Abstract
The transition from fetal to postnatal life involves clearance of liquid from the lung and airways, and rapid formation of a functional residual capacity. Despite the importance of the diaphragm in this process, the impact of birth on the mechanical and functional activity of its muscle fibers is not known. This study determined the contractile characteristics of individual “skinned” diaphragm fibers from 70 days (0.47) gestation to after birth in sheep. Based on differential sensitivity to the divalent ions calcium (Ca2+) and strontium (Sr2+), all fibers in the fetal diaphragm were classified as “fast,” whereas fibers from the adult sheep diaphragm exhibited a “hybrid” phenotype where both “fast” and “slow” characteristics were present within each single fiber. Transition to the hybrid phenotype occurred at birth, was evident after only 40 min of spontaneous breathing, and could be induced by simple mechanical stretch of diaphragm fibers from near-term fetuses (∼147 days gestation). Both physical stretch of isolated fibers, and mechanical ventilation of the fetal diaphragm in situ, significantly increased sensitivity to Ca2+ and Sr2+, maximum force generating capacity, and decreased passive tension in near-term and preterm fetuses; however, only fibers from near-term fetuses showed a complete transition to a “hybrid” activation profile. These findings suggest that stretch associated with the transition from a liquid to air-filled lung at birth induces physical changes of proteins determining the activation and elastic properties of the diaphragm. These changes may allow the diaphragm to meet the increased mechanical demands of breathing immediately after birth.
Keywords: calcium–strontium activated contraction, skinned muscle fibers, hybrid fiber type, contractile proteins, diaphragm fibers
Introduction
Before birth the lungs are filled with liquid and take no part in gas exchange, which occurs between the maternal and fetal circulations in the placenta. At birth, the respiratory system undergoes important changes to ensure a successful transition of the lungs from a liquid-filled to an air-filled environment – this involves removal of liquid from the airways and alveoli, and the formation of a functional residual capacity (Bland et al., 1980; Olver et al., 2004; Siew et al., 2009). These processes provoke further changes necessary for the lungs to become efficient in gas exchange, including: increased pulmonary blood flow, a reduction in intrapleural pressure, lung recoil, and closure of the ductus arteriosus initiated, in part, by the increase in blood oxygenation (Rudolph, 1985; Hooper and Harding, 2005; Crossley et al., 2009). Failure to remove liquid and aerate the lungs is a major cause of dyspnea and respiratory-related morbidity in neonates (Jain and Eaton, 2006).
These respiratory and cardiopulmonary changes are largely dependent on a series of deep inspiratory efforts or gasps that occur soon after the fetus emerges from the birth canal (Olver et al., 2004; Siew et al., 2009); these inspiratory efforts depend almost entirely on contractions of the diaphragm (Guslits et al., 1987), which must adapt to the change in chest wall and lung compliances, whilst also establishing and sustaining a regular breathing pattern. The stiff, liquid-filled lungs, and high compliance of the chest wall increases the trans-diaphragmatic pressure necessary to inflate the lungs at birth, placing high metabolic, and mechanical demands on the diaphragm in sustaining regular breathing at this time (Guslits et al., 1987; Mantilla and Sieck, 2008). It is known that mechanical load has significant and rapid effects on diaphragm muscle fibers, as shown by the fiber atrophy, proteolysis, and decrease in force generating capacity which can appear within hours of starting mechanical ventilation (Shanely et al., 2004). We therefore suggest that the onset of air breathing at birth could induce rapid change in the force generating capacity of muscle fibers in the diaphragm. While there has been extensive research into the structural and functional changes of the diaphragm that occur during postnatal maturation in species that are immature at birth (Sieck et al., 1991; Watchko et al., 1998; Geiger et al., 1999), there is relatively limited information available on the development of diaphragm for species where lung development is advanced, and rapid displacement of liquid by air is essential for survival. In baboons, Maxwell et al. (1983) showed that the phenotype of respiratory muscle fibers changed gradually over the last trimester of pregnancy and continued into postnatal life. However, little is understood about the impact of birth itself on the activation properties of fibers within the diaphragm.
Previous studies have shown that individual fibers of the adult diaphragm have functional characteristics typical of both fast- and slow-twitch fibers, as shown by activation of single “skinned” fibers with Ca2+ and Sr2+ (Bortolotto et al., 2000; O’Connell et al., 2004a,b). It is not known when this “hybrid” property emerges during development, or what effect the transition to air breathing, or the mechanics of regular forceful breathing might have on the activation properties of the diaphragm fibers. In fetal sheep, limb muscle fibers initially have a “fast-twitch” phenotype, whether characterized by the contraction speed of whole muscle groups (Walker and Luff, 1995), or by the Ca2+ and Sr2+ activation properties of single “skinned” muscle fibers (West et al., 1999). We hypothesized that, as for hind limb muscle fibers in fetal sheep, all diaphragm fibers would initially have a fast-twitch phenotype, and that slow-twitch characteristics would emerge either late in gestation or at birth. Furthermore, we hypothesized that increased longitudinal stretch associated with the onset of breathing would accentuate the slow-twitch characteristics of individual diaphragm fibers, because it is known that stretch induces expression of slow myosin heavy chain (MHC) isoforms in other skeletal muscle (Goldspink et al., 1992, 2002; West et al., 2000).
Therefore, the aims of this study were to: (i) determine the developmental changes of the activation properties of single fibers obtained from the costal portion of the diaphragm of fetal and newborn sheep; (ii) identify changes in diaphragm fibers that occur as a result of the transition to air breathing at birth; and (iii) determine the effects of stretch and rhythmic ventilation, whether spontaneous or applied mechanically, on the activation properties of diaphragm fibers in preterm and term lambs. Given that many of the cardiopulmonary changes occurring at birth depend on the prompt onset of effective ventilation, obtaining insight into how the developing diaphragm adapts to sudden demands imposed upon it is important, particularly for infants born prematurely where the immature diaphragm may be unable to adapt, thereby contributing to the high incidence of respiratory-related problems that occur in this patient group.
Materials and Methods
Animals
All experiments were approved in advance by Monash University School of Biomedical Sciences Animal Ethics Committee and conducted in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. Pregnant Border-Leicester × Merino ewes were killed by intravenous injection of an overdose of pentobarbitone (Lethabarb; Virbac Pty Ltd., Australia) at 70 (n = 3), 116 (n = 2), 127(n = 8) days of gestation, and at term when labor had just begun (147 days of gestation, n = 5). Two lambs were killed using pentobarbitone 8 h after birth, and strips of the diaphragm from four adult ewes were also obtained at other post-mortems. In five pregnant ewes carrying twins, signs of the commencement of labor were monitored closely from 145 days gestation, and the first lamb was allowed to deliver naturally and to establish breathing for ∼40 min before being killed by pentobarbitone injection and the diaphragm excised (n = 5). Immediately after delivery of this lamb the ewe was killed by intravenous injection of pentobarbitone, and the other fetus was rapidly removed, cardiac arrest was produced by injection of 5 mL pentobarbitone solution into the heart, and then the diaphragm was excised; thus, the second fetus never got to breathe air or clear liquid from its lungs. Diaphragm tissue was also obtained from fetuses at 127 day gestation (n = 8) that had been delivered by hysterotomy when the ewe was anesthetized using isoflurane (1.5–2.5%) in oxygen. The fetal head and neck were exposed and the trachea was intubated with a 3.5-mm cuffed endotracheal tube and lung liquid drained passively before the umbilical cord was clamped and cut. These fetuses were then either killed immediately using 5 mL sodium pentobarbitone (n = 5), or were delivered, dried, placed under a radian theater and ventilated using a Babylog 8000+ ventilator (Dräger, Lubeck, Germany) set to deliver 60 inflations/min, using “volume guarantee” tidal volume (VT) of 5 mL/kg, and a positive end expiratory pressure (PEEP) of 5 cmH2O; this method of resuscitation is similar to that used for premature human infants and has been described previously (Polglase et al., 2005). The fraction of inspired oxygen (FiO2) was initially set at 1.0 and altered to maintain arterial pH between 7.30 and 7.45. These fetuses (n = 3) received an intravenous infusion of 5% dextrose and were sedated using the anesthetic steroid alfaxalone (Alfaxan, 15 mg/h; Jurox, Rutherford, NSW, Australia) to prevent spontaneous breathing. They were then ventilated for 2.5 h before being killed using an intravenous overdose of sodium pentobarbitone, after which the diaphragm was excised.
