Abstract
Background and Purpose
Functional tissue engineering of the gastrointestinal (GI) tract is a complex process aiming to aid the regeneration of structural layers of smooth muscle, intrinsic enteric neuronal plexuses, specialized mucosa and epithelial cells as well as interstitial cells. The final tissue engineered construct is intended to mimic the native GI tract anatomically and physiologically. Physiological functionality of tissue engineered constructs is of utmost importance while considering clinical translation. The construct comprises of cellular components as well as biomaterial scaffolding components. Together, these determine the immune-response a tissue engineered construct would elicit from a host upon implantation. Over the last decade, significant advances have been made to mitigate adverse host reactions. These include a quest for identifying autologous cell sources like embryonic and adult stem cells, bone marrow-derived cells, neural crest-derived cells and muscle-derived stem cells. Scaffolding biomaterials have been fabricated with increasing biocompatibility and biodegradability. Manufacturing processes have advanced to allow for precise spatial architecture of scaffolds in order to mimic in vivo milieu closely and achieve neovascularization. This review will focus on the current concepts and the future vision of functional tissue engineering of the diverse neuromuscular structures of the GI tract from the esophagus to the internal anal sphincter.
Keywords: Smooth muscle, enteric nervous system, biomaterials, functional tissue engineering, autologous cells
1. Introduction
The gastrointestinal (GI) tract is a structurally complex hollow organ that displays diverse motility patterns to perform a variety of functions that aid ingestion, digestion, absorption of nutritive elements and excretion of waste. GI motility is a result of chemical and electrical interactions between smooth muscle, intramural innervation, interstitial cells and mucosal epithelial layers. This innate anatomical and physiological complexity dictates the requirement for a multi-disciplinary approach to regeneration of functional tissue replacements.
Anatomic complexity is recreated by engineering biomaterial micro-environments with characterized porosity and stiffness. Remodeling of the scaffold occurs upon cellular seeding according to the microenvironment, thereby dictating the functionality of the final bioengineered product. The primary goal of tissue engineering is to manufacture/engineer a physiological functional replacement tissue, using materials with appropriate biologic activity and biodegradability. Various strategies, including flow/perfusion or mechanical conditioning, are employed to maximize the functionality of the engineered “replacement tissue” before implantation. Although the flow of the process is fairly logical, there are multiple hurdles involved in each step. Figure 1 shows a schematic representation of the complexity involved in intestinal tissue engineering.
Figure 1. Schematic of tissue engineered tubular constructs for replacement of GI neuromuscular structures.
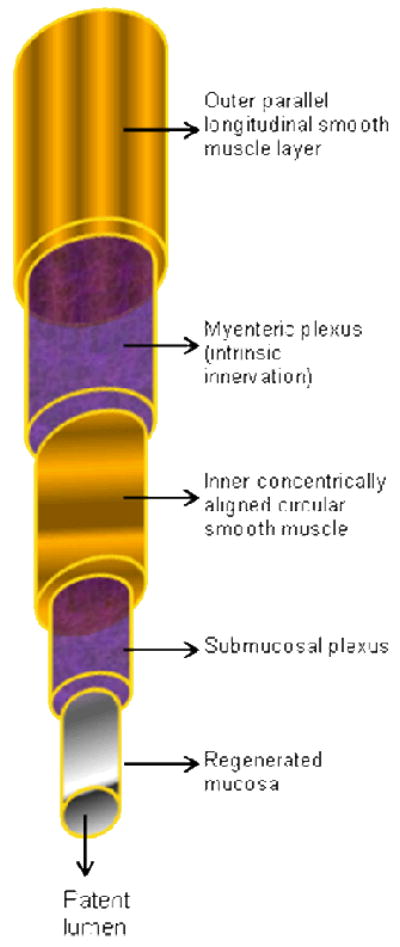
The chosen biomaterial scaffold must be adequately porous to allow neovascularization when implanted. Ideally, the tissue engineered replacement is envisioned to contain different cellular components in different layers namely, the circular and longitudinal smooth muscle components with intramuscular ICC. Additionally, the tubular construct would also contained localized enteric neuronal plexuses (myenteric and submucosal). The scaffolding material along with its cellular component must allow reepithelialization to regenerate mucosal layers. Most importantly, the bioengineered tubular constructs must be able to maintain a patent lumen and maintain integrity so as not to allow leakage during use in the body as a replacement. The ideal tissue engineered construct, when implanted, would integrate with the existing muscular, neuronal and mucosal layers as well as receive cues from the central nervous system, to facilitate peristalsis, gut motility, digestion and excretion. For the stomach, different shaped molds can be envisioned with the same modular approach to repopulation of different cellular layers.
This review will focus on the multiple approaches used to reconstruct the neuromusculature of the gut, classified under the following functional segments: esophagus, stomach, small intestine and colon that are interspersed with “sphincters” – closure zones that prevent backflow and reflux. These sphincteric regions include the lower esophageal sphincter (LES), pyloric sphincter, and the internal anal sphincter (IAS). Table 1 summarizes different GI neuromuscular disorders that could directly benefit from intestinal tissue engineering.
Table 1. Future vision of Intestinal Tissue Engineering.
| Organ system | Disoder | Regenerative Medicine alternative |
|---|---|---|
| Esophagus | Congenital long gap atresia Esophageal cancer | Tubular constructs that allow neomucosa formation and perform conduit-function; Muscular layers and enteric plexuses for motility; Integrate with central nervous system for “at-will” peristalsis |
| Lower Esophageal Sphincter | Gastroesophageal reflux disease | Sphincteric augmentation with sphincter-specialized aligned circular smooth muscle Maintenance of pressure to prevent reflux Enteric neuronal plexuses for receptive relaxation to allow passage of luminal contents to stomach |
| Stomach | Gastroparesis, Altered gastric emptying | Elastic reservoir for accomodation of food (Fundus); Intrinsically innervated smooth muscle layer for propulsive mixing (Antrum); Neomucosa for secretion; Repopulation of Interstitial Cells of Cajal for adequate pacemaking. |
| Pyloric Sphincter | Hypertensive Pylorus, Dumping syndrome | Sphincteric augmentation with sphincter-specialized aligned circular smooth muscle to maintain high-pressure closure. Enteric neuronal plexuses for adequate relaxation to allow passage of chyme |
| Small Intestine | Short Bowel Syndrome | Tubular constructs with mucosal villi and crypts for adquate nutrient absorption; Circular and longitudinal smooth muscle and enteric plexuses for peristalsis, absorption and motility. |
| Large Intestine | Aganglionosis, Inflammatory Bowel Disorders | Tubular constructs with concentrically aligned circular and longitudinal smooth muscle for peristalsis, motility and emptying. Enteric neuronal plexuses for neurotransmission and motility. |
| Internal Anal Sphincter | Fecal Incontinence, Hypertensive sphincter | Sphincteric augmentation with sphincter specialized aligned circular smooth muscle to maintain anorectal closure pressure. Enteric neuronal plexuses to mediate relaxation of the sphincter to allow defecation. |
2. Challenges in Intestinal Tissue Engineering
2.1 Cell Source & Proliferation
The first hurdle in bioengineering any tissue is the cell source. Due to the complexity of the gut, procuring cells of multiple phenotypes remains a challenge. Many individual types of cells need to be isolated by enzymatic dissociation of a single full-thickness biopsy. In this process of identifying a cell source, one also has to pay heed to the challenge of expansion of these cells to adequate numbers. Biopsy of native gut may not be an efficient source of highly proliferative cells. Therefore, cell expansion of isolated intestinal smooth muscle cells, enteric neuronal and glial precursor cells, interstitial cells of Cajal, or epithelial cells is an on-going challenge in intestinal tissue engineering.
