Abstract
Background
The global burden of injury is receiving recognition as a major public health problem but inadequate information delays many proposed solutions. Many attempts to collect reliable data on orthopaedic trauma have been unsuccessful. The Surgical Implant Generation Network (SIGN) database is one of the largest collections of fracture cases from lower and middle income countries.
Questions/purposes
We describe the information in the SIGN database then address two questions: In the context of the design and implementation of a global trauma database, what lessons does the SIGN database teach? Does the SIGN program have a role in the evolution of a wider global system?
Methods
The SIGN database is Internet based. After treating a patient with a SIGN nail surgeons enter radiographs and details of the case.
Results
Over 26000 cases are in the SIGN database. The database has been used as a source of cases for followup studies. Analysis shows the data are of sufficient quality to allow studies of fracture patterns but not for outcome studies or bone measurement.
Where do we need to go?
A global database with more comprehensive coverage of injuries, causes, treatment modalities and outcomes is needed.
How do we get there?
The SIGN database itself will not become a global trauma database (GTD) but the personnel of the SIGN program have much to offer in the design and adoption of a GTD. Studies of suitable methods of data collection and the incentive to use them are required.
Electronic supplementary material
The online version of this article (doi:10.1007/s11999-010-1442-1) contains supplementary material, which is available to authorized users.
Introduction
According to Gosselin, the global burden of injury is recognized as of “increasing importance … as a major contributor to the overall burden of ill-health” [10]. The extent of the problem is poorly documented, and the review concluded “resources allocated to researching, prevention, treatment and rehabilitation of injuries in developing countries is woefully inadequate” [10]. Road traffic accidents (RTAs) are already the 9th leading cause of disability worldwide [4]. The burden of trauma as a whole is probably twice that of trauma caused by RTAs [7, 23]; that burden is heaviest on the most poverty-stricken segments of the lower- and middle-income countries (LMICs) [37]. Many cost-effective methods exist for the prevention and treatment of trauma but are not being applied [11]. This major public health problem is not receiving the attention it deserves [35], reflecting the overall lack of information on the subject. Quite simple information can make a major impact on public policies [31]. Collecting information on the incidence, causes, treatment, and outcomes of injury cases in LMICs should therefore be a high priority. However, designing and implementing a data collection system to document the problems will not be easy, especially in LMICs [21, 24, 40, 42]. The intended use of a database is a critical element in the design [13]. Databases on trauma have been difficult to maintain even in developed medical systems. The Musculoskeletal Outcomes Data Evaluation and Management System (MODEMS) was discontinued [3, 28]. The Trauma Registry [5] is an ambitious multi-center attempt to collect standardized orthopaedic trauma information with content specific point and click data entry and help with classification [14]; however, the number of contributing institutions has declined steadily; privacy issues and many other factors have inhibited its use [5]. The Orthopaedic Trauma Association (OTA) maintains a database system for its members [25] but the information recorded is quite sparse with no provision for followup. The only incentive to use these systems is academic. Many individual institutions maintain a database of their trauma cases [13, 18]; these are mainly used to find cases to enter into retrospective and followup studies. Although some of these databases are large, no studies have emerged considering fracture patterns using thousands of comparable fracture cases.
The Surgical Implant Generation Network (SIGN) [35, 43] designs and manufactures IM nails for use in LMICs and distributes them free of charge. Surgeons who use the implants report the cases to the SIGN database [36] over the internet. The institutions are resupplied with implants after 20 cases have been submitted to the database. The database has also been used extensively for collecting cases for studies on the union rate and complications of this treatment method [15, 32, 33]; many other studies have been presented to the annual SIGN conferences or in the country of origin. The December 2009 issue of Techniques in Orthopaedics [44] is devoted to reports about the SIGN surgical system [34].
