Abstract
Background and Purpose
Studies have demonstrated that CT angiography source images(CTA-SI) acquired under near-steady state contrast concentration provide infarct core estimates equivalent to diffusion-weighted images(DWI). We sought to test this relationship using our current CTA protocol optimized for faster scan acquisition.
Methods
Forty-eight consecutive acute ischemic stroke patients met the following criteria: fast-acquisition CTA and MRI within nine hours of symptom onset, CTA-to-MRI interval under two hours, and anterior circulation vessel occlusion. Collaterals were graded on CTA, and lesion volumes were calculated on CTA-SI, DWI and MR mean transit time(MTT) maps.
Results
The mean CTA-to-MRI interval was 36 minutes(± 18 minutes). In paired analysis, lesion volumes on CTA-SI were significantly larger than on DWI(45.6 cm3 vs. 29.9 cm3; P<0.0001). In 14(29.2%) cases, there was major CTA-SI overestimation(>25 cm3 difference) of the DWI lesion. Lower collateral score(P=0.001), higher NIHSS score(P=0.01), older age(P=0.01), and proximal occlusion(P<0.05) were univariate predictors of major overestimation, with collateral score being the only independent predictor. The interobserver agreement was worse for CTA-SI than for DWI(P<0.001 for limits of agreement).
Conclusions
CTA-SI performed using a fast-acquisition protocol overestimates the infarct core on DWI. Substantial differences are observed in over 25% of cases, and are associated with reduced collateralization.
Keywords: Acute Ischemic Stroke, Infarct Core, Diffusion Magnetic Resonance Imaging, CT Angiography Source Images
Introduction
In AIS patients, early imaging is important to identify underlying pathology and to provide information for treatment decisions. The advantage of CT-based methods is that they are more widely available than MRI in the emergent setting. Of the CT techniques, CTA-SI has numerous advantages. It is highly specific and more sensitive than non-enhanced CT for detecting early irreversible ischemia,[1] and allows for evaluation of the whole brain which is not routinely available with CT perfusion.
CTA-SI hypoattenuation primarily denotes regions with decreased contrast opacification. Under a near steady-state concentration of arterial and tissue contrast, CTA-SI is blood-volume weighted,[2] and areas of hypoattenuation are thought to approximate infarct core.[3–4] Studies using single- and 4-slice CT scanners have supported this view by demonstrating a high correlation and no difference between CTA-SI and DWI lesion volume in acute stroke.[5–6] However, we have noticed that using our current CTA acquisition protocol which has been optimized for fast, arterial-phase imaging, the CTA-SI lesion markedly overestimates the DWI lesion in a high number of cases.
We sought to determine whether CTA-SI hypoattenuation using our fast acquisition protocol significantly overestimates the DWI lesion size obtained soon afterwards.
Methods
This study was approved by our Institutional Review Board. We retrospectively reviewed the clinical and imaging findings of consecutive cases of anterior circulation AIS that presented to our hospital between February 2007 and April 2008. Inclusion criteria were: 1) admission CTA performed on a 64-slice CT scanner using our optimized fast acquisition protocol and subsequent MRI with DWI within 9 hours of symptom onset, 2) CTA-to-MRI interval of less than 2 hours, and 3) documented anterior circulation vessel occlusion on CTA. Most patients also underwent MR PWI. Medical records were reviewed for clinical information, and were HIPAA (Health Insurance Portability and Accountability Act) compliant.
Imaging
CT Angiography
In 2005, our institutional CTA protocol was optimized for faster scan acquisition with the goals of speeding treatment decisions and lowering radiation and contrast dose. This fast scan protocol incorporated a shortened imaging delay triggered by a region of interest (ROI) in the aortic arch, a vertex-to-arch scanning direction and a faster table speed in order to acquire images during the arterial phase of contrast enhancement. Several centers have employed similar imaging protocols using vertex-to-arch imaging[7–11] and a shortened delay time.[12]
Specific imaging parameters for our current protocol are 120–140 kVp, auto mA 300–800, 0.5-second rotation time, 1.25-mm section thickness reconstructed to 5mm, 9.37 to 20.62 mm/rotation table speed and pitch 0.938:1 to 0.516:1 from the vertex to the aortic arch. SmartPrep (GE Medical Systems, Waukesha, WI) is used with an ROI centered over the aortic arch and a monitoring delay of 10 seconds. Scanning is triggered once the ROI reaches Δ50–100 Hounsfield units with a 3-second diagnostic delay. Fifty-five to 90mL of Isovue 370 contrast (Bracco Diagnostics, Princeton, NJ) are injected, followed by a 40mL normal saline bolus at 3–4mL/s. Contrast is injected through an 18-gauge intravenous line using a power injector(Medrad, Warrendale, PA).
MRI
MR examinations were performed on a 1.5-Tesla Signa whole-body scanner(GE Medical Systems, Milwaukee, WI). DWI was performed using a single-shot echo-planar spin-echo sequence with two 180-degree radiofrequency pulses to minimize eddy current warping. Five images/slice were acquired at b=0 s/mm2, followed by five at b=1000 s/mm2 in six directions, for a total 35 images/slice. Twenty-three to 27 slices covered the entire brain. Imaging parameters were TR/TE 5000/80–110 ms, FOV 22-cm, matrix 128×128 zero-filled to 256×256, 5-mm section thickness, 1-mm gap.
