Abstract
AMPK is an important sensor of cellular energy levels.
Objective
The aim of these studies was to investigate whether cardiac KATP channels, which couple cellular energy metabolism to membrane excitability, are regulated by AMPK activity.
Research Design and Methods
We investigated effects of AMPK on rat ventricular KATP channels using electrophysiological and biochemical approaches
Results
Whole-cell KATP channel current was activated by metabolic inhibition; this occurred more rapidly in the presence of AICAR (an AMPK activator). AICAR had no effects on KATP channel activity recorded in the inside-out patch clamp configuration, but ZMP (the intracellular intermediate of AICAR) strongly activated KATP channels. An AMPK-mediated effect is demonstrated by the finding that ZMP had no effect on KATP channels in the presence of Compound C (an AMPK inhibitor). Recombinant AMPK activated Kir6.2/SUR2A channels in a manner that was dependent on the AMP concentration, whereas heat-inactivated AMPK was without effect. Using mass-spectrometry and co-immunoprecipitation approaches, we demonstrate that the AMPK α-subunit physically associates with KATP channel subunits.
Conclusions
Our data demonstrate that the cardiac KATP channel function is directly regulated by AMPK activation. During metabolic stress, a small change in cellular AMP that activates AMPK can be a potential trigger for KATP channel opening.
Keywords: ATP-sensitive K+ channels, AMP-activated protein kinase, Potassium channels
INTRODUCTION
ATP-sensitive K+ (KATP) channels are found in most tissue beds, including the heart, skeletal and smooth muscle, brain, kidney and pancreatic β-cells [1]. Although the various subtypes of KATP channels differ from each other in terms of their gating properties and pharmacological sensitivities, a common property is their regulation by intracellular nucleotides. The cellular ATP:ADP ratio is considered to be the prime regulator of KATP channels in cardiac muscle and the pancreatic β-cell [2]. As such, KATP channels function to couple intracellular metabolic events to membrane excitability and cellular effector responses. The cloning of the molecular subunits of KATP channels have revealed them to be hetero-octameric complexes, consisting of four pore-forming inward rectifier subunits (Kir6.1 or Kir6.2) and four regulatory subunits (SUR1 or SUR2) [2]. Since the discovery of KATP channels over two decades ago, much work has gone into the delineation of mechanisms that regulate their activity, including their modulation by nucleotides, cellular metabolic events, pharmacological agents and regulation by signaling pathways. Despite these advances, the triggers for KATP channel opening during myocardial ischemia and metabolic impairment are not fully understood.
AMP-activated protein kinase (AMPK) represents the mammalian form of the core component of a kinase cascade that is conserved between fungi, plants, and animals [3]. When activated, AMPK switches off ATP consuming pathways (e.g. biosynthetic pathways) while switching on a variety of pathways to enhance ATP production and cell survival. The latter includes increased β-oxidation of free fatty acids, increased formation of creatine phosphate, and enhanced membrane glucose transport by membrane translocation of GLUT-4 [3]. AMPK is a heterotrimeric protein composed of a catalytic α-subunit and regulatory β and γ subunits, which are important for protein stability and substrate specificity. Each subunit has two or more different isoforms [3]. The α1 subunit is widely expressed, while the α2 subunit is predominantly found in liver, heart and skeletal muscle [4]. AMPK is activated through Thr172 phosphorylation by one or more upstream kinases (AMPKK) and allosterically by increases in the ATP:AMP and creatine:phosphocreatine ratios [3, 5]. AMPK has previously been proposed to regulate KATP channels trafficking in a cellular model of hypoxia-induced “ischemic preconditioning” [6], but there are no reports that AMPK directly affects cardiac KATP channel function. Our data demonstrate that rat ventricular KATP channel activity is regulated by AMPK, and that this kinase therefore directly connects alterations in cellular energy metabolism (i.e. the ATP:AMP ratio) with the cardiac KATP channel function. Furthermore, the AMPK α-subunit associates with KATP channel subunits, suggesting that AMPK may be a local signalling component of the KATP channel macromolecular complex.
METHODS
Preparation of single ventricular myocytes
Ventricular myocytes were enzymatically isolated from male Sprague-Dawley rats (~200 g). Hearts were rapidly excised after pentobarbital overdose (60 mg/kg), rinsed with ice-cold Tyrode’s solution (in mM: NaCl 137, KCl 5.4, HEPES 10, MgCl2 1, NaH2PO4 0.33, CaCl2 1.8; glucose 10; pH 7.4), cannulated and retrogradedly perfused with oxygenated Tyrode’s solution for 3–5 min at 37°C. The perfusate was switched to nominally Ca2+-free Tyrode’s buffer for 5 min, followed by perfusion for 11–13 min with the same solution containing collagenase (type I, 3 mg/mL; Sigma) and protease (type XIV, 0.44 U/mL; Sigma). The enzyme was washed out by 5 min perfusion with KB solution (in mM: taurine 20, L-glutamic acid 50, HEPES 10, EGTA 0.5, MgSO4 3, KH2PO4 30, KCl 30, KOH 78; pH 7.2 adjusted with KOH). The heart was removed from the cannula, and cells were isolated by gentle titration, filtered (150 µm mesh) and kept in KB buffer for at least 30 min at room temperature before experimentation. All data were collected within 8 hours of cell isolation.
Whole-cell patch clamping
Whole-cell recordings were performed as previously described [7]. Pipettes (3–4 MΩ) were filled with (in mM): L-Aspartic acid (K+ salt) 115, KCl 20, EGTA 5, HEPES 10, Na2ATP 5, MgCl2 1, pH 7.2. Cardiomyocytes were superfused with Tyrode’s solution. The following compounds were added to Tyrode’s solution as needed: NaCN, 2-deoxy-glucose, 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR) and/or 8-p-sulfophenyl-theophyline (all from Sigma). Experiments were conducted at 37°C.
Excised patch recordings
KATP channels were recorded in excised patches using the inside-out configuration as described [8]. Pipettes (2–4 MΩ) were filled with (in mM): KCl, 140; MgCl2, 1; HEPES, 10; EGTA, 1; CaCl2, 2; pH 7.4 adjusted with KOH. The bath solution contained (in mM) KCl, 140; EGTA, 1; HEPES, 10; MgCl2, 1.2; pH 7.2 adjusted with KOH. Experiments were performed at room temperature at a holding potential of −60 or −80 mV. Solution changes were performed using a multi-barrel rapid solution changer (RSC-200; Molecular kinetics, Inc., IN). Recombinant AMPK (Upstate Biotechnology, Lake Placid, NY) was used at a final concentration of 1 U/mL.
Solutions containing ATP, AMP or ZMP were freshly prepared from frozen stocks (100 mM). Compound C (10 µM) was prepared from a 10 mM stock solution made in DMSO. Channel open probability (N.Po) was calculated using all points histograms (using 15s of continuous recording) or by dividing the mean patch current by the unitary current amplitude.
Heterologous Expression of KATP channel subunits
For electrophysiological studies, COS7L cells (Invitrogen, Carlsbad CA) were transfected (Fugene 6, Roche Applied Science) with mouse Kir6.2 and SUR2A cDNA (a gift from Dr. S. Seino, Kobe University) as well as a GFP reporter vector. For biochemical experiments, we used Kir6.2-HA cDNA, which has four C-terminal HA epitopes [9].
Antibodies
Rabbit polyclonal anti-Kir6.2 antibodies (W62 or W62b) were raised against the N terminal region of human Kir6.2 [10]. We also used goat anti-Kir6.2 (G-16; Santa Cruz, CA) and anti-SUR2A antibodies (M-19; Santa Cruz, CA), rabbit polyclonal anti-AMPKα (U; α-pan; Upstate Biotechnology, Billerica, MA), rabbit polyclonal anti-AMPKα (C; α-pan; Cell Signaling Technology, Beverly, MA), mouse monoclonal anti-GFP antibodies (BD Biosciences, San Jose, CA), mouse monoclonal anti-HA (12CA5) and rat monoclonal anti-HA-peroxidase (3F10; both from Roche Applied Science, IN).
