Abstract
Background
Bipolar disorders and alcohol use disorders commonly co-occur, yet little is known about the proximal impact of bipolar symptoms on alcohol use in patients with this comorbidity. The present study examined the impact of depressive symptoms and alcohol craving on proximal alcohol use in patients with co-occurring bipolar disorder and alcohol dependence.
Methods
Data were collected during an 8-week randomized controlled trial of acamprosate for individuals with co-occurring bipolar disorder and alcohol dependence (n = 30). Depressive symptoms and alcohol craving were assessed biweekly using the Montgomery Asberg Depression Rating Scale (MADRS) and the Obsessive Compulsive Drinking Scale (OCDS), respectively. Daily alcohol use data were available via administration of the Time-line Follow-back interview at baseline and at subsequent weekly study visits. Correlational analyses and hidden Markov modeling were used to examine the prospective relationships between depressive symptoms, alcohol craving, and alcohol use.
Results
Depressive symptoms and alcohol craving were significantly correlated with proximal (i.e., one-week later) alcohol use across a variety of alcohol consumption summary measures. In hidden Markov models, depressive symptoms (OR = 1.3, 95% Credible Interval=[1.1,1.5]) and alcohol craving (OR = 1.6, 95% Credible Interval=[1.4,1.9]) significantly predicted transitioning from a light to a heavy drinking state, or remaining in a heavy drinking state.
Conclusions
The results from the present study suggest that depressive symptoms and alcohol craving increase proximal risk for alcohol use in individuals with co-occurring bipolar and alcohol use disorders.
Keywords: bipolar disorder, alcohol, alcoholism, comorbidity, craving, dependence, depression, drinking, mania, relapse
Introduction
Alcohol use disorders (AUD) co-occur with a lifetime prevalence of roughly 50% in people with bipolar disorder (Regier et al., 1990; Merikangas et al., 2007). As reviewed previously (Tohen et al., 1998; Salloum and Thase, 2000; Frye and Salloum, 2006), considerable evidence indicates that comorbid AUD complicates the treatment of bipolar disorder and is generally associated with a more severe course of bipolar illness. Bipolar patients with co-occurring substance use disorders (SUD) have decreased treatment adherence (Manwani et al., 2007), more frequent mood episodes (Schneck et al., 2004), increased likelihood of mixed episodes (Himmelhoch et al., 1976), more suicide attempts (Oquendo et al., 2010), more hospitalizations (Sonne et al., 1994), and lower responsiveness to treatment (Goldberg et al., 1999) compared to patients with bipolar disorder alone. However, worsened mood outcomes in bipolar patients with AUD have not been observed uniformly in all studies (Fleck et al., 2006; Ostacher et al., 2010; Van Zaane et al., 2010). Further, course of illness in this population may be associated with relative order and/or age of onset of bipolar disorder and substance abuse (Winokur et al., 1995; Strakowski et al., 2005; Fossey et al., 2006) suggesting that alcohol and drug use disorders may be a marker, rather than a determinant, of severe illness course. In support of this, bipolar patients with a history of SUD exhibit impaired syndromic and functional recovery relative to those with no history of SUD whether alcohol and drug use is current or in the past (Goldberg et al., 1999; Weiss et al., 2005). Together, these findings suggest that the relationship between bipolar disorder and AUD is complex, challenging previous conceptualizations that alcohol use by bipolar patients represents primarily an attempt at self-medication, mainly during manic episodes and rarely during depressive episodes (Reich et al., 1974).
Unfortunately, remarkably little research has been conducted on the concurrent trajectories of mood symptoms and alcohol use in people with co-occurring bipolar disorder and AUD. Existing data primarily address the impact of drinking on mood stability and are limited by a number of methodological issues. First, many studies have evaluated associations between retrospective self-reported lifetime diagnosis and illness course variables for bipolar disorder, AUD, or both (Fossey et al., 2006; Weiss et al., 2005; Reich et al., 1974). Other studies have used cross-sectional designs to compare mood symptom severity at the time of presentation in bipolar subjects who reported variable amounts of recent or remote alcohol use (Salloum et al., 2002; McKowen et al., 2005), but did not track longitudinal changes in mood or drinking outcomes. In each of these cases, the study design does not allow interpretation of the temporal relationships between drinking and mood symptoms. Second, several studies that prospectively tracked both drinking and mood symptoms concurrently used categorical measures of alcohol use such as investigator-rated severity scales (Reich et al., 1974; Salloum et al., 2002), presence or absence of DSM diagnostic criteria for AUD (Strakowski et al., 1998; Strakowski et al., 2000), and/or scores on the Addiction Severity Index over time (Fleck et al., 2006; Strakowski et al., 2000), but did not quantify actual amounts of alcohol use. Similarly, many of these previous studies have used graded categorical measures of mood symptom severity (e.g. syndromal relapse, remission, or recovery) instead of assessment instruments of depressive and manic symptoms (e.g. Montgomery Asberg Depression Rating Scale [MADRS; Montgomery and Asberg, 1979], Young Mania Rating Scale [YMRS; Young et al., 1978]) commonly used in clinical trials that allow more precise tracking of subsyndromal mood symptoms (Strakowski et al., 2005; Strakowski et al., 2000). Finally, sampling intervals and duration of mood and drinking outcomes have varied considerably across studies, some of which have included only participants with bipolar I disorder and/or have excluded data from subjects whose mood or alcohol use symptoms did not change appreciably over time (Fleck et al., 2006), introducing the potential for selection bias. Given these methodologic challenges, it is perhaps not surprising that divergent results on the relationship between affective and alcohol use outcomes have been reported in the recent literature (Fleck et al., 2006; Van Zaane et al., 2010; Baethge et al., 2008; Jaffee et al., 2009).
