Abstract
The maximum capability of a muscle can be estimated from simple measurements of muscle architecture such as muscle belly mass, fascicle length and physiological cross-sectional area. While the hindlimb anatomy of the non-human apes has been studied in some detail, a comparative study of the forelimb architecture across a number of species has never been undertaken. Here we present data from chimpanzees, bonobos, gorillas and an orangutan to ascertain if, and where, there are functional differences relating to their different locomotor repertoires and habitat usage. We employed a combination of analyses including allometric scaling and ancovas to explore the data, as the sample size was relatively small and heterogeneous (specimens of different sizes, ages and sex). Overall, subject to possible unidentified, confounding factors such as age effects, it appears that the non-human great apes in this sample (the largest assembled to date) do not vary greatly across different muscle architecture parameters, even though they perform different locomotor behaviours at different frequencies. Therefore, it currently appears that the time spent performing a particular behaviour does not necessarily impose a dominating selective influence on the soft-tissue portion of the musculoskeletal system; rather, the overall consistency of muscle architectural properties both between and within the Asian and African apes strengthens the case for the hypothesis of a possible ancient shared evolutionary origin for orthogrady under compressive and/or suspensory loading in the great apes.
Keywords: locomotion, positional behaviour, primate, scaling
Introduction
It is generally agreed that the living apes form a biological lineage defined by characters of the locomotor system (trunk and limbs) rather than the cranial and dental features which define many other mammalian groups (see review in Crompton et al. 2008). Due to interest in the evolution of hominin bipedalism, morphological studies of the apes have frequently focused on hindlimb anatomy (e.g. Thorpe et al. 1999; Carlson, 2006; Payne et al. 2006; Channon et al. 2009). Recently, a thorough phylogenetic analysis of gross muscle morphology (presence/absence, origin/insertion) in the primate upper limb has been undertaken, with the result that the hominids cluster in consensus with the molecular evidence (Diogo & Wood, 2011). However, forelimb muscle architecture (muscle mass, fascicle length and physiological cross-sectional area) has been studied to a lesser extent and in fewer species (e.g. chimpanzee: Thorpe et al. 1999; Carlson, 2006; orangutan and chimpanzee: Oishi et al. 2008, 2009; gibbon: Michilsens et al. 2009). Non-human apes are considered to be united predominantly by shared features in the thorax and upper limb, such as short lumbar spines, craniocaudal increase in area of lumbar centra, broad ilia, broad, shallow trunks, dorsally placed scapulae, and shoulder joints otherwise adapted for highly abducted arm postures (e.g. Larson, 1998; Ward, 2007). While past comparative studies of ape forelimb anatomy have been carried out under a paradigm that held that apes were united by their use of brachiation (e.g. Ashton & Oxnard, 1962a,b;), fossil evidence (e.g. Pierolapithecus; Moyà-Solà et al. 2004) now suggests that forelimb-suspensory locomotion arose independently in several ape lineages. An increasing number of field studies have also revealed that it is generalised orthograde clambering, where the trunk is upright and both fore- and hindlimbs are used in varying degrees to support body mass in suspensory or compressive loading regimes (e.g. Hunt, 1991a, 1996; Fleagle, 1999), which is the locomotor behaviour that characterises the non-human great apes (e.g. Hunt, 1992; Doran, 1993a,b; Fleagle, 1999; Thorpe & Crompton, 2006; reviewed in Crompton et al. 2008). A comparison of the functional morphology of the forelimbs could therefore add greatly to our understanding of locomotor diversity in the hominoids and the evolution of such diversity. As muscle architecture might be expected to respond relatively strongly to changes in locomotor behaviour, the overall aim of this study was to compare the forelimb muscle architecture of the non-human great apes, with the goal of expanding our understanding of the relationship between form and function in these species and furthering our knowledge of the extent and evolution of locomotor diversity in the hominoids.
The measurement of basic soft-tissue parameters, including muscle belly mass and muscle fascicle length, enables functional parameters to be estimated. Muscle fascicle length reflects the number of sarcomeres in series, and the longer the muscle fascicle length, the greater the maximum shortening velocity of the muscle fascicles (see Wickiewicz et al. 1984; Thorpe et al. 1999). Physiological cross-sectional area (PCSA), on the other hand, reflects the number of sarcomeres in parallel, and provides an indication of the maximum force which a muscle can produce (Sacks & Roy, 1982; Zajac, 1992).Together, muscle fascicle length and PCSA provide a way to determine the maximum capability of a muscle (e.g. Thorpe et al. 1999; Payne et al. 2006; Carlson, 2006). By relating these measurements of muscle architecture (anatomical form) to the locomotor behaviours performed by the animal (function and performance), we can determine whether and where differences exist in the properties of the muscle architecture of non-human great apes.
Even though the locomotor repertoires of the non-human great apes overlap, the proportions of the different locomotor modes/behaviours, and their kinematics, do differ between species and we might expect this to be reflected in their morphology. One of the major behavioural differences is the percentage of time spent in the arboreal milieu. The Asian great ape, the orangutan, is predominantly arboreal, spending nearly 100% of its time in the forest canopy (although Bornean flanged male orangutans will travel on the ground; see Galdikas, 1988), as reflected by much longer forelimbs relative to body size compared with the African apes (chimpanzees, bonobos and gorillas). African apes primarily use terrestrial quadrupedalism/knuckle-walking when travelling (89.9% of locomotor behaviour in chimpanzees, 35.3% in bonobos, 64.4% in lowland gorillas, but up to 96% in Virunga mountain gorillas: see Hunt, 2004), and enter the canopy predominantly to feed and sleep (in the case of chimpanzees; Hunt, 1992). We might therefore expect that the greater use of arboreal locomotor behaviour in orangutans would be reflected in their morphology, e.g. digital flexor muscles of greater mass with longer muscle fascicles (providing the ability to produce force over a greater range of motion) might be beneficial when gripping variously angled supports in suspension (Alexander et al. 1981).
However, African ape morphology might be expected to exhibit stronger adaptations to terrestrial quadrupedalism. Chimpanzees and other primates support the majority of their body mass on their hindlimbs during quadrupedalism (Reynolds, 1985; Kimura, 1992; Demes et al. 1994; Li et al. 2004; Raichlen et al. 2009) and this might be expected to facilitate non-supportive use of the forelimbs in an arboreal habitat. Nevertheless, due to the greater use of terrestrial behaviour in the African apes than in orangutans, we might expect that their forelimbs would be less specialised for arboreal behaviours. African apes do use suspensory behaviours, but most frequently as static postural activity during feeding, where the forelimb stresses must be lower than those for suspensory locomotion: we should, therefore, anticipate differences in their muscle use from that in orangutans, where suspensory locomotion is much more common.
As all non-human apes use vertical climbing to a greater or lesser extent to access food in the canopy, one would expect all to show adaptations for large force production in the elbow flexors for pulling-up (e.g. Isler, 2005). However, vertical climbing is performed in a kinematically distinct manner in the African apes compared with orangutans (Isler & Thorpe, 2003; Isler, 2005). We therefore expect that such distinctions will be reflected in the forelimb muscle architecture (e.g. the elbow flexors) of the different species.
Materials and methods
The material obtained for this study comprised cadavers of one chimpanzee (Pan troglodytes: Ptsm), two bonobos (Pan paniscus: Ppam, Pp), five gorillas (Gorilla gorilla gorilla: Gsm, Gam, Gp, Gj; Gorilla gorilla graueri: Gm), and three orangutans (Pongo abelii: Oaf, Ojf, Ojm) (see Table 1 for subject information). Additional data for three chimpanzees (chimps 93, 94 and 95) from Thorpe (1997) were also incorporated. Cadavers were obtained from The Zoological Society, London (Ptsm, chimp 95), The North of England Zoological Society (chimp 93, chimp 94, Gj), Twycross Zoo (Gsm, Gam), Apenheul Zoo (Ppam), The Royal Zoological Society of Antwerp (Pp, Gm), the Anthropological Institute and Museum, Zurich (Ojf, Ojm, Gp) and Paignton Zoo (Oaf). Although the use of captive animals is not ideal, it is the only ethically acceptable option when studying (critically) endangered non-human apes. However, in an attempt to reduce the possible confounding effects of captivity, we ensured that all individuals had been active and healthy immediately prior to death and had suffered no illnesses that would result in muscle wastage. We make extensive use of data collected from previous studies, as individual datasets are necessarily very small because of the problems of obtaining ape cadavers: combining datasets enables more robust statistics to be performed. All specimens had been eviscerated during autopsy and were fresh-frozen in the anatomical position until needed. Only one limb from each of the specimens was available for the collection of muscle architecture data.
Table 1.
Subject information
| Subject | Species | Sex | Body mass (kg) | Age at death (years) | Cause of death |
|---|---|---|---|---|---|
| Ptsm | Pan troglodytes | M | 50.2 | 11 | Group violence |
| Chimp 93 | M | 27.6 | Sub-adult | PE | |
| Chimp 94 | M | 41.7 | Adult | Euthanized | |
| Chimp 95 | M | 37.0 | 6 | Peritonitis | |
| Ppam | Pan paniscus | M | 41.92 | 22 | Euthanized |
| Pp | M | 64.0 | 29 | Cardiovascular | |
| Gsm | Gorilla gorilla gorilla | M | 152.0 | 18 | BH |
| Gam | M | 175.0 | 30 | Cardiovascular | |
| Gp | M | 130.0 | 35 | Cardiovascular | |
| Gj | M | 120.0 | 30 | Cardiovascular | |
| Gm | Gorilla gorilla graueri | M | 120.0 | 33 | Cardiovascular |
| Oaf | Pongo abelii | F | 54.0 | 45 | Euthanized |
| Ojf | F | 12.5 | 5 | Viral | |
| Ojm | M | 18.7 | 6 | Cardiovascular |
M, male; F, female.