PWI was performed using a dynamic susceptibility technique. Serial echo-planar gradient-echo images were acquired with TR/TE 1500/40 ms, FOV 22-cm, matrix 128×128, 5-mm slice thickness, 1-mm gap. Fourteen to 16 slices were acquired every 1.5s, for a total 46–80 images/slice. Ten seconds after image acquisition began, 20mL of gadopentetate dimeglumine 0.5 mmol/mL (Magnevist, Bayer HealthCare Pharmaceuticals) was injected via a peripheral intravenous catheter at 5mL/s using a power injector(Medrad, Warrendale, PA), followed by a 20mL normal saline bolus. Signal intensity-versus-time curves for each pixel were converted to concentration-versus-time curves, which were integrated to yield maps of CBV. CBF was calculated by singular value decomposition deconvolution.[13] A global arterial input function was derived from the MCA ipsilateral to each patient’s infarct. MTT was calculated by dividing CBV by CBF.
Image Analysis
All imaging analysis was performed by two experienced neuroradiologists(A.Y., P.S.) who were blinded to all clinical and imaging information except for side of involvement. Collateral circulation was graded on CTA at the levels of the sylvian fissure and the hemispheric convexity using collapsed maximum intensity projection images. At each level, collateral vessels were given one point if they were equal to or greater than the normal hemisphere, or no points if they were less than the other side.[14] All discrepancies were resolved by consensus. The points were combined for both levels for a total score between 0 and 2.
Volume measurements of the ischemic lesion on CTA-SI and DWI and MTT maps were performed with a semi-automated commercially available image analysis program(Analyze, Biomedical Imaging Resource, Mayo Foundation). CTA-SI were reconstructed to 5-mm slice thickness for analysis; variable window width and center level settings were used to accentuate differences between normal and ischemic brain. Lesion volumes were recorded for each reviewer, and used to determine interobserver agreement. Average lesion volumes were used for all other statistical analyses. Lesion volume calculations incorporated the imaging gap between the MR images.
Statistical Analysis
All variables were tested for normality using the Kolmogorov-Smirnov test. Continuous variables were reported as mean ± standard deviation, or median with IQR. Categorical variables were reported as proportions. The admission CTA-SI lesion volume was tested for correlation with DWI and MTT lesion volumes using the Spearman rank correlation, and paired comparisons were performed using the Wilcoxon signed-rank test. The paired comparisons were performed for the whole group, as well as the subsets of patients with proximal artery(ICA terminus and MCA M1 segment) occlusions and with distal artery(MCA M2 and M3 segment) occlusions.
Additionally, the difference between CTA-SI and DWI lesion volumes was analyzed as a dichotomous variable, defined as major(> 25 cm3) versus minor(≤ 25cm3) overestimation. The 25-cm3 threshold was chosen because two recent studies have demonstrated the importance of this infarct size in predicting outcome after intravenous tissue plasminogen activator (IV tPA).[15–16] These two groups were compared for several variables in a univariate analysis. Variables with a univariate P-value <0.10 were included in a multiple logistic regression analysis comparing major versus minor overestimation. ROC analysis was performed to identify the optimal threshold for predicting major overestimation on CTA-SI.
Interobserver agreement was examined for both CTA-SI and DWI using Bland-Altman analysis;[17] mean differences were compared using the Welch test(t-test for unequal variances), and limits of agreement were compared using the Fisher’s test for equal variances. Statistical analyses were performed using MedCalc, version 11.2.1.0(MedCalc Software, Mariakerke, Belgium). Statistical significance was considered at P<0.05.
Results
Forty-eight patients satisfied our inclusion criteria (Table 1). There were 26(54.2%) females. The mean age was 72.0±13.5 years. The median NIHSS score was 15(IQR 8–19). There were 30(62.5%) patients with proximal artery occlusions: 10 intracranial ICA and 20 M1 segment. Eighteen(37.5%) patients had distal occlusions: 14 M2 and 4 M3 segment.
Table 1.
Baseline clinical and demographic variables and imaging characteristics
| Sex (female) | 54.2% |
| Age (years) | 72.0 ± 13.5 |
| Occlusion Site | |
| Internal carotid artery | 10 |
| M1 segment, middle cerebral artery | 20 |
| M2 segment, middle cerebral artery | 14 |
| M3 segment, middle cerebral artery | 4 |
| Hemisphere (left) | 62.5% |
| NIHSS | 15 (8–19) |
| Time onset to admission CT (hr) | 3:58 ± 1:49 |
| Time admission CT to MR (hr) | 0:36 ± 0:18 |
| Admission CTA-SI volume (cm3) | 45.6 (25.5–96.0) |
| Admission DWI volume (cm3) | 29.9 (13.6–61.5) |
| Admission MTT volume (cm3) | 122.3 (61.2–180.8) |
Continuous variables are reported as means(± standard deviation) or as medians(interquartile range).
The MRI was always performed after the CTA. The mean time from symptom onset to admission CTA was 3 hours, 58 minutes(± 1 hour, 49 minutes). The mean time from CTA to MRI was 36 minutes(± 18 minutes). Forty-five(93.7%) patients had a CTA-to-MRI time of less than one hour. The remaining three patients had CTA-to-MRI times of 71, 84 and 119 minutes.
Treatments varied within the study population: 15 patients were treated with IV tPA; 6 patients underwent combined IV tPA and IAT; 2 patients underwent IAT alone; 1 patient underwent NeuroFlo(CoAxia, Maple Grove, MN) treatment;[18] and 24 patients underwent no treatment. Of 39 patients with follow-up 3-month mRS, 17(43.6%) had an independent outcome(mRS≤2), and 16(41%) died(mRS 6).