Because integrated treatment of both bipolar disorder and alcohol dependence may be optimal (Drake et al., 2004), understanding how bipolar symptoms impact drinking outcomes is important for clinical management of this population. To our knowledge, no studies to date have examined the proximal influence of depressive symptoms on alcohol use in individuals with co-occurring bipolar and alcohol use disorders. In the present study, participants' depressive symptoms and alcohol craving were assessed biweekly for 8 weeks; daily alcohol consumption data were available for the entire course of the study. Following correlational analysis of proximal (i.e., one-week), lagged associations between depressive symptoms, alcohol craving, and weekly alcohol consumption summary measures, hidden Markov modeling (HMM; MacDonald and Zucchini, 1997) was employed to investigate the prospective impact of depressive symptoms and alcohol craving on transitions between empirically defined heavy and light alcohol use states. Our hypothesis was that depressive symptoms and alcohol craving would each uniquely predict remaining in, or transitioning to, the heavy alcohol consumption state.
Materials and Methods
Participants and procedures
Data were collected as part of an 8-week, randomized, double-blind placebo-controlled trial of acamprosate treatment for individuals with co-occurring bipolar disorder and alcohol dependence (Tolliver et al., submitted). Briefly, participants were 30 treatment-seeking individuals aged 18-65, with primary DSM-IV diagnoses of bipolar I or bipolar II disorder and alcohol dependence who reported alcohol use in the past 90 days. Exclusion criteria included severe mania (score > 25 on the YMRS), severe depression (score > 35 on the MADRS), any imminent risk of suicide or homicide as determined by the study psychiatrist, or any Axis I diagnoses considered to be primary other than bipolar disorder and alcohol dependence. Other exclusions included significant cognitive impairment that would interfere with capacity to give informed consent, or other significant medical/neurological conditions such as epilepsy, human immunodeficiency virus, renal failure, hepatic failure, unstable angina, or chronic obstructive pulmonary disease. Females of childbearing age who were pregnant, breastfeeding, or who refused adequate forms of contraception were also excluded.
Participants were required to (1) be receiving psychiatric management for bipolar disorder outside of the study setting, (2) provide written consent for the study psychiatrist to exchange diagnostic and clinical status information at any time with the outside treating psychiatrist, and (3) be taking stable doses of mood stabilizing medications as prescribed by the outside treating psychiatrist for one month prior to randomizations. Accepted mood stabilizing medications included lithium, the anticonvulsant medicines carbamazepine, lamotrigine, or valproic acid, and/or first- or second-generation antipsychotic medicines. Adjustments to concomitant medications by the outside treating psychiatrist were allowed as clinically indicated and assessed weekly. Participants were permitted and encouraged, but not required, to participate in outpatient addiction treatment and/or support group (e.g. Alcoholics Anonymous) attendance. Use of FDA-approved medications for alcohol dependence (disulfiram, naltrexone, or acamprosate) was not permitted during study participation due to potential confounding of the results. Each participant was required to remain abstinent from alcohol for three consecutive days immediately preceding the baseline visit.
Following baseline assessment, participants were randomized to receive either acamprosate (1998 mg daily) or matching placebo. Participants were asked to return weekly for 8 visits during the active phase of the trial to assess alcohol use and depressive and manic symptoms. Psychosocial interventions consisted of non-manualized individual counseling and education provided at each weekly visit by the study psychiatrist and were consistent with those generally available in primary care outpatient settings. Urine drug screening and serum biomarkers of alcohol use (percent carbohydrate-deficient transferrin (CDT) and gamma-glutamyltransferase (GGT) were collected at the screening visit and again at the final active phase (week 8) visit. Participants with evidence of persistent alcohol and/or drug abuse throughout or at the completion of the study were referred for further treatment. All study procedures were approved by the Medical University of South Carolina Institutional Review Board, and written informed consent was obtained from participants at the initial study appointment.
