In this timely review, Coussens and colleagues delve into the current landscape of tumor inflammation. They survey the current literature on cancer-associated immune microenvironments and assess the effects of various types of immune cells—from both the adaptive and immune arms—on tumor growth. This review further discusses the immune-based mechanisms that regulate the response to conventional cytotoxic therapy and evaluates combinational strategies for cancer therapy that combine cytotoxins with immune-modulatory approaches.
Keywords: leukocyte, inflammation, chemotherapy, radiation therapy, cytotoxic therapy, cancer
Abstract
Leukocytes and their soluble mediators play important regulatory roles in all aspects of solid tumor development. While immunotherapeutic strategies have conceptually held clinical promise, with the exception of a small percentage of patients, they have failed to demonstrate effective, consistent, and durable anti-cancer responses. Several subtypes of leukocytes that commonly infiltrate solid tumors harbor immunosuppressive activity and undoubtedly restrict the effectiveness of these strategies. Several of these same immune cells also foster tumor development by expression of potent protumor mediators. Given recent evidence revealing that immune-based mechanisms regulate the response to conventional cytotoxic therapy, it seems reasonable to speculate that tumor progression could be effectively diminished by combining cytotoxic strategies with therapies that blunt protumor immune-based effectors and/or neutralize those that instead impede development of desired anti-tumor immunity, thus providing synergistic effects between traditional cytotoxic and immune-modulatory approaches.
Despite expanded appreciation for the diversity of cellular mechanisms fostering solid tumor development, anti-cancer therapy remains heavily reliant on cytotoxic modalities—including chemotherapy (CTX) and radiation therapy (RT)—that kill rapidly proliferating (neoplastic) cells within tumors. Emerging clinical and experimental data indicate that clinical responses to cytotoxic therapy can be improved if immunogenic cell death pathways are also concurrently activated (Ma et al. 2010). Evidence for simultaneous engagement of immunogenic cell death programs has been provided for some tumors following conventional cytotoxic therapy, based on the increased presence of molecules released by dying cells thought to be “sensed” by leukocytes (Kepp et al. 2011), the result of which leads to enhanced “killing” of target cells. While an obvious clinical strategy has been to bolster these anti-tumor mechanisms, achieving clinical success has been limited. Possible mechanisms underlying these clinical failures include the underappreciated properties of some immune cell types that can harbor both immunosuppressive activity—e.g., blunting malignant cell killing by CD8+ cytotoxic T lymphocytes (CTLs) or natural killer (NK) cells—simultaneously with protumor activities that promote survival, invasion, and dissemination of malignant cells (Ruffell et al. 2010). Experimental studies in immune-competent murine models of human cancer have provided support for this concept by revealing that blockade of some protumor immune-based pathways effectively bolsters anti-tumor immunity (neoplastic cell killing) when combined with cytotoxic therapy (DeNardo et al. 2011).
Cancer and chronic inflammation
In homeostatic tissue, resident immune cells serve as sentinels that safeguard tissue and organ integrity. Following acute damage (e.g., infiltration/infection by pathogens or physical trauma), one activity of resident leukocytes is to limit tissue damage while engaging tissue repair programs (e.g., activation of stromal fibroblasts and vasculature for matrix resynthesis and angiogenesis, respectively, and recruitment of leukocytes from peripheral blood to remove damaged cells and debris) and facilitate re-epithelialization, all without inducing autoimmunity. Following resolution of wound responses, tissue damage is (hopefully) minimal and homeostatic maintenance programs return such that organ physiology is unperturbed.
In cancer, immune cells play dual roles with potential to either eliminate or promote malignancy. Premalignant tissues contain proliferating cells harboring genomic damage (e.g., “initiated” cells) that typically activate critical proliferation/survival pathways. In these tissues, chronic engagement/activation of immune cells, stromal fibroblasts, and vascular and mesenchymal support cells together fosters survival of “initiated” cells, culminating in tissue expansion and development of premalignant lesions via a process reminiscent of typical “inflammatory-type” responses observed in tissues responding to acute damage/trauma (Coussens and Werb 2002). When these chronic inflammatory-type events are sustained, neoplastic progression can ensue. Unresolved chronic immune responses thus resemble the resolution phase of wound healing, where the tumor microenvironment contains significant infiltrations of cells with immunosuppressive activity akin to a wound failing to heal (Coussens and Werb 2002).
Consistent with this, studies evaluating leukocyte complexity by flow cytometry in human (and murine) tumors have identified multiple immune cell types that variably contain immunosuppressive activity (e.g., block anti-tumor CTL- or NK T-cell-mediated killing of malignant cells)—including regulatory T cells (Tregs), immature monocytes (iMCs), alternatively activated macrophages (AAMs), mast cells, neutrophils, Tie2+ monocytes, dendritic cells (DCs), and T helper 2 (TH2)-CD4+ effector T cells (DeNardo et al. 2011; Rolny et al. 2011; Ruffell et al. 2011)—and thus afford developing malignancies a mechanism to escape killing by T cells. Mouse modeling studies indicate that the net effect of these assemblages results in favoring tumor expansion (Fig. 1; DeNardo et al. 2010; Grivennikov et al. 2010; Qian and Pollard 2010; Ruffell et al. 2010). Three types of leukocytes in particular have emerged as playing significant roles in suppressing anti-tumor immune responses: Treg cells, iMCs, and AAMs.
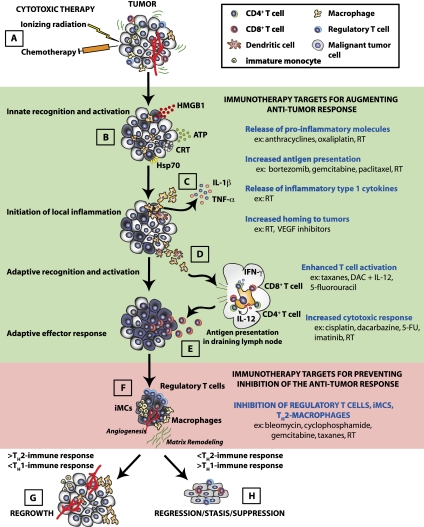
Figure 1.
Schematic of immune response pathways induced following cytotoxic therapy. Traditional cytotoxic therapies (e.g., CTX and RT) trigger an immune response in tissues (A), leading to the release of inflammatory mediators (including HMGB1, calreticulin, ATP, and Hsp70) from tumor cells (B). (C) These molecules activate resident immune cells such as DCs and tissue macrophages through cognate receptors, including TLR4 and P2RX7, which triggers the release of TNF-α and IL-1, which further recruits peripheral blood leukocytes (PBLs) from the circulation. (D) Activation of resident DCs and tissue macrophages stimulates their migration to the lymphoid tissue bearing tumor antigens. In the lymphoid tissue, the DCs and macrophages present antigen to CD4+ and CD8+ T cells, leading to their activation. (E) Activated CD4+ and CD8+ T cells then re-enter the circulation and return to the tumor to eliminate tumor cells. (F) Throughout this process, a portion of the PBLs recruited from the circulation into the tumor also possess suppressive function (e.g., Treg cells and various subtypes of myeloid cells), and these become increasingly dominant as the tumor is cleared by a cytotoxic response (primarily CD8+ T cells and NK cells) and function to reduce the anti-tumor cytotoxic response by a diverse array of mechanisms. (H) If the malignant cells are completely eradicated, the tissue can return to a normal, homeostatic state. (G) However, if incomplete eradication of malignant cells occurs, then, over time, tumor regrowth is evident in the form of recurrent disease at the primary site or metastases at distal sites. Events in the immune response that might serve as targets to enhance the immune response (shaded green) or prevent suppression of the immune response (shaded red) are outlined on the right with examples of cytotoxic agents that can mediate each of these events.