Admission CTA-SI versus DWI lesion volumes
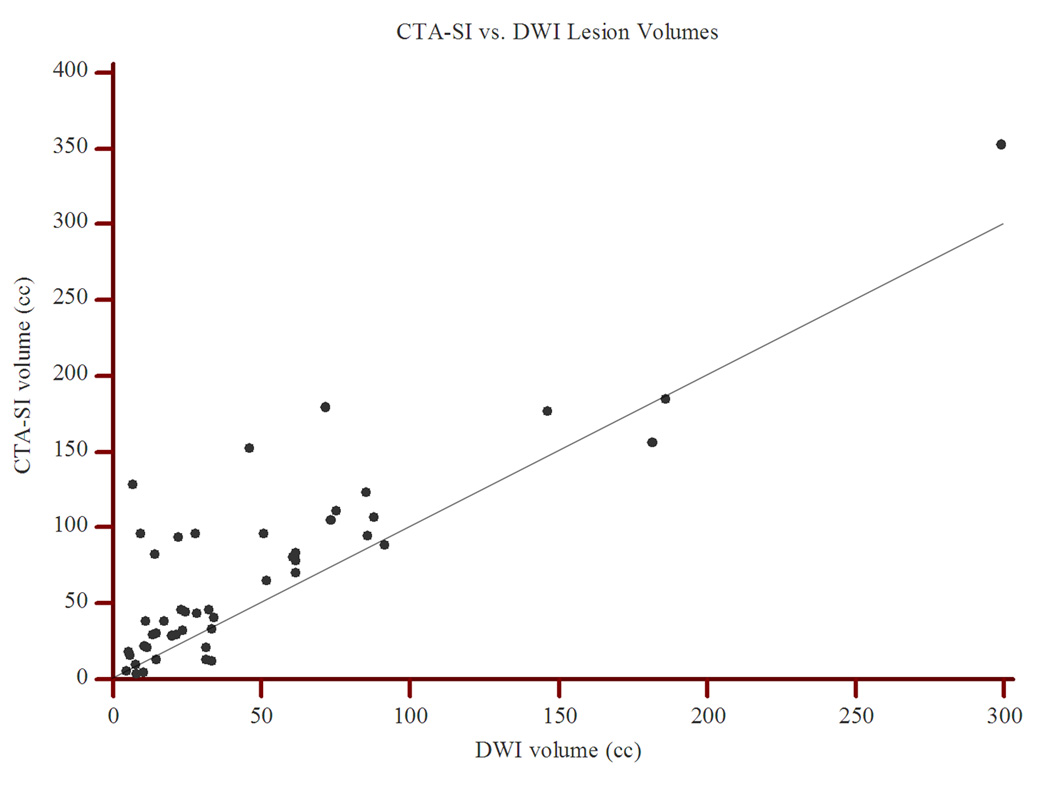
There was a significant correlation between the admission CTA-SI and DWI lesion volumes(Spearman rho: 0.71; P<0.0001; Figure 1). However, in 38(79.2%) cases, the CTA-SI lesion was larger than the DWI lesion(P=0.0001; Figure 2). Furthermore, in pairwise comparison, the lesion volumes on CTA-SI were significantly larger than on the corresponding DWI(45.6 cm3 [IQR, 25.5–96.0 cm3] vs. 29.9 cm3 [IQR, 13.6–61.5 cm3]; P<0.0001). This relationship remained statistically significant for both the proximal artery occlusions(85.4 cm3 [IQR, 37.9–122.6 cm3] vs. 48.3 cm3 [IQR, 13.1–85.2 cm3]; P<0.0001) and the distal artery occlusions(30.5 cm3 [IQR, 18.1–45.5 cm3] vs. 25.7 cm3 [IQR, 17.3–32.9 cm3]; P=0.03).
Figure 1.
Scatter plot of CTA-SI vs. DWI lesion volumes. The correlation is significant with Spearman rho of 0.71(P<0.0001). However, note that most points lie above the line of equality(slope=1).
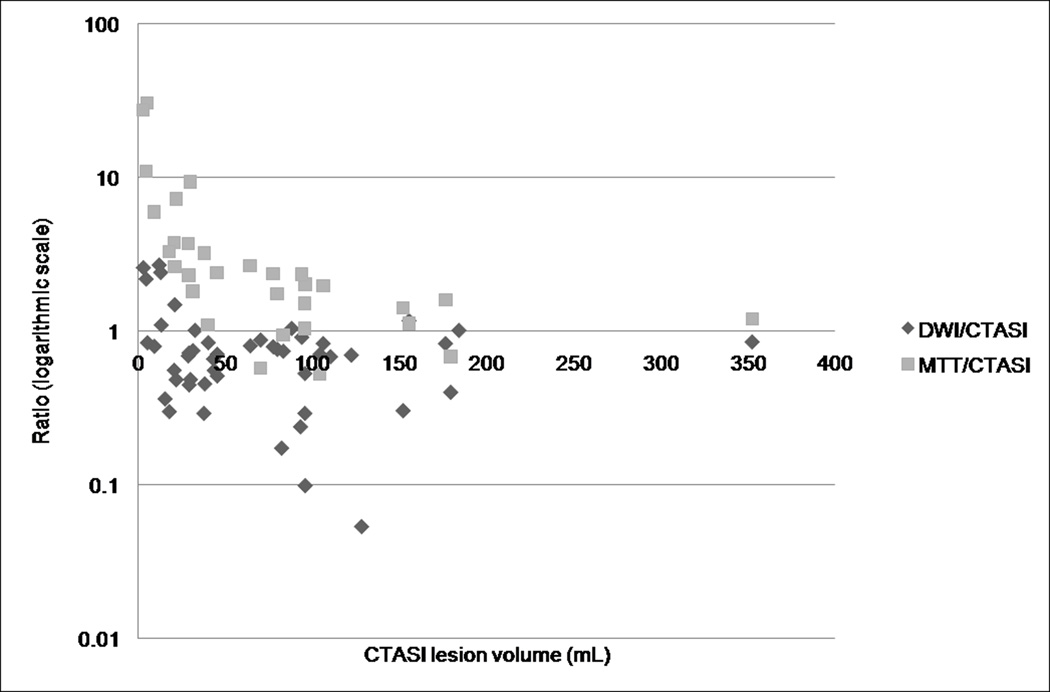
Figure 2.