PE, death due to a pulmonary embolism; BH, death due to a brain haemorrhage.
Anatomical measurements
Muscle fascia were removed, muscles separated and identified, and their points of bony origin and insertion recorded before muscles were removed systematically with complete tendons of origin and insertion attached. Muscle–tendon unit lengths were measured, including separate measurements for external tendon lengths at the origin and insertion, and for muscle belly length. External tendon length was measured as the distance from either the most proximal (tendon of origin) or distal (tendon of insertion) muscle fibres to the point of tendon attachment to the bone. Any external tendon was then removed and muscle belly mass (including internal tendons if present) recorded. Finally, the muscle belly was cut, either along the line of the internal tendon (for pennate muscles) or along the belly (for parallel-fibred muscles) to reveal the full length of the muscle fibres. Three measurements of muscle fascicle length were made at different locations throughout the belly: muscle fascicle length assesses the length of the bundle of muscle fibres that are visible to the naked eye. All lengths were measured to the nearest millimetre using a metal rule. Muscle mass was measured to the nearest 0.1 g.
To provide an estimate of maximum muscle force production, PCSA was calculated using the equation:
| (1) |
where m is muscle belly mass in grams, ρ is the density of fresh muscle (1.06 g cm−3, Mendez & Keys, 1960), l is muscle fascicle length in cm, and θ is the angle of the fascicles to the tendon, i.e. the pennation angle. In apes, θ is generally < 30° in both fore- and hindlimb muscles (see Thorpe et al. 1999), thus cosθ is approximately one and can be omitted from the equation (also discussed in Calow & Alexander, 1973).
To enable comparisons, muscles were grouped according to their primary functions, based on the classical convention of joint movement as used in previous studies of ape muscle anatomy (Swindler & Wood, 1973; Michilsens et al. 2009; see Table 2 for groupings). The use of EMG data to ascertain the role of muscles during different locomotor modes would provide more specific details of their function; however, sufficient data are not available at present during naturalistic behaviours (although see Tuttle & Basmajian, 1974, 1978, Tuttle et al. 1972; Stern & Larson, 2001 for examples), to be able to classify groupings based on EMG. However, grouping data into functional muscle groups in this way is adequate when studying locomotor adaptation and enables the data to be interpreted at a sufficiently broad level. If significant differences are identified between species in a large muscle group, the functional group can then be divided into individual muscles to ascertain the specific muscle(s) responsible.
Table 2.
Functional muscle groups
| Muscle group | Muscles |
|---|---|
| Shoulder rotators* | Latissimus dorsi, infraspinatus, teres major, teres minor, subscapularis, deltoid |
| Shoulder adductors | Coracobrachialis, teres major |
| Shoulder abductors | Deltoid, supraspinatus |
| Shoulder flexors | Deltoid, biceps brachii, coracobrachialis |
| Shoulder extensors | Triceps brachii, teres major, latissimus dorsi, deltoid |
| Elbow flexors | Biceps brachii, brachialis, brachioradialis |
| Elbow extensors | Triceps brachii, dorsoepitrochlearis, anconeus |
| Supinators | Supinator |
| Pronators | Pronator teres, pronator quadratus |
| Wrist flexors | Flexor carpi ulnaris, flexor carpi radialis, flexor digitorum superficialis, flexor digitorum profundus |
| Wrist extensors | Extensor carpi ulnaris, extensor carpi radialis brevis, extensor carpi radialis longus |
| Digital flexors | Flexor pollicis longus, flexor digitorum superficialis, flexor digitorum profundus, abductor pollicis longus |
| Digital extensors | Extensor pollicis brevis, extensor digitorum communis, extensor pollicis longus, extensor digiti minimi, extensor indicis |
For description of shoulder muscle group actions see Michilsens et al. (2009).
Obtaining measurements for muscles at the shoulder was problematic as often these muscles could not be separated into their separate functional units. For example, the deltoid muscle (see Ashton & Oxnard, 1962a,b; for a description of the primate deltoid) consists of three components arising from three different points on the shoulder girdle that insert together onto the shaft of the humerus. Each part performs a different action, but it was not possible to separate the functional units anatomically, and as a result the deltoid was removed and measured as one muscle. Such muscles were therefore placed into multiple functional groups, even though only a proportion of the muscle was likely involved in each separate function. Estimating the proportion of each muscle that contributed to a specific function was not a viable option: without detailed EMG analysis of the different regions of a muscle we cannot ascertain accurately what proportion contributes to what function, and indeed such relationships may be more complex than expected (e.g. Michiels & Bodem, 1992). Intrinsic hand muscles were not included in the analysis and dissection of the shoulder girdle was not complete in all species due to damage during evisceration or autopsy (particularly of the pectoral muscles). Pectoralis major was missing from all individuals because of the autopsy requirements. Therefore, total values for the shoulder flexors, shoulder adductors and shoulder rotators were underestimated, as pectoralis major contributes to each of these groups. Otherwise, in cases where we were not able to sample all muscles in a muscle group for a particular individual, those individuals were excluded from the analysis of that particular muscle group.
To obtain overall values for muscle groups, muscle belly masses and PCSA values within a group were simply added together. However, muscle fascicle length was calculated as a weighted harmonic mean to take into account the different sizes of the muscles in a group, using the equation:
| (2) |
where L is the group fascicle length for a group where the jth member has a mass mj and a fascicle length of lj (Alexander et al. 1981).
Data analysis
For species comparisons we have previously recommended both normalising the data using scaling exponents calculated from regression analysis of the data (to determine whether the relationship is geometric or allometric) and using ancova with body mass as a covariate to explore whether interspecific differences are present (Myatt et al. 2011a). This approach was also used here. Ordinary least squares (OLS) regression analysis of log-transformed data was performed by plotting each muscle architecture variable (i.e. muscle belly mass, fascicle length and PCSA) against body mass (kg) using minitab® version 15 (USA). We intended thereby to establish the form of relationships based on our collected measurements of muscle architecture, rather than assume geometric similarity, as has frequently been done in previous studies (e.g. Thorpe et al. 1999; Payne et al. 2006).
From the regression analysis, the relationships between body mass and muscle belly mass, fascicle length and PCSA were explored and scaling exponents established for the functional muscle groups, where a significant linear relationship was found to exist. Scaling exponents were determined based on the equation Y = aMb, where Y is the muscle architecture variable, M is body mass in kg, a and b are constants, and b is the scaling exponent (see Alexander et al. 1981; Schmidt-Nielson, 1984; Pollock & Shadwick, 1994). Exponents [together with standard errors (SE) and confidence intervals (CI)] for the individual muscle groups were calculated, as were exponents for the proximal and distal forelimb muscles and for the limb as a whole. Mean scaling exponents were established from the individual group values to enable comparison with previous studies. Raw data were normalised using the individual muscle group scaling exponents for muscle belly mass, fascicle length and PCSA, except in instances where there was no significant relationship with body mass.
ancovas
ancovas were employed using minitab 15 (GLM function; type III hypotheses) using log-transformed data, with body mass as a covariate, to compare muscle belly mass, fascicle length and PCSA between the different species. To achieve a model of best fit, the main effects ‘species’ and ‘body mass’ and the interaction ‘species*body mass’ were first included. The interaction between the variable of interest and the covariate was included to test for homogeneity (Engqvist, 2005). If the interaction term is significant, then the slopes are heterogeneous and it is inappropriate to continue with the ancova (Engqvist, 2005). If the interaction term is non-significant (as was the case in all models in the present study), backward elimination can be used to remove each non-significant term (significance taken at the P=0.05 level), one at a time, until the best fitting model remains (see Grafen & Hails, 2002). In instances where species was found to have a significant effect, Tukey's post-hoc tests were performed to establish which species were significantly different (P=0.05). Multiple individuals of each species are needed to adopt this approach. Models for the shoulder extensors, shoulder abductors and shoulder rotators could therefore not be established, as the data available were reduced to one individual per species in these groups because of missing data. Missing data also resulted in Ptsm, Gp and Gj being excluded from the supinator model, chimp 93 being excluded from the shoulder flexor model, and Gp and Gj being excluded from the shoulder adductor model.
Results
Descriptive anatomy
Raw data from all subjects are presented in Supporting Information Appendix S1. The general gross anatomy of non-human ape arm and shoulder muscles is similar to that of humans, except for the presence of an additional muscle, the dorsoepitrochlearis, in the upper arm of the non-human primates, which is only present as fascia in humans (Ashton & Oxnard, 1962a,b; Oxnard & Franklin, 2008). The qualitative anatomy of the ape forelimb has been described elsewhere (e.g. Sonntag, 1924; Miller, 1952; Ashton & Oxnard, 1962a,b; Kimura & Takai, 1970; Swindler & Wood, 1973; Gibbs et al. 2002; Michilsens et al. 2009) and thus this section is limited to the description of those anomalies which may be functionally important.
The biceps brachii usually arises from the coracoid process of the scapula (short head) and the supraglenoid tuberosity of the scapula (long head) in primates (Sonntag, 1924; Miller, 1952; Kimura & Takai, 1970; Swindler & Wood, 1973; Youlatos, 2000). However, in the orangutan specimen Oaf the long head was monoarticular, arising from the top of the lateral side of the humerus, just below the bicipital groove. The short head originated as normal from the coracoid process of the scapula. The origin of biceps brachii in the gorilla specimens Gsm and Gam also differed in that the tendon of the long head originated from below the supraglenoid tuberosity on the dorsum of the scapula.