Immune-based programs that blunt anti-tumor immunity
Treg cells
Treg cells, a subset of the CD4+ T-cell population, constitutively express the high-affinity interleukin-2 (IL-2) receptor (CD25), CTL antigen-4 (CTLA-4), glucocorticoid-induced tumor necrosis factor receptor (GITR), and the lineage-specific transcription factor Foxp3 and play an important physiological role in suppressing responses to self-antigens, thereby preventing autoimmunity (Hori et al. 2003). As many malignant cell types express self-antigens (Kawakami and Rosenberg 1997), it follows that Treg cells in their physiologic capacity hamper anti-tumor immunity and that tumors may evade immune detection by engaging or activating Treg cell-based pathways. This notion has been borne out by studies evaluating peripheral blood, tumor-draining lymph nodes, and tumors—e.g., breast (Bates et al. 2006) and gastrointestinal (Sasada et al. 2003)—where increased presence of Tregs is prominent. Importantly, increased frequency of Tregs also correlates with poor outcome for several cancer types (Sasada et al. 2003; Curiel et al. 2004; Bates et al. 2006). Further support for the notion of tumors activating development of Treg cells comes from studies showing that stromal cells produce chemokines such as CCL22 (Curiel et al. 2004) and cytokines such as transforming growth factor-β (TGFβ) (Ghiringhelli et al. 2005) that enhance Treg infiltration.
The ability of Tregs to block anti-tumor immunity has been confirmed in vivo, where adoptive transfer of CD3+CD25− T cells from patients into NOD/SCID mice was found to retard tumor growth, while simultaneous transfer of Tregs abrogated the protective effect (Curiel et al. 2004). Mechanistically, in vitro studies have revealed that leukocytes isolated from melanoma and ovarian cancer patients depleted of Treg cells ex vivo enabled remaining leukocytes to respond to selective tumor antigens (Nishikawa et al. 2005). Also significant is the observation that Tregs directly promote malignant cell proliferation and dissemination via soluble mediators they express (Tan et al. 2011). Given evidence demonstrating that Tregs block anti-tumor immunity (Dunn et al. 2004), it stands to reason that, in order to augment anti-tumor immunity therapeutically, neutralizing pathways that bolster the presence or activity of Tregs would likely provide a survival advantage.
AAMs
Unlike Tregs, macrophages derived from circulating immature myeloid precursors play a more complex role in regulating immune responses owing to their ability to possess both pro- and anti-tumor bioactivity, depending on the cytokine milieu they encounter once within tissue (Qian and Pollard 2010). Classically activated macrophages (CAMs) regulated by TH1 cytokines—e.g., interferon γ (IFNγ), tumor necrosis factor α (TNFα), or granulocyte/monocyte colony-stimulating factor (GM-CSF)—possess enhanced cytotoxic activity, produce proinflammatory (TH1) cytokines, and have antigen presentation capability (Mosser and Edwards 2008). In contrast, macrophages exposed to TH2 cytokines (IL-4, IL-13, etc.), immune complexes, or immunosuppressive cytokines become alternatively activated (AAMs) (Qian and Pollard 2010) and instead typically lack cytotoxic activity, block CD8+ T-cell proliferation or infiltration, and express a diverse assortment of proliferative, proangiogenic, and tissue remodeling mediators (DeNardo et al. 2009, 2011; Andreu et al. 2010; Qian and Pollard 2010; Ruffell et al. 2010). Experimental data from murine models indicate that AAMs become TH2-skewed due to high levels of type 2 cytokines (IL-4 and IL-13) released by infiltrating CD4+ T cells and neoplastic epithelial cells (DeNardo et al. 2009; Gocheva et al. 2010) or TSLP (thymic stromal lymphopoietin) also produced by neoplastic epithelial cells (Pedroza-Gonzalez et al. 2011). While AAMs are typical constituents of tissue repair processes, in solid tumors, rather than aiding in “healing,” they instead foster neoplasia (Qian and Pollard 2010). AAMs produce a multitude of factors—including epidermal growth factor (EGF), TGFβ, and cathepsin proteases—that together provide a survival advantage to malignant epithelia and regulate their response to cytotoxic therapies (DeNardo et al. 2011; Shree et al. 2011). Data from human tumors support this hypothesis, since the presence of AAMs that are CD163+CD204+ correlate with reduced survival for patients with breast cancer, non-small-cell lung cancer, and Hodgkin's lymphoma (Kawai et al. 2008; Steidl et al. 2010; DeNardo et al. 2011). Owing to lack of specificity for CD68 as a macrophage-specific marker, however, some of these findings may need to be revisited (Ruffell et al. 2011).
The importance of macrophages in tumor progression is further underscored by mouse modeling data revealing that genetic loss of CSF1/CSF1 receptors (Lin et al. 2001) or blockade of M-CSF-induced signaling cascades (DeNardo et al. 2011) reduces macrophage presence in tumors and correlates with reduced mammary tumor metastasis. Thus, AAMs, through their ability to differentially regulate immunity and express molecules that support angiogenesis/tissue remodeling and proliferation, profoundly affect the development, maintenance, and dissemination of malignant tumors.
Immunosuppressive monocytes
Sharing the same common myeloid progenitor as macrophages, immunosuppressive monocytes in rodent tumor models encompass a diverse population of cells characterized by expression of surface markers, including CD11b and Gr1 (Ostrand-Rosenberg 2008; Gabrilovich and Nagaraj 2009), and include monocytes variably referred to as myeloid-derived suppressor cells (MDSCs), iMCs, inflammatory monocytes, and neutrophils (Ostrand-Rosenberg 2008). Human equivalents have been identified as LIN−/Lo human leukocyte antigen (HLA)-DR−CD33+CD11b+ and CD14+HLA-DR−/Lo cells (Serafini et al. 2006); however, as with mice, these share markers with multiple mature granulocytic subtypes and thus likely represent a mixed population in which some cells contain immune-suppressive properties. MDSCs and iMCs are functionally characterized by their T-cell-suppressive activity; e.g., the ability to suppress T- and NK cell proliferation via arginase I, inducible nitric oxide synthase expression, and perioxynitrite, and, at the same time, promote generation of Treg cells (Mazzoni et al. 2002; Gabrilovich and Nagaraj 2009; Doedens et al. 2010; Lu et al. 2011).
In mice, systemic increases in the presence of MDSCs and iMCs have been observed when syngeneic mice are transplanted with or develop spontaneous tumors (Ostrand-Rosenberg 2008). Significant increases in MDSCs in peripheral blood are also a common feature for patients with several types of cancer (Almand et al. 2001). Moreover, in murine models of cancer, MDSCs/iMCs have also been found to mediate resistance to some forms of anti-angiogenic therapy (Shojaei et al. 2007; Priceman et al. 2010). Thus, strategies aiming to eliminate MDSCs/iMCs may result in shifting the immune microenvironment to instead favor anti-tumor type responses that improve survival.
Cytotoxic therapy and immune cells
Cytotoxic therapy and immunogenic cell death
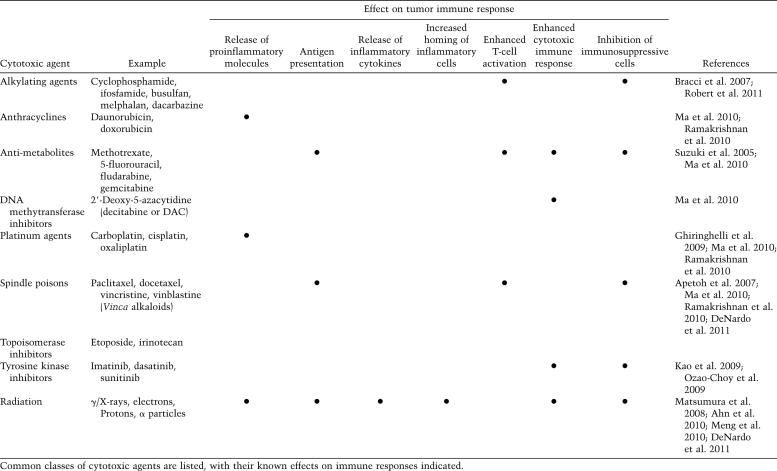
Cytotoxic therapy (CTX and RT), in combination with surgery, forms the cornerstone of systemic treatment for most clinically detectable solid tumors. Significantly, most cytotoxic therapies result in immune suppression due to a higher sensitivity of bone marrow-derived stem cells and many leukocyte subsets, especially lymphocytes, to their cytotoxic effects. Through specialized cell death pathways, including Fas–FasL, lymphocytes respond to DNA damage induced by CTX and RT by undergoing early apoptosis at doses significantly lower than other cell types, especially epithelial or neural cell types. Bone marrow-derived stem cells are also uniquely sensitive to CTX and RT (Apetoh et al. 2007; Ghiringhelli et al. 2009), and their early destruction is likely a dose-limiting toxicity for many of these modalities; thus, administration of cytotoxic agents can lead to systemic immune suppression. That said, there is increasing evidence that within tumors, cell death generated by these agents also triggers activation of other immune response pathways that serendipitously also regulate therapeutic efficacy of the particular cytotoxic agent/modality (Table 1).