Semi-logarithmic plot of DWI/CTA-SI and MTT/CTA-SI lesion volume ratios. In the majority of cases, CTA-SI lesions are larger than the corresponding DWI lesions and smaller than the MTT lesions.
The percent overestimation by CTA-SI(relative to the DWI lesion volume) was not different for proximal versus distal occlusions(43.1% [IQR, 14.5–151.3%] vs. 37.4% [IQR, 19.3–80.4%]; P=0.43). However, the absolute increase in lesion volume on CTA-SI was greater for proximal occlusions(17.4 cm3 [IQR, 8.9–45.3 cm3] vs. 8.6 cm3 [IQR, 0.9–21.0 cm3]; P=0.04; Figure 3).
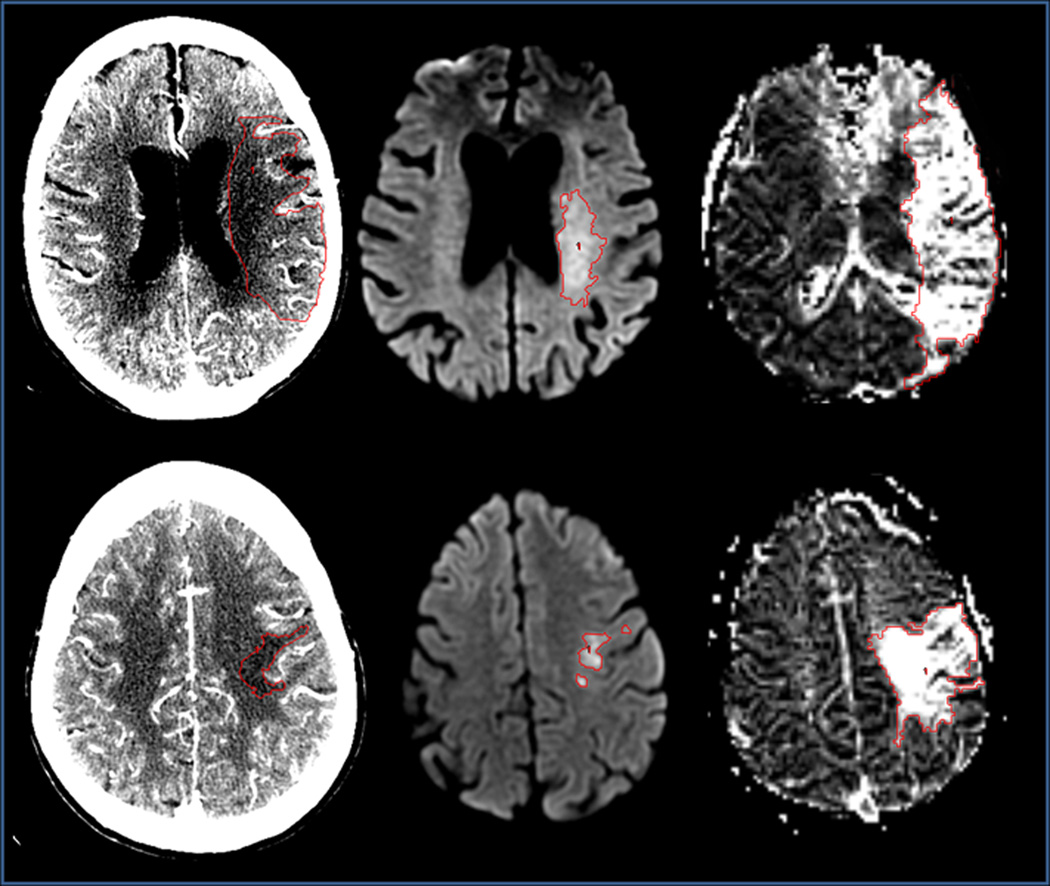
Figure 3.
Two cases comparing CTA-SI(left), DWI(middle) and MTT(right) lesions. Top row: 92-year-old female with left M1 occlusion, NIHSS score=15, CTA-to-MRI time=27 minutes, collateral score=1. Lesion volumes for CTA-SI, DWI and MTT are 96cm3, 9.4cm3 and 193.5cm3. Bottom row: 69-year-old female with left M3 occlusion, NIHSS=12, CTA-to-MRI time=27 minutes, collateral score=2. Lesion volumes for CTA-SI, DWI and MTT are 9.5cm3, 7.5cm3 and 56.3cm3.