Because the SIGN database has been successful in collecting early information from trauma cases in LMICs, we present an overview of the type of information that can be obtained from the SIGN database and further examine the following questions: (1) In the context of the design and implementation of a GTD, what can we learn from the SIGN database? (2) Does the SIGN program have a role in the evolution of a wider global system?
Materials and Methods
The Surgical Implant Generation Network (SIGN) has been a “disruptive innovation” [12], changing the landscape by providing specially designed, high-quality, intramedullary nail implants free of charge to surgeons and hospitals treating indigent trauma victims in LMICs. SIGN is a not-for-profit corporation supported by donations from private individuals, service clubs, and industry. The SIGN Nail is solid and is inserted with a rigidly attached targeting side arm, which allows the placement of interlocking screws distally. These technical innovations distinguish SIGN nails from other intramedullary rods and allow them to be inserted without fluoroscopic control. Manufacture and distribution has been accompanied by teaching of surgical technique [9] and a two-way process of improvement. Initially an e-mail feedback system was set up to make sure the complication rate was acceptable and to discuss the surgical technique. This early system developed into an Internet-based database [34, 36] to which the surgeons in the field report basic demographic information, preoperative and postoperative radiographs, and the sizes of the implants used. (Supplemental materials are available with the online version of CORR; Appendix 1.) Supply and resupply of SIGN nails requires the surgeons to have access to the Internet. Each case receives a comment from SIGN staff and questions about the case are answered. Reporting to the database is a requirement for resupply. Shipments of replacement nails and screws are sent after 20 surgeries have been reported. The sizes of the replacements correspond to the sizes of the nails and screws reported to the database Thus, there is a strong incentive to keep up the database. The expected use of the database to the management of the SIGN program was built into the database design from the onset.
The preliminary goal of the SIGN project was to design, introduce, distribute, and evaluate the SIGN Nail as a treatment for long bone fractures. The SIGN database [36] must be considered in light of these aims. It is primarily a method of communication within the project.
Results
The SIGN database includes entries from over 100 hospitals worldwide. The SIGN database has allowed evaluation of reduction and fixation; it serves as a check to ensure the implants are not being diverted to a private hospital; it is used for determination of future shipments of nails. The comments section provides an excellent opportunity for discussion of fracture treatment and feedback about the implant. The SIGN database has resulted in continuous improvement in the instruments and implants of the SIGN system. Shaft fractures of the femur and tibia predominate with 3% of cases involving the humerus (Table 1). The high proportion of cases that required open reduction reflects the realities of fracture treatment in developing medical systems. Many patients present late or have to wait several days for surgery. In this review of the database, 9% of cases had followup radiographs (compared with 12.6% “followup” in the previous study [34]). In view of these low figures and the probability that followup cases in the database are biased toward difficult and unexpected outcomes, we suggest the data in the SIGN database itself cannot be used for outcome studies. It is, however, a source of cases that were used for followup studies [15, 32, 33].
Table 1.
Global statistics
| Variable | Number | Percentage |
|---|---|---|
| Total number of cases | 26,014 | 100% |
| Males | 21,689 | 83.4% |
| Females | 4325 | 16.6% |
| Tibia | 10,220 | 39.3% |
| Antegrade femur | 9484 | 36.5% |
| Retrograde femur | 5544 | 21.3% |
| Total femur | 15,028 | 57.8% |
| Humerus | 751 | 2.9% |
| Hip | 39 | 0.15% |
| Cases with followup radiographs | 2449 | 9.4% |
| Open fractures (all grades) | 5154 | 19.8% |
| Open reduction | 17,865 | 69.2% |
| Closed reduction | 8018 | 30.8% |
A pilot project indicates the pre- and postoperative radiographs in the SIGN database are of sufficient quality to allow classification of the fractures according to the OTA Classification system [19]. (Supplemental materials are available with the online version of CORR; Appendix 2.) The size of the database and its global coverage would allow studies of fracture pattern related to age, gender, and ethnic origin. Measurement of bone length, width and cortical thickness (after treatment) might also be undertaken, but a preliminary examination of the quality of the radiographs available showed a very high proportion did not include both ends of the bone and were therefore unsuitable for bone length measurement. (Supplemental materials are available with the online version of CORR; Appendix 2.) Among the more subtle lessons learned from the current state of the SIGN database is the relationship between incentive and the quality of information (Table 2).