Measures
Substance use disorders were assessed using the Structured Clinical Interview for DSM-IV (First et al., 1996); all other disorders were evaluated using the Mini International Neuropsychiatric Interview (Sheehan et al., 1998). Alcohol use was assessed at baseline and weekly for 8 weeks using the Time-line Follow-back method (TLFB; Sobell and Sobell 1996); at baseline, participants were asked to recount the number of standard alcoholic beverages they consumed each day for the past 60 days. Subsequent TLFB data were assessed for the past week at each visit. This assessment strategy resulted in 120 continuous days of drinking data that were subsequently divided into one to two week summary variables (i.e., percent days alcohol consumed, average daily number of drinks) for subsequent statistical modeling. Depressive symptoms and alcohol craving were evaluated at baseline, and at weeks 2, 4, 6, and 8 using the MADRS and the Obsessive Compulsive Drinking Scale (OCDS; Anton et al., 2006), respectively. Manic symptoms were evaluated in the present study using the YMRS, but because subjects were selected to be relatively free of manic symptoms as a basis for inclusion, scores were uniformly low throughout the trial and had little variability across participants (for example, at baseline, mean YMRS = 6.50, SD = 4.55), and thus were not included in the analyses.
Data analysis
Correlational analysis
Prior to estimating the HMM, spearman correlations were calculated between depressive symptoms (i.e., MADRS) and alcohol craving (i.e., OCDS) on the one hand, and weekly alcohol use summary variables (i.e., number of drinks, % drinking days, and % heavy drinking days [≥ 5 standard drinks for men, ≥ 4 for women]) on the other. All correlations were calculated at a time-lag of one-week (e.g., baseline MADRS and OCDS with week 1 alcohol use summary variables).
Hidden Markov model
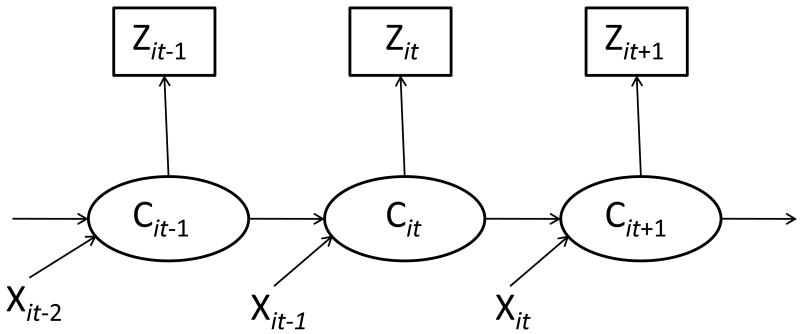
Hidden Markov modeling (MacDonald and Zucchini, 1997) has been prominent in recent alcohol (Shirley et al., 2010; Wall and Li, 2009), and cocaine (DeSantis et al., 2009; DeSantis et al., in press) research. As described by Shirley and colleagues (2010), HMM provides an ideal statistical model for representing drinking behavior in alcohol use disorder treatment trials. Specifically, unlike other commonly used statistical models (e.g., univariate summary statistics, failure time models, linear mixed models), HMM reduces the impact of measurement error on parameter estimates and standard errors that originates from patients' self-report of alcohol use, and HMM readily accommodates the chaotic nature of drinking behavior in clinical trials (i.e., consistent stretches of alcohol use interspersed with unusual bursts; Shirley et al., 2010). Additionally, unlike other approaches that attempt to reduce measurement error and/or the influence of outliers on longitudinal alcohol use data (e.g., coding alcohol consumption as an ordinal, univariate summary variable; Shirley et al., 2010), HMM retains the original resolution of the collected data, thereby maximizing statistical power. Due to these considerations, a hidden Markov model was fit to the data in order to assess the temporal effect of depressive symptoms and alcohol craving on alcohol consumption. Using this approach, weekly alcohol consumption, in terms of average count per week, was assumed to be a manifestation of two underlying latent states, heavy and light drinking. Note that in the HMM framework, heavy and light drinking states are not defined a priori, but are instead empirically-defined through a procedure analogous to latent class analysis. Patients were assumed to transition among these latent states over time, where transitions were modeled as functions of time invariant (i.e., gender and acamprosate group status) and time-varying (i.e., MADRS, OCDS) covariates according to a logistic regression model. Specifically, the first order autoregressive nature of the weekly drinking data was modeled (as the value of the hidden state at week t was allowed to depend on the value at week t-1), and the unique impact of covariates on the probability of transitioning between latent drinking classes was evaluated. Figure 1 (adapted from Wall and Li, 2009) presents a graphical depiction of the estimated model; Zit represents the number of alcoholic beverages consumed by participant i during study week t, Cit represents the unobserved drinking state of patient i at study week t, and Xit represents a vector of covariates (e.g., depressive symptoms, alcohol craving) for patient i at study week t. Bayesian Markov Chain Monte Carlo (MCMC; Gelfand and Smith, 1990) estimation, which uses all available data and yields unbiased parameters when missing data are missing at random, was implemented in WinBUGS software. To determine convergence of parameter estimates from the MCMC sampler, the MCMC chains for each parameter were observed in order to ensure the parameter space was adequately sampled, and the Gelman and Rubin convergence diagnostic was calculated; as this value was close to 1 for the HMM estimated for the present study, adequate convergence of the MCMC sampler was indicated. Results were reported in terms of posterior mean odds ratios with 95% credible intervals (which are analogous to 95% confidence intervals under frequentist estimation). Odds ratios with credible intervals that do not overlap with 1 are interpreted as statistically significant. The resultant odds ratios of interest are interpreted as the odds of remaining in, or transitioning to, the high use state for a standard deviation increase in a given covariate.