Table 1.
Immune effects of cytotoxic agents
Whereas neoplastic cells have long been thought to undergo an “immunologically silent” demise following cytotoxic therapy, whereby apoptotic machinery eliminates them (Albert et al. 1998), recent studies have challenged this notion (Ma et al. 2011) and revealed that nonapoptotic and biochemically distinct cell death pathways are also activated following RT and some forms of CTX (e.g., anthracyclines and oxaliplatin) (Fig. 1). Mechanistically, leukocytes detect cell death through immune-based receptors selective for molecules released by dying cells (often termed “danger signals”), including Toll-like receptor-4 (TLR-4) and its ligand, the high-mobility group box protein 1 (HMGB1) (Apetoh et al. 2007). Detection of danger signals by resident leukocytes results in subsequent activation of both innate (myeloid and NK cells) and adaptive (T and B) cell lineages. Molecular mechanisms underlying immunogenic cell death following cytotoxic therapy involve activation of several critical sequential checkpoints. These include (1) exposure of endoplasmic reticulum (ER)-resident protein complexes, comprised of calreticulin/ERp57 on plasma membranes of neoplastic cells that serve as “eat me” signals for DCs; (2) release of the chromatin-binding HMGB1 protein, which by a TLR4/MyD88-dependent mechanism inhibits degradation of DC phagosomes, thereby facilitating antigen presentation (Apetoh et al. 2007); (3) ATP release from dying neoplastic cells and subsequent engagement of DC P2RX7 purinergic receptors, leading to IL-1β release (Ghiringhelli et al. 2009); and (4) effective antigen cross-presentation by DCs with increased production of IFNγ/IFNγ receptors and CD8+ CTL-dependent killing responses. Experimental evidence supporting these pathways emanates from in vivo evaluation in murine tumor models where the immune response induced by CTX or RT efficiently prevents tumor growth dependent on activation of these pathways (Apetoh et al. 2007; Ghiringhelli et al. 2009). Clinical evidence for the importance of these mechanisms is provided by human breast cancer patients harboring Asp299Gly TLR4 polymorphisms or loss-of-function mutations in the P2RX7 gene, both of which disrupt DC–T-cell functional interactions by impairing DCs' ability to sense HMGB1 and ATP release by dying cells, and correlates clinically with resistance to CTX (anthracyclines) and RT (Ghiringhelli et al. 2009).
Recognition that immune-based mechanisms modulate the response to cytotoxic therapy implies that the ultimate effectiveness of cytotoxic modalities could be improved by combinatorial approaches that also engage immunogenic death programs. Thus, strategies improving antigen presentation (to T cells) and/or increasing macrophage cytolytic activity would theoretically impede tumorigenesis if the protumorigenic properties of those leukocytes following cytotoxic therapy could also be effectively blunted. Requisite for success of this scenario is that TH1-based immune programs would be fostered, and dominant TH2-type programming would be blunted. TH1 programming in response to increased expression of type 1 cytokines (TNFα, IFNγ, and IL-2) activates cell-mediated responses that are “anti-tumor” in nature. TH2 programming, on the other hand, is mediated by expression of type 2 cytokines (IL-4, IL-10, and TGFβ) that instead initiate tissue remodeling, angiogenesis, and (sometimes) humoral immunity, and together foster a protumorigenic state (Yang et al. 2008; DeNardo et al. 2010; Ruffell et al. 2010).
Evidence that forced TH1 polarization of tumor microenvironments can improve response to cytotoxic therapy has been observed. For example, immunization with plasmacytoma supernatant plus IL-1 resulted in decreased tissue/tumor levels of IL-10 and TGFβ and increased levels of IFNγ and IL-2, thus favoring TH1 immunity and tumor regression (Li et al. 1998). Other studies reported that combined CTX or RT with DC vaccination, which augments the initial TH1 response through enhanced antigen presentation, also resulted in tumor regression (Koike et al. 2008; Matsumura et al. 2008). A general conclusion from these studies is that cytotoxic therapy indeed fosters an anti-tumor immune microenvironment; however, this response tends not to be durable, likely due to protumor, immunosuppressive programs that become dominant, thereby fueling tumor recurrence and subsequent resistance to therapy.
Cytotoxic therapy and cancer immunotherapy—a different approach
Harnessing the body's own immune system to fight cancer has long been considered the ultimate treatment for cancer because of its potential to specifically and durably target antigen-positive neoplastic cells while limiting damage to normal tissue. Given the immunogenic potential of cytotoxic therapies alone, it follows that strategies augmenting the immune response to cancer would synergize with anti-tumor immunity generated by cytotoxic therapy. That said, current cancer immunotherapies use a variety of strategies, including therapeutic monoclonal antibodies (mAbs) and adoptive cell transfer (ACT) involving transfer of ex vivo expanded autologous or allogeneic tumor-reactive lymphocytes and cancer vaccines, and thus attempt to stimulate anti-tumor immunity (Table 2). A critical appraisal of these approaches reveals limited overall objective response rates (3.6%) across several early-phase trials (Klebanoff et al. 2011). Although positive results with surrogate immunological endpoints have been reported, the vast majority of phase III immunotherapy trials in patients with solid tumors have failed to demonstrate improved overall survival. Analysis of these reveals that the strategies do indeed initiate and/or prime anti-tumor immunity; however, their limited success lies in their failure to also inhibit the pathways that block CTL and NK T-cell-mediated killing. As new data emerge expanding on our understanding of these complex immune-based mechanisms, new approaches are sure to develop that not only enhance generation of anti-tumor immunity, but also prevent its suppression.
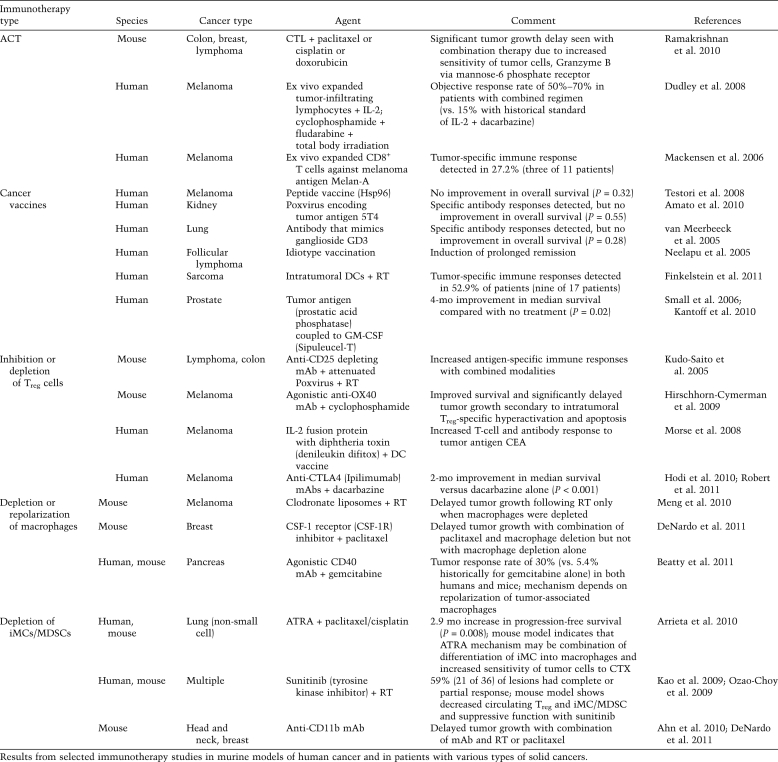
Table 2.
