Abstract
2, 2-Bis (bromomethyl)-1, 3-propanediol (BMP) is an extensively used brominated flame retardant found in urethane foams and polyester resins. In a two year dietary study conducted by the National Toxicology Program, BMP caused neoplastic lesions at multiple sites including the urinary bladder in both rats and mice. The mechanism of its carcinogenic effect is unknown. In the present study, using SV-40 immortalized human urothelial cells (UROtsa), endpoints associated with BMP induced DNA damage and oxidative stress were investigated. The effects of time (1–24 h) and concentration (5–100 μM) on BMP induced DNA strand breaks were assessed via the alkaline comet assay. The results revealed evidence of DNA strand breaks at 1 and 3h following incubation of cells with non-cytotoxic concentrations of BMP. Strand breaks were not present after 6h of incubation. Evidences for BMP associated oxidative stress include: an elevation of intracellular ROS formation as well as induction of Nrf2 and HSP70 protein levels. In addition, DNA strand breaks were attenuated when cells were pre-treated with N-acetyl-L-cysteine (NAC) and oxidative base modifications were revealed when a lesion specific endonuclease, human 8-hydroxyguanine DNA glycosylase 1 (hOGG1) was introduced into the comet assay. In conclusion, these results demonstrate that BMP induces DNA strand breaks and oxidative base damage in UROtsa cells. Oxidative stress is a significant, determinant factor in mediating these DNA lesions. These early genotoxic events may, in part, contribute to BMP-induced carcinogenesis observed in rodents.
Keywords: Brominated flame retardants; 2, 2-Bis (bromomethyl)-1, 3-propanediol; DNA damage; Oxidative stress; UROtsa cell
1. Introduction
2, 2-Bis (bromomethyl)-1, 3-propanediol (BMP), or as it is known commercially dibromoneopentyl-glycol, is a brominated flame retardant used in unsaturated polyester resins, molded products, rigid polyurethane foam, and as an additive in the manufacture of plastic polymers(Larsen,1973). BMP is also used as a chemical intermediate in the production of other flame retardants. Between 1986 and 2002, the estimated annual production of BMP in the United States was as high as 10 million pounds (EPA, 2002). This production has resulted in the environmental contamination, as BMP has been identified in dust particles and wastewater (EPA, 1983). Once in the environment, BMP is slowly degraded. Its half life is estimated to be over 100 years in groundwater (Ezra et al., 2006, 2010). Therefore, humans may be exposed to BMP by multiple routes (NTP, 1996). The widespread production and use of BMP, as well as evidence that it is a contaminate present in dust and water, heighten the importance of understanding the potential risk of mammalian exposure to BMP.
When administered orally, the acute toxicity of BMP (LD50: 3458 mg/kg) in rats is low (Keyes et al., 1979). In a thirteen-week sub-chronic toxicity study, results obtained in rats and mice that received BMP daily either in feed or by oral gavage demonstrated transitional cell hyperplasia in their kidneys and urinary bladders (Elwell et al., 1989). Results of a two year dietary bioassay conducted by the National Toxicology Program (NTP) reported that BMP is a multisite carcinogen in both sexes of rats and mice (Dunnick et al., 1997; NTP, 1996). Increased incidences of neoplasms were observed in a variety of tissues including kidney, urinary bladder, skin, mammary gland, oral cavity, esophagus, forestomach, small and large intestine, mesothelium, lung, thyroid gland, hematopoietic system, seminal vesicle and pancreas after BMP exposure. Such a wide spread distribution of tissues that developed neoplasms suggests that BMP might be a direct acting carcinogen. Based on these animal studies, BMP is reasonably anticipated to be a human carcinogen (NTP, 2011). Although BMP is carcinogenic, results of microbial mutagenicity assays are uniformly negative, except for a positive response in one study (with 30% Aroclor 1254-induced hamster liver S9) (NTP, 1996; Zeiger et al., 1992).
Induction of DNA lesions is believed to be one of the initial steps in chemical induced carcinogenesis. DNA can be damaged by such mechanisms as a direct attack of the chemical or its metabolites or via events associated with oxidative stress. Oxidation products formed in DNA include strand breaks, abasic sites and oxidized bases. Within the last group, attention has been focused on 7, 8-dihydro-8-oxo-guanine (8-OHgua) as a major maker for oxidative DNA damage with a clear mutagenic potential (Hwang et al., 2007; Van Long et al., 2010). The single cell gel electrophoresis assay (alkaline version), which measures DNA strand breaks and alkali-labile sites i.e. AP-sites or abasic sites, can be modified to measure oxidative DNA base modifications. For example, human 8-hydroxyguanine DNA glycosylase 1 (hOGG1) is an endonuclease that specifically removes 8-OHgua and is used for the detection of oxidative DNA lesions (Mihaljevic et al., 2011; Smith et al., 2006). Due to the lack of significant data on the mechanism of BMP induced carcinogenesis and since the urinary bladder was one of the target tissues in rat and mice, the present study investigated if BMP caused DNA damage and oxidative stress in an immortalized, non-malignant, p53 deficient human urothelial cell line (UROtsa cells). This cell line has been used as an effective model for studies on human bladder transitional epithelium and for investigation of both acute and chronic arsenic-induced cellular insults (Eblin et al., 2008; Rossi et al., 2001; Sens et al., 2004). In the studies reported here the ability of BMP to cause DNA strand breaks and oxidative base lesions in these cells was characterized via both the standard and the hOGG1 modified comet assay. Oxidative stress and its involvement in BMP mediated genotoxicity were evaluated by intracellular ROS generation, expression of NF-E2 related factor 2 (Nrf2) and Heat shock protein 70 (HSP70), as well as the protective capability of N-acetyl-L-cysteine (NAC), a well-established antioxidant on BMP induced DNA strand breaks.
