has been observed for both CD4[23] and CD8[32] T cells, and is potentially reflected here by the relative accumulation of antigen-specific CD8 T cells in lymphoid organs of double-transgenic animals (Figure 1B). These data also suggest that human oncogenesis may be accompanied by T cell recognition of evolving tumors, the responding lymphocytes providing a substrate upon which vaccine or other tumor immunotherapy strategies may be based. In summary, our results are encouraging in terms of tumor immunology for prostate cancer, as they suggest that deletion of tumor-specific CD8 T cells may not be a universal occurrence, and that functional tolerance may be mitigated by manipulations that reduce tumor burden in vivo.
ACKNOWLEDGEMENTS
CGD is a Damon Runyon-Lilly Clinical Investigator. These studies were supported by National Institutes of Health R01 CA127153, 1P50CA58236-15, the Patrick C. Walsh Fund, the OneInSix Foundation and the Prostate Cancer Foundation.
We would like to thank Dr. Linda Sherman for her gift of Clone 4 mice. We would also like to thank Christopher Nirschl for his critical reading of the manuscript as well as Roula Albadine for her assistance in scoring the pathology.
Footnotes
DISCLOSURES The authors have no financial conflicts of interest relevant to this work.
Reference List
- 1.Bogen B, Schenck K, Munthe LA, Dembic Z. Deletion of idiotype (Id)-specific T cells in multiple myeloma. Acta Oncol. 2000;39:783–788. doi: 10.1080/028418600750063505. [DOI] [PubMed] [Google Scholar]
- 2.Lauritzsen GF, Hofgaard PO, Schenck K, Bogen B. Clonal deletion of thymocytes as a tumor escape mechanism. Int J Cancer. 1998;78:216–222. doi: 10.1002/(sici)1097-0215(19981005)78:2<216::aid-ijc16>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 4.Staveley-O'Carroll K, Sotomayor E, Montgomery J, Borrello I, Hwang L, Fein S, Pardoll D, Levitsky H. Induction of antigen-specific T cell anergy: An early event in the course of tumor progression. Proc Natl Acad Sci U S A. 1998;95:1178–1183. doi: 10.1073/pnas.95.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drake CG, Doody AD, Mihalyo MA, Huang CT, Kelleher E, Ravi S, Hipkiss EL, Flies DB, Kennedy EP, Long M, McGary PW, Coryell L, Nelson WG, Pardoll DM, Adler AJ. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell. 2005;7:239–249. doi: 10.1016/j.ccr.2005.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shrikant P, Khoruts A, Mescher MF. CTLA-4 blockade reverses CD8+ T cell tolerance to tumor by a CD4+ T cell- and IL-2-dependent mechanism. Immunity. 1999;11:483–493. doi: 10.1016/s1074-7613(00)80123-5. [DOI] [PubMed] [Google Scholar]
- 7.Bai A, Higham E, Eisen HN, Wittrup KD, Chen J. Rapid tolerization of virus-activated tumor-specific CD8+ T cells in prostate tumors of TRAMP mice. Proc Natl Acad Sci U S A. 2008;105:13003–13008. doi: 10.1073/pnas.0805599105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Getnet D, Maris CH, Hipkiss EL, Grosso JF, Harris TJ, Yen HR, Bruno TC, Wada S, Adler A, Georgantas RW, Jie C, Goldberg MV, Pardoll DM, Drake CG. Tumor recognition and self-recognition induce distinct transcriptional profiles in antigen-specific CD4 T cells. J Immunol. 2009;182:4675–4685. doi: 10.4049/jimmunol.0803400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell PJ, Yachida S, Mudie LJ, Stephens PJ, Pleasance ED, Stebbings LA, Morsberger LA, Latimer C, McLaren S, Lin ML, McBride DJ, Varela I, Nik-Zainal SA, Leroy C, Jia M, Menzies A, Butler AP, Teague JW, Griffin CA, Burton J, Swerdlow H, Quail MA, Stratton MR, Iacobuzio-Donahue C, Futreal PA. