Abstract
Antibody mediated rejection is a significant clinical problem encountered in a subset of renal transplant recipients. This type of rejection has a variable pathogenesis from the presence of donor specific antibodies with no overt disease to immediate hyperacute rejection and many variations between. Antibody mediated rejection is more common in human leukocyte antigen sensitized patients. In general, transplant graft survival after antibody mediated rejection is jeopardized, with less than 50% graft survival 5 years after this diagnosis. A variety of agents have been utilized singly and in combinations to treat antibody mediated rejection with differing results and significant research efforts are being placed on developing new targets for intervention. These same agents have been used in desensitization protocols with some success. In this review, we describe the biology of antibody mediated rejection, review the available agents to treat this form of rejection, and highlight areas of ongoing and future research into this difficult clinical problem.
Scope of the Problem
Renal transplantation is the treatment of choice for end-stage renal disease (ESRD) in appropriately selected patients, providing both survival and quality of life benefit to renal transplant recipients. Unfortunately, demands for renal transplantation have vastly outstripped the supply of organs with more than 90,000 ESRD patients in the United States on the renal transplant waiting list as of May 2011, but just under 17,000 renal transplants performed in calendar year 2010, of which roughly two-thirds were derived from deceased donors and one-third from living donors[1]. Approximately 14,000 individuals (16%) awaiting a renal transplant are prior organ transplant recipients. Increasing access to renal transplantation by accepting marginal donor organs, expanding living donation, and performing paired donor exchange transplants have increased overall transplant numbers but have not been able to match the demand for transplantation.
Waiting times for renal transplantation have continued to increase, with waits in excess of 6 years common in some regions of the United States, depending upon blood type. Waiting times are even longer in potential recipients for whom it is difficult to find a compatible organ match due to human leukocyte antigen (HLA)-specific alloantibodies. Patients sensitized to HLA account for about 30% of the kidney wait list. Median waiting time for renal transplant recipients listed in 2001-2 is 1329 days for those with panel-reactive antibody (PRA) 0-9%, 1920 days for those with PRA 10-79%, and 3649 days for those with PRA 80% or greater[1]. Organ Procurement and Transplantation Network (OPTN) data has shown that any degree of sensitization has a detrimental impact on transplantation rate, meaning greater likelihood of never being transplanted or being delisted due to co-morbidities prior to obtaining a transplant in this group[2]. Sensitized patients not only have diminished access to transplantation but also have been shown to have inferior outcomes after transplantation, with higher rates of rejection and graft loss than unsensitized patients, even when compatible organs are utilized [1, 3]. Desensitization protocols have been developed in a variety of centers with some notable successes but also high rates of rejection, particularly antibody mediated rejection (AMR)[4-11]. Additionally, renal transplantation across blood group incompatibility has been accomplished under some protocols with goal directed therapy to reduce anti-blood group antigen titers, often using modifications of protocols used for patient desensitization[12].
AMR can occur with a spectrum of clinical manifestations, from hyperacute rejection leading to immediate graft loss, AMR with acute impairment in renal function, and a more indolent course of chronic rejection that may not be associated with acute graft dysfunction but rather a more gradual loss of function over time[13-16]. More than 40% of patients with AMR go on to develop transplant glomerulopathy regardless of whether initial treatment is able to reverse the acute renal functional impairment and the development of glomerulopathy is associated with less than a 50% 5-year graft survival from the time of identification [15]. AMR may also be associated with concurrent cellular rejection. Alloantibodies preferentially bind to the peritubular and glomerular capillaries in contrast to the typical injury pattern of acute cellular rejection (ACR) by T cells which tends to infiltrate renal tubules and the arterial endothelial layer[17-19]. AMR is associated with greater acute graft loss than ACR, with 15-20% losing their grafts within a year, despite typical mainstay immunosuppressive therapies[17]. The gold standard criteria identifying AMR remains a constellation of features seen on analysis of renal biopsy including C4d deposition and histological features of inflammation, allograft dysfunction, and serologic evidence of circulating antibodies to donor HLA or other non-HLA DSA[20]. C4d is a complement split product of C4b which can form covalent bonds with proteins in the setting of the complement pathway initiation via antibody binding and association with C1. C4d does not appear to be pathogenic in and of itself, but rather appears to be a fingerprint of antibody binding and complement deposition[21].
The presence of donor specific antibody (DSA) in the recipient serum can be assessed by ELISA or by bead-based fluorometric assays (Luminex or flow cytometry). Despite significant advances in the ability to detect, specify, and quantify the strength of HLA-specific and non-HLA DSA, it is not yet clear how effective these methods are at predicting AMR when assessed pre-transplant or serially over time. It does appear that the presence of DSA at the time of transplant is an independent risk factor for AMR and that patients who develop anti-HLA DSA tend to have inferior long term graft survival compared to patients who do not develop DSA[22-28]. It is apparent that a degree of DSA can be detected in some recipients without apparent clinical pathology in the transplanted kidney. It is possible that this DSA with unknown acute significance may have more obvious significance over a longer observed graft life.
Potential Targets for Therapy
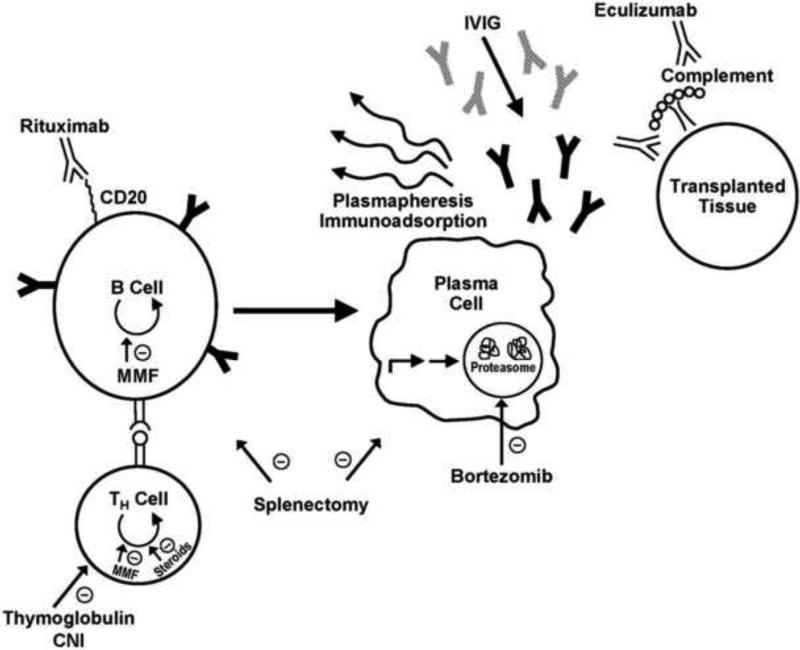
There are multiple steps in the mechanism of AMR that have served as potential intervention points in known therapies to counteract AMR and that serve as promising targets for further investigation (Figure 1). A direct approach can be utilized to inhibit or delete B cells. This includes metabolic inhibitors of B cell division (mycophenolate mofetil) or antibody directed depletion on the basis of B cell surface molecules (rituximab). An additional target to diminishing AMR is to inhibit T cells and by extension, diminish T cell help to B cells. This approach utilizes inhibitors of T cell division (mycophenolate mofetil (MMF) and steroids), inhibitors of IL-2 signaling to T cells (calcinuerin inhibitors (CNI)), or T cell depleting agents such as antithymocyte globulin (ATG). Polyclonal ATG may also have some B cell depleting effects. A third approach to controlling B cell responses in transplantation involves the removal or dilution of the B cell effector arm: antibodies. Antibody removal can be done by plasmapheresis (PP) or immunoadsorption (IA). Graft-directed antibodies can be diluted by administration of IVIG, which can also have more direct effects on B cell function through Fc receptors. Recent data has indicated a fourth target for diminishing antibody responses – targeting the antibody-secreting plasma cell (PC) with bortezomib, a proteasome inhibitor. This is the first agent available that appears to directly inhibit the cell considered to be the primary mediator of AMR. Lastly, a fifth target for therapy has recently been developed which involves the final step in AMR, the fixation of complement by antibodies that have targeted the graft. Eculizumab is a C5 inhibitor that diminishes the propagation of complement cascade even after antibodies have bound to the graft. There are also some therapies that appear to act at multiple steps in the process, including the nonspecific action of splenectomy, which seems to inhibit the process of B cell development in a nonspecific fashion.