2. Material and Methods
2.1 Chemicals
BMP (Lot No. 04119MD) with a purity of 98% was obtained from Sigma-Aldrich (St. Louis, MO). Hydrogen peroxide was purchased from JT baker (Phillipsburg, NJ) and was diluted with sterilized distilled water (dH2O) before use. Dulbecco's Modified Eagle Medium (DMEM), Penicillin/Streptomycin, trypsin-EDTA and trypan blue were acquired from Gibco Invitrogen Corporation (Carlsbad, CA) and Fetal Bovine Serum (FBS) from Atlanta Biologicals (Lawrenceville, GA). Nrf2 (rabbit polyclonal) and HSP70 (mouse monoclonal) antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA) and EMD Bioscience, Inc. (Calbiochem®, San Diego, CA) respectively. 2', 7'-dichlorodihydrofluorescein diacetate (H2DCFDA) and 5-(and-6)-carboxy-2', 7'-dichlorofluorescein diacetate (carboxy-DCFDA) were obtained from Molecular Probes, Invitrogen Detection Technology (Eugene, OR). Other generally used chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless stated otherwise, and used without further purification.
2.2 Cell culture and BMP exposure
The UROtsa cells were generously provided by Drs. Donald and Maryann Sens (University of North Dakota). Cell culture conditions were as previously described (Wnek et al., 2010). Particularly, stock cultures were maintained in a growth medium of DMEM containing 5% (v/v) FBS, 50 unit/ml penicillin and 50 μg/ml streptomycin. Growth medium was changed every 3 days. Cultured cells were incubated in a humidified atmosphere containing 5% CO2 at 37 °C. Confluent cells were removed from plates with trypsin-EDTA (0.25%) and sub-cultured at approximately 5 × 105 cells/flask in 75-cm2 culture flasks. Results of the periodical screening test for mycoplasma contamination indicated all UROtsa cells used in this study were negative.
BMP was dissolved in and serially diluted with 100% Ethanol(EtOH). Incubations were carried out from 0 to 24 h in growth medium. All control and treated cultures received the same final concentration of vehicle (0.5% EtOH).
2.3 Cytotoxicity assay
Cell viability was determined by Cell Counting Kit-8 (CCK-8, Dojindo Molecular Technologies, Rockville, MD) which measures the conversion of the highly water-soluble tetrazolium salt to formazan. The amount of formazan is proportional to the number of living cells. Briefly, UROtsa cells were seeded in 96-well plates (1 × 10 4 cells/well) and incubated in fresh DMEM medium at 37°C for 24 h. Then the cells were treated with vehicle or various concentration of BMP (10–1500 μM). After incubation for 1, 6 and 24 h, 10 μL of CCK-8 solution were added to each well and the plates were further incubated for 2 h at 37°C. The absorbance at 450 nm was measured with a microplate reader (BioTek, Winooski, VT). Cell viability following BMP and vehicle exposure compared to untreated (naïve) control. The results were expressed as the percentage of viability with respect to naïve control.
2. 4 Standard and hOGG1 modified comet assay
Standard and modified comet assays were done using the CometAssay® and hOGG1 FlARE® Assay kit (Trevigen, Gaithersburg, MD). Cells were seeded in 12-well plate at 5 × 104 cells/well and maintained for 48 h until chemical exposure. In some cases, cells were pretreated with NAC (2mM) for 1h before incubation with vehicle or BMP. Agents known to produce effects in the comet assay (H2O2 or KBrO3) were used as positive controls. Following the different treatments, cells were collected and quantified. 30 μL of cell suspensions were added to 200 μL low melting agarose and the cell/agarose mixture (50 μL) was then added to the comet slide. The slides were placed in the dark at 4°C for 30 min after which they were immersed in the lysis solution for 35 min. Next, the slides were placed in fresh alkali solution (300 mM NaOH and 1mM Na2EDTA in dH2O, PH>13) for 35 min at room temperature. Electrophoresis was conducted in the same buffer at 4°C for 35 min at 25V (0.8V/cm), 300 mA. Following electrophoresis, the slides were washed using dH2O and then immersed in 70% EtOH for 5 min. The slides were air dried and stained with 100 μL diluted SYBR® green I for 1 h. In the hOGG1 modified assay, following the incubation with the lysis buffer, the slides were immersed in three changes of FLARE buffer® for 10 min, each time at room temperature. Then 75 μl of hOGG1 enzyme solution (0.08 U/ gel) was added to the gel area and at the same time, a buffer-only control was also included. Gels were covered with Parafilm® and incubated in a humidified chamber for 1h at 37°C. The slides were then placed in the alkaline solution and processed as in the standard comet assay.
Comet slides were analyzed at 40× magnifications under an Olympus ImT-2 epifluorescence microscope (Center Valley, PA) equipped with an excitation filter of 460–500 nm, a 100W mercury lamp, a long pass filter at 515 nm and a Hamamatsu Orca 100 digital camera (Bridgewater, NJ). The images were collected using Hamamatsu SimplePCI digital imaging software (Sewickey, PA) and the analysis of DNA damage was performed by Comet Score program (V1.5, Tritek Corp). Computer generated % tail DNA (T% DNA) was used as the parameter to assess DNA damage. In the hOGG1 modified comet assay, the difference in the extent of DNA strand breaks (Net T% DNA) between hOGG1-treated and buffer-treated control slides was determined to give a quantitative measurement of hOGG1-sensitive sites. For each exposure group a total of 100 cells were analyzed using systemic random sampling (McArt et al., 2009). All experiments were repeated at least three times independently.
2.5 Intracellular ROS measurement
Intracellular ROS were determined by using H2DCFDA and carboxy-DCFDA as the sensitive non-fluorescent precursor dyes according to Tome et al (2006). Both dyes are taken into the cells and enzymatically hydrolyzed by intracellular esterases. Carboxy-DCFDA will fluoresce once the acetate groups are removed, but H2DCFDA fluoresces only in the presence of ROS (H2O2, HO•). When used in cellular systems, H2DCFDA is generally considered as a marker of oxidative stress rather than an indicator of specific reactive species (Gomes et al., 2005). Cells were seeded in 96-well plates at 5 × 103 cells/ well and incubated for 1h at 37°C in serum free DMEM medium containing 20 μM H2DCFDA or 1 μM carboxy-DCFDA. Afterwards, cells were washed twice with PBS and exposed to vehicle, BMP (10–100 μM) or H2O2 (100 μM) in PBS for 1h. ROS were measured periodically using a fluorescent plate reader (BioTek, Winooski, VT) at an emission wavelength of 538 nm and an excitation wavelength of 485 nm. Fluorescence due to H2DCFDA was corrected for the relative carboxy-DCFDA fluorescence to account for differences in dye uptake. ROS level was expressed as percentage of the fluorescence in vehicle control.
