Abstract
The MRL/MpJ mouse displays the rare ability amongst mammals to heal injured ear tissue without scarring. Numerous studies have shown that the formation of a blastema-like structure leads to subsequent tissue regeneration in this model, indicating many parallels with amphibian limb regeneration and mammalian embryogenesis. We have recently shown that the MRL/MpJ mouse also possesses an enhanced capacity for peripheral nerve regeneration within the ear wound. Indeed, nerves are vital for the initial phase of blastema formation in the amphibian limb. In this study we investigated the capacity for wound regeneration in a denervated ear. The left ears of MRL/MpJ mice and C57BL/6 (a control strain known to have a poorer regenerative capacity) were surgically denervated at the base via an incision and nerve transection, immediately followed by a 2-mm ear punch wound. Immunohistochemical analysis showed a lack of neurofilament expression in the denervated ear wound. Histology revealed that denervation prevented blastema formation and chrondrogenesis, and also severely hindered normal healing, with disrupted re-epithelialisation, increasing wound size and progressive necrosis towards the ear tip. Denervation of the ear obliterated the regenerative capacity of the MRL/MpJ mouse, and also had a severe negative effect on the ear wound repair mechanisms of the C57BL/6 strain. These data suggest that innervation may be important not only for regeneration but also for normal wound repair processes.
Keywords: denervation, regeneration, repair, wound healing, skin, MRL
Introduction
Peripheral nerves are known to play an important role in adult mammalian wound healing (Westerman et al. 1993). Following wounding the tissue develops a dense nerve network known as the cicatricial plexus. Here axons form close associations with cells within the wound environment, including endothelial cells, macrophages and myofibroblasts (Liu et al. 1999). Sensory nerves mediate their action on wound repair by the release of neuropeptides, which function as mediators of neurogenic inflammation, regulating blood flow (Wallengren, 1997) and modulating local immune responses (Maggi, 1997). Impaired neurological function is thought to be a major cause of chronic cutaneous wounds (Barker et al. 2006). Numerous studies in wound models have shown that denervation by either neurotomy or capsaicin causes severe defects in all phases of the wound-healing process, resulting in: delayed wound contraction and re-epithelialisation; reduced microvascular response and neovascularisation; hypertrophic scarring and keloids; decreased wound tensile strength; and a delayed and prolonged inflammatory phase (reviewed by Barker et al. 2006).
Peripheral nerves are also vital for the process of amphibian limb regeneration as they provide the initial trophic stimulus for blastema formation (Kumar et al. 2007; Brockes & Kumar, 2008; reviewed in Whited & Tabin, 2009). The ‘blastema’ is a proliferative mass of mesenchymal progenitor cells that accumulate at the injury site beneath a secretory apical epidermal cap; a self-organising system based on positional information inherited from parent cells that then gives rise to new structures, for example, the regrowth of a severed limb (Stocum, 2004). As originally reported by Todd (1823), denervation of the limb either immediately prior to amputation or before the blastema reaches the late bud stage prevents or arrests limb regeneration, respectively.
Previous reports (Clark et al. 1998; Rajnoch et al. 2003; Metcalfe & Ferguson, 2005, 2007) have revealed that the MRL/MpJ mouse heals a biopsy punch wound by the formation of a blastema-like structure, leading to regeneration of the pinna architecture without scarring. Superficially this structure bears remarkable resemblance to the regenerating newt forelimb blastema. Collagen, vascularisation, hair follicles, sebaceous glands and even cartilage are regenerated, with no visible evidence of scarring (Heber-Katz et al. 2003; Beare et al. 2006).
We have recently shown that the MRL/MpJ mouse displays an enhanced capacity for peripheral nerve regeneration in an ear punch wound compared with the control strain (C57BL/6) (Buckley et al. 2011). We propose that the mechanism of blastema formation and subsequent regeneration of MRL/MpJ ear tissue may also be under the influence of peripheral nerves present at the wound site. In this study we aimed to determine the effect of pinna denervation on ear wound regeneration in MRL/MpJ and C57BL/6 mice by comparing the rate of ear punch closure and histological characteristics in denervated vs. non-denervated ear wounds. This study provides the first mechanistic evidence that, similar to amphibian limb regeneration, MRL/MpJ ear wound regeneration is also highly dependent on wound innervations. Denervation also had a severe negative effect on the ear wound repair mechanisms of the C57BL/6 strain.
Materials and methods
All procedures involving animals were carried out in accordance with UK Home Office regulations of the 1986 Animals in Scientific Procedures Act, under appropriate project and personal license authority. Female MRL/MpJ and C57BL/6 mice aged between 7 and 12 weeks were used throughout the study. Buprenorphrine (Vetergesic, Rhodia Organique Fine, Bristol, UK) was administered (100 μL) subcutaneously 40 min prior to wounding, at a concentration of 3 μg mL−1. Anaesthesia was maintained using a Bain anaesthetic circuit, 2.5% Isoflurane in oxygen and nitrous oxide at a flow rate of 2.5 L min−1. Mice were wounded with a hole to the centre of each ear using a 2-mm sterile clinical biopsy punch (Stiefel Laboratories, Woodburn Green, UK).
Surgical denervation of the ear
In order to assess the importance of peripheral nerves in the process of regeneration, the nerve supply to the left ear was transected prior to the mouse receiving an ear punch wound. Under appropriate anaesthesia, an area approximately 2 cm2 at the base of the left ear was shaved and swabbed with 70% alcohol. To each side, 200 μL of sterile saline was injected intra-dermally in the centre of the shaved area to form a raised bleb. A 1-cm incision was then made through the raised bleb using a size 11 scalpel, in order to access the rostral auricular nerve innervating the ear from the base. Under an operating microscope, microsurgical scissors were used, under direct vision, to cut precisely the auricular nerves without damage to the surrounding tissues or blood vessels.