Fig. 1.
A schematic pathway of the steps needed to develop AMR after transplantation and the discrete activities of the various therapeutic modalities against AMR are depicted. Pathogenic antibodies are depicted in black, IVIG is depicted in gray, and antibody-based drugs are depicted in white.
It should be noted that several fairly desirable pathways to target in the attempt to control B cell responses are currently not addressed in the arsenal of options to treat AMR. These include inhibitors to control the development of plasma cells from less mature B cells and direct inhibitors or depleting agents for plasma cells. This is an important gap in the treatment algorithm for AMR as plasma cells are durable cells and AMR would likely be easier to control if the process did not proceed to plasma cell responses before therapy was initiated. These important steps in B cell development serve as potential targets for future experimentation and development. In part due to a lack of specific plasma cell effectors, many treatments for AMR utilize multiple drugs or processes to simultaneously attack multiple steps in this process in order to control these challenging responses.
Antithymocyte Globulin (ATG)
ATG is a polyclonal antibody preparation derived from rabbits immunized with human thymic tissue. While it is generally thought that ATG has primarily anti-T cell effects, it also can inhibit the interaction of CD4+ helper T cells with B cells and thereby diminish B cell activation. ATG also can have direct B cell antibody derived cytotoxicity and may have effects on both antibody production of B cells and may be able to induce B cell apoptosis[29]. ATG is commonly included in treatment algorithms for AMR, especially when the transplant biopsy identifies mixed features of cellular and antibody-mediated rejection.
Typical dosing for ATG involves four divided doses of 1.5 mg/kg/day to yield a total treatment dose of 6 mg/kg. Platelet and white blood cell counts can diminish to a degree that the divided doses must be spread out to allow for recovery between doses in some cases. One study utilized lower dose ATG (0.75 – 1.0 mg/kg/d) for 5-10 days along with a mean of 7 treatments of plasmapheresis in seven renal transplant patients diagnosed with AMR[29]. Improvement in graft function was seen in 6 of the 7 patients and the serum creatinine level at one year in these six patients was not statistically different from their larger group of 60 patients that did not develop AMR. These AMR events were all discovered within the first month of transplant and it should be noted that only one patient of the seven with AMR was given ATG for immunosuppression induction at the time of transplantation, potentially limiting the applicability of this study to groups that use ATG routinely at the time of transplantation.
IVIG
IVIG inhibition of the cytotoxic effects of anti-HLA antibody was recognized in the 1990's [30, 31]. IVIG is currently used in desensitization protocols and for the treatment of AMR. It is derived from the pooled plasma of thousands of blood donors and is primarily composed of IgG. There are several proposed mechanisms of action (reviewed in [32]). Immunoglobulin molecules are well known for their ability to activate complement and complement is an important mediator of ischemia reperfusion injury. However evidence suggests that immunoglobulin molecules can also limit antibody-mediated complement activation in experimental models[33] [34]. IVIG may have the ability to regulate long-term alloimmune response through interactions with cell mediated immunity by binding to Fc receptors and preventing binding of alloantibody complexes as well as inducing the inhibitory FcgRIIb receptor. It is believed that IVIG can regulate innate immune responses by inhibiting the dendritic cell inflammatory response important to allograft rejection and the dendritic activation of alloreactive T-cells[35, 36]. IVIG may also have direct effects on the adaptive immune response through inhibition of T-cell induction of B-cell apoptosis[37].
Two general treatment protocols have been developed utilizing IVIG. The first is the use of high dose IVIG (2 gm/kg) alone and the second is to combine lower dose IVIG with other modalities, usually plasmapheresis (PP). High dose IVIG with methylprednisolone was reported for treating AMR in 1998 by Jordan et al[38]. Subsequently IVIG was tested prior to transplant in order to inhibit cross match positivity in kidney recipients. The Cedars-Sinai group performed in vitro IVIG assays to determine if crossmatch positivity could be inhibited. Those patients with a negative inhibition cross match received 2 g/kg of IVIG followed by kidney transplantation. Graft survival was 89.1% at 24 months[39]. In a randomized multicenter placebo-controlled trial conducted by the NIH (the NIH G02 study) in highly sensitized patients, 4 monthly infusions of IVIG significantly lowered anti-HLA antibody levels and improved rates of transplantation compared to placebo (35% vs. 17%, P=0.02) in patients with a PRA>50%. Projected waiting time on the IVIG group was 4.8 years and 10.3 years for placebo. The reduction in PRA was transient and moderate. Acute rejection episodes were more common in the IVIG group, but 3 year allograft survival rates were similar between IVIG and placebo[7].
Due to the length of isolated IVIG treatment, and in order to improve effectiveness, the Cedars-Sinai group conducted an open label phase 1-2 single-center study examining whether the addition of rituximab to IVIG was effective in reducing anti-HLA antibodies. 2 doses of IVIG (2 g/kg) and 2 doses of rituximab (1 g) were given prior to transplant. The protocol proved efficacious in reducing mean PRA compared to pretreatment level (P<0.001) and appeared to reduce the waiting time for transplant. With short term follow-up the mean graft survival was 94%[5].
Low dose IVIG (100mg/kg) in combination with PP represents an alternative to desensitize individuals with alloantibody prior to transplant as well as treat AMR. A small retrospective study compared combined therapy with PP, IVIG, and rituximab to high dose IVIG alone for AMR. After 36 months, the graft survival for the combined therapy group was 91.7% compared to 50% in the monotherapy group[40]. Other groups have reported successful desensitization outcomes with a variety of combined IVIG and PP protocols in the setting of HLA and anti-agglutinin antibodies [41] [6] [11] [42].
In general, high doses of IVIG are relatively safe. However, serious side effects have been reported including acute renal dysfunction likely related to high osmotic load, thrombotic events with rapid infusions, and aseptic meningitis[43]. Slowing the infusion rate and using iso-osmolar preparations may reduce the risk of side effects[44]. IVIG has the potential benefit of replacing antibodies lost during PP.
