Abstract
Chronic infusion of angiotensin II (AngII) augments atherosclerosis and abdominal aortic aneurysm (AAAs) formation in hypercholesterolemic mice. AngII-induced AAAs are associated with medial macrophage accumulation and matrix metalloproteinase (MMP) activation. Inhibition of calpain, a calcium-activated neutral cysteine protease, by overexpression of its endogenous inhibitor, calpastatin, attenuates AngII-induced leukocyte infiltration, perivascular inflammation, and MMP activation in mice. The purpose of this study was to define whether pharmacological inhibition of calpain influences AngII-induced AAAs in hypercholesterolemic mice. Male LDL receptor −/− mice were fed a fat-enriched diet and administered with either vehicle or a calpain-specific inhibitor, BDA-410 (30 mg/kg/day) for 5 weeks. After 1 week of feeding, mice were infused with AngII (1,000 ng/kg/min) for 4 weeks. AngII-infusion profoundly increased aortic calpain protein and activity. BDA-410 administration had no effect on plasma cholesterol concentrations or AngII-increased systolic blood pressure. Calpain inhibition significantly attenuated AngII-induced AAA formation and atherosclerosis development. BDA-410 administration attenuated activation of MMP12, pro-inflammatory cytokines (IL-6, MCP-1) and macrophage infiltration into the aorta. BDA-410 administration significantly attenuated thioglycollate-elicited macrophage accumulation in the peritoneal cavity. We conclude that calpain inhibition using BDA-410 attenuated AngII-induced AAA formation and atherosclerosis development in LDL receptor −/− mice.
Keywords: Angiotensin II, aneurysm, calpain, BDA-410
INTRODUCTION
Abdominal aortic aneurysms (AAAs) are permanent arterial dilations and rupture of AAAs is the 13th leading cause of death in United States.1,2 AAAs are characterized by chronic inflammation, matrix degradation, and vascular remodeling.3 The only current approach to prevent aortic rupture is restricted to surgical repair.4 In an experimental animal model, angiotensin II (AngII) plays a pivotal role in AAA development.5,6 Chronic infusion of AngII into hypercholesterolemic mice promotes atherosclerosis and leads to development of AAAs.5 AngII-induced AAAs show characteristics similar to human AAAs, including inflammation associated with leukocyte infiltration and medial destruction.7 Proteolytic enzymes like matrix metalloproteinases (MMPs),8,9 chymase10 and cathepsins11,12 have been suggested to promote medial elastic laminal disruption and macrophage infiltration during the development of AngII-induced AAAs. Although there is no proven pharmacological therapy to treat AAAs, selective inhibition of MMPs,9 and chymase10 has been found to impair aneurysm formation in animal models.
Recently, calpain, a calcium-dependent cysteine protease gained attention as a critical mediator of various diseases including Alzheimer's,13,14,15 lissencephaly,16 diabetes,17 and muscular dystrophy.18 The two major isoforms of the calpain family, calpain-1 and calpain-2, are ubiquitously expressed along with their endogenous inhibitor, calpastatin, whereas the other isoforms (e.g. −3, −8 and −9) are tissue-specific.19 Upon activation by calcium, calpain causes damage to cells by selectively degrading intracellular proteins including signaling proteins (e.g. protein kinase C, cyclin dependent kinase),20,21 cytoskeletal proteins (e.g. spectrin)19 and transcription factors (e.g. c-Jun, IkB).19,22,23 In addition, calpains play a critical role in cellular apoptosis through the activation of both caspase dependent and independent pathways.24,25 Calpains are involved in acute inflammatory processes via the activation of nuclear factor kappa B (NF-kB).26,27 In both cultured aortic smooth muscle cells (SMCs) and AngII-infused rat aortas, activated calpain mediates MMP activation, which may play a key role in vessel wall degradation.28 Calpain activation is also shown to mediate AngII-induced cardiac hypertrophy, aortic medial hypertrophy, and perivascular inflammation in mice.27 Furthermore, inhibition of calpain by overexpression of its endogenous inhibitor, calpastatin, also attenuated AngII-induced vascular inflammation by suppressing macrophage infiltration and NF-kB activation.27 However, the role of calpain activation in the development of AngII-induced AAA formation has not been determined.
As calpain activation plays a critical role in AngII-induced vascular inflammation, we sought to determine whether pharmacological inhibition of calpain would modulate the development of AngII-induced AAA formation in LDL receptor −/− mice. (2S)-N-(1S)-1- [(S)-Hydroxy (3-oxo-2-phenyl-1-cyclopropen-1-yl) methyl]-2-methyl propyl-2- benzene sulfonylamino-4-methyl pentanamide, (C26H32N2O5S, MW 484.61) or BDA-410 is a novel, orally active and potent inhibitor of calpain. BDA-410 has a selective inhibitory action on calpain over other proteases.29,15 In cultured SHSY5Y cells, the inhibitory effects (IC50) of BDA-410 on various proteases are: calpain = 21 nM, papain = 400 nM, cathepsin B = 16,000 nM, thrombin > 100 μM, cathepsin G > 100 μM, cathepsin D = 91 μM, and proteasome 20S > 100 μM. BDA-410 has been evaluated and shown to be protective against neuronal cell death 13 and improved memory function in a mouse model of Alzheimer's disease,15 and reduced malarial infection in mice.29 By using BDA-410, we demonstrate that calpain inhibition significantly reduces the incidence and development of AAAs in LDL receptor −/− mice.
METHODS
Mice
Ten to twelve weeks old, male LDL receptor −/− mice (stock # 002207) were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were 10 times backcrossed into a C57BL/6J background. The mice were housed in a barrier facility and fed with normal laboratory diet and water ad libitum. The studies were approved by the University of Kentucky Institutional Animal Care and Use Committee.
Diet
To induce hypercholesterolemia, mice were fed a diet supplemented with saturated fat (21% wt/wt milk fat; TD88137, Harlan Teklad, Indianapolis, IN) for 2–5 weeks.
Calpain Inhibitor BDA-410 Administration
BDA-410 was a kind gift from the Mitsubishi Tanabe Pharma Corporation, Osaka, Japan. The BDA-410 compound was pulverized and suspended in 1% Tween 80 in saline and administered daily for 2, 3 or 5 weeks by gavage at a dose of 30 mg/kg/day.15
AngII Infusion
After an initial week of high-fat diet feeding and daily calpain inhibitor administration, mice were implanted with Alzet osmotic minipumps (model 1004, Durect Corporation, Cupertino, CA), subcutaneously into the right flank, and infused with AngII (1,000 ng/kg/min, Bachem, Torrance, CA) continuously for a period of 7,14 or 28 days, as described previously.5 The mice were continued on high fat-enriched diet and gavaged daily with the calpain inhibitor throughout the study.
Blood Pressure Measurement
Systolic blood pressure (SBP) was measured noninvasively on conscious mice by volume pressure recording of the tail using a computerized tail cuff blood pressure system (Kent Scientific Corp, Torrington, CT).30 SBP was measured on 5 consecutive days prior to pump implantation, and during the last 5 days of the AngII infusion.
Measurement of Plasma Components
Plasma cholesterol concentrations were measured using a commercially available enzymatic kit (Wako Chemicals, Richmond, VA) and lipoprotein cholesterol distribution was determined as described previously.5,31
Ultrasound Imaging of AAA
Luminal dilation of the abdominal aorta was measured by a high frequency ultrasound imaging system (Vevo 660, Visual Sonics, Toronto, Canada) using a RMV 704 scanhead with a frequency of 40 MHz and a focal length of 6 mm.32 Mice were anaesthetized and restrained in a supine position to acquire the ultrasonic images. Short axis scans of abdominal aortas were performed from the left renal arterial branch level to the suprarenal region.32 Images of abdominal aortas were acquired and measured to determine the maximal diameter in the suprarenal region of the abdominal aorta. Aortic images were acquired at day 0 and 28 of AngII-infusion.