Immunoprecipitation
Forty-eight hour post-transfection, each 100 mm dish was lysed in ice-cold immunoprecipitation lysis (IP) buffer containing 25 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA, 1 % Triton X-100, plus protease inhibitors [1 mM phenylmethanesulfonyl fluoride and 10 µl/ml protease inhibitor cocktail (Sigma-Aldrich)]. Immunoprecipitation was performed as described [10]. Briefly, anti-Kir6.2 (W62), anti-Kir6.2 (G-16), anti-SUR2A (M-19), anti-AMPKα (U), anti-AMPKα (C), anti-HA, or anti-GFP antibodies were added to 400µg of the cell lysates and incubated overnight at 4°C. Protein G beads (25 µl; Amersham Biosciences, NJ) was added and incubated at 4°C with rotation for 3 hours. As negative controls we used unrelated antibodies (e.g. anti-GFP). The immunoprecipitates were washed (4x) with the IP buffer, modified to contain 0.1% Triton X-100. Washed beads were resuspended in 40 µl of 2× Laemmli sample buffer.
For native immunoprecipitations, enzymatically isolated ventricular myocytes from rat heart were lysed in ice cold buffer containing 250 mM sucrose, 10 mM HEPES, 1 mM EDTA, 1 mM DTT, pH 7.4, supplemented with protease inhibitors (Roche Complete cocktail). Membranes were prepared essentially as described [11]. Anti-Kir6.2 (W62b) antibody or unrelated IgG (as control) were bound to protein A/G beads (Invitrogen). Membrane proteins (120µg) were added to the antibody-bead complexes and incubated overnight at 4°C with rotation. Beads were washed, proteins were eluted and subjected to SDS-PAGE using AMPK antibodies.
Western blotting
Samples were fractionated by 12% SDS-PAGE, transferred to PVDF membranes (Bio-Rad) and incubated overnight at 4°C in blocking solution, consisting of 5% non-fat milk in TN-buffer (100 mM Tris-HCl, pH 7.5, 150 mM NaCl containing 0.1% Tween-20). Membranes were incubated (1 h) with primary antibodies at room temperature, washed with TN-buffer (3 times), and incubated (1 h) at room temperature in peroxidase-linked donkey anti-rabbit IgG (Amersham Biosciences) or monoclonal anti-goat IgG-peroxidase (Sigma-Aldrich) in TN-buffer. For HA tagged proteins, we used anti-HA peroxidase (1:800 dilution). Detection was with chemiluminesence (Supersignal West Pico, Pierce Biotechnology, Rockford, IL). A minimum of three independent experiments were performed.
RESULTS
We recently characterized the cardiac KATP channel macromolecular complex using proteomic approaches and found the enzymes of the glycolytic pathway to be important associated proteins [8]. These mass spectrometry experiments also identified 5’-AMP-activated protein kinase (AMPK) catalytic α subunit in an immunoprecipitate obtained with an anti-Kir6.2 antibody (data not shown). Experiments were therefore performed to investigate the functional relevance of this observation.
AMPK activation predisposes to KATP channel opening during metabolic stress
We used the membrane-permeable 5-aminoimidazole-4-carboxyamide (AICAR) to activate AMPK [12] in initial experiments with intact cells. After being taken up into cells by a nucleoside transporter, AICAR is phosphorylated to the monophosphate form, 5-amino-4-imidazolecarboxamide (also known as AICA ribotide or ZMP), which is a normal intermediate in the purine nucleotide synthesis pathway. ZMP accumulates inside the cell because of the slow rate of purine metabolism and, as an AMP analog, activates AMPK signalling [13–14].
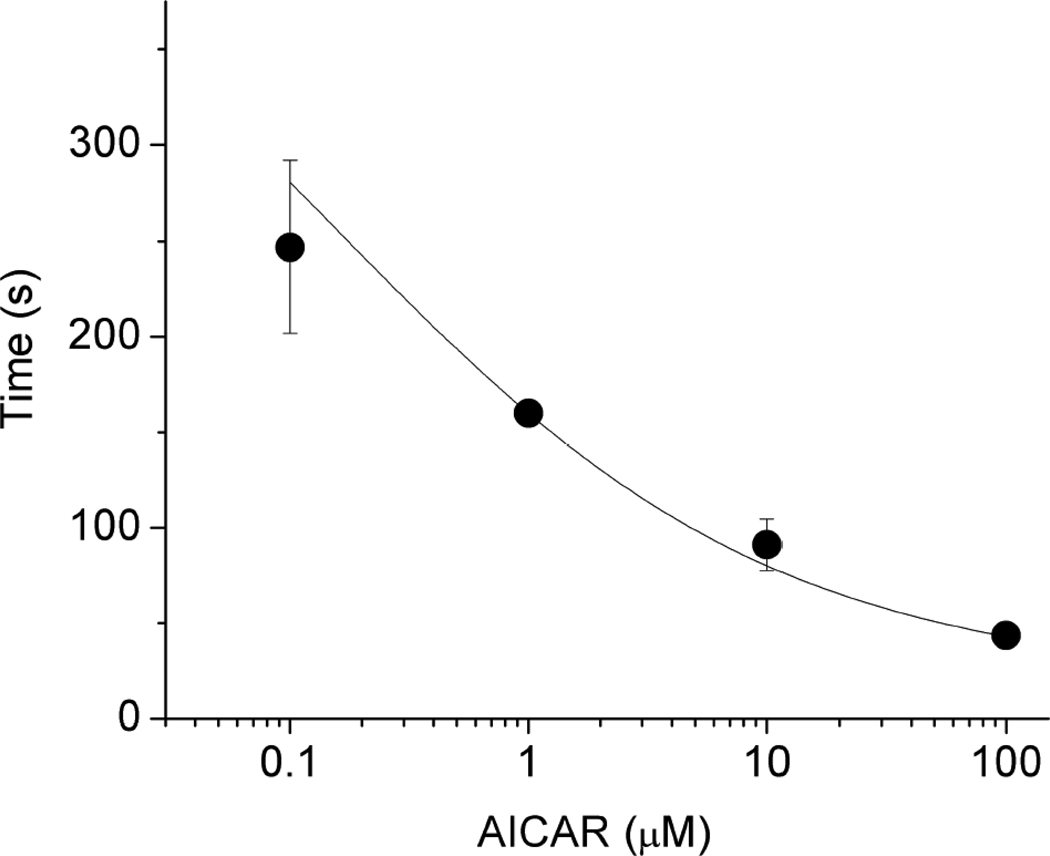
Isolated rat ventricular myocytes were subjected to whole-cell voltage clamping and KATP channel opening was induced by metabolic inhibition with 2-deoxyglucose plus cyanide (Fig. 1). The time to half-maximal activation was 76±9.17s (n=5). Another group of cells were pre-treated for 10 minutes with 100 µM AICAR before the onset of the experiment. Upon metabolic inhibition the KATP channel current activated more rapidly in the AICAR-treated cells, with maximal activation occurring at 24±3.42 s (p<0.05; n=9). The time required for the KATP channel to activate during metabolic inhibition dose-dependently decreased with increasing AICAR concentrations (Fig. 2).