Plasmapheresis
The goal of plasmapheresis is to remove DSA from the circulation. PP is utilized in desensitization protocols and for the treatment of AMR following transplant. It is the fastest method to decrease DSA. There are several different modalities: plasma exchange, double filtration plasmapheresis, and immunoadsorption plasmapheresis. Plasma exchange is the most frequent modality applied in the United States and generally involves 1.0-1.5 volume exchange, using albumin as replacement. Immunoadsorption is a more selective modality that uses adsorbent membranes for antibody elimination. When utilized for desensitization, the clinician usually aims to decrease DSA below a threshold before transplant. After transplant, PP is often continued for a variable period of time.
Often PP is used in combination with other antibody blocking (IVIG), suppression (rituximab, mycophenolate, calcineurin inhibitors), or depleting (bortezomib) modalities, as antibody levels have a tendency to rebound if PP therapy is performed in isolation[45]. Few studies have been published where PP modalities are the sole or primary form of antibody reducing therapy[46, 47]
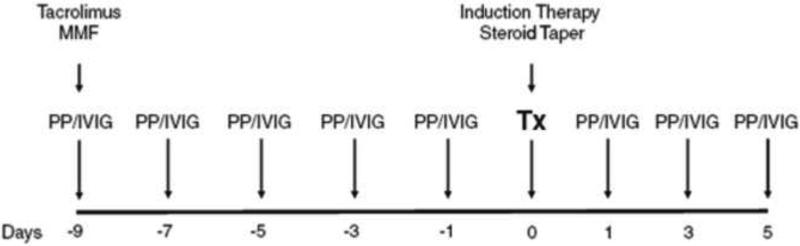
The Johns Hopkins’ desensitization protocol consist of every other day PP followed by 100 mg/kg IVIG after each PP session (Figure 2)[48]. The objective is to decrease DSA or iso-agglutinin titers to a pre-specified level prior to transplant. The number of pre-transplant PP/IVIG sessions depends on the starting titer. Patients are started on tacrolimus and MMF at the time PP/IVIG is initiated. PP/IVIG is continued post transplant, with the number of treatments governed by antibody levels and the clinical course[41, 48].
Fig. 2.
The Johns Hopkins PP/IVIG desensitization protocol. The protocol utilizes every other day plasmapheresis along with low-dose IVIG (100 mg/kg). The number of pre-transplant PP/IVIG treatments can be predicted from the starting donor reactive antibody titer. Several post-transplant PP/IVIG treatments are performed by protocol. PP/IVIG treatments are added as needed to reduce antibody levels to pre-transplant targets or to treat an episode of antibody mediated rejection. In selective high-risk cases, anti-CD20 is given the night before the transplant. About 5% of +XM patients will require rescue splenectomy as part of the treatment for severe AMR. Used with permission[48].
In a retrospective comparison of different desensitization strategies performed at the Mayo Clinic, Stegall et al noted that a negative preoperative crossmatch could be achieved in 85% of patients treated with a combination of PP, low dose IVIG, and rituximab compared to 36% treated with high does IVIG alone. The incidence of AMR was 80% in the IVIG only group, but 29-37% in the groups treated with three agents[49].
The incidence of side-effects from PP is relatively low ranging from 5-12% and most are considered mild or moderate in nature. Commonly reported symptoms are due to allergic reactions that present as rigors and urticaria, symptoms of hypocalcemia such as parasthesias, and hypovolemia which can manifest as muscle cramps and hypotension. The incidence and side effects relate to the use of anticoagulants during PP, type of replacement fluid, and complications related to vascular access. There is a small risk of blood borne pathogen transmission[50].
Rituximab
Rituximab is a chimeric anti-CD20 monoclonal antibody composed of human IgG1 heavy chain and kappa light chain constant regions fused with mouse variable regions. A transmembrane protein, CD20 is expressed on pre-B and mature B-lymphocytes throughout the antigen independent stage of development until early stages of antigen-dependent B-cell activation. CD20 is absent from plasma cells. Cells bound by rituximab are eliminated by traditional antibody-mediated mechanisms; antibody-dependent cell mediated cytotoxicity, complement dependent cytotoxicity, and cell-mediated apoptosis via CD20.
Rituximab was originally approved to treat lymphoma and it is also used for rheumatoid arthritis and other autoimmune disorders[51]. This agent exerts a profound depletion in circulating B cells as well as a less marked reduction in B cell numbers in spleen and lymph nodes[52]. A single dose in renal transplant recipients can result in prolonged B cell depletion, with populations remaining suppressed for 1-2 years[53]. Non-transplant studies of rituximab demonstrate a delayed recovery of the CD27+ memory B cell population[54].
Ramos et al. have investigated the in-vivo effect of rituximab on B-cell populations in individuals who underwent splenectomy[55]. Spleens removed for trauma victims and from transplant recipients who received multiple rounds of pre-transplant PP plus low-dose IVIG showed similar numbers of naïve B cells (CD20+ and CD79+), plasma cells (CD138+), and memory B cells (CD27+). However in transplant recipients that underwent splenectomy and where rituximab was added to the PP/IVIG regimen, the number of naïve B cells was reduced but no notable difference in the memory or plasma cell populations was seen. As rituximab is ineffective for reducing plasma cells, the source of DSA, its therapeutic benefit may be related to modifications of cellular immunity rather than antibody reduction. Evidence exists that rituximab may impact the important antigen presenting cell activity provided by antigen specific B-cells to T-cells[56, 57].
Rituximab has been used as induction therapy in patients with HLA antibodies and in the setting of ABO incompatible kidney transplantation as well as to treat AMR. Becker et al published the initial report describing the use of rituximab to treat AMR. 27 patients with refractory rejection were given a single dose of rituximab and a majority also received PP and ATG. In short term follow-up 24 patients had good graft function, but 3 grafts were lost[58]. Several other studies have reported results with use of rituximab for AMR. Zarkhin reported the 1 year outcome of a randomized trial of rituximab compared to thymoglobulin and/or pulse steroids for acute rejection in 20 pediatric renal recipients. There was some benefit for recovery of graft function and histology at 1 and 6 months after treatment[59]. There was no change in DSA in either group, although reappearance of C4d deposition was absent after rituximab, but was observed in 30% of control patients. Similarly Steinmetz et al. noted that rituximab removed intra-renal B cells in patients with vascular rejection compared to those who had received conventional immunosuppression[60]. In a retrospective study of 54 patients with AMR, Kaposztas et al compared 26 patients treated with PP plus rituximab to 28 patients who underwent PP without rituximab. The 2 year graft survival for the rituximab group was 90% compared to 60% in the PP cohort[61].
Bortezomib
Bortezomib, a proteasome inhibitor, has recently received attention as a possible agent to reduce alloantibody levels through its direct effect on plasma cells. Approved by the FDA in 2003 for the treatment of relapsed refractory multiple myeloma, it binds selectively and reversibly to the 26S proteasome. Proteasomes are located in the cell nucleus and cytoplasm and are the primary proteolytic mechanism in eukaryotic cells[62]. The 26S proteasome is part of an enzyme complex that plays a role in degradation of super-numerous, misfolded, or defective proteins targeted for degradation by ubiquitinylation[63]. Besides damaged proteins, proteasomes degrade proteins involved in cell cycle regulation, oncogenesis, and apoptosis [64, 65].