Quantification of Atherosclerosis and AAA
Atherosclerosis was quantified on the aortic arch as lesion area on the intimal surface by en face analysis as described previously.33,34 AAAs were quantified ex vivo at 28 days by measuring maximum external width of the suprarenal abdominal aortic diameter using computerized morphometry (Image-Pro Cybernetics, Bethesda, MD) as described previously.35
Thioglycollate Elicitation of Peritoneal Macrophages
Male LDL receptor −/− mice fed high-fat enriched diet were gavaged with either vehicle or BDA-410 throughout the study. After 7 days of drug administration, mice were injected intraperitoneally with thioglycollate broth (1 ml; 3% wt/vol) and maintained with fat-enriched diet and BDA-410 administration. Seventy two hours after thioglycollate injection, mice were sedated and peritoneal macrophages were harvested as described earlier.36 Red blood cells were lysed using a solution of ammonium chloride. Cell numbers were calculated using a hemacytometer and stained with fluorescent labeled CD68-FITC (1:10, catalog No: MCA1957F, Serotec, Raleigh, NC) and analyzed by fluorescent activated cell sorter (FACS).
Tissue Immunostaining
Macrophages were detected in tissue sections of AAA using a rat anti-mouse CD68 (1:200, catalog No. MCA1957; Serotec, Raleigh, NC). Immunostaining was performed on formalin-fixed frozen sections, with appropriate negative controls, as described previously.31,37
mRNA Abundance
RNA was harvested from mouse aortic tissues, using the RNeasy fibrous tissue kit (catalog No: 74704; Qiagen, Valencia, CA). RNA (100 ng) was reverse transcribed using the iScript cDNA synthesis kit (catalog No: 170–8891; Bio-Rad, Hercules, CA). qRT-PCR was performed as described previously.31,37 mRNA abundance was calculated by normalization to β-actin. Non-template and no RT reactions were used as negative controls. The primers used are detailed in Table 1.
Table 1.
Primers used for real-time PCR
| Gene | Primers | Product size (bp) |
|---|---|---|
| MCP-1 | 5'-CAGCCAGATGCAGTTAACGC 5'-TCTGGACCCATTCCTTCTTG |
175 |
| IL-6 | 5'-GGGAAATCGTGGAAATGAGAAA 5'-AAGTGCATCATCGTTGTTCATACA |
167 |
| IL-10 | 5'-CCAAGCCTTATCGGAAATGA 5'-TCTCACCCAGGGAATTCAAA |
190 |
| ICAM-1 | 5'-AGATCACATTCACGGTGCTG 5'-CTTCAGAGGCAGGAAACAGG |
170 |
| IKKα | 5'-GTCAGGACCGTGTTCTCAAGG 5'-GCTTCTTTGATGTTACTGAGGGC |
118 |
| IKKβ | 5'-ACAGCCAGGAGATGGTACG 5'-AGGGTGACTGAGTCGAGAC |
296 |
| IKKε | 5'-ACCACTAACTACCTGTGGCAT 5'-CCTCCACTGCGAATAGCTTC |
214 |
| β-actin | 5'-CGTGGGCCGCCCTAGGCAACCA 5'-TTGGCCTTAGGGTTCAGGGGGG |
220 |
Western Blot Analyses
Aortic tissue lysates were extracted in RIPA lysis buffer and protein content was measured using the Bradford assay (Bio-Rad, Hercules, CA). Protein extracts (25 μg) were resolved by SDS-PAGE (6 % wt/vol) and transferred electrophoretically to PVDF membranes. After blocking with non-dry fat milk (5 % wt/vol), the membranes were probed with antibodies against calpain-1 domain IV (catalog No: ab39170, Abcam, Cambridge, MA), calpain-2 (catalog No: ab39165, Abcam, Cambridge, MA), α-spectrin (catalog No: MAB1622, Millipore, Billerica, MA), and β-actin (catalog No: A5441, Sigma-Aldrich, St. Louis, MO). Membranes were then incubated with appropriate secondary antibodies, and immune complexes were visualized by chemiluminescence (Pierce, Rockford, IL) and quantified using a Kodak Imager.
Calpain, Cathepsin B, and Proteasome Activity Assays
Calpain, cathepsin B, and proteasome activities were measured in aortic tissue lysates fluorimetrically using commercially-available activity assay kits (Calpain, catalog No: K240-100; Cathepsin B, catalog No: K140-100; Proteasome, catalog No: K245-100; BioVision, Mountain View, CA). Aortic protein extracts (20 μg) were incubated with fluorogenic (4-trifluoromethyl coumarin labeled) substrate specific to calpain, cathepsin B or proteasome for 60–120 min at 37°C. The mean fluorescent signals were measured using a microplate fluorescent plate reader (Spectramax M2; Molecular Devices, Sunnyvale, CA) as per manufacturer instructions.
Zymography
Aortic protein extracts (5 μg) were resolved under non-reducing conditions by SDS-PAGE (10% wt/vol) polymerized in the presence of gelatin (2 mg/ml) or casein (1 mg/ml) to measure the activities of MMP2, MMP9, or MMP12 respectively. Gels were washed with Triton X-100 (2.5% vol/vol) and distilled water for 30 min each. Gels were then incubated overnight at 37°C in Tris buffer containing calcium chloride (5 mM) and sodium azide (0.02% w/w), pH 8.0 for MMP2/MMP9 or pH 8.5 for MMP12. After incubation, gels were stained with Coomassie Brilliant Blue followed by destaining with acetic acid (7% vol/vol) and methanol (40% vol/vol). Gel images were captured using a Kodak Imager, the unstained, translucent digested regions represented areas of MMP activity.
Statistical Analyses
Data are represented as either mean ± SEM or median ± CI. Statistical analyses were performed using Sigmastat (SPSS Inc) or SAS version 8.2 (SAS Institute). Blood pressure data were analyzed by repeated measures of ANOVA as a quadratic polynomial in time for each treatment. Ultrasound measurements of abdominal aortic diameter were analyzed using the nonparametric repeated measures of ANOVA on Ranks with Tukey post hoc test. Atherosclerosis and abdominal aortic diameter data were analyzed using the nonparametric Mann-Whitney Rank sum test. Fisher's exact test was used to determine differences between groups in the incidence of AAA. Western blot, qRT-PCR and activity assay data were analyzed using Students t test, or two-way ANOVA with Holm-Sidak post hoc tests as appropriate. Values of P<0.05 were considered to be statistically significant.
RESULTS
AngII infusion increased calpain activity in aortas
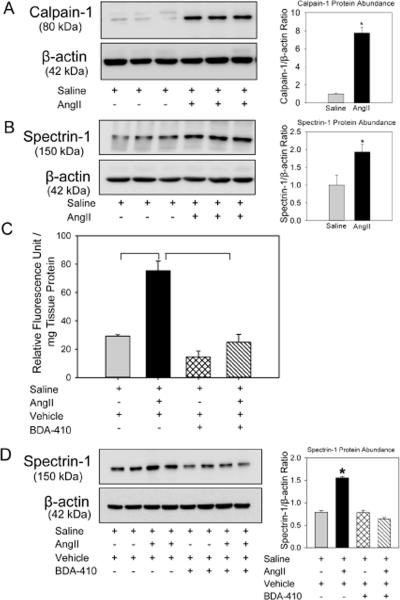
To determine whether AngII increases calpain activity in aortic tissue, male LDL receptor −/− mice were infused with either saline or AngII (1000 ng/kg/min) for 14 days. Aortas were minced and protein was extracted using lysis buffer. Western blot analyses demonstrated that AngII-infusion significantly increased calpain-1 protein abundance (Figure 1A). Increased calpain activity was demonstrated by increased breakdown product of spectrin, the major substrate of calpain (Figure 1B). The increased calpain activity was further confirmed by measuring calpain activity in aortic tissue extracts using fluorescent labeled calpain substrate, Ac-LLY-AFC. AngII-infusion showed a 2 fold increase in calpain activity compared to saline group (Figure 1C).