Tissue engineered replacements can be autologous, heterologous or allogenic. The cellular component as well as the material component of the tissue engineered construct contributes to its biocompatibility and its immunogenicity. Advancement in our understanding of immunology has led to various immune-suppression and immune-tolerance treatments for patients receiving xenografts. However, this prospect of eliciting an adverse host immune response has driven regenerative medicine towards acquiring autologous cell sources.
The quest for autologous cell sources led to the discovery of populations of tissue-resident, organ-associated adult stem cells (1). Recent reports suggest that bone marrow-derived cells can be used for vascular and bladder smooth muscle regeneration by manipulating the soluble factors in their growth medium (2-4). This is an optimistic step towards producing tissue engineered smooth muscle from autologous bone marrow-derived cells. Multiple populations of muscle derived-stem cells have been identified with evidence of self-renewal and multi-lineage differentiation capabilities (5, 6). These cells have displayed the ability to differentiate into myotubes as well as smooth muscle phenotypes (7).
Neuronal progenitor cells have been identified to reside within both the central nervous system as well the enteric nervous system in embryonic as well as post-natal rodents and humans (8). Neural crest-derived stem cells have been shown to persist through adult development (9) and are a potential source of autologous neuronal cells required to re-engineer the gut neuromusculature (10). Advances in cell culture techniques have allowed the isolation of enteric neuronal and glial progenitor cells by cell sorting that express markers such as Ret and p75 (11). A recent report by Kulkarni et al. focuses on nudging CNS-derived neuronal progenitor cells into an enteric phenotype by culturing them in the presence of gut derived soluble factors (12). Metzger et al. (13) also demonstrated the reliable and reproducible isolation of enteric neuronal progenitor cells from adult human gut up to 84 years of age. These cells demonstrated the ability to differentiate into a number of mature enteric neuronal subtypes. Thus, advances in cell culture techniques have expanded the pool of available cells for autologous intestinal tissue engineering.
2.2 Biomaterials
Cells cultured within a three-dimensional environment demonstrate better cell-cell contact. The cellular microenvironment mimics in vivo milieu more closely. Tissue engineering aims to re-engineer this environment using multiple approaches, including the use of porous biocompatible scaffolds or spinner flasks maintained in bioreactor cultures. Biomaterials act as a surface to direct cell-cell interactions. They support the adhesion, proliferation and differentiation of cells seeded on to them. Scaffolding substances are typically polymeric biomaterials that are either bioinert or are adequately biodegradable. Many biomaterials have been widely used in tissue engineering applications for decades. Porous three-dimensional scaffolds provide a matrix for seeding a high density of cells to promote reorganization into a functional tissue. During this process, the cells secrete and deposit their own extra cellular matrix, while the biomaterial degrades ideally into non-toxic products. Biodegradable materials or bioinert materials are chosen depending on the end objective of the tissue engineered construct (14). Biodegradable materials need to provide adequate mechanical support until remodeling by cellular components over time can support the engineered structure structurally and mechanically. Ideally, when neuronal and muscular ingrowth and mechanical activity are required, biodegradable materials that degrade slowly over time while being replaced by cellular extracellular matrix are preferred. Moreover, bioinert materials prolong the body's exposure time with the biomaterial, which may trigger an inflammatory or foreign body response due to its permanent presence (15, 16).
Factors to keep in mind while designing scaffolds for intestinal tissue engineering are: i) mechanical properties of the scaffold itself; ii) porosity to promote gas and nutrient exchange; iii) degradation rates and iv) biocompatibility with respect to adhesion, proliferation as well as host-immune response (14, 16). Commonly used biomaterials for intestinal tissue engineering have been naturally derived materials (collagen scaffolds, small-intestinal submucosa derived scaffolds) or synthetic polymer based scaffolds (poly-L-lactic acid, poly glycolic acid (PGA), poly ε-caprolactone, etc.).
While designing scaffolds for smooth muscle, the end objectives are to provide the cells a microenvironment that specifically permits the following: i) maintenance of a contractile phenotype; ii) formation of cellular syncytium connections; and iii) directional self-organization.
Overall function directly translates from the structural cellular alignment as well as the maintenance of the contractile muscle phenotype. The template scaffold must permit circular smooth muscle of the gut to concentrically align in syncytium and form a hollow tube that can contract to propel luminal contents. Similarly, the scaffold must also allow the alignment of longitudinal smooth muscle in parallel sheets orthogonal to the circular smooth muscle layer. Scaffold chemistry and mechanical properties highly influence self-organization and regeneration of functional smooth muscle (17).
2.3 Challenges in Vascularization
Vascularization is the limiting step in the survival of a tissue engineered construct. It determines the optimal size and porosity of the scaffolding backbone. Neovascularization of implanted de novo re-engineered tissues remains a challenge to the success of tissue engineering constructs. Generation of vasculature is key to the survival of implanted tissues in vivo, because oxygen diffusion is limited to ∼200μm in tissues. Beyond this limit, vascular in-growth is required to facilitate nutrient exchange and survival. Lack of vascular in-growth into the thickness of engineered tissue will result in hypoxia, necrosis and loss of viability and subsequently functionality.
Prevascularization, either in vivo or in vitro, has been a commonly used strategy. Tissue engineered grafts are often initially implanted into a region with an artery suitable for microsurgery like the omentum for in vivo prevascularization. Alternately, tissue engineered grafts are often combined with endothelial cells that form pre-vascular structures in order to speed up vascular in-growth upon implantation (18-20). For the regeneration of complex tissues with multiple layers, like GI neuromuscular structures, thicker tissues will require more extensive vascular networks to provide nutrients to every cellular layer. Prevascularization provides the advantage of providing a vascular network-ready tissue engineered construct that can be readily perfused upon implantation, although an additional surgical step is often involved. Moreover, prevascularization offers a distinct advantage only when anastomosis to the host vasculature is available at the final site of implantation for ready perfusion (21, 22).
Delivery of angiogenic growth factors, like FGF-2, VEGF, TGF-β and more recently PDGF-BB (approved by the FDA), promote mobilization and recruitment of endothelial cells as well as stabilization of newly formed vessels (23). Delivery is achieved either by the use of micro-osmotic pumps, polymeric carrier systems, growth factor loaded collagen microspheres or even adenoviral vectors that stimulate the secretion of growth factors in situ. Polymeric carrier systems can have precisely defined spatio-temporal release patterns to locally deliver a cocktail of angiogenic growth factors to tissue engineered grafts (24). Collagen microspheres encapsulating FGF-2 have been successfully used in intestinal smooth muscle maintenance for grafts implanted in the small bowel of a mouse model (25). Recently, our group compared the use of FGF-2, VEGF and PDGF-BB in maintenance of bioengineered sphincteric smooth muscle implanted in a mouse animal model. Our data indicated that there was no significant difference with the use of any of these growth factors on neovascularization, smooth muscle fiber diameter, viability and phenotype (26).
3. Functional tissue engineering of phasic GI neuromusculature: Esophagus, Stomach, Small Intestine & Colon
Phasic neuromuscular structures of the GI tract contain orthogonal layers of smooth muscle, interlaced with enteric neuronal plexuses. They are also associated with the interstitial cells of Cajal (ICC) and specialized mucosal layers. The propagating peristaltic wave defines the phasic nature of this neuromusculature. It encompasses contraction and relaxation of both the circular and longitudinal smooth muscle layers. The neuronal components as well as the ICC generate electrical activity for the coordination of peristalsis. This activity is coupled in a highly coordinated manner to intracellular biochemical events in the smooth muscle layers to result in gut motility. These mechanisms are additionally segmentally modulated by the release of different neurotransmitters from the enteric neuronal plexuses as well as the electrical activity from the ICCs (27, 28). While designing tissue engineering replacements, it is important to keep in mind that motility patterns of the GI tract, though segmental are inherently linked to one another and work in a highly coordinated fashion (29). An example of this phenomenon is the entry of the food into the esophagus leading to the relaxation of the LES to allow passage of food into the stomach (30). Table 2A-D summarizes literature cited in the following section.