Upon dissection, it was noticed that in addition to the rostral auricular nerve, several other nerve bundles could be observed entering the ear base, which were also transected. It is unclear whether these additional axon bundles were branches of the rostral auricular nerve or separate and distinct nerves; however, the pattern of innervation at the base of the ear was similar in all mice used in this study. It is thought that this branch of the facial nerve provides innervation to the central pinna (Nieuwenhuys et al. 1998) All nerves found entering the base of the ear were transected with sharp microsurgical scissors, removing a section approximately 2–4 mm in length. The incised skin edges were re-apposed and sutured closed using discontinuous stitches with 8–0 non-absorbable prolene sutures (Ethicon nylon monofilament, Johnson & Johnson, USA). The centre of the left ear was then wounded with a 2-mm through-and-through clinical biopsy punch.
A contra-lateral control (non-denervated ear wound) was created on the right ear. As described above, a 1-cm incision was made over a raised bleb of saline, and the underlying tissue was parted in order to locate the nerve supply to the ear. However, the nerves were left intact in the contra-lateral control. The control incision was sutured closed as before, and the centre of the right ear received an identical 2-mm biopsy punch wound.
Post-operative care
Post-surgery, mice were individually housed in cages and observed until fully recovered with the resumption of normal behavior, such as foraging and grooming. Sutures were removed from incisional wounds at 7 days post-wounding. In order to verify that the ear remained denervated throughout the course of the experiment, the skin incision was re-incised and the area inspected under the microscope, after death, to confirm that the nerve stump had not regenerated.
Tissue processing
Mice were killed using CO2 followed by cervical dislocation at 7 or 50 days post-wounding (n = 6). Prior to mounting or processing of tissue, all wounds were photographed alongside a graded scale bar using a digital camera (Nikon Coolpix 5400). Wound areas were then quantified using Image Pro Plus software (Media Cybernetics, version 4.1.0.0). Ear wounds were excised, bisected, cryo-preserved in optimum cutting temperature embedding matrix (Cell Path, Powys, Wales, UK) and cryo-sectioned (7 μm). Samples of sections from each wound were stained with Masson's Trichrome.
Dual-labelling immunohistochemistry
Cryo-sections were rehydrated in 0.1% polyoxyethylenesorbitan monoalurate (Tween20®) in phosphate-buffered saline (PBST) for 10 min. Sections were placed in a light-excluding humidified chamber and incubated in goat serum block (10% serum, 5% bovine serum albumin in PBS) for 1 h at room temperature (RT). Sections were incubated overnight at 4 °C with a rabbit polyclonal cocktail to neurofilaments (Biomol NA1297) diluted 1 : 500 serum block. Following incubation, the slides were washed in three changes of PBST for 15 min, and biotinylated anti-rabbit (Vector BA1001) secondary antibody diluted in serum block (2.5 μg mL−1) was applied to the sections (200 μL per slide) and incubated for 1 h at RT. Sections were washed in three changes of PBST for 15 min and incubated in the dark for 40 min at RT in streptavidin-conjugated fluorescein label (Chemicon, CA, USA) applied at a concentration of 2.5 μg mL−1. The slides were again rinsed in three changes of PBST for 15 min excluding light. The rat monoclonal antibody to CD31 (BD Biosciences, 553370) was applied to the sections (2 μg mL−1) for 1 h at RT in a humidified chamber, followed by rinsing in three changes of PBST for 15 min excluding light. A secondary TRITC-conjugated donkey anti-rat antibody (Jackson ImmunoResearch, 712 025 153) was applied to the sections (1.5 μg mL−1) and incubated for a further 1 h at RT in a humidified chamber, and again rinsed in three changes of PBST for 15 min excluding light. Finally, sections were mounted in Gelvatol before visualisation.
Masson's Trichrome histology
Rehydrated sections were immersed in filtered Harris's Haematoxylin for 4 min, and then rinsed in running tap water for 5 min until sections appeared blue in colour. The sections were placed in 1% (v/v) picric acid solution for 30 s and rinsed in water. Slides were then transferred to 0.1% (w/v) Biebrich scarlet for 1 min, dipped in water and immersed in 50% PMA/50% PTA acid for 10 min. Finally, slides were placed in 2.5% (w/v) fast green for 6 min. Following rinsing in water, sections were rapidly dehydrated in graded alcohols (50–100%) then in two changes of xylene for 10 min each, before being mounted in Pertex™ (CellPath, Newton, Powys, UK). Nuclei stained purple/black, muscle, red blood cells, fibrin and cytoplasmic granules stained orange/red, and collagen a light green colour.
Images were captured and processed with a Spot RT Slider Digital camera with a 1 × magnification lens and Spot RT-v.3.5 software running on a silicon graphics Pentium III 230MHz PC (Diagnostic instruments, MI, USA).
Results
Nerve infiltration and vascularisation of the denervated pinna post-wounding
MRL/MpJ and C57BL/6 mouse ears were surgically denervated at the base of the left ear and immediately wounded with a biopsy punch to create an ear punch hole. The right (non-denervated) ear also received a skin incision to the base of the ear and an ear punch, but the nerve supply remained intact. To determine if the denervation procedure was successful, tissue sections were dual-stained for the peripheral nerve marker, pan-neurofilament, and endothelial cell marker, CD31, using immunohistochemistry. Figure 1a shows that at 7 days post-wounding in the non-denervated MRL/MpJ mouse ear, a thickened wound epidermis, characteristic of early blastema formation, had formed, and nerves and blood vessels were prevalent throughout the ear wound. The denervated MRL/MpJ ear wound, however, had failed to re-epithelialise and exhibited a complete lack of neurofilament expression (Fig. 1b). This indicates that peripheral axons distal to the transected nerve had degenerated and the ear was successfully denervated. Equivalent histological observations in the denervated MRL/MpJ ear wound were seen in the denervated C57BL/6 ear wounds.