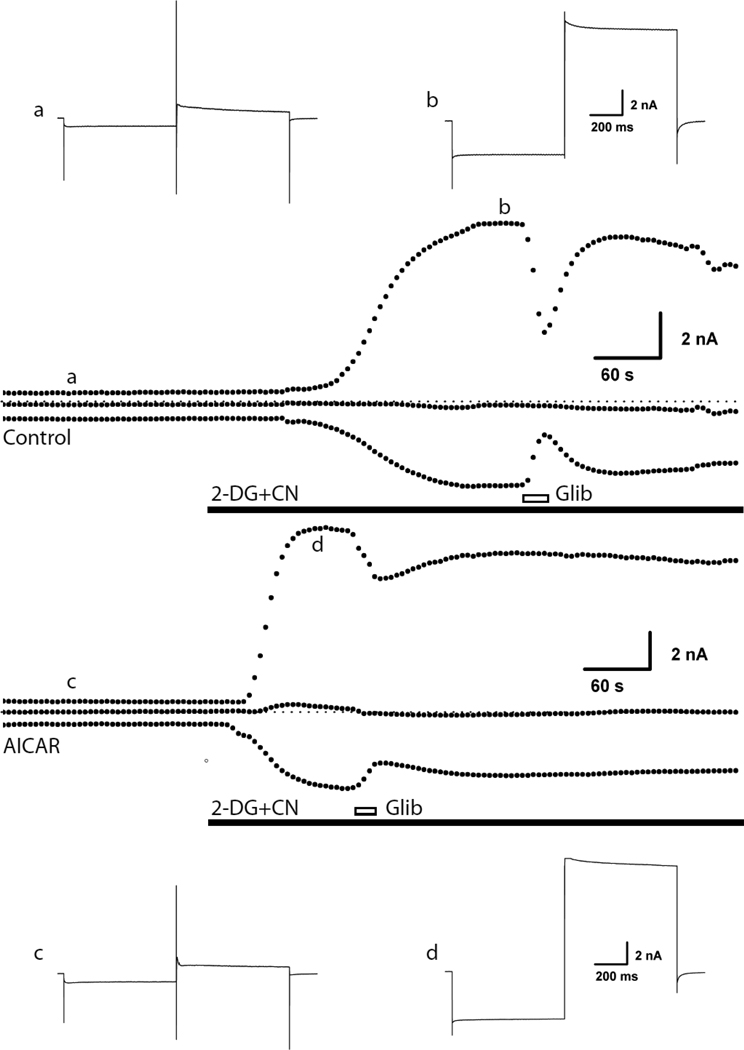
Fig 1.
Pretreatment of rat ventricular myocytes with AICAR shortened the time required for KATP channels to be activated by metabolic inhibition. Ventricular myocytes were voltage-clamped in whole-cell mode. From a holding potential of −70 mV, a dual voltage step was applied repeatedly (every 5s), consisting of a 700 ms hyperpolarization step to −100 followed by a 700 ms depolarization step to 0 mV. Two examples of currents recorded are shown at a collapsed time scale and each point represents a voltage clamp episode. For each recording, the top points are current at 0 mV, the middle are the holding current and the bottom points represent the current at −100 mV. The dotted lines represent the zero current level. The insets depict currents of individual voltage clamp episodes recorded at the points indicated. KATP channel current was activated with metabolic inhibition (2-deoxyglycose plus cyanide; 2-DG+CN) and blocked by glibenclamide (10µM; Glib). One of the cells was pre-incubated with AICAR (100 µM) for 10 min, whereas the control cell was perfused with Tyrode’s solution for an identical time. The adenosine receptor antagonist [8 (p-sulfophenyl) theophyline); 100µM] was included in the bath solution since AICAR may be a weak adenosine receptor agonist. Consistent with a previous report of incomplete sulfonylurea block of KATP channels during metabolic impairment [56], the current was only partially blocked by glibenclamide.
Fig 2.
AMPK dose-dependently shortened the time to maximal activation of KATP channels by metabolic inhibition. Cells (n=5–9) were incubated with various concentrations of AICAR before metabolic inhibition (as in the previous figure). The time to maximal activation of KATP channels is plotted as a function of the AMPK concentration. The line was drawn by fitting data points to a Boltzman distribution.
To exclude the possibility that AICAR directly activated KATP channels (instead of acting though ZMP formation), we examined its effect in the inside-out patch clamp configuration. Robust KATP channel activity was observed following patch excision with typical dose-dependent inhibition by ATP (Fig. 3). AICAR (1 mM) was applied in the presence of an ATP concentration (100 M) that produces sub-maximal channel inhibition, which allows us to observe either stimulatory or inhibitory effects. However, AICAR had no effect on the mean patch current (Fig. 3A), demonstrating that neither the ATP-sensitivity nor the channel open probability was affected. This result is supportive of a secondary action of AICAR on KATP currents, possibly mediated by intracellular formation of the ZMP metabolite.
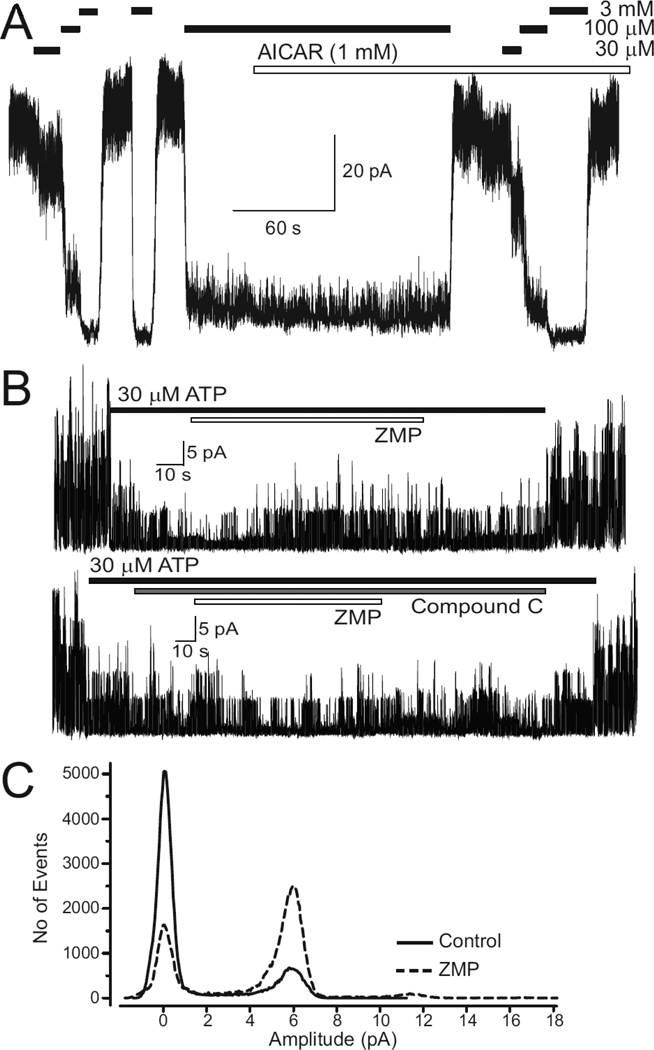
Fig 3.
ZMP (the AICAR metabolite) activates rat ventricular KATP channels without an effect on the unitary conductance. A, Inside-out recordings demonstrating that KATP channels are dose-dependently blocked by cytosolic ATP. There was no effect of 1 mM AICAR when applied in the presence of 100 µM ATP. Similar results were observed in 4 other patches. B, Upper panel: KATP channel activity was partially blocked by 30 µM ATP; subsequent addition of ZMP (100 µM) led to a progressive and reversible increase in KATP channel activity. Lower panel: pre-application of Compound C (10 µM) prevented the ZMP-induced activation of KATP channel activity. C, All-points histograms of KATP channel activity before (control) and after application of ZMP (100 µM) demonstrates that there was no effect on the unitary current amplitude (~5.9pA in this patch). The patch potential was −80mV. The result depicted is representative of 10 similar recordings.
We next investigated the effects of ,intracellular’ monophosphate nucleosides (AMP and ZMP) on KATP channel activity. Experiments were performed as described above (i.e. nucleotides were applied in the presence of ATP at a concentration that produces sub-maximal block). First, we examined effects of AMP. We consistently saw that KATP channel activity recorded in inside-out patches of rat ventricular myocytes was significantly increased by AMP (100 M; data not shown; see also [15–7]). This activation may either be the result of a phosphotransfer reaction mediated by adenylate kinase [17] and/or AMP-induced activation of AMPK. We therefore used ZMP (the AICAR metabolite), which is not an adenylate kinase substrate [18–19]. ZMP (100 M) led to a significant activation of KATP channel activity within ~40s (Fig. 3B). This effect was reversible upon washout. Overall, ZMP increased channel open probability (N.Po) from 2.3±0.61 to 4.8±1.68 (p<0.01; n=10; paired t-test). An AMPK-mediated effect is demonstrated by the finding that ZMP was without effect on KATP channel activity in the presence of the specific AMPK inhibitor, Compound C (10 M; Fig 3C; the N.Po respectively was 3.1±0.93 and 3.4±1.06; n=10; p>0.05 before and after application of ZMP). Compound C itself was without effect on KATP channel activity (Fig 3; also see Supplemental Figure S1). This result is consistent with our whole-cell experiments, demonstrating that AMPK regulates KATP channel function.