Ca2+- and Sr2+-activation of single skinned fibers
This study was performed on chemically skinned, isolated muscle fibers as described previously for fetal sheep limb muscles (West et al., 1999). Strips of the costal diaphragm were dissected from the diaphragms collected at each pre- and postnatal age described above, and placed directly into a relaxing solution (mM; propionic acid, 150; 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 20; ethylene glycol bis(2-aminoethyl ether)-N,N,N′,N′-tetra acetic acid (EGTA), 10; MgCl2, 3; ATP, 2) containing 50% glycerol (v/v) under slight tension, by tying the ends of the muscle strips with surgical silk (Deknatel, 5.0, USA) to small wooden sticks and stored at −20°C until use. Muscle fibers are “chemically skinned” by this exposure to glycerol (Stephenson and Williams, 1982; Balnave and Allen, 1996).
Solutions used to activate and relax the muscle fibers have been documented elsewhere (Ashley and Moisescu, 1977) and used by us previously (West et al., 1999; Cannata et al., 2009, 2010; see Table 1). Previously determined apparent affinity constants (Kapp) were used to calculate the calcium and strontium ion concentrations of the solutions (expressed as pCa and pSr, the −log10 ionic concentration), and the amount of free EGTA in each solution determined by titration. Solutions containing Ca2+ (0.02–14.4 μM) or Sr2+ (0.03–363 μM) were obtained through combination of relaxing solution containing 50 mM EGTA (solution A) with the solutions containing Ca2+(solution B) or Sr2+ (solution S; see Table 1).
Table 1.
Composition of solutions used for relaxing and activating skinned fibers.
| Solution | [K+] | [EGTA] | [HDTA] | [Mg2+]total | [Ca]total | [Ca2+]free | [Sr]total | [Sr2+]free |
|---|---|---|---|---|---|---|---|---|
| A | 117 | 50 | – | 10.3 | – | <10−6 | – | – |
| B | 117 | 50 | – | 8.12 | 49.5 | 0.02 | – | – |
| H | 117 | 0.2 | 49.8 | 8.51 | – | <1.7 × 10−5 | – | – |
| S | 114 | 50 | – | 5.95 | – | – | 40 | 0.283 |
All solutions contained [mmol]: Na+ [36]; HEPES [60]; total ATP [8]; creatine phosphate (CP) [10]; and sodium azide [1]. pH was 7.10 ± 0.01 at 23–25°C. A = Relaxing solution. B = Ca2+ containing activating solution. H = Pre-activating solution. S = Sr2+ containing activating solution. Solutions were titrated using a pH metric method (Ashley and Moisescu, 1977).
Fibers were activated using the “jump-technique” (West et al., 1999). Briefly, each fiber was connected to stainless steel pins using surgical silk (Deknatel 10), and one pin was attached to a micromanipulator and the other to a micro force transducer (AE 801, SensoNor, Horten, Norway). The micromanipulator was adjusted to place the fiber under slight tension, and it was then activated in a series of solutions of increasing Ca2+ or Sr2+concentrations until a maximum activated force was reached. To accommodate any time-dependent decrease in force capacity during activation in increasing concentrations of either divalent ion, maximum force response was determined at the beginning and end of a series of contractions. Any decrease in force (<10%) over the duration of the experiment was assumed to have declined linearly with time, thus the force measured at each sub-maximal Ca2+ and Sr2+ concentration was normalized to the estimated maximum force response at that time. Maximum Ca2+-activated force was obtained from the first maximum activation in the staircase protocol.
Stretch-induced activation profiles of diaphragm fibers
To observe the effect of stretch on the activation properties of each fiber, a slightly modified protocol to the one described above was employed. Diaphragm fibers were dissected out and activated using Ca2+ and Sr2+ as per the normal protocol, under slight tension. After the activation was completed the fiber was then mechanically stretched using a micromanipulator attached to the apparatus. The stretch that was induced was always calibrated to ∼0.5 μm in sarcomere length using a He–Ne laser. The fiber was activated again in the entire range of Ca2+ and Sr2+ solutions while the increased length was maintained.
Passive tension and maximum Ca2+-activated force of single fibers
A change in the passive tension that is produced by a single skinned fiber is directly related to changes that occur to the elastic properties within the contractile apparatus. Single skinned fibers were prepared as described for Ca2+- and Sr2+-activation, mounted to the pins of the force recording apparatus at a length that produced no tension (slack length). Sarcomere length was measured using a He–Ne laser. While continually submerged in the relaxing solution (solution A) passive tension was initiated by stretching the fiber by 10% of its starting length with at least 60 s hold time to allow for stress relaxation. After each hold time, sarcomere length was measured in the center of the fiber using the He–Ne laser, and passive tension was recorded on the chart recorder and normalized to the cross-sectional area (CSA) of the fiber. Fibers were stretched in eight steps to a final fiber length of 180% of the starting length. At the start and after every second induced stretch, the fiber was submerged in a solution containing a sufficient amount of Ca2+ (1.55 × 10−5 M; pCa 4.83) to maximally activate the fiber (solution B). Once a plateau has been reached in the maximum calcium activating solution the fiber was placed back in the relaxing solution. The fiber was not stretched again until the activated force had returned to the baseline produced by the passive tension.
Analysis of force–pCa and force–pSr curves
The analysis of force–pCa and force–pSr curves has been previously described (Cannata et al., 2010). Force–pCa curves were always described by a simple sigmoid function. The curve was fitted to data using a Marquardt non-linear regression algorithm, and the parameters derived from this were: pCax (the amount of Ca2+ needed to produce “x” amount of force) and nCa (maximum slope of the force–pCa curve; see Table 2). The force–pSr relationship could not always be described by a single sigmoid curve, but showed a discontinuity in the central region and was then significantly better described as the sum of two sigmoid curves. “Goodness of fit” by a single curve or double sigmoid curves was determined using an F-test, and a double sigmoid curve fit was used if P < 0.05. The quantitative measures obtained from the force–pSr curves that were fitted by a double sigmoid were; pSr501, pSr502 (the amount of Sr2+ needed to produce 50% of the maximum force described by the first and second sigmoid, respectively), nSr1, nSr2 (maximum slope of the first and second sigmoid of the force–pSr curve, respectively) and F1% (the portion of the entire force–pSr curve described by the first sigmoid curve).
Table 2.
Ca2+- and Sr2+-activation parameters in diaphragm fibers of the sheep during pre- and postnatal development.
| Prenatal |
Term 0 min (N = 5, n = 25) | Postnatal |
||||||
|---|---|---|---|---|---|---|---|---|
| 70 days GA (N = 3, n = 12) | 116 days GA (N = 2, n = 6) | 127 days GA (N = 5, n = 20) | 127 days GA (2.5 h MV; N = 3, n = 15) | 40 min (N = 5, n = 26) | +8 h (N = 2, n = 6) | Adult (N = 4, n = 16) | ||
| pCa10 | 6.93 ± 0.06 | 7.10 ± 0.03a | 6.67 ± 0.03ab | 6.80 ± 0.02c | 6.61 ± 0.01ab | 6.70 ± 0.02abd | 6.69 ± 0.04abd | 6.60 ± 0.10ab |
| pCa50 | 6.43 ± 0.05 | 6.67 ± 0.03a | 6.30 ± 0.02ab | 6.39 ± 0.0c | 6.40 ± 0.02abc | 6.48 ± 0.01bcd | 6.37 ± 0.14b | 6.41 ± 0.08b |
| pCa90 | 5.94 ± 0.09 | 6.24 ± 0.04a | 5.90 ± 0.02b | 6.00 ± 0.0c | 6.12 ± 0.02abc | 6.17 ± 0.02acd | 6.05 ± 0.02bcde | 6.20 ± 0.12cd |
| nCa | 1.94 ± 0.09 | 2.23 ± 0.11 | 2.55 ± 0.05ab | 2.38 ± 0.10 | 3.66 ± 0.15abc | 3.83 ± 0.12abc | 3.02 ± 0.27abe | 4.51 ± 0.88abcf |
| pSr501 | N/A | N/A | 5.36 ± 0.04 | 5.70 ± 0.06c | 5.64 ± 0.06c | 5.94 ± 0.02cd | 6.17 ± 0.01cd | 6.18 ± 0.06abcd |
| nSr1 | N/A | N/A | 1.57 ± 0.01 | 1.60 ± 0.08 | 1.78 ± 0.12c | 2.72 ± 0.14cd | 2.47 ± 0.39c | 4.85 ± 0.33cdef |
| pSr502 | N/A | N/A | 4.95 ± 0.03 | 4.94 ± 0.02 | 4.85 ± 0.05 | 4.91 ± 0.02 | 4.96 ± 0.05 | 4.88 ± 0.04 |
| nSr2 | N/A | N/A | 4.13 ± 0.40 | 4.10 ± 0.46 | 3.77 ± 0.36 | 3.02 ± 0.14cd | 2.77 ± 0.48c | 3.88 ± 0.52e |
| F1 (%) | N/A | N/A | 48.57 ± 7.69 | 47.32 ± 4.85 | 38.61 ± 2.32 | 40.36 ± 4.26 | 42.00 ± 5.00 | 44.30 ± 8.10 |
| SL (μm) | 2.19 ± 0.08 | 2.29 ± 0.03 | 2.28 ± 0.04 | 2.33 ± 0.05 | 2.42 ± 0.02abc | 2.49 ± 0.03abcd | 2.50 ± 0.04abcd | 2.64 ± 0.12abcde |
GA, days gestational age; MV, mechanical ventilation; N, number of animals; n, the number of fibers analyzed. Values presented are mean ± SEM. Superscript letter indicates significant differences (P < 0.05) between the following groups: asignificantly different from 70 days GA; b116 days GA; c127 days GA; d0 min of breathing at term; e40 min of breathing at term, f+8 h of spontaneous breathing after term birth. N/A = not applicable, as the force–pSr curve of fibers at 70 and 116 day GA were fitted by a single exponential function. pCax = indicates the amount of Ca2+ needed to produce “x” amount of force; nCa = the maximum slope of the force–pCa curve. pSr501 = amount of Sr2+ needed to produce 50% of the force–pSr curve described by the first exponential. nSr1 = The maximum slope of the first exponential. F1 (%) = the proportion of the force–pSr curve described by the first exponential. SL, sarcomere length.