Table 2.
Lessons learned from the SIGN database
| Number | Conclusion |
|---|---|
| 1 | Data collection on trauma cases from LMICs on a very large scale is possible |
| 2 | The SIGN database is best viewed as a component of a larger project, providing two-way feedback; it was not designed as a step in the direction of a global trauma database |
| 3 | The incentive to supply the information to the database must be strong |
| 4 | The quality of the incentive to supply specific information is related to the quality of the information |
| 5 | The SIGN database could be used for studies of global patterns of long bone fracture |
LMIC = lower and middle income countries.
Discussion
Without valid data all representations about the seriousness of the global burden of trauma may be discounted. A strategy for collecting good data on a massive scale would therefore seem to be urgent. By reporting some of the features of the SIGN database we can define the lessons learned on the road to a GTD (Table 2) and delineate the role the SIGN program and database may have. A Global Database is feasible; we have shown that the SIGN database has successfully captured a variety of data. (Supplemental materials are available with the online version of CORR; Appendix 1.) from thousands of trauma cases treated in LMIC institutions and related the quality of the data collected to the incentive. Where the incentive is strong (resupply of implants) the data are present in nearly all cases. Radiographs were required and were usually provided by the surgeon sending copies of photographs of the film(s); there are no stringent quality criteria. We found the radiographs were adequate for fracture classification in most cases but not adequate for bone measurement. (Supplemental materials are available with the online version of CORR; Appendix 2.) For the study period, followup data entries were voluntary; approximately 10% of cases have followup data so we conclude that the SIGN database cannot be used on its own for outcome studies.
The SIGN database is subject to a number of limitations and will not evolve into a GTD. First, it is a tool of the SIGN program that is focused on a subset of trauma—long bone fractures treated by an IM nail. Second, SIGN requires access to the Internet, and not all potential users have access. Third, data entry is cumbersome and extra work; Internet connections in LMICs are slow and often unreliable [40]. Some form of integration of data collection with the medical records system (MRS) (Table 3) is the most likely way to provide an incentive to capture the needed information. Fourth, interaction with the SIGN database is conducted exclusively in English; this would not be appropriate for a global system. To provide standardization, data entry and the MRS output needs to be in the local language but mapped to a standard term in the GTD. Lastly, the scope of the problem is too large for an organization like SIGN to manage on its own. However, the personnel of the SIGN program, particularly the surgeons in over 50 LMICs, have much to offer the development of a GTD. They are leading traumatologists in their own countries and are familiar with the use of a trauma database and the benefits that accrue. Their contribution through database design and early adoption could be invaluable.
Table 3.
Methods for collecting data
| Method | Ease of use | Speed* | Legibility | Standard terms | Comment |
|---|---|---|---|---|---|
| Handwritten notes | Familiar | Standard | Poor | Rarely | Current practice |
| Forms with checkboxes | Easier to enter; less easy to read | Fast (tearoff form) | Variable | Yes | Can be transcribed; needs a form for every condition |
| Computer workstation | Point and click entry | Fast if printer available | Legible | Yes | Expensive; not portable |
| Handheld device | Easy | Fast | Legible | Yes | Batch uploading may create problems |
| SMS messaging | Easy | Fast | Legible | Yes | In development |
| Online entry | Easy | Slow | Legible | Yes | Speed and potential for feedback depend on Internet connection |
* Including the speed with which a report can be generated and entered into the patient’s chart.
Where are we now?