Fourteen(29.2%) cases had major overestimation of the DWI lesion by CTA-SI(>25 cm3 difference). Lower collateral score(P=0.001), higher NIHSS score(P=0.01), older age(P=0.01), and proximal occlusion(P<0.05) were univariate predictors of major CTA-SI overestimation(Table 2). In multiple logistic regression, only collateral score was an independent predictor(O.R. 0.19, 95% C.I. 0.06–0.58; P=0.0006). In ROC analysis, a collateral score of ≤1 had the highest accuracy(100% sensitivity, 61.8% specificity; AUC=0.801, P=0.0001) for detecting major overestimation by CTA-SI, with a positive predictive value of 51.9% and a negative predictive value of 100%. In comparison, an NIHSS score >14 had 90% sensitivity and 59.4% specificity(AUC=0.761, P=0.006) for detecting major overestimation, with a positive predictive value of 40.9% and a negative predictive value of 95%. There was no difference in the areas under the curve for the collateral and NIHSS scores(P=0.48).
Table 2.
Predictors of major CTA-SI overestimation (> 25 cm3) in the entire study population (48 patients)
| No major overestimation (n= 34) |
Major overestimation (n= 14) |
Univariate Significance |
Multivariate Significance |
|
|---|---|---|---|---|
| Age (years) | 68.9 ± 13.5 | 79.3 ± 10.8 | P = 0.01 * | |
| Sex (female) | 50.0% | 64.3% | P = 0.52 † | |
| Hemisphere (left) | 55.9% | 78.6% | P = 0.20 † | |
| NIHSS | 12 (8–18.5) | 19.5 (15–23) | P = 0.01 ‡ | |
| Proximal Occlusion | 52.9% | 85.7% | P < 0.05 † | |
| Admission DWI lesion volume (cm3) | 26.3 (13.1–60.7) | 48.3 (14.2–75.1) | P = 0.29 ‡ | |
| Collateral score (0–2) | 2 (1–2) | 0.5 (0–1) | P = 0.001‡ | P = 0.0006 § |
| Onset to CT (minutes) | 236.2 ± 103.7 | 243.6 ± 124.4 | P = 0.83* | |
| CT to MR (minutes) | 37.3 ± 18.7 | 34.1 ± 18.0 | P = 0.59 * |
Continuous variables are reported as means(±SD) or medians(IQR);
Student’s T-test;
Fisher’s Exact test;
Mann-Whitney U test;
Multiple logistic regression analysis
Admission CTA-SI versus MTT lesion volumes
Thirty-one(64.6%) patients underwent PWI. There was a significant correlation between CTA-SI and MTT lesion volumes(Spearman rho: 0.51; P=0.005). In 27(87.1%) cases, the CTA-SI lesion was smaller than the MTT lesion(P=0.0001; Figure 2). In pairwise comparison, the lesion volumes were significantly smaller on CTA-SI versus the corresponding MTT map(64.6 cm3 [IQR, 23.7–96.0 cm3] vs. 122.3 cm3 [IQR, 61.2–180.8 cm3]; P<0.0001). This remained true for both the proximal(P=0.0008) and distal(P=0.0007) occlusions. The percent decrease in lesion size(relative to the MTT lesion volume) was not different for proximal versus distal occlusions(−45.9% [IQR, −62.4 to −10.2%] vs. − 61.8% [IQR, −73.1 to −42.0%]; P=0.21). Likewise, the absolute reduction in lesion volume was not different between proximal and distal occlusions(−76.7 cm3 [IQR, −105.6 to −17.7 cm3] vs. −46.8 cm3 [IQR, −67.2 to −32.0 cm3]; P=0.23).
Interobserver agreement for CTA-SI versus DWI
The mean inter-reader difference in lesion volume was significantly larger for CTA-SI(11.6 cm3 [95%CI, 7.4–15.7 cm3]) than for DWI(−1.9 cm3 [95%CI, −3.2 to −0.7 cm3]; P<0.0001; Suppl. Figs 1 and 2). Furthermore, the limits of agreement[17] (mean ± 1.96 standard deviations) were significantly broader for CTA-SI(−16.5 to 39.6 cm3, range 56.1 cm3) than for DWI(−10.4 to 6.5 cm3, range 16.9 cm3; P<0.001).
Discussion
In acute ischemic stroke patients imaged within nine hours of symptom onset, the ischemic lesion on CTA-SI performed using our fast acquisition protocol is significantly larger than on DWI performed soon afterwards. This is true for both proximal and distal artery occlusions. Lesion overestimation can be sizeable(>25 cm3) in more than 25% of patients. Reduced collaterals, older age, higher NIHSS score, and proximal artery occlusion are associated with major CTA-SI overestimation, with lower collateral score being the only independent predictor.
Clinical outcome following recanalization is strongly dependent on the pretreatment infarct size.[19–20] DWI is widely considered the gold standard for identifying the infarct core in the acute setting.[19] However, a major disadvantage is its limited availability outside of major stroke centers. This has provided the impetus to identify CT-based methods for accurately determining core versus penumbral tissue. While dynamic CT perfusion has been widely studied, it has known limitations including poor standardization,[21] questionable reproducibility[22] and limited brain coverage.[23] Another approach currently used in the clinical setting is delineation of the hypoattenuating lesion on CTA-SI.[19] Previous studies have demonstrated that this method provides a clinically useful estimate of the infarct core,[1, 4] and is comparable to DWI.[5–6] Our findings reveal that this approximation is not invariably true.