The flexor pollicis longus, a separate muscle in humans, is frequently absent as a separate muscle in non-human apes, although there may be an additional tendon from the belly of flexor digitorum profundus running to the pollex (Mangini, 1960). A separate belly with a tendon to digit one was present in some specimens in the present study (Ppam, Gam and Oaf), with the same origin and insertion as the flexor pollicis longus in Homo (Mangini, 1960). In the bonobo specimen, Ppam, the flexor pollicis longus also had an additional tendon to digit two and the muscle belly was more tightly fused to flexor digitorum profundus. Specimens Ptsm and Gsm also had separate muscle bellies to flexor digitorum profundus, but rather than giving a tendon to digit one, gave a tendon to digit two (and so were analogous to flexor pollicis longus, but only inserting on digit two).
Scaled data
Equation components (together with SEs and CIs) from regressions of log-transformed data for allometric scaling are given in Table 3 for muscle belly mass, in Table 4 for fascicle length and in Table 5 for PCSA. Muscle belly masses and PCSAs for all individual muscle groups were found to have significant linear relationships with body mass (M). However, fascicle length did not have a significant relationship with body mass for the shoulder abductors, pronators, wrist flexors or wrist extensors, although fascicle length for the other muscle groups did have a significant linear relationship with body mass. Scaling exponents with confidence intervals overlapping the exponents that would be predicted by isometry (belly mass: M1.0; fascicle length: M0.33; PCSA: M0.67; see Alexander et al. 1981) were identified in some instances and are highlighted in bold. The amount of overlap with those exponents predicted by isometry is notable because of the number of studies using these exponents to scale their data (e.g. Payne et al. 2006; Channon et al. 2009). The overall mean scaling exponent for muscle mass was M0.89, individual muscle groups ranging from M0.71 to M1.04, compared to the isometric prediction of M1.0. Overall, eight of the 13 individual muscle group exponents had CIs overlapping the exponent predicted by isometry, although some muscle groups, e.g. the elbow flexors, scaled below that predicted by isometry (labelled negatively isometric). The mean scaling exponent for fascicle length was M0.23 (range: M0.12–M0.35), compared with the isometric prediction of M0.33. Although all 13 individual muscle group exponent CIs overlapped the exponent predicted by isometry, two functional groups (pronators and digital flexors) were negatively isometric. Furthermore, three of the overlapping exponents did not actually scale significantly to body mass in a linear relationship and thus should not be used to scale the data. The PCSA mean scaling exponent was M0.67 (range: M0.50–M0.85) and all CIs overlapped the value predicted by isometry.
Table 3.
Allometric equation constants for forelimb muscle group belly mass (g) ± SE using logged data (exponents in bold have CIs overlapping those predicted by isometry; Mb, where b = 1.0)
| Muscle group | a (± SE) | CI (a) | b (± SE) | CI (b) | R2 | P |
|---|---|---|---|---|---|---|
| Overall | ||||||
| Total forelimb | 4.74 (± 0.74) | 1.74 | 0.99 (± 0.18) | 0.42 | 0.90 | 0.033 |
| Proximal forelimb | 4.55 (± 0.68) | 1.45 | 1.00 (± 0.17) | 0.36 | 0.90 | 0.009 |
| Distal forelimb | 3.61 (± 0.23) | 0.41 | 0.78 (± 0.06) | 0.11 | 0.94 | < 0.001 |
| Proximal muscles* | ||||||
| Shoulder rotators | 3.05 (± 0.59) | 1.19 | 1.05 (± 0.14) | 0.28 | 0.92 | 0.002 |
| Shoulder adductors | 1.32 (± 0.36) | 0.65 | 1.00 (± 0.09) | 0.16 | 0.92 | < 0.001 |
| Shoulder abductors | 2.71 (± 0.76) | 1.48 | 0.87 (± 0.20) | 0.39 | 0.75 | 0.007 |
| Shoulder flexors | 2.97 (± 0.44) | 0.78 | 0.88 (± 0.11) | 0.20 | 0.85 | < 0.001 |
| Shoulder extensors | 3.15 (± 0.37) | 0.70 | 1.04 (± 0.09) | 0.17 | 0.95 | < 0.001 |
| Elbow flexors | 3.42 (± 0.39) | 0.69 | 0.72 (± 0.10) | 0.18 | 0.81 | < 0.001 |
| Elbow extensors | 2.12 (± 0.21) | 0.37 | 1.01 (± 0.05) | 0.09 | 0.97 | < 0.001 |
| Distal muscles | ||||||
| Supinators | 0.80 (± 0.55) | 0.99 | 0.78 (± 0.14) | 0.25 | 0.72 | < 0.001 |
| Pronators | 0.79 (± 0.28) | 0.50 | 0.84 (± 0.07) | 0.12 | 0.92 | < 0.001 |
| Wrist flexors | 1.05 (± 0.51) | 0.90 | 0.96 (± 0.12) | 0.21 | 0.82 | < 0.001 |
| Wrist extensors | 1.57 (± 0.27) | 0.48 | 0.79 (± 0.07) | 0.12 | 0.92 | < 0.001 |
| Digital flexors | 3.10 (± 0.25) | 0.44 | 0.71 (± 0.06) | 0.11 | 0.92 | < 0.001 |
| Digital extensors | 1.05 (± 0.25) | 0.44 | 0.87 (± 0.06) | 0.11 | 0.94 | < 0.001 |
| Mean exponent | 0.89 (± 0.03) | 0.05 | ||||
Individuals not included in particular muscle groups due to missing data include: shoulder rotators: C93, C94, C95, Gp, Gj, Gm, Oaf, Ojf; shoulder adductors: Gp, Gm; shoulder abductors: C93, C94, C95, Gsm, Gp, Gj, Gm; shoulder flexors: C93, shoulder extensors: C93. C94, Gp, Gm, Oaf, Ojf; supinators: Gj, Gp.
Table 4.
Allometric equation constants for forelimb muscle group fascicle length (cm) ± SE using logged data (exponents in bold have CIs overlapping those predicted by isometry; Mb, where b = 0.33)
| Muscle group | a (± SEM) | CI (a) | b (± SEM) | CI (b) | R2 | P |
|---|---|---|---|---|---|---|
| Overall | ||||||
| Total forelimb | 0.68 (± 0.77) | 1.81 | 0.27 (± 0.19) | 0.45 | 0.21 | 0.248 |
| Proximal forelimb | 1.22 (± 0.47) | 1.00 | 0.30 (± 0.12) | 0.26 | 0.48 | 0.052 |
| Distal forelimb | 1.37 (± 0.29) | 0.52 | 0.12 (± 0.07) | 0.13 | 0.15 | 0.149 |
| Proximal muscles* | ||||||
| Shoulder rotators | 1.41 (± 0.24) | 0.48 | 0.27 (± 0.06) | 0.12 | 0.81 | 0.009 |
| Shoulder adductors | 0.89 (± 0.41) | 0.74 | 0.35 (± 0.10) | 0.18 | 0.52 | 0.008 |
| Shoulder abductors | 1.36 (± 0.40) | 0.78 | 0.20 (± 0.10) | 0.19 | 0.31 | 0.114 |
| Shoulder flexors | 1.46 (± 0.32) | 0.57 | 0.22 (± 0.08) | 0.14 | 0.36 | 0.017 |
| Shoulder extensors | 1.65 (± 0.31) | 0.59 | 0.22 (± 0.07) | 0.13 | 0.53 | 0.025 |
| Elbow flexors | 1.74 (± 0.31) | 0.55 | 0.22 (± 0.08) | 0.14 | 0.36 | 0.014 |
| Elbow extensors | 0.91 (± 0.23) | 0.41 | 0.34 (± 0.05) | 0.09 | 0.74 | < 0.001 |
| Distal muscles | ||||||
| Supinators | 0.30 (± 0.27) | 0.48 | 0.24 (± 0.07) | 0.14 | 0.52 | 0.005 |
| Pronators | 1.06 (± 0.36) | 0.64 | 0.12 (± 0.09) | 0.16 | 0.06 | 0.199 |
| Wrist flexors | 1.14 (± 0.40) | 0.71 | 0.17 (± 0.10) | 0.18 | 0.12 | 0.117 |
| Wrist extensors | 0.47 (± 0.77) | 1.36 | 0.27 (± 0.19) | 0.34 | 0.07 | 0.180 |
| Digital flexors | 1.43 (± 0.30) | 0.53 | 0.16 (± 0.07) | 0.12 | 0.22 | 0.050 |
| Digital extensors | 1.19 (± 0.32) | 0.57 | 0.20 (± 0.08) | 0.14 | 0.30 | 0.026 |
| Mean exponent | 0.23 (± 0.02) | 0.04 | ||||
Individuals not included in particular muscle groups due to missing data include: shoulder rotators: C93, C94, C95, Gp, Gj, Gm, Oaf, Ojf; shoulder adductors: Gp, Gm; shoulder abductors: C93, C94, C95, Gsm, Gp, Gj, Gm; shoulder flexors: C93, shoulder extensors: C93. C94, Gp, Gm, Oaf, Ojf; supinators: Gj, Gp.
Table 5.