Table 2. Current Concepts in Intestinal Tissue Engineering.
| A. Esophagus | ||||||
|---|---|---|---|---|---|---|
| Author | Animal model | Biomaterial component | Cellular/Acellular approach | Type of defect | Period of follow up | Study outcomes |
| Takimoto et al. (1998) | Dogs | Silicone tube with porcine collagen | Acellular | Segmental | 1,3,6 months | Constriction of esophagus, high rates of scar tissue formation; repopulation of striated muscle and epithelialization upon removal of silicone stent. |
| Freud et al. (1999) | Dogs | Polytetrafluoroethylene, Dacron | Acellular | Patch and circumferential | 1,2,4,6,7 months | Good epithelialization but no muscular ingrowth; Circular and longitudinal stenosis. |
| Jansen et al. (2004) | Rabbits | Polyvinylidene fluoride and Polyglactic 910 | Acellular | Semicircular wall | 3 months | Reepithelialization and minimal muscular ingrowth in polyglactic; high rates of anastomotic leakage. |
| Badylak et al. (2000) | Dogs | Porcine submucosal extracellular matrix scaffold | Acellular | Patch and segmental | 4 days to 15 months | Contiguous muscle ingrowth in patch defects and good reepithelialization, stricture formations in tube grafts. |
| Lopes et al. (2006) | Rats | Porcine small intestinal submucosa derived collagen matrix | Acellular | Semi circumferential | 5 months | Minimal nerve ingrowth, good reepithelialization and muscular ingrowth. |
| Saxena et al. (2009) | In vitro | Collagen scaffolds with a Matrigen® coating | Rat esophageal epithelial cells, aortic smooth muscle cells | In vitro three-dimensional cultures | 1-8 weeks | Unidirectionally aligned tissue engineered smooth muscle strands as well as esophageal epithelium. |
| Hayashi et al. (2005) | Athymic rats | Porcine Type I Collagen | Human esophageal epithelial cells, dermal fibroblasts, aortic smooth muscle cells | Subcutaneous transplantation on dorsum | 1,2 weeks | Preservation of aligned epithelial, submucosal and smooth muscle layers, neovascularization. |
| Sato et al. (1994) | Athymic rats | Polyglycolic acid and collagen | Human esophageal epithelial cells | Subcutaneous implantation on dorsum | 4,8, 20 and 28 days | Healthy epithelium and neovascularization in tubular structures. |
| Nakase et al. (2008) | Dog | Polyglycolic acid | Canine oral fibroblasts, keratinocytes and gastric smooth muscle | Tubular esophageal replacement | 10,20, 50 and 70 weeks | Repopulation of epithelium, muscularis mucosa and smooth muscle; stricture formation in tubular grafts; report absence of peristaltic activity in tissue engineered esophagus. |
| B. Stomach | ||||||
|---|---|---|---|---|---|---|
| Author | Animal model | Biomaterial component | Cellular/Acellular approach | Type of defect | Period of follow up | Study outcomes |
| Hori et al. (2002) | Dogs | Collagen sponge with polyglycolic acid | Acellular | Wall defect | 16 weeks | Moderate repopulation of mucosal and smooth muscle layers, minimal proton pump staining and no acetylcholine induced contraction in tissue engineered gastric wall. |
| Araki et al. (2009) | Dogs | Collagen sponge reinforced with polylactide and caprolactone | Scaffolds dipped in bone marrow and peripheral blood | Circular defect | 16 weeks | Ulcerations before minimal mucosal regeneration, no smooth muscle regeneration, shrinkage of tissue engineered constructs |
| Maemura et al. (2003) | Rats | Composite poly- lactic and glycolid acid meshes | Rodent gastric organoid units | Full stomach | 12 weeks | Neomucosa, stratified smooth muscle, vascularization, good reservoir function, no stenosis. |
| C. Small Instestine | ||||||
|---|---|---|---|---|---|---|
| Author | Animal model | Biomaterial component | Cellular/Acellular approach | Type of defect | Period of follow up | Study outcomes |
| Nakase et al. (2006) | Dogs | Collagen sponge | With and without intestinal smooth muscle | Ileal patch graft | 4,8,12 weeks | Very short villi and very minimal muscular repopulation in the acellular group; Well populated epithelial layer with villi and a circular smooth muscle layer in the cellular group. Shrinkage of graft. |
| Kaihara et al. & Kim et al. (1999) | Rats | Polyglycolic acid meshes | Intestinal organoid units | Side to side grafts in jejunum, tubular replacements | 10 weeks | Prevascularized in the omentum, Continuous neomucosa formation with crypt-villi structures, no report on muscular ingrowth. |
| Chen et al. (2001) | Dogs | Small intestinal submucosa | Acellular | Partial wall defect | 2 weeks to 1 year | Reepithelialization and formation of neomucosa, moderate repopulation of smooth muscle, Intestinal leakage and obstruction. |
| D. Colon | ||||||
|---|---|---|---|---|---|---|
| Author | Animal model | Biomaterial component | Cellular/Acellular approach | Type of defect | Period of follow up | Study outcomes |
| Grikscheit et al. (2003) | Rats | Polyglycolic acid mesh | Colonic organoid units | Omentum implantation | 2-14 weeks | Repopulation of muscosal, muscularis propria layers and enteric plexuses |
| Metzger et al. (2009) | Organotypic human and chick hindgut cultures | No biomaterial | Human neural crest progenitor cells | Injection into organotypic aganglionic gut tissue cultures | 12 days | Neurosphere-like bodies localized and repopulated myenteric and submucosal plexuses, differentiated into glial and neuronal phenotypes (serotonergic, nitrergic, cholinergic, VIP-ergic). |
| Pan et al. (2011) | Rats | No biomaterial | Neural crest progenitor cells | Intramuscular injection into distal colon | 1,4,8 weeks | Progenitor cells differentiated into neurons and glia in the host colon, moderate reversal of denervation on colonic function |
| E. Sphincters | ||||||
|---|---|---|---|---|---|---|
| Author | Animal model | Biomaterial component | Cellular/Acellular approach | Type of defect | Period of follow up | Study outcomes |
| Feretis et al. (2001) | Human | Polymethylmethacrylate microspheres | Acellular | Submucosal injection into esophageal folds | 5-11 months | Decrease in reflux episodes and symptoms, no granulomas or ulcerations at the site of injection; Require long-term follow up studies as well as establishment of a clear method for reduction of reflux. |
| Fockens et al. (2004) | Human | Polyacrylonitrile based hydrogel | Acellular | Submucosal injection into Lower Esophageal Sphincter | 1,3 and 6 months | Significant improvement in reflux symptoms, reduced acid exposure of the LES-esophagus junction; Requires long-term efficacy as well as migration of prosthetic material studies. |
| Bonavina et al. (2008) | Human | Titanium wiring encasing magnetic beads | Acellular | Laproscopic implantation of device around Lower Esophageal Sphincter | 3 months – 1 year | Symptomatic improvement, reduced acid exposure; risk of device erosion and no significant change in manometric pressure recordings pre- and post- surgery. |
| Pasricha et al. (2009) | Rats and Dogs | No biomaterial | Skeletal muscle derived cells | Bilateral injection into pyloric wall or Lower Esophageal Sphincter | 3- 4 weeks | Cells localized in muscularis mucosa and muscular layers, differentiated into skeletal muscle and not smooth muscle, improved baseline LES pressure but no change in Pyloric pressure; Increased cholinergic contraction but lacks adequate controls or long-term follow up. |
| Kang et al. (2008) | Rats | No biomaterial | Muscle-derived stem cells | Intramucular injection into the Internal Anal Sphicnter | 1 week | Ambiguity over whether injected stem cells perform a bulking function or augment injured sphincteric muscle function; Moderately improved cholinergic contractility, but lack of long-term follow up of migration of injected cells. |
| Raghavan et al. (2009) | Mice | Fibrin-based hydrogel | Murine Internal Anal Sphincter circular smooth muscle cells | Dorsal implantation | 4 weeks | Good neovascularization and maintenance of smooth muscle phenotype; adequate physiological response compared to pre-implant control bioengineered tissues; lack of enteric neuronal population. |
| Raghavan et al. (2010) | Mice | Collagen-based hydrogel | Human Internal Anal Sphincter circular smooth muscle and Mouse enteric neuronal progenitor cells | Dorsal implantation | 4 weeks | Formation of enteric neuronal network, Neovascularization and maintenance of neuronal network upon implantation; Maintenance of aspects of neuronal and myogenic IAS physiology. |
3.1 Esophagus
The esophageal conduit extends from the pharynx to the gastroesophageal junction. Due to coordinated motility in the GI tract, the lack of peristalsis in the esophagus leads to hypertensive LES. Surgical interventions to remedy long-gap esophageal atresia are usually followed by dysmotility and impaired quality of life. In the case of bioengineered esophageal replacements, the restoration of physiological functionality must meet the requirement of both gravitational and peristaltic food transport. This becomes challenging due to the phenomenon of at-will “primary peristalsis”; a complex interplay between the central and enteric nervous systems (31).