Figure 1.
Hidden Markov model schematic. Zit represents the number of alcoholic beverages consumed by participant i during study week t. Cit represents the unobserved drinking state of patient i at study week t. Xit represents a vector of covariates (e.g., depressive symptoms, alcohol craving) for patient i at study week t.
Results
Participant characteristics
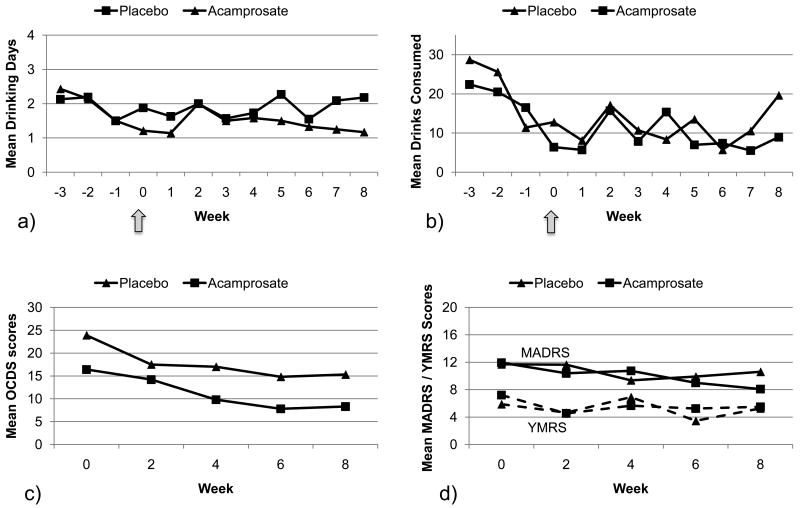
Of 33 subjects randomized, 30 returned for at least one visit and were thus considered evaluable for analysis. Participants were 19 men and 11 women, with a mean age of 42.33 (SD = 9.41). Most participants were caucasian (90.0%, n = 27), unemployed (70.0%, n = 21), and had completed some post-high-school education (59%, n = 18). Just under half (43.3%, n = 13) were current cigarette smokers. In terms of comorbid psychiatric disorders, 76.7% (n = 23) of participants were diagnosed with at least one anxiety disorder, and 60% (n = 18) were assigned at least one drug dependence diagnosis. Study retention was relatively high: 76.7% (n = 23) of evaluable participants attended all study sessions. Seven participants (23%) missed at least the last 4 weekly study visits; three participants did not return following their 1st weekly visit, two participants did not return following their 2nd weekly visit, and two participants did not return following their 3rd weekly visit. There were no other missing data points for any participants. The reasons for study discontinuation were identifiable in four subjects; one subject in each group was removed due to hospitalization, one subject in the acamprosate group was removed due to an anaphylactoid skin reaction, and one subject in the placebo group was removed due to incarceration. All other subjects who dropped out were lost to follow-up, so missing data cannot be assumed to be missing completely at random. The full range of available participant characteristics is presented in Table 1. Participants' weekly average alcohol consumption (i.e., drinking days, drinks consumed), OCDS, MADRS, and YMRS scores are displayed in Figure 2.