Allometric equation constants for forelimb muscle group PCSA (cm2) ± SE using logged data (exponents in bold have CIs overlapping those predicted by isometry; Mb, where b = 0.67)
| Muscle group | a (± SEM) | CI (a) | b (± SEM) | CI (b) | R2 | P |
|---|---|---|---|---|---|---|
| Overall | ||||||
| Total forelimb | 3.33 (± 0.57) | 1.34 | 0.74 (± 0.14) | 0.33 | 0.90 | 0.034 |
| Proximal forelimb | 3.13 (± 0.79) | 1.68 | 0.73 (± 0.20) | 0.43 | 0.76 | 0.034 |
| Distal forelimb | 2.34 (± 0.32) | 0.57 | 0.66 (± 0.08) | 0.14 | 0.86 | < 0.001 |
| Proximal muscles* | ||||||
| Shoulder rotators | 1.58 (± 0.66) | 1.33 | 0.78 (± 0.16) | 0.32 | 0.83 | 0.008 |
| Shoulder adductors | −0.13 (± 0.40) | 0.72 | 0.75 (± 0.10) | 0.18 | 0.83 | < 0.001 |
| Shoulder abductors | 1.29 (± 0.75) | 1.46 | 0.67 (± 0.19) | 0.37 | 0.65 | 0.018 |
| Shoulder flexors | 1.47 (± 0.56) | 1.00 | 0.66 (± 0.16) | 0.29 | 0.66 | < 0.001 |
| Shoulder extensors | 1.31 (± 0.55) | 1.04 | 0.85 (± 0.13) | 0.25 | 0.85 | 0.001 |
| Elbow flexors | 1.62 (± 0.47) | 0.83 | 0.50 (± 0.11) | 0.19 | 0.59 | 0.001 |
| Elbow extensors | 1.11 (± 0.30) | 0.53 | 0.68 (± 0.07) | 0.12 | 0.87 | < 0.001 |
| Distal muscles | ||||||
| Supinators | 0.49 (± 0.53) | 0.95 | 0.52 (± 0.13) | 0.23 | 0.56 | 0.003 |
| Pronators | −0.33 (± 0.41) | 0.73 | 0.72 (± 0.10) | 0.18 | 0.80 | < 0.001 |
| Wrist flexors | 0.72 (± 0.41) | 0.73 | 0.73 (± 0.10) | 0.18 | 0.80 | < 0.001 |
| Wrist extensors | 0.51 (± 0.32) | 0.57 | 0.55 (± 0.08) | 0.14 | 0.79 | < 0.001 |
| Digital flexors | 1.53 (± 0.39) | 0.69 | 0.56 (± 0.10) | 0.18 | 0.72 | < 0.001 |
| Digital extensors | −0.60 (± 0.47) | 0.83 | 0.79 (± 0.11) | 0.19 | 0.78 | < 0.001 |
| Mean exponent | 0.67 (± 0.03) | 0.05 | ||||
Individuals not included in particular muscle groups due to missing data include: shoulder rotators: C93, C94, C95, Gp, Gj, Gm, Oaf, Ojf; shoulder adductors: Gp, Gm; shoulder abductors: C93, C94, C95, Gsm, Gp, Gj, Gm; shoulder flexors: C93, shoulder extensors: C93. C94, Gp, Gm, Oaf, Ojf; supinators: Gj, Gp.
Muscle group data for fascicle length and PCSA, scaled using individual muscle-group allometric exponents, are presented in Figs 1 and 2, respectively (muscle belly mass is not presented as it is not the focus of this study). In all species, (scaled) muscle fascicles were longest in the majority of proximal muscle groups (i.e. the shoulder flexors, shoulder extensors and elbow flexors). Within the distal muscle groups, the digital flexors and extensors had the longest scaled fascicle lengths. Overall, PCSA was generally greater in the proximal than the distal muscle groups, particularly in the shoulder flexors and elbow flexors, with the exception of the digital flexors, which had by far the largest PCSAs of the distal muscle groups.
Fig. 1.
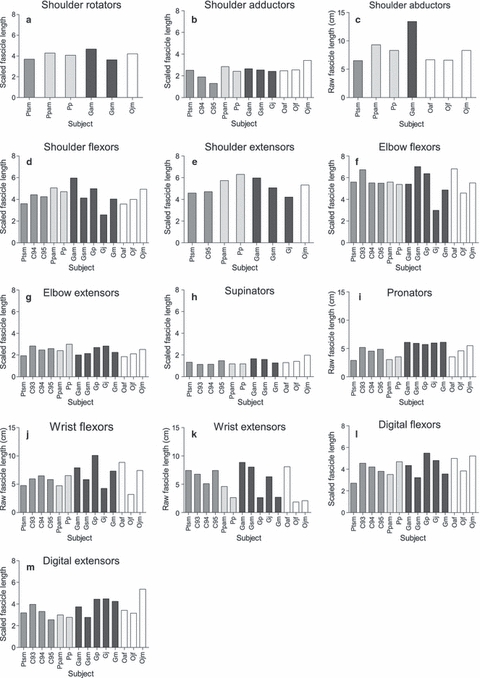
Raw fascicle length (cm) data scaled using individual group allometric exponents in instances where there was a significant relationship to body mass. In instances where this was not the case, raw fascicle length data are presented, although a direct comparison between the two forms of data cannot be made. (a) Shoulder rotators (M0.27), (b) shoulder adductors (raw data), (c) shoulder abductors (M0.20), (d) shoulder flexors (M0.22), (e) shoulder extensors (M0.22), (f) elbow flexors (M0.22), (g) elbow extensors (M0.34), (h) supinators (M0.24), (i) pronators (raw data), (j) wrist flexors (raw data), (k) wrist extensors (raw data), (l) digital flexors (M0.16), (m) digital extensors (M0.20). Chimpanzees are shaded in medium grey, bonobos in light grey, gorillas in dark grey and orangutans in white.
Fig. 2.
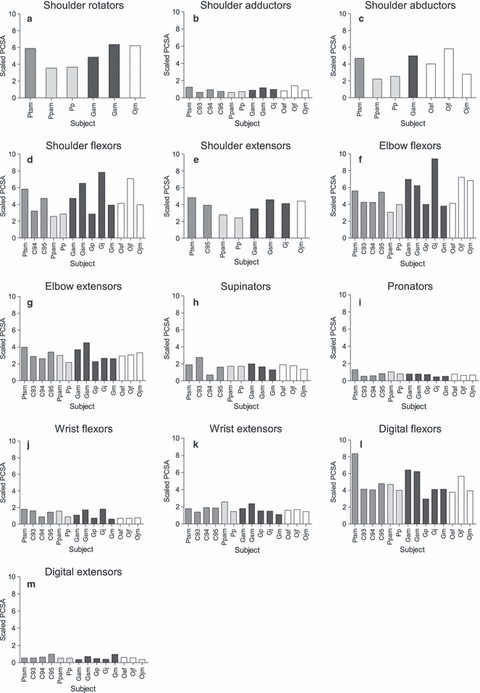
Raw PCSA (cm2) data scaled using individual group allometric exponents. (a) Shoulder rotators (M0.78) (b) shoulder adductors (M0.75), (c) shoulder abductors (M0.67), (d) shoulder flexors (M0.66), (e) shoulder extensors (M0.85), (f) elbow flexors (M0.50), (g) elbow extensors (M0.68), (h) supinators (M0.52), (i) pronators (M0.72), (j) wrist flexors (M0.73), (k) wrist extensors (M0.55), (l) digital flexors (M0.56), (m) digital extensors (M0.79). Chimpanzees are shaded in medium grey, bonobos in light grey, gorillas in dark grey and orangutans in white.
The most notable observation is the high level of intraspecific variation, which makes it difficult to ascertain whether interspecific differences actually exist. Intraspecific variation was particularly prevalent in the gorillas, even though, except for specimen Gsm, they were all male and were of a similar age (see Figs 1 and 2; Table 1). When considering age-related differences in captive animals, one should take into account that captive animals often mature more rapidly and thus sub-adults (that is, independent individuals, often sexually active although, in the case of females, not having borne offspring yet) are often more similar to adult individuals than may be expected in wild individuals. Therefore, differences in the gorilla, chimpanzee and bonobo samples are unlikely to be related to age–sex differences, as all were male and either sub-adult or adult (except for the juvenile chimpanzee, chimp 95). For example, although the sub-adult chimpanzee Ptsm was found to have a larger digital flexor PCSA than the other chimpanzees, the remaining chimpanzees contained both adults and a juvenile, thus reducing the likelihood that this difference was due to ontogenetic variation during growth. However, there might be an effect of age in the orangutan specimens: the juvenile orangutans (Ojf, Ojm) had slightly shorter fascicle lengths in the elbow flexors and much larger PCSAs than did the adult orangutan (Oaf). In the wrist extensors, the adult orangutan had much longer fascicle lengths than the juvenile orangutans, although there was no apparent difference in PCSA. These particular differences observed in the orangutan sample, therefore, may be attributed to ontogeny; not only to ontogenetic changes in the musculoskeletal properties but also to changes in locomotor behaviour (e.g. Thorpe & Crompton, 2005, 2006; Thorpe et al. 2009).
ancovas
Table 6 shows the results obtained from the ancova models for muscle belly mass, fascicle length and PCSA comparisons between the different species. For all belly mass and PCSA models, only body mass was found to have a significant effect on the variation observed, species being non-significant. For fascicle length, two muscle groups were found to differ significantly between species: the pronators and wrist extensors. For the pronators, the main effect ‘species’ resulted in a significant model but ‘body mass’ did not (although it remained in the model as a covariate). Tukey's post-hoc test revealed that the pronator muscle of bonobos had significantly shorter muscle fascicles than that in both chimpanzees and gorillas, but it did not differ significantly from that in orangutans (Fig. 3a). For the wrist extensors, body mass alone was significant, but this significance was only achieved, together with a better fitting model, when species was also included, although the species effect itself was non-significant (P=0.086). As species was necessary to produce the best fitting model for the wrist extensors, Tukey's post-hoc test was performed. Although no species were found to be significantly different, Fig. 3b suggests that gorillas tend to have the shortest fascicle lengths in the wrist extensors, whereas chimpanzees (and orangutans) tend to have the longest.
Table 6.