The issue of data collection underlies all attempts to study trauma in LMICs. National Trauma Registers have been set up or called for in several developed countries [1, 5, 6, 8, 20, 26]. In LMICs most of the work has been on a regional or institutional scale [2, 16, 17, 21, 24, 27, 29–31, 38, 39, 42]. The Moscow Declaration [22] of the First Global Ministerial Conference on Road Safety (Nov 2009) includes the resolution (with reference to the health aspects of injury) to “Improve national data collection and comparability at the international level. . .; and . . international cooperation to develop reliable and harmonized data systems”. There is more awareness, determination and even funding to tackle this problem than ever before. The SIGN database should be taken as an example, a feasibility study and an educational experience.
Where do we need to go?
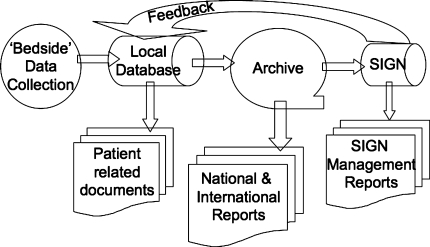
There is an urgent need to design and implement a GTD. This system should be available for low or no cost to users to document injuries, treatments, and outcomes in all countries, but particularly in LMICs. It would consist of collection of descriptive clinical data after admission, treatment procedures, investigations, and followup visits (Fig. 1). These data would be passed to a local hub (computer), which would produce documentation to go in the (paper or electronic) chart. A mature system would eliminate handwritten records so no extra work is required.
Fig. 1.
The components and interrelationships of a Global Trauma Database (GTD) are shown.
The local hub would pass the information through to a national or international archive. This database could be used to prepare reports on the incidence, treatment, and outcome of trauma on a much larger scale. The SIGN data management system could then query the archive for the subset of information relating to SIGN cases. Thus, in the mature system, SIGN would be a client of a larger data collection system and would not collect the information directly. SIGN would be one of a potentially large number of organizations that might use the GTD for governmental, nongovernmental, and commercial purposes. These inquiries to the database might be a potential source of funding.
How do we get there?
The essential transaction and limiting step is the collection of data at the bedside with the related provision of a patient record (Fig. 1). The desiderata at this stage are ease of use, speed, legibility, standardized data and the substitution of this activity for an existing one (such as dictating or writing notes). Making more work is an obvious disincentive. SMS messaging, sending a text message from a mobile phone to a computer hub, is receiving a lot of attention in other fields; a trauma implementation has not been reported. There are open source electronic MRS systems specifically developed for use in developing medical systems [41]. Because there is so little existing MRS infrastructure it is possible that LMIC health institutions could leapfrog over the “paper” stage of research and record keeping, instituting cheaper and more effective electronic MRS. Research and practical demonstrations should initially be directed at this element of the system. Although there are major issues with Internet access, standardization of the data set, organization, feedback, and funding, at the “archive” level (Fig. 1), there are no serious technical problems. However, if accurate data cannot be collected at the bedside level, a major undertaking of this nature will rapidly join the list of failures. Although the ultimate benefit of this system may be in the area of research on the global burden of injury, it must function as an electronic records system at the local level and deliver benefits at that level.
Improvements in the SIGN database are planned. Batch entry of cases, instead of the more laborious point by point data entry, is being introduced. In an attempt to improve the quality of followup material in the database, resupply will be linked to the provision of followup information as well as the acute stage information. A GTD, as envisaged, would reduce the burden of reporting that SIGN surgeons currently carry. The new system would substitute the slow process of reporting cases over the Internet with a more rapid process of accumulating patient data at a local level as part of a records system, then periodically uploading data, including SIGN specific data, to the archive (Fig. 1).
The need for a GTD collecting data on the incidence, causes, variety, management, and outcome of trauma cases is apparent. Equally apparent are the barriers to setting up such a system. The SIGN database has been successful in collecting basic data on a subset of trauma cases in LMICs because of the incentive, resupply of implants. The lessons learned from examination of the SIGN database indicate that a more comprehensive data collection system is feasible, but only if adequate incentives to using it are built in. There are widespread calls for more research on trauma in LMICs; a usable data collection system is a prerequisite for such research; funding agencies will need to consider the information infrastructure including hardware, software, network capabilities, training and implementation costs in any research projects in this arena. With a suitable information base it should be possible to show that improved trauma care is cost-effective [11] as well as medically indicated.