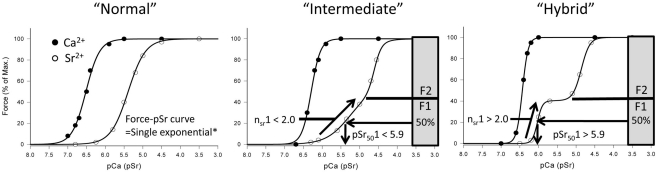
Note: The two sigmoid curves that make up the complete force–pSr curve are termed F1 and F2. The first sigmoid curve describing the data at the lower range of Sr2+concentrations (F1) is assumed to reflect the activation of slow isoforms because the small separation of the force–pCa and force–pSr curves is typical of that obtained from “pure” slow-twitch fibers (West et al., 1999; Bortolotto et al., 2000; see Figure 1). The second sigmoid (F2) is likely to reflect the activation of fast isoforms, as the greater separation of the force–pCa and force–pSr curves is typical of “pure” fast-twitch fibers. In the analysis of the force–pSr curves of fetal and postnatal diaphragm it became clear that the force–pSr curve changed from a simple, single sigmoid function to a discontinuous function best described by a double sigmoid curve fit. We therefore arbitrarily classified the force–pSr curve as “normal” when best described by a single sigmoid; “intermediate” when significantly better described by a double function even though the separation between the upper and lower parts of the curve was not always distinct; and “hybrid” when the force–pSr curve was significantly better described by a double sigmoid and there was clear distinction between the lower and upper parts of the force–pSr relationship; in all cases the decision to categorize the data as intermediate or hybrid was based on the F-test for “Goodness of Fit.” The basis of classification used in this study was that when pSr501 > 5.90 and nSr1 value > 2.0, then the fiber was truly “hybrid”; when the force–pSr curve was described by a single sigmoid, then the fiber was “normal”; and when a double sigmoid function was required and pSr501 < 5.90 and nSr1 < 2.0, then the fiber was deemed to be “intermediate.” Figure 1 displays the three different activation profiles based on this classification system and demonstrates how the parameters govern the shape of the force–pSr curve. This classification was used to identify the gradual changes that occur in the activation properties of diaphragm fibers during development (see Figures 1 and 2 and Results). Diaphragm muscle fibers have previously been described as “hybrid”; for example, see, O’Connell et al. (2004b).
Figure 1.
Classification of diaphragm fibers based on Sr2+-activation parameters. “Normal” refers to Ca2+- and Sr2+-activation profiles where both functions are described by a single exponential function, and is normally observed in skeletal muscle – separation between the force–pCa and force–PSr curves is greater for “fast” twitch fibers, and least for “slow” twitch fibers. “Hybrid” activation profile of the force–pSr curve is typical for fibers from adult diaphragm – the separation between the lower portion of the force–pSr curve and force–pCa curve is typical of that for “slow” twitch fibers, while the separation between the upper part of the force–pSr and force–pCa curves is typical of that for “fast” twitch fibers. “Intermediate” fibers were identified in the developing diaphragm when the force–pSr curve could no longer be fitted by a single exponential; Marquardt curve fitting was used to derive parameters from a double exponential curve, and also to determine the F1 and F2 portions of the complete force–pSr relationship.
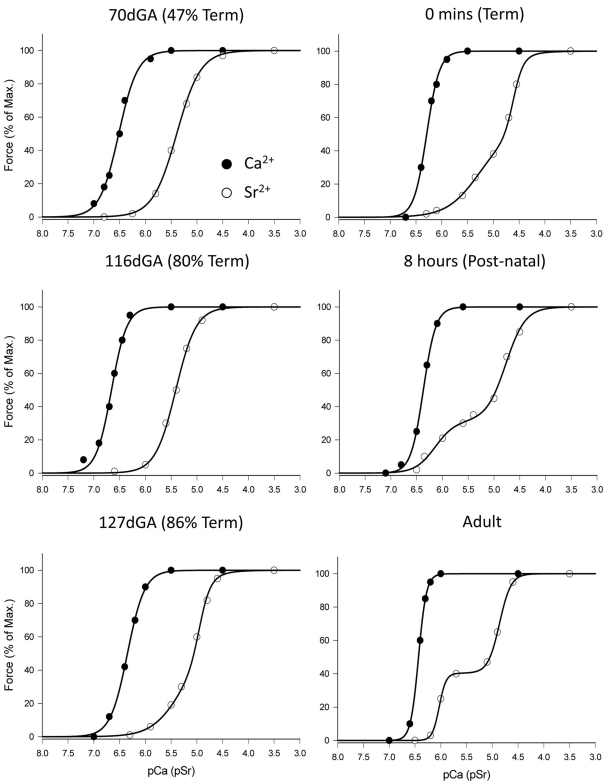
Figure 2.
Ca2+- and Sr2+-activation profiles of diaphragm fibers from fetal newborn and adult sheep. Zero minutes (Term) fibers were taken from fetuses where the ewe was in labor at 147 days gestation, but no breathing had occurred and the lungs remained filled with fluid. Eight hours (Postnatal) refers to a lamb that had been delivered and allowed to auto-resuscitate and breathe for 8 h until autopsy. Note the change of the force–pSr relationship from fetal to postnatal life; full description is given in Section “Results.”
Myosin heavy chain isoform expression
The MHC isoform composition of each of the single “skinned” muscle fibers used in the studies described above was then determined by SDS-PAGE using the method described by Talmadge and Roy (1993) with a few modifications. After the Ca2+/Sr2+ activation procedures had been completed, the fibers were removed from the force recording apparatus and placed in a SDS reducing buffer (62.5 mM Tris–HCl (pH 6.8); 12.5% glycerol, 2.3% SDS) overnight at room temperature (20–23°C). Samples were then boiled for 5 min at 94°C in the reducing buffer, and then stored at −20°C. MHC separation was carried out using a Bio-Rad mini-Protean III cell apparatus. Stacking gels were composed of 30% glycerol, 4% acrylamide: N,N′-methylenebisacrylamide (37.5:1), 70 mM Tris (pH 6.7), 4 mM ethylenediaminetetraacetic acid (EDTA; pH 7.0), 0.4% SDS, 0.1% ammonium persulfate (APS), and 0.05% N,N,N′,N′-tetramethylethylenediamine (TEMED). Separating gels were composed of 30% glycerol, 8% acrylamide: N,N′-methylenebisacrylamide (37.5:1), 0.2 M Tris (pH 8.8), 0.1 M Glycine, 0.4% SDS, 0.1% APS, and 0.05% TEMED. The upper running buffer consisted of 0.1 M Tris (base), 150 mM glycine, 0.1% SDS, and 0.1% β-mercaptoethanol. The lower running buffer consisted of 50 mM Tris (base), 75 mM glycine, and 0.05% SDS. The pH values of the running buffers were not adjusted. The gel was run in a cold room (∼4°C) at a constant voltage (110 V) for 24 h. Samples were run with MHC standards; MHC I (from the soleus) and MHC IIa (from the EDL). Bands were visualized using a silver-staining kit (161-0443, Bio-Rad) before gels were photographed using a Chemdoc LAS 3000 program (Fujifilm). The density of the bands was obtained using densitometry computer software (Multi-gage).
Statistical Analysis
All values presented are expressed as the mean ± SEM. Statistical difference (P < 0.05) was determined using a one-way ANOVA and subsequent Tukey’s HSD post hoc test (SPSS 17.1). When only two groups were compared (stretch-induced activation profiles and the determination of passive tension) the student’s t-test were used. A total of 29 sheep were studied, from which 223 fibers were analyzed, with 99 fibers undergoing dual-analysis for Ca2+- and Sr2+-activation as well as MHC isoform expression.