Early reports in esophageal wall replacement demonstrated no muscular ingrowth with non-absorbable materials like polytetrafluoroethylene or Dacron. Surface functionalization of these bioinert prosthetic materials with antigenic collagen resulted in a moderate cellular repopulation. However, major side-effects associated with the use of these materials were stricture formation and inflammatory reaction (32-35). Acellular absorbable biomaterials were developed in order to improve the biocompatibility and minimize the host-inflammatory response of the existing prosthetic materials. These were typically extra cellular matrix patches or collagen matrices derived from the urinary bladder or intestinal submucosa. The use of acellular xenogenic extra cellular matrix scaffolds to repair patch defects in the esophageal wall of canine models demonstrated neovascularization and neo-innervation, but no repopulation of esophageal smooth muscle (35, 36).
Acellular approaches were improved by seeding biomaterials with cells. A modular approach to the regeneration of the esophagus by Saxena et al. used basement membrane matrix coated scaffolds to promote survival and unidirectional alignment of both epithelial cells as well as smooth muscle (37). Autologous neo-esophagus constructs have been engineered using composite cells (human esophageal epithelial cells, aortic smooth muscle cells and dermal fibroblasts) all embedded into porcine tendon collagen or PGA meshes (38, 39). More recently, Nakase et al. replaced a small portion of resected esophagus using keratinocytes, fibroblasts and smooth muscle cells seeded on human amniotic membrane and PGA sheets (40).
Although these tissue engineering attempts display significant repopulation of constituent cell types and similarities to native esophagus morphology, most segments remain aperistaltic and may cause dysmotility related problems during long-term implantation. In order to externally induce peristalsis, artificial esophagus have been engineered using nickel-titanium shape memory alloys, and programmed to display peristaltic patterns when implanted in a goat model (41). Independent experiments using these materials for esophageal reconstruction, however, resulted in stenosis to different degrees (42). The paradigm of functional esophageal tissue engineering, if clinically intended to replace long segments, must mandatorily include peristalsis mediated by the intramural and myogenic esophageal components.
3.2 Stomach
The stomach acts as a food reservoir. Gastric motility breaks down food mechanically and enhances mixing with digestive enzymes. This further breaks down food chemically. Gastric emptying into the duodenum is mediated by mechanical, neuronal and hormonal influences. This ensures delivery of nutrients in an orderly manner to facilitate absorption. Disruption of motility in the stomach, due to diabetes, depletion of ICCs or weakened smooth muscle function results in either delayed or rapid gastric emptying.
Hori et al. used acellular collagen sponge scaffolds to repair gastric wall defects in a canine model. They demonstrated the regeneration of a mucosal layer with proton pump positive cells. However, the regenerated muscular layer did not display the ability to contract upon treatment with Acetylcholine (43). Araki et al. seeded collagen sponge scaffolds with autologous bone marrow and mesenchymal cells. They additionally supported the scaffold with a biodegradable composite of poly-L-lactic acid and polycaprolactone, to prevent shrinkage of the scaffold upon implantation (44). Gastric epithelial organoid units were seeded on composite PLGA meshes to replace native stomach of rats (45). While all the above techniques repopulated a functional mucosa, the regeneration of stratified smooth muscle layers with the proper orientation remains a challenge. Moreover, restoration of functional motility was not demonstrated in the study, highlighting the biggest challenge yet again in functional tissue engineering of the GI neuromusculature.
Tissue engineering of the stomach needs to primarily focus on creating a hollow elastic reservoir. Aspects of bladder tissue engineering, whereby de-novo bladder reservoirs are manufactured with a variety of biomaterials, can be used as templates to re-engineer the musculature of the stomach. These can be paced by implantable gastric stimulation units, already commonly used in bariatric surgeries and in gastroparesis to stimulate enteric neurons or simulate gastric electrical rhythm. A report by Micci et al. demonstrates that the transplantation of CNS-derived neuronal progenitor cells can repopulate nitrergic neurons as well as improve gastric function in the pylorus of a rodent model of gastroparesis (46). Transplantation of the progenitor cells offers an alternate route to be explored in mimicking aspects of functional gut motility.
3.3 Small Intestine
The small intestine is the primary nutrient absorptive structure of the GI tract. Peristalsis and segmental contractions of the small intestine increase the surface area to facilitate greater absorption by the villi of the intestinal epithelium. Loss of intestinal segments due to congenital defects or multiple surgical resections due to inflammation or cancer result in short bowel syndrome. Short segments of small bowel result in malabsorption, malnutrition and adaptive alteration of motility patterns.
Tissue engineering has offered an elegant solution to the bowel lengthening surgeries commonly carried out in short bowel syndrome. Collagen sponge scaffolds seeded with autologous smooth muscle cells have been successfully implanted as a patch graft in a canine model (47). This patch graft regenerated the mucosal and intestinal epithelial layers along with the villi structures. The major problem encountered with these grafts was shrinkage. Dunn et al. used pseudo-tubular structures formed from collagen sponge scaffolds seeded with intestinal smooth muscle cells. These were neovascularized within a month after prevascularization in the omentum (25). These techniques did not regenerate the enteric neuronal layers, and the smooth muscle cells demonstrated a phenotypic switch to their non-contractile forms.
In order to preserve the epithelium-mesenchyme interaction to aid survival, intestinal organoid units were cultured and seeded onto tubular polymer scaffolds (48, 49). Vacanti and colleagues implanted tissue-engineered intestine comprised of neonatal rat intestinal organoid units into the omentum of adult rats, and then subsequently implanted these constructs to rescue morbidity resulting from a massive bowel resection. (50). Scaffolds made of small intestinal submucosa and wrapped with omentum were implanted in canine models of short bowel syndrome. These scaffolds repaired patch defects and replaced tubular segments of short bowel, thereby increasing the length of the short bowel (51). Tissue engineered small intestinal constructs regenerated enteric neuronal plexuses and met basic physiological demands. However, these techniques did not regenerate the alignment of the circular and longitudinal smooth muscle that is crucial to generating appropriate force and motility to facilitate nutrient absorption.
3.4 Colon
The colon is contiguous with the small intestine, facilitating water absorption and excretion of stool. Loss of colonic segments by surgical resections to treat aganglionosis (Hirschsprung's Disease) or inflammation significantly alters colonic motility. Disruption of colonic motility alters transit time, resulting in constipation or diarrhea. The idiopathic nature of some of these disease states poses a strong demand for in vitro tissue engineered models of colon, where investigations can be carried out on individual components (smooth muscle, enteric neurons, interstitial cells and mucosa) to understand alterations in pathophysiological conditions. Moreover, alterations in peristalsis and segmental contractions of the colon have direct implications on an individual's quality of life.