Fig. 1.
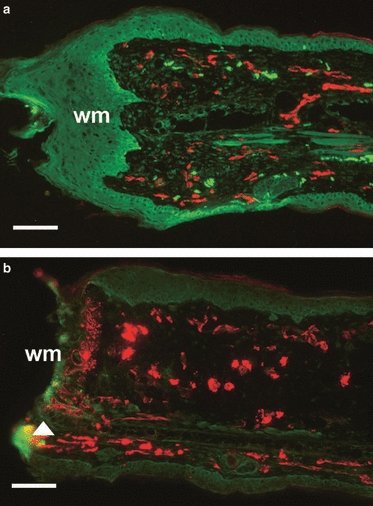
Re-innervation and vascularisation of denervated vs. non-denervated MRL/MpJ ear wounds 7 days post-wounding. Immunohistochemistry assessed the presence of pan-neurofilament (FITC) and CD31 (TRITC) in both non-denervated ear wounds (a) and denervated ear wounds (b) in the MRL/MpJ mouse 7 days post-wounding. (a) Section from the centre of the regenerating ear showing the presence of nerves and blood vessels advancing beyond the cut edge of the cartilage (arrow) and a thickened wound epithelium at the wound margin (wm). (b) Section from the denervated ear showing the denervation procedure was successful as no nerves were present and the blood vessels appear dilated. Note the speckled staining of CD31 (arrow), indicating disintegration of vessels at the necrosing wound margin (wm). Scale bars: 100 μm.
By day 50 post-wounding, the non-denervated MRL/MpJ ear wound exhibited vascularised regenerating tissue; newly formed cartilage islands had started to elongate and connect, and target structures such as hair follicles and sebaceous glands were present (Fig. 2a). The denervated MRL/MpJ ear wound showed no closure of the initial punch hole and only a few regenerated nerves in the proximal wound margin, but not to the same extent as the non-denervated ear (Fig. 2b).
Fig. 2.
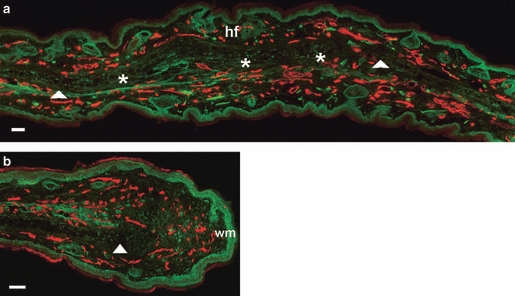
Re-innervation and vascularisation of denervated vs. innervated non-denervated MRL/MpJ ear wounds 50 days post-wounding. Immunohistochemistry was used to detect the presence of neurofilament (FITC) and CD31 (TRITC) in both non-denervated ear wounds (a) and denervated ear wounds (b) in the MRL/MpJ mouse 50 days post-wounding. (a) Section taken from regenerating ear tissue showing cartilage island formation (*), regenerated hair follicles (hf) beyond the original wound margin (wm; arrows). (b) Section from proximal wound margin of a denervated ear showing re-vascularisation, but only a small influx of regenerating nerves between the cut edge of cartilage (arrow) and the wound margin (wm). Scale bars: 100 μm.
Impact of denervation on gross morphology and wound histology
The non-denervated MRL/MpJ ear wound demonstrated minimal necrosis, and shedding of eschar (scab) occurred at 5 days post-wounding to produce a re-epithelialised uniform circular wound margin by 10 days post-wounding (Fig. 3). This was followed by the development of an opaque annular swelling of regenerating tissue and a visible reduction in wound size by day 35 post-wounding (Fig. 3). The non-denervated wound continued to regenerate, nearly closing the ear punch hole by day 50 post-wounding.
Fig. 3.
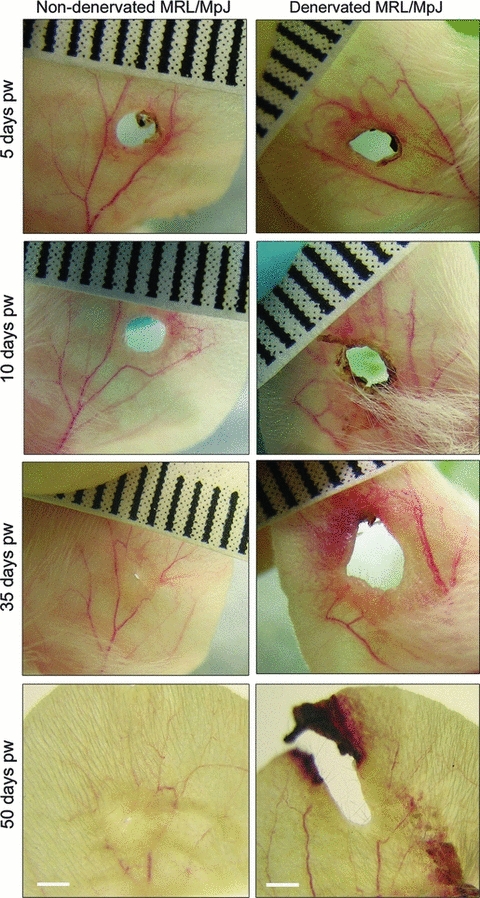
Macroscopic photographs of MRL/MpJ ear wound healing in a non-denervated (left) vs. denervated ear (right) 5–50 days post-wounding (pw). MRL/MpJ ear wounds photographed at days 5, 10, 35 and 50 post-wounding (representative examples shown). The non-denervated wound re-epithelialised and regenerated to nearly close the wound hole by day 50. The denervated ear wound became increasingly necrotic, failed to re-epithelialise distally, increased in size and extended out toward the ear tip. Ruler graduations: 1 mm; scale bars: 2 mm.