Results
Activation properties of diaphragm fibers during prenatal and postnatal development
The Ca2+- and Sr2+-activation parameters for all age groups is shown in Table 2. At 70 and 116 days of fetal age (0.48 and 0.80 full-term), both the force–pSr and force–pCa curves could be described by single sigmoid functions (F-test, P > 0.05), as shown in Figure 2. The separation of the force–pCa and force–pSr curves was typical of a fast-twitch skeletal muscle fiber (i.e., the curves were separated by >1.0 units). At 127 days gestational age (0.87 full-term) the force–pCa curve remained unchanged compared to data from the earlier fetal ages, but the force–pSr curve of diaphragm fibers now presented with two distinct phases, each of which had significantly different slopes (nSr1, 1.57 ± 0.01; nSr2, 4.95 ± 0.03; Table 2), and the entire data was best described by a double sigmoid function (F-test, P < 0.05; Figure 2). For fetuses near to full-term the force–pSr curve was also best described by a double sigmoid, but the parameters describing the lower portion of the force–pSr curve (pSr501 = 5.64 ± 0.06; nSr1 = 1.78 ± 0.12, Table 2) were significantly higher than the values calculated at 127 days gestation (P < 0.05, Table 2). At birth, and after 8 h of spontaneous breathing, the force–pSr curve had changed so that a distinct sub-maximal plateau could be seen (Figure 2) and, as for the adult diaphragm, the force–pSr curve was divided into upper and lower portions by a distinct mid-range plateau (Figure 2). Thus, from shortly after birth and the establishment of spontaneous breathing, diaphragm fibers were classified a truly “hybrid” based on the force–pSr activation profiles, because values for pSr1 pSr501 and nSr1 were similar (Table 2). In fibers obtained from the adult diaphragm the increased slope (nSr1) was related to the more pronounced sub-maximal plateau that appears in the force–pSr activation profile between birth and adulthood (Table 2).
The force–pCa curve was described as a single sigmoid throughout fetal and postnatal development, but the slope (nCa) of the force–pCa curve increased significantly with age (P < 0.05, Table 2). The greatest increase in the slope of force–pCa curve occurred after birth, i.e., after the onset of breathing.
Influence of spontaneous air breathing and stretch on passive and activation properties of diaphragm fibers
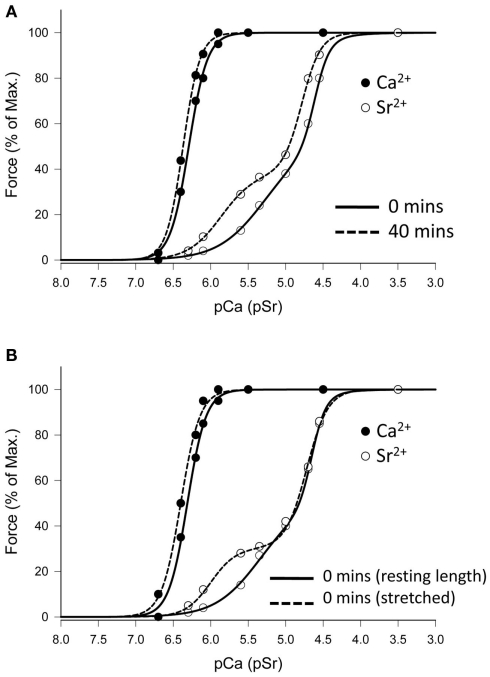
To investigate the impact of the commencement of air breathing on the diaphragm at birth, activation profiles were obtained for diaphragm fibers dissected from fetuses at term that had not commenced breathing (0 min), and from age-matched (twin) lambs that had breathed air for 40 min. After 40 min of spontaneous breathing there was a significant increase in sensitivity to both Ca2+ and Sr2+, and the emergence of a sub-maximal plateau in the force–pSr (Figure 3A), so that fibers could be classified as hybrid based on pSr501 value > 5.90 (5.94 ± 0.02, Table 2) and nSr1 values > 2.0 (2.72 ± 0.14; Table 2). Thus, birth and air breathing resulted in increased sensitivity of fibers to both Ca2+ and Sr2+, but the greatest change occurred in the first phase of the force–pSr curve.
Figure 3.
Effect of ventilation or mechanical stretch on Ca2+- and Sr2+-activation profiles of diaphragm fibers at term. (A) Representative data from twins where one lamb was delivered but not permitted to breathe (0 min), and the other lamb was allowed to establish ventilation and breathe for 40 min before autopsy. Breathing induces changes in the lower part of force–pSr curve that are similar to that occurring after 8 h of spontaneous ventilation (see Figure 2). (B) Representative data for Ca2+- and Sr2+-activation in a fiber from a term fetus where no breathing had occurred, and where the fiber was studied before (sarcomere length = 2.38 μm) and after (sarcomere length = 2.88 μm) mechanical stretch, in vitro. Stretch induces a change in the force–pSr curve similar to that induced by breathing.
It is possible that the transition from a liquid-filled to an air-filled lung increases the tension of the diaphragm, and the ensuing stretch of fibers results in conformational changes to the contractile proteins. To test this hypothesis we studied single fibers from term fetuses that had not breathed using the normal protocol to obtain data as described above, and then applied a stretch of approx. 0.5 μm in sarcomere length to the fibers and repeated the Ca2+- and Sr2+-activation protocol. A representative activation profile of a diaphragm fiber that was activated under normal conditions and again after stretching is shown in Figure 3B. Activating the fiber under increased tension resulted in increased fiber sensitivity to Ca2+ and Sr2+ (P < 0.05, Table 3), and the force–pSr activation profile was then almost identical to that of a fiber obtained from a lamb after 40 min of unassisted breathing (Figures 3A,B). Accordingly to the criteria outlined in Section “Materials and Methods,” fibers from the term fetal diaphragm activated at resting length produced an intermediate activation profile that was neither like that found at earlier fetal ages or in postnatal lambs and adult sheep. However when activated under an applied stretch, the force–pSr activation profile was similar to that of a true hybrid fiber (pSr501 = 5.93 ± 0.10, nSr1 = 2.59 ± 0.22, Table 3) typical of that observed in the postnatal lamb and adult sheep diaphragm.
Table 3.
Ca2+- and Sr2+-activation parameters of diaphragm fibers activated at resting length and then with a stretch applied.
| 0 min |
127dGA |
|||
|---|---|---|---|---|
| Resting length | Stretched | Resting length | Stretched | |
| pCa10 | 6.62 ± 0.03 | 6.74 ± 0.02* | 6.65 ± 0.04 | 6.74 ± 0.01* |
| pCa50 | 6.40 ± 0.03 | 6.49 ± 0.00* | 6.27 ± 0.02 | 6.35 ± 0.01* |
| pCa90 | 6.10 ± 0.02 | 6.13 ± 0.03 | 5.89 ± 0.02 | 5.93 ± 0.02 |
| nCa | 3.62 ± 0.10 | 3.54 ± 0.08 | 2.51 ± 0.13 | 2.36 ± 0.09 |
| pSr50l | 5.55 ± 0.04 | 5.93 ± 0.10† | 5.40 ± 0.07 | 5.60 ± 0.03* |
| nSrl | 1.68 ± 0.14 | 2.59 ± 0.22† | 1.52 ± 0.14 | 1.64 ± 0.06 |
| pSr502 | 4.70 ± 0.05 | 4.78 ± 0.02 | 4.96 ± 0.01 | 4.92 ± 0.04 |
| nSr2 | 3.60 ± 0.24 | 3.25 ± 0.13 | 4.40 ± 0.47 | 4.52 ± 0.48 |
| Fl (%) | 33.82 ± 4.36 | 29.88 ± 6.85 | 52.76 ± 3.54 | 52.68 ± 1.69 |
| SL (μm) | 2.40 ± 0.05 | 2.92 ± 0.06† | 2.29 ± 0.04 | 2.75 ± 0.08† |
Values presented are the mean ± SEM. n = 3 animals (12 fibers) for both term and 127 day GA fetal sheep. Diaphragm fibers were first activated at resting length, and then under an induced stretch of ∼0.5 μm in sarcomere length (SL). *P < 0.05 and †P < 0.01 indicates significant differences compared to the data obtained at resting length.