Results from ancova models for forelimb functional muscle groups
| Muscle belly mass |
Muscle fascicle length |
Muscle PCSA |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscle group | Model* | Fdegrees of freedom | R2 | P | Model | Fdegrees of freedom | R2 | P | Model | Fdegrees of freedom | R2 | P |
| Proximal muscles | ||||||||||||
| Shoulder rotators | –** | – | – | – | – | – | – | – | – | – | – | – |
| Shoulder adductors | 3 | 123.251,11 | 0.92 | < 0.001 | 3 | 11.751,11 | 0.52 | 0.008 | 3 | 55.801,11 | 0.83 | < 0.001 |
| Shoulder abductors | – | – | – | – | – | – | – | – | – | – | – | – |
| Shoulder flexors | 3 | 69.221,12 | 0.85 | < 0.001 | 3 | 7.851,12 | 0.36 | 0.017 | 3 | 23.941,12 | 0.66 | < 0.001 |
| Shoulder extensors | – | – | – | – | – | – | – | – | – | – | – | – |
| Elbow flexors | 3 | 57.121,13 | 0.81 | < 0.001 | 3 | 8.261,13 | 0.36 | 0.014 | 3 | 19.691,13 | 0.59 | 0.001 |
| Elbow extensors | 3 | 376.271,14 | 0.97 | < 0.001 | 3 | 38.261,13 | 0.74 | < 0.001 | 3 | 85.581,11 | 0.87 | < 0.001 |
| Distal muscles | ||||||||||||
| Supinators | 3 | 32.441,12 | 0.72 | < 0.001 | 3 | 12.931,11 | 0.52 | 0.005 | 3 | 15.251,11 | 0.56 | 0.003 |
| Pronators | 3 | 154.531,13 | 0.92 | < 0.001 | 2 | S: 7.633,13B: 4.041,13 | 0.65 0.65 | 0.008 0.075 | 3 | 51.671,13 | 0.80 | < 0.001 |
| Wrist flexors | 3 | 59.261,13 | 0.82 | < 0.001 | 3 | 2.841,13 | 0.12 | 0.117 | 3 | 53.071,13 | 0.80 | < 0.001 |
| Wrist extensors | 3 | 147.861,13 | 0.92 | < 0.001 | 2 | S: 3.023,13B: 6.261,13 | 0.38 0.38 | 0.086 0.034 | 3 | 50.131,13 | 0.79 | < 0.001 |
| Digital flexors | 3 | 140.201,13 | 0.91 | < 0.001 | 3 | 4.741,13 | 0.22 | 0.050 | 3 | 34.871,13 | 0.72 | < 0.001 |
| Digital extensors | 3 | 199.821,13 | 0.94 | < 0.001 | 3 | 6.501,13 | 0.30 | 0.026 | 3 | 48.381,13 | 0.78 | < 0.001 |
Model 1 refers to a model where the interaction term was significant, model 2 where species was significant and model 3 where only body mass was the significant term in the model. In instances where model 2 was significant (i.e. species) the results for body mass (the covariate) are also presented as they were included in the model.
Results were not available for the shoulder rotators, shoulder abductors or shoulder extensors due to a reduction to one or less per species in some cases.
Fig. 3.
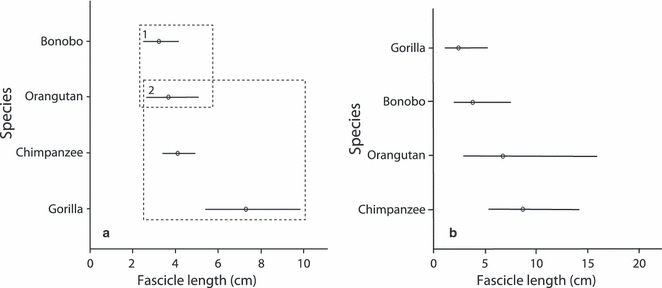
Results from Tukey's post-hoc tests displaying mean values and 95% confidence intervals for the different species for (a) pronator fascicle length and (b) wrist extensor fascicle length. Values presented are back-transformed from logged data. Dashed boxes represent the significantly different sub-sets as highlighted from Tukey's post-hoc tests; if there are no dashed boxes, Tukey's post-hoc tests did not reveal a significant difference between a specific pair.
Discussion
Data collection and method of analysis
Previously, there has been an extensive study of forelimb anatomy in gibbons (Michilsens et al. 2009) and data are available for a number of chimpanzees and orangutans (Thorpe et al. 1999; Carlson, 2006; Oishi et al. 2008, 2009). However, to our knowledge, gorilla and bonobo forelimb muscle architecture data have not been published and an overall comparison of the forelimb musculature of the apes has not been undertaken. Our study therefore provides the first comprehensive overview of the variation in forelimb muscle architecture across the non-human great apes.
A discussion of the different methods available for comparing muscle architecture parameters (see Myatt et al. 2011a) has indicated that allometric scaling using exponents from the collected data is more appropriate than the use of geometric scaling (as has been employed previously, e.g. Thorpe et al. 1999; Payne et al. 2006; Oishi et al. 2009).This is because the scaling exponent established may vary from that predicted by isometry for some muscle groups and furthermore, in some instances, the physiological variable, e.g. fascicle length in particular, may not actually have a significant linear relationship with body mass, as has been found in the hindlimb muscles of great apes (Myatt et al. 2011a). Some studies have also questioned any use of ratios for data comparison (e.g. Packard & Boardman, 1999). ancovas (a form of general linear model) were therefore additionally employed to establish significant interspecific differences, although the method ideally requires a large sample size to increase the power of the test (Grafen & Hails, 2002). Although the sample size in this study was much larger than has been possible in studies of great ape anatomy to date, our sample is still relatively small and further constitutes a range of age–sex classes, thus limiting the interpretations that can be made. Thus to explore our dataset, analyses were undertaken using both allometric scaling and ancovas.
In some instances, the allometric exponents obtained from the data in the present study were found to have CIs overlapping those predicted by isometry yet, once again, fascicle length did not always scale to body mass, thus rendering it un-scalable. Although there was an overlap with isometry in a number of instances, we still highlight the need for studies to check the scaling exponents of their data prior to analysis, as even a small variation in exponent can affect the magnitude of the differences observed (Myatt et al. 2011a). Furthermore, in some cases there was no significant linear relationship between the variable and body mass, further highlighting the problems that can be faced when data are scaled geometrically without reference to the actual relationships observed.
The allometric scaling exponents obtained in the present study were similar to those found for primate hindlimb muscles (see Alexander et al. 1981; Pollock & Shadwick, 1994; Myatt et al. 2011a), which is what one might expect for quadrupedal animals which use both their fore- and hindlimbs equally. However, as discussed previously, when quadrupedal, chimpanzees at least tend to rely on their hindlimbs for propulsion and steering, while the forelimb tends to act just as a prop (Li et al. 2004; Raichlen et al. 2009). In general, the non-human apes are considered adapted for forelimb-propelled behaviours, e.g. climbing and suspensory locomotion. Thus, one might expect the forelimbs to scale with greater exponents than the hindlimbs, as was found with relative forelimb length in ‘climbing’ primates (see Jungers, 1984). Therefore, the similarity in allometric scaling exponents between the fore- and hindlimbs may reflect the range of behaviours performed by the non-human apes which involve use of both the fore- and hindlimbs during locomotion (e.g. Cant, 1987; Hunt, 1992; Doran, 1993a,b; Remis, 1995; Fleagle, 1999; Thorpe & Crompton, 2005, 2006).
Descriptive anatomy
One of the most significant variations in this study was the presence of a mono-articular long head in the biceps brachii muscle of orangutan Oaf, rather than the bi-articular arrangement that is normally observed in non-human apes (e.g. Swindler & Wood, 1973) and was observed in the two juvenile orangutans in the present study (Ojf and Ojm). No such observation was described by Sonntag (1924) or Oishi et al. (2008, 2009) for orangutans, although Oishi et al. presented only a small amount of descriptive data. A similar, but opposite, situation has been observed in gibbons, where it was the short head which was mono-articular, originating from the lesser tubercle of the humerus instead of the coracoid process, with the long head originating from the supraglenoid as normal (Michilsens et al. 2009). This apparent reduction in shoulder flexion capacity in gibbons has been suggested to be compensated for by the larger site of origin of the biceps brachii, extending more proximally on the humerus, and its relatively large PCSA, thus increasing force production during elbow flexion. Such a capacity would be more beneficial during suspensory behaviours in gibbons, when they pull themselves up from hanging beneath a branch with an extended shoulder by flexing the elbow (Jungers & Stern, 1980; Aiello & Dean, 2002; Michilsens et al. 2009). The situation present in the single orangutan in the current study could, however, simply be a random anomaly. Such anatomical variation in muscles is frequently observed in humans and indeed, the biceps brachii muscle is commonly one of the most variable muscles in humans, including the presence of additional heads and variations in origin and insertion (e.g. Hyman & Warren, 2001; Emeka & Emmanuel, 2009).
The presence of a separate flexor pollicis longus muscle with a tendon to digit one has not been commonly reported in the non-human apes, except in gibbons (see Aziz & Dunlap, 1986), but in the present study it was observed in the bonobo, one gorilla and one orangutan (specimens Ppam, Gam and Oaf). A separate belly was also observed in a chimpanzee and another gorilla specimen (specimens Ptsm and Gsm), although the tendon of insertion was to digit two, not one. A separate flexor pollicis longus muscle enables the production of greater forces when a large object, such as a rock or cylinder, is held in a power grasp and used for hammering or pounding, behaviours observed when stone tools are employed (Boesch & Boesch, 1993; Hamrick et al. 1998). Such grips may be employed by apes when using stone tools, e.g. chimpanzees using hammer-stones to crack nuts (e.g. Boesch & Boesch, 1981, 1993), but may also be important during arboreal behaviours, for example, the breaking and bending of branches during nest building in orangutans (J. P. Myatt, personal observation). It is unlikely, however, that flexor pollicis longus plays a major role when moving through the canopy, grasping branches, as a separate muscle belly and tendon is not a common feature of arboreal dwelling apes. Rather, the occurrence of a separate muscle belly in a minority of individuals reflects individual variation on which selective pressures could act to increase or decrease the presence of a feature if it provided an advantage or disadvantage. Interestingly, of the 14 individuals in this study, four of the five showing marked functional anomalies demonstrated more than one. This may indicate that particular individuals are more prone to variation, although the sample size present here is too small to propose a trend.