By contrast, by 5 days post-wounding the denervated MRL/MpJ ear wound had become elliptical in shape, with a ring of eschar tissue and blood clot at the margin (Fig. 3). Not only was the normal regenerative phenotype severely disrupted, but in all mice the necrosing distal half of the ear wound failed to undergo re-epithelialisation and repair by day 10 post-wounding (Fig. 3). Healing of the denervated ear continued to deteriorate 35 days post-wounding, particularly in the distal half of the ear wound (Fig. 3). By 50 days post-wounding the hole had widened and extended out to the ear tip behind an advancing band of necrotic tissue. The proximal half of the denervated wound, however, underwent limited repair and produced a re-epithelialised edge free from necrosis, although no regenerative features were observed.
A marked difference between the wound size of the denervated MRL/MpJ ear and the non-denervated MRL/MpJ ear became apparent within 5 days of wounding. From day 7 post-wounding onwards the denervated MRL/MpJ wound area became significantly larger (P < 0.05) than the non-denervated MRL/MpJ ear wound, and from day 10 post-wounding remained at least double the size of the original 2-mm hole punch for the remainder of the experiment up to day 50 (Fig. 4). The C57BL/6 strain, known to be a poorer healer than the MRL/MpJ strain (Clark et al. 1998), exhibited a similar phenomenon, with the denervated ear wound remaining at least double the original wound size from day 21 and continuing to increase over time (Fig. 4). The non-denervated C57BL/6 ear wound, however, had only closed by up to half of its original size by day 50 post-wounding.
Fig. 4.
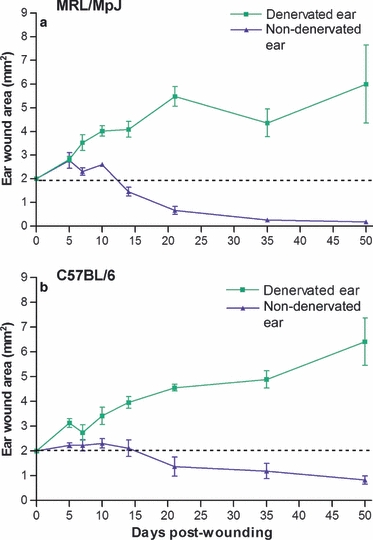
Effect of denervation on wound area. Both denervated and non-denervated ears were wounded with a 2-mm clinical biopsy punch. Denervation of ear wounds caused a significant increase in punch hole area (*P ≤0.05) compared with non-denervated ear wounds from day 7 post-wounding in MRL/MpJ (a) and day 10 in C57BL/6 (b). Ear hole diameter was measured at intervals of 5, 7, 10, 14, 21, 35 and 50 days post-wounding. Image pro plus software was used to calculate wound area. Data shown represent means ± SEM, MRL/MpJ; n = 6, C57BL/6; n = 6 for each time point.
Histological analysis of the non-denervated MRL/MpJ ear wound demonstrated near closure of the wound hole by 50 days post-wounding and showed the classic hallmarks of ear regeneration, as described in previous studies (Clark et al. 1998; Rajnoch et al. 2003; Metcalfe et al. 2006). Such features include: dermal extension; epithelial fusion of opposing wound margins; and regeneration of structures such as hair follicles and sebaceous glands (Fig. 5A). In addition, islands of cartilage aggregates had formed de novo in line with existing cartilage (Fig. 5B,C).
Fig. 5.
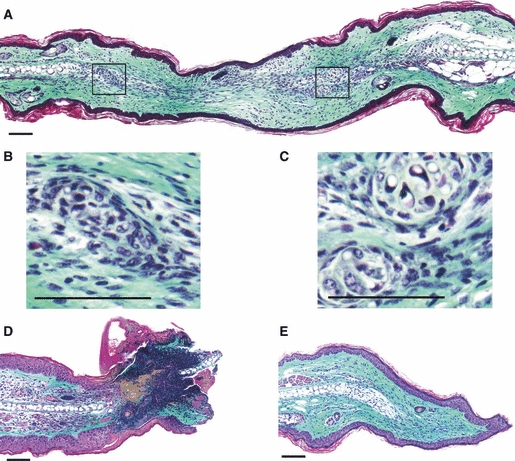
Histology of the denervated and non-denervated MRL/MpJ ear wounds 50 days post-wounding. Masson's Trichrome stained tissue sections. Non-denervated ear: (A) section through the centre of the regenerating wound displaying collagen deposition, epithelial fusion of opposing wound margins, and chondrogenesis with the formation of numerous cartilage islands (magnified in B and C). Denervated ear: (D) section through the distal edge of the wound at the necrosing ear tip, displaying severe regression of the wound with necrosis and failure to re-epithelialise; (E) section through the proximal wound edge showing re-epithelialisation, collagen deposition and proliferation at the cartilage stump (arrow). However, no characteristic features of a regenerative blastema-like structure were observed in the denervated MRL/MpJ ear wound. Scale bars: 100 μm.