Table 1. Participant characteristics (n = 30).
| Demographics | |
| Age (M, SD) | 42.33 (9.41) |
| Female, % | 36.67 |
| Caucasian | 90.00 |
| ≤ High school graduate, % | 40.00 |
| Employed, % | 30.00 |
| Current smoker, % | 43.33 |
| Past smoker, % | 40.00 |
| Bipolar subtype, BPI % | 50.00 |
| Comorbid Anxiety Disorders | |
| Generalized Anxiety Disorder, % | 50.00 |
| Obsessive-Compulsive Disorder, % | 10.00 |
| Panic Disorder, % | 13.04 |
| Posttraumatic Stress Disorder, % | 6.67 |
| Social Phobia, % | 30.00 |
| Comorbid Drug Dependence (Lifetime) | |
| Cannabis Dependence, % | 43.33 |
| Amphetamine Dependence, % | 13.33 |
| Cocaine Dependence, % | 43.33 |
| Opioid Dependence, % | 23.33 |
| Baseline Assessments (M, SD) | |
| Montgomery Asberg Depression Rating Scale | 11.80 (5.97) |
| Young Mania Rating Scale | 6.50 (4.55) |
| # of drinks consumed in past month | 97.88 (103.66) |
| % days alcohol consumed in past month | 31.67 (27.72) |
| Obsessive Compulsive Drinking Scale | 20.40 (10.83) |
Figure 2.
Time course of drinking and mood outcomes in each treatment group across the entire trial. Values correspond to weekly average drinking days (a), drinks consumed (b), biweekly OCDS scores (c) and biweekly MADRS and YMRS scores (d) and include all observed cases without imputation of missing data. Arrows represent time of randomization and initiation of acamprosate or placebo. OCDS = Obsessive Compulsive Drinking Scale, MADRS = Montgomery Asberg Depression Rating Scale, YMRS = Young Mania Rating Scale.
Correlational analysis
Results of the correlation analysis are presented in Table 2. With few exceptions, participants' MADRS and OCDS scores on a given week were significantly correlated with their alcohol use over the subsequent week, across summary measures of weekly alcohol consumption (e.g., number of drinks, % drinking days). The average correlations between current MADRS and OCDS and subsequent alcohol use, across alcohol use summary measures, were 0.44 and 0.61, respectively.
Table 2. Cross-lagged spearman correlations between depressive symptoms, alcohol craving, and alcohol use summary variables.
| Alcohol Use Variable | MADRS | OCDS | n | |
|---|---|---|---|---|
| Week | Baseline | Baseline | 30 | |
| Number of Drinks | 1 | 0.33† | 0.50** | |
| % Drinking Days | 0.33† | 0.43* | ||
| % HDD | 0.45* | 0.49** | ||
| Week | 2 | 2 | 25 | |
| Number of Drinks | 3 | 0.59** | 0.61** | |
| % Drinking Days | 0.57** | 0.60** | ||
| % HDD | 0.63*** | 0.51** | ||
| Week | 4 | 4 | 23 | |
| Number of Drinks | 5 | 0.38† | 0.73*** | |
| % Drinking Days | 0.44* | 0.78*** | ||
| % HDD | 0.31 | 0.64** | ||
| Week | 6 | 6 | 23 | |
| Number of Drinks | 7 | 0.48* | 0.65*** | |
| % Drinking Days | 0.31 | 0.74*** | ||
| % Heavy Drinking | 0.45* | 0.59** |
Note. Variable n reflects participant dropout at each comparison point. MADRS = Montgomery Asberg Depression Rating Scale; OCDS = Obsessive-Compulsive Drinking Scale; HDD = Heavy Drinking Days.
P<0.05
P<0.01
P<0.001
P<0.10
Hidden Markov model
Parameters for the hidden Markov model are presented in Table 3. The light latent drinking state (which included individuals who abstained from using alcohol) was characterized by a model-estimated mean of 0.1 (SE=1.1) drinks per day versus a mean of 9.3 (SE=1.3) drinks per day for the heavy latent drinking state. Current drinking was significantly, and strongly, associated with drinking on the prior day (OR=19.5 [15.2, 25.1]), thereby reinforcing the appropriateness of the HMM model for the present data (Shirley et al., 2009; Wall and Li, 2009; DeSantis et al., 2009; DeSantis et al., 2011). In addition to the robust impact of prior drinking on current drinking, the odds of remaining in or transitioning to the heavy drinking state was 1.3 [1.1, 1.5] for each SD increase in MADRS and 1.6 [1.4, 1.9] for each SD increase in OCDS. Thus, both depression severity and craving were significantly, uniquely associated with increased odds of detrimental transitional drinking behavior. Finally, overall transitional drinking was significantly lower after versus before the start of the study (OR = 0.7 [0.6, 0.9]), independent of treatment group. Neither gender nor acamprosate group status were associated with transitional drinking behavior.
Table 3. Hidden Markov model of drinking behavior with effects of lagged covariates on current drinking state.
| Predictor | Odds Ratio | Standard Error | 95% Credible Intervals |
|---|---|---|---|
| Prior state | 19.5 | 1.1 | (15.2, 25.1) |
| Gender (F:M) | 1.1 | 1.1 | (0.8, 1.4) |
| Acamprosate | 1.0 | 1.2 | (0.6, 1.4) |
| Study (PostB:PreB) | 0.7 | 1.2 | (0.6, 0.9) |
| MADRS | 1.3 | 1.1 | (1.1, 1.5) |
| OCDS | 1.6 | 1.1 | (1.4, 1.9) |
Note. Odds Ratios reflect odds for transitioning into or remaining in the high use state. Odds ratios with 95% Credible Intervals that do not overlap with 1 are interpreted as statistically significant. PostB = Post baseline visit; PreB = Pre baseline visit; MADRS = Montgomery Asberg Depression Rating Scale (SD = 7.2); OCDS = Obsessive Compulsive Drinking Scale (SD=10.0).