Intraspecific variation
In general, there was a large amount of intraspecific variation, which is likely to be due to differences in age–sex class, the nature of the captive environments in which the animals had been housed, and individual or random variation. If different environments encourage more activity in some individuals and less in others, this may explain the high level of variation seen, as in humans increased amount of inactivity results in a decrease in muscle volume and PCSA (e.g. Kawakami et al. 2000). Although all the animals in this study were captive, modern enclosures better reflect the environments found in the natural habitats of these species and higher levels of activity are encouraged by species-specific enrichment programmes, for example, by placing food in harder-to-reach places (reviewed in Britt, 1996). However, activity levels may vary between individuals. This may explain the degree of variation observed in the gorillas even though they were all of the same age–sex class (except for the sub-adult male Gsm). Gorilla Gsm was from the same enclosure as Gam: the age difference may therefore have been responsible for different levels of activity between the two individuals, although one would not expect large differences resulting from growth variation due to the early maturation of captive animals. Age–sex differences were also evident in the orangutan sample, in particular for the increased force-producing potential (larger scaled PCSA) in the juvenile orangutan's elbow flexors compared with that of the adult orangutan, relative to body mass. This may reflect the more explorative/playful nature of juvenile orangutans and their increased use of arboreal climbing behaviours in both captive and wild environments (Thorpe & Crompton, 2005, 2006; Thorpe et al. 2009). Although the high level of individual variation was not attributable predominantly to age–sex variation in this study, greater sample sizes may in the future allow species comparisons to be made within age–sex classes to control for this potentially confounding factor.
Interspecific variation
In all non-human ape species, the proximal muscles had the greatest PCSAs, in particular the shoulder muscle groups, except the shoulder adductors (although the latter will have been influenced by the absence of pectoralis major from the current dataset). The latissimus dorsi, an extremely large back muscle, is likely responsible for the large mass and PCSA of the shoulder muscles. Latissimus dorsi is active during the support phase of vertical climbing and brachiation (Fleagle et al. 1981; Bogduk et al. 1998). Vertical climbing is an important component of the locomotion of all non-human apes, accounting for between 6.5 and 50.4% of their locomotor behaviour (Hunt, 1992, 2004; Doran, 1993a,b; Remis, 1995; Thorpe & Crompton, 2005, 2006). However, as pectoralis major was missing from the shoulder rotators, adductors and flexors, the PCSA of these groups is likely to have been much larger than any other group, and the remaining shoulder muscles may have been more equal. Fascicle lengths were also generally longer in the proximal muscles, particularly the shoulder flexors, shoulder extensors and elbow flexors, which suggests the need for forces to be exerted over a wide range of motion in these groups, particularly at the shoulder, which would be particularly beneficial during arboreal suspensory and climbing behaviours (Isler, 2005).
For all species measured here, the digital flexors had both the longest fascicles and the greatest PCSAs among the distal muscles. The importance of this muscle group in non-human great apes was to be expected as it enables greater grip strength. Grip strength is important when moving in an arboreal environment and it also enables fine manipulation of objects, necessary for tool use and dexterity when feeding, e.g. ant-dipping in chimpanzees (McGrew et al. 2005), leaf-rolling in gorillas (Sawyer & Robbins, 2009) and seed and insect extraction in orangutans (Fox et al. 2004). Oishi et al. (2009) observed that their orangutan specimens had longer fascicles and smaller PCSAs in their digital flexors compared with chimpanzees. In the present study, only chimpanzee Ptsm appeared to follow this pattern (from the scaled data), whereas all other chimpanzees were more similar to the orangutans and no differences were highlighted in the ancova model. Oishi et al. (2009) speculated that their apparent difference was likely due to the increased need for mobility in the wrist joint during arboreal behaviours in orangutans, and a greater emphasis on power in the chimpanzees during quadrupedal locomotion. Although this seems a reasonable conclusion, the absence of such interspecific differences in our study highlights the importance of interindividual/intraspecific variation. Further, it underlines the need to continue increasing the dataset of ape muscle architecture to give a better appraisal of this variation, as the small sample sizes in both the present study and Oishi et al.’s (2009) have yielded different conclusions.
The high intraspecific variation in this study made it difficult to assess from the scaled data if, and where, interspecific differences were present, thus rendering the results inconclusive in many respects. Using ancovas we were able to discern a statistically significant interspecific difference in the pronator muscles and, although species was included in the significant model for wrist extensor fascicle length, no significant species differences were highlighted. The significantly longer muscle fascicles in the gorilla and chimpanzee pronator muscles, compared with those of bonobos, imply that they can produce force over a greater range of motion during pronation of the forearm. The need for this increased range of motion in gorillas and chimpanzees may be related to the greater use of terrestrial quadrupedalism in gorillas and chimpanzees, but a greater level of arboreal quadrupedalism in bonobos (Hunt, 1991b). Palmigrade arboreal quadrupedalism (i.e. placing the palm on the support with the hands turned out) requires greater force production to maintain stability of the dorsi-flexed wrist and a more flexed elbow – particularly in the unstable arboreal habitat – compared with the terrestrial knuckle-walking employed more frequently by gorillas (Tuttle et al. 1972). Therefore, an increased frequency of arboreal quadrupedalism may lead to an increased need for shorter fascicles, and thus increased force production, in pronators (although no significant difference in the PCSA was observed in this small sample). This result also grouped together the two most arboreal non-human apes, bonobos and orangutans. This possible separation (although non-significant in the case of orangutans, chimpanzees and gorillas) between the arboreal and more terrestrial species implies that an increased ability to apply force during pronation is important when moving through the forest canopy, grasping branches in orientations distinct from those during knuckle-walking. This result is perhaps surprising, as one may expect arboreal species to also require an ability to produce forces over a greater range of motion in comparison with more terrestrial species, for example, during suspensory/clambering behaviours where rotation about the wrist enables a change in body orientation. However, the range of motion of a muscle with short fascicles can be increased by the presence of a smaller moment arm, and thus further investigation is required to see whether this is indeed the case in this muscle group. Although only one muscle group, for one muscle architecture parameter, was highlighted as being significantly different between species, the power of tests such as an ancova is reduced when the sample sizes are small. The results obtained from the present study, therefore, are preliminary and we urge future studies to combine data from previous studies to increase the sample size and thus enable more robust conclusions to be obtained.
Neither the greater behavioural arboreality, and putatively more frequent suspensory activity, of orangutans, nor the differences in kinematics of vertical climbing between African apes and orangutans, were reflected in the forelimb muscle architecture of the apes in the present study, using either form of analysis. The small sample size and the large individual variation may be masking differences that may come to light once a larger sample is available: but it is also possible that the lack of a significant variation will remain. Any interpretations must as yet be made with caution. However, within the African apes the degree of arboreality may have contributed to differences between the (more arboreal) bonobos and the (more terrestrial) gorillas and chimpanzees. Further, the very presence in the African ape dataset of more arboreal species (bonobos, lowland gorillas) would act to reduce any African-ape vs. Asian-ape distinction. Overall, we cautiously suggest that the non-human apes may indeed have a rather generalised forelimb morphology, in terms of muscle architecture, adapted to the entire locomotor repertoire. This is also suggested by the postural and locomotor behaviours directly observed in the wild which are characterised by generally torso-orthograde behaviour (whether compressive or suspensory; Thorpe & Crompton, 2006; Crompton et al. 2008) in all the non-human apes, rather than specialisations to particular, selectively important behaviours, e.g. brachiation. This implies that, in the non-human great apes, muscles are adapted to perform all the behaviours required, whether they form a large proportion of an animal's locomotor repertoire or not (Alexander et al. 1981), and there is a greater advantage for great apes in having a generalised morphology than one specialised to a specific behaviour, e.g. leaping in galagos. Skeletal morphology also shows a range of similarities within the apes that are thought to represent shared, derived features (synapomorphies) of the hominoids linked to truncal orthogrady (e.g. a dorsally placed scapula: Larson, 1998), but, equally, features of the appendicular skeleton of all living great apes adaptive to suspension may have been acquired independently in different lineages (see Moyà-Solà et al. 2004).
Nevertheless, assessment of other muscle architecture parameters than those examined herein, such as moment arms, may yet reveal clearer interspecific differences reflecting functional adaptations. Muscles that in this study were found to have a small PCSA (and thus have been inferred as producing smaller forces), may in fact be able to produce greater torque due to their utilisation of a larger moment arm (see Lieber & Friden, 2001). Furthermore, as studies increase in sample size, the variation between individual muscles within a functional muscle group can also be statistically assessed to increase our understanding of the differences present (see Eng et al. 2008). In addition to these variations in macro-architecture, adaptations to more frequently used behaviours may well be reflected in muscle micro-architecture (i.e. different fibre type proportions/distributions), and it may be this which fine-tunes muscles to the output required by a given species, or different populations of the same species (see Myatt et al. 2011b). It is thus a detailed knowledge of both macro- and micro-architecture of muscles that is required to appreciate the more subtle links between form, function and performances in the non-human apes.