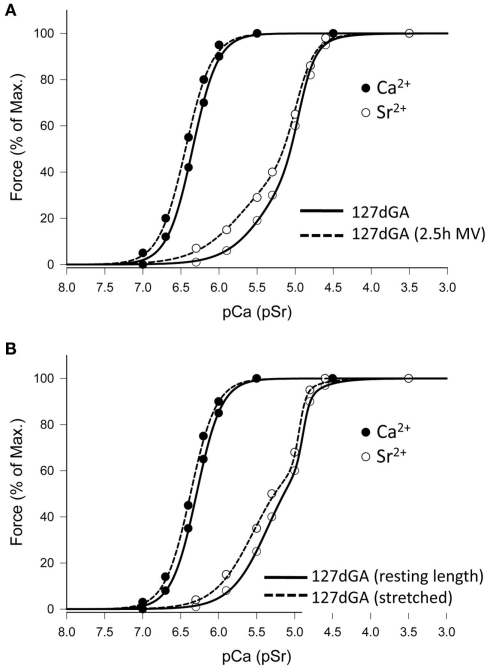
To determine if the same effects could be induced in preterm lambs, fetuses were either delivered at 127 days gestation and mechanically ventilated for 2.5 h, or fibers obtained from non-ventilated fetuses of 127 days gestation and stretched in vitro as described above. Mechanical ventilation was used in these experiments because at this age fetal sheep, like many preterm infants, cannot sustain an adequate level of ventilation. As shown in Figure 4A, 2.5 h of mechanical ventilation induced changes to the activation properties which result in an increased sensitivity to Ca2+ and Sr2+ (P < 0.05, Table 2), but it is notable that while mechanical ventilation yielded an increase in pSr501 of approx. 0.34 pSr units (P < 0.05, Table 2), the pSr501 value remained below that obtained from fully “hybrid” fibers in the postnatal diaphragm (where pSr501 > 5.90). In addition, 2.5 h of mechanical ventilation had no effect on the slope of the force–pSr curve (nSr1 < 2.0), and so these 127 day gestation fetal fibers were still classified as having an intermediate profile. Physical stretch of a diaphragm muscle fiber of this developmental age had a similar effect as mechanical ventilation; sensitivity to both Ca2+ and Sr2+ increased (P < 0.05, Figure 4B), but despite a significant increase of pSr501 of approx. 0.20 pSr units, this parameter remained less than the value found in true hybrid fibers of the postnatal diaphragm where pSr501 > 5.90 (see Table 3). The nSr1 value did not change (Table 3). Thus, while stretch applied physically or as a result of mechanical ventilation induced significant change, the activation profile of fibers obtained from the 127 day gestation fetus do not exhibit the true hybrid activation profile that is present in the full-term fetal sheep diaphragm.
Figure 4.
Effect of ventilation or mechanical stretch on Ca2+- and Sr2+-activation profiles of diaphragm fibers at 127 days gestation. (A) Representative data where fibers were obtained either immediately after delivery of the fetus (127 dGA), or after 2.5 h of mechanical ventilation (see Methods for details). Ventilation induces a small change in the lower part of force–pSr curve. (B) Representative data for Ca2+- and Sr2+-activation profiles in a fiber from a 127-day gestation fetus where the fiber was studied before (sarcomere length = 2.27 μm) and after (sarcomere length = 2.75 μm) mechanical stretch, in vitro. Stretch induces a modest change in the lower portion of the force–pSr curve.
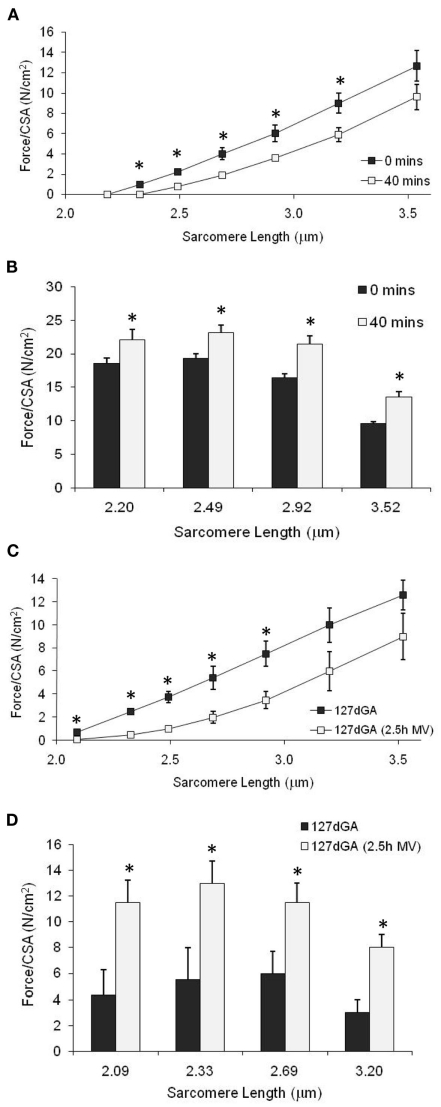
The effect of spontaneous breathing, mechanical ventilation, or stretch on passive tension and maximum force production of single fibers was also determined. For fibers obtained from the term diaphragm where the lambs had been allowed to breathe for 40 min, alteration of sarcomere length across the physiological range (2.20–3.20 μm) resulted in a decrease of passive tension (Figure 5A), and an increase in Ca2+-activated force corrected for CSA (P < 0.05, Figure 5B), in comparison to fibers obtained from term fetuses where no breathing had occurred. Similar observations were obtained for the preterm (127 days gestation) fetal diaphragm – after 2.5 h of mechanical ventilation, fibers showed significantly reduced passive tension (P < 0.05, Figure 5C) and increased Ca2+-activated force (P < 0.05, Figure 5D) compared to fibers obtained from non-ventilated fetuses of the same age.
Figure 5.
Effect of spontaneous air breathing or mechanical ventilation on passive tension and maximum Ca2+-activated tension in diaphragm fibers from 127-day gestation and term fetuses. (A,B) Passive tension and maximum force at increasing sarcomere length for fibers from term diaphragm where fetuses either did not breathe (0 min) or had been spontaneously breathing for 40 min; n = 4 fetuses, 12 fibers, each treatment group. (C,D) Passive tension and maximum force at increasing sarcomere length for fibers from 127 day gestation diaphragm where the fetuses had been subjected to either no, or 2.5 h mechanical ventilation; n = 3 fetuses, 12 fibers, each treatment group. *P < 0.05, between treatment group comparison.
Myosin heavy chain isoform expression
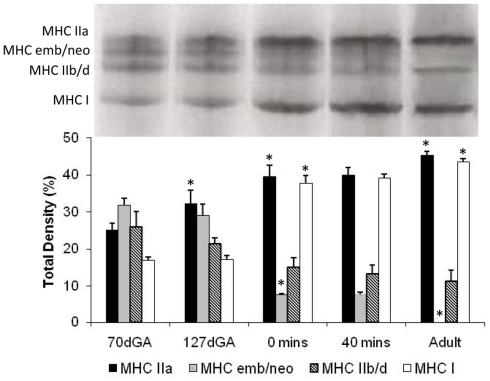
All fibers activated under the standard Ca2+- and Sr2+-activation protocol were then placed in a solubilizing buffer and the MHC isoforms separated using SDS-PAGE. MHC isoforms I, IIa, and IIb/d, identified by molecular weights consistent with these native MHCs, were present in the diaphragm from 70 days gestation (Figure 6). A further isoform was present at 70 and 127 days gestation, which was tentatively identified as the transitional embryonic/neonatal MHC, based on its equivalent molecular size and relation to the other MHCs. Using densitometry, expression of MHC I was increased after birth relative to the term fetal diaphragm, and MHC IIa expression increased during late fetal life and then postnatally (Figure 6). In contrast, MHC IIb/d expression decreased after birth compared to the amount of protein present in the fetal diaphragm (Figure 6). Consistent with it being a transitional isoform, the MHC embryonic/neonatal isoform was decreased at term compared to expression in the fetal diaphragm, and was not present in the adult diaphragm. Spontaneous breathing for 40 min after birth had no effect on the relative abundance of these MHC isoforms in the diaphragm (Figure 6). This indicates that the difference observed between term fetuses that had not taken a breath and lambs that had breathed for 40 min was not the result of differences in abundance of MHCs, as fibers from both age groups have the same relative expression of the different MHC isoforms.
Figure 6.
Myosin heavy chain isoforms in single fibers of the diaphragm from fetal, newborn, and adult sheep. Fibers used in the Ca2+, Sr2+ activation studies were then treated with solubilizing buffer and proteins separated by SDS-PAGE. Upper panel: identification of MHC isoforms based on molecular weight. Lower panel: Relative density of each MHC isoform, expressed as a percentage of the total density of all MHC protein in the fiber. Data shown as mean ± SEM, where at 70 days gestation [dGA] n = 3 fetuses, 12 fibers; 127 dGA n = 5 fetuses, 20 fibers; term fetuses no breathing [0 min] n = 5 fetuses, 25 fibers; term fetuses + 40 min breathing n = 5 fetuses, 26 fibers; adult, n = 4 ewes, 16 fibers. *P < 0.05, comparison of the same MHC isoform to the previous age or treatment group.