Vacanti et al. generated tissue engineered colon on composite poly lactic and glycolic acid polymers from organoid units isolated from the sigmoid colon (52). These approaches demonstrated that the tissue engineered conduits have significant absorptive capacity when implanted, but there was no direct measurement of peristalsis or motility. Our group has successfully bioengineered three-dimensional fibrin-based models of colon that allow self-alignment of circular smooth muscle layers concentrically around a patent lumen. These bioengineered tissues mimic native smooth muscle alignment and maintain aspects of colonic physiology like peristalsis, contraction and relaxation (53). In a recent study by Pan et al. (54), neural crest progenitor cells isolated from neonatal rats were transplanted into the distal colon of a rat model of Hirschsprung's Disease. These cells differentiated into neurons and glia in the host colon. They also demonstrated rescue of neuronal mediated motility in the aganglionic host colon. Metzger et al. (55) demonstrated that adult human gut derived enteric progenitor cells can repopulate segments of human aganglionic colon grown in organotypic cultures. Transplantation of enteric neuronal progenitor cells therefore offer successful alternate routes to regeneration and improvement of gut function.
Although significant advances have been made in tissue engineering of phasic neuromuscular structures, focus is required on the identifiable gaps that exist in regeneration of functional smooth muscle and enteric neuronal plexuses.
4. Functional tissue engineering of sphincteric neuromusculature: Lower Esophageal, Pyloric & Internal Anal Sphincters
Sphincters of the GI tract segregate the different phasic segments. They are made of concentrically aligned specialized circular smooth muscle with dense enteric neuronal plexuses. Sphincters remain tonically closed, creating a high pressure (basal tone) closure zone. They mediate transfer of luminal contents between esophagus and stomach (Lower Esophageal Sphincter; LES), stomach and small intestine (Pyloric sphincter) or from the rectum to the outside (Internal Anal Sphincter; IAS). Efferent innervation pathways automatically signal to the sphincter during the passage of food. This allows sphincters to transiently relax their pressure and allow luminal contents to pass and then subsequently return to their basal closure pressure. Sphincter specialized circular smooth muscles express higher levels of proteins involved in contractility like RhoA, PKCα, smooth muscle actin, myosin isoforms, etc. to myogenically generate basal tone in the absence of innervation (56-58). Inhibitory neurotransmission, in particular mediated by nitric oxide, is more important in sphincters than in adjoining phasic muscle segments to mediate transient relaxation. Age-related weakening of the mechanical efficiency of the sphincters, diabetes-induced neuropathy, idiopathic degeneration of sphincter muscle all result in altered closure pressures of the sphincter leading to reflux (LES), gastroparesis (Pylorus) or incontinence (IAS). Ideally, the paradigm for functional sphincteric tissue engineering should focus on manufacturing innervated smooth muscle replacement structures. These replacements must be made of structurally sound biomaterials that do not alter mechanotransduction while allowing biocompatible integration, neovascularization and neuronal in-growth from the central and peripheral nervous system. Table 2E summarizes literature cited in the following section.
4.1 Lower Esophageal Sphincter
The lower esophageal sphincter is responsible for preventing reflux of acidic gastric contents back to the esophagus which can lead to severe conditions ranging from Gastroesophageal reflux disease (GERD) to Barretts esophagus and adenocarcinomas. Pharmacotherapies do not directly address the issue of weakened sphincter efficiency, while surgical manipulations significantly alter the GI tract anatomy at the gastroesophageal junction.
Initial solutions for reflux management focused on augmenting the bulk of the LES by submucosal injection of bioinert materials like polymethylmethacrylate beads (59), polyacrylonitrile based hyrdrogels (60), etc. However, there was no evidence of long-term efficacy or improvement in sphincteric physiology due to a lack of follow-up. Bioinert magnetic titanium beads looped in a roman arch were implanted laprascopically to augment LES closure pressure (61). Feasibility trials with this device however did not rule out the propensity for device erosion. Recently, a cellular transplantation approach to improving baseline LES pressure was achieved by injecting skeletal muscle-derived stem cells in the LES region of canine models. These cells integrated within the underlying GI smooth muscle, but did not demonstrate differentiation into contractile smooth muscle phenotypes (62).
Tissue engineered LES constructs must possess the ability to generate myogenic basal tone. They must also additionally be highly biocompatible and wire into the existing neuronal network to transiently relax to allow food to pass from the esophagus to stomach, upon esophageal peristalsis.
4.2 Internal Anal Sphincter
The Internal Anal Sphincter (IAS) contributes to 70% of the anal canal closure pressure, maintaining continence. Weakened mechanical efficiency of the IAS due to idiopathic sphincteric degeneration, surgical or obstetric trauma all lead to passive and active incontinence. Similar to the LES, current therapeutic mainstays do not address the direct issue of muscular degeneration of the sphincter itself.
A biomedical device approach to restore sphincteric function involved the manufacture of artificial anal sphincters. These were prone to device failure and had significant morbidity associated with them (63). Bulking of the IAS, similar to the LES, was carried out by injecting biocompatible materials like microspheres of hydroxyapatite or cross-linked collagen. This approach was associated with the potential of migration of biomaterials into the lymph drainage and did not significantly improve sphincter contractility. These measures of introducing biomaterials or biomedical devices have had limited success in their outcome in overcoming incontinence or augmenting sphincteric function.
Recently, Kang et al. used a cellular transplantation approach to augment IAS function in a rat incontinence model. The authors transplanted autologous skeletal muscle-derived stem cells in cryo-injured IAS. These injected cells were integrated within the muscle with minimal inflammation, but demonstrated no evidence of developing into functional muscle (64). Our lab has demonstrated the functional bioengineering of self-aligned IAS smooth muscle tissue constructs from rodents, rabbits and humans. These constructs generate myogenic basal tone, display native smooth muscle alignment and contractile smooth muscle phenotype. These constructs were neovascularized upon implantation on the dorsum of mice. Additionally, they also maintained key aspects of IAS physiology like myogenic basal tone generation and transient relaxation (65). More recently, our group also demonstrated the ability to bioengineer “pre-wired” sphincters with an intrinsic enteric neuronal population associated with the smooth muscle. Implantation of these constructs preserved neuronal networking as well myogenic and neuronal functionality (66).
5. Conclusion
The evolution of tissue engineering has advanced from its novelty as an experimental therapeutic paradigm to an immensely clinically translatable therapy. Examples of GI motility-related pathologies that are inclined to tissue engineering solutions are short bowel syndrome, fecal incontinence and GERD among others. Bioengineering of GI neuromuscular structures offers a solution to improve quality of life and reduce morbidity associated with the use of external devices or conventional surgical and pharmacological approaches that have formed the mainstay of current clinical management for many of these conditions. Additionally, de novo generation of three-dimensional neuromuscular structures of the GI tract in vitro can be used to investigate molecular mechanisms that contribute to pathophysiology of functional GI disorders.
Acknowledgments
This work was supported by NIH/NIDDK DK071614 and 1RC1DK08715.
Footnotes
Competing interests: The authors have no competing interests.