Current immunotherapy
Cancer immunotherapy I: augmenting the anti-tumor immune response
ACT
ACT has been reported to induce objective tumor regression and long-term responses for a small fraction of melanoma patients (Rosenberg et al. 2008). Although first described in the 1980s, therapeutic efficacy and increased patient survival were only reported following addition of immuno-depleting CTX prior to ACT, which was subsequently further improved by myeloablative lympho-depleting regimens (Dudley et al. 2008). Mechanistically, (limited) removal of endogenous lymphocytes that act as “sinks” for homeostatic cytokines and elimination of immunosuppressive Tregs and iMCs underlay these objective clinical responses (Gattinoni et al. 2005; Dudley et al. 2008).
Gabrilovich and colleagues (Ramakrishnan et al. 2010) evaluated several cancer vaccines with ACT in murine models in combination with several widely used chemotherapeutic drugs. These researchers reported that CTX rendered tumor cells more susceptible to the cytolytic effects of CTLs via increased perforin-independent permeability to granzyme B, mediated by up-regulation of mannose-6-phosphate receptors on malignant cells (Ramakrishnan et al. 2010). When combined with CTX, CTLs raised against specific tumor antigens induced apoptosis in neighboring tumor cells that did not express the antigens. Thus, small numbers of CTLs can mediate potent anti-tumor effects when combined with CTX and provide a rationale for combining these modalities for treatment of patients with advanced cancer.
Cancer vaccines
Inspired by success with vaccination against bacterial and some viral pathogens, a variety of approaches have been explored in an attempt to immunize patients against their own cancers, some of which include use of whole (killed) tumor cells, proteins, peptides, or DNA vaccines (Giaccone et al. 2005; Testori et al. 2008; Dougan and Dranoff 2009; Amato et al. 2010). In spite of limited success with these, there is renewed interest following recent positive clinical results in prostate cancer and lymphoma. Sipuleucel-T (Provenge), a cellular vaccine comprising autologous antigen-presenting cells (APCs) cultured with a fusion protein of prostatic acid phosphatase with GM-CSF, extended median survival in two independent phase III trials, leading to FDA approval for treatment of advanced prostate cancer (Small et al. 2006; Kantoff et al. 2010). Other encouraging results have been reported, most notably idiotype vaccination for follicular lymphoma in a phase III trial that demonstrated a prolonged period of CTX-induced remission (Neelapu et al. 2005).
DCs link innate and adaptive immunity and can induce contrasting states, including immunity and tolerance to self. Multiple populations of DCs are recognized in vivo in both human and murine tumors, each with distinct properties that variably regulate humoral and cellular immunity (Hashimoto et al. 2011). While antibody responses are preferentially mediated by CD14+ dermal DCs, CTL responses are instead preferentially mediated by Langerhans cells (Hashimoto et al. 2011), thus indicating that DC-mediated mechanisms inducing humoral and/or cellular immunity are fundamentally distinct. Early clinical trials testing vaccination with ex vivo generated DCs pulsed with tumor antigens provided proof-of-principle evidence that therapeutic immunity could be elicited; however, clinical benefit measured by regression of established tumors in patients with stage IV cancer was observed in only a small percentage of patients (Palucka et al. 2008). Patients with soft tissue sarcoma who received fractionated external beam radiation in combination with administration of intratumoral DCs demonstrated an increased T-cell infiltration, with tumoral CD4+ T cells positively correlating with tumor-specific immune responses (Finkelstein et al. 2011). Thus, new-generation DC vaccines are needed that generate large numbers of high avidity effector anti-tumor T cells able to overcome suppressive mechanisms in the tumor microenvironment. These, combined with therapies blunting TH2-based protumor immunity and CTX or RT, would thus be anticipated to provide much more durable tumor repression.
Cancer immunotherapy II: targeting immunosuppressive pathways and cells
Treg cells
Evidence for the role of Tregs in anti-tumor immunity was first provided by Sakaguchi and colleagues (Shimizu et al. 1999; Sakaguchi 2005) using a syngeneic murine heterotopic transplant model. This was later reproduced in several murine tumor models, all of which demonstrated that depletion of Tregs via anti-CD25 mAb prior to tumor inoculation led to syngeneic tumor rejection (Casares et al. 2003). In conjunction with cytotoxic therapy, strategies targeting CD25, such as depleting mAbs or an IL-2-diphtheria toxin fusion protein, enhanced anti-tumor immune responses in both murine models and humans (Kudo-Saito et al. 2005; Mackensen et al. 2006; Morse et al. 2008). However, strategies targeting CD25 lack specificity, in that activated T cells also express CD25; thus, these agents may also blunt formation of robust anti-tumor T-cell responses while also depleting Treg cells (Curtin et al. 2008). Therefore, other strategies targeting the Tregs—such as the agonistic antibody against OX40, a TNR receptor (TNFR) family costimulatory molecule expressed on T cells and DCs—in combination with cyclophosphamide minimize this paradox by inducing Treg-specific apoptosis (Hirschhorn-Cymerman et al. 2009).
Targeting Tregs with CTLA-4 antagonists has been perhaps the most successful of the strategies targeting an immunosuppressive pathway, although others such as B7-H3, PD-L1, and CD73 are currently under investigation (Yi and Chen 2009; Ascierto et al. 2010; Jin et al. 2011). CTLA-4 is a negative costimulatory molecule expressed on both activated T cells and Treg cells that helps dampen ongoing immune response, is frequently up-regulated on chronically activated and exhausted T cells (Engelhardt et al. 2006; Wherry et al. 2007), and not only inhibits T-cell activation, but also promotes Treg function (Teft et al. 2006). Results from a phase III trial evaluating the CTLA-4-blocking mAb ipilimumab, recently approved for the treatment of advanced malignant melanoma by the FDA, revealed extended overall survival of previously treated melanoma patients, correlating with increased CD8+ T-cell activation and Treg inhibition (Hodi et al. 2010). A subsequent landmark study demonstrated improved survival in patients with untreated advanced melanoma who received ipilimumab combined with the CTX agent dacarbazine, as compared with those receiving CTX alone (Robert et al. 2011). This study supports the hypothesis that the combination of CTX with a reduction in the suppressive environment—in this case, elimination of Tregs—is a strategy that can lead to more effective anti-tumor immunity.
AAMs
CTX and RT stimulate recruitment of macrophages (and monocytes) into tissues through direct induction of myeloid cell chemoattractant molecules. Epithelial cells rapidly respond to CTX (paclitaxel, cisplatin, and carboplatin) and RT by direct mRNA induction of monocyte-promoting chemokines such as csf-1, IL-34, ccl2/MCP-1, ccl5, cxcl10, cxcl11, cx3cl1, and HIF1 (Kioi et al. 2010; DeNardo et al. 2011; Ruffell et al. 2011). Cyclophosphamide, oxaliplatin, and RT induce TH1 (Bracci et al. 2007; Ghiringhelli et al. 2009) as well as TH2 cytokines (Gremy et al. 2008), indicating that CTX and RT have the potential to skew macrophage phenotypes to either anti- or protumorigenic states. Thus, while CTX and RT may initially mediate a cytotoxic/cytolytic macrophage response (Lambert and Paulnock 1987), enhanced presence of TH2 cytokines may contribute to ongoing skewing and maintenance of AAMs in tumors and subsequent repulsion of CD8+ T-cell-mediated anti-tumor immunity (Doedens et al. 2010).