Discussion
The three major findings in this study are: (1) there is transformation of the activation profile of diaphragm muscle fibers from a “fast” to the “hybrid” phenotype between late gestation and birth; (2), the onset of air breathing at birth causes changes in fiber activation that may result directly from the increased stretch placed on the diaphragm due to expansion of the thoracic cavity, since these changes can also be produced by passive stretch of term fetal diaphragm fibers; and (3), these changes can only be partially produced by stretch or mechanical ventilation earlier in gestation. The change in activation profile occurs with concurrent changes in MHC expression in which the MHC I and IIa expression increases while that of MHC IIb/d expression decreases. The finding that the preterm diaphragm cannot fully adapt to a sudden transition to air breathing has implications for the clinical management of the preterm infant.
“Fast to slow” transformation of skeletal muscle is a fundamental response to chronic change of load or the pattern of neural activity, as shown originally by Buller et al. (1960). In such circumstances fiber switching usually involves the repression and/or up-regulation of sets of genes that determine MHC isoform expression (Campbell et al., 2001); post-translational modification of regulatory proteins involved in muscle contraction may also be involved (reviewed by Harridge, 2011). In cardiac and skeletal muscle, post-translational modifications such as oxidation and phosphorylation can occur rapidly after stretch (Chambers et al., 2009; Monasky et al., 2010; Prosser et al., 2011), thus it is possible that these mechanisms also play a role in the adaptation of diaphragm fibers at birth. However, their contributions to the changes in activation properties observed in this study are likely to be small, because we showed that a single passive stretch of a skinned fiber could elicit changes to activation that were almost identical to those seen after the diaphragm had been engaged in spontaneous breathing for 40 min or mechanical ventilation for 2.5 h. Also, chemically skinning or permeabilizing the membrane removes the sarcoplasmic reticulum and T-tubules and washes away most phosphatases and kinases needed for signaling cascades that result in post-translation modification of protein function. Thus under this protocol, oxidation and phosphorlyation are unlikely to contribute to the effects caused by passive stretch. However it is still possible that they may be involved in the increased force generating capacity observed after breathing or ventilation in intact diaphragm fibers.
The effect of sarcomere length on Ca2+and Sr2+ sensitivity
Stretching a fiber increases the sarcomere length and results in increased sensitivity of the contractile apparatus to Ca2+ (Stephenson and Wendt, 1984). This finding has been shown in single skeletal muscle fibers in the adult rat, mouse, and rabbit (Stephenson and Williams, 1982; Moss et al., 1983; Balnave and Allen, 1996). The stretch-induced change in sensitivity differs between fiber types, with slow-twitch soleus fibers showing a greater increase in Ca2+ sensitivity than fast-twitch EDL fibers (Stephenson and Williams, 1982).
The effect of stretch on “hybrid” muscle fibers of the diaphragm and, in particular, the effect on Sr2+ sensitivity has not been previously addressed. Based on the results of Stephenson and Williams (1982), it was predicted that stretching diaphragm fibers would affect the F1 portion of the force–pSr curve (due to activation of slow MHC isoforms) to a greater degree than for the F2 portion (due to activation of fast MHC isoforms). Indeed, this was the outcome when fibers from the full-term fetal diaphragm were passively stretched. Mechanical ventilation for 2.5 h at 127 days (0.87) gestation also had the effect of altering the F1 phase of the force–pSr curve, suggesting that gaseous ventilation places increased tension on the diaphragm. The effects of mechanical ventilation at 127 days gestation, where the diaphragm was stretched by repeated tidal lung inflations of appropriate volume, were less marked than at term, suggesting that muscle fibers in the diaphragm are as yet incompletely developed, despite the presence of vigorous fetal breathing movements at this time (Dawes et al., 1972; Clewlow et al., 1983). The observed changes in relative expression of MHC isoforms is consistent with this conclusion, as expression of the embryo/neonatal isoform decreases, whereas MHC IIa expression increases over the last weeks of gestation and MHC I expression increases mainly after birth, perhaps as a result of the increased stress placed on the diaphragm by air breathing.
Changes in the elastic properties of the contractile apparatus
Muscle elasticity is governed by both intracellular and extracellular components, and it is possible that extracellular structural proteins such as collagen contribute to physical remodeling of the diaphragm as a result of the increased load placed on it. However, in this study, in which single skinned fibers were used, the changes we have observed are likely to arise from interactions of contraction-related proteins only. The passive elastic properties and structural integrity of sarcomeres within muscle fibers is determined by conformational changes of a number of large proteins, perhaps the most important of which is the >3 MDa muscle-specific protein titin, which spans half the length of a sarcomere from the Z-line to the M-line (Fukuda et al., 2008). Titin is structured so that it contains an extensible region – the “spring element” – located in the I-band, and which is comprised of an immunoglobulin (Ig)-like and PEVK (proline[P], glutamate [E], valine[V], lysine [K]) segments [14]. During contraction, titin controls the structure and stability of the sarcomere by keeping myosin in a central position to maximize myosin head attachment to actin (Fukuda et al., 2005, 2008). When the spring element extends or increases in tension, it decreases the interfilament lattice spacing, resulting in an increased force generating capacity and sensitivity to Ca2+, improving contractile function (Fukuda et al., 2008). A decrease in passive tension is consistent with the possibility that titin is physically extended or unwound. Diaphragm dysfunction has been associated with increased stiffness due to the loss of titin (Ottenheijm et al., 2006).
Fibers isolated from the fetal diaphragm produced significantly less passive tension and greater maximum Ca2+-activated force after lung inflation, whether being stretched by mechanical ventilation at 127 days gestation or after 40 min of spontaneous breathing at term. Lower passive force is consistent with the length of the “spring element” of titin being extended by lung inflation, reducing the interfilament lattice spacing and enhancing myosin attachment, both of which would increase the force generating capacity. If the magnitude of these findings for single muscle fibers could be amplified for the entire muscle, improved contractile function of the diaphragm as a whole might be an expected outcome.
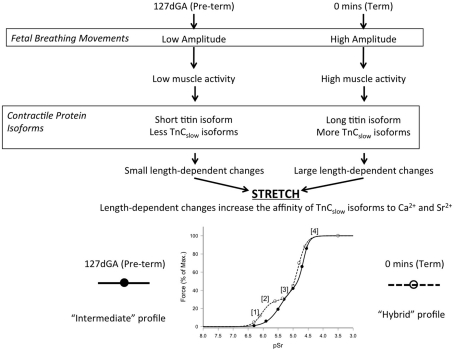
Length-dependent changes for other regulatory proteins may also occur within the contractile apparatus. There are two isoforms of troponin C (TnC)–TnCfast (expressed in fast-twitch skeletal muscle fibers, also referred to as TnCskeletal) and TnCslow (expressed in cardiac and slow-twitch skeletal muscle fibers, also referred to as TnCcardiac; Ohtsuki and Morimoto, 2008). The difference in structure of TnCfast and TnCslow isoforms is the main reason cardiac and slow-twitch fibers are more sensitive to Ca2+ and Sr2+ than fast-twitch fibers (Ashley et al., 1991; Palmer and Kentish, 1994; Ottenheijm et al., 2009). Previous studies have shown both force and activation-dependent changes alter the structure of the TnCslow binding sites which are in turn influenced by sarcomere length; i.e., an increase in length increases the binding affinity to Ca2+ (Martyn and Gordon, 2001). Individual diaphragm fibers express both TnCfast and TnCslow isoforms (O’Connell et al., 2004b). In the current study, an increase in sarcomere length not only increased the sensitivity of the contractile apparatus to Ca2+,but also to Sr2+and notably, significantly more so in the F1 portion of the force–-pSr curve (slow isoform activation) than the F2 portion (fast isoform activation). These findings therefore support the conclusion, summarized in Figure 7, that breathing causes diaphragm fibers to stretch, resulting in the unwinding of titin, which reduces the passive tension and leads to an increase in sarcomere length. This increase in sarcomere length may also induce physical changes to the TnCslow molecule which increases its affinity to Ca2+ but does not affect the structure or the Ca2+ affinity of the TnCfast binding sites. The result of this interaction can be observed in the activation profiles to Ca2+ and Sr2+after only 40 min of breathing in term lambs, where the leftward shift was greater in the F1 than the F2 portion of the force–pSr curve (see Figure 7).
Figure 7.