The denervated MRL/MpJ distal wound margin, however, demonstrated a mass of necrotic tissue still present at 50 days post-wounding, with little inflammatory cell infiltrate (Fig. 5D). The epidermis had failed to migrate across this wound margin (Fig. 5D). The proximal half of the denervated wound showed a greater healing capacity, producing a re-epithelialised edge free from necrosis and demonstrated extensive collagen deposition with some limited proliferation at the cartilage stump (Fig. 5E). Unlike the non-denervated ear wound, however, there were no apparent features of a regenerative blastema-like structure, and no regeneration of hair follicles, sebaceous or sweat glands.
The denervation experiment was repeated in the C57BL/6 mouse strain, which has previously been demonstrated to possess a reduced tissue regenerative capacity in the ear wound compared with the MRL/MpJ strain (Rajnoch et al. 2003). We have confirmed in this study that regenerative features were far more pronounced and accelerated in the MRL/MpJ strain. Furthermore, unlike the MRL/MpJ strain, non-denervated C57BL/6 ear wounds failed to close completely by 4 months post-wounding. At days 5–10 post-wounding the denervated C57BL/6 ear wound exhibited similar characteristics to that observed in the denervated MRL/MpJ ear wound (data not shown). The wound became increasingly elliptical in shape with a thickening band of persistent necrosis around the margin, which also continued to enlarge well beyond the original biopsy wound and advanced towards the tip of the ear in a similar manner to the MRL/MpJ. As observed in the MRL/MpJ, the proximal half of the denervated C57BL/6 wound, however, appeared to have some limited healing capacity and produced a smooth re-epithelialised margin, although the hole remained wider than the original punch wound (Fig. 6). The non-denervated C57BL/6 ear healed in a similar fashion to that described in Rajnoch et al. (2003), as the ear wound had closed to up to half its original size by day 50 post-wounding (Fig. 6a). Histological analysis of the non-denervated C57BL/6 ear wound demonstrated characteristic features of limited regenerative capacity of the C57BL/6 (Fig. 6a). Both proximal and distal margins had re-epithelialised, but the proximal margin produced only a bulbous cartilage aggregate instead of the spatially organised islands observed in the MRL/MpJ. In the distal denervated C57BL/6 wound margin Masson's Trichrome staining indicated persistent necrosis and a delayed repair process (Fig. 6b). The proximal wound margin had undergone re-epithelialisation, but no regenerative features, such as cartilage formation, were present (Fig. 6b).
Fig. 6.

Macroscopic photographs and histology of the denervated and non-denervated C57BL/6 ear wound 50 days post-wounding. (a) Macroscopic photograph of the non-denervated C57BL/6 wound 50 days post-wounding showing limited wound closure (figures and arrows correspond to cross-sections sampled for histology displayed below). Masson's Trichrome stained tissue sections; (a1) through distal wound margin and (a2) through proximal wound margin showing a bulbous aggregate of cartilage. (b) Photograph of the C57BL/6 denervated ear wound 50 days post-wounding showing extension of wound area towards the ear tip (b1) through the distal tip of the ear wound displaying extensive necrosis and failure to re-epithelialise, and (b2) through the proximal ear wound margin showing re-epithelialisation, but no evidence of cartilage formation or dermal extension. Scale bars: 100 μm.
Discussion
For the first time we report that surgical denervation of the pinna has profound and significant effects on the regenerative ability of the MRL/MpJ mouse. We initially hypothesised that denervation would inhibit regeneration and blastema formation, and instead drive the wound down a default pathway of repair. However, we demonstrate that denervation not only halted regeneration in the MRL/MpJ strain, but also had a severe negative effect on normal ear wound repair in the C57BL/6 strain. This observation was particularly apparent in the distal portion of the ear punch wound. In both strains, denervation caused a significantly (P <0.05) increased wound area, extensive necrosis and, as a result, prolonged eschar retention and an inability to re-epithelialise the distal wound margin of the ear hole. Smith & Liu (2002) showed that chemical denervation with capsaicin treatment also resulted in an increase in wound area, prolonged scab retention and delayed re-epithelialisation in rat dorsal excisions. The enlarged wound tissue volume was thought to arise as a result of increased granulation cell proliferation without a proportionate increase in apoptosis. The report proposed that nociceptor innervation plays a critical role by regulating wound cellularity (Smith & Liu, 2002). Souza et al. (2005) later confirmed these findings, and also showed that denervation caused a decrease in mast cell migration.
Far less necrosis was observed in the proximal half of the denervated wounds in both strains; collagen deposition and successful re-epithelialisation indicated that the tissue was indeed attempting to repair itself. Unlike the non-denervated ear wound, however, there was no evidence of any blastema-like features such as a thickened epidermis or cartilage island formation. These findings correlate with observations made following amputation of a denervated amphibian limb. Re-epithelialisation of the stump still occurs, but a thickened secretory apical epidermal cap and subsequent blastema fail to form and the wound heals by repair rather than regeneration (Mescher & Tassava, 1975; Maden, 1978).
Both macroscopically and histologically, the day 50 denervated C57BL/6 ear wounds revealed a similar morphology to the MRL/MpJ ear wound equivalents with tissue decay localised to the distal half of the wound. The non-denervated C57BL/6 ear wound also demonstrated a limited regenerative capacity but lacked cartilage island formation, instead taking the form of a bulbous aggregate emanating from the original cartilage wound margin and showed only limited dermal extension. This delay in wound healing between the proximal and distal wound margins has also been described in our previous study (Buckley et al. 2011), where we demonstrated that the proximal wound margin healed at a faster rate and contained a higher density of regenerating nerve fibres than the distal margin. This observation in healing capacity is suggestive of a gradient effect across the ear and warrants further investigation. The act of ear punching itself will create a small amount of denervation immediately distal to the wound, and this may contribute to this gradient effect.