The duality of macrophages as mediators of cytotoxic therapy responses has been demonstrated in experimental murine models showing that macrophage depletion significantly slows tumor growth, but only when provided in combination with either CTX or RT. Selective depletion of macrophages using clodronate liposomes in an orthotopic murine melanoma model given before RT increased latency and slowed tumor regrowth, whereas coimplantation of malignant cells along with bone marrow-derived macrophages increased tumor radioresistance mediated by TNFα signaling pathways (Meng et al. 2010). Macrophage depletion strategies in combination with CTX or RT slow tumor development in murine models of sarcoma and melanoma in part by altering, or perhaps “normalizing,” tumor vasculature (Meng et al. 2010; Rolny et al. 2011). Vascular normalization in this context likely improves tumor hemodynamics, thereby increasing delivery of chemotherapeutic agents and oxygenation of tumor parenchyma, and thereby reducing hypoxia.
Given the evidence that cytotoxic agents are also potent immune adjuvants, it would not be surprising that strategies abolishing or reprogramming AAMs would enhance both cell killing by cytotoxics and immunogenic cell death. Thus, in order to overcome the immunosuppressive barriers established by tumors, it may be important to not only provide antigenic stimulus in the form of cytotoxic therapy, but also neutralize myeloid-based pathways established in the tumor that blunt effective anti-tumor immune responses. To address this possibility, we recently used a mouse model of mammary carcinogenesis (MMTV-PyMT mice) and reported that CSF1R blockade depleted CD11b+Ly6G−Ly6CLoF4/80+ macrophages, but not the less abundant population of granulocytic CD11b+Ly6G+-expressing myeloid cells, and resulted in slowed primary tumor growth and diminished metastasis, but only when given in combination with CTX, by CD8+ T-cell-dependent mechanisms (DeNardo et al. 2011). Cathepsin protease-expressing macrophages have been found to mediate many of these effects, and cathepsin B and S protect malignant mammary epithelial cells from Taxol-induced (as well as etoposide and doxorubicin) tumor cell death in coculture (Shree et al. 2011). Combining Taxol with cathepsin inhibition in vivo significantly enhanced efficacy against primary and metastatic mammary tumors, supporting the therapeutic relevance for this effect (Shree et al. 2011). These experimental studies provide a compelling rationale for clinical evaluation of combinatorial approaches inhibiting macrophage recruitment or altering macrophage response pathways and mediator expression/activity in combination with “standard of care” CTX for treatment of some solid tumors in order to overcome inherent resistance to CTX. These strategies are an active area of clinical research in the phase I and II setting, testing a variety of agents designed to either block macrophage recruitment or stimulate alternative macrophage programming (Anthony et al. 2011) with the hope that combinations will provide improved clinical outcomes.
In addition to targeting macrophage recruitment, it is also possible to target macrophage polarization in an attempt to elicit the presence of more favorable TH1-polarized cytotoxic macrophages in tumors. One such strategy currently being explored is via targeting CD40, a member of the TNFR superfamily and a costimulatory molecule expressed on a diverse assortment of cells, including DCs, B cells, and macrophages, as well as endothelial, mesenchymal, and epithelial cells. Binding of the CD40 ligand (CD40L) CD154 to CD40 mediates distinct effects on cells, depending on cell type and the tissue and microenvironment in which they reside. On immune cells, CD40 regulates humoral and cellular immunity, while apoptotic and anti-proliferative pathways are regulated by CD40 on some neoplastic cells (Fonsatti et al. 2010). Activation of APCs requires binding of CD40L on TH cells to CD40, whereas macrophage activation requires IFNγ produced by TH1-CD4+ T cells in addition to CD40L–CD40 interaction. This results in macrophage up-regulation of CD40 and TNFR and induction of cytotoxic activity, including increased expression of nitric oxide and reactive oxygen species (Fonsatti et al. 2010). To investigate whether agonist CD40 mAbs would thwart tumor-induced immune suppression and instead invoke productive T-cell-dependent anti-tumor immunity, Beatty et al. (2011) treated 21 patients with pancreatic ductal adenocarcinoma (PDA) with a fully humanized agonistic CD40 mAb in combination with gemcitabine and reported tumor regression in some patients. Using a mouse model of PDA to reveal the molecular/cellular mechanisms underlying the improved response, tumor regression was found to be dependent on CD40-activated MHC-IIhiCD86+ tumoricidal macrophages as opposed to CD8+ T cells (Beatty et al. 2011).
In addition to these approaches, others have investigated the efficacy of CD47 blockade to foster macrophage and DC phagocytic activity (Jaiswal et al. 2010). CD47, also known as integrin-associated protein (IAP), encodes a membrane protein mediating intracellular calcium levels following cell adhesion to extracellular matrix. CD47 binds to the SIRPα inhibitor receptor on macrophages and DCs and thereby inhibits phagocytosis; in autoimmune processes, these interactions limit tissue damage (Jaiswal et al. 2010). Expression of CD47 has been found to be significantly increased on some malignant tumor cells, especially in non-Hodgkin's lymphoma, thus rendering malignant cells resistant to macrophage and DC phagocytosis (Chao et al. 2010). Since agonistic CD40 mAb in combination with gemcitabine provides a survival advantage for PDA dependent on tumoricidal macrophages, it seems reasonable to speculate that combining similar approaches with therapies blocking CD47 may be efficacious in solid tumors where CD47 is up-regulated. Taken together, the experience with immunotherapy makes a compelling case for integrating strategies that restrain and/or reprogram tumor immune microenvironments, resulting in bolstering of diverse anti-tumor pathways to achieve meaningful therapeutic gains.
Immunosuppressive myeloid cells
Minimizing suppressive iMCs/MDSCs in tumors has been investigated using all-trans retinoic acid (ATRA), which induces differentiation of iMCs into macrophages and correlates with enhanced anti-tumor immunity in murine models (Kusmartsev and Gabrilovich 2003). In human clinical trials, addition of retinoic acid to standard CTX improved outcome for patients with advanced non-small-cell lung cancer (Arrieta et al. 2010). While ATRA decreased accumulation of iMCs in both tumor-bearing mice and humans, exposure to this agent also increased sensitivity of malignant cells to CTX, likely accounting for at least some of its anti-tumor efficacy (Arrieta et al. 2010). Other strategies to eliminate iMCs have used c-KIT antagonists that decrease accumulation of iMCs in murine and human tumors but have only improved anti-tumor immunity when given in the presence of tumor vaccines (Ozao-Choy et al. 2009). A phase I/II clinical study revealed that concurrent administration of sunitinib—an oral, small-molecule, multi-targeted receptor tyrosine kinase inhibitor of the vascular endothelial growth factor receptor (VEGFR), c-KIT, and platelet-derived growth factor receptor approved by the FDA for treatment of renal cell carcinoma and imatinib-resistant gastrointestinal stromal tumor—with RT in patients with one to five distant oligometastases improved progression-free survival; responses correlated with decreased peripheral blood monocyte levels 7 d following start of therapy (Kao et al. 2009). While evidence supporting the use of c-KIT antagonists and cytotoxic therapy is encouraging, agents that target c-KIT can also have effects on many other cell types, including hematopoietic stem cells, mast cells, and melanocytes, due to activity also against other related kinases, thus posing a significant challenge for interpreting data in terms of effects on iMC subtypes. Although many of the agents used for targeting iMCs lack specificity, data from agents such ATRA and c-KIT antagonists provide suggestive evidence that immature myeloid populations may have important roles in regulating anti-tumor immune responses.
Another approach for depletion of immunosuppressive myeloid cells has been treatment of tumor-bearing mice with αCD11b mAbs. CD11b is an integrin cell adhesion molecule involved in transendothelial migration expressed predominantly by myeloid lineage cells, including neutrophils, macrophages, monocytes, and DCs. Bone marrow-derived CD11b+ myeloid cells are recruited to tumors following RT, where they restore vascular programming via VEGF secretion, thus aiding subsequent tumor (re)growth. Neutralizing CD11b mAbs inhibit recruitment of CD11b+ myeloid cells into RT-treated tumors, slowing tumor regrowth and thus improving RT response (Ahn et al. 2010). Similarly, mice bearing syngeneic 4T1 mammary tumors treated with CTX and αCD11b mAbs demonstrated significantly slowed primary tumor growth as well as reduced pulmonary metastases (DeNardo et al. 2011). Gr1+CCR2+CX3CR1Lo iMCs are highly responsive to CCL2 (Zhang et al. 2010), and CCL2/MCP1 is expressed at high levels in mammary tumors and is now mechanistically demonstrated to potentiate pulmonary metastasis (Qian et al. 2011).