Proteasome inhibition induces apoptotic cell death as a result of activation of the terminal unfolded protein response[66, 67]. Bortezomib induces apoptosis in various malignant cells, but the sensitivity of myeloma cells to bortezomib may be related to high synthesis rates of immunoglobulin associated with accumulation of unfolded proteins and the subsequent stress in the endoplasmic reticulum[68]. Other mechanisms include modification of cytokine signaling pathways through the inhibitor of Kappa B (ikB) and nuclear factor Kappa B (NF-kB) signal transduction.
Bortezomib is primarily metabolized by cytochrome P450 enzymes. Adverse events include fatigue, malaise, weakness, nausea, diarrhea, vomiting, peripheral neuropathy, thrombocytopenia, and neutropenia. Drug related side effects generally can be managed with dose reduction and supportive care[69].
A few case reports and case series have been published where bortezomib was used to modify pre-transplant anti-HLA antibodies or as therapy for AMR[70-75]. The impact of bortezomib in reducing anti-HLA in these studies is difficult to assess as most of the studies have been complicated by the addition of other therapies to modify alloantibody, including IVIG, rituximab, and plasmapheresis. Furthermore these studies primarily report short term follow-up. Long-term studies will be needed to determine the durability of proteasome inhibition to prevent return of donor specific antibody and the impact on allograft survival and function.
Only two studies have examined the use of bortezomib as isolated therapy for donor specific antibody. In one study of 2 sensitized kidney transplant candidates receiving two cycles of bortezomib administered without further anti-humoral measures, proteasome inhibition did not affect or only modestly affected allosensitization[76]. In a post-renal transplant study among four patients with DSA, one cycle of bortezomib as isolated therapy was unable to reduce DSA titers[77]. While the PC-targeting of bortezomib is a logical approach to treat AMR, given the mixed results of small studies using bortezomib, properly designed and controlled studies are clearly needed and caution exercised with the off-label use of this drug[78].
Eculizumab
Eculizumab (Soliris, Alexion Pharmaceuticals) is a humanized monoclonal antibody against the C5 complement protein which has been approved by the FDA for treating paroxysmal nocturnal hemoglobinemia. By preventing C5 cleavage by C5 convertase into C5a and C5b, formation of the C5b-C9 membrane attack complex is prevented. Complement activation plays a critical role in the development of AMR after kidney transplantation. As such, the complement cascade represents a potential target for therapy. A small series presented by Stegall et al. reported no incidence of AMR in the year following transplant in 3 patients who underwent desensitization with IVIG and PP with eculizumab after transplant[79]. This absence of AMR is substantially lower than a historic rate for desensitized recipients, although this is a small series. A handful of case reports exist which document success treating AMR with eculizumab[80, 81]. There are several ongoing clinic trials enrolling transplant candidates or recipients (clinicaltrials.gov). Despite the appeal of treating the complement cascade, further investigation may be hampered by the cost of this antibody, one of the most expensive medications in the world.
Potential New Targets
The survival signals that govern PC persistence are currently emerging from the basic science literature[82-84]. Understanding these signals will be critical to developing successful therapies to permanently eliminate alloantibodies. A major shortcoming in the therapies reviewed above (IVIG, rituximab, PP, splenectomy, and eculizumab with the exception of bortezomib) is their inability to target PCs. PC-directed therapy aims to eliminate alloantibody-secreting PCs permanently. Berek and colleagues made the recent discovery that eosinophils are required for the maintenance of PCs[85]. Desensitization protocols targeting eosinophils have not been investigated and eosinophil-directed immunotherapy maybe a potential desensitization strategy. Furthermore, in mice TACI-Ig decreases PC numbers substantially by sequestering both BLyS and APRIL and could be translated into a novel desensitization strategy as well[86]. Additionally, PCs reliance on RANKL, IL-6, CXCR4, and CXCL12 could also be targeted in novel PC directed therapies[87-92]. Without the targeted elimination of alloantibody-secreting PCs, DSA cannot be expected to be fully eliminated in the sensitized recipient and will continue to challenge long-term allograft survival.
Summary
AMR remains a significant clinical problem in a minority of recipients of kidney transplants. Outcomes after AMR are less than ideal. In part, this is due to the fact that no optimal treatment modality currently exists to treat AMR and antibody responses, once established, can be difficult or impossible to extinguish. Thus, nearly all current strategies to address AMR rely on a mixture of partially effective strategies as outlined above. These combined approaches are difficult to compare due to the fact that few studies directly compare different complex approaches to one another but rather generally compare a mixed strategy with or without an additional agent to determine the added benefit of that agent to the strategy. While this is a valid approach, it is clear that AMR is in need of protocolized studies to compare regimens and seek an optimal approach while minimizing toxicity. This is not an easy task, as AMR is an intrinsically heterogeneous process and it is well-known that controlling AMR when detected early after transplant before a mature B cell response is established is more easily accomplished than eliminating late-onset AMR or established sensitization to HLA antigens. Future studies must be careful to clearly define the type of AMR that is being treated. In addition, AMR is a rare enough event after transplantation that it will be difficult to accumulate sufficient patient numbers at a single center level to allow comparison of different treatment strategies. Multicenter trials will need to be established but will require standardized cross-institutional diagnoses of AMR..
Interest in post transplant B cell responses and AMR has increased substantially in the past decade associated with the appreciation of the clinical relevance of this phenomenon. New agents developed for non-transplant purposes, such as bortezomib and eculizumab have shown promise in addressing some of the downstream targets in the AMR process and have been added to a number of desensitization strategies. Future targets of relevance include the transition from immature B cells to mature B cells and plasma cells and development of agents with more direct and specific effects on plasma cell numbers or function. With increased interest and attention to the problem of AMR and sensitization, there is renewed promise that such approaches may be developed to augment the armamentarium against this difficult clinical problem.
Highlights.
>Antibody mediated rejection is a significant clinical problem after renal transplant. >We review the biological basis and pathways of antibody mediated rejection. >We review available treatments and future directions for therapy. >We review clinical trials results dealing with antibody mediated rejection.
Acknowledgements
Thank you to Robert Redfield III and Robin Noel for assistance with the preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no conflicts of interest as described in the author's guide for Seminars in Immunology
References
- 1.United States Organ Procurement and Transplantation Network (OPTN) Database. US Department of Health and Human Services, Health Resources and Services Administration. May 11, 2011.