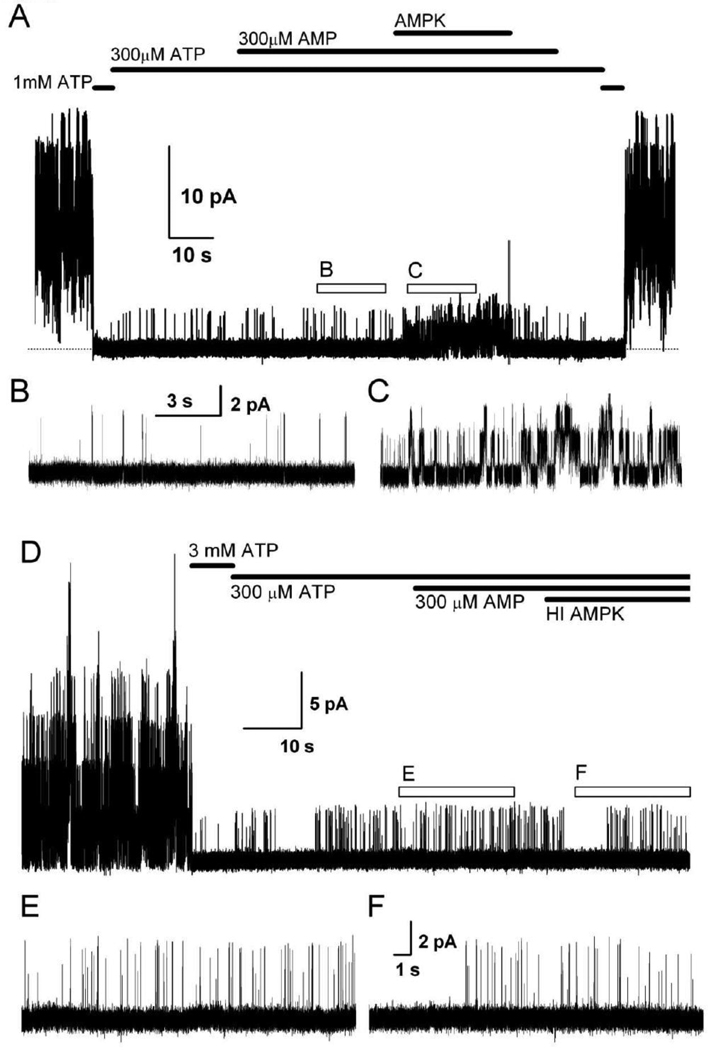
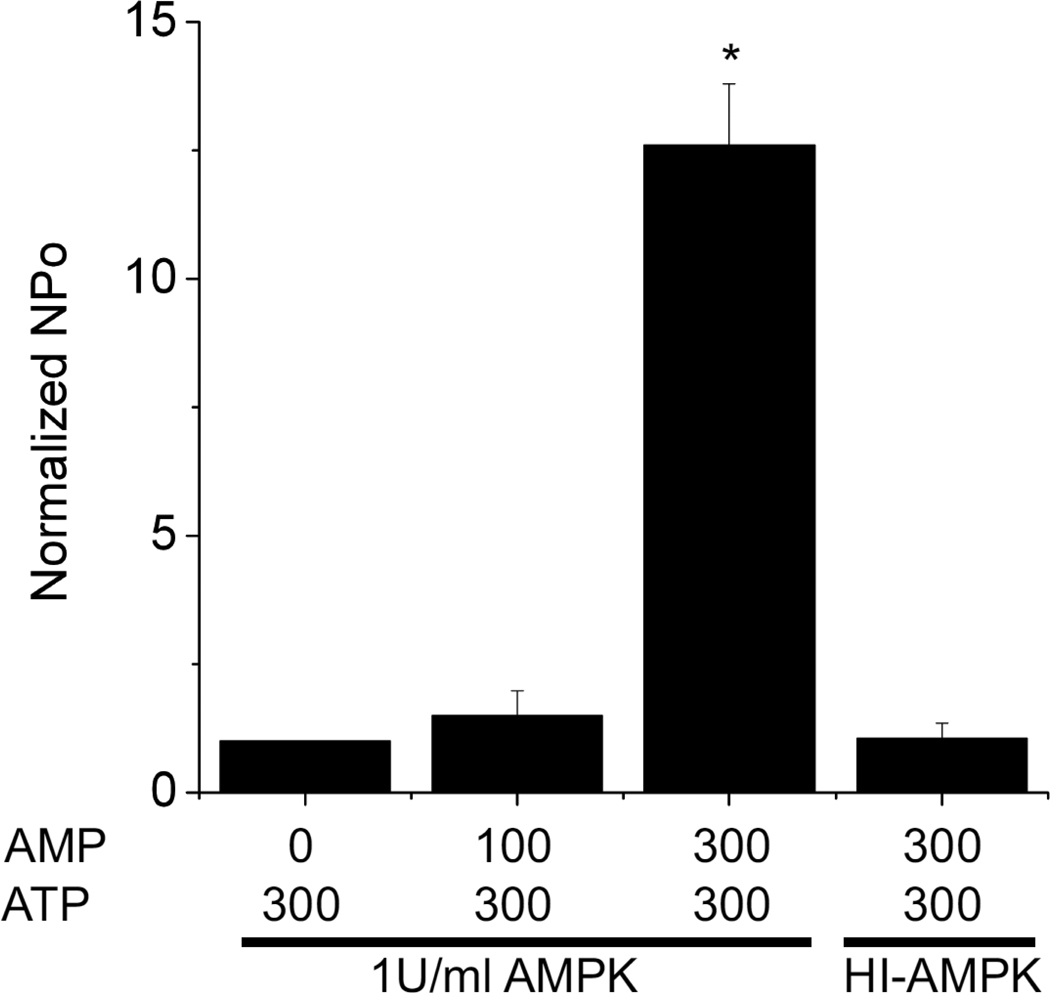
To further investigate the effect of this kinase, we used recombinantly purified AMPK. Experiments were performed using COS-7 cells transfected with mouse Kir6.2 and rat SUR2A cDNAs and KATP channels were recorded in the inside-out patch configuration. In the absence of “cytosolic” ATP, high levels of channel activity occurred, which were completely blocked by ATP (Fig. 4), characteristic of Kir6.2/SUR2A channels. Channels were then partially blocked by application of 300 µM ATP. In most patches, further application of AMP (100–300 µM) had little effect on channel activity. This is in contrast to patches obtained from cardiac myocytes, where AMP stimulates KATP channel activity under similar conditions [our observations; also see [16]]. The reasons for the different AMP response between KATP channels in heterologous expression systems and ventricular myocytes are not obvious, but may involve differences in the regulation by AMP-activated processes in the two cell types. To investigate the direct effect of AMPK on Kir6.2/SUR2A channels, we applied purified AMPK protein (Upstate Biotechnology) together with AMP to the cytosolic face of the same patch. A significant stimulation of KATP channel activity was observed (Fig. 4). The degree of stimulation of Kir6.2/SUR2A channels by AMPK was dependent on the AMP concentration. With 100 µM AMP, recombinant AMPK stimulated KATP channel mean patch current only mildly (by about 20%; Fig. 5), whereas AMPK enhanced the mean patch current several fold in the presence of 300 µM AMP (Figs. 4 and 5). The unusual AMP dose-response might reflect the possibility that AMPK becomes dephosphorylated in our in-vitro system [20]. Heat-inactivated AMPK did not stimulate KATP channel activity (Figs. 4 and 5).
Fig 4.