2.6 Western immunoblotting analysis
Cells were rinsed with ice cold Tris Buffer Saline (TBS) twice and then lysed in lysis buffer (10 mM Tris-HCl pH 7.4, 100 mM NaCl, 0.5% sodium deoxycholate, 1 mM EDTA, 1 mM EGTA, 20 mM Na4P2O7, 1mM PMSF, 200 μM NaF, 230 nM Aprotinin, 21 μM Leupeptin and 5 mM Na orthovanadate). Protein assays (Bio-Rad, Hercules, CA) were performed on cell lysates to correct for loading. Equal amounts of protein (30 μg) were resolved by SDS-PAGE, transferred onto a PVDF membrane (Bio-Rad, Hercules, CA) and immunoblotted for Nrf2, HSP70 and β-actin. Proteins were visualized by Visiglo HRP chemilum substrate kit (Amresco, Solon, OH) on genemate blue autoradiography films (ISC Bioexpress, Kaysville, UT). Images were quantified using the ImageJ software (NIH, USA). Each measured protein was normalized to the loading control β-actin.
2.7 Statistical analysis
All data are presented as means ± SEM. Data sets were subjected to ANOVA (one-way or two-way) followed by Dunnett's post-hoc analysis or unpaired t- test (GraphPad Prism 5, GraphPad software, Inc). P≤ 0.05 was accepted as the level of significance.
3. Results
3.1 Cytotoxicity of BMP in UROtsa cell culture
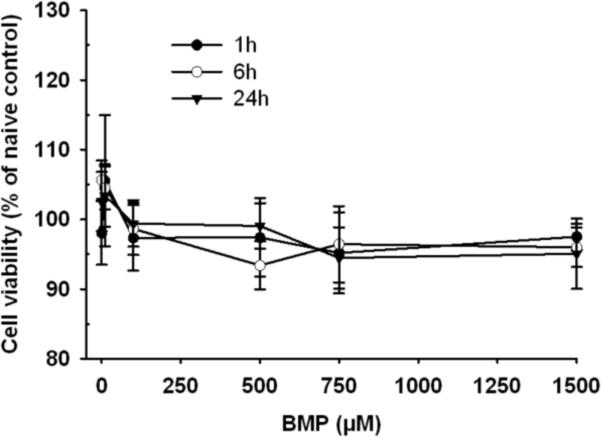
Since xenobiotic induced cytotoxicity can confound interpretation of DNA damage evaluated in the comet assay, cell viability was determined by the CCK-8 assay following BMP exposure for up to 24 h at concentrations ranging from 10 to 1500 μM. No significant reductions in the number of viable cells were detected (Fig 1). Viability (mean value of 5 independent experiments) was greater than 90% in all incubations compared with naïve control. Therefore, significant reductions in cell viability were not observed under the conditions of these studies.
Figure 1.
Viability of UROtsa cells after 1, 6 and 24h of incubation with vehicle or BMP (10–1500 μM). Data represents the mean ± SEM of 5 determinations.
3.2 Induction of time and concentration dependent DNA stand breaks by BMP
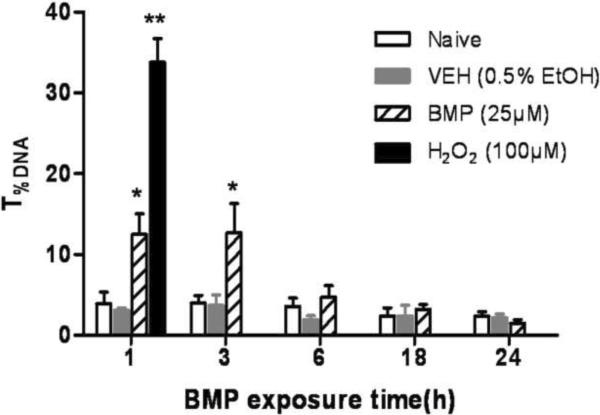
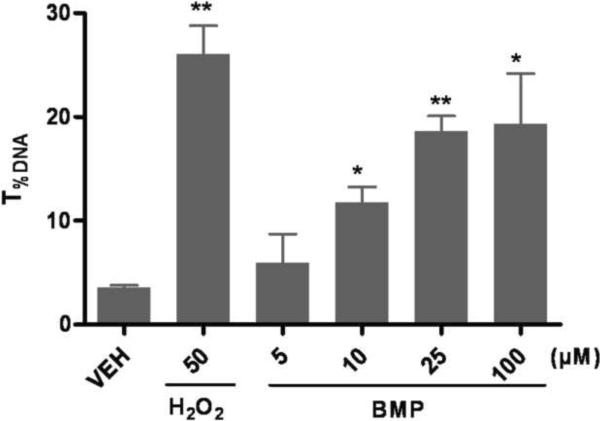
To test the ability of BMP to induce DNA strand breaks, UROtsa cells were incubated with BMP (25 μM) or dosing vehicle (0.5% EtOH) for 1, 3, 6, 18 and 24 h and subjected to the alkaline comet assay. When cells were incubated in the presence of BMP, a statistically significant increase in T%DNA was observed at 1 and 3 h. No significant increases were detected at later time points (Fig 2 A and B). Since the DNA damage was observed early after BMP exposure, the concentration dependent effects of BMP after 1h exposure were determined. Results indicated that 1h of BMP exposure (5–100μM) induced a concentration dependent increase on T%DNA in UROtsa cells (Fig 2C).
Figure 2.