Discussion
The present study investigated the proximal associations between depressive symptoms, alcohol craving, and alcohol consumption in a sample of individuals with co-occurring bipolar and alcohol use disorders. In both correlational and hidden Markov analyses, depressive symptoms and alcohol craving significantly predicted subsequent alcohol use. The present study is the first to demonstrate a significant proximal impact of depressive symptoms on alcohol consumption in individuals with co-occurring bipolar and alcohol use disorders.
Results from the present study are not entirely consistent with past findings. For example, unlike the present study, Baethge et al (2008) did not find evidence for a prospective association from depressive symptoms to alcohol use, and both Baethge et al (2008) and Jaffee et al (2009) found support for a prospective association from alcohol use to depressive symptoms. However, only 45% of Baethge et al (2008) participants received a diagnosis of substance use disorder. The impact of depressive symptoms on alcohol use may be more consistent in individuals with an alcohol use disorder. Furthermore, Jaffee et al (2009) did not examine the proximal impact of depressive symptoms on alcohol use; their investigation only looked at the impact of alcohol use on depressive symptoms. Also, the present study examined the relationship between depressive symptoms and alcohol use over a shorter time interval (i.e., 2 weeks) than did past investigations. In contrast to Baethge et al (2008) and Jaffee et al (2009), other studies have reported no significant association between bipolar symptoms and alcohol use (Fleck et al., 2006; Van Zaane et al., 2010). Overall, there has been a great deal of heterogeneity in the results from investigations of the relationship between bipolar symptoms and alcohol use. As discussed earlier, these discrepancies may largely be due to methodological differences across studies.
Future research should examine the relationship between bipolar symptoms and substance use over brief intervals (e.g., daily). Although the present study collected daily drinking data, mood and craving assessments were taken biweekly. Because it is unclear over what interval depressive symptoms and alcohol use influence each other, a sound strategy would be to collect high resolution (e.g., daily) measurements of both mood and alcohol use and explicitly test a variety of time-lagged associations (e.g., daily, weekly, monthly) within the same data set. Although the present study utilized state-of-the-art clinical assessments of mood and alcohol use, more objective assessments (e.g., actigraphy, breathalyzer) may help to reduce measurement error and further clarify the true association between bipolar symptoms and alcohol use in future studies. Finally, potential limitations concerning the generalizability of our results to dually diagnosed patients with bipolar disorder at large deserves consideration. The present findings were obtained from a small sample of participants in a single-site randomized controlled trial who were required to meet stringent inclusion and exclusion criteria, including relative euthymia, ongoing maintenance therapy with mood stabilizing medications, and absence of imminent risk of suicide or homicide. Therefore, our results may not generalize to alcohol-dependent patients with bipolar disorder likely to be encountered in clinical practice, and should be considered preliminary until replicated in a larger sample recruited from diverse clinical settings.
These limitations aside, the present study is the first to demonstrate a proximal prospective association between current depressive symptoms and future alcohol use in individuals with co-occurring bipolar and alcohol use disorders. These findings potentially provide an important caution to clinicians that their dually-diagnosed patients are at acutely elevated risk for intensified alcohol consumption when they experience increased depressive symptomatology. Because integrated treatment of bipolar disorder and alcohol dependence may improve outcomes (Drake et al., 2004, Weiss et al. 2007), understanding how bipolar symptoms impact drinking outcomes is vital for optimal clinical management of this population.
Acknowledgments
Funding/support: This study was funded by an investigator-initiated research grant to Dr. Tolliver from Forest Laboratories, Inc.; Forest Laboratories had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. Dr. Prisciandaro was supported by NIDA T32 DA007288.