The effects of AMPK on recombinant KATP (Kir6.2/SUR2A) channels. A, Representative recordings of Kir6.2/SUR2A currents from excised inside-out membrane patches from transiently transfected COS7L cells. Substances were applied as indicated. The functional expression of KATP channels was confirmed by the presence of large currents that were inhibited by 1 mM ATP. In the presence of 300 µM ATP and 300 µM AMP, recombinant AMPK (1U/ml), applied to the cytosolic face of the membrane, significantly increased channel activity. The holding potential was −60mV. B. and C. are expanded current sections from the regions in the upper panel as marked by the open bars. D. Similar experiment as in the upper panel, but here we used heat-inactivated AMPK (HI AMPK). E, F: Expanded sections of the recording depicted in panel D, as indicated.
Fig 5.
The effect of AMPK is dependent on the AMP concentration. The bar graph depicts KATP channel current recorded at −60 mV in the presence of 300 µM ATP and 1U/ml AMPK. In control, no AMP was present. Increasing the AMP concentration dose-dependently increased KATP channel activity. The right-most bar represents recordings made with 300 µM AMP and heat-inactivated AMPK. In all cases, NPo was normalized to recordings made in the presence of 300 µM ATP and absence of AMP.
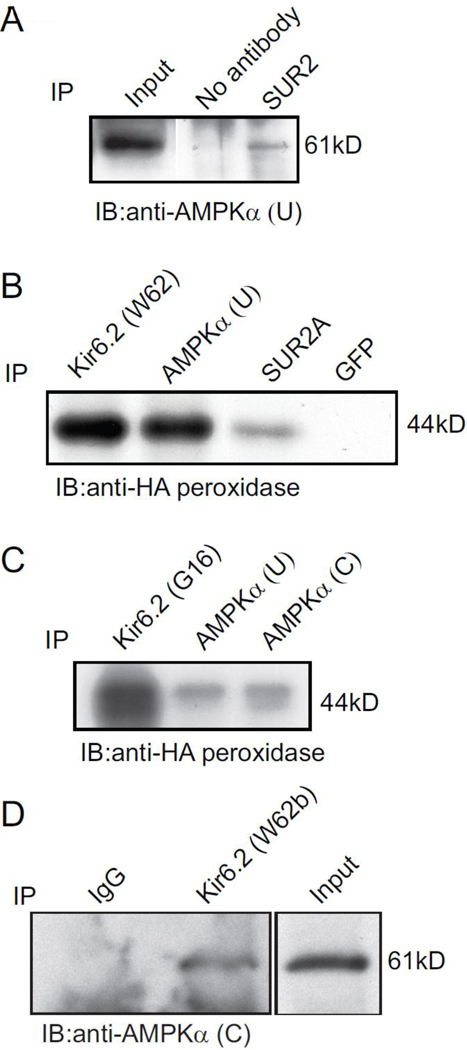
To determine whether AMPK and KATP channel subunits form protein complexes in mammalian cells, COS7L cells were transiently transfected with Kir6.2-HA and SUR2A cDNAs. Whole cell extracts were immunoprecipitated with two separate anti-AMPK antibodies, with anti-Kir6.2 or anti-SUR2A antibodies (anti-GFP antibodies were used as a negative control). The immunoprecipitates were assayed in immunoblotting using a peroxidase-conjugated anti-HA antibody and AMPK antibody (Fig. 6). As expected, Kir6.2-HA subunits were detected as a ~44 kDa band in the positive controls, but not in the negative control (Fig 6B). Kir6.2-HA was detected in the immunoprecipitate obtained with two separate antibodies against the AMPK α-subunit (Fig 6 B and C). Reciprocally, AMPK α-subunits are detected in immunoprecipitates containing SUR2 subunits. These data demonstrate that AMPK α-subunits physically interact with KATP channel subunits.
Fig 6.
Co-immunoprecipitation of AMPK α-subunits and KATP channel subunits. Co-immunoprecipitation of cell lysates from cells transfected with Kir6.2-HA and SUR2A cDNAs and rat ventricular tissue. Detection of AMPKα in the immunoprecipitate obtained with anti-SUR2A antibodies. B, Kir6.2-HA protein was detected in the immunoprecipitate obtained with the anti-Kir6.2 (W62), anti-AMPKα (U) and anti-SUR2A antibodies, but not with anti-GFP antibodies that were used as a negative control. C, Detection of Kir6.2-HA protein in immunoprecipitates obtained with a different anti-Kir6.2 (G16) antibody, as well as the immunoprecipitate obtained with two separate anti-AMPKα antibodies. D, Detection of AMPKα subunits in immunoprecipitates obtained with an anti-Kir6.2 (W62b) antibody. An unrelated antibody (rabbit IgG) was used as a negative control. The results shown are representative of 2–3 separate experiments, which showed similar results.
To investigate the physiological relevance of this interaction, the co-immunoprecipitation experiment was repeated using membranes prepared from enzymatically isolated rat ventricular myocytes. Immunoprecipitation was performed using an anti-Kir6.2 antibody (or unrelated IgG as a control). Immunoblotting of the immunoprecipitates confirmed detection of AMPK α-subunits in the experimental (but not negative control) lane (Fig 6D). These data confirm the mass-spectrometry data (see earlier) and demonstrate that AMPK and KATP channel subunits physically interact.
DISCUSSION
Physiological actions of AMPK and its effect on KATP channels
Biochemically, AMPK has been identified over 2 decades ago as an ultra-sensitive sensor of cellular stress. Even small rises in cellular AMP levels activate AMPK, which results in ATP consumption pathways to be inhibited and ATP synthesis pathways to be activated [3]. The activity of AMPK is increased during muscle contraction, hypoxia, ischemia, heat shock, a decrease in pH, inhibition of glycolysis and by uncouplers of oxidative phosphorylation [21–22]. In addition to acting as a fuel gauge under conditions of stress, AMPK has been assigned other roles, including stimulation of glucose-stimulated insulin secretion from pancreatic islets [23], stimulation of vascular endothelial growth factor (VGEF) expression and angiogenesis in skeletal muscle [24] and stimulation of nitric-oxide synthesis in human aortic endothelial cells [25]. Some ion channels have also been described to be targets of AMPK. Epithelial sodium channels, for example, are inhibited by AMPK [26] and AMPK plays a physiological role in modulating CFTR activity [27–29]. Until now, however, there have been few reports of AMPK affecting the expression or function of cardiac KATP channels. An inhibitory role for AMPK on KATP channel activity has been suggested by the finding that AICAR stimulates insulin release from pancreatic β-cells [23]. Furthermore, anti-diabetic drugs (such as rosiglitazone and phenformin) also activate AMPK, and stimulate insulin release [30–31]. The picture is clouded by the observation that some of these anti-diabetic drugs have direct inhibitory effects on the pancreatic β-cell subtype of the KATP channel independent of AMPK activation [32] and that AMPK stimulation actually increases β-cell KATP channel activity, in part by enhanced surface trafficking [33]. Effects of AMPK on the cardiac ventricular KATP channel are less well documented. One report demonstrated that whole-cell KATP channel density in isolated mouse ventricular myocytes is increased by repeated hypoxic episodes, and that this increase does not occur in myocytes isolated from a transgenic mouse with cardiac-specific expression of a dominant negative AMPK α2 subunit [6]. The same study demonstrated that repeated hypoxia leads to elevated KATP channel subunit levels in sarcolemmal membrane fractions and that this increase does not occur in the transgenic mouse hearts. These studies support the concept that hypoxia-induced activation of AMPK leads to increased surface expression of KATP channel subunits, but do not address the question whether KATP channels are direct targets of the AMPK signalling cascade. Our data demonstrate that AMPK does have a direct activating role in the rat ventricular KATP channel function. In whole-cell patch clamp conditions, we found that the KATP channel current was activated more readily during metabolic inhibition in the presence of AMPK activation by AICAR. This compound had no effect on KATP channels in inside-out membrane patches, whereas ZMP (the downstream metabolite of AICAR) activated KATP channels. Moreover, the stimulatory effect of ZMP was prevented by the AMPK inhibitor, Compound C. Heterologously expressed Kir6.2/SUR2A channels were activated by recombinant AMPK in an AMP-dependent manner, but not by heat-inactivated AMPK. Our co-immunoprecipitation data demonstrate that the AMPK α-subunit associates with KATP channel subunits, which raises the possibility of localized signalling of the KATP channel subunits and/or proteins with which they associate.