- 2.Jordan SC, Reinsmoen N, Peng A, Lai CH, Cao K, Villicana R, et al. Advances in diagnosing and managing antibody-mediated rejection. Pediatr Nephrol. 2010;25(10):2035–45. doi: 10.1007/s00467-009-1386-4. quiz 2045-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paramesh AS, Zhang R, Baber J, Yau CL, Slakey DP, Killackey MT, et al. The effect of HLA mismatch on highly sensitized renal allograft recipients. Clin Transplant. 2010;24(6):E247–52. doi: 10.1111/j.1399-0012.2010.01306.x. [DOI] [PubMed] [Google Scholar]
- 4.Montgomery RA, Zachary AA. Transplanting patients with a positive donor-specific crossmatch: a single center's perspective. Pediatr Transplant. 2004;8(6):535–42. doi: 10.1111/j.1399-3046.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- 5.Vo AA, Lukovsky M, Toyoda M, Wang J, Reinsmoen NL, Lai CH, et al. Rituximab and intravenous immune globulin for desensitization during renal transplantation. N Engl J Med. 2008;359(3):242–51. doi: 10.1056/NEJMoa0707894. [DOI] [PubMed] [Google Scholar]
- 6.Gloor JM, DeGoey SR, Pineda AA, Moore SB, Prieto M, Nyberg SL, et al. Overcoming a positive crossmatch in living-donor kidney transplantation. Am J Transplant. 2003;3(8):1017–23. doi: 10.1034/j.1600-6143.2003.00180.x. [DOI] [PubMed] [Google Scholar]
- 7.Jordan SC, Tyan D, Stablein D, McIntosh M, Rose S, Vo A, et al. Evaluation of intravenous immunoglobulin as an agent to lower allosensitization and improve transplantation in highly sensitized adult patients with end-stage renal disease: report of the NIH IG02 trial. J Am Soc Nephrol. 2004;15(12):3256–62. doi: 10.1097/01.ASN.0000145878.92906.9F. [DOI] [PubMed] [Google Scholar]
- 8.Jordan SC, Pescovitz MD. Presensitization: the problem and its management. Clin J Am Soc Nephrol. 2006;1(3):421–32. doi: 10.2215/CJN.01651105. [DOI] [PubMed] [Google Scholar]
- 9.Montgomery RA, Cooper M, Kraus E, Rabb H, Samaniego M, Simpkins CE, et al. Renal transplantation at the Johns Hopkins Comprehensive Transplant Center. Clin Transpl. 2003:199–213. [PubMed] [Google Scholar]
- 10.Glotz D, Antoine C, Julia P, Pegaz-Fiornet B, Duboust A, Boudjeltia S, et al. Intravenous immunoglobulins and transplantation for patients with anti-HLA antibodies. Transpl Int. 2004;17(1):1–8. doi: 10.1007/s00147-003-0674-3. [DOI] [PubMed] [Google Scholar]
- 11.Schweitzer EJ, Wilson JS, Fernandez-Vina M, Fox M, Gutierrez M, Wiland A, et al. A high panel-reactive antibody rescue protocol for cross-match-positive live donor kidney transplants. Transplantation. 2000;70(10):1531–6. doi: 10.1097/00007890-200011270-00023. [DOI] [PubMed] [Google Scholar]
- 12.Montgomery RA, Locke JE, King KE, Segev DL, Warren DS, Kraus ES, et al. ABO incompatible renal transplantation: a paradigm ready for broad implementation. Transplantation. 2009;87(8):1246–55. doi: 10.1097/TP.0b013e31819f2024. [DOI] [PubMed] [Google Scholar]
- 13.Kissmeyer-Nielsen F, Olsen S, Petersen VP, Fjeldborg O. Hyperacute rejection of kidney allografts, associated with pre-existing humoral antibodies against donor cells. Lancet. 1966;2(7465):662–5. doi: 10.1016/s0140-6736(66)92829-7. [DOI] [PubMed] [Google Scholar]
- 14.Williams GM, Hume DM, Hudson RP, Jr., Morris PJ, Kano K, Milgrom F. “Hyperacute” renal-homograft rejection in man. N Engl J Med. 1968;279(12):611–8. doi: 10.1056/NEJM196809192791201. [DOI] [PubMed] [Google Scholar]
- 15.Gloor JM, Cosio FG, Rea DJ, Wadei HM, Winters JL, Moore SB, et al. Histologic findings one year after positive crossmatch or ABO blood group incompatible living donor kidney transplantation. Am J Transplant. 2006;6(8):1841–7. doi: 10.1111/j.1600-6143.2006.01416.x. [DOI] [PubMed] [Google Scholar]
- 16.Rafiq MA, de Boccardo G, Schroppel B, Bromberg JS, Sehgal V, Dinavahi R, et al. Differential outcomes in 3 types of acute antibody-mediated rejection. Clin Transplant. 2009;23(6):951–7. doi: 10.1111/j.1399-0012.2009.01036.x. [DOI] [PubMed] [Google Scholar]
- 17.Lucas JG, Co JP, Nwaogwugwu UT, Dosani I, Sureshkumar KK. Antibody-mediated rejection in kidney transplantation: an update. Expert Opin Pharmacother. 2011;12(4):579–92. doi: 10.1517/14656566.2011.525219. [DOI] [PubMed] [Google Scholar]
- 18.Mauiyyedi S, Colvin RB. Humoral rejection in kidney transplantation: new concepts in diagnosis and treatment. Curr Opin Nephrol Hypertens. 2002;11(6):609–18. doi: 10.1097/00041552-200211000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Montgomery RA, Hardy MA, Jordan SC, Racusen LC, Ratner LE, Tyan DB, et al. Consensus opinion from the antibody working group on the diagnosis, reporting, and risk assessment for antibody-mediated rejection and desensitization protocols. Transplantation. 2004;78(2):181–5. doi: 10.1097/01.tp.0000129256.84027.d6. [DOI] [PubMed] [Google Scholar]
- 20.Racusen LC, Colvin RB, Solez K, Mihatsch MJ, Halloran PF, Campbell PM, et al. Antibody-mediated rejection criteria - an addition to the Banff 97 classification of renal allograft rejection. Am J Transplant. 2003;3(6):708–14. doi: 10.1034/j.1600-6143.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 21.Bohmig GA, Exner M, Habicht A, Schillinger M, Lang U, Kletzmayr J, et al. Capillary C4d deposition in kidney allografts: a specific marker of alloantibody-dependent graft injury. J Am Soc Nephrol. 2002;13(4):1091–9. doi: 10.1681/ASN.V1341091. [DOI] [PubMed] [Google Scholar]
- 22.Riethmuller S, Ferrari-Lacraz S, Muller MK, Raptis DA, Hadaya K, Rusi B, et al. Donor-specific antibody levels and three generations of crossmatches to predict antibody-mediated rejection in kidney transplantation. Transplantation. 2010;90(2):160–7. doi: 10.1097/tp.0b013e3181e36e08. [DOI] [PubMed] [Google Scholar]
- 23.Lefaucheur C, Loupy A, Hill GS, Andrade J, Nochy D, Antoine C, et al. Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J Am Soc Nephrol. 2010;21(8):1398–406. doi: 10.1681/ASN.2009101065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lefaucheur C, Suberbielle-Boissel C, Hill GS, Nochy D, Andrade J, Antoine C, et al. Clinical relevance of preformed HLA donor-specific antibodies in kidney transplantation. Am J Transplant. 2008;8(2):324–31. doi: 10.1111/j.1600-6143.2007.02072.x. [DOI] [PubMed] [Google Scholar]