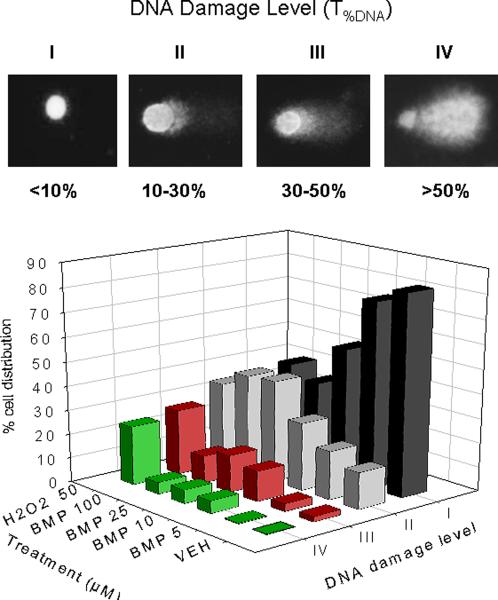
Effects of time and concentration on DNA strand breaks induced by BMP in UROtsa cells by standard comet assay.(A) Representative comet images after 1h of exposure to BMP (B) DNA strand breaks following BMP (25μM) exposure for 1, 3, 6, 18 and 24h and H2O2 (100 μM) for 1h. (C) DNA strand breaks following exposure to various concentrations of BMP (5–100μM) for 1 h (D) The distribution of UROtsa cells with respect to different DNA damage levels (I ~IV) following BMP (5–100 μM) and H2O2 (50μM) treatment for 1h. T%DNA represents % of DNA in the comet tail. Data are shown as mean ± SEM, N=3–7. *P<0.05, **P<0.01 versus vehicle control at each time point.
To evaluate the extent of this DNA damage, cells were classified according to T%DNA in four categories, ranging from DNA damage level I (no visible tail, T%DNA <10%) to DNA damage level IV (still a detectable head of the comet but most of the DNA in the tail, T%DNA>50%). The distribution of cells in each DNA damage level as a function of BMP concentration are presented in Fig 2D. Results showed that as the concentration of BMP increases, there is a shift of cells from level I (minimal to no DNA damage) to level II and III (low to moderate DNA damage). The distribution of cells to level IV was less than 5% at any concentration of BMP.
3.3 Detection of intracellular ROS in BMP exposed UROtsa cells
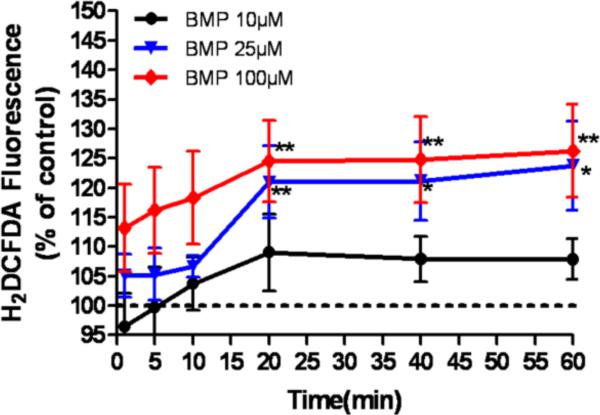
To investigate whether BMP can cause oxidative stress in UROtsa cells, intracellular ROS was assessed by H2DCFDA fluorescence between 0 and 60 min of incubation with BMP. BMP (10–100μM) induced a time and concentration dependent increase in intracellular H2DCFDA fluorescence that reached maximum within 20 min (Fig 3). When the cells were incubated with H2O2 (100μM), the fluorescence signal was increased significantly by 10 min (data not shown).
Figure 3.
Generation of intracellular ROS in UROtsa cells exposed to BMP (10–100μM) for up to 1h. H2DCFDA fluorescence signal was normalized for carboxy-DCFDA fluorescence using the following equation: H2DCFDA Signal = H2DCFDAtreatment × (carboxy-DCFDAcontrol / carboxy-DCFDAtreatment). The results are expressed as the percentage of H2DCFDA signal with respect to vehicle control. Data are expressed as mean ± SEM, N= 6, *P<0.05, **P<0.01 versus vehicle control.
3.4 Western analysis of stress proteins following BMP exposure
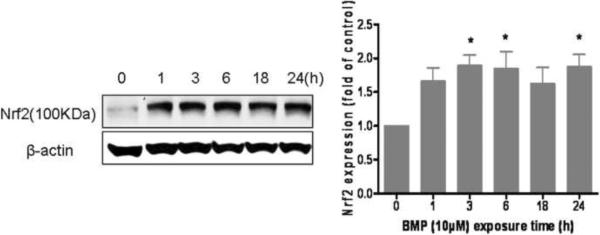
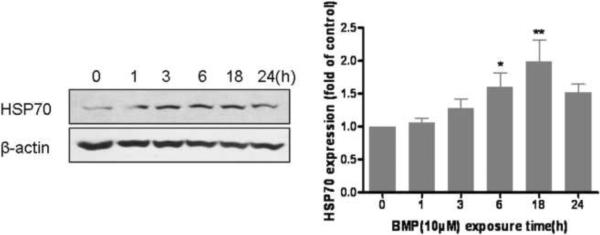
To provide additional evidence that BMP induced an oxidative stress in UROtsa cells, subsequent cellular responses were evaluated through stress protein expression. UROtsa cells were incubated with BMP (10μM) for 1, 3, 6, 18 or 24 h and the protein levels of Nrf2 and HSP70 in whole cell lysate were detected by western blotting analysis. Results showed both Nrf2 and HSP70 were induced by BMP (Fig 4). The expression of Nrf2 was increased early after BMP exposure (within 3h) and remained elevated throughout the time course (Fig 4A). The expression of HSP70 protein was delayed and peaked after 6–18 h of exposure (Fig 4B). These data demonstrated that a cellular stress response occurs in UROtsa cells at a relatively low concentration (10μM) of BMP.
Figure 4.
The expression of (A) Nrf2 and (B) HSP70 protein in UROtsa cells following BMP (10μM) exposure for 1, 3, 6, 18 and 24h. Data are shown as mean ± SEM, N= 4. *P<0.05 versus control (0h).