Kir6 and SUR K+ channel subunits as possible substrates for AMP-activated protein kinase
AMPK is a serine /threonine kinase. In a recent study, the Kir6.2 subunit of the pancreatic β-cell KATP channel was shown to be phosphorylated at Ser-385 [31], which does not conform to a typical AMPK consensus sequence [34]. It is likely that this Ser residue is also AMPK phosphorylated in the cardiac KATP channel, which is thought to be composed of a Kir6.2/SUR2A subunit combination [35]. It is interesting that the SUR2A subunit also contains several consensus sequences for AMPK phosphorylation, including Ser-401 (located within the intracellular linker between TM7 and TM8), Ser-1468 and Ser-1508 (both contained within the intracellular distal C-terminus). Our ongoing experiments are directed to map the KATP channel subunit phosphorylation sites and whether KATP channel associated subunits are also substrates for AMPK.
Dual regulation of KATP channels by AMP
AMP inhibits KATP channels at high millimolar concentrations [36]. This inhibition is likely to be due to interaction of AMP with the Kir6.2 ATP-binding site and is different from the stimulation of KATP channel activity observed at low AMP concentrations [15–17]. An interesting possibility was recently reported to explain the mechanism by which AMP activates KATP channels. Since AMP was found to stimulate KATP channel activity only in the presence of Mg2+ and a hydrolysable analog of ATP [16], a kinase-mediated phosphorylation event was suggested. Further, P1,P5-di-adenosine-5’-pentaphosphate (Ap5A), which blocks adenylate kinase activity (albeit rather non-specifically [37]), prevented AMP-dependent stimulation of KATP channel activity. This led to the suggestion that AMP effects occur through the action of phosphotransfer reactions that are mediated in part by adenylate kinase [16]. This represents an important manner by which metabolism can be coupled to KATP channel activity. Our data demonstrate that AMP might have dual regulatory role in that it may additionally activate KATP channels by acting through AMPK.
Role of AMPK in ischemia – effects on KATP channels?
The concept that AMP at low micromolar levels may provoke KATP channel opening during ischemia is interesting and important, since AMP levels are almost undetectably low in normal, well-oxygenated heart tissue (due to the action of adenylate kinase). However, AMP levels rise rapidly early during ischemia [38–39]. In dog heart, for example, the ATP:AMP ratio decreases from 78 to 46 within just 1 minute of regional ischemia [38] – a change mediated predominantly by changes in the AMP levels. Since the ATP:AMP ratio is approximately the square of the ATP:ADP ratio [40], it is a far more sensitive indicator of alterations in the cellular metabolic status. An increase in cytosolic AMP is therefore an ideal candidate as a signaling molecule to indicate cellular stress. Stimulation of KATP channel activity by AMP could thus represent one of the earliest events in the ischemic heart. It is unlikely for AMP to act via adenylate kinase under ischemic conditions for the following reasons. First, the balance of the adenylate kinase reaction may not be favorable to produce AMP from ADP under ischemic conditions. Second, adenylate kinase activity is inhibited during myocardial ischemia [41]. Third, unlike the protective effect of KATP channel openers [42], inhibitors of adenylate kinase do not affect functional recovery of the ischemic heart [43]. Other potential mechanisms should therefore be considered by which AMP might signal metabolic stress and regulate the activity of key proteins, such as KATP channels.
AMPK is emerging as an important signaling protein during myocardial ischemia. In the isolated heart, ischemia strongly activates AMPK [44]. Low-flow or regional ischemia also rapidly activates the activity of the upstream kinase, AMPKK, which further promotes phosphorylation of AMPK [45–46]. Support for a protective role of AMPK during ischemia comes from transgenic mice expressing a kinase dead AMPK subunit, which demonstrated significantly impaired recovery of post-ischemic contractile function in the setting of ischemia and reperfusion [47]. In rabbit heart, ischemic preconditioning also activates AMPK [48]. Historically, the literature provides ample support for the concept that AMPK activation may be protecting the heart from ischemic episodes. The widely used AMPK activator, AICAR (also known as acadesine), has been known for more than a decade to be cardioprotective by improving post-ischemic heart recovery and preconditioning [49–51]. Initially it was used in an attempt to enhance the rate of repletion of the nucleotide pool in post-ischemic myocardium [49], but has since been found to be without major effects on the cellular nucleotide complement [50]. Given our preliminary data that AMPK activates KATP channels and the known protective role of KATP channels in ischemic damage, we hypothesize that the protective effect of AICAR may be mediated (at least in part) through activation of AMPK and stimulation of KATP channel activity. Thus, AMP may act as a signaling molecule early during ischemia to stimulate KATP channel opening through activation of AMPK. There are two catalytic AMPK α-subunits, both of which are expressed in the heart. The AMPK α1-subunit is expressed widely, whereas the α2 subunit is expressed predominantly in the heart, liver and skeletal muscle [4, 40, 52]. It is likely that, similar to the regulation of CFTR channels [27], cardiac KATP channels may be regulated by both α1- and α2-subunits.
AMPK as a membrane-associated protein
There are reports to indicate that membrane proteins or ion channels are affected by AMPK [23, 26–27, 53]. Our co-immunoprecipitation show that the AMPK α-subunit physically interacts with KATP channel subunits. The AMPK α-subunit has also been described to interact with the Cl− channel subunit CFTR [27], which is part of the same ABC cassette family of proteins to which SUR subunits belong. In the case of CFTR, the interaction domain has been mapped to residues 1420–1457 [27], which corresponds to the distal C-terminus of SUR (amino acids 1523–1549 of SUR2 and 1555–1581 or SUR1). This corresponds to the alternatively spliced region in SUR2 and it would be extremely interesting if the AMPK α-subunit also interacts with SUR in this region, since this raises the possibility of isoform-specific interaction. Of course, it remains possible that interaction is not direct and that unidentified adaptor proteins are involved, akin to the regulation of PKA that interacts with membrane channels through specific anchoring proteins (e.g. AKAPs) to target kinases close to their effector sites [54–55].
Significance of these studies
KATP channels are strongly regulated by the ATP:ADP ratio: the channel is activated both by decreasing ATP levels and increasing ADP levels during metabolic demand. Our data show that elevated AMP levels positively regulate KATP channels, hence adding another layer of regulation during metabolic stress (in addition to the well described changes in ATP:ADP ratio). In the cardiovascular system, the coupling between AMPK activation and KATP channel opening (due to alterations in the ATP:AMP ratio) may be particularly relevant during increased metabolic demand (such as intense exercise) and under pathophysiological conditions, such as hypoxia and ischemia.
HIGHLIGHTS.
During metabolic impairment, a change in the ATP:ADP ratio stimulates KATP channels opening.
The ATP:AMP ratio is an acquisitively sensitive indicator of alterations in the metabolic status.
We show that AMP-activated protein kinase (AMPK) activity promotes KATP channel opening.
AMPK physically interacts with KATP channel subunits, suggestive of local signalling.
Thus, small changes in AMP may trigger KATP channel availability under ischemic conditions.