Electronic supplementary material
Acknowledgments
We thank the surgeons of the SIGN program for their diligence in entering data into the SIGN database.
Footnotes
SIGN (Surgical Implant Generation Network) is registered as a nonprofit, tax-exempt corporation in the state of Washington and in the United States with IRS 501(c)(3) status. LGZ is the President of SIGN. RJS is employed by SIGN.
This work was performed in Canada using the SIGN database located in the United States.
References
- 1.Aharonson-Daniel L, Avitzour M, Giveon A, Peleg K. A decade to the Israel National Trauma Registry. Isr Med Assoc J. 2007;9:347–351. [PubMed] [Google Scholar]
- 2.Al Naami MY, Sadik AA, Adam MA. Evaluation of trauma registry data in Asir region. Saudi Med J. 2001;22:438–443. [PubMed] [Google Scholar]
- 3.Alexander I. The impact of future trends in electronic data collection on musculoskeletal research and evidence-based orthopaedic care. Arthroscopy. 2003;19:1007–1011. doi: 10.1016/j.arthro.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 4.Begg S, Tomijima N. Global Burden of Injury in the Year 2000: An Overview of Methods. Geneva, Switzerland: World Health Organization; 2006:1–22.
- 5.Burman W. The Trauma Register Experience - A Quest for Better Quality Data through Direct Physician and Patient Structured Data (i.e. point-and-click, menu-driven) Entry. Available at: http://www.hwbf.org/oko/. Accessed May 3, 2010.
- 6.Davey TM, Pollard CW, Aitken LM, Fitzgerald M, Bellamy N, Cass D, Danne PD, Griggs WM, Cameron PA, Atkinson RN, Hamill J, Rao S, Richardson DB, O’Connor C. Tackling the burden of injury in Australasia: developing a binational trauma registry. Med J Aust. 2006;185:512–514. doi: 10.5694/j.1326-5377.2006.tb00668.x. [DOI] [PubMed] [Google Scholar]
- 7.Demyttenaere SV, Nansamba C, Nganwa A, Mutto M, Lett R, Razek T. Injury in Kampala, Uganda: 6 years later. Can J Surg. 2009;52:E146–E150. [PMC free article] [PubMed] [Google Scholar]
- 8.Fantus RJ, Fildes J. NTDB data points: NTDB breaks the 1 million record mark. Bull Am Coll Surg. 2005;90:39. [PubMed] [Google Scholar]
- 9.Feibel R, Zirkle LG., Jr Use of interlocking intramedullary tibial nails in developing countries. Tech Orthop. 2009;24:233–246. doi: 10.1097/BTO.0b013e3181c2d0f9. [DOI] [Google Scholar]
- 10.Gosselin RA. The increasing burden of injuries in developing countries; direct and indirect consequences. Tech Orthop. 2009;24:230–232. doi: 10.1097/BTO.0b013e3181bfd56c. [DOI] [Google Scholar]
- 11.Gosselin RA, Heitto M, Zirkle LG., Jr Cost-effectiveness of replacing skeletal traction by interlocked intramedullary nailing for femoral shaft fractures in a provincial trauma hospital in Cambodia. Int Orthop. 2009;33:1445–1448. doi: 10.1007/s00264-009-0798-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen E, Bozic KJ. The impact of disruptive innovations in orthopaedics. Clin Orthop Relat Res. 2009;467:2512–2520. doi: 10.1007/s11999-009-0865-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrast JJ, Poss R. The value and promise of patient databases in orthopaedic surgery. J Bone Joint Surg Am. 2000;82:1506–1509. [PubMed] [Google Scholar]
- 14.Hippocrates, Winslow & Babbage Foundation. Orthopaedic Trauma Data Entry. Available at: http://www.hwbf.org/ota/trdemo/trdx.htm. Accessed May 3, 2010.