- 25.Lee PC, Terasaki PI, Takemoto SK, Lee PH, Hung CJ, Chen YL, et al. All chronic rejection failures of kidney transplants were preceded by the development of HLA antibodies. Transplantation. 2002;74(8):1192–4. doi: 10.1097/00007890-200210270-00025. [DOI] [PubMed] [Google Scholar]
- 26.Worthington JE, Martin S, Al-Husseini DM, Dyer PA, Johnson RW. Posttransplantation production of donor HLA-specific antibodies as a predictor of renal transplant outcome. Transplantation. 2003;75(7):1034–40. doi: 10.1097/01.TP.0000055833.65192.3B. [DOI] [PubMed] [Google Scholar]
- 27.Terasaki PI, Ozawa M. Predicting kidney graft failure by HLA antibodies: a prospective trial. Am J Transplant. 2004;4(3):438–43. doi: 10.1111/j.1600-6143.2004.00360.x. [DOI] [PubMed] [Google Scholar]
- 28.Einecke G, Sis B, Reeve J, Mengel M, Campbell PM, Hidalgo LG, et al. Antibody-mediated microcirculation injury is the major cause of late kidney transplant failure. Am J Transplant. 2009;9(11):2520–31. doi: 10.1111/j.1600-6143.2009.02799.x. [DOI] [PubMed] [Google Scholar]
- 29.Shah A, Nadasdy T, Arend L, Brennan J, Leong N, Coppage M, et al. Treatment of C4d-positive acute humoral rejection with plasmapheresis and rabbit polyclonal antithymocyte globulin. Transplantation. 2004;77(9):1399–405. doi: 10.1097/01.tp.0000122187.76518.bc. [DOI] [PubMed] [Google Scholar]
- 30.Tyan DB, Li VA, Czer L, Trento A, Jordan SC. Intravenous immunoglobulin suppression of HLA alloantibody in highly sensitized transplant candidates and transplantation with a histoincompatible organ. Transplantation. 1994;57(4):553–62. [PubMed] [Google Scholar]
- 31.Glotz D, Haymann JP, Sansonetti N, Francois A, Menoyo-Calonge V, Bariety J, et al. Suppression of HLA-specific alloantibodies by high-dose intravenous immunoglobulins (IVIg). A potential tool for transplantation of immunized patients. Transplantation. 1993;56(2):335–7. doi: 10.1097/00007890-199308000-00015. [DOI] [PubMed] [Google Scholar]
- 32.Jordan SC, Toyoda M, Vo AA. Intravenous immunoglobulin a natural regulator of immunity and inflammation. Transplantation. 2009;88(1):1–6. doi: 10.1097/TP.0b013e3181a9e89a. [DOI] [PubMed] [Google Scholar]
- 33.Arumugam TV, Tang SC, Lathia JD, Cheng A, Mughal MR, Chigurupati S, et al. Intravenous immunoglobulin (IVIG) protects the brain against experimental stroke by preventing complement-mediated neuronal cell death. Proc Natl Acad Sci U S A. 2007;104(35):14104–9. doi: 10.1073/pnas.0700506104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Basta M, Van Goor F, Luccioli S, Billings EM, Vortmeyer AO, Baranyi L, et al. F(ab)'2-mediated neutralization of C3a and C5a anaphylatoxins: a novel effector function of immunoglobulins. Nat Med. 2003;9(4):431–8. doi: 10.1038/nm836. [DOI] [PubMed] [Google Scholar]
- 35.Bayry J, Lacroix-Desmazes S, Carbonneil C, Misra N, Donkova V, Pashov A, et al. Inhibition of maturation and function of dendritic cells by intravenous immunoglobulin. Blood. 2003;101(2):758–65. doi: 10.1182/blood-2002-05-1447. [DOI] [PubMed] [Google Scholar]
- 36.Tha-In T, Metselaar HJ, Tilanus HW, Boor PP, Mancham S, Kuipers EJ, et al. Superior immunomodulatory effects of intravenous immunoglobulins on human T-cells and dendritic cells: comparison to calcineurin inhibitors. Transplantation. 2006;81(12):1725–34. doi: 10.1097/01.tp.0000226073.20185.b1. [DOI] [PubMed] [Google Scholar]
- 37.Toyoda M, Pao A, Petrosian A, Jordan SC. Pooled human gammaglobulin modulates surface molecule expression and induces apoptosis in human B cells. Am J Transplant. 2003;3(2):156–66. doi: 10.1034/j.1600-6143.2003.00011.x. [DOI] [PubMed] [Google Scholar]
- 38.Jordan SC, Quartel AW, Czer LS, Admon D, Chen G, Fishbein MC, et al. Posttransplant therapy using high-dose human immunoglobulin (intravenous gammaglobulin) to control acute humoral rejection in renal and cardiac allograft recipients and potential mechanism of action. Transplantation. 1998;66(6):800–5. doi: 10.1097/00007890-199809270-00017. [DOI] [PubMed] [Google Scholar]
- 39.Jordan SC, Vo A, Bunnapradist S, Toyoda M, Peng A, Puliyanda D, et al. Intravenous immune globulin treatment inhibits crossmatch positivity and allows for successful transplantation of incompatible organs in living-donor and cadaver recipients. Transplantation. 2003;76(4):631–6. doi: 10.1097/01.TP.0000080685.31697.FC. [DOI] [PubMed] [Google Scholar]
- 40.Lefaucheur C, Nochy D, Andrade J, Verine J, Gautreau C, Charron D, et al. Comparison of combination Plasmapheresis/IVIg/anti-CD20 versus high-dose IVIg in the treatment of antibody-mediated rejection. Am J Transplant. 2009;9(5):1099–107. doi: 10.1111/j.1600-6143.2009.02591.x. [DOI] [PubMed] [Google Scholar]
- 41.Montgomery RA, Zachary AA, Racusen LC, Leffell MS, King KE, Burdick J, et al. Plasmapheresis and intravenous immune globulin provides effective rescue therapy for refractory humoral rejection and allows kidneys to be successfully transplanted into cross-match-positive recipients. Transplantation. 2000;70(6):887–95. doi: 10.1097/00007890-200009270-00006. [DOI] [PubMed] [Google Scholar]
- 42.Sonnenday CJ, Ratner LE, Zachary AA, Burdick JF, Samaniego MD, Kraus E, et al. Preemptive therapy with plasmapheresis/intravenous immunoglobulin allows successful live donor renal transplantation in patients with a positive cross-match. Transplant Proc. 2002;34(5):1614–6. doi: 10.1016/s0041-1345(02)03044-0. [DOI] [PubMed] [Google Scholar]
- 43.Rault R, Piraino B, Johnston JR, Oral A. Pulmonary and renal toxicity of intravenous immunoglobulin. Clin Nephrol. 1991;36(2):83–6. [PubMed] [Google Scholar]
- 44.Jordan S, Cunningham-Rundles C, McEwan R. Utility of intravenous immune globulin in kidney transplantation: efficacy, safety, and cost implications. Am J Transplant. 2003;3(6):653–64. doi: 10.1034/j.1600-6143.2003.00121.x. [DOI] [PubMed] [Google Scholar]