References
- Anton RF, Moak DH, Latham P. The Obsessive Compulsive Drinking Scale: A self-rated instrument for the quantification of thoughts about alcohol and drinking behavior. Alcohol Clin Exp Res. 2006;19:92–99. doi: 10.1111/j.1530-0277.1995.tb01475.x. [DOI] [PubMed] [Google Scholar]
- Baethge C, Hennen J, Khalsa HMK, Salvatore P, Tohen M, Baldessarini RJ. Sequencing of substance use and affective morbidity in 166 first-episode bipolar I disorder patients. Bipolar Disord. 2008;10:738–741. doi: 10.1111/j.1399-5618.2007.00575.x. [DOI] [PubMed] [Google Scholar]
- DeSantis SM, Bandyopadhyay D. Hidden Markov Models for zero-inflated poisson counts with an application to substance use. Stat Med. doi: 10.1002/sim.4207. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis SM, Bandyopadhyay D, Back SE, Brady KT. Laboratory stress- and cue-reactivity studies are associated with decreased substance use among drug-dependent individuals. Drug Alcohol Depend. 2009;105:227–233. doi: 10.1016/j.drugalcdep.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake RE, Xie H, McHugo GJ, Shumway M. Three year outcomes of long-term patients with co-occurring bipolar and substance use disorders. Biol Psychiatry. 2004;56:749–756. doi: 10.1016/j.biopsych.2004.08.020. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Patient Edition. New York State Psychiatric Institute; New York: 1996. [Google Scholar]
- Fleck DE, Arndt S, DelBello MP, Strakowski SM. Concurrent tracking of alcohol use and bipolar disorder symptoms. Bipolar Disord. 2006;8:338–344. doi: 10.1111/j.1399-5618.2006.00332.x. [DOI] [PubMed] [Google Scholar]
- Fossey MD, Otto MW, Yates WR, Wisniewski SR, Gyulai L, Allen MH, Miklowitz DJ, Coon KA, Ostacher MJ, Neel JL, Thase ME, Sachs GS, Weiss RD. Validity of the distinction between primary and secondary substance use disorder in patients with bipolar disorder: data from the first 1000 STEP-BD participants. Am J Addict. 2006;15:138–143. doi: 10.1080/10550490500528423. [DOI] [PubMed] [Google Scholar]
- Frye MA, Salloum IM. Bipolar disorder and comorbid alcoholism: prevalence rate and treatment considerations. Bipolar Disorders. 2006;8:677–685. doi: 10.1111/j.1399-5618.2006.00370.x. [DOI] [PubMed] [Google Scholar]
- Gelfand AE, Smith AFM. Sampling-based approaches to calculating marginal densities. J Am Stat Assoc. 1990;85:398–409. [Google Scholar]
- Goldberg JF, Garno JL, Leon AC, Kocsis JH, Portera L. A history of substance abuse complicates remission from acute mania in bipolar disorder. J Clin Psychiatry. 1999;60:733–740. doi: 10.4088/jcp.v60n1103. [DOI] [PubMed] [Google Scholar]
- Himmelhoch JM, Mulla D, Neil JF, Detre TP, Kupfer DJ. Incidence and significance of mixed affective states in a bipolar population. Arch Gen Psychiatry. 1976;33:1062–1066. doi: 10.1001/archpsyc.1976.01770090052004. [DOI] [PubMed] [Google Scholar]
- Jaffee WB, Griffin ML, Gallop R, Meade CS, Graff F, Bender RE, Weiss RD. Depression precipitated by alcohol use in patients with co-occurring bipolar and substance use disorders. J Clin Psychiatry. 2009;70:171–176. doi: 10.4088/jcp.08m04011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald IL, Zucchini W. Hidden Markov and Other Methods for Discrete-valued Time Series. New York: Chapman and Hall; 1997. [Google Scholar]
- Manwani SG, Szilagyi KA, Zablotsky B, Hennen J, Griffin ML, Weiss RD. Adherence to pharmacotherapy in bipolar disorder patients with and without co-occurring substance use disorders. J Clin Psychiatry. 2007;68:1172–1176. doi: 10.4088/jcp.v68n0802. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RM, Petukhova M, Kessler RC. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64:543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKowen JW, Frye MA, Altshuler LL, Gitlin MJ. Patterns of alcohol consumption in bipolar patients comorbid for alcohol abuse or dependence. Bipolar Disord. 2005;7:377–381. doi: 10.1111/j.1399-5618.2005.00208.x. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg MA. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Currier D, Liu SM, Hasin DS, Grant BF, Blanco C. Increased risk for suicidal behavior in comorbid bipolar disorder and alcohol use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) J Clin Psychiatry. 2010;71:902–909. doi: 10.4088/JCP.09m05198gry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostacher MJ, Perlis RH, Nierenberg AA, Calabrese J, Stange JP, Salloum I, Weiss RD, Sachs GS. Impact of substance use disorders on recovery from episodes of depression in bipolar disorder patients: prospective data from the systematic treatment enhancement program for bipolar disorder (STEP-BD) Am J Psychiatry. 2010;167:289–297. doi: 10.1176/appi.ajp.2009.09020299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264:2511–2518. [PubMed] [Google Scholar]