Figure 1. Abundance of calpain protein and its activity was increased in AngII-infused aortas.
Calpain-1 (A) and spectrin-1 (B,D) protein were detected by Western blotting in tissue extracts from aortas in LDL receptor −/− mice infused with either saline or AngII for 14 days. β-actin was shown as loading control. Calpain-1 (A) and spectrin-1 (B,D) protein abundance was quantified by image analysis. Images are representative out of 4 or 5 independent experiments. Results are represented as means ± SEMs; Calpain activity (C) were measured by fluorimetric assay in aortic tissue extracts from saline and AngII infused mice administered either vehicle or BDA-410 (n=4). Statistical analyses were performed using Students t test (A,B) or two-way ANOVA with a Holm-Sidak multiple comparison post-hoc test (C,D). * and horizontal bars represent significance of P<0.05.
BDA-410 inhibited AngII-induced calpain activation in aortas
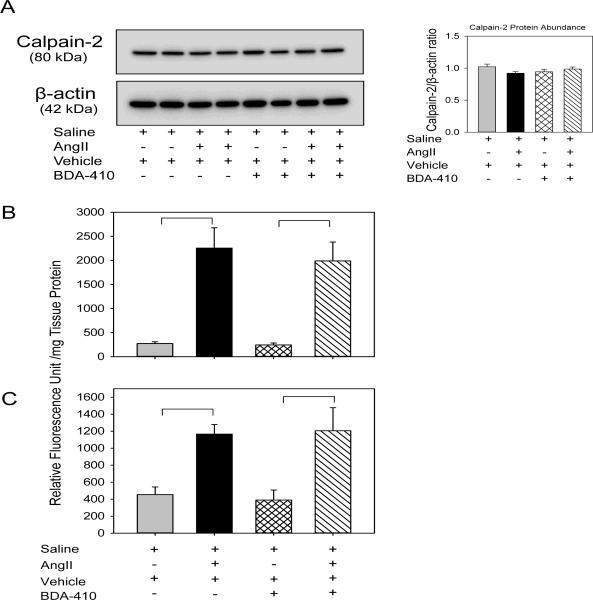
To determine whether BDA-410 administration was sufficient to inhibit calpain activity, male LDL receptor −/− mice fed with high fat-enriched diet were infused with either saline or AngII for 14 days. The calpain inhibitor, BDA-410, was administered at the dose of 30 mg/kg/day by gavage 1 week prior to infusions and throughout the subsequent 14 days. Administration of BDA-410 significantly attenuated AngII-induced calpain activation as demonstrated by suppressed breakdown of fluorescent labeled calpain substrate (Figure 1C) and reduced breakdown of spectrin in aortas from mice infused with AngII and BDA-410, compared to AngII + vehicle (Figure 1D). Western blot analyses of protein extracted from aortic tissue using calpain-2 antibodies showed that neither AngII-infusion nor BDA-410 administration had an effect on calpain-2 protein suggesting that calpain-1 is more active than calpain-2 during AngII-infusion and BDA-410 has more specificity to calpain-1 (Figure 2A). The specificity of BDA-410 to calpain was demonstrated further by measuring its effect on cysteine protease, cathepsin B (Figure 2B), and proteasome activity (Figure 2C) in aortic tissue extracts. AngII-infusion significantly increased cathepsin B and proteasome activity, whereas BDA-410 administration had no effect on their activities both at basal and AngII-infused conditions.
Figure 2. BDA-410 administration did not change calpain-2, cathepsin B and proteasome in AngII-infused aortas.
Calpain-2 (A) protein was detected by Western blotting in tissue extracts from aortas in LDL receptor −/− mice infused with either saline or AngII for 14 days. β-actin was shown as loading control. Calpain-2 (A) protein abundance was quantified by image analysis. Images are representative out of 4 independent experiments. Cathepsin B (B) and proteasome activity (C) were measured by fluorimetric assay in aortic tissue extracts from saline and AngII infused mice administered either vehicle or BDA-410 (n=4). Results are represented as means ± SEMs; Statistical analyses were performed using Students t test or two-way ANOVA with a Holm-Sidak multiple comparison post-hoc test. * and horizontal bars represent significance of P<0.05.
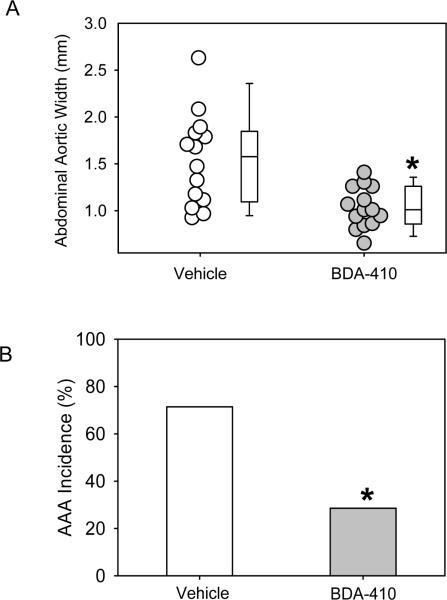
Calpain inhibition attenuated AngII-induced AAA formation
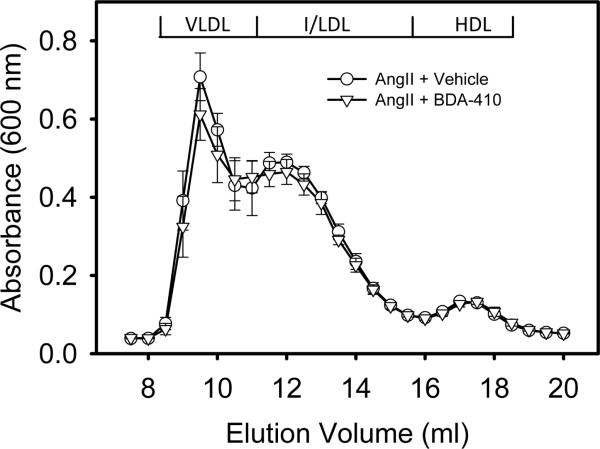
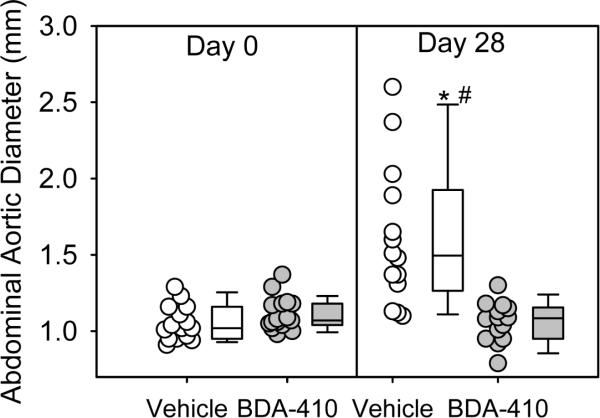
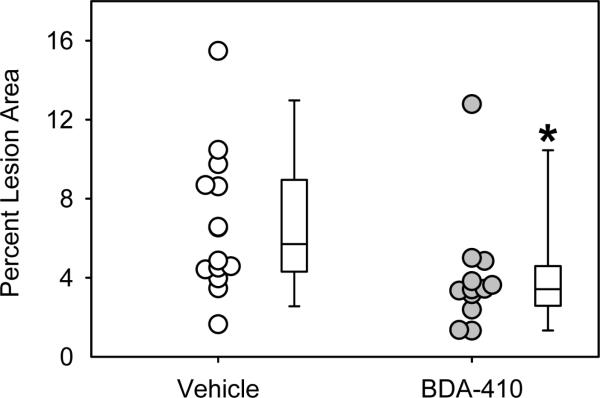
AngII-infusion for 28 days significantly increased systolic blood pressure in both groups of LDL receptor −/− mice (Table 2). Administration of BDA-410 had no effect on body weight, plasma total cholesterol concentrations (Table 2), or lipoprotein-cholesterol distributions (Figure 3). AngII-infusion significantly increased luminal dilation of abdominal aortas in vehicle administered group, as measured by ultrasound on day 28 (Figure 4). In contrast, administration of BDA-410 significantly attenuated AngII-induced luminal dilation of abdominal aortas (Figure 4). Calpain inhibition using BDA-410 significantly attenuated the formation (Figure 5A) and incidence (Figure 5B) of AngII-induced AAA in LDL receptor −/− mice (vehicle: 71% vs BDA-410: 20%, P<0.05, Figure 4B). Moreover, calpain inhibition also significantly attenuated development of AngII-induced atherosclerosis in aortic arches (P<0.05, Figure 6).