Proposed relationship between breathing, stretch, and gestation age in determining the activation properties of diaphragm fibers in preterm and term fetal sheep. At 127 days gestation, low amplitude FBMs and low neural input result in expression of short titin and little TnCslow isoform, so that effects of mechanical stretch at this age is minimal – hence little change occurs in the force–pSr curve. At term, maturational changes together with FBMs of increased amplitude result in expression of long titin and greater expression of TnCslow, so that stretch has greater effects on the lower portion of the force–pSr curve (see dotted line, lower panel). Lower panel: (1) At very low concentration Sr2+ binds only to TnCslow isoforms that have an increased stretch-induced affinity which, if increased at term, allow for greater force production. (2) At term low Sr2+ concentration bind to the constitutively expressed TnCslow isoforms resulting in increased force production. (3) At higher concentrations Sr2+ binds all TnCslow isoforms so that no new cross-bridges (regulated by TnCslow) are created, producing a plateau in force production. (4) With further increase in concentrations, Sr2+ binds to TnCfast isoforms, creating a second increase in force production.
Factors influencing a complete transition to a typical hybrid profile
In the full-term sheep fetus, diaphragm fibers successfully transform to a hybrid activation profile typical of the adult diaphragm after only 40 min of spontaneous breathing, or when a stretch was applied to isolated, skinned fibers. A less complete transition occurred at 127 days gestation when isolated skinned fibers were activated under an applied stretch, or after 2.5 h of rhythmic movement of the diaphragm induced by mechanical ventilation similar to that used by neonatologists in the resuscitation of preterm infants. While Ca2+ sensitivity was clearly increased, these procedures produced only modest changes in the pSr501 value of the force–pSr activation curve, so that at 127 days gestation diaphragm fibers have a phenotype that is intermediate between the earlier fetal “fast” phenotype and the “hybrid” phenotype eventually attained after term birth.
One developmental factor that may affect the diaphragm are fetal breathing movements which occur from at least mid-gestation in sheep and become more forceful (i.e., the fall in intrathoracic pressure is greater) and organized in pattern by term (Dawes et al., 1972; Clewlow et al., 1983). Changes in neural activity have a strong influence on the expression of the different MHC isoforms (Buller et al., 1960; Sieck and Zhan, 2000). The results of this study revealed that a significant increase in the expression of MHC I and IIa, decrease in MHC IIb/d, and almost complete loss of MHCembryo/neonate expression occurred between 127 day gestation and term, consistent with findings in another precocially developed species, the baboon (Maxwell et al., 1983). As shown for the adult rat diaphragm, fibers expressing MHCs that include I and IIa are known to express a greater proportion of TnCslow isoforms and produce a more distinct sub-maximal plateau in the force–pSr curve than do fibers with a predominant expression of MHC IIb/d (Bortolotto et al., 2000; O’Connell et al., 2004a). Therefore, a major difference between the fibers from preterm and full-term lambs may be the ratio of TnCslow and TnCfast expression within individual fibers of the diaphragm. Although the expression of TnC isoforms was not examined in this study, increased expression of MHCI has been associated with increased expression of TnCslow, and decreased expression of TnCfast isoforms (O’Connell et al., 2004a). MHC transformation from late gestation to early postnatal life has been well documented in the rat (Sieck and Zhan, 2000; Geiger et al., 2006).
Combinations of different tropomyosin subunits and troponin isoforms are known to alter the slope of the force–pCa (pSr) curves (Schachat et al., 1987). Since the slope of the force–pSr curve in the F1 portion (nSr1) was the factor indicating that full transition to a hybrid activation profile had not been attained in preterm lambs, it is possible that changes in the expression of tropomyosin subunits and/or troponin isoforms also influence the activation profile in the developing diaphragm. It is important to note that the activation profile of a skinned fiber is the result of the interaction of all the contractile and regulatory proteins; thus, a change observed in the activation properties is unlikely to be due to change in the expression of just one particular contractile protein isoform.
Conclusion
These findings provide evidence for the first time that the activation properties of the perinatal diaphragm are changed when lung compliance is altered in the transition from a liquid-filled lung to gaseous ventilation at birth. These changes occur rapidly after the commencement of air breathing, can be induced by stretch of isolated skinned diaphragm fibers, and may therefore result from increased load and tension as soon as ventilation is established. We suggest that the fast to slow transition at the level of contractile apparatus involves physical changes of proteins (e.g., titin unwinding, myofilament re-positioning, altered TnCslow structure) that influence the elastic and activation properties within the fiber, rather than changes in gene expression or post-translational modification of protein function. The increase in Ca2+ sensitivity and the force generating capacity of diaphragm fibers after stretching may be an important adaptation that allows the diaphragm to quickly meet the increased mechanical demand of breathing at birth. These changes can be only partly triggered in preterm lambs, indicating that complete transition to the typical hybrid activation profile in this important muscle may require modification of other factors such as the motor neuron pool and expression of contractile protein isoforms in the latter part of gestation. Future studies should investigate the role of accessory contractile proteins such as titin and TnC in diaphragm fibers during development, and the contribution of post-translational protein modification to the adaptation of the diaphragm at birth.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This project was funded by a grant from the National Health and Medical Research Council of Australia to David W. Walker, and the Australian Research Council to Jan M. West, and the Victorian Government’s Operational Infrastructure Support Program. We thank Dr. Phil Dooley (La Trobe University) for help with the skinned-fiber activation data, Prof Stuart Hooper (Ritchie Centre) for providing the subsidiary data from the ventilated preterm lambs, and Ana Baburamani (Ritchie Centre) for assistance with the fetal and newborn sheep ventilation studies.
References
- Ashley C. C., Lea T. J., Hoar P. E., Kerrick W. G., Strang P. F., Potter J. D. (1991). Functional characterization of the two isoforms of troponin C from the arthropod Balanus nubilus. J. Muscle Res. Cell. Motil. 12, 532–542 10.1007/BF01738441 [DOI] [PubMed] [Google Scholar]
- Ashley C. C., Moisescu D. G. (1977). Effect of changing the composition of the bathing solutions upon the isometric tension-pCa relationship in bundles of crustacean myofibrils. J. Physiol. (Lond.) 270, 627–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balnave C. D., Allen D. G. (1996). The effect of muscle length on intracellular calcium and force in single fibre from the mouse skeletal muscle. J. Physiol. 492, 705–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland R. D., McMillan D. D., Bressack M. A., Dong L. (1980). Clearance of liquid from lungs of newborn rabbits. J. Appl. Physiol. 49, 171–177 [DOI] [PubMed] [Google Scholar]
- Bortolotto S. K., Cellini M., Stephenson D. G., Stephenson G. M. (2000). MHC isoform composition and Ca(2+)- or Sr(2+)-activation properties of rat skeletal muscle fibers. Am. J. Physiol. Cell Physiol. 279, C1564–C1577 [DOI] [PubMed] [Google Scholar]
- Buller A. J., Eccles J. C., Eccles R. M. (1960). Interactions between motoneurones and muscles in respect of the characteristic speeds of their responses. J. Physiol. (Lond.) 150, 417–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell W. G., Gordon S. E., Carlson C. J., Pattison J. S., Hamilton M. T., Booth F. W. (2001). Differential global gene expression in red and white skeletal muscle. Am. J. Physiol. Cell Physiol. 280, C763–C768 [DOI] [PubMed] [Google Scholar]
- Cannata D. J., Finkelstein D. I., Gantois I., Teper Y., Drago J., West J. M. (2009). Altered fast- and slow-twitch muscle fibre characteristics in female mice with a (S248F) knock-in mutation of the brain neuronal nicotinic acetylcholine receptor. J. Muscle Res. Cell. Motil. 30, 73–83 10.1007/s10974-009-9177-x [DOI] [PubMed] [Google Scholar]