- Reich LH, Davis RK, Himmelhoch JM. Excessive alcohol use in manic-depressive illness. Am J Psychiatry. 1974;131:83–86. doi: 10.1176/ajp.131.1.83. [DOI] [PubMed] [Google Scholar]
- Salloum IM, Cornelius JR, Mezzich JE, Kirisci L. Impact of concurrent alcohol misuse on symptom presentation of acute mania at initial evaluation. Bipolar Disord. 2002;4:418–421. doi: 10.1034/j.1399-5618.2002.01194.x. [DOI] [PubMed] [Google Scholar]
- Salloum IM, Thase ME. Impact of substance abuse on the course and treatment of bipolar disorder. Bipolar Disord. 2000;2:269–289. doi: 10.1034/j.1399-5618.2000.20308.x. [DOI] [PubMed] [Google Scholar]
- Schneck CD, Miklowitz DJ, Calabrese JR, Allen MH, Thomas MR, Wisniewski SR, Miyahara S, Shelton MD, Ketter TA, Goldberg JF, Bowden CL, Sachs GS. Phenomenology of rapid-cycling bipolar disorder: data from the first 500 participants in the Systematic Treatment Enhancement Program. Am J Psychiatry. 2004;161:1902–1908. doi: 10.1176/ajp.161.10.1902. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavas J, Weiller E, Hergueta T, Baker R, Dunbar GC. The mini-international neuropsychiatric interview (M.I.N.I): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Shirley KE, Small DS, Lynch KG, Maisto SA, Oslin DW. Hidden Markov models for alcoholism treatment trial data. Ann Appl Stat. 2010;4:366–395. [Google Scholar]
- Sobell LC, Sobell MB. Timeline followback user's guide: a calendar method for assessing alcohol and drug use. Toronto: Addiction Research Foundation; 1996. [Google Scholar]
- Sonne SC, Brady KT, Morton WA. Substance abuse and bipolar affective disorder. J Nerv Ment Disease. 1994;182:349–352. doi: 10.1097/00005053-199406000-00007. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, DelBello MP, Fleck DE, Adler CM, Anthenelli RM, Keck PE, Arnold LM, Amicone J. Effects of co-occurring alcohol abuse on the course of bipolar disorder following a first hospitalization for mania. Arch Gen Psychiatry. 2005;62:851–858. doi: 10.1001/archpsyc.62.8.851. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, DelBello MP, Fleck DE, Arndt S. The impact of substance abuse on the course of bipolar disorder. Biol Psychiatry. 2000;48:477–485. doi: 10.1016/s0006-3223(00)00900-8. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, Sax KW, McElroy SL, Keck PE, Hawkins JM, West SA. Course of psychiatric and substance abuse syndromes co-occurring with bipolar disorder after a first psychiatric hospitalization. J Clin Psychiatry. 1998;59:465–471. doi: 10.4088/jcp.v59n0905. [DOI] [PubMed] [Google Scholar]
- Tohen M, Greenfield SF, Weiss RD, Zarate CA, Jr, Vagge LM. The effect of comorbid substance use disorders on the course of bipolar disorder: a review. Harv Rev Psychiatry. 1998;6:133–141. doi: 10.3109/10673229809000321. [DOI] [PubMed] [Google Scholar]
- Van Zaane J, van den Brink W, Draisma S, Smit JH, Nolen WA. The effect of moderate and excessive alcohol use on the course and outcome of patients with bipolar disorders: a prospective cohort study. J Clin Psychiatry. 2010;71:885–893. doi: 10.4088/JCP.09m05079gry. [DOI] [PubMed] [Google Scholar]
- Wall MM, Li R. Multiple indicator hidden Markov model with an application to medical utilization data. Stat Med. 2009;28:293–310. doi: 10.1002/sim.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, Ostacher MJ, Otto MW, Calabrese JR, Fossey M, Wisniewski SR, Bowden CL, Nierenberg AA, Pollack MH, Salloum IM, Simon NM, Thase ME, Sachs GS. Does recovery from substance use disorder matter in patients with bipolar disorder? J Clin Psychiatry. 2005;66:730–735. doi: 10.4088/jcp.v66n0609. [DOI] [PubMed] [Google Scholar]
- Weiss RD, Griffin ML, Kolodziej ME, Greenfield SF, Najavits LM, Daley DC, Doreau HR, Hennen JA. A randomized trial of integrated group therapy versus group drug counseling for patients with bipolar disorder and substance dependence. Am J Psychiatry. 2007;164:100–107. doi: 10.1176/ajp.2007.164.1.100. [DOI] [PubMed] [Google Scholar]
- Winokur G, Coryell W, Akiskal HS, Maser JD, Keller MB, Endicott J, Mueller T. Alcoholism in manic-depressive (bipolar) illness: familial illness, course of illness, and the primary-secondary distinction. Am J Psychiatry. 1995;152:365–372. doi: 10.1176/ajp.152.3.365. [DOI] [PubMed] [Google Scholar]
- Young R, Biggs J, Meyer D. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]