Conclusion
This study contributes to the description of forelimb anatomy and muscle architecture in non-human apes and, in particular, provides the first data on gorilla and bonobo forelimb muscle architecture. Overall, we found that the non-human apes in this (limited) sample do not vary greatly in the forelimb across different muscle architecture variables at the macro-level, possibly reflecting that the Hominoidea as a whole are characterised by adaptations in these properties for orthograde behaviours (Thorpe & Crompton, 2006), as indeed is their thoracic skeletal anatomy reflecting a similar ecological history over much of the Miocene (Crompton et al. 2008), although further evidence is needed to substantiate this. This study does, however, highlight the difficulty of comparing different species across a small sample size. With limited sample sizes, substantial intraspecific variation may swamp the signal of even real interspecific distinctions and caution must be taken when drawing conclusions, as methods used to scale or analyse the data may exert untoward influence on results. It is imperative and indeed urgent that more studies are carried out, preferably with fresh cadavers and utilising the same or at least directly comparable methods to increase sample size and thus provide more robust conclusions.
Acknowledgments
The cadaveric specimens were kindly provided by The Zoological Society of London, The North of England Zoological Society, Twycross Zoo, Apenheul Zoo, The Royal Zoological Society of Antwerp, the Anthropological Institute and Museum, Zurich, and Paignton Zoo, in many cases through collaboration with Dr Andrew Kitchener of the National Museum of Scotland. We thank Dr Robert Ker, Dr Anthony Channon, Dr Sam Coward and Katy Wareing for their assistance with the dissections. Additional thanks go to the Centre for Research and Conservation (Royal Zoological Society of Antwerp), structurally supported by the Flemish Government. Finally, thanks to Dr Steven Portugal for his assistance with the statistical analysis.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Appendix S1. Raw forelimb data.
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
References
- Aiello L, Dean C. An Introduction to Human Evolutionary Anatomy. London, UK: Elsevier Academic Press; 2002. [Google Scholar]
- Alexander RM, Jayes AA, Maloiy GMO, et al. Allometry of the leg muscles of mammals. J Zool Lond. 1981;194:539–552. [Google Scholar]
- Ashton EH, Oxnard CE. Functional adaptations in the primate shoulder girdle. Proc Zool Soc Lond. 1962a;142:49–66. [Google Scholar]
- Ashton EH, Oxnard CE. The musculature of the primate shoulder. Trans Zool Soc Lond. 1962b;29:553–650. [Google Scholar]
- Aziz MA, Dunlap SS. The human extensor digitorum profundus muscle with comments on the evolution of the primate hand. Primates. 1986;27:293–319. [Google Scholar]
- Boesch C, Boesch H. Sex differences in the use of natural hammers by wild chimpanzees: a preliminary report. J Hum Evol. 1981;19:583–593. [Google Scholar]
- Boesch C, Boesch H. Different hand postures for pounding nuts with natural hammers by wild chimpanzees. In: Preuschoft H, Chivers DJ, editors. Hands of Primates. Vienna: Springer-Verlag; 1993. pp. 31–43. [Google Scholar]
- Bogduk N, Johnson G, Spalding D. The morphology and biomechanics of latissimus dorsi. Clin Biomech. 1998;13:377–385. doi: 10.1016/s0268-0033(98)00102-8. [DOI] [PubMed] [Google Scholar]
- Britt A. Environmental Influences on the Behavioural Ecology of the Black-and-White Ruffed Lemur. Liverpool: University of Liverpool; 1996. Unpublished PhD Dissertation. [Google Scholar]
- Calow LJ, Alexander RM. A mechanical analysis of a hind leg of a frog (Rana temporaria) J Zool Lond. 1973;171:293–321. [Google Scholar]
- Cant JGH. Positional behavior of female Bornean orangutans (Pongo pygmaeus) Am J Primatol. 1987;12:71–90. doi: 10.1002/ajp.1350120104. [DOI] [PubMed] [Google Scholar]
- Carlson KJ. Muscle architecture of the common chimpanzee (Pan troglodytes): perspectives for investigating chimpanzee behavior. Primates. 2006;47:218–229. doi: 10.1007/s10329-005-0166-4. [DOI] [PubMed] [Google Scholar]
- Channon AJ, Gunther MM, Crompton RH, et al. Mechanical constraints on the functional morphology of the gibbon hind limb. J Anat. 2009;215:383–400. doi: 10.1111/j.1469-7580.2009.01123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton RH, Vereecke EE, Thorpe SKS. Locomotion and posture from the common hominoid ancestor to fully modern hominins, with special reference to the last common panin/hominin ancestor. J Anat. 2008;212:501–543. doi: 10.1111/j.1469-7580.2008.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demes B, Larson SG, Stern JT, Jr, et al. The kinetics of ‘hind limb drive’ reconsidered. J Hum Evol. 1994;26:353–374. [Google Scholar]
- Diogo R, Wood B. Soft-tissue anatomy of the primates: phylogenetic analyses based on the muscles of the head, neck, pectoral region and upper limb, with notes on the evolution of these muscles. J Anat. 2011;219:273–359. doi: 10.1111/j.1469-7580.2011.01403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran DM. Comparative locomotor behaviour of chimpanzees and bonobos – the influence of morphology on locomotion. Am J Phys Anthropol. 1993a;91:83–98. doi: 10.1002/ajpa.1330910106. [DOI] [PubMed] [Google Scholar]
- Doran DM. Sex differences in adult chimpanzee positional behaviour: the influence of body size on locomotion and posture. Am J Phys Anthropol. 1993b;91:99–115. doi: 10.1002/ajpa.1330910107. [DOI] [PubMed] [Google Scholar]
- Emeka AG, Emmanuel OB. Variations of the proximal attachment of the biceps brachii muscle in a Nigerian population. Int J Anat Var. 2009;2:91–92. [Google Scholar]
- Eng CM, Smallwood LH, Rainiero MP, et al. Scaling of muscle architecture and fiber types in the rat hindlimb. J Exp Biol. 2008;211:2336–2345. doi: 10.1242/jeb.017640. [DOI] [PubMed] [Google Scholar]
- Engqvist L. The mistreatment of covariate interaction terms in linear model analyses of behavioural and evolutionary ecology studies. Anim Behav. 2005;70:967–971. [Google Scholar]
- Fleagle JG, Stern JT, Jr, Jungers WL, et al. Climbing: a biomechanical link with brachiation and with bipedalism. In: Day MH, editor. Vertebrate Locomotion. New York: Academic Press; 1981. pp. 359–375. [Google Scholar]
- Fleagle JR. Primate Adaptation and Evolution. New York, USA: Academic Press; 1999. [Google Scholar]
- Fox EA, van Schaik CP, Sitompul A, et al. Intra- and interpopulational differences in orangutan (Pongo pygmaeus) activity and diet: implications for the invention of tool use. Am J Phys Anthropol. 2004;125:162–174. doi: 10.1002/ajpa.10386. [DOI] [PubMed] [Google Scholar]
- Galdikas BMF. Orangutan diet, range, and activity at Tanjung Puting, Central Borneo. Int J Primatol. 1988;9:1–35. [Google Scholar]
- Gibbs S, Collard M, Wood B. Soft-tissue anatomy of the extant hominoids: a review and phylogenetic analysis. J Anat. 2002;200:3–49. doi: 10.1046/j.0021-8782.2001.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafen A, Hails R. Modern Statistics for the Life Sciences. Oxford: Oxford University Press; 2002. [Google Scholar]
- Hamrick MW, Churchill SE, Schmitt D, et al. EMG of the human flexor pollicis longus muscle: implications for the evolution of hominid tool use. J Hum Evol. 1998;34:123–136. doi: 10.1006/jhev.1997.0177. [DOI] [PubMed] [Google Scholar]
- Hunt KD. Mechanical implications of chimpanzee positional behavior. Am J Phys Anthropol. 1991a;86:521–536. doi: 10.1002/ajpa.1330860408. [DOI] [PubMed] [Google Scholar]
- Hunt KD. Positional behavior in the Hominoidea. Int J Primatol. 1991b;12:95–118. [Google Scholar]
- Hunt KD. Positional behavior of Pan troglodytes in the Mahale Mountains and Gombe Stream National Parks, Tanzania. Am J Phys Anthropol. 1992;87:83–105. doi: 10.1002/ajpa.1330870108. [DOI] [PubMed] [Google Scholar]
- Hunt KD. The postural feeding hypothesis: an ecological model for the evolution of bipedalism. S Afr J Sci. 1996;92:77–90. [Google Scholar]
- Hunt KD. The special demands of great ape locomotion and posture. In: Russon AE, Begun DR, editors. The Evolution of Thought: Evolutionary Origins of Great Ape Intelligence. Cambridge: Cambridge University Press; 2004. pp. 172–189. [Google Scholar]
- Hyman JL, Warren RF. Extra-articular origin of biceps brachii. Arthroscopy. 2001;17:E29. doi: 10.1053/jars.2001.24691. [DOI] [PubMed] [Google Scholar]
- Isler K. 3D-kinematics of vertical climbing in hominoids. Am J Phys Anthropol. 2005;126:66–81. doi: 10.1002/ajpa.10419. [DOI] [PubMed] [Google Scholar]
- Isler K, Thorpe SKS. Gait parameters in vertical climbing of captive, rehabilitant and wild Sumatran orang-utans (Pongo pygmaeus abelii) J Exp Biol. 2003;206:4081–4096. doi: 10.1242/jeb.00651. [DOI] [PubMed] [Google Scholar]