References
- 1.Weiner LP. Definitions and criteria for stem cells. Methods Mol Biol. 2008;438:3–8. doi: 10.1007/978-1-59745-133-8_1. Epub 2008/03/29. [DOI] [PubMed] [Google Scholar]
- 2.Tian H, Bharadwaj S, Liu Y, Ma PX, Atala A, Zhang Y. Differentiation of human bone marrow mesenchymal stem cells into bladder cells: potential for urological tissue engineering. Tissue Eng Part A. 16(5):1769–79. doi: 10.1089/ten.TEA.2009.0625. Epub 2009/12/22. PubMed Central PMCID: PMC2952115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar AH, Metharom P, Schmeckpeper J, Weiss S, Martin K, Caplice NM. Bone marrow-derived CX3CR1 progenitors contribute to neointimal smooth muscle cells via fractalkine CX3CR1 interaction. FASEB J. 24(1):81–92. doi: 10.1096/fj.09-132225. Epub 2009/09/12. doi: fj.09-132225 [pii] [DOI] [PubMed] [Google Scholar]
- 4.Metharom P, Kumar AH, Weiss S, Caplice NM. A specific subset of mouse bone marrow cells has smooth muscle cell differentiation capacity-brief report. Arterioscler Thromb Vasc Biol. 30(3):533–5. doi: 10.1161/ATVBAHA.109.200097. Epub 2009/12/17. doi: ATVBAHA.109.200097 [pii] [DOI] [PubMed] [Google Scholar]
- 5.Deasy BM, Jankowski RJ, Huard J. Muscle-derived stem cells: characterization and potential for cell-mediated therapy. Blood Cells Mol Dis. 2001;27(5):924–33. doi: 10.1006/bcmd.2001.0463S1079-9796(01)90463-2 [pii]. Epub 2002/01/11. [DOI] [PubMed] [Google Scholar]
- 6.Cao B, Huard J. Muscle-derived stem cells. Cell Cycle. 2004;3(2):104–7. Epub 2004/01/09. doi: 644 [pii] [PubMed] [Google Scholar]
- 7.Hwang JH, Yuk SH, Lee JH, Lyoo WS, Ghil SH, Lee SS, et al. Isolation of muscle derived stem cells from rat and its smooth muscle differentiation [corrected] Mol Cells. 2004;17(1):57–61. Epub 2004/04/02. [PubMed] [Google Scholar]
- 8.Kulkarni S, Becker L, Pasricha PJ. Stem cell transplantation in neurodegenerative disorders of the gastrointestinal tract: future or fiction? Gut. 2011 doi: 10.1136/gut.2010.235614. Epub 2011/08/06. doi: gut.2010.235614 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kruger GM, Mosher JT, Bixby S, Joseph N, Iwashita T, Morrison SJ. Neural crest stem cells persist in the adult gut but undergo changes in self-renewal, neuronal subtype potential, and factor responsiveness. Neuron. 2002;35(4):657–69. doi: 10.1016/s0896-6273(02)00827-9. Epub 2002/08/27. doi: S0896627302008279 [pii] PubMed Central PMCID: PMC2728576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schafer KH, Micci MA, Pasricha PJ. Neural stem cell transplantation in the enteric nervous system: roadmaps and roadblocks. Neurogastroenterol Motil. 2009;21(2):103–12. doi: 10.1111/j.1365-2982.2008.01257.x. Epub 2009/02/14. doi: NMO1257 [pii] [DOI] [PubMed] [Google Scholar]
- 11.Young HM. Neural stem cell therapy and gastrointestinal biology. Gastroenterology. 2005;129(6):2092–5. doi: 10.1053/j.gastro.2005.10.033. Epub 2005/12/14. doi: S0016-5085(05)02192-X [pii] [DOI] [PubMed] [Google Scholar]
- 12.Kulkarni S, Zou B, Hanson J, Micci MA, Tiwari G, Becker L, et al. Gut-derived factors promote neurogenesis of CNS-neural stem cells and nudge their differentiation to an enteric-like neuronal phenotype. Am J Physiol Gastrointest Liver Physiol. 2011 doi: 10.1152/ajpgi.00123.2011. Epub 2011/08/06. doi: ajpgi.00123.2011 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Metzger M, Bareiss PM, Danker T, Wagner S, Hennenlotter J, Guenther E, et al. Expansion and differentiation of neural progenitors derived from the human adult enteric nervous system. Gastroenterology. 2009;137(6):2063–73 e4. doi: 10.1053/j.gastro.2009.06.038. Epub 2009/06/25. doi: S0016-5085(09)00931-7 [pii] [DOI] [PubMed] [Google Scholar]
- 14.Hubbell JA. Biomaterials in tissue engineering. Biotechnology (N Y) 1995;13(6):565–76. doi: 10.1038/nbt0695-565. Epub 1995/06/01. [DOI] [PubMed] [Google Scholar]
- 15.Shin H, Jo S, Mikos AG. Biomimetic materials for tissue engineering. Biomaterials. 2003;24(24):4353–64. doi: 10.1016/s0142-9612(03)00339-9. Epub 2003/08/19. [DOI] [PubMed] [Google Scholar]
- 16.Kim BS, Mooney DJ. Development of biocompatible synthetic extracellular matrices for tissue engineering. Trends in biotechnology. 1998;16(5):224–30. doi: 10.1016/s0167-7799(98)01191-3. Epub 1998/06/11. [DOI] [PubMed] [Google Scholar]
- 17.Kim BS, Nikolovski J, Bonadio J, Smiley E, Mooney DJ. Engineered smooth muscle tissues: regulating cell phenotype with the scaffold. Experimental cell research. 1999;251(2):318–28. doi: 10.1006/excr.1999.4595. Epub 1999/09/02. [DOI] [PubMed] [Google Scholar]
- 18.Schultheiss D, Gabouev AI, Cebotari S, Tudorache I, Walles T, Schlote N, et al. Biological vascularized matrix for bladder tissue engineering: matrix preparation, reseeding technique and short-term implantation in a porcine model. J Urol. 2005;173(1):276–80. doi: 10.1097/01.ju.0000145882.80339.18. Epub 2004/12/14. S0022-5347(05)60838-5 [pii] [DOI] [PubMed] [Google Scholar]
- 19.Levenberg S, Rouwkema J, Macdonald M, Garfein ES, Kohane DS, Darland DC, et al. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005;23(7):879–84. doi: 10.1038/nbt1109. Epub 2005/06/21. doi: nbt1109 [pii] [DOI] [PubMed] [Google Scholar]
- 20.Schauerte P, Schimpf T, Mischke K, Zarse M, Schmid M, Plisiene J, et al. Morphology and function of the intrinsic cardiac nervous system. Herz. 2006;31(2):96–100. doi: 10.1007/s00059-006-2781-2. Epub 2006/06/02. [DOI] [PubMed] [Google Scholar]
- 21.Lovett M, Lee K, Edwards A, Kaplan DL. Vascularization strategies for tissue engineering. Tissue engineering Part B, Reviews. 2009;15(3):353–70. doi: 10.1089/ten.TEB.2009.0085. Epub 2009/06/06. PubMed Central PMCID: PMC2817665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rouwkema J, Rivron NC, van Blitterswijk CA. Vascularization in tissue engineering. Trends in biotechnology. 2008;26(8):434–41. doi: 10.1016/j.tibtech.2008.04.009. Epub 2008/07/01. [DOI] [PubMed] [Google Scholar]
- 23.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19(11):1029–34. doi: 10.1038/nbt1101-1029nbt1101-1029 [pii]. Epub 2001/11/02. [DOI] [PubMed] [Google Scholar]
- 24.Lee KY, Peters MC, Anderson KW, Mooney DJ. Controlled growth factor release from synthetic extracellular matrices. Nature. 2000;408(6815):998–1000. doi: 10.1038/35050141. Epub 2001/01/05. [DOI] [PubMed] [Google Scholar]