In a previous study with a design similar to ours but using a 4-slice scanner,[5] Schramm et al. found that 30%(6/20) of patients had CTA-SI lesion volumes that were greater than the corresponding DWI volumes, a rate significantly lower than the 79.2% rate found in our study(P=0.0002, two-tailed Fisher’s exact test). Also contrary to our study, they found no statistically significant difference between the two volumes. Their study examined patients imaged within six hours of stroke onset, 80% of whom had documented vessel occlusion. In our study, 75%(36/48) of patients were imaged within six hours, and all had vessel occlusion. Their average CTA-to-MRI time was 33 ±15 minutes, similar to our interval of 36 ±18 minutes. A follow-up study by the same group reported similar findings to their initial study.[6]
We hypothesize that this variable relationship between the CTA-SI and DWI lesions likely reflects protocol-based differences in time delay between contrast injection and CTA imaging. Under near steady-state conditions whereby the arteries, capillaries and veins are maximally opacified with contrast, the degree of hypoattenuation is proportional to the CBV deficit[2] and indicates tissue with a high probability of infarction.[3] With early arterial-phase imaging, there is less time for the contrast to traverse the collaterals and opacify the ischemic bed. Thus, the region of hypoattenuation will appear larger than images obtained with greater delay, and will overestimate the infarct core. In this case, the CTA-SI lesion will be at least partially CBF-weighted and incorporate some fraction of the ischemic penumbra. Consistent with this idea is our finding that the CTA-SI lesion volume was between the DWI and MTT lesion volumes. Unfortunately, we cannot compare our current protocol with those in the Schramm et al. studies[5–6] because the table speed and scan direction are not described in their manuscripts. However, it should be noted that in their second study, CTA-SI was acquired after CT perfusion imaging such that contrast was already circulating with a great delay by the time of CTA acquisition.
Furthermore, this idea helps to explain our finding that reduced collaterals were the strongest predictor of major CTA-SI overestimation. A decrease in collateralization necessitates a longer time for the contrast to reach the affected tissue, and likely accentuates differences in apparent lesion size with shorter delays. Because the NIHSS score is inversely correlated with collateral strength,[24] it follows that a higher NIHSS score should also be predictive of major overestimation, another finding in our study.
Until this issue is fully addressed, our findings suggest that CTA-SI performed using faster multidetector protocols may not be reliable for infarct core evaluation, particularly in patients who have reduced collaterals or NIHSS score >14. In our study, none of the 21 patients with a collateral score of 2(normal or exuberant collaterals) had major overestimation of the DWI lesion by CTA-SI, versus 51.9%(14/27) of patients with any reduction in collaterals(scores 0–1). Using the NIHSS, ninety-five percent(19/20) of patients with a score ≤14 did not have a major CTA-SI overestimation, while 40.9%(9/22) of patients with a score >14 did. These thresholds are particularly relevant for IAT-eligible patients because the majority of ICA or M1 occlusions in our study had reduced collaterals(76.7%) as well as NIHSS score >14(76%).
Another issue that limits the utility of CTA-SI is the poor interobserver agreement in delineating the ischemic lesion. In this study, the 95% confidence interval range for inter-reader differences was 56.1 cm3 for CTA-SI versus 16.9 cm3 for DWI. Such large differences can lead to significant errors in interpretation for patients who are potentially eligible for reperfusion therapy, especially in light of recent studies[20, 25] demonstrating that the pretreatment infarct volume threshold for predicting a good clinical response to reperfusion may be as low as 70 cm3. This poor interobserver agreement is likely related to variable lesion conspicuity in white matter, where attenuation differences between normal and ischemic brain may be as low as 3–4 Hounsfield units and obscured by image noise.[26] In this case, lesion detection may be improved by reconstructing the source images to a greater slice thickness(e.g., 5 mm). Compared to white matter, ischemic involvement of gray matter is more easily discernible.
Limitations of this study included its retrospective design and relatively small sample size. However, there were enough cases to demonstrate a significant difference between the lesion volumes on CTA-SI, DWI and MTT. Also, because we did not examine CTA cases performed using older acquisition protocols, we were not able to determine whether image acquisition delay contributed to the discrepancy between our findings and those of previous studies. Finally, the time delay between CTA and MRI in this study (mean 36 minutes) could result in varying amounts of infarct growth between the two scans. However, because the CTA preceded the MRI in all cases, any infarct growth would be expected to minimize the lesion overestimation that we found on CTA source images. Additionally, the CTA-to-MRI time in our study was similar to the interval (33 minutes) seen in the study by Schramm et al.,[5] which would make this an unlikely explanation for the different findings in the two studies.
Conclusion
CTA-SI performed using our fast acquisition protocol significantly overestimates the ischemic lesion (infarct core) on concurrent DWI, with substantial differences seen in over 25% of cases. Reduced collaterals and NIHSS score >14 are predictive of major CTA-SI overestimation. Further studies are necessary to confirm whether CTA-SI is CBF-weighted under the current, faster CTA protocols, and whether this relationship is mediated by the image acquisition delay after contrast administration.
Supplementary Material
Acknowledgments
Grant support:
The following sources of funding were utilized, in part, for the preparation of this manuscript: Neuroradiology Education and Research Foundation/Boston Scientific Fellowship in Cerebrovascular Disease Research (A.J.Y.), National Institutes of Health through a grant from the National Institute of Neurological Disorders and Stroke NS050041 (R.G.G).