- 45.Rocha PN, Butterly DW, Greenberg A, Reddan DN, Tuttle-Newhall J, Collins BH, et al. Beneficial effect of plasmapheresis and intravenous immunoglobulin on renal allograft survival of patients with acute humoral rejection. Transplantation. 2003;75(9):1490–5. doi: 10.1097/01.TP.0000060252.57111.AC. [DOI] [PubMed] [Google Scholar]
- 46.Madan AK, Slakey DP, Becker A, Gill JI, Heneghan JL, Sullivan KA, et al. Treatment of antibody-mediated accelerated rejection using plasmapheresis. J Clin Apher. 2000;15(3):180–3. doi: 10.1002/1098-1101(2000)15:3<180::aid-jca5>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 47.Lennertz A, Fertmann J, Thomae R, Illner WD, Hillebrand GE, Feucht HE, et al. Plasmapheresis in C4d-positive acute humoral rejection following kidney transplantation: a review of 4 cases. Ther Apher Dial. 2003;7(6):529–35. doi: 10.1046/j.1526-0968.2003.00101.x. [DOI] [PubMed] [Google Scholar]
- 48.Warren DS, Montgomery RA. Incompatible kidney transplantation: lessons from a decade of desensitization and paired kidney exchange. Immunol Res. 2010;47(1-3):257–64. doi: 10.1007/s12026-009-8157-y. [DOI] [PubMed] [Google Scholar]
- 49.Stegall MD, Gloor J, Winters JL, Moore SB, Degoey S. A comparison of plasmapheresis versus high-dose IVIG desensitization in renal allograft recipients with high levels of donor specific alloantibody. Am J Transplant. 2006;6(2):346–51. doi: 10.1111/j.1600-6143.2005.01178.x. [DOI] [PubMed] [Google Scholar]
- 50.Okafor C, Ward DM, Mokrzycki MH, Weinstein R, Clark P, Balogun RA. Introduction and overview of therapeutic apheresis. J Clin Apher. 25(5):240–9. doi: 10.1002/jca.20247. [DOI] [PubMed] [Google Scholar]
- 51.Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350(25):2572–81. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 52.Reff ME, Carner K, Chambers KS, Chinn PC, Leonard JE, Raab R, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83(2):435–45. [PubMed] [Google Scholar]
- 53.Genberg H, Hansson A, Wernerson A, Wennberg L, Tyden G. Pharmacodynamics of rituximab in kidney transplantation. Transplantation. 2007;84(12 Suppl):S33–6. doi: 10.1097/01.tp.0000296122.19026.0f. [DOI] [PubMed] [Google Scholar]
- 54.Anolik JH, Barnard J, Owen T, Zheng B, Kemshetti S, Looney RJ, et al. Delayed memory B cell recovery in peripheral blood and lymphoid tissue in systemic lupus erythematosus after B cell depletion therapy. Arthritis Rheum. 2007;56(9):3044–56. doi: 10.1002/art.22810. [DOI] [PubMed] [Google Scholar]
- 55.Ramos EJ, Pollinger HS, Stegall MD, Gloor JM, Dogan A, Grande JP. The effect of desensitization protocols on human splenic B-cell populations in vivo. Am J Transplant. 2007;7(2):402–7. doi: 10.1111/j.1600-6143.2006.01632.x. [DOI] [PubMed] [Google Scholar]
- 56.Liossis SN, Sfikakis PP. Rituximab-induced B cell depletion in autoimmune diseases: potential effects on T cells. Clin Immunol. 2008;127(3):280–5. doi: 10.1016/j.clim.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 57.Stasi R, Cooper N, Del Poeta G, Stipa E, Laura Evangelista M, Abruzzese E, et al. Analysis of regulatory T-cell changes in patients with idiopathic thrombocytopenic purpura receiving B cell-depleting therapy with rituximab. Blood. 2008;112(4):1147–50. doi: 10.1182/blood-2007-12-129262. [DOI] [PubMed] [Google Scholar]
- 58.Becker YT, Becker BN, Pirsch JD, Sollinger HW. Rituximab as treatment for refractory kidney transplant rejection. Am J Transplant. 2004;4(6):996–1001. doi: 10.1111/j.1600-6143.2004.00454.x. [DOI] [PubMed] [Google Scholar]
- 59.Zarkhin V, Li L, Kambham N, Sigdel T, Salvatierra O, Sarwal MM. A randomized, prospective trial of rituximab for acute rejection in pediatric renal transplantation. Am J Transplant. 2008;8(12):2607–17. doi: 10.1111/j.1600-6143.2008.02411.x. [DOI] [PubMed] [Google Scholar]
- 60.Steinmetz OM, Lange-Husken F, Turner JE, Vernauer A, Helmchen U, Stahl RA, et al. Rituximab removes intrarenal B cell clusters in patients with renal vascular allograft rejection. Transplantation. 2007;84(7):842–50. doi: 10.1097/01.tp.0000282786.58754.2b. [DOI] [PubMed] [Google Scholar]
- 61.Kaposztas Z, Podder H, Mauiyyedi S, Illoh O, Kerman R, Reyes M, et al. Impact of rituximab therapy for treatment of acute humoral rejection. Clin Transplant. 2009;23(1):63–73. doi: 10.1111/j.1399-0012.2008.00902.x. [DOI] [PubMed] [Google Scholar]
- 62.Brooks P, Fuertes G, Murray RZ, Bose S, Knecht E, Rechsteiner MC, et al. Subcellular localization of proteasomes and their regulatory complexes in mammalian cells. Biochem J. 2000;346(Pt 1):155–61. [PMC free article] [PubMed] [Google Scholar]
- 63.Ciechanover A. The ubiquitin-proteasome proteolytic pathway. Cell. 1994;79(1):13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 64.Rock KL, Gramm C, Rothstein L, Clark K, Stein R, Dick L, et al. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78(5):761–71. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 65.Pagano M, Tam SW, Theodoras AM, Beer-Romero P, Del Sal G, Chau V, et al. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995;269(5224):682–5. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- 66.Obeng EA, Carlson LM, Gutman DM, Harrington WJ, Jr., Lee KP, Boise LH. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood. 2006;107(12):4907–16. doi: 10.1182/blood-2005-08-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Neubert K, Meister S, Moser K, Weisel F, Maseda D, Amann K, et al. The proteasome inhibitor bortezomib depletes plasma cells and protects mice with lupus-like disease from nephritis. Nat Med. 2008;14(7):748–55. doi: 10.1038/nm1763. [DOI] [PubMed] [Google Scholar]
- 68.Meister S, Schubert U, Neubert K, Herrmann K, Burger R, Gramatzki M, et al. Extensive immunoglobulin production sensitizes myeloma cells for proteasome inhibition. Cancer Res. 2007;67(4):1783–92. doi: 10.1158/0008-5472.CAN-06-2258. [DOI] [PubMed] [Google Scholar]