- Jungers WL. Aspects of size and scaling in primate biology with special reference to the locomotor skeleton. Yearb Phys Anthropol. 1984;27:73–97. [Google Scholar]
- Jungers WL, Stern JT. Telemetered electromyography of forelimb muscle chains in gibbons (Hylobates-lar) Science. 1980;208:617–619. doi: 10.1126/science.7367886. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Muraoka Y, Kubo K, et al. Changes in muscle size and architecture following 20 days of bed rest. J Gravit Physiol. 2000;7:53–59. [PubMed] [Google Scholar]
- Kimura K. Hindlimb dominance during primate high-speed locomotion. Primates. 1992;33:465–476. [Google Scholar]
- Kimura K, Takai S. On the musculature of the fore limb of the crab-eating macaque. Primates. 1970;11:145–170. [Google Scholar]
- Larson SG. Parallel evolution in the hominoid trunk and forelimb. Evol Anthropol. 1998;6:87–89. [Google Scholar]
- Li Y, Crompton RH, Wang W, et al. Hind limb drive, hind limb steering? Functional differences between fore and hind limbs in chimpanzee quadrupedalism. In: Anapol F, German RZ, Jablonski NG, editors. Shaping Primate Evolution. Cambridge: Cambridge University Press; 2004. pp. 258–277. [Google Scholar]
- Lieber RL, Friden J. Clinical significance of skeletal muscle architecture. Clin Orthop Relat Res. 2001;383:140–151. doi: 10.1097/00003086-200102000-00016. [DOI] [PubMed] [Google Scholar]
- Mangini U. Flexor pollicis longus muscle – its morphology and clinical significance. J Bone Joint Surg Am. 1960;42:467–470. [PubMed] [Google Scholar]
- McGrew WC, Pruetz JD, Fulton SJ. Chimpanzees use tools to harvest social insects at Fongoli, Senegal. Folia Primatol. 2005;76:222–226. doi: 10.1159/000086023. [DOI] [PubMed] [Google Scholar]
- Mendez J, Keys A. Density and composition of mammalian muscle. Metabolism. 1960;9:184–188. [Google Scholar]
- Michiels I, Bodem F. The deltoid muscle – an electromyographic analysis of its activity in arm abduction in various body postures. Int Orthop. 1992;16:268–271. doi: 10.1007/BF00182709. [DOI] [PubMed] [Google Scholar]
- Michilsens F, Vereecke EE, D'Août K, et al. Functional anatomy of the gibbon forelimb: adaptations to a brachiating lifestyle. J Anat. 2009;215:335–354. doi: 10.1111/j.1469-7580.2009.01109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA. The musculature of Pan paniscus. Am J Anat. 1952;91:183–232. doi: 10.1002/aja.1000910202. [DOI] [PubMed] [Google Scholar]
- Moyà-Solà S, Köhler M, Alba DM, et al. Pierolapithecus catalaunicus, a new Middle Miocene great ape from Spain. Science. 2004;306:1339–1344. doi: 10.1126/science.1103094. [DOI] [PubMed] [Google Scholar]
- Myatt JP, Crompton RH, Thorpe SKS. Hindlimb muscle architecture in non-human great apes and a comparison of methods for analysing inter-species variation. J Anat. 2011a;219:150–166. doi: 10.1111/j.1469-7580.2011.01383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myatt JP, Schilling N, Thorpe SKS. Distribution patterns of fibre types in the triceps surae muscle groups of chimpanzees and orangutans. J Anat. 2011b;218:402–412. doi: 10.1111/j.1469-7580.2010.01338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi M, Ogihara N, Endo H, et al. Muscle architecture of the upper limb in the orangutan. Primates. 2008;49:204–209. doi: 10.1007/s10329-008-0082-5. [DOI] [PubMed] [Google Scholar]
- Oishi M, Ogihara N, Endo H, et al. Dimensions of forelimb muscles in orangutans and chimpanzees. J Anat. 2009;215:373–382. doi: 10.1111/j.1469-7580.2009.01125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxnard CE, Franklin D. Ghosts of the past, II: muscles and fasciae in the primate forelimb domain. Folia Primatol. 2008;79:441–457. doi: 10.1159/000151357. [DOI] [PubMed] [Google Scholar]
- Packard CG, Boardman TJ. The use of percentages and size-specific indices to normalize physiological data for variation in body size: wasted time, wasted effort? Comp Biochem Physiol A. 1999;122:37–44. [Google Scholar]
- Payne RC, Crompton RH, Isler K, et al. Morphological analysis of the hindlimb in apes and humans. I. Muscle architecture. J Anat. 2006;208:709–724. doi: 10.1111/j.1469-7580.2006.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock CM, Shadwick RE. Allometry of muscle, tendon, and elastic energy storage capacity in mammals. Am J Physiol. 1994;266:1022–1031. doi: 10.1152/ajpregu.1994.266.3.R1022. [DOI] [PubMed] [Google Scholar]
- Raichlen DA, Pontzer H, Shapiro LJ, et al. Understanding hind limb weight support in chimpanzees with implications for the evolution of primate locomotion. Am J Phys Anthropol. 2009;138:395–402. doi: 10.1002/ajpa.20952. [DOI] [PubMed] [Google Scholar]
- Remis M. Effects of body size and social context on the arboreal activities of lowland gorillas in the Central African Republic. Am J Phys Anthropol. 1995;97:413–433. doi: 10.1002/ajpa.1330970408. [DOI] [PubMed] [Google Scholar]
- Reynolds TR. Stresses on the limbs of quadrupedal primates. Am J Phys Anthropol. 1985;67:351–362. doi: 10.1002/ajpa.1330670407. [DOI] [PubMed] [Google Scholar]
- Sacks RD, Roy RR. Architecture of the hindlimb muscles of cats – functional significance. J Morphol. 1982;173:185–195. doi: 10.1002/jmor.1051730206. [DOI] [PubMed] [Google Scholar]
- Sawyer SC, Robbins MM. A novel food processing technique by a wild mountain gorilla (Gorilla beringei beringei) Folia Primatol. 2009;80:83–88. doi: 10.1159/000217680. [DOI] [PubMed] [Google Scholar]
- Schmidt-Nielson K. Scaling, Why Is Animal Size So Important? Cambridge: Cambridge University Press; 1984. pp. 1–245. [Google Scholar]
- Sonntag CR. On the anatomy, physiology, and pathology of the orang-outan. Proc Zool Soc Lond. 1924;1924:349+. [Google Scholar]
- Stern JT, Jr, Larson SG. Telemetered electromyography of the supinators and pronators of the forearm in gibbons and chimpanzees: implications for the fundamental positional adaptation of hominoids. Am J Phys Anthropol. 2001;115:253–268. doi: 10.1002/ajpa.1080. [DOI] [PubMed] [Google Scholar]
- Swindler DR, Wood CD. An Atlas of Primate Gross Anatomy. Seattle: University of Washington Press; 1973. [Google Scholar]
- Thorpe SKS. Bipedal Locomotion in Humans and Chimpanzees: Biomechanics and Implications for Hominid Evolution. Leeds: University of Leeds; 1997. PhD thesis. [Google Scholar]
- Thorpe SKS, Crompton RH. Locomotor ecology of wild orangutans (Pongo abelii) in the Gunung Leuser ecosystem, Sumatra, Indonesia: a multivariate analysis using log-linear modelling. Am J Phys Anthropol. 2005;127:58–78. doi: 10.1002/ajpa.20151. [DOI] [PubMed] [Google Scholar]
- Thorpe SKS, Crompton RH. Orangutan positional behavior and the nature of arboreal locomotion in Hominoidea. Am J Phys Anthropol. 2006;131:384–401. doi: 10.1002/ajpa.20422. [DOI] [PubMed] [Google Scholar]
- Thorpe SKS, Crompton RH, Gunther MM, et al. Dimensions and moment arms of the hind- and forelimb muscles of common chimpanzees (Pan troglodytes) Am J Phys Anthropol. 1999;110:179–199. doi: 10.1002/(SICI)1096-8644(199910)110:2<179::AID-AJPA5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Thorpe SKS, Holder RL, Crompton RH. Orangutans employ unique strategies to control branch flexibility. Proc Natl Acad Sci USA. 2009;212:2403–2410. doi: 10.1073/pnas.0811537106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle RH, Basmajian JV. Electromyography of brachial muscles in Pan gorilla and hominoid evolution. Am J Phys Anthropol. 1974;41:71–90. doi: 10.1002/ajpa.1330610108. [DOI] [PubMed] [Google Scholar]
- Tuttle RH, Basmajian JV. Electromyography of pongid shoulder muscles, 3. Quadrupedal positional behavior. Am J Phys Anthropol. 1978;49:57–69. doi: 10.1002/ajpa.1330490110. [DOI] [PubMed] [Google Scholar]
- Tuttle RH, Basmajian JV, Regenos E, et al. Electromyography of knuckle-walking: results of four experiments on the forearm of Pan gorilla. Am J Phys Anthropol. 1972;37:255–266. doi: 10.1002/ajpa.1330370210. [DOI] [PubMed] [Google Scholar]
- Ward CV. Postcranial and locomotor adaptations of hominoids. In: Henke W, Tattersall I, editors. Handbook of Paleoanthropology Vol. 2: Primate Evolution and Human Origins. Heidelberg: Springer; 2007. pp. 1011–1030. [Google Scholar]
- Wickiewicz TL, Roy RR, Powell PL, et al. Muscle-architecture and force-velocity relationships in humans. J App Physiol. 1984;57:435–443. doi: 10.1152/jappl.1984.57.2.435. [DOI] [PubMed] [Google Scholar]
- Youlatos D. Functional anatomy of forelimb muscles in Guanian atelines (Platyrrhini: primates) Ann Sci Nat Zool. 2000;21:137–151. [Google Scholar]
- Zajac FE. How musculotendon architecture and joint geometry affect the capacity of muscles to move and exert force on objects – a review with application to arm and forearm tendon transfer design. J Hand Surg Am. 1992;17A:799–804. doi: 10.1016/0363-5023(92)90445-u. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