Abbreviation Key
- AIS
acute ischemic stroke
- CTA-SI
CT angiography source imaging
- DWI
MRI diffusion-weighted imaging
- MTT
mean transit time
- PWI
MR perfusion-weighted imaging
- CBV
cerebral blood volume
- CBF
cerebral blood flow
- IQR
interquartile range
- ICA
internal carotid artery
- MCA
middle cerebral artery
- ROC
receiver-operating curve
- IV tPA
intravenous tissue plasminogen activator
- IAT
intra-arterial therapy
- mRS
modified Rankin scale score
- NIHSS
National Institutes of Health Stroke Scale
- AUC
area under the ROC curve
- SD
standard deviation
Footnotes
Presentation: This work was presented in whole at the International Stroke Conference, San Antonio, TX, February 2010.
Conflicts of Interest:
Albert J. Yoo: Penumbra, Inc. (research funding: core imaging lab for the START trial; significant)
Ranliang Hu: None
Reza Hakimelahi: None
Michael H. Lev: NIH SPOTRIAS research grant (significant); GE Healthcare (research funding; modest); Consultant for CoAxia, Inc., GE Healthcare, Millennium Pharmaceuticals, Vernalis (all modest)
Raul G. Nogueira: Physician advisory board for Concentric Medical, Inc.; ev3 Neurovascular, Inc.; CoAxia, Inc.; Rapid Medical, Inc.
Joshua A. Hirsch: None
R. Gilberto Gonzalez: NIH research grant (significant); Penumbra, Inc. (research funding: core imaging lab for the START trial; significant)
Pamela W. Schaefer: None
References
- 1.Camargo EC, Furie KL, Singhal AB, Roccatagliata L, Cunnane ME, Halpern EF, Harris GJ, Smith WS, Gonzalez RG, Koroshetz WJ, Lev MH. Acute brain infarct: detection and delineation with CT angiographic source images versus nonenhanced CT scans. Radiology. 2007;244:541–548. doi: 10.1148/radiol.2442061028. [DOI] [PubMed] [Google Scholar]
- 2.Hamberg LM, Hunter GJ, Kierstead D, Lo EH, Gilberto Gonzalez R, Wolf GL. Measurement of cerebral blood volume with subtraction three-dimensional functional CT. AJNR Am J Neuroradiol. 1996;17:1861–1869. [PMC free article] [PubMed] [Google Scholar]
- 3.Hunter GJ, Silvennoinen HM, Hamberg LM, Koroshetz WJ, Buonanno FS, Schwamm LH, Rordorf GA, Gonzalez RG. Whole-brain CT perfusion measurement of perfused cerebral blood volume in acute ischemic stroke: probability curve for regional infarction. Radiology. 2003;227:725–730. doi: 10.1148/radiol.2273012169. [DOI] [PubMed] [Google Scholar]
- 4.Lev MH, Segal AZ, Farkas J, Hossain ST, Putman C, Hunter GJ, Budzik R, Harris GJ, Buonanno FS, Ezzeddine MA, Chang Y, Koroshetz WJ, Gonzalez RG, Schwamm LH. Utility of perfusion-weighted CT imaging in acute middle cerebral artery stroke treated with intra-arterial thrombolysis: prediction of final infarct volume and clinical outcome. Stroke. 2001;32:2021–2028. doi: 10.1161/hs0901.095680. [DOI] [PubMed] [Google Scholar]
- 5.Schramm P, Schellinger PD, Fiebach JB, Heiland S, Jansen O, Knauth M, Hacke W, Sartor K. Comparison of CT and CT angiography source images with diffusion-weighted imaging in patients with acute stroke within 6 hours after onset. Stroke. 2002;33:2426–2432. doi: 10.1161/01.str.0000032244.03134.37. [DOI] [PubMed] [Google Scholar]
- 6.Schramm P, Schellinger PD, Klotz E, Kallenberg K, Fiebach JB, Kulkens S, Heiland S, Knauth M, Sartor K. Comparison of perfusion computed tomography and computed tomography angiography source images with perfusion-weighted imaging and diffusion-weighted imaging in patients with acute stroke of less than 6 hours' duration. Stroke. 2004;35:1652–1658. doi: 10.1161/01.STR.0000131271.54098.22. [DOI] [PubMed] [Google Scholar]
- 7.Josephson SA, Bryant SO, Mak HK, Johnston SC, Dillon WP, Smith WS. Evaluation of carotid stenosis using CT angiography in the initial evaluation of stroke and TIA. Neurology. 2004;63:457–460. doi: 10.1212/01.wnl.0000135154.53953.2c. [DOI] [PubMed] [Google Scholar]
- 8.Morhard D, Pellkofer H, Reiser MF, Ertl-Wagner B. Inadvertent intra-arterial contrast agent injection mimicking bilateral occlusion of the internal carotid arteries in a patient with suspected stroke on maximum-slope, nondeconvolution perfusion computed tomography. Stroke. 2009;40:e46–e49. doi: 10.1161/STROKEAHA.108.526186. [DOI] [PubMed] [Google Scholar]
- 9.Sliker CW. Blunt cerebrovascular injuries: imaging with multidetector CT angiography. Radiographics. 2008;28:1689–1708. doi: 10.1148/rg.286085521. discussion 709-10. [DOI] [PubMed] [Google Scholar]