3.5 Induction of oxidative DNA lesions by BMP
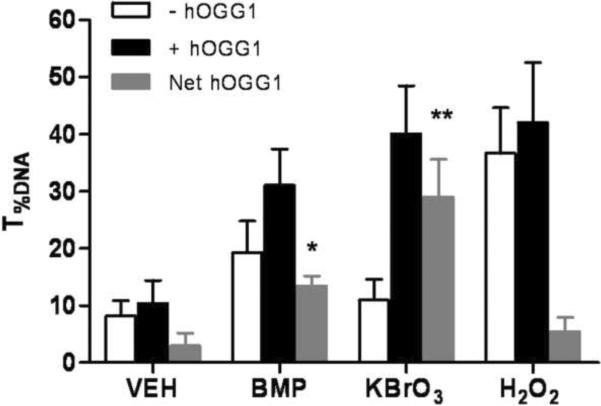
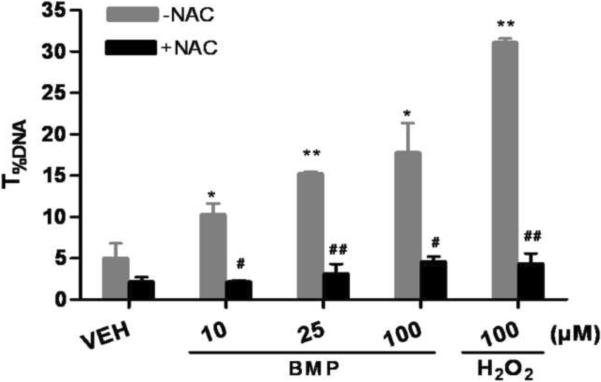
To further elucidate the potential role of oxidative stress in BMP induced DNA damage, oxidative base damage was measured by the hOGG1 modified comet assay. hOGG1 specifically removes the oxidized guanine (primarily 8-OHgua) and leaves apurinic sites which are converted to breaks in the alkaline comet assay. When hOGG1 was incorporated into the assay, additional DNA strand breaks between the hOGG1-treated and buffer-treated control slides were detected at 1h after exposure of BMP (25μM) and KBrO3 (2mM). There were not additional breaks in the DNA of H2O2 (100μM) exposed UROtsa cells (Fig 5A). In addition, the protective capability of NAC was also investigated. NAC significantly decreased the extent of DNA strand breaks induced by BMP at all concentrations tested in the standard comet assay (Fig 5B).
Figure 5.
Involvement of oxidative stress in BMP mediated DNA damage. (A) Oxidative base modifications in UROtsa cells following 1h of BMP (25μM), KBrO3(2mM) and H2O2(100μM) exposure measured by hOGG1 modified comet assay. Gray Bars represent the net increase in T%DNA after incubation with hOGG1. (B) Protective effects of NAC (2mM) on BMP (10–100μM) induced DNA strand breaks in UROtsa cells. Data are shown as mean ± SEM, N=3. *P<0.05 versus vehicle control, #P<0.05 versus cells without NAC pre-treatment.
4. Discussion
BMP is currently regulated by the US Environmental Protection Agency (EPA) under the Toxic Substance Control Act. On Nov 26, 2010, EPA added BMP to the Community Right-to-know Toxic Chemical Release Reporting List (TRI) based on the reviewing of NTP Report on Carcinogens document (EPA, 2010). However, the current toxicology database of BMP is very limited. Little information on the mode of action for BMP induced carcinogenesis is available. In the present study, the effects of BMP induced DNA damage and oxidative stress were investigated as potential mechanisms of its carcinogenic effects. Using human urinary bladder epithelium cells as an in vitro model, BMP was found to induce DNA strand breaks and oxidative DNA lesions after acute exposure. Our findings demonstrate that BMP can cause genotoxic effects in a cell line of human origin and that oxidative stress plays a major role in mediating these effects.
The BMP induced DNA damage observed in UROtsa cells was time and concentration dependent. Under the conditions used here, DNA strand breaks were maximal between 1 and 3h and were not evident by 6h following BMP exposure in UROtsa cells. Importantly, the response was concentration dependent, which underscores that the strand breaks are BMP related. It is unlikely that the observed DNA fragmentation was a result of oncosis/apoptosis since the cytotoxic assay indicated that BMP was not acutely cytotoxic even at much higher concentrations. Also, these in vitro concentrations of BMP are in the range expected for plasma concentrations of BMP following oral administration of tumorigenic doses (100 mg/kg/day and above) to rats. The estimated Cmax following a single dose of BMP (100 mg/kg) is 15 μM, based on the Cmax of 1.5 μM determined directly in rats administered 10 mg/kg of BMP orally by gavage (Hoehle et al., 2009).
These BMP associated strand breaks are not persistent as evidenced by the return of T%DNA to basal levels by 6h. Also, the fact that the degree of BMP associated DNA damage was in the mild to moderate level (levels II and III), indicates that the cells survive the initial injury and that the fragmented DNA strands are rapidly rejoined. Such results are not unexpected as DNA strand breaks detected by the standard comet assay represent a stage of imbalance between the rate of DNA damage and the rate of repair (Fortini et al., 1996). This has been demonstrated for alkylating agents and oxidants. For example, Siman et al. (2000) reported a transient response in the comet assay when they investigated the genotoxic effects of fern spore extracts. Similarly, Collins et al. (1995) reported that most H2O2 induced strand breaks in HeLa cells are repaired within 1h. In the present study, this imbalance was evident in the UROtsa cells between 1 and 3 h of exposure to BMP. It should be emphasized that this rapid repair of strand breaks does not mean that BMP induced mutations are not present. Events that might lead to mutations could have occurred before or during the repair processes, particularly if the repair was mediated by an error-prone polymerase (Crespan et al., 2010; Deem et al., 2011; Wang et al., 2001). Such mutations may become fixed with subsequent cell divisions, leading to clones of transformed cells that are ultimately expressed as neoplasms.
It is well documented that ROS can damage DNA by producing a variety of lesions such as oxidized bases, AP-sites and strand breaks (Cadet et al., 2010; Goetz et al., 2008; Sedelnikova et al., 2010). Results of studies presented here provide considerable evidence that ROS contributes to the DNA damage associated with exposure of UROtsa cells to BMP. First, incubation of cells with BMP led to a concentration and time dependent increase in ROS generation as assessed by H2DCFDA fluorescence. This increase in ROS reached a maximum by 20 min of incubation and was maintained for the 1 h of incubation. This time frame coincided with the production of DNA strand breaks. Second, when the cells were pre-incubated with the antioxidant, NAC, the production of BMP associated strand breaks was nearly completely inhibited. The mechanism by which NAC inhibited DNA strand breaks most likely relates to its antioxidant action, as BMP was not consumed and new metabolite peaks were not observed when NAC and BMP were co-incubated in the cell culture medium (data not shown). Finally, direct evidence of BMP-induced oxidative base alteration was obtained in the modified Comet assay. When hOGG1 was included in the assay additional strand breaks were expressed. These most likely represent breaks induced during the excision of 8-OHgua. Additional breaks were also present in the modified Comet assay when cells were exposed to the oxidizing agent KBrO3, which served as a positive control. On the other hand, additional breaks were not observed in the modified Comet assay when H2O2 served as a positive control. At the concentration of H2O2 (100μM) used, a high level of strand breaks was induced under non-hOGG1 conditions, which precluded quantification of additional strand breaks in the modified assay.