A neutralizing antibody specific for human CD11b–CD18 integrin heterodimers, rovelizumab (LeukArrest), has previously been investigated and was found to have an excellent safety profile, but lacked therapeutic efficacy in inflammatory diseases such as multiple sclerosis. However, based on murine studies, it seems reasonable to speculate that a drug like rovelizumab could be administered safely for transient blockade of myeloid cell infiltration following local RT or systemic CTX and thereby provide a window of opportunity when tumors could be prevented from efficient revascularization and anti-tumor immunity could be bolstered. Thus, extrapolating to the clinical scenario, it will be important to stratify human tumors containing predominately high levels of mature tissue macrophages, as compared with those containing iMCs/MDSCs, as these tumors would likely be less responsive to therapy directed at CSF1R-positive macrophages, but might be expected to instead respond to drugs like rovelizumab.
Conclusions
The relatively modest gains provided by immunotherapy despite intense investigation can be in part attributed to the presence of pathways that suppress anti-tumor immunity. These mechanisms likely evolved as part of tumor development where the local microenvironment contains an immune set point skewed favoring TH2-type pathways, as compared with homeostatic tissue. Cell types including Tregs, AAMs, and iMCs form an inhibitory network that suppresses local immunity, thereby limiting the efficacy of many forms of anti-cancer therapy reliant on formation of productive anti-tumor immune responses.
Studies investigating the mechanism of action for CTX and RT have historically focused on cell-intrinsic molecules regulated by these cytotoxic agents; however, recent evidence indicates the importance of cell-extrinsic factors, particularly for induction of anti-tumor immunity. Given this, inhibitory mechanisms that stymied development of effective immunotherapy may also play an important role in regulating response to cytotoxic agents. Emerging data indicate that targeting immune inhibitory/stimulatory pathways, in conjunction with conventional cytotoxic therapy and current immunotherapy, significantly enhances the effectiveness of cytotoxic therapy by augmenting anti-tumor immunity and preventing its suppression. Further exploration to better characterize and understand inhibitory immune pathways will aid in identification of new targets that redefine our understanding of the anti-tumor mechanism of traditional cytotoxic therapies and direct us to new strategies that improve the efficacy of standard therapy.
Acknowledgments
We thank Drs. Brian Ruffell and Anna Wasiuk for comments, and acknowledge support from the American Board of Radiology (ABR) and Conquer Cancer Foundation of the American Society for Clinical Oncology (ASCO) to S.L.S., support from Cancer Research UK to A.P.G., grants from the Breast Cancer Research Foundation to H.S.R., grants from the NIH/NCI and Department of Defense (W81XWH-11-1-0702 and PR080717) to L.M.C., and a grant from the Susan G. Komen for the Cure Foundation (KG111084) to H.S.R. and L.M.C.
Footnotes
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.169029.111.
References
- Ahn GO, Tseng D, Liao CH, Dorie MJ, Czechowicz A, Brown JM 2010. Inhibition of Mac-1 (CD11b/CD18) enhances tumor response to radiation by reducing myeloid cell recruitment. Proc Natl Acad Sci 107: 8363–8368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert ML, Sauter B, Bhardwaj N 1998. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 392: 86–89 [DOI] [PubMed] [Google Scholar]
- Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI 2001. Increased production of immature myeloid cells in cancer patients: A mechanism of immunosuppression in cancer. J Immunol 166: 678–689 [DOI] [PubMed] [Google Scholar]
- Amato RJ, Hawkins RE, Kaufman HL, Thompson JA, Tomczak P, Szczylik C, McDonald M, Eastty S, Shingler WH, de Belin J, et al. 2010. Vaccination of metastatic renal cancer patients with MVA-5T4: A randomized, double-blind, placebo-controlled phase III study. Clin Cancer Res 16: 5539–5547 [DOI] [PubMed] [Google Scholar]
- Andreu P, Johansson M, Affara NI, Pucci F, Tan T, Junankar S, Korets L, Lam J, Tawfik D, DeNardo DG, et al. 2010. FcRγ activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell 17: 121–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony SP, Puzanov I, Lin PS, Nolop KB, West B, Von Hof DD 2011. Pharmacodynamic activity demonstrated in phase I for PLX3397, a selective inhibitor of FMS and Kit. J Clin Oncol 29: (suppl; abstr 3093). [Google Scholar]
- Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, et al. 2007. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 13: 1050–1059 [DOI] [PubMed] [Google Scholar]
- Arrieta O, Gonzalez-De la Rosa CH, Arechaga-Ocampo E, Villanueva-Rodriguez G, Ceron-Lizarraga TL, Martinez-Barrera L, Vazquez-Manriquez ME, Rios-Trejo MA, Alvarez-Avitia MA, Hernandez-Pedro N, et al. 2010. Randomized phase II trial of All-trans-retinoic acid with chemotherapy based on paclitaxel and cisplatin as first-line treatment in patients with advanced non-small-cell lung cancer. J Clin Oncol 28: 3463–3471 [DOI] [PubMed] [Google Scholar]
- Ascierto PA, Simeone E, Sznol M, Fu YX, Melero I 2010. Clinical experiences with anti-CD137 and anti-PD1 therapeutic antibodies. Semin Oncol 37: 508–516 [DOI] [PubMed] [Google Scholar]
- Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, Banham AH 2006. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol 24: 5373–5380 [DOI] [PubMed] [Google Scholar]
- Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, et al. 2011. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 331: 1612–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracci L, Moschella F, Sestili P, La Sorsa V, Valentini M, Canini I, Baccarini S, Maccari S, Ramoni C, Belardelli F, et al. 2007. Cyclophosphamide enhances the antitumor efficacy of adoptively transferred immune cells through the induction of cytokine expression, B-cell and T-cell homeostatic proliferation, and specific tumor infiltration. Clin Cancer Res 13: 644–653 [DOI] [PubMed] [Google Scholar]
- Casares N, Arribillaga L, Sarobe P, Dotor J, Lopez-Diaz de Cerio A, Melero I, Prieto J, Borras-Cuesta F, Lasarte JJ 2003. CD4+/CD25+ regulatory cells inhibit activation of tumor-primed CD4+ T cells with IFN-γ-dependent antiangiogenic activity, as well as long-lasting tumor immunity elicited by peptide vaccination. J Immunol 171: 5931–5939 [DOI] [PubMed] [Google Scholar]
- Chao MP, Alizadeh AA, Tang C, Myklebust JH, Varghese B, Gill S, Jan M, Cha AC, Chan CK, Tan BT, et al. 2010. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell 142: 699–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Werb Z 2002. Inflammation and cancer. Nature 420: 860–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, et al. 2004. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 10: 942–949 [DOI] [PubMed] [Google Scholar]
- Curtin JF, Candolfi M, Fakhouri TM, Liu C, Alden A, Edwards M, Lowenstein PR, Castro MG 2008. Treg depletion inhibits efficacy of cancer immunotherapy: Implications for clinical trials. PLoS One 3: e1983 doi: 10.1371/journal.pone.0001983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM 2009. CD4+ T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell 16: 91–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNardo DG, Andreu P, Coussens LM 2010. Interactions between lymphocytes and myeloid cells regulate pro- versus anti-tumor immunity. Cancer Metastasis Rev 29: 309–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNardo DG, Brennan D, Rexhapaj E, Ruffel B, Shiao S, Gallagher WM, Wadhani N, Kial SD, Junaid SA, Rugo HS, et al. 2011. Leukocyte complexity in breast cancer predicts overall survival and functionally regulates response to chemotherapy. Cancer Discovery 1: 54–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doedens AL, Stockmann C, Rubinstein MP, Liao D, Zhang N, Denardo DG, Coussens LM, Karin M, Goldrath AW, Johnson RS 2010. Macrophage expression of hypoxia-inducible factor-1α suppresses T-cell function and promotes tumor progression. Cancer Res 70: 7465–7475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougan M, Dranoff G 2009. Immune therapy for cancer. Annu Rev Immunol 27: 83–117 [DOI] [PubMed] [Google Scholar]
- Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, et al. 2008. Adoptive cell therapy for patients with metastatic melanoma: Evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol 26: 5233–5239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn GP, Old LJ, Schreiber RD 2004. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 21: 137–148 [DOI] [PubMed] [Google Scholar]
- Engelhardt JJ, Sullivan TJ, Allison JP 2006. CTLA-4 overexpression inhibits T cell responses through a CD28-B7-dependent mechanism. J Immunol 177: 1052–1061 [DOI] [PubMed] [Google Scholar]
- Finkelstein SE, Iclozan C, Bui MM, Cotter MJ, Ramakrishnan R, Ahmed J, Noyes DR, Cheong D, Gonzalez RJ, Heysek RV, et al. 2011. Combination of external beam radiotherapy (EBRT) with intratumoral injection of dendritic cells as neo-adjuvant treatment of high-risk soft tissue sarcoma patients. Int J Radiat Oncol Biol Phys doi: 10.1016/j.ijrobp.2010.12.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonsatti E, Maio M, Altomonte M, Hersey P 2010. Biology and clinical applications of CD40 in cancer treatment. Semin Oncol 37: 517–523 [DOI] [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S 2009. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 9: 162–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, Hwang LN, Yu Z, Wrzesinski C, Heimann DM, et al. 2005. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med 202: 907–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiringhelli F, Puig PE, Roux S, Parcellier A, Schmitt E, Solary E, Kroemer G, Martin F, Chauffert B, Zitvogel L 2005. Tumor cells convert immature myeloid dendritic cells into TGF-β-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J Exp Med 202: 919–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, et al. 2009. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1β-dependent adaptive immunity against tumors. Nat Med 15: 1170–1178 [DOI] [PubMed] [Google Scholar]
- Giaccone G, Debruyne C, Felip E, Chapman PB, Grant SC, Millward M, Thiberville L, D'Addario G, Coens C, Rome LS, et al. 2005. Phase III study of adjuvant vaccination with Bec2/bacille Calmette-Guerin in responding patients with limited-disease small-cell lung cancer (European Organisation for Research and Treatment of Cancer 08971-08971B; Silva Study). J Clin Oncol 23: 6854–6864 [DOI] [PubMed] [Google Scholar]
- Gocheva V, Wang HW, Gadea BB, Shree T, Hunter KE, Garfall AL, Berman T, Joyce JA 2010. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes Dev 24: 241–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremy O, Benderitter M, Linard C 2008. Acute and persisting Th2-like immune response after fractionated colorectal γ-irradiation. World J Gastroenterol 14: 7075–7085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M 2010. Immunity, inflammation, and cancer. Cell 140: 883–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto D, Miller J, Merad M 2011. Dendritic cell and macrophage heterogeneity in vivo. Immunity 35: 323–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschhorn-Cymerman D, Rizzuto GA, Merghoub T, Cohen AD, Avogadri F, Lesokhin AM, Weinberg AD, Wolchok JD, Houghton AN 2009. OX40 engagement and chemotherapy combination provides potent antitumor immunity with concomitant regulatory T cell apoptosis. J Exp Med 206: 1103–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. 2010. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363: 711–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science 299: 1057–1061 [DOI] [PubMed] [Google Scholar]
- Jaiswal S, Chao MP, Majeti R, Weissman IL 2010. Macrophages as mediators of tumor immunosurveillance. Trends Immunol 31: 212–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D, Fan J, Wang L, Thompson LF, Liu A, Daniel BJ, Shin T, Curiel TJ, Zhang B 2011. CD73 on tumor cells impairs antitumor T-cell responses: A novel mechanism of tumor-induced immune suppression. Cancer Res 70: 2245–2255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, et al. 2010. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 363: 411–422 [DOI] [PubMed] [Google Scholar]
- Kao J, Packer S, Vu HL, Schwartz ME, Sung MW, Stock RG, Lo YC, Huang D, Chen SH, Cesaretti JA 2009. Phase 1 study of concurrent sunitinib and image-guided radiotherapy followed by maintenance sunitinib for patients with oligometastases: Acute toxicity and preliminary response. Cancer 115: 3571–3580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai O, Ishii G, Kubota K, Murata Y, Naito Y, Mizuno T, Aokage K, Saijo N, Nishiwaki Y, Gemma A, et al. 2008. Predominant infiltration of macrophages and CD8+ T Cells in cancer nests is a significant predictor of survival in stage IV nonsmall cell lung cancer. Cancer 113: 1387–1395 [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Rosenberg SA 1997. Human tumor antigens recognized by T-cells. Immunol Res 16: 313–339 [DOI] [PubMed] [Google Scholar]
- Kepp O, Galluzzi L, Martins I, Schlemmer F, Adjemian S, Michaud M, Sukkurwala AQ, Menger L, Zitvogel L, Kroemer G 2011. Molecular determinants of immunogenic cell death elicited by anticancer chemotherapy. Cancer Metastasis Rev 30: 61–69 [DOI] [PubMed] [Google Scholar]
- Kioi M, Vogel H, Schultz G, Hoffman RM, Harsh GR, Brown JM 2010. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest 120: 694–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff CA, Acquavella N, Yu Z, Restifo NP 2011. Therapeutic cancer vaccines: Are we there yet? Immunol Rev 239: 27–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike N, Pilon-Thomas S, Mule JJ 2008. Nonmyeloablative chemotherapy followed by T-cell adoptive transfer and dendritic cell-based vaccination results in rejection of established melanoma. J Immunother 31: 402–412 [DOI] [PubMed] [Google Scholar]
- Kudo-Saito C, Schlom J, Camphausen K, Coleman CN, Hodge JW 2005. The requirement of multimodal therapy (vaccine, local tumor radiation, and reduction of suppressor cells) to eliminate established tumors. Clin Cancer Res 11: 4533–4544 [DOI] [PubMed] [Google Scholar]
- Kusmartsev S, Gabrilovich DI 2003. Inhibition of myeloid cell differentiation in cancer: The role of reactive oxygen species. J Leukoc Biol 74: 186–196 [DOI] [PubMed] [Google Scholar]
- Lambert LE, Paulnock DM 1987. Modulation of macrophage function by γ-irradiation. Acquisition of the primed cell intermediate stage of the macrophage tumoricidal activation pathway. J Immunol 139: 2834–2841 [PubMed] [Google Scholar]
- Li L, Okino T, Sugie T, Yamasaki S, Ichinose Y, Kanaoka S, Kan N, Imamura M 1998. Cyclophosphamide given after active specific immunization augments antitumor immunity by modulation of Th1 commitment of CD4+ T cells. J Surg Oncol 67: 221–227 [DOI] [PubMed] [Google Scholar]
- Lin EY, Nguyen AV, Russell RG, Pollard JW 2001. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med 193: 727–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Ramakrishnan R, Altiok S, Youn JI, Cheng P, Celis E, Pisarev V, Sherman S, Sporn MB, Gabrilovich D 2011. Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice. J Clin Invest 121: 4015–4029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Kepp O, Ghiringhelli F, Apetoh L, Aymeric L, Locher C, Tesniere A, Martins I, Ly A, Haynes NM, et al. 2010. Chemotherapy and radiotherapy: Cryptic anticancer vaccines. Semin Immunol 22: 113–124 [DOI] [PubMed] [Google Scholar]
- Ma Y, Conforti R, Aymeric L, Locher C, Kepp O, Kroemer G, Zitvogel L 2011. How to improve the immunogenicity of chemotherapy and radiotherapy. Cancer Metastasis Rev 30: 71–82 [DOI] [PubMed] [Google Scholar]
- Mackensen A, Meidenbauer N, Vogl S, Laumer M, Berger J, Andreesen R 2006. Phase I study of adoptive T-cell therapy using antigen-specific CD8+ T cells for the treatment of patients with metastatic melanoma. J Clin Oncol 24: 5060–5069 [DOI] [PubMed] [Google Scholar]