- 25.Lee M, Wu BM, Stelzner M, Reichardt HM, Dunn JC. Intestinal smooth muscle cell maintenance by basic fibroblast growth factor. Tissue Eng Part A. 2008;14(8):1395–402. doi: 10.1089/ten.tea.2007.0232. Epub 2008/08/06. [DOI] [PubMed] [Google Scholar]
- 26.Miyasaka EA, Raghavan S, Gilmont RR, Mittal K, Somara S, Bitar KN, et al. In vivo growth of a bioengineered internal anal sphincter: comparison of growth factors for optimization of growth and survival. Pediatr Surg Int. doi: 10.1007/s00383-010-2786-z. Epub 2010/11/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarna SK. Physiology and pathophysiology of colonic motor activity (1) Dig Dis Sci. 1991;36(6):827–62. doi: 10.1007/BF01311244. Epub 1991/06/01. [DOI] [PubMed] [Google Scholar]
- 28.Thomsen L, Robinson TL, Lee JC, Farraway LA, Hughes MJ, Andrews DW, et al. Interstitial cells of Cajal generate a rhythmic pacemaker current. Nature medicine. 1998;4(7):848–51. doi: 10.1038/nm0798-848. Epub 1998/07/14. [DOI] [PubMed] [Google Scholar]
- 29.Sarna SK, Otterson MF. Gastrointestinal motility: some basic concepts. Pharmacology. 1988;36 1:7–14. doi: 10.1159/000138415. Epub 1988/01/01. [DOI] [PubMed] [Google Scholar]
- 30.Castell DO. The lower esophageal sphincter. Physiologic and clinical aspects. Annals of internal medicine. 1975;83(3):390–401. doi: 10.7326/0003-4819-83-3-390. Epub 1975/09/01. [DOI] [PubMed] [Google Scholar]
- 31.Goyal RK, Chaudhury A. Physiology of normal esophageal motility. J Clin Gastroenterol. 2008;42(5):610–9. doi: 10.1097/MCG.0b013e31816b444d. Epub 2008/03/28. PubMed Central PMCID: PMC2728598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takimoto Y, Nakamura T, Yamamoto Y, Kiyotani T, Teramachi M, Shimizu Y. The experimental replacement of a cervical esophageal segment with an artificial prosthesis with the use of collagen matrix and a silicone stent. J Thorac Cardiovasc Surg. 1998;116(1):98–106. doi: 10.1016/S0022-5223(98)70247-8. Epub 1998/07/22. doi: S0022522398002633 [pii] [DOI] [PubMed] [Google Scholar]
- 33.Freud E, Efrati I, Kidron D, Finally R, Mares AJ. Comparative experimental study of esophageal wall regeneration after prosthetic replacement. J Biomed Mater Res. 1999;45(2):84–91. doi: 10.1002/(SICI)1097-4636(199905)45:2<84(x02237)AID-JBM2>3.0.CO;2-O [pii]. Epub 1999/07/09. [DOI] [PubMed] [Google Scholar]
- 34.Lynen Jansen P, Klinge U, Anurov M, Titkova S, Mertens PR, Jansen M. Surgical mesh as a scaffold for tissue regeneration in the esophagus. Eur Surg Res. 2004;36(2):104–11. doi: 10.1159/000076650ESR2004036002104 [pii]. Epub 2004/03/10. [DOI] [PubMed] [Google Scholar]
- 35.Badylak S, Meurling S, Chen M, Spievack A, Simmons-Byrd A. Resorbable bioscaffold for esophageal repair in a dog model. J Pediatr Surg. 2000;35(7):1097–103. doi: 10.1053/jpsu.2000.7834. Epub 2000/08/05. doi: S0022-3468(00)17439-1 [pii] [DOI] [PubMed] [Google Scholar]
- 36.Lopes MF, Cabrita A, Ilharco J, Pessa P, Patricio J. Grafts of porcine intestinal submucosa for repair of cervical and abdominal esophageal defects in the rat. J Invest Surg. 2006;19(2):105–11. doi: 10.1080/08941930600569621. Epub 2006/03/15. doi: VWX25L16U131163H [pii] [DOI] [PubMed] [Google Scholar]
- 37.Saxena AK, Kofler K, Ainodhofer H, Hollwarth ME. Esophagus tissue engineering: hybrid approach with esophageal epithelium and unidirectional smooth muscle tissue component generation in vitro. J Gastrointest Surg. 2009;13(6):1037–43. doi: 10.1007/s11605-009-0836-4. Epub 2009/03/12. [DOI] [PubMed] [Google Scholar]
- 38.Hayashi K, Ando N, Ozawa S, Kitagawa Y, Miki H, Sato M, et al. A neo-esophagus reconstructed by cultured human esophageal epithelial cells, smooth muscle cells, fibroblasts, and collagen. ASAIO J. 2004;50(3):261–6. doi: 10.1097/01.mat.0000123688.45717.a4. Epub 2004/06/03. [DOI] [PubMed] [Google Scholar]
- 39.Sato M, Ando N, Ozawa S, Miki H, Kitajima M. An artificial esophagus consisting of cultured human esophageal epithelial cells, polyglycolic acid mesh, and collagen. ASAIO J. 1994;40(3):M389–92. doi: 10.1097/00002480-199407000-00028. Epub 1994/07/01. [DOI] [PubMed] [Google Scholar]
- 40.Nakase Y, Nakamura T, Kin S, Nakashima S, Yoshikawa T, Kuriu Y, et al. Intrathoracic esophageal replacement by in situ tissue-engineered esophagus. J Thorac Cardiovasc Surg. 2008;136(4):850–9. doi: 10.1016/j.jtcvs.2008.05.027. Epub 2008/10/29. doi: S0022-5223(08)01046-5 [pii] [DOI] [PubMed] [Google Scholar]
- 41.Watanabe M, Sekine K, Hori Y, Shiraishi Y, Maeda T, Honma D, et al. Artificial esophagus with peristaltic movement. ASAIO J. 2005;51(2):158–61. doi: 10.1097/01.mat.0000154644.44891.f1. Epub 2005/04/21. [DOI] [PubMed] [Google Scholar]
- 42.Xie YC, Zhang BG, Peng PX, Liang JH. Application of artificial prosthesis constructed by titanium-nickel alloy and silicone in repairing esophageal defects. Journal of Clinical Rehabilitative Tissue Engineering Research. 2009;13(34):6675–9. [Google Scholar]
- 43.Hori Y, Nakamura T, Kimura D, Kaino K, Kurokawa Y, Satomi S, et al. Functional analysis of the tissue-engineered stomach wall. Artif Organs. 2002;26(10):868–72. doi: 10.1046/j.1525-1594.2002.07006.x. Epub 2002/09/26. doi: aor7006[pii] [DOI] [PubMed] [Google Scholar]
- 44.Araki M, Tao H, Sato T, Nakajima N, Hyon SH, Nagayasu T, et al. Development of a new tissue-engineered sheet for reconstruction of the stomach. Artif Organs. 2009;33(10):818–26. doi: 10.1111/j.1525-1594.2009.00808.x. Epub 2009/10/21. [DOI] [PubMed] [Google Scholar]
- 45.Maemura T, Shin M, Sato M, Mochizuki H, Vacanti JP. A tissue-engineered stomach as a replacement of the native stomach. Transplantation. 2003;76(1):61–5. doi: 10.1097/01.TP.0000068903.63554.1B. Epub 2003/07/17. [DOI] [PubMed] [Google Scholar]
- 46.Micci MA, Kahrig KM, Simmons RS, Sarna SK, Espejo-Navarro MR, Pasricha PJ. Neural stem cell transplantation in the stomach rescues gastric function in neuronal nitric oxide synthase-deficient mice. Gastroenterology. 2005;129(6):1817–24. doi: 10.1053/j.gastro.2005.08.055. Epub 2005/12/14. doi: S0016-5085(05)01773-7 [pii] [DOI] [PubMed] [Google Scholar]
- 47.Nakase Y, Hagiwara A, Nakamura T, Kin S, Nakashima S, Yoshikawa T, et al. Tissue engineering of small intestinal tissue using collagen sponge scaffolds seeded with smooth muscle cells. Tissue Eng. 2006;12(2):403–12. doi: 10.1089/ten.2006.12.403. Epub 2006/03/22. [DOI] [PubMed] [Google Scholar]