Supplementary Material
ACKNOWLEDGEMENTS
These studies were supported by the National Institutes of Health (HL064838 and HL085820), the American Heart Association (Established Investigator Award to WAC) and in part by the New York Masonic Seventh District Association, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ashcroft FM. Adenosine 5'-triphosphate-sensitive potassium channels. Annual Review of Neuroscience. 1988;11:97–118. doi: 10.1146/annurev.ne.11.030188.000525. [DOI] [PubMed] [Google Scholar]
- 2.Nichols CG, Lopatin AN. Inward rectifier potassium channels. Annual Review of Physiology. 1997;59:171–191. doi: 10.1146/annurev.physiol.59.1.171. [DOI] [PubMed] [Google Scholar]
- 3.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007 Oct;8(10):774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 4.Stapleton D, Mitchelhill KI, Gao G, Widmer J, Michell BJ, Teh T, et al. Mammalian AMP-activated protein kinase subfamily. J Biol Chem. 1996;271:611–614. doi: 10.1074/jbc.271.2.611. [DOI] [PubMed] [Google Scholar]
- 5.Kemp BE, Mitchelhill KI, Stapleton D, Michell BJ, Chen ZP, Witters LA. Dealing with energy demand: the AMP-activated protein kinase. Trends Biochem Sci. 1999;24(1):22–25. doi: 10.1016/s0968-0004(98)01340-1. [DOI] [PubMed] [Google Scholar]
- 6.Sukhodub A, Jovanovic S, Du Q, Budas G, Clelland AK, Shen M, et al. AMP-activated protein kinase mediates preconditioning in cardiomyocytes by regulating activity and trafficking of sarcolemmal ATP-sensitive K(+) channels. J Cell Physiol. 2007 Jan;210(1):224–236. doi: 10.1002/jcp.20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coetzee WA. Regulation of ATP sensitive potassium channel of isolated guinea pig ventricular myocytes by sarcolemmal monocarboxylate transport. Cardiovascular Research. 1992;26:1077–1086. doi: 10.1093/cvr/26.11.1077. [DOI] [PubMed] [Google Scholar]
- 8.Hong M, Kefalogianni E, Bao L, Malester B, Delaroche D, Neubert TA, et al. The cardiac ATP-Sensitive K+ channel associates with the glycolytic enzyme complex. FASEB J. 2011 doi: 10.1096/fj.10-176669. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pountney DJ, Sun ZQ, Porter LM, Nitabach MN, Nakamura TY, Holmes D, et al. Is the molecular composition of K(ATP) channels more complex than originally thought? Biochemical and electrophysiological evidence for heteromultimeric assembly of the K ATP channel subunits Kir6.1 and Kir6.2. Journal of Molecular and Cellular Cardiology. 2001;33(8):1541–1546. doi: 10.1006/jmcc.2001.1407. [DOI] [PubMed] [Google Scholar]
- 10.Yoshida H, Feig J, Ghiu IA, Artman M, Coetzee WA. K(ATP) channels of primary human coronary artery endothelial cells consist of a heteromultimeric complex of Kir6.1, Kir6.2, and SUR2B subunits. Journal of Molecular and Cellular Cardiology. 2004;37(4):857–869. doi: 10.1016/j.yjmcc.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 11.Bao L, Hadjiolova K, Coetzee WA, Rindler MJ. Endosomal KATP channels as a reservoir after myocardial ischemia: a role for SUR2 subunits. Am J Physiol Heart Circ Physiol. 2011 Jan;300(1):H262–H270. doi: 10.1152/ajpheart.00857.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardie DG. AMP-activated protein kinase: the guardian of cardiac energy status. Journal of Clinical Investigation. 2004;114(4):465–468. doi: 10.1172/JCI22683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? European Journal of Biochemistry. 1995;229(2):558–565. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- 14.Henin N, Vincent MF, Van den BG. Stimulation of rat liver AMP-activated protein kinase by AMP analogues. Biochimica et biophysica acta. 1996;1290(2):197–203. doi: 10.1016/0304-4165(96)00021-9. [DOI] [PubMed] [Google Scholar]
- 15.Larsson O, Ammala C, Bokvist K, Fredholm B, Rorsman P. Stimulation of the KATP channel by ADP and diazoxide requires nucleotide hydrolysis in mouse pancreatic beta- cells. Journal of Physiology (London) 1993;463:349–365. doi: 10.1113/jphysiol.1993.sp019598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elvir-Mairena JR, Jovanovic A, Gomez LA, Alekseev AE, Terzic A. Reversal of the ATP-liganded state of ATP-sensitive K + channels by adenylate kinase activity. J Biol Chem. 1996;271:31903–31908. doi: 10.1074/jbc.271.50.31903. [DOI] [PubMed] [Google Scholar]
- 17.Dzeja PP, Terzic A. Phosphotransfer reactions in the regulation of ATP-sensitive K+ channels. FASEB Journal. 1998;12:523–529. doi: 10.1096/fasebj.12.7.523. [DOI] [PubMed] [Google Scholar]
- 18.Hardie DG, Salt I, Hawley SA, Davies SP. AMP-activated protein kinase: an ultrasensitive system for monitoring cellular energy charge. Biochemical Journal. 1999;338:717–722. [PMC free article] [PubMed] [Google Scholar]
- 19.Font B, Gautheron DC. General and kinetic properties of pig heart mitochondrial adenylate kinase. Biochim Biophys Acta. 1980;611(2):299–308. doi: 10.1016/0005-2744(80)90065-0. [DOI] [PubMed] [Google Scholar]
- 20.Xiao B, Sanders MJ, Underwood E, Heath R, Mayer FV, Carmena D, et al. Structure of mammalian AMPK and its regulation by ADP. Nature. 2011 Apr 14;472(7342):230–233. doi: 10.1038/nature09932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardie DG, Hawley SA. AMP-activated protein kinase: the energy charge hypothesis revisited. Bioessays. 2001 Dec;23(12):1112–1119. doi: 10.1002/bies.10009. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi T, Hirshman MF, Fujii N, Habinowski SA, Witters LA, Goodyear LJ. Metabolic stress and altered glucose transport: activation of AMP-activated protein kinase as a unifying coupling mechanism. Diabetes. 2000 Apr;49(4):527–531. doi: 10.2337/diabetes.49.4.527. 2000; 49: 527-31. [DOI] [PubMed] [Google Scholar]
- 23.Wang CZ, Wang Y, Di A, Magnuson MA, Ye H, Roe MW, et al. 5-amino-imidazole carboxamide riboside acutely potentiates glucose-stimulated insulin secretion from mouse pancreatic islets by KATP channel-dependent and -independent pathways. Biochem Biophys Res Commun. 2005 May 20;330(4):1073–1079. doi: 10.1016/j.bbrc.2005.03.093. [DOI] [PubMed] [Google Scholar]
- 24.Ouchi N, Shibata R, Walsh K. AMP-activated protein kinase signaling stimulates VEGF expression and angiogenesis in skeletal muscle. Circ Res. 2005 Apr 29;96(8):838–846. doi: 10.1161/01.RES.0000163633.10240.3b. Epub 2005 Mar 24. 2005; 96: 838-46. [DOI] [PubMed] [Google Scholar]
- 25.Morrow VA, Foufelle F, Connell JM, Petrie JR, Gould GW, Salt IP. Direct activation of AMP-activated protein kinase stimulates nitric-oxide synthesis in human aortic endothelial cells. J Biol Chem. 2003 Aug 22;278(34):31629–31639. doi: 10.1074/jbc.M212831200. Epub 2003 Jun 4. 2003; 278: 31629-39. [DOI] [PubMed] [Google Scholar]
- 26.Carattino MD, Edinger RS, Grieser HJ, Wise R, Neumann D, Schlattner U, et al. Epithelial sodium channel inhibition by AMP-activated protein kinase in oocytes and polarized renal epithelial cells. J Biol Chem. 2005 May 6;280(18):17608–17616. doi: 10.1074/jbc.M501770200. Epub 2005 Mar 7. 2005; 280: 17608-16. [DOI] [PubMed] [Google Scholar]
- 27.Hallows KR, Raghuram V, Kemp BE, Witters LA, Foskett JK. Inhibition of cystic fibrosis transmembrane conductance regulator by novel interaction with the metabolic sensor AMP-activated protein kinase. J Clin Invest. 2000;105(12):1711–1721. doi: 10.1172/JCI9622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hallows KR, Kobinger GP, Wilson JM, Witters LA, Foskett JK. Physiological modulation of CFTR activity by AMP-activated protein kinase in polarized T84 cells. Am J Physiol Cell Physiol. 2003;284(5):C1297–C1308. doi: 10.1152/ajpcell.00227.2002. [DOI] [PubMed] [Google Scholar]