- 15.Ikem IC, Ogunlusi JD, Ine HR. Achieving interlocking nails without using an image intensifier. Int Orthop. 2007;31:487–490. doi: 10.1007/s00264-006-0219-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karbakhsh M, Zandi NS, Rouzrokh M, Zarei MR. Injury epidemiology in Kermanshah: the National Trauma Project in Islamic Republic of Iran. East Mediterr Health J. 2009;15:57–64. [PubMed] [Google Scholar]
- 17.Kobusingye OC, Lett RR. Hospital-based trauma registries in Uganda. J Trauma. 2000;48:498–502. doi: 10.1097/00005373-200003000-00022. [DOI] [PubMed] [Google Scholar]
- 18.Lefaivre KA, Guy P, Chan H, Blachut PA. Long-term follow-up of tibial shaft fractures treated with intramedullary nailing. J Orthop Trauma. 2008;22:525–529. doi: 10.1097/BOT.0b013e318180e646. [DOI] [PubMed] [Google Scholar]
- 19.Marsh JL, Slongo TF, Agel J, Broderick JS, Creevey W, DeCoster TA, Prokuski L, Sirkin MS, Ziran B, Henley B, Audigé L. Fracture and dislocation classification compendium—2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21(Suppl):S1–S133. doi: 10.1097/00005131-200711101-00001. [DOI] [PubMed] [Google Scholar]
- 20.McLellan BA. A Canadian National Trauma Registry: the time is now. J Trauma. 1997;42:763–768. doi: 10.1097/00005373-199705000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Mehmood A, Razzak JA. Trauma registry–needs and challenges in developing countries. J Pak Med Assoc. 2009;59:807–808. [PubMed] [Google Scholar]
- 22.Moscow Declaration: First Global Ministerial Conference on Road Safety Nov 2009. Available at: http://www.makeroadssafe.org/Documents/final_declaration_en.pdf. Accessed May 3, 2010.
- 23.Naddumba EK. Musculoskeletal trauma services in Uganda. Clin Orthop Relat Res. 2008;466:2317–2322. doi: 10.1007/s11999-008-0369-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nwomeh BC, Lowell W, Kable R, Haley K, Ameh EA. World History and development of trauma registry: lessons from developed to developing countries. J Emerg Surg. 2006;1:32. doi: 10.1186/1749-7922-1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orthopaedic Trauma Association Trauma Database. Available at: https://consulting.dataharborsolutions.com/ota. Accessed April 17, 2010.
- 26.Probst C, Richter M, Haasper C, Lefering R, Otte D, Oestern HJ, Krettek C, Hüfner T, Traumaregister der Deutschen Gesellschaft für Unfallchirurgie Trauma and accident documentation in Germany compared with elsewhere in Europe. Chirurg. 2008;79:650–656. doi: 10.1007/s00104-008-1498-6. [DOI] [PubMed] [Google Scholar]
- 27.Sabariah FJ, Ramesh N, Mahathar AW. National Trauma Database (NTrD)–improving trauma care: first year report. Med J Malaysia. 2008;63 Suppl C:45–49. [PubMed]
- 28.Saleh KJ, Bershadsky B, Cheng E, Kane R. Lessons learned from the hip and knee musculoskeletal outcomes data evaluation and management system. Clin Orthop Relat Res. 2004;429:272–278. doi: 10.1097/01.blo.0000137589.23853.61. [DOI] [PubMed] [Google Scholar]