Table 2.
Effects of BDA-410 administration in male LDL receptor −/− mice during infusion of AngII.
| Groups | Vehicle | BDA-410 |
|---|---|---|
| N | 14 | 14 |
| Body Weight (g) | 26 ± 1 | 27 ± 1 |
| Plasma Cholesterol Concentrations (mg/dL) | 1047 ± 60 | 1084 ± 67 |
| Systolic BP Pre-infusion (mmHg) | 132 ± 1 | 130 ± 1 |
| Systolic BP Post-infusion (mmHg) | 149 ± 2* | 152 ± 2* |
Values are represented as means ± SEMs.
Denotes P<0.001 systolic BP post-infusion vs pre-infusion, by one-way ANOVA.
Figure 3. BDA-410 did not affect lipoprotein cholesterol distributions.
Lipoproteins were resolved by size-exclusion chromatography. Total cholesterol concentrations are expressed as mean absorbance per fraction. Symbols represent the means and bars are SEMs of 5 individual mice per group: Vehicle (circles) and BDA-410 (triangles).
Figure 4. BDA-410 administration attenuated AngII-induced aortic luminal dilation in LDL receptor −/− mice.
Ultrasonic measurements of abdominal aortic diameters were measured on day 0 and after 28 days of AngII-infusion (n=14). Open circles (vehicle) and gray circles (BDA-410) represent individual mice. The length of each box represents the interquartile range (difference between 75th percentile, top of box, and 25th percentile, bottom of box), the dash inside box depicts the median, and the whiskers extending from the top to the bottom of each box indicate the 95th and 5th percentiles, respectively. Statistical analyses were performed using the nonparametric repeated measures of ANOVA on Ranks with Tukey post hoc test. *P<0.05 vs day 0; #P<0.05 vs day 28 BDA-410.
Figure 5. BDA-410 administration attenuated AngII-induced abdominal aortic aneurysms in LDL receptor −/− mice.
A. Measurements of maximal external width of abdominal aortas (n=14). Open circles (vehicle) and gray circles (BDA-410) represents values from individual mice. The length of each box represents the interquartile range (difference between 75th percentile, top of box, and 25th percentile, bottom of box), the dash inside box depicts the median, and the whiskers extending from the top to the bottom of each box indicate the 95th and 5th percentiles, respectively. Statistical analyses were performed using the nonparametric Mann-Whitney Rank sum test. B. Percent incidence of AAAs based on 50% increase in luminal diameter compared to Day 0. Fisher's exact test was used to determine differences between groups in the incidence of AAA. *P<0.05 BDA-410 vs vehicle.
Figure 6. BDA-410 administration attenuated AngII-induced atherosclerosis in LDL receptor −/− mice.
Atherosclerotic lesion areas were measured on aortic arch intimal surfaces (n = 12–14). Open circles (vehicle) and gray circles (BDA-410) represents values from individual mice. The length of each box represents the interquartile range (difference between 75th percentile, top of box, and 25th percentile, bottom of box), the dash inside box depicts the median, and the whiskers extending from the top to the bottom of each box indicate the 95th and 5th percentiles, respectively. Statistical analyses were performed using the nonparametric Mann-Whitney Rank sum test. *P<0.05 BDA-410 vs vehicle.
BDA-410 administration attenuated AngII-induced MMP12 activation in aortas
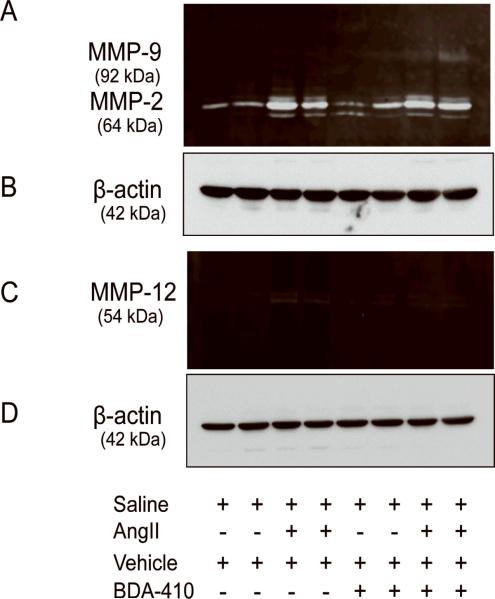
Pharmacological inhibition of MMPs using doxycycline was shown to attenuate AngII-induced AAA formation.9 A recent study also showed that macrophage elastase, MMP12 is involved in AngII-induced aneurysm progression and rupture.38 Since overexpression of calpastatin, an endogenous inhibitor of calpain, attenuated AngII-induced MMP2 activation in aortas,27 we sought to determine whether BDA-410 administration influences MMPs activation in the present study. AngII-infusion increased aortic both MMP2 (gelatinolytic) and MMP12 (caseinolytic) activity, in aortas as analyzed by zymography. BDA-administration did not influence AngII-induced MMP2 activation (Figure 7A) whereas it blunted AngII-induced MMP12 activation (Figure 7C). Western blot analyses, using β-actin antibodies, with the same concentration of aortic protein lysate confirmed equal amount of loading in all samples (Figure 7B and 7D).
Figure 7. BDA-410 administration attenuated AngII-induced MMP12 activity in aortas from LDL receptor −/− mice.
Gelatin (A) and caseinolytic (C) zymography detected MMP2,−9 and MMP12 in tissue extracts from aortas (n=4). β-actin protein (B,D), a loading control, was detected by Western blotting in aortic tissue extracts (n=4).