Sparse nerve infiltration of the denervated ear was noted 50 days post-wounding. It is possible that these axons originated from a transected nerve that had regenerated. A denervation study in rats determined that transected dorsal nerves regenerated within 3 weeks post-transection (Ranne et al. 2000). However, upon harvesting the mice were autopsied, but there was no evidence of nerve reconnection at the ear base. This suggests that these few axons identified in the ear wound may have arisen from smaller collateral branches of the rostral auricular nerve. Furthermore, an increased nerve infiltration was observed in the proximal wound margin than the distal (data not shown).
The proximal wound margin is directly above the base of the ear, with an abundant supply of larger capillaries and a thicker cartilage layer compared with the distal wound. At distal wound margin, where the main branches of nerves and blood vessels running down the centre of the ear have been rendered non-functional following surgery, the wound is reliant on local subsidiary branches to supply oxygen, nutrients and important regenerative signals. Rageh et al. (2002) claimed that the main oxygen supply to an amphibian limb blastema is derived from the newly developing vasculature.
In the denervated ear wounds of both MRL/MpJ and C57BL/6 strains there was a progressive deterioration of tissue, spanning from the initial ear punch wound out towards the tip of the ear, which often became discoloured and cyanosed. A similar observation has also been described in denervated larval amphibian limb stumps where nerve transection at the time of amputation prevented regeneration but, unlike adult limbs, also caused mass death of dedifferentiated cells leading to a distal–proximal regression of the stump (Singer, 1952). Bedelbaeva et al. (2010) have recently shown that MRL/MpJ ear fibroblasts are poised in G2 arrest due to a defect in the cell cycle G1 checkpoint protein, p21, and display a heightened basal and wound site damage/repair response that is also common to regenerating axolotl limbs and mammalian embryonic cells. Extensive necrosis and disintegration of the denervated ear tip may have changed the structural dynamics of the wound. This could have altered the mechanical stress across the whole wound and prevented the usual purse-string closure observed during ear regeneration, as the wound is effectively splinted open by the relative rigidity of the cartilage.
It is interesting to note that denervation appeared to affect both MRL/MpJ and C57BL/6 strains in a similar manner. We have demonstrated that the C57BL/6 strain also possesses a limited regenerative capacity, suggesting that the strain may be sub-optimally utilising some of the molecular signalling pathways involved in regeneration (Rajnoch et al. 2003). It is not surprising therefore, that denervation also disrupted the healing process observed in the C57BL/6 ear wound, particularly as even basic wound repair failed to occur.
Several other studies also report that denervation impairs the wound repair process (Peskar et al. 1995; Reinshagen et al. 1996; Engin, 1998). Denervation decreases levels of potent vasodilatory and chemotactic neuropeptides involved in neurogenic inflammation, such as substance P, which stimulates leukocyte chemotaxis, neutrophil activation, synthesis of transforming growth factor alpha and induces expression of intracellular adhesion molecule-1 (Richards et al. 1999); and calcitonin gene-related peptide, a neuropeptide found in C and Aδ sensory axons that stimulates T-lymphocyte and neutrophil migration (Chiang et al. 2005). Immunohistochemical studies in rats have reported significantly reduced monocyte, macrophage and T-lymphocyte counts in denervated wounds 4 days post-operatively (Richards et al. 1999). The action of debriding the wound and removing injured cells may be paramount for creating an appropriate environment for blastema-like formation. Amphibians are known to possess a poor immune system; however, an acute and tightly regulated period of inflammation is observed prior to blastema formation and de-differentiation of cells (Flajnik et al. 1986; Sicard, 2002; Harty et al. 2003; Mescher & Neff, 2005, 2006). This study indicates that innervation is important for the regenerative process, but is not wholly responsible for promoting full regeneration of the ear wound. It seems other factors that are inherently different between MRL/MpJ and C57BL/6 are also governing the regenerative capacity of the strain. Further investigation is required to determine how denervation may hinder inflammatory cell recruitment and delay healing in both MRL/MpJ and C57BL/6 wounds.
It is unclear how the nerves are capable of exerting different effects on the surrounding cells depending on the local tissue environment. Several studies have indicated that trophic factors released from the nerves are thought to stimulate blastema formation and regeneration (Maden, 1978; Meschar, 1996; Nye et al. 2003). Shepherd et al. (2005) propose that these blastema-promoting factors, thought to include neuropeptides such as substance P (Stocum, 1995), may actually exert their trophic effects by acting on local immune cells present at the wound site, although this is yet to be established. Nerves may promote an environment compatible with active cell proliferation and movement. Kumar et al. (2007) have determined that nAG, an anterior gradient protein family member and a secreted ligand for Prod 1 important for proximodistal identity in amphibian limb regeneration, is expressed in regenerating nerves and the wound epidermis. Furthermore, nAG expression is halted by denervation, and interestingly its re-expression following electroporation is capable of rescuing a denervated blastema. Kumar et al. (2007) suggest that Schwann cells rather than the axons are the source of this stimulatory factor for regeneration. Schwann cells, which wrap around and myelinate the axons, respond to injury by forming a pathway to guide axons as they regenerate (Raisman, 2001). Interestingly, the expression of an nAG is upregulated by regenerating axons in both the nerve sheath and by gland cells under (newt) or within (axolotl) the wound epithelium, which discharge by a holocrine mechanism (Kumar et al. 2010). More recently, Kumar et al. (2011) have shown that nerve-free aneurogenic limbs demonstrate increased expression of nAG in the epidermis, and are able to regenerate without nerves but become nerve-dependent after innervation. Future studies will investigate the role of Schwann cells, inflammation, the wound epidermis, as well as gland cells and potential mitogens within the MRL/MpJ regeneration process. It may be that an increased soluble factor released from the intact nerves of the MRL/MpJ mouse following injury enables the strain to elicit a greater regenerative capacity.