- 69.Jackson G, Einsele H, Moreau P, Miguel JS. Bortezomib, a novel proteasome inhibitor, in the treatment of hematologic malignancies. Cancer Treat Rev. 2005;31(8):591–602. doi: 10.1016/j.ctrv.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 70.Everly MJ, Everly JJ, Susskind B, Brailey P, Arend LJ, Alloway RR, et al. Bortezomib provides effective therapy for antibody- and cell-mediated acute rejection. Transplantation. 2008;86(12):1754–61. doi: 10.1097/TP.0b013e318190af83. [DOI] [PubMed] [Google Scholar]
- 71.Trivedi HL, Terasaki PI, Feroz A, Everly MJ, Vanikar AV, Shankar V, et al. Abrogation of anti-HLA antibodies via proteasome inhibition. Transplantation. 2009;87(10):1555–61. doi: 10.1097/TP.0b013e3181a4b91b. [DOI] [PubMed] [Google Scholar]
- 72.Walsh RC, Everly JJ, Brailey P, Rike AH, Arend LJ, Mogilishetty G, et al. Proteasome inhibitor-based primary therapy for antibody-mediated renal allograft rejection. Transplantation. 2010;89(3):277–84. doi: 10.1097/TP.0b013e3181c6ff8d. [DOI] [PubMed] [Google Scholar]
- 73.Lonze BE, Dagher NN, Simpkins CE, Singer AL, Segev DL, Zachary AA, et al. The fate of anti-HLA antibody among renal transplantation recipients treated with bortezomib. Clin Transpl. 2009:377–84. [PubMed] [Google Scholar]
- 74.Manitpisitkul W, Wilson N, Cooper M, Gurk-Turner C, Hurley H, Rasetto F, et al. Rescue therapy for early antibody mediated rejection with a proteasome inhibitor: a case report. Clin Transpl. 2009:461–3. [PubMed] [Google Scholar]
- 75.Perry DK, Burns JM, Pollinger HS, Amiot BP, Gloor JM, Gores GJ, et al. Proteasome inhibition causes apoptosis of normal human plasma cells preventing alloantibody production. Am J Transplant. 2009;9(1):201–9. doi: 10.1111/j.1600-6143.2008.02461.x. [DOI] [PubMed] [Google Scholar]
- 76.Wahrmann M, Haidinger M, Kormoczi GF, Weichhart T, Saemann MD, Geyeregger R, et al. Effect of the proteasome inhibitor bortezomib on humoral immunity in two presensitized renal transplant candidates. Transplantation. 2010;89(11):1385–90. doi: 10.1097/TP.0b013e3181d9e1c0. [DOI] [PubMed] [Google Scholar]
- 77.Sberro-Soussan R, Zuber J, Suberbielle-Boissel C, Candon S, Martinez F, Snanoudj R, et al. Bortezomib as the sole post-renal transplantation desensitization agent does not decrease donor-specific anti-HLA antibodies. Am J Transplant. 2010;10(3):681–6. doi: 10.1111/j.1600-6143.2009.02968.x. [DOI] [PubMed] [Google Scholar]
- 78.Kirk AD. What should work, may not. Am J Transplant. 2010;10(3):447–8. doi: 10.1111/j.1600-6143.2010.03025.x. [DOI] [PubMed] [Google Scholar]
- 79.Stegall MD, Diwan TS, Burns JM, Dean PG, Cornell LD, Gandhi MJ, et al. Prevention of Acute Humoral Rejection with C5 Inhibition. Am J Transplant. 2009;9(Suppl 2)(s2):241–2. [Google Scholar]
- 80.Lonze BE, Dagher NN, Simpkins CE, Locke JE, Singer AL, Segev DL, et al. Eculizumab, bortezomib and kidney paired donation facilitate transplantation of a highly sensitized patient without vascular access. Am J Transplant. 2010;10(9):2154–60. doi: 10.1111/j.1600-6143.2010.03191.x. [DOI] [PubMed] [Google Scholar]
- 81.Locke JE, Magro CM, Singer AL, Segev DL, Haas M, Hillel AT, et al. The use of antibody to complement protein C5 for salvage treatment of severe antibody-mediated rejection. Am J Transplant. 2009;9(1):231–5. doi: 10.1111/j.1600-6143.2008.02451.x. [DOI] [PubMed] [Google Scholar]
- 82.Fairfax KA, Kallies A, Nutt SL, Tarlinton DM. Plasma cell development: from B-cell subsets to long-term survival niches. Semin Immunol. 2008;20(1):49–58. doi: 10.1016/j.smim.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 83.Good-Jacobson KL, Shlomchik MJ. Plasticity and heterogeneity in the generation of memory B cells and long-lived plasma cells: the influence of germinal center interactions and dynamics. J Immunol. 2010;185(6):3117–25. doi: 10.4049/jimmunol.1001155. [DOI] [PubMed] [Google Scholar]
- 84.Minges Wols HA, Underhill GH, Kansas GS, Witte PL. The role of bone marrow-derived stromal cells in the maintenance of plasma cell longevity. J Immunol. 2002;169(8):4213–21. doi: 10.4049/jimmunol.169.8.4213. [DOI] [PubMed] [Google Scholar]
- 85.Chu VT, Frohlich A, Steinhauser G, Scheel T, Roch T, Fillatreau S, et al. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat Immunol. 2011;12(2):151–9. doi: 10.1038/ni.1981. [DOI] [PubMed] [Google Scholar]
- 86.Benson MJ, Dillon SR, Castigli E, Geha RS, Xu S, Lam KP, et al. Cutting edge: the dependence of plasma cells and independence of memory B cells on BAFF and APRIL. J Immunol. 2008;180(6):3655–9. doi: 10.4049/jimmunol.180.6.3655. [DOI] [PubMed] [Google Scholar]
- 87.Cassese G, Arce S, Hauser AE, Lehnert K, Moewes B, Mostarac M, et al. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J Immunol. 2003;171(4):1684–90. doi: 10.4049/jimmunol.171.4.1684. [DOI] [PubMed] [Google Scholar]
- 88.Cyster JG. Homing of antibody secreting cells. Immunol Rev. 2003;194:48–60. doi: 10.1034/j.1600-065x.2003.00041.x. [DOI] [PubMed] [Google Scholar]
- 89.Hargreaves DC, Hyman PL, Lu TT, Ngo VN, Bidgol A, Suzuki G, et al. A coordinated change in chemokine responsiveness guides plasma cell movements. J Exp Med. 2001;194(1):45–56. doi: 10.1084/jem.194.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hauser AE, Debes GF, Arce S, Cassese G, Hamann A, Radbruch A, et al. Chemotactic responsiveness toward ligands for CXCR3 and CXCR4 is regulated on plasma blasts during the time course of a memory immune response. J Immunol. 2002;169(3):1277–82. doi: 10.4049/jimmunol.169.3.1277. [DOI] [PubMed] [Google Scholar]
- 91.Klein B, Tarte K, Jourdan M, Mathouk K, Moreaux J, Jourdan E, et al. Survival and proliferation factors of normal and malignant plasma cells. Int J Hematol. 2003;78(2):106–13. doi: 10.1007/BF02983377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Quinn W, Stohl W, Choi Y, Cancro M. RANK-L expression marks long-lived plasma cells. J Immunol. 2010;184(132.5 (suppl)) [Google Scholar]