- 29.Samuel JC, Akinkuotu A, Baloyi P, Villaveces A, Charles A, Lee CN, Miller W, Hoffman IF, Muyco AP. Hospital-based injury data in Malawi: strategies for data collection and feasibility of trauma scoring tools. Trop Doct. 2010;40:98–99. doi: 10.1258/td.2009.090009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schultz CR, Ford HR, Cassidy LD, Shultz BL, Blanc C, King-Schultz LW, Perry HB. Development of a hospital-based trauma registry in Haiti: an approach for improving injury surveillance in developing and resource-poor settings. J Trauma. 2007;63:1143–1154. doi: 10.1097/TA.0b013e31815688e3. [DOI] [PubMed] [Google Scholar]
- 31.Shaban S, Ashour M, Bashir M, El-Ashaal Y, Branicki F, Abu-Zidan FM. The long term effects of early analysis of a trauma registry. World J Emerg Surg. 2009;4:42. doi: 10.1186/1749-7922-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah RK, Moehring HD, Singh RP, Dhakal A. Surgical Implant Generation Network (SIGN) intramedullary nailing of open fractures of the tibia. Int Orthop. 2004;28:163–166. doi: 10.1007/s00264-003-0535-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shah RK, Singh RP, Quasem MF, Faraquee SR, Harrison J. SIGN interlocking nail for the treatment of infected nonunion. Tech Orthop. 2009;24:289–296. doi: 10.1097/BTO.0b013e3181bfd597. [DOI] [Google Scholar]
- 34.Shearer D, Cunningham B, Zirkle LG., Jr Population characteristics and clinical outcomes from the SIGN online surgical database. Tech Orthop. 2009;24:273–276. doi: 10.1097/BTO.0b013e3181c3e761. [DOI] [Google Scholar]
- 35.Shearer D, Zirkle LG., Jr Future directions for assisting orthopaedic surgery in the developing world. Tech Orthop. 2009;24:312–315. doi: 10.1097/BTO.0b013e3181c3e8e6. [DOI] [Google Scholar]
- 36.SIGN Database. Available at: http://www.signsurgery.org. Accessed April 17, 2010 (registration and password available only from the SIGN program).
- 37.Spiegel DA, Gosselin RA, Coughlin RR, Joshipura M, Browner BD, Dormans JP. The burden of musculoskeletal injury in low and middle-income countries: challenges and opportunities. J Bone Joint Surg Am. 2008;90:915–923. doi: 10.2106/JBJS.G.00637. [DOI] [PubMed] [Google Scholar]
- 38.Taye M, Munie T. Trauma registry in Tikur Anbessa Hospital, Addis Ababa, Ethiopia. Ethiop Med J. 2003;41:221–226. [PubMed] [Google Scholar]
- 39.Ward E, Arscott-Mills S, Gordon G, Ashley D, McCartney T, Jamaican Injury Surveillance System The establishment of a Jamaican all-injury surveillance system. Inj Control Saf Promot. 2002;9:219–225. doi: 10.1076/icsp.9.4.219.13677. [DOI] [PubMed] [Google Scholar]
- 40.Wild M, Candrian A, Wenda K. Possibilities and limits of Internet-based registers. Inform Health Soc Care. 2009;34:81–90. doi: 10.1080/17538150902865078. [DOI] [PubMed] [Google Scholar]
- 41.Wolfe BA, Mamlin BW, Biondich PG, Fraser HS, Jazayeri D, Allen C, Miranda J, Tierney WM. The OpenMRS system: collaborating toward an open source EMR for developing countries. AMIA Annu Symp Proc. 2006:1146. [PMC free article] [PubMed]
- 42.Zafar H, Rehmani R, Raja AJ, Ali A, Ahmed M. Registry based trauma outcome: perspective of a developing country. Emerg Med J. 2002;19:391–394. doi: 10.1136/emj.19.5.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zirkle LG., Jr Injuries in developing countries—how can we help? The role of orthopaedic surgeons. Clin Orthop Relat Res. 2008;466:2443–2450. doi: 10.1007/s11999-008-0387-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zirkle LG., Jr Introduction. Tech Orthop. 2009;24:224. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.