Summary
We have shown that denervation of MRL/MpJ ears leads to a dramatic decline in the ability of an ear punch wound to develop a blastema-like structure and successfully close an ear punch hole. This study suggests that the nerves within the ear wound influence the formation of a blastema-like structure and subsequent tissue regeneration. Denervated ear wounds degenerate, expand and have still not healed by 50 days post wounding. Further investigations are required to establish the identity of the trophic stimulus exerted by nerves within this regenerative model. Ultimately, a greater understanding of how peripheral nerve networks regenerate into wounds and successfully re-innervate appropriate target structures is required and, further still, how nerves themselves can promote regeneration rather than repair.
References
- Barker AR, Rosson GD, Dellon AL. Wound healing in denervated tissue. Ann Plast Surg. 2006;57:339–342. doi: 10.1097/01.sap.0000221465.69826.b7. [DOI] [PubMed] [Google Scholar]
- Beare AH, Metcalfe AD, Ferguson MW. Location of injury influences the mechanisms of both regeneration and repair within the MRL/MpJ mouse. J Anat. 2006;209:547–559. doi: 10.1111/j.1469-7580.2006.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedelbaeva K, Snyder A, Gourevitch D, et al. Lack of p21 expression links cell cycle control and appendage regeneration in mice. PNAS. 2010;30:107. doi: 10.1073/pnas.1000830107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockes JP, Kumar A. Comparative aspects of animal regeneration. Annu Rev Cell Dev Biol. 2008;24:525–549. doi: 10.1146/annurev.cellbio.24.110707.175336. [DOI] [PubMed] [Google Scholar]
- Buckley G, Metcalfe AD, Ferguson MWJ. Peripheral nerve regeneration in the MRL/MpJ ear wound. J Anat. 2011;218:163–172. doi: 10.1111/j.1469-7580.2010.01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang HY, Chen CT, Chien HF, et al. Skin denervation, neuropathology, and neuropathic pain in a laser-induced focal neuropathy. Neurobiol Dis. 2005;18:40–53. doi: 10.1016/j.nbd.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Clark LD, Clark RK, Heber-Katz E. A new murine model for mammalian wound repair and regeneration. Clin Immunol Immunopathol. 1998;88:35–45. doi: 10.1006/clin.1998.4519. [DOI] [PubMed] [Google Scholar]
- Engin C. Effects of calcitonin gene-related peptide on wound contraction in denervated and normal rat skin: a preliminary report. Plast Reconstr Surg. 1998;101:1887–1890. doi: 10.1097/00006534-199806000-00017. [DOI] [PubMed] [Google Scholar]
- Flajnik MF, Kaufman JF, Hsu E, et al. Major histocompatibility complex-encoded class I molecules are absent in immunologically competent Xenopus before metamorphosis. J Immunol. 1986;137:3891–3899. [PubMed] [Google Scholar]
- Harty M, Neff AW, King MW, et al. Regeneration or scarring: an immunological perspective. Dev Dyn. 2003;226:268–279. doi: 10.1002/dvdy.10239. [DOI] [PubMed] [Google Scholar]
- Heber-katz E, Leferovich JM, Bedelbaeva K, et al. Spallanzani's mouse: a model of restoration and regeneration. Curr Top Microbiol Immunol. 2003;280:165–190. doi: 10.1007/978-3-642-18846-6_5. [DOI] [PubMed] [Google Scholar]
- Kumar A, Godwin JW, Gates PB, et al. Molecular basis for the nerve dependence of limb regeneration in an adult vertebrate. Science. 2007;318:772–777. doi: 10.1126/science.1147710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Neville G, Brockes JP, et al. A comparative study of gland cells implicated in the nerve dependence of salamander limb regeneration. J Anat. 2010;217:16–25. doi: 10.1111/j.1469-7580.2010.01239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Delgado JP, Gates PB, et al. The aneurogenic limb identifies developmental cell interactions underlying vertebrate limb regeneration. PNAS. 2011;103:13 588–13 593. doi: 10.1073/pnas.1108472108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Warn JD, Fan Q, et al. Relationships between nerves and myofibroblasts during cutaneous wound healing in the developing rat. Cell Tissue Res. 1999;297:423–433. doi: 10.1007/s004410051369. [DOI] [PubMed] [Google Scholar]
- Maden M. Neurotrophic control of the cell cycle during amphibian limb regeneration. J Embryol Exp Morphol. 1978;48:169–175. [PubMed] [Google Scholar]
- Maggi CA. The effects of tachykinins on inflammatory and immune cells. Regul Pept. 1997;70:75–90. doi: 10.1016/s0167-0115(97)00029-3. [DOI] [PubMed] [Google Scholar]
- Mescher AL. The cellular basis of limb regeneration in urodeles. Int J Dev Biol. 1996;40:785–795. [PubMed] [Google Scholar]
- Mescher AL, Neff AW. Regenerative capacity and the developing immune system. Adv Biochem Eng Biotechnol. 2005;93:39–66. doi: 10.1007/b99966. [DOI] [PubMed] [Google Scholar]