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature. 2010;467:1109–1113. doi: 10.1038/nature09460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramsdell F, Fowlkes BJ. Maintenance of in vivo tolerance by persistence of antigen. Science. 1992;257:1130–1134. doi: 10.1126/science.257.5073.1130. [DOI] [PubMed] [Google Scholar]
- 13.den Boer AT, van Mierlo GJ, Fransen MF, Melief CJ, Offringa R, Toes RE. The tumoricidal activity of memory CD8+ T cells is hampered by persistent systemic antigen, but full functional capacity is regained in an antigen-free environment. J Immunol. 2004;172:6074–6079. doi: 10.4049/jimmunol.172.10.6074. [DOI] [PubMed] [Google Scholar]
- 14.Nichols LA, Chen Y, Colella TA, Bennett CL, Clausen BE, Engelhard VH. Deletional self-tolerance to a melanocyte/melanoma antigen derived from tyrosinase is mediated by a radio-resistant cell in peripheral and mesenteric lymph nodes. J Immunol. 2007;179:993–1003. doi: 10.4049/jimmunol.179.2.993. [DOI] [PubMed] [Google Scholar]
- 15.Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, Dellemijn TA, Antony PA, Spiess PJ, Palmer DC, Heimann DM, Klebanoff CA, Yu Z, Hwang LN, Feigenbaum L, Kruisbeek AM, Rosenberg SA, Restifo NP. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen LT, Elford AR, Murakami K, Garza KM, Schoenberger SP, Odermatt B, Speiser DE, Ohashi PS. Tumor growth enhances cross-presentation leading to limited T cell activation without tolerance. J Exp Med. 2002;195:423–435. doi: 10.1084/jem.20010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whitmore WF, Gittes RF. Studies on the prostate and testis as immunologically privileged sites. Cancer Treat Rep. 1977;61:217–222. [PubMed] [Google Scholar]
- 18.Grosso JF, Goldberg MV, Getnet D, Bruno TC, Yen HR, Pyle KJ, Hipkiss E, Vignali DA, Pardoll DM, Drake CG. Functionally distinct LAG-3 and PD-1 subsets on activated and chronically stimulated CD8 T cells. J Immunol. 2009;182:6659–6669. doi: 10.4049/jimmunol.0804211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wada S, Yoshimura K, Hipkiss EL, Harris TJ, Yen HR, Goldberg MV, Grosso JF, Getnet D, DeMarzo AM, Netto GJ, Anders R, Pardoll DM, Drake CG. Cyclophosphamide augments antitumor immunity: studies in an autochthonous prostate cancer model. Cancer Res. 2009;69:4309–4318. doi: 10.1158/0008-5472.CAN-08-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mihalyo MA, Hagymasi AT, Slaiby AM, Nevius EE, Adler AJ. Dendritic cells program non-immunogenic prostate-specific T cell responses beginning at early stages of prostate tumorigenesis. Prostate. 2007;67:536–546. doi: 10.1002/pros.20549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morgan DJ, Liblau R, Scott B, Fleck S, McDevitt HO, Sarvetnick N, Lo D, Sherman LA. CD8(+) T cell-mediated spontaneous diabetes in neonatal mice. J Immunol. 1996;157:978–983. [PubMed] [Google Scholar]
- 22.Gingrich JR, Barrios RJ, Kattan MW, Nahm HS, Finegold MJ, Greenberg NM. Androgen-independent prostate cancer progression in the TRAMP model. Cancer Res. 1997;57:4687–4691. [PubMed] [Google Scholar]