- Matsumura S, Wang B, Kawashima N, Braunstein S, Badura M, Cameron TO, Babb JS, Schneider RJ, Formenti SC, Dustin ML, et al. 2008. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol 181: 3099–3107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni A, Bronte V, Visintin A, Spitzer JH, Apolloni E, Serafini P, Zanovello P, Segal DM 2002. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J Immunol 168: 689–695 [DOI] [PubMed] [Google Scholar]
- Meng Y, Beckett MA, Liang H, Mauceri HJ, van Rooijen N, Cohen KS, Weichselbaum RR 2010. Blockade of tumor necrosis factor α signaling in tumor-associated macrophages as a radiosensitizing strategy. Cancer Res 70: 1534–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse MA, Hobeika AC, Osada T, Serra D, Niedzwiecki D, Lyerly HK, Clay TM 2008. Depletion of human regulatory T cells specifically enhances antigen-specific immune responses to cancer vaccines. Blood 112: 610–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP 2008. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8: 958–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelapu SS, Gause BL, Nikcevich DA, Schuster SJ, Winter J, Gockerman JP, Loughran T Jr, Takeshita K, Inghirami G, McGaughey D, et al. 2005. Phase III randomized trial of patient-specific vaccination for previously untreated patients with follicular lymphoma in first complete remission: Protocol summary and interim report. Clin Lymphoma 6: 61–64 [DOI] [PubMed] [Google Scholar]
- Nishikawa H, Jager E, Ritter G, Old LJ, Gnjatic S 2005. CD4+ CD25+ regulatory T cells control the induction of antigen-specific CD4+ helper T cell responses in cancer patients. Blood 106: 1008–1011 [DOI] [PubMed] [Google Scholar]
- Ostrand-Rosenberg S 2008. Immune surveillance: A balance between protumor and antitumor immunity. Curr Opin Genet Dev 18: 11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozao-Choy J, Ma G, Kao J, Wang GX, Meseck M, Sung M, Schwartz M, Divino CM, Pan PY, Chen SH 2009. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res 69: 2514–2522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palucka AK, Ueno H, Fay J, Banchereau J 2008. Dendritic cells: A critical player in cancer therapy? J Immunother 31: 793–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedroza-Gonzalez A, Xu K, Wu TC, Aspord C, Tindle S, Marches F, Gallegos M, Burton EC, Savino D, Hori T, et al. 2011. Thymic stromal lymphopoietin fosters human breast tumor growth by promoting type 2 inflammation. J Exp Med 208: 479–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priceman SJ, Sung JL, Shaposhnik Z, Burton JB, Torres-Collado AX, Moughon DL, Johnson M, Lusis AJ, Cohen DA, Iruela-Arispe ML, et al. 2010. Targeting distinct tumor-infiltrating myeloid cells by inhibiting CSF-1 receptor: Combating tumor evasion of antiangiogenic therapy. Blood 115: 1461–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian BZ, Pollard JW 2010. Macrophage diversity enhances tumor progression and metastasis. Cell 141: 39–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW 2011. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475: 222–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan R, Assudani D, Nagaraj S, Hunter T, Cho HI, Antonia S, Altiok S, Celis E, Gabrilovich DI 2010. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J Clin Invest 120: 1111–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, et al. 2011. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 364: 2517–2526 [DOI] [PubMed] [Google Scholar]
- Rolny C, Mazzone M, Tugues S, Laoui D, Johansson I, Coulon C, Squadrito ML, Segura I, Li X, Knevels E, et al. 2011. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell 19: 31–44 [DOI] [PubMed] [Google Scholar]
- Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME 2008. Adoptive cell transfer: A clinical path to effective cancer immunotherapy. Nat Rev Cancer 8: 299–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffell B, Denardo DG, Affara NI, Coussens LM 2010. Lymphocytes in cancer development: Polarization towards pro-tumor immunity. Cytokine Growth Factor Rev 21: 3–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffell B, Au A, Rugo HS, Esserman LJ, Hwang ES, Coussens LM 2011. Leukocyte composition in human breast cancer. Proc Natl Acad Sci doi: 10.1073/pnas.1104303108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S 2005. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol 6: 345–352 [DOI] [PubMed] [Google Scholar]
- Sasada T, Kimura M, Yoshida Y, Kanai M, Takabayashi A 2003. CD4+CD25+ regulatory T cells in patients with gastrointestinal malignancies: Possible involvement of regulatory T cells in disease progression. Cancer 98: 1089–1099 [DOI] [PubMed] [Google Scholar]
- Serafini P, Borrello I, Bronte V 2006. Myeloid suppressor cells in cancer: Recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol 16: 53–65 [DOI] [PubMed] [Google Scholar]
- Shimizu J, Yamazaki S, Sakaguchi S 1999. Induction of tumor immunity by removing CD25+CD4+ T cells: A common basis between tumor immunity and autoimmunity. J Immunol 163: 5211–5218 [PubMed] [Google Scholar]
- Shojaei F, Wu X, Malik AK, Zhong C, Baldwin ME, Schanz S, Fuh G, Gerber HP, Ferrara N 2007. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol 25: 911–920 [DOI] [PubMed] [Google Scholar]
- Shree T, Olson OC, Elie BT, Kester JC, Garfall AL, Simpson K, Bell-McGuinn KM, Zabor EC, Brogi E, Joyce JA 2011. Macrophages and cathepsin proteases blunt chemotherapeutic response in breast cancer. Genes Dev 25:2465–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH, Verjee SS, Jones LA, Hershberg RM 2006. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol 24: 3089–3094 [DOI] [PubMed] [Google Scholar]
- Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T, Delaney A, Jones SJ, Iqbal J, Weisenburger DD, et al. 2010. Tumor-associated macrophages and survival in classic Hodgkin's lymphoma. N Engl J Med 362: 875–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM 2005. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res 11: 6713–6721 [DOI] [PubMed] [Google Scholar]
- Tan W, Zhang W, Strasner A, Grivennikov S, Cheng JQ, Hoffman RM, Karin M 2011. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature 470: 548–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teft WA, Kirchhof MG, Madrenas J 2006. A molecular perspective of CTLA-4 function. Annu Rev Immunol 24: 65–97 [DOI] [PubMed] [Google Scholar]
- Testori A, Richards J, Whitman E, Mann GB, Lutzky J, Camacho L, Parmiani G, Tosti G, Kirkwood JM, Hoos A, et al. 2008. Phase III comparison of vitespen, an autologous tumor-derived heat shock protein gp96 peptide complex vaccine, with physician's choice of treatment for stage IV melanoma: The C-100-21 Study Group. J Clin Oncol 26: 955–962 [DOI] [PubMed] [Google Scholar]
- van Meerbeeck JP, Gaafar R, Manegold C, Van Klaveren RJ, Van Marck EA, Vincent M, Legrand C, Bottomley A, Debruyne C, Giaccone G 2005. Randomized phase III study of cisplatin with or without raltitrexed in patients with malignant pleural mesothelioma: An intergroup study of the European Organisation for Research and Treatment of Cancer Lung Cancer Group and the National Cancer Institute of Canada. J Clin Oncol 23: 6881–6889 [DOI] [PubMed] [Google Scholar]
- Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R 2007. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 27: 670–684 [DOI] [PubMed] [Google Scholar]
- Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, Carbone DP, Matrisian LM, Richmond A, Lin PC, et al. 2008. Abrogation of TGF β signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell 13: 23–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi KH, Chen L 2009. Fine tuning the immune response through B7-H3 and B7-H4. Immunol Rev 229: 145–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Patel L, Pienta KJ 2010. CC chemokine ligand 2 (CCL2) promotes prostate cancer tumorigenesis and metastasis. Cytokine Growth Factor Rev 21: 41–48 [DOI] [PMC free article] [PubMed] [Google Scholar]