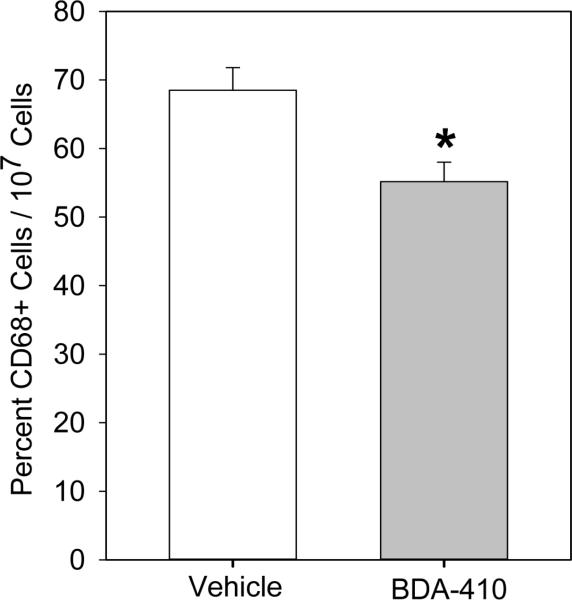
Calpain inhibition attenuated thioglycollate-induced peritoneal macrophage accumulation
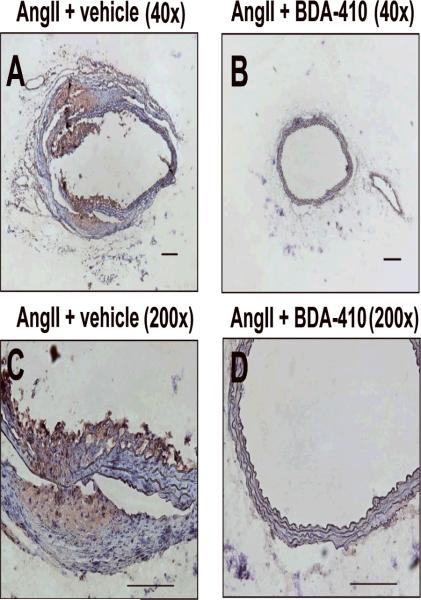
Immunostaining of abdominal aortas using anti-CD68 antibodies showed increased CD68+ macrophage accumulation in the suprarenal aorta of mice infused with AngII, but not in the group of mice administered with BDA-410 and AngII (Figure 8). To further confirm the effect of calpain inhibition on macrophage recruitment, we used a thioglycollate-induced peritonitis as a model of inflammation.36 Thioglycollate-induced peritonitis is used extensively as a model that promotes the recruitment of leukocytes into the peritoneal cavity. In this model, peritoneal macrophage accumulation occurs at a slow rate, reaching a maximum number at 72–96 hours, whereas neutrophils reach a maximum number at less than 10 hours. Male LDL receptor −/− mice, administered with either vehicle or BDA-410, were injected with a single dose of 3% thioglycollate. Administration of calpain inhibitor, BDA-410, significantly reduced the number of macrophages elicited to the peritoneal cavity after 72 hours of thioglycollate injection (P<0.05, Figure 9).
Figure 8. BDA-410 administration reduced macrophage accumulation in abdominal aortas from LDL receptor −/− mice.
Representative immunostaining of tissue-sections of AngII -(A,C) and AngII+BDA -(B,D) infused suprarenal aortas with rat anti-mouse CD68 demonstrates macrophage infiltration. Scale bars corresponds to 50μm. A and B - 40× C and D - 200×.
Figure 9. BDA-410 administration reduced thioglycollate-elicited peritoneal macrophage accumulation in LDL receptor −/− mice.
Thioglycollate-elicited CD68+ peritoneal macrophages were quantified from LDL receptor −/− mice administered with vehicle or BDA-410 (n=5). *P<0.05 BDA-410 vs vehicle.
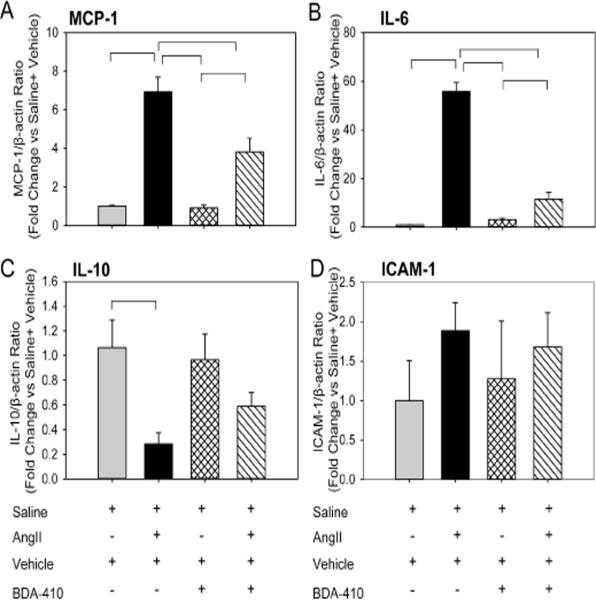
Calpain inhibition attenuated AngII-induced proinflammatory gene expression in the aorta
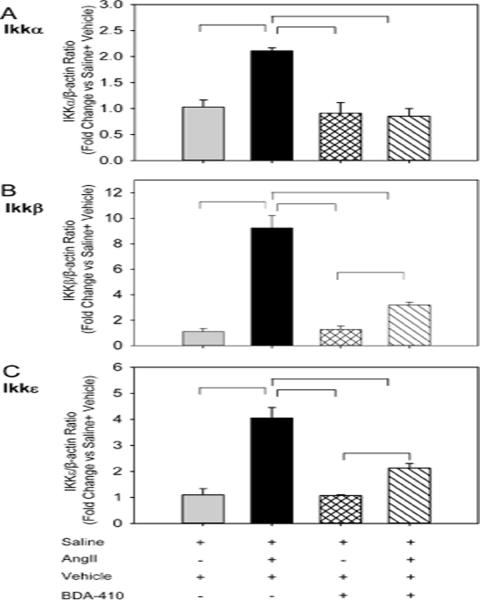
To further understand the mechanism by which BDA-410 administration attenuates macrophage accumulation, we examined mRNA abundance of monocyte chemoattractant protein (MCP-1), inflammatory cytokines (IL-6, IL-10), adhesion molecule (ICAM-1) and NF-kB-related IkappaB kinases (IKKα, β and ε) in aortas. Male LDL receptor −/− mice fed a high fat-enriched diet were infused with either saline or AngII for 7 days. The calpain inhibitor, BDA-410, was administered at a dose of 30 mg/kg/day by gavage 1 week prior to infusions and throughout the subsequent 7 days. Ang II-infusion significantly increased MCP-1, IL-6 (Figure 10 A, 10 B), reduced IL-10 (Figure 10 C) and had no effect on ICAM-1 (Figure 10 D) in aorta compared to saline infused mice. In addition, Ang II-infusion significantly increased mRNA abundance of NF-kB-related IkappaB kinases (IKKα, β and ε) in the aorta (Figure 11 A–C). BDA-410 administration significantly attenuated AngII-induced alterations in the level of inflammatory genes.
Figure 10. BDA-410 administration attenuated AngII-induced MCP-1, IL-6, and IL-10 gene in aortas from LDL receptor −/− mice.
Total RNA was extracted and mRNA abundance of MCP-1 (A), IL-6 (B), IL-10 (C), and ICAM-1 (D) genes were analyzed by real-time PCR using β-actin as an internal control (n=4). Values are represented as mean ± SEM. All horizontal bars represent significance of P<0.05 by two-way ANOVA followed by Holm-Sidak post hoc tests.
Figure 11. BDA-410 administration attenuated AngII-induced NF-kB dependent inflammatory genes in aortas from LDL receptor −/− mice.