BMP mediated oxidative stress was also evidenced by the up-regulation of cellular stress responses in UROtsa cells. The expression of both Nrf2 and HSP70 was significantly increased within 6 h of exposure to BMP. Both proteins are known to respond to oxidant induced cellular injury. These responses were particularly evident in UROtsa cells since the basal expression level for Nrf2 (Du et al., 2008) and HSP70 (Eblin et al., 2006; Rossi et al., 2002) is low under normal conditions. These results verified that UROtsa cells recognized the BMP induced insults even at a concentration of 10μM. Together, these data, coupled with those mentioned above; support a major contribution of oxidative damage to the effects observed in the comet assay after acute exposure of UROtsa cells to BMP.
Interestingly, increased expression of HSP70 occurred later in the incubation (6h) than that of Nrf2. This delayed response is possibly due to a rapid and reversible translational inhibition in cells to protect against oxidative stress (Grant 2011; Lu et al., 2001). Cells can reduce protein synthesis to prevent continued gene expression during potentially error-prone conditions as well as allow for the turnover of existing mRNA and proteins. On the other hand, Nrf2 accumulates under stress conditions as the results of inhibition of its degradation (McMahon et al., 2003) and a cap-independent translation initiation mechanism (Purdom-Dickinson et al., 2007; Li et al., 2010).
The molecular mechanism(s) by which BMP causes oxidative stress in UROtsa cells is unknown. Yang et al. have shown that the intracellular glutathione (GSH) content in normal human urothelium mucosa is relatively low (Yang et al., 1997). Similar results were observed by us: the GSH level in UROtsa cells is about 5 fold lower than that in mouse hepatocytes (data not shown). This could be one reason that UROtsa cells are susceptible to oxidant injury. Also, since the functions of p53 are altered in immortalized cell lines as a result of SV40 large T-antigen transfection, UROtsa cells may have inadequate antioxidant defense mechanisms. This occurs because p53 is reported to exhibit antioxidant activities at low levels of oxidative stress (Liu and Xu, 2011). How these possible deficits in antioxidant activities of UROtsa cells relate to the BMP induced carcinogenesis observed in rodents remain to be established.
At present, there is no direct evidence for formation of a reactive/toxic intermediate of BMP that could promote oxidative stress. In fact, studies with isolated rat hepatic microsomes and hepatocytes have demonstrated nearly stoichiometric conversion of BMP to a BMP monoglucuronide (Rad et al., 2010). Importantly, in rat hepatocytes no other metabolites were observed. In vivo studies in rat also demonstrated extensive glucuronidation of BMP, with no evidence of other metabolites being formed (Hoehle et al., 2009). When BMP was incubated with UROtsa cells, no BMP glucuronide was formed and no other metabolites were detected via HPLC (data not shown). Taken together, these results suggest that it may be the parent compound that is responsible for the oxidative damage to the DNA observed in UROtsa cells. Although parent BMP was not detected in the urine following oral administration of a single dose, it may be present following repeated high dose administrated as in the condition of the NTP two year bioassay. These doses (100 mg/kg/day to 400 mg/kg/day) would produce high amounts of BMP glucuronide, which when delivered to bladder, could undergo partial hydrolysis to yield parent BMP. Importantly, in this dose range it was noted that the elimination rate of BMP decreased dose dependently (Hoehle et al., 2009). At high doses, this slower rate of elimination, most likely as a result of saturation of relevant UGTs, may expose the tissues to higher levels of parent compound.
The relevance of these results to humans is not known at this time as exposure data are not available (NTP, 2011). However, it is important to note that human hepatic microsomes and hepatocytes are extremely inefficient at glucuronidation of BMP (the rate is about 100 fold lower than that observed for rat or mice microsomes/hepatocytes) (Rad et al., 2010). If indeed, it is parent BMP that is responsible for oxidative DNA damage, this metabolic deficiency should be considered in any human risk assessment.
In conclusion, the results of this study demonstrate BMP is a genotoxic agent. It induces DNA strand breaks and oxidative base damages through generation of oxidative stress shortly after its exposure in UROtsa cells. Although the induced damage seems rapidly repaired, these early genotoxic events may, in part, contribute to the BMP-induced carcinogenesis observed in rodents.
Highlights
-
➢
BMP induces DNA strand breaks and oxidative base damage in UROtsa cells.
-
➢
BMP induced DNA strand breaks are time and concentration dependent.
-
➢
Oxidative stress is a determinant factor in mediating these DNA lesions.
Acknowledgments
We thank Dr. Michael Cuningham (National Toxicology Program and National Center for Toxicogenomics, NIEHS), Dr. Amanda Baker (Arizona Cancer Center) and Gabriel Knudsen (Department of Pharmacology, University of Arizona) for advice and support; Douglas W. Cromey (Southwest Environmental Science Center Cellular Imaging Facility Core) for assistance in data analysis. This research was supported by the NIEHS Grant No. N01-ES-45529 and NIEHS-sponsored Southwest Environmental Science Center Grant No. P30-ES-06694.
Uncommon Abbreviations
- AP-sites
apurinic/apyrimidinic sites
- BMP
2, 2-Bis (bromomethyl)-1, 3-propanediol
- CCK-8
Cell Counting Kit-8
- carboxy-DCFDA
5-(and-6)-carboxy-2', 7'-dichlorofluorescein diacetate
- EPA
U.S. Environmental Protection Agency
- H2DCFDA
2', 7'-dichlorodihydrofluorescein diacetate
- hOGG1
human 8-hydroxyguanine DNA glycosylase 1
- NAC
N-acetyl-L-cysteine
- NTP
National Toxicology Program
- 8-OHgua
7, 8-dihydro-8-oxo-guanine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest statement The authors declare that there are no conflicts of interest.