- 10.Sliker CW, Mirvis SE. Imaging of blunt cerebrovascular injuries. Eur J Radiol. 2007;64:3–14. doi: 10.1016/j.ejrad.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Smith WS, Roberts HC, Chuang NA, Ong KC, Lee TJ, Johnston SC, Dillon WP. Safety and feasibility of a CT protocol for acute stroke: combined CT, CT angiography, and CT perfusion imaging in 53 consecutive patients. AJNR Am J Neuroradiol. 2003;24:688–690. [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy BD, Fox AJ, Lee DH, Sahlas DJ, Black SE, Hogan MJ, Coutts SB, Demchuk AM, Goyal M, Aviv RI, Symons S, Gulka IB, Beletsky V, Pelz D, Hachinski V, Chan R, Lee TY. Identification of penumbra and infarct in acute ischemic stroke using computed tomography perfusion-derived blood flow and blood volume measurements. Stroke. 2006;37:1771–1777. doi: 10.1161/01.STR.0000227243.96808.53. [DOI] [PubMed] [Google Scholar]
- 13.Ostergaard L, Weisskoff RM, Chesler DA, Gyldensted C, Rosen BR. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: Mathematical approach and statistical analysis. Magn Reson Med. 1996;36:715–725. doi: 10.1002/mrm.1910360510. [DOI] [PubMed] [Google Scholar]
- 14.Maas MB, Lev MH, Ay H, Singhal AB, Greer DM, Smith WS, Harris GJ, Halpern E, Kemmling A, Koroshetz WJ, Furie KL. Collateral vessels on CT angiography predict outcome in acute ischemic stroke. Stroke. 2009;40:3001–3005. doi: 10.1161/STROKEAHA.109.552513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parsons MW, Christensen S, McElduff P, Levi CR, Butcher KS, De Silva DA, Ebinger M, Barber PA, Bladin C, Donnan GA, Davis SM. Pretreatment diffusion- and perfusion-MR lesion volumes have a crucial influence on clinical response to stroke thrombolysis. J Cereb Blood Flow Metab. 2010 doi: 10.1038/jcbfm.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lansberg MG, Thijs VN, Bammer R, Olivot JM, Marks MP, Wechsler LR, Kemp S, Albers GW. The MRA-DWI mismatch identifies patients with stroke who are likely to benefit from reperfusion. Stroke. 2008;39:2491–2496. doi: 10.1161/STROKEAHA.107.508572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 18.Uflacker R, Schonholz C, Papamitisakis N. Interim report of the SENTIS trial: cerebral perfusion augmentation via partial aortic occlusion in acute ischemic stroke. J Cardiovasc Surg (Torino) 2008;49:715–721. [PubMed] [Google Scholar]
- 19.Latchaw RE, Alberts MJ, Lev MH, Connors JJ, Harbaugh RE, Higashida RT, Hobson R, Kidwell CS, Koroshetz WJ, Mathews V, Villablanca P, Warach S, Walters B. Recommendations for Imaging of Acute Ischemic Stroke. A Scientific Statement From the American Heart Association. Stroke. 2009 doi: 10.1161/STROKEAHA.108.192616. [DOI] [PubMed] [Google Scholar]
- 20.Yoo AJ, Verduzco LA, Schaefer PW, Hirsch JA, Rabinov JD, Gonzalez RG. MRI-based selection for intra-arterial stroke therapy: value of pretreatment diffusion-weighted imaging lesion volume in selecting patients with acute stroke who will benefit from early recanalization. Stroke. 2009;40:2046–2054. doi: 10.1161/STROKEAHA.108.541656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wintermark M, Albers GW, Alexandrov AV, Alger JR, Bammer R, Baron JC, Davis S, Demaerschalk BM, Derdeyn CP, Donnan GA, Eastwood JD, Fiebach JB, Fisher M, Furie KL, Goldmakher GV, Hacke W, Kidwell CS, Kloska SP, Kohrmann M, Koroshetz W, Lee TY, Lees KR, Lev MH, Liebeskind DS, Ostergaard L, Powers WJ, Provenzale J, Schellinger P, Silbergleit R, Sorensen AG, Wardlaw J, Wu O, Warach S. Acute stroke imaging research roadmap. AJNR Am J Neuroradiol. 2008;29:e23–e30. doi: 10.1161/STROKEAHA.107.512319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiorella D, Heiserman J, Prenger E, Partovi S. Assessment of the reproducibility of postprocessing dynamic CT perfusion data. AJNR Am J Neuroradiol. 2004;25:97–107. [PMC free article] [PubMed] [Google Scholar]
- 23.Konstas AA, Goldmakher GV, Lee TY, Lev MH. Theoretic basis and technical implementations of CT perfusion in acute ischemic stroke, part 1: Theoretic basis. AJNR Am J Neuroradiol. 2009;30:662–668. doi: 10.3174/ajnr.A1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan IY, Demchuk AM, Hopyan J, Zhang L, Gladstone D, Wong K, Martin M, Symons SP, Fox AJ, Aviv RI. CT angiography clot burden score and collateral score: correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. AJNR Am J Neuroradiol. 2009;30:525–531. doi: 10.3174/ajnr.A1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanak D, Nosal V, Horak D, Bartkova A, Zelenak K, Herzig R, Bucil J, Skoloudik D, Burval S, Cisarikova V, Vlachova I, Kocher M, Zapletalova J, Kurca E, Kanovsky P. Impact of diffusion-weighted MRI-measured initial cerebral infarction volume on clinical outcome in acute stroke patients with middle cerebral artery occlusion treated by thrombolysis. Neuroradiology. 2006;48:632–639. doi: 10.1007/s00234-006-0105-0. [DOI] [PubMed] [Google Scholar]
- 26.Kucinski T, Vaterlein O, Glauche V, Fiehler J, Klotz E, Eckert B, Koch C, Rother J, Zeumer H. Correlation of apparent diffusion coefficient and computed tomography density in acute ischemic stroke. Stroke. 2002;33:1786–1791. doi: 10.1161/01.str.0000019125.80118.99. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.