Total RNA was extracted and mRNA abundance of IKKα (A), IKKβ (B), and IKKε (C) genes were analyzed by real-time PCR using β-actin as an internal control (n=4). Values are represented as mean ± SEM. All horizontal bars represent significance of P<0.05 by two-way ANOVA followed by Holm-Sidak post hoc tests.
DISCUSSION
AngII-induced AAAs in mice are a well-established, model of generating AAA.5 In this present study, we demonstrated that pharmacological inhibition of calpain attenuates development of both AngII-induced AAAs and atherosclerosis in male LDL receptor −/− mice. The protective effect of calpain inhibition was associated with reduction of macrophage accumulation, inflammation, and elastase-mediated extracellular matrix degradation in the aorta. This is the first report that reveals a causal effect of calpain in the development of AngII-induced atherosclerosis and AAAs.
In this present study, AngII infusion into hypercholesterolemic mice profoundly increased aortic calpain-1, but not calpain-2, protein and activity. BDA-410 administration significantly attenuated AngII-induced calpain-1 activity as evidenced by suppressed breakdown of fluorescent labeled calpain substrate and reduced breakdown of aortic spectrin. Consistent with this observation, an earlier study showed that AngII-infusion increased calpain activity in kidneys of normolipidemic mice.20 In that study, overexpression of calpastatin, an endogenous inhibitor of calpains, attenuated AngII-induced calpain activity as evidenced by reduced breakdown product of spectrin. The observed, beneficial effect of BDA-410 on inhibiting calpain activity is possibly due to its effect on calpain-1, since the compound has a higher selectivity (Ki value of 130 nM) compared to calpain-2 (Ki value of 630 nM).29
Calpain inhibition by BDA-410 did not show any effect on AngII-induced blood pressure elevation. This result is in agreement with an earlier study in which overexpression of calpastatin attenuated AngII-induced cardiac hypertrophy without affecting blood pressure.27 In a recent study, our laboratory also demonstrated that the AngII-induced AAA formation is independent of its effect on systolic blood pressure.39
The first discernable event in development of AngII-induced AAAs is elastin fiber fragmentation associated with leukocytic accumulation in the aneurysm- prone region.7 Destruction of elastin fibers in the aortic media may be due to activation of various proteases that have been detected in AngII-induced AAA tissue, including MMPs.9 Similarly, several MMPs have been shown to present in atherosclerotic lesions.40,41,42 However, these activated MMPs have been reported to exert divergent effects on atherosclerotic lesion formation and stability in mice.43,44,45 Doxycycline, a broad spectrum inhibitor of MMPs has been shown to attenuate AngII-induced AAA formation, but has no effect on atherosclerosis in mice.9 Recent studies using MMP12 (macrophage elastase) deficient mice showed that AngII-induced MMP12 promotes aneurysm progression and rupture.38 Consistent with other studies, AngII-infusion in the present study showed an increase in both MMP2 and MMP12 in the aortas. However, the inhibition of calpain by BDA-410 attenuated only MMP12 but did not change the activation of MMP2. In contrast, an earlier study using cultured aortic SMCs showed that calpain inhibition by overexpression of adenoviral delivered calpastatin partially reduced AngII-induced MMP2 activity.28 The possible mechanism for the specific effect of calpain inhibition on MMP12 may be due to the suppression of macrophage accumulation upon BDA-410 administration. In addition, MMP2 can be synthesized by all major cell types of aorta including endothelial cells, smooth muscle cells and adventitial fibroblasts. Furthermore, AngII-infusion activates MMPs, primarily gelatinases, as early as day 3 post-infusion, whereas MMP12 appears at later stages (day 9) post infusion.38 MMP12 deficiency was also shown to limit aortic dilation in CaCl2 model46 whereas it showed partial effect in the elastase model of aortic aneurysm.47 Selective inhibition of MMP12 using a phosphinic peptide, RXP470.1, retarded atherosclerotic development in mice by attenuating monocyte/macrophage invasion and reducing macrophage apoptosis.48 However, the observed beneficial effect of calpain inhibition on AngII-induced atherosclerosis and AAA formation, irrespective of MMP2 activation, suggests that calpain activation may play a pivotal role in MMP12 activation in pathogenesis of these AngII-induced vascular pathologies.
AngII-infusion causes vascular inflammation at an early stage by promoting medial macrophage accumulation.7 Here, our data demonstrate BDA-410 administration reduced macrophage accumulation in abdominal aneurysm tissue, and thereby reduced the incidence and severity of AAA formation. These results are consistent with calpain activation mediating the early stage of AngII-induced vascular inflammation by promoting macrophage migration and accumulation into aortic tissue. In agreement, transgenic mice overexpressing calpastatin showed marked reductions in monocyte/macrophage infiltration into aortic adventitia in response to AngII-infusion.27 Furthermore in our study, BDA-410 also significantly suppressed AngII-induced MCP-1, IL-6 and NF-kB-related IkappaB kinases (IKKα, β and ε) in aortic tissue. Calpastatin transgenic mice also showed a defect in MCP-1 secretion, that attributed as a mechanism of defective leukocyte recruitment.27 Calpain activation was also shown to promote NF-kB translocation to the nucleus by targeting inhibitor of NF-kB (IkB).19 BDA-410 administration suppressed AngII-induced IKKs expression which suggested that activated calpain promotes NF-kB translocation by degrading IkB through the activation of IKKs. In agreement, calpastatin transgenic mice also suppressed AngII-induced perivascular inflammation by reducing NF-kB activation.27 Thus, in our study, BDA-410 may attenuate AngII-induced AAA formation by suppressing the initial key event of macrophage infiltration into the aortic media, thereby reducing inflammation and elastase-mediated extracellular matrix degradation. BDA-410 administration also significantly attenuated AngII-induced atherosclerosis in the present study. Calpain inhibition had no effect on changes in plasma lipid concentrations which suggests that the inhibitory effect on macrophage migration and accumulation may be the key mechanism by which calpain inhibition mediates the attenuation of atherosclerosis development.
In conclusion, this study demonstrated for the first time that inhibition of calpain by a novel calpain inhibitor, BDA-410, significantly attenuated development of AngII-induced AAA and atherosclerosis in LDL receptor deficient mice. Inhibition of calpain might offer a new therapeutic target to prevent AAA formation. Further studies are warranted to determine the role of specific calpain isoforms in the development of these AngII-induced vascular pathologies which will require mice with genetic deficiency of specific calpain isoforms.
ACKNOWLEDGMENTS
We thank Dr. Alan Daugherty for his helpful suggestions and discussions of this project. We acknowledge Debra Rateri for technical and editorial assistance, and Mitsubishi Tanabe Pharma Corporation, Osaka, Japan for providing BDA-410.
Support and Disclosure - The study was supported by the National Institute of Health (HL80100) and an AHA Great Rivers Affiliate Postdoctoral Fellowship (0825592D).