- 29.Hallows KR, McCane JE, Kemp BE, Witters LA, Foskett JK. Regulation of channel gating by AMP-activated protein kinase modulates cystic fibrosis transmembrane conductance regulator activity in lung submucosal cells. J Biol Chem. 2003;278(2):998–1004. doi: 10.1074/jbc.M210621200. [DOI] [PubMed] [Google Scholar]
- 30.Boshell BR, Roddam RF, McAdams GL. Effects of phenformin on insulin reserve and release. Ann N Y Acad Sci. 1968 Mar 26;148(3):756–767. doi: 10.1111/j.1749-6632.1968.tb27748.x. [DOI] [PubMed] [Google Scholar]
- 31.Chang TJ, Chen WP, Yang C, Lu PH, Liang YC, Su MJ, et al. Serine-385 phosphorylation of inwardly rectifying K+ channel subunit (Kir6.2) by AMP-dependent protein kinase plays a key role in rosiglitazone-induced closure of the K(ATP) channel and insulin secretion in rats. Diabetologia. 2009 Jun;52(6):1112–1121. doi: 10.1007/s00125-009-1337-4. [DOI] [PubMed] [Google Scholar]
- 32.Aziz Q, Thomas A, Khambra T, Tinker A. Phenformin has a direct inhibitory effect on the ATP-sensitive potassium channel. Eur J Pharmacol. 2010 May 25;634(1–3):26–32. doi: 10.1016/j.ejphar.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 33.Lim A, Park SH, Sohn JW, Jeon JH, Park JH, Song DK, et al. Glucose deprivation regulates KATP channel trafficking via AMP-activated protein kinase in pancreatic beta-cells. Diabetes. 2009 Dec;58(12):2813–2819. doi: 10.2337/db09-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008 Apr 25;30(2):214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Babenko AP, Gonzalez G, Aguilar-Bryan L, Bryan J. Reconstituted human cardiac KATP channels: functional identity with the native channels from the sarcolemma of human ventricular cells. Circulation Research. 1998;83(11):1132–1143. doi: 10.1161/01.res.83.11.1132. [DOI] [PubMed] [Google Scholar]
- 36.Kakei M, Noma A, Shibasaki T. Properties of adenosine-triphosphate-regulated potassium channels in guinea-pig ventricular cells. Journal of Physiology (London) 1985;363:441–462. doi: 10.1113/jphysiol.1985.sp015721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flores NA, Stavrou BM, Sheridan DJ. The effects of diadenosine polyphosphates on the cardiovascular system. Cardiovasc Res. 1999;42(1):15–26. doi: 10.1016/s0008-6363(99)00004-8. [DOI] [PubMed] [Google Scholar]
- 38.Opie LH. Effects of regional ischemia on metabolism of glucose and fatty acids. Relative rates of aerobic and anaerobic energy production during myocardial infarction and comparison with effects of anoxia. Circulation Research. 1976;38 Suppl 1:I-52–I-74. [PubMed] [Google Scholar]
- 39.Opie LH, Nathan D, Lubbe WF. Biochemical aspects of arrhythmogenesis and ventricular fibrillation. The American Journal of Cardiology. 1979;43:131–148. doi: 10.1016/0002-9149(79)90055-9. [DOI] [PubMed] [Google Scholar]
- 40.Hardie DG, Carling D, Carlson M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annual Review of Biochemistry. 1998;67:821–855. doi: 10.1146/annurev.biochem.67.1.821. [DOI] [PubMed] [Google Scholar]
- 41.Vitkevicius K, Dzeja P. Changes in adenylate kinase isoenzyme activity in ischemic rabbit heart. Byull Eksp Biol Med. 1990;110:1039–1040. [Google Scholar]
- 42.Hearse DJ. Activation of ATP-sensitive potassium channels: A novel pharmacological approach to myocardial protection. Cardiovascular Research. 1995;30:1–17. [PubMed] [Google Scholar]
- 43.Humphrey SM, Holliss DG, Cartner LA. The influence of inhibitors of the ATP degradative pathway on recovery of function and high energy phosphate after transient ischemia in the rat heart. J Mol Cell Cardiol. 1986;18 Suppl 4:55–59. doi: 10.1016/s0022-2828(86)80027-x. [DOI] [PubMed] [Google Scholar]
- 44.Kudo N, Gillespie JG, Kung L, Witters LA, Schulz R, Clanachan AS, et al. Characterization of 5'AMP-activated protein kinase activity in the heart and its role in inhibiting acetyl-CoA carboxylase during reperfusion following ischemia. Biochim Biophys Acta. 1996;1301(1–2):67–75. doi: 10.1016/0005-2760(96)00013-6. [DOI] [PubMed] [Google Scholar]
- 45.Baron SJ, Li J, Russell RR, III, Neumann D, Miller EJ, Tuerk R, et al. Dual mechanisms regulating AMPK kinase action in the ischemic heart. Circ Res. 2005;96(3):337–345. doi: 10.1161/01.RES.0000155723.53868.d2. [DOI] [PubMed] [Google Scholar]
- 46.Altarejos JY, Taniguchi M, Clanachan AS, Lopaschuk GD. Myocardial ischemia differentially regulates LKB1 and an alternate 5'-AMP-activated protein kinase kinase. J Biol Chem. 2005 Jan 7;280(1):183–190. doi: 10.1074/jbc.M411810200. Epub 2004 Oct 26. 2005; 280: 183-90. [DOI] [PubMed] [Google Scholar]
- 47.Russell RR, III, Li J, Coven DL, Pypaert M, Zechner C, Palmeri M, et al. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest. 2004 Aug;114(4):495–503. doi: 10.1172/JCI19297. 2004; 114: 495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nishino Y, Miura T, Miki T, Sakamoto J, Nakamura Y, Ikeda Y, et al. Ischemic preconditioning activates AMPK in a PKC-dependent manner and induces GLUT4 up-regulation in the late phase of cardioprotection. Cardiovasc Res. 2004 Feb 15;61(3):610–619. doi: 10.1016/j.cardiores.2003.10.022. 2004; 61: 610-9. [DOI] [PubMed] [Google Scholar]
- 49.Swain JA, Hines JJ, Sabina RL, Holmes EW. Accelerated repletion of ATP and GTP pools in postischemic canine myocardium using a precursor of purine de novo synthesis. Circulation Research. 1983;51(1):102–105. doi: 10.1161/01.res.51.1.102. [DOI] [PubMed] [Google Scholar]
- 50.Galinanes M, Mullane KM, Bullough D, Hearse DJ. Acadesine and myocardial protection. Studies of time of administration and dose-response relations in the rat. Circulation. 1992;86(2):598–608. doi: 10.1161/01.cir.86.2.598. [DOI] [PubMed] [Google Scholar]
- 51.Burckhartt B, Yang XM, Tsuchida A, Mullane KM, Downey JM, Cohen MV. Acadesine extends the window of protection afforded by ischaemic preconditioning in conscious rabbits. Cardiovascular Research. 1995 May;29(5):653–657. [PubMed] [Google Scholar]
- 52.Stapleton D, Woollatt E, Mitchelhill KI, Nicholl JK, Fernandez CS, Michell BJ, et al. AMP-activated protein kinase isoenzyme family: subunit structure and chromosomal location. FEBS letters. 1997;409:452–456. doi: 10.1016/s0014-5793(97)00569-3. [DOI] [PubMed] [Google Scholar]
- 53.Light PE, Wallace CH, Dyck JR. Constitutively active adenosine monophosphate-activated protein kinase regulates voltage-gated sodium channels in ventricular myocytes. Circulation. 2003;107(15):1962–1965. doi: 10.1161/01.CIR.0000069269.60167.02. [DOI] [PubMed] [Google Scholar]
- 54.Fraser ID, Scott JD. Modulation of ion channels: a "current" view of AKAPs. Neuron. 1999;23(3):423–426. doi: 10.1016/s0896-6273(00)80795-3. [DOI] [PubMed] [Google Scholar]
- 55.Li Z, Rossi EA, Hoheisel JD, Kalderon D, Rubin CS. Generation of a novel A kinase anchor protein and a myristoylated alanine-rich C kinase substrate-like analog from a single gene. J Biol Chem. 1999;274(38):27191–27200. doi: 10.1074/jbc.274.38.27191. [DOI] [PubMed] [Google Scholar]
- 56.Findlay I. Effects of glibenclamide upon ATP-sensitive K channels during metabolic inhibition of isolated rat cardiac myocytes. Cardiovascular Drugs and Therapy. 1993;7:495–497. doi: 10.1007/BF00877613. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.