- 23.Huang CT, Huso DL, Lu Z, Wang T, Zhou G, Kennedy EP, Drake CG, Morgan DJ, Sherman LA, Higgins AD, Pardoll DM, Adler AJ. CD4+ T cells pass through an effector phase during the process of in vivo tolerance induction. J Immunol. 2003;170:3945–3953. doi: 10.4049/jimmunol.170.8.3945. [DOI] [PubMed] [Google Scholar]
- 24.Speiser DE, Miranda R, Zakarian A, Bachmann MF, McKall-Faienza K, Odermatt B, Hanahan D, Zinkernagel RM, Ohashi PS. Self antigens expressed by solid tumors Do not efficiently stimulate naive or activated T cells: implications for immunotherapy. J Exp Med. 1997;186:645–653. doi: 10.1084/jem.186.5.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Degl'Innocenti E, Grioni M, Boni A, Camporeale A, Bertilaccio MT, Freschi M, Monno A, Arcelloni C, Greenberg NM, Bellone M. Peripheral T cell tolerance occurs early during spontaneous prostate cancer development and can be rescued by dendritic cell immunization. Eur J Immunol. 2005;35:66–75. doi: 10.1002/eji.200425531. [DOI] [PubMed] [Google Scholar]
- 26.Anderson MJ, Shafer-Weaver K, Greenberg NM, Hurwitz AA. Tolerization of tumor-specific T cells despite efficient initial priming in a primary murine model of prostate cancer. J Immunol. 2007;178:1268–1276. doi: 10.4049/jimmunol.178.3.1268. [DOI] [PubMed] [Google Scholar]
- 27.Lees JR, Charbonneau B, Hayball JD, Diener K, Brown M, Matusik R, Cohen MB, Ratliff TL. T-cell recognition of a prostate specific antigen is not sufficient to induce prostate tissue destruction. Prostate. 2006;66:578–590. doi: 10.1002/pros.20307. [DOI] [PubMed] [Google Scholar]
- 28.Grosso JF, Kelleher CC, Harris TJ, Maris CH, Hipkiss EL, De MA, Anders R, Netto G, Getnet D, Bruno TC, Goldberg MV, Pardoll DM, Drake CG. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J Clin Invest. 2007;117:3383–3392. doi: 10.1172/JCI31184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lyman MA, Aung S, Biggs JA, Sherman LA. A spontaneously arising pancreatic tumor does not promote the differentiation of naive CD8+ T lymphocytes into effector CTL. J Immunol. 2004;172:6558–6567. doi: 10.4049/jimmunol.172.11.6558. [DOI] [PubMed] [Google Scholar]
- 30.Lyman MA, Nugent CT, Marquardt KL, Biggs JA, Pamer EG, Sherman LA. The Fate of Low Affinity Tumor-Specific CD8+ T Cells in Tumor-Bearing Mice. J Immunol. 2005;174:2563–2572. doi: 10.4049/jimmunol.174.5.2563. [DOI] [PubMed] [Google Scholar]
- 31.Danna EA, Sinha P, Gilbert M, Clements VK, Pulaski BA, Ostrand-Rosenberg S. Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Cancer Res. 2004;64:2205–2211. doi: 10.1158/0008-5472.can-03-2646. [DOI] [PubMed] [Google Scholar]
- 32.Huang X, Yang Y. Transient gain of effector function by CD8+ T cells undergoing peripheral tolerance to high-dose self-antigen. Eur J Immunol. 2004;34:1351–1360. doi: 10.1002/eji.200324734. [DOI] [PubMed] [Google Scholar]
- 33.Goldberg MV, Maris CH, Hipkiss EL, Flies AS, Zhen L, Tuder RM, Grosso JF, Harris TJ, Getnet D, Whartenby KA, Brockstedt DG, Dubensky TW, Jr., Chen L, Pardoll DM, Drake CG. Role of PD-1 and its ligand, B7-H1, in early fate decisions of CD8 T cells. Blood. 2007 doi: 10.1182/blood-2006-12-062422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DuPage M, Cheung AF, Mazumdar C, Winslow MM, Bronson R, Schmidt LM, Crowley D, Chen J, Jacks T. Endogenous T cell responses to antigens expressed in lung adenocarcinomas delay malignant tumor progression. Cancer Cell. 2011;19:72–85. doi: 10.1016/j.ccr.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]