- Mescher AL, Neff AW. Limb regeneration in amphibians: immunological considerations. Scientific World Journal. 2006;6:1–11. doi: 10.1100/tsw.2006.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher AL, Tassava RA. Denervation effects on DNA replication and mitosis during the initiation of limb regeneration in adult newts. Dev Biol. 1975;44:187–197. doi: 10.1016/0012-1606(75)90386-3. [DOI] [PubMed] [Google Scholar]
- Metcalfe AD, Ferguson MWJ. Harnessing wound healing and regeneration for tissue engineering. Biochem Soc Trans. 2005;33:413–417. doi: 10.1042/BST0330413. [DOI] [PubMed] [Google Scholar]
- Metcalfe AD, Ferguson MWJ. Tissue engineering of replacement skin: the crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J R Soc Interface. 2007;4:413–437. doi: 10.1098/rsif.2006.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe AD, Willis H, Beare A, et al. Characterizing regeneration in the vertebrate ear. J Anat. 2006;209:439–446. doi: 10.1111/j.1469-7580.2006.00632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuys R, Donkelaar HJ, Nicholson C. The Central Nervous System of Vertebrates. Vol. 1. Berlin, London: Springer; 1998. ISBN 3-540-56013-0. [Google Scholar]
- Nye HL, Cameron JA, Chernoff EA, et al. Regeneration of the urodele limb: a review. Dev Dyn. 2003;226:280–294. doi: 10.1002/dvdy.10236. [DOI] [PubMed] [Google Scholar]
- Peskar BM, Lambrecht N, Stroff T, et al. Functional ablation of sensory neurons impairs healing of acute gastric mucosal damage in rats. Dig Dis Sci. 1995;40:2460–2464. doi: 10.1007/BF02063255. [DOI] [PubMed] [Google Scholar]
- Rageh MA, Mendenhall L, Moussad EE, et al. Vasculature in pre-blastema and nerve-dependent blastema stages of regenerating forelimbs of the adult newt, Notophthalmus viridescens. J Exp Zool. 2002;292:255–266. doi: 10.1002/jez.10015. [DOI] [PubMed] [Google Scholar]
- Raisman G. Olfactory ensheathing cells – another miracle cure for spinal cord injury? Nat Rev Neurosci. 2001;2:369–375. doi: 10.1038/35072576. [DOI] [PubMed] [Google Scholar]
- Rajnoch C, Ferguson S, Metcalfe AD, et al. Regeneration of the ear after wounding in different mouse strains is dependent on the severity of wound trauma. Dev Dyn. 2003;226:388–397. doi: 10.1002/dvdy.10242. [DOI] [PubMed] [Google Scholar]
- Ranne J, Kalimo H, Pyykko K, et al. Wound healing in denervated rat groin skin flap. Eur Surg Res. 2000;32:197–202. doi: 10.1159/000008763. [DOI] [PubMed] [Google Scholar]
- Reinshagen M, Patel A, Sottili M, et al. Action of sensory neurons in an experimental at colitis model of injury and repair. Am J Physiol. 1996;270:G79–G86. doi: 10.1152/ajpgi.1996.270.1.G79. [DOI] [PubMed] [Google Scholar]
- Richards AM, Floyd DC, Terenghi G, et al. Cellular changes in denervated tissue during wound healing in a rat model. Br J Dermatol. 1999;140:1093–1099. doi: 10.1046/j.1365-2133.1999.02908.x. [DOI] [PubMed] [Google Scholar]
- Shepherd AJ, Downing JE, Miyan JA. Without nerves, immunology remains incomplete –in vivo veritas. Immunology. 2005;116:145–163. doi: 10.1111/j.1365-2567.2005.02223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicard RE. Differential inflammatory and immunological responses in tissue regeneration and repair. Ann N Y Acad Sci. 2002;961:368–371. doi: 10.1111/j.1749-6632.2002.tb03126.x. [DOI] [PubMed] [Google Scholar]
- Singer M. The influence of the nerve in regeneration of the amphibian extremity. Q Rev Biol. 1952;27:169–200. doi: 10.1086/398873. [DOI] [PubMed] [Google Scholar]
- Smith PG, Liu M. Impaired cutaneous wound healing after sensory denervation in developing rats: effects on cell proliferation and apoptosis. Cell Tissue Res. 2002;307:281–291. doi: 10.1007/s00441-001-0477-8. [DOI] [PubMed] [Google Scholar]
- Souza BR, Cardoso JF, Amadeu TP, et al. Sympathetic denervation accelerates wound contraction but delays reepithelialization in rats. Wound Repair Regen. 2005;13:498–505. doi: 10.1111/j.1067-1927.2005.00070.x. [DOI] [PubMed] [Google Scholar]
- Stocum DL. Wound Repair, Regeneration and Artificial Tissues. Georgetown, Texas, USA: R.G. Landes; 1995. [Google Scholar]
- Stocum DL. Amphibian regeneration and stem cells. Curr Top Microbiol Immunol. 2004;280:1–70. doi: 10.1007/978-3-642-18846-6_1. [DOI] [PubMed] [Google Scholar]
- Todd TJ. On the process of reproduction of the members of the aquatic salamander. Q J Sci Lit Arts. 1823;16:84–96. [Google Scholar]
- Wallengren J. Vasoactive peptides in the skin. J Investig Dermatol Symp Proc. 1997;2:49–55. doi: 10.1038/jidsymp.1997.11. [DOI] [PubMed] [Google Scholar]
- Westerman RA, Carr RW, Delaney CA, et al. The role of skin nociceptive afferent nerves in blister healing. Clin Exp Neurol. 1993;30:39–60. [PubMed] [Google Scholar]
- Whited JL, Tabin CJ. Limb regeneration revisited. J Biol. 2009;8:5. doi: 10.1186/jbiol105. [DOI] [PMC free article] [PubMed] [Google Scholar]