- 48.Kaihara S, Kim SS, Benvenuto M, Choi R, Kim BS, Mooney D, et al. Successful anastomosis between tissue-engineered intestine and native small bowel. Transplantation. 1999;67(2):241–5. doi: 10.1097/00007890-199901270-00009. Epub 1999/02/12. [DOI] [PubMed] [Google Scholar]
- 49.Kim SS, Kaihara S, Benvenuto MS, Choi RS, Kim BS, Mooney DJ, et al. Regenerative signals for intestinal epithelial organoid units transplanted on biodegradable polymer scaffolds for tissue engineering of small intestine. Transplantation. 1999;67(2):227–33. doi: 10.1097/00007890-199901270-00007. Epub 1999/02/12. [DOI] [PubMed] [Google Scholar]
- 50.Grikscheit TC, Siddique A, Ochoa ER, Srinivasan A, Alsberg E, Hodin RA, et al. Tissue-engineered small intestine improves recovery after massive small bowel resection. Ann Surg. 2004;240(5):748–54. doi: 10.1097/01.sla.0000143246.07277.73. Epub 2004/10/20. doi: 00000658-200411000-00004 [pii] PubMed Central PMCID: PMC1356478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen MK, Badylak SF. Small bowel tissue engineering using small intestinal submucosa as a scaffold. J Surg Res. 2001;99(2):352–8. doi: 10.1006/jsre.2001.6199S0022-4804(01)96199-2 [pii]. Epub 2001/07/27. [DOI] [PubMed] [Google Scholar]
- 52.Grikscheit TC, Ochoa ER, Ramsanahie A, Alsberg E, Mooney D, Whang EE, et al. Tissue-engineered large intestine resembles native colon with appropriate in vitro physiology and architecture. Ann Surg. 2003;238(1):35–41. doi: 10.1097/01.SLA.0000074964.77367.4a00000658-200307000-00005 [pii]. Epub 2003/07/02. PubMed Central PMCID: PMC1422658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hecker L, Baar K, Dennis RG, Bitar KN. Development of a three-dimensional physiological model of the internal anal sphincter bioengineered in vitro from isolated smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2005;289(2):G188–96. doi: 10.1152/ajpgi.00335.2004. Epub 2005/03/19. doi: 00335.2004 [pii] [DOI] [PubMed] [Google Scholar]
- 54.Pan WK, Zheng BJ, Gao Y, Qin H, Liu Y. Transplantation of neonatal gut neural crest progenitors reconstructs ganglionic function in benzalkonium chloride-treated homogenic rat colon. J Surg Res. 2011;167(2):e221–30. doi: 10.1016/j.jss.2011.01.016. Epub 2011/03/12. doi: S0022-4804(11)00013-8 [pii] [DOI] [PubMed] [Google Scholar]
- 55.Metzger M, Caldwell C, Barlow AJ, Burns AJ, Thapar N. Enteric nervous system stem cells derived from human gut mucosa for the treatment of aganglionic gut disorders. Gastroenterology. 2009;136(7):2214–25 e1-3. doi: 10.1053/j.gastro.2009.02.048. Epub 2009/06/10. doi: S0016-5085(09)00288-1 [pii] [DOI] [PubMed] [Google Scholar]
- 56.Somara S, Gilmont RR, Dennis RG, Bitar KN. Bioengineered internal anal sphincter derived from isolated human internal anal sphincter smooth muscle cells. Gastroenterology. 2009;137(1):53–61. doi: 10.1053/j.gastro.2009.03.036. Epub 2009/03/31. doi: S0016-5085(09)00464-8 [pii] [DOI] [PubMed] [Google Scholar]
- 57.Rattan S. The internal anal sphincter: regulation of smooth muscle tone and relaxation. Neurogastroenterol Motil. 2005;17 1:50–9. doi: 10.1111/j.1365-2982.2005.00659.x. Epub 2005/04/20. doi: NMO659 [pii] [DOI] [PubMed] [Google Scholar]
- 58.Szymanski PT, Chacko TK, Rovner AS, Goyal RK. Differences in contractile protein content and isoforms in phasic and tonic smooth muscles. Am J Physiol. 1998;275(3 Pt 1):C684–92. doi: 10.1152/ajpcell.1998.275.3.C684. Epub 1998/09/09. [DOI] [PubMed] [Google Scholar]
- 59.Feretis C, Benakis P, Dimopoulos C, Dailianas A, Filalithis P, Stamou KM, et al. Endoscopic implantation of Plexiglas (PMMA) microspheres for the treatment of GERD. Gastrointest Endosc. 2001;53(4):423–6. doi: 10.1067/mge.2001.113912. Epub 2001/03/29. doi: S0016-5107(01)14275-6 [pii] [DOI] [PubMed] [Google Scholar]
- 60.Fockens P, Bruno MJ, Gabbrielli A, Odegaard S, Hatlebakk J, Allescher HD, et al. Endoscopic augmentation of the lower esophageal sphincter for the treatment of gastroesophageal reflux disease: multicenter study of the Gatekeeper Reflux Repair System. Endoscopy. 2004;36(8):682–9. doi: 10.1055/s-2004-825665. Epub 2004/07/29. [DOI] [PubMed] [Google Scholar]
- 61.Bonavina L, Saino GI, Bona D, Lipham J, Ganz RA, Dunn D, et al. Magnetic augmentation of the lower esophageal sphincter: results of a feasibility clinical trial. J Gastrointest Surg. 2008;12(12):2133–40. doi: 10.1007/s11605-008-0698-1. Epub 2008/10/11. [DOI] [PubMed] [Google Scholar]
- 62.Pasricha PJ, Ahmed I, Jankowski RJ, Micci MA. Endoscopic injection of skeletal muscle-derived cells augments gut smooth muscle sphincter function: implications for a novel therapeutic approach. Gastrointest Endosc. 2009;70(6):1231–7. doi: 10.1016/j.gie.2009.05.014. Epub 2009/08/04. doi: S0016-5107(09)01916-6 [pii] [DOI] [PubMed] [Google Scholar]
- 63.Hajivassiliou CA, Carter KB, Finlay IG. Assessment of a novel implantable artificial anal sphincter. Dis Colon Rectum. 1997;40(6):711–7. doi: 10.1007/BF02140902. Epub 1997/06/01. [DOI] [PubMed] [Google Scholar]
- 64.Kang SB, Lee HN, Lee JY, Park JS, Lee HS. Sphincter contractility after muscle-derived stem cells autograft into the cryoinjured anal sphincters of rats. Dis Colon Rectum. 2008;51(9):1367–73. doi: 10.1007/s10350-008-9360-y. Epub 2008/06/10. PubMed Central PMCID: PMC2517093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Raghavan S, Miyasaka EA, Hashish M, Somara S, Gilmont RR, Teitelbaum DH, et al. Successful implantation of physiologically functional bioengineered mouse internal anal sphincter. Am J Physiol Gastrointest Liver Physiol. 2010;299(2):G430–9. doi: 10.1152/ajpgi.00269. Epub 2010/06/19. doi: ajpgi.00269.2009 [pii] PubMed Central PMCID: PMC2928530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Raghavan S, Gilmont RR, Miyasaka EA, Somara S, Srinivasan S, Teitelbaum DH, et al. Successful implantation of bioengineered, intrinsically innervated, human internal anal sphincter. Gastroenterology. 2011;141(1):310–9. doi: 10.1053/j.gastro.2011.03.056. Epub 2011/04/06. doi: S0016-5085(11)00439-2 [pii] PubMed Central PMCID: PMC3129458. [DOI] [PMC free article] [PubMed] [Google Scholar]