References
- Cadet J, Douki T, Ravanat JL. Oxidatively generated base damage to cellular DNA. Free Radic. Biol. Med. 2010;49:9–21. doi: 10.1016/j.freeradbiomed.2010.03.025. [DOI] [PubMed] [Google Scholar]
- Collins AR, Ma AG, Duthie SJ. The kinetics of repair of oxidative DNA damage (strand breaks and oxidised pyrimidines) in human cells. Mutat. Res. 1995;336:69–77. doi: 10.1016/0921-8777(94)00043-6. [DOI] [PubMed] [Google Scholar]
- Crespan E, Amoroso A, Maga G. DNA polymerases and mutagenesis in human cancers. Subcell. Biochem. 2010;50:165–188. doi: 10.1007/978-90-481-3471-7_9. [DOI] [PubMed] [Google Scholar]
- Deem A, Keszthelyi A, Blackgrove T, Vayl A, Coffey B, Mathur R, Chabes A, Malkova A. Break-induced replication is highly inaccurate. PLoS Biol. 2011;9:e1000594. doi: 10.1371/journal.pbio.1000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Villeneuve NF, Wang XJ, Sun Z, Chen W, Li J, Lou H, Wong PK, Zhang DD. Oridonin confers protection against arsenic-induced toxicity through activation of the Nrf2-mediated defensive response. Environ. Health Perspect. 2008;116:1154–1161. doi: 10.1289/ehp.11464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnick JK, Heath JE, Farnell DR, Prejean JD, Haseman JK, Elwell MR. Carcinogenic activity of the flame retardant, 2, 2-bis (bromomethyl) -1, 3-propanediol in rodents, and comparison with the carcinogenicity of other NTP brominated chemicals. Toxicol. Pathol. 1997;25:541–548. doi: 10.1177/019262339702500602. [DOI] [PubMed] [Google Scholar]
- Eblin KE, Bowen ME, Cromey DW, Bredfeldt TG, Mash EA, Lau SS, Gandolfi AJ. Arsenite and monomethylarsonous acid generate oxidative stress response in human bladder cell culture. Toxicol. Appl. Pharmacol. 2006;217:7–14. doi: 10.1016/j.taap.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Eblin KE, Bredfeldt TG, Gandolfi AJ. Immortalized human urothelial cells as a model of arsenic-induced bladder cancer. Toxicology. 2008;248:67–76. doi: 10.1016/j.tox.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Elwell MR, Dunnick JK, Brown HR, Montgomery CA. Kidney and urinary bladder lesions in F344/N rats and B6C3F1 mice after 13 weeks of 2, 2-bis (bromomethyl) -1, 3-propanediol administration. Fundam. Appl. Toxicol. 1989;12:480–490. doi: 10.1016/0272-0590(89)90022-5. [DOI] [PubMed] [Google Scholar]
- Ezra S, Feinstein S, Yakirevich A, Adar E, Bilkis I. Retardation of organo-bromides in a fractured chalk aquitard. J. Contam. Hydrol. 2006;86:195–214. doi: 10.1016/j.jconhyd.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Ezra S, Bilkis I, Feinstein S, Adar E, Ganor J. Chemical degradation of 2, 2-bis (bromomethyl) propan-1, 3-diol (DBNPG) in alkaline conditions. Chemosphere. 2010;79:476–481. doi: 10.1016/j.chemosphere.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Fortini P, Raspaglio G, Falchi M, Dogliotti E. Analysis of DNA alkylation damage and repair in mammalian cells by the comet assay. Mutagenesis. 1996;11:169–175. doi: 10.1093/mutage/11.2.169. [DOI] [PubMed] [Google Scholar]
- Goetz ME, Luch A. Reactive species: a cell damaging rout assisting to chemical carcinogens. Cancer Lett. 2008;266:73–83. doi: 10.1016/j.canlet.2008.02.035. [DOI] [PubMed] [Google Scholar]
- Gomes A, Fernandes E, Lima JL. Fluorescence probes used for detection of reactive oxygen species. J. Biochem. Biophys. Methods. 2005;65:45–80. doi: 10.1016/j.jbbm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Grant CM. Regulation of translation by hydrogen peroxide. Antioxid. Redox Signal. 2011;15:191–203. doi: 10.1089/ars.2010.3699. [DOI] [PubMed] [Google Scholar]
- Hoehle SI, Knudsen GA, Sanders JM, Sipes IG. Absorption, distribution, metabolism, and excretion of 2, 2-bis (bromomethyl) -1, 3-propanediol in male fischer-344 rats. Drug Metab. Dispos. 2009;37:408–416. doi: 10.1124/dmd.108.023937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang ES, Kim GH. Biomarkers for oxidative stress status of DNA, lipids, and proteins in vitro and in vivo cancer research. Toxicology. 2007;229:1–10. doi: 10.1016/j.tox.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Keyes DG, Kociba RJ, Schwetz RW, Wade CE, Dittenber DA, Quinn T, Gorzinski SJ, Hermann EA, Momany JJ, Schwetz BA. Results of a two-year toxicity and oncogenic study of rat ingesting diets containing dibromoneopentyl glycol (FR-1138) J. Combust. Toxicol. 1979:777–798. [Google Scholar]
- Larsen ER. 2, 2-bis (bromomethyl)-1, 3-propanediol: A light stable fire retardant monomer for condensation polymers. Org. Coatings. Plast. Chem. 1973;29:375. [Google Scholar]
- Li W, Thakor N, Xu EY, Huang Y, Chen C, Yu R, Holcik M, Kong AN. An internal ribosomal entry site mediates redox-sensitive translation of Nrf2. Nucleic Acids Res. 2010;38:778–788. doi: 10.1093/nar/gkp1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Xu Y. p53, Oxidative Stress, and Aging. Antioxid. Redox Signal. 2011;15:1669–1678. doi: 10.1089/ars.2010.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Han AP, Chen JJ. Translation initiation control by heme-regulated eukaryotic initiation factor 2alpha kinase in erythroid cells under cytoplasmic stresses. Mol. Cell. Biol. 2001;21:7971–7980. doi: 10.1128/MCB.21.23.7971-7980.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArt DG, Wasson GR, McKerr G, Saetzler K, Reed M, Howard CV. Systematic random sampling of the comet assay. Mutagenesis. 2009;24:373–378. doi: 10.1093/mutage/gep020. [DOI] [PubMed] [Google Scholar]