Footnotes
- Bo Liu, University of Wisconsin-Madison, liub@surgery.wisc.edu
- 2. Masanori Aikawa, Brigham and Women's Hospital, Harvard Medical School, maikawa@rics.bwh.harvard.edu
- Neal L. Weintraub, University of Cincinnati, neal.weintraub@uc.edu
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Alcorn HG, Wolfson SK, Jr, Sutton-Tyrrell K, Kuller LH, O'Leary D. Risk factors for abdominal aortic aneurysms in older adults enrolled in The Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 1996;16:963–970. doi: 10.1161/01.atv.16.8.963. [DOI] [PubMed] [Google Scholar]
- 2.Sakalihasan N, Limet R, Defawe OD. Abdominal aortic aneurysm. Lancet. 2005;365:1577–1589. doi: 10.1016/S0140-6736(05)66459-8. [DOI] [PubMed] [Google Scholar]
- 3.Daugherty A, Cassis L. Chronic angiotensin II infusion promotes atherogenesis in low density lipoprotein receptor −/− mice. Ann NY Acad Sci. 1999;892:108–118. doi: 10.1111/j.1749-6632.1999.tb07789.x. [DOI] [PubMed] [Google Scholar]
- 4.Thompson RW, Geraghty PJ, Lee JK. Abdominal aortic aneurysms: basic mechanisms and clinical implications. Curr Probl Surg. 2002;39:110–230. doi: 10.1067/msg.2002.121421. [DOI] [PubMed] [Google Scholar]
- 5.Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, Podowski M, Neptune ER, Halushka MK, Bedja D, Gabrielson K, Rifkin DB, Carta L, Ramirez F, Huso DL, Dietz HC. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saraff K, Babamusta F, Cassis LA, Daugherty A. Aortic dissection precedes formation of aneurysms and atherosclerosis in angiotensin II-infused, apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:1621–1626. doi: 10.1161/01.ATV.0000085631.76095.64. [DOI] [PubMed] [Google Scholar]
- 8.Eagleton MJ, Ballard N, Lynch E, Srivastava SD, Upchurch GR, Jr, Stanley JC. Early increased MT1-MMP expression and late MMP-2 and MMP-9 activity during Angiotensin II induced aneurysm formation. J Surg Res. 2006;135:345–351. doi: 10.1016/j.jss.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 9.Manning MW, Cassis LA, Daugherty A. Differential effects of doxycycline, a broad-spectrum matrix metalloproteinase inhibitor, on angiotensin II-induced atherosclerosis and abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2003;23:483–488. doi: 10.1161/01.ATV.0000058404.92759.32. [DOI] [PubMed] [Google Scholar]
- 10.Miyazaki M, Takai S, Jin D, Muramatsu M. Pathological roles of angiotensin II produced by mast cell chymase and the effects of chymase inhibition in animal models. Pharmacol Ther. 2006;112:668–676. doi: 10.1016/j.pharmthera.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Sun J, Zhang J, Lindholt JS, Sukhova GK, Liu J, He A, Abrink M, Pejler G, Stevens RL, Thompson RW, Ennis TL, Gurish MF, Libby P, Shi GP. Critical role of mast cell chymase in mouse abdominal aortic aneurysm formation. Circulation. 2009;120:973–982. doi: 10.1161/CIRCULATIONAHA.109.849679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schulte S, Sun J, Libby P, Macfarlane L, Sun C, Lopez-Ilasaca M, Shi GP, Sukhova GK. Cystatin C deficiency promotes inflammation in angiotensin II-induced abdominal aortic aneurisms in atherosclerotic mice. Am J Pathol. 2010;177:456–463. doi: 10.2353/ajpath.2010.090381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Battaglia F, Trinchese F, Liu S, Walter S, Nixon RA, Arancio O. Calpain inhibitors, a treatment for Alzheimer's disease: position paper. J Mol Neurosci. 2003;20:357–62. doi: 10.1385/JMN:20:3:357. [DOI] [PubMed] [Google Scholar]
- 14.Saito K, Elce JS, Hamos JE, Nixon RA. Widespread activation of calcium-activated neutral proteinase (calpain) in the brain in Alzheimer disease: a potential molecular basis for neuronal degeneration. Proc Natl Acad Sci U S A. 1993;90:2628–2632. doi: 10.1073/pnas.90.7.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trinchese F, Fa' M, Liu S, Zhang H, Hidalgo A, Schmidt SD, Yamaguchi H, Yoshii N, Mathews PM, Nixon RA, Arancio O. Inhibition of calpains improves memory and synaptic transmission in a mouse model of Alzheimer disease. J Clin Invest. 2008;118:2796–2807. doi: 10.1172/JCI34254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsubakimoto Y, Yamada H, Yokoi H, Kishida S, Takata H, Kawahito H, Matsui A, Urao N, Nozawa Y, Hirai H, Imanishi J, Ashihara E, Maekawa T, Takahashi T, Okigaki M, Matsubara H. Bone marrow angiotensin AT1 receptor regulates differentiation of monocyte lineage progenitors from hematopoietic stem cells. Arterioscler Thromb Vasc Biol. 2009;29:1529–1536. doi: 10.1161/ATVBAHA.109.187732. [DOI] [PubMed] [Google Scholar]
- 17.Horikawa Y, Oda N, Cox NJ, Li X, Orho-Melander M, Hara M, Hinokio Y, Lindner TH, Mashima H, Schwarz PE, del Bosque-Plata L, Horikawa Y, Oda Y, Yoshiuchi I, Colilla S, Polonsky KS, Wei S, Concannon P, Iwasaki N, Schulze J, Baier LJ, Bogardus C, Groop L, Boerwinkle E, Hanis CL, Bell GI. Genetic variation in the gene encoding calpain-10 is associated with type 2 diabetes mellitus. Nat Genet. 2000;26:163–175. doi: 10.1038/79876. [DOI] [PubMed] [Google Scholar]
- 18.Ojima K, Kawabata Y, Nakao H, Nakao K, Doi N, Kitamura F, Ono Y, Hata S, Suzuki H, Kawahara H, Bogomolovas J, Witt C, Ottenheijm C, Labeit S, Granzier H, Toyama-Sorimachi N, Sorimachi M, Suzuki K, Maeda T, Abe K, Aiba A, Sorimachi H. Dynamic distribution of muscle-specific calpain in mice has a key role in physical-stress adaptation and is impaired in muscular dystrophy. J Clin Invest. 2010;120:2672–2683. doi: 10.1172/JCI40658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 20.Smolock AR, Mishra G, Eguchi K, Eguchi S, Scalia R. Protein kinase C upregulates intercellular adhesion molecule-1 and leukocyte-endothelium interactions in hyperglycemia via activation of endothelial expressed calpain. Arterioscler Thromb Vasc Biol. 2011:289–296. doi: 10.1161/ATVBAHA.110.217901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sato K, Minegishi S, Takano J, Plattner F, Saito T, Asada A, Kawahara H, Iwata N, Saido TC, Hisanaga S. Calpastatin, an endogenous calpain-inhibitor protein, regulates the cleavage of the Cdk5 activator p35 to p25. J Neurochem. 2011;117:504–515. doi: 10.1111/j.1471-4159.2011.07222.x. [DOI] [PubMed] [Google Scholar]
- 22.Carillo S, Pariat M, Steff AM, Roux P, Etienne-Julan M, Lorca T, Piechaczyk M. Differential sensitivity of FOS and JUN family members to calpains. Oncogene. 1994;9:1679–1689. [PubMed] [Google Scholar]
- 23.Lin YC, Brown K, Siebenlist U. Activation of NF-kappa B requires proteolysis of the inhibitor I kappa B-alpha: signal-induced phosphorylation of I kappa B-alpha alone does not release active NF-kappa B. Proc Natl Acad Sci U S A. 1995;92:552–556. doi: 10.1073/pnas.92.2.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bevers MB, Neumar RW. Mechanistic role of calpains in postischemic neurodegeneration. J Cereb Blood Flow Metab. 2008:655–673. doi: 10.1038/sj.jcbfm.9600595. [DOI] [PubMed] [Google Scholar]