- McMahon M, Itoh K, Yamamoto M, Hayes JD. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J. Biol. Chem. 2003;278:21592–21600. doi: 10.1074/jbc.M300931200. [DOI] [PubMed] [Google Scholar]
- Mihaljevic Z, Ternjej I, Stankovic I, Ivkovic M, Zeljezic D, Mladinic M, Kopjar N. Assessment of genotoxic potency of sulfate-rich surface waters on medicinal leech and human leukocytes using different versions of the Comet assay. Ecotoxicol. Environ. Saf. 2011;74(5):1416–1426. doi: 10.1016/j.ecoenv.2011.04.001. [DOI] [PubMed] [Google Scholar]
- National Toxicology Program NTP Toxicology and Carcinogenesis Studies of 2, 2-Bis (Bromomethyl) -1, 3-Propanediol (FR-1138(R)) (CAS No. 3296-90-0) in F344 Rats and B6C3F1 Mice (Feed Studies) Natl. Toxicol. Program. Tech. Rep. Ser. 1996;452:1–465. [PubMed] [Google Scholar]
- National Toxicology Program NTP 12th Report on Carcinogens. Rep. Carcinog. 2011;12:70–1. [PubMed] [Google Scholar]
- Purdom-Dickinson SE, Sheveleva EV, Sun H, Chen QM. Translational control of nrf2 protein in activation of antioxidant response by oxidants. Mol. Pharmacol. 2007;72:1074–1081. doi: 10.1124/mol.107.035360. [DOI] [PubMed] [Google Scholar]
- Rad G, Hoehle SI, Kuester RK, Sipes IG. In vitro glucuronidation of 2, 2-bis (bromomethyl) -1, 3-propanediol by microsomes and hepatocytes from rats and humans. Drug Metab. Dispos. 2010;38:957–962. doi: 10.1124/dmd.110.032110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi MR, Masters JR, Park S, Todd JH, Garrett SH, Sens MA, Somji S, Nath J, Sens DA. The immortalized UROtsa cell line as a potential cell culture model of human urothelium. Environ. Health Perspect. 2001;109:801–808. doi: 10.1289/ehp.01109801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi MR, Somji S, Garrett SH, Sens MA, Nath J, Sens DA. Expression of hsp 27, hsp 60, hsc 70, and hsp 70 stress response genes in cultured human urothelial cells (UROtsa) exposed to lethal and sublethal concentrations of sodium arsenite. Environ. Health Perspect. 2002;110:1225–1232. doi: 10.1289/ehp.021101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedelnikova OA, Redon CE, Dickey JS, Nakamura AJ, Georgakilas AG, Bonner WM. Role of oxidatively induced DNA lesions in human pathogenesis. Mutat. Res. 2010;704:152–159. doi: 10.1016/j.mrrev.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sens DA, Park S, Gurel V, Sens MA, Garrett SH, Somji S. Inorganic cadmium- and arsenite-induced malignant transformation of human bladder urothelial cells. Toxicol. Sci. 2004;79:56–63. doi: 10.1093/toxsci/kfh086. [DOI] [PubMed] [Google Scholar]
- Siman SE, Povey AC, Ward TH, Margison GP, Sheffield E. Fern spore extracts can damage DNA. Br. J. Cancer. 2000;83:69–73. doi: 10.1054/bjoc.2000.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CC, O`Donovan MR, Martin EA. hOGG1 recognizes oxidative damage using the comet assay with greater specificity than FPG or ENDOIII. Mutagenesis. 2006;21:185–190. doi: 10.1093/mutage/gel019. [DOI] [PubMed] [Google Scholar]
- Tome ME, Johnson DB, Samulitis BK, Dorr RT, Briehl MM. Glucose 6-phosphate dehydrogenase overexpression models glucose deprivation and sensitizes lymphoma cells to apoptosis. Antioxid. Redox Signal. 2006;8:1315–1327. doi: 10.1089/ars.2006.8.1315. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency . Contract No. 68-01-6239. Dynamic Corp Rockville; MD: 1983. An overview of the exposure potential of commercial flame retardants; pp. 192–201. [Google Scholar]
- U.S. Environmental Protection Agency . CAS No. 3296-90-0, High Production Volume (HPV) Chemical Challenge Program. Washington, DC: 2002. Test plan for 2, 2-bis (bromomethyl) -1, 3-propanediol. [Google Scholar]
- U.S. Environmental Protection Agency Federal Register/Rules and Regulations. 2010;75:72727–72734. Available at: http://www.epa.gov/tri/lawsandregs/ntp_chemicals/final.html. [PubMed] [Google Scholar]
- Van Loon B, Markkanen E, Hubscher U. Oxygen as a friend and enemy: How to combat the mutational potential of 8-oxo-guanine. DNA Repair (Amst) 2010;9:604–616. doi: 10.1016/j.dnarep.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Wang Z. DNA damage-induced mutagenesis: a novel target for cancer prevention. Mol. Interv. 2001;1:269–281. [PubMed] [Google Scholar]
- Wnek SM, Jensen TJ, Severson PL, Futscher BW, Gandolfi AJ. Monomethylarsonous acid produces irreversible events resulting in malignant transformation of a human bladder cell line following 12 weeks of low-level exposure. Toxicol. Sci. 2010;116:44–57. doi: 10.1093/toxsci/kfq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CR, Ou YC, Kuo JH, Kao YL, Chen CL, Yean SY, Horng YY, Yang CS. Intracellular glutathione content of urothelial cancer in correlation to chemotherapy response. Cancer Lett. 1997;119:157–162. doi: 10.1016/s0304-3835(97)00274-7. [DOI] [PubMed] [Google Scholar]
- Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K. Salmonella mutagenicity tests: V. Results from the testing of 311 chemicals. Environ. Mol. Mutagen. 1992;19(Suppl 21):2–141. doi: 10.1002/em.2850190603. [DOI] [PubMed] [Google Scholar]