- 25.Nakagawa T, Yuan J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J Cell Biol. 2000:887–894. doi: 10.1083/jcb.150.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDonald MC, Mota-Filipe H, Paul A, Cuzzocrea S, Abdelrahman M, Harwood S, Plevin R, Chatterjee PK, Yaqoob MM, Thiemermann C. Calpain inhibitor I reduces the activation of nuclear factor-kappaB and organ injury/dysfunction in hemorrhagic shock. FASEB J. 2001;15:171–186. doi: 10.1096/fj.99-0645com. [DOI] [PubMed] [Google Scholar]
- 27.Letavernier E, Perez J, Bellocq A, Mesnard L, de Castro Keller A, Haymann JP, Baud L. Targeting the calpain/calpastatin system as a new strategy to prevent cardiovascular remodeling in angiotensin II-induced hypertension. Circ Res. 2008;102:720–728. doi: 10.1161/CIRCRESAHA.107.160077. [DOI] [PubMed] [Google Scholar]
- 28.Jiang L, Wang M, Zhang J, Monticone RE, Telljohann R, Spinetti G, Pintus G, Lakatta EG. Increased aortic calpain-1 activity mediates age-associated angiotensin II signaling of vascular smooth muscle cells. PLoS ONE. 2008;3:e2231. doi: 10.1371/journal.pone.0002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X, Chen H, Jeong JJ, Chishti AH. BDA-410: a novel synthetic calpain inhibitor active against blood stage malaria. Mol Biochem Parasitol. 2007;155:26–32. doi: 10.1016/j.molbiopara.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daugherty A, Rateri D, Lu H, Balakrishnan A. Measuring blood pressure in mice using volume pressure recording, a tail-cuff method. J Vis Exp. 2009:1291. doi: 10.3791/1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daugherty A, Rateri DL, Lu H, Inagami T, Cassis LA. Hypercholesterolemia stimulates angiotensin peptide synthesis and contributes to atherosclerosis through the AT1A receptor. Circulation. 2004;110:3849–3857. doi: 10.1161/01.CIR.0000150540.54220.C4. [DOI] [PubMed] [Google Scholar]
- 32.Barisione C, Charnigo R, Howatt DA, Moorleghen JJ, Rateri DL, Daugherty A. Rapid dilation of the abdominal aorta during infusion of angiotensin II detected by noninvasive high-frequency ultrasonography. J Vasc Surg. 2006;44:372–376. doi: 10.1016/j.jvs.2006.04.047. [DOI] [PubMed] [Google Scholar]
- 33.Daugherty A, Whitman SC. Quantification of atherosclerosis in mice. Methods Mol Biol. 2003;209:293–309. doi: 10.1385/1-59259-340-2:293. [DOI] [PubMed] [Google Scholar]
- 34.Daugherty A, Rateri DL. Development of experimental designs for atherosclerosis studies in mice. Methods. 2005;36:129–138. doi: 10.1016/j.ymeth.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 35.Daugherty A, Cassis LA. Mouse models of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2004;24:429–434. doi: 10.1161/01.ATV.0000118013.72016.ea. [DOI] [PubMed] [Google Scholar]
- 36.Sendobry SM, Cornicelli JA, Welch K, Grusby MJ, Daugherty A. Absence of T lymphocyte-derived cytokines fails to diminish macrophage 12/15-lipoxygenase expression in vivo. J Immunol. 1998;161:1477–82. [PubMed] [Google Scholar]
- 37.Lu H, Boustany-Kari CM, Daugherty A, Cassis LA. Angiotensin II increases adipose angiotensinogen expression. Am J Physiol Endocrinol Metab. 2007;292:E1280–1287. doi: 10.1152/ajpendo.00277.2006. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, Ait-Oufella H, Herbin O, Bonnin P, Ramkhelawon B, Taleb S, Huang J, Offenstadt G, Combadiere C, Renia L, Johnson JL, Tharaux PL, Tedgui A, Mallat Z. TGF-beta activity protects against inflammatory aortic aneurysm progression and complications in angiotensin II-infused mice. J Clin Invest. 2010;120:422–432. doi: 10.1172/JCI38136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cassis LA, Gupte M, Thayer S, Zhang X, Charnigo R, Howatt DA, Rateri DL, Daugherty A. Angiotensin II infusion promotes abdominal aortic aneurysms independent of increased blood pressure in hypercholesterolemic mice. Am J Physiol Heart Circ Physiol. 2009;296:H1660–1665. doi: 10.1152/ajpheart.00028.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silence J, Lupu F, Collen D, Lijnen HR. Persistence of atherosclerotic plaque but reduced aneurysm formation in mice with stromelysin-1 (MMP-3) gene inactivation. Arterioscler Thromb Vasc Biol. 2001;21:1440–1445. doi: 10.1161/hq0901.097004. [DOI] [PubMed] [Google Scholar]
- 41.Lemaitre V, O'Byrne TK, Borczuk AC, Okada Y, Tall AR, D'Armiento J. ApoE knockout mice expressing human matrix metalloproteinase-1 in macrophages have less advanced atherosclerosis. J. Clin. Invest. 2001;107:1227–1234. doi: 10.1172/JCI9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laxton RC, Hu Y, Duchene J, Zhang F, Zhang Z, Leung KY, Xiao Q, Scotland RS, Hodgkinson CP, Smith K, Willeit J, Lopez-Otin C, Simpson IA, Kiechl S, Ahluwalia A, Xu Q, Ye S. A role of matrix metalloproteinase-8 in atherosclerosis. Circ Res. 2009;105:921–929. doi: 10.1161/CIRCRESAHA.109.200279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ouyang X, Le TH, Roncal C, Gersch C, Herrera-Acosta J, Rodriguez-Iturbe B, Coffman TM, Johnson RJ, Mu W. Th1 inflammatory response with altered expression of profibrotic and vasoactive mediators in AT1A and AT1B double-knockout mice. Am J Physiol Renal Physiol. 2005;289:F902–10. doi: 10.1152/ajprenal.00141.2005. [DOI] [PubMed] [Google Scholar]
- 44.Deguchi JO, Aikawa E, Libby P, Vachon JR, Inada M, Krane SM, Whittaker P, Aikawa M. Matrix metalloproteinase-13/collagenase-3 deletion promotes collagen accumulation and organization in mouse atherosclerotic plaques. Circulation. 2005:2708–2715. doi: 10.1161/CIRCULATIONAHA.105.562041. [DOI] [PubMed] [Google Scholar]
- 45.Schneider F, Sukhova GK, Aikawa M, Canner J, Gerdes N, Tang SM, Shi GP, Apte SS, Libby P. Matrix metalloproteinase-14 deficiency in bone marrow-derived cells promotes collagen accumulation in mouse atherosclerotic plaques. Circulation. 2008;117:931–939. doi: 10.1161/CIRCULATIONAHA.107.707448. [DOI] [PubMed] [Google Scholar]
- 46.Longo GM, Buda SJ, Fiotta N, Xiong W, Griener T, Shapiro S, Baxter BT. MMP-12 has a role in abdominal aortic aneurysms in mice. Surgery. 2005;137:457–462. doi: 10.1016/j.surg.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 47.Pyo R, Lee JK, Shipley JM, Curci JA, Mao D, Ziporin SJ, Ennis TL, Shapiro SD, Senior RM, Thompson RW. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J Clin Invest. 2000;105:1641–1649. doi: 10.1172/JCI8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnson JL, Devel L, Czarny B, George SJ, Jackson CL, Rogakos V, Beau F, Yiotakis A, Newby AC, Dive V. A selective matrix metalloproteinase-12 inhibitor retards atherosclerotic plaque development in apolipoprotein E-knockout mice. Arterioscler Thromb Vasc Biol. 2011;31:528–535. doi: 10.1161/ATVBAHA.110.219147. [DOI] [PMC free article] [PubMed] [Google Scholar]