- Cannata D. J., Ireland Z., Dickinson H., Snow R. J., Russell A. P., West J. M., Walker D. W. (2010). Maternal creatine supplementation from mid-pregnancy protects the diaphragm of the newborn spiny mouse from intrapartum hypoxia-induced damage. Pediatr. Res. 68, 393–398 [DOI] [PubMed] [Google Scholar]
- Chambers M. A., Moylan J. S., Smith J. D., Goodyear L. J., Reid M. B. (2009). Stretch-stimulated glucose uptake in skeletal muscle is mediated by reactive oxygen species and p38 MAP-kinase. J. Physiol. (Lond.) 587, 3363–3373 10.1113/jphysiol.2008.165639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewlow F., Dawes G. S., Johnston B. M., Walker D. W. (1983). Changes in breathing, electrocortical and muscle activity in unanaesthetized fetal lambs with age. J. Physiol. (Lond.) 341, 463–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley K. J., Allison B. J., Polglase G. R., Morley C. J., Davis P. G., Hooper S. B. (2009). Dynamic changes in the direction of blood flow through the ductus arteriosus at birth. J. Physiol. (Lond.) 587, 4695–4704 10.1113/jphysiol.2009.174870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes G. S., Fox H. E., Leduc B. M., Liggins G. C., Richards R. T. (1972). Respiratory movements and rapid eye movement sleep in the foetal lamb. J. Physiol. (Lond.) 220, 119–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda N., Granzier H. L., Ishiwata S., Kurihara S. (2008). Physiological functions of the giant elastic protein titin in mammalian striated muscle. J. Physiol. Sci. 58, 151–159 10.2170/physiolsci.RV005408 [DOI] [PubMed] [Google Scholar]
- Fukuda N., Wu Y., Farman G., Irving T. C., Granzier H. (2005). Titin-based modulation of active tension and interfilament lattice spacing in skinned rat cardiac muscle. Pflugers Arch. 449, 449–457 10.1007/s00424-004-1354-6 [DOI] [PubMed] [Google Scholar]
- Geiger P. C., Bailey J. P., Mantilla C. B., Zhan W. Z., Sieck G. C. (2006). Mechanisms underlying myosin heavy chain expression during development of the rat diaphragm muscle. J. Appl. Physiol. 101, 1546–1555 10.1152/japplphysiol.00221.2006 [DOI] [PubMed] [Google Scholar]
- Geiger P. C., Cody M. J., Sieck G. C. (1999). Force-calcium relationship depends on myosin heavy chain and troponin isoforms in rat diaphragm muscle fibers. J. Appl. Physiol. 87, 1894–1900 [DOI] [PubMed] [Google Scholar]
- Goldspink G., Scutt A., Loughna P. T., Wells D. J., Jaenicke T., Gerlach G. F. (1992). Gene expression in skeletal muscle in response to stretch and force generation. Am. J. Physiol. 262, R356–R563 [DOI] [PubMed] [Google Scholar]
- Goldspink G., Williams P., Simpson H. (2002). Gene expression in response to muscle stretch. Clin. Orthop. Relat. Res. 403 (Suppl.), S146–S152 10.1097/00003086-200210001-00017 [DOI] [PubMed] [Google Scholar]
- Guslits B. G., Gaston S. E., Bryan M. H., England S. J., Bryan A. C. (1987). Diaphragmatic work of breathing in premature human infants. J. Appl. Physiol. 62, 1410–1415 [DOI] [PubMed] [Google Scholar]
- Harridge S. (2011). Plasiticity of human skeletal muscle: gene expression to in vivo function. Exp. Physiol. 92, 783–797 10.1113/expphysiol.2006.036525 [DOI] [PubMed] [Google Scholar]
- Hooper S. B., Harding R. (2005). Role of aeration in the physiological adaptation of the lung to air-breathing at birth. Curr. Respir. Med. Rev. 1, 185–195 [Google Scholar]
- Jain L., Eaton D. C. (2006). Physiology of fetal lung fluid clearance and the effect of labor. Semin. Perinatol. 30, 34–43 10.1053/j.semperi.2006.01.006 [DOI] [PubMed] [Google Scholar]
- Mantilla C. B., Sieck G. C. (2008). Key aspects of phrenic motoneuron and diaphragm muscle development during the perinatal period. J. Appl. Physiol. 104, 1818–1827 10.1152/japplphysiol.01192.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martyn D. A., Gordon A. M. (2001). Influence of length on force and activation-dependent changes in troponin c structure in skinned cardiac and fast skeletal muscle. Biophys. J. 80, 2798–2808 10.1016/S0006-3495(01)76020-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell L. C., McCarter R. J., Kuehl T. J., Robotham J. L. (1983). Development of histochemical and functional properties of baboon respiratory muscles. J. Appl. Physiol. 54, 551–561 [DOI] [PubMed] [Google Scholar]
- Monasky M. M., Biesiadecki B. J., Janssen P. M. (2010). Increased phosphorylation of tropomyosin, troponin I, and myosin light chain-2 after stretch in rabbit ventricular myocardium under physiological conditions. J. Mol. Cell. Cardiol. 48, 1023–1028 10.1016/j.yjmcc.2010.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss R. L., Swinford A. E., Greaser M. L. (1983). Alterations in the Ca2+ sensitivity of tension development by single skeletal muscle fibers at stretched lengths. Biophys. J. 43, 115–119 10.1016/S0006-3495(83)84329-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell B., Nguyen L. T., Stephenson G. M. (2004a). A single-fibre study of the relationship between MHC and TnC isoform composition in rat skeletal muscle. Biochem. J. 378, 269–274 10.1042/BJ20031170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell B., Stephenson D. G., Blazev R., Stephenson G. M. (2004b). Troponin C isoform composition determines differences in Sr(2+)-activation characteristics between rat diaphragm fibers. Am. J. Physiol. Cell Physiol. 287, C79–C87 10.1152/ajpcell.00555.2003 [DOI] [PubMed] [Google Scholar]
- Ohtsuki I., Morimoto S. (2008). Troponin: regulatory function and disorders. Biochem. Biophys. Res. Commun. 369, 62–73 10.1016/j.bbrc.2007.11.187 [DOI] [PubMed] [Google Scholar]
- Olver R. E., Walters D. V., Wilson S. M. (2004). Developmental regulation of lung liquid transport. Annu. Rev. Physiol. 66, 77–101 10.1146/annurev.physiol.66.071702.145229 [DOI] [PubMed] [Google Scholar]
- Ottenheijm C. A., Heunks L. M., Hafmans T., van der Ven P. F., Benoist C., Zhou H., Labeit S., Granzier H. L., Dekhuijzen P. N. (2006). Titin and diaphragm dysfunction in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 173, 527–534 10.1164/rccm.200507-1056OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottenheijm C. A., Knottnerus A. M., Buck D., Luo X., Greer K., Hoying A., Labeit S., Granzier H. (2009). Tuning passive mechanics through differential splicing of titin during skeletal muscle development. Biophys. J. 97, 2277–2286 10.1016/j.bpj.2009.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer S., Kentish J. C. (1994). The role of troponin C in modulating the Ca2+ sensitivity of mammalian skinned cardiac and skeletal muscle fibre. J. Physiol. (Lond.) 480(Pt 1), 45–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polglase G. R., Morley C. J., Crossley K. J., Dargaville P., Harding R., Morgan D. L., Hooper S. B. (2005). Positive end-expiratory pressure differentially alters pulmonary hemodynamics and oxygenation in ventilated, very premature lambs. J. Appl. Physiol. 99, 1453–1461 10.1152/japplphysiol.00055.2005 [DOI] [PubMed] [Google Scholar]
- Prosser B. L., Ward C. W., Lederer W. J. (2011). X-ROS signaling: rapid mechano-chemo transduction in heart. Science 333, 1440–1445 10.1126/science.1202768 [DOI] [PubMed] [Google Scholar]
- Rudolph A. M. (1985). Distribution and regulation of blood flow in the fetal and neonatal lamb. Circ. Res. 57, 811–821 [DOI] [PubMed] [Google Scholar]
- Schachat F. H., Diamond M. S., Brandt P. W. (1987). Effect of different troponin T-tropomyosin combinations on thin filament activation. J. Mol. Biol. 198, 551–554 10.1016/0022-2836(87)90300-7 [DOI] [PubMed] [Google Scholar]
- Shanely R. A., Van Gammeren D., Deruisseau K. C., Zergeroglu A. M., McKenzie M. J., Yarasheski K. E., Powers S. K. (2004). Mechanical ventilation depresses protein synthesis in the rat diaphragm. Am. J. Resp. Crit. Care Med. 170, 994–999 10.1164/rccm.200304-575OC [DOI] [PubMed] [Google Scholar]
- Sieck G. C., Fournier M., Blanco C. E. (1991). Diaphragm muscle fatigue resistance during postnatal development. J. Appl. Physiol. 71, 458–464 [DOI] [PubMed] [Google Scholar]
- Sieck G. C., Zhan W. Z. (2000). Denervation alters myosin heavy chain expression and contractility of developing rat diaphragm muscle. J. Appl. Physiol. 89, 1106–1113 [DOI] [PubMed] [Google Scholar]
- Siew M. L., Wallace M. J., Kitchen M. J., Lewis R. A., Fouras A., Te Pas A. B., Yagi N., Uesugi K., Siu K. K., Hooper S. B. (2009). Inspiration regulates the rate and temporal pattern of lung liquid clearance and lung aeration at birth. J. Appl. Physiol. 106, 1888–1895 10.1152/japplphysiol.91526.2008 [DOI] [PubMed] [Google Scholar]
- Stephenson D. G., Wendt I. R. (1984). Length dependence of changes in sarcoplasmic calcium concentration and myofibrillar calcium sensitivity in striated muscle fibre. J. Muscle Res. Cell. Motil. 5, 243–272 10.1007/BF00713107 [DOI] [PubMed] [Google Scholar]
- Stephenson D. G., Williams D. A. (1982). Effects of sarcomere length on the force-pCa relation in fast- and slow-twitch skinned muscle fibre from the rat. J. Physiol. (Lond.) 333, 637–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge R. J., Roy R. R. (1993). Electrophoretic separation of rat skeletal muscle myosin heavy-chain isoforms. J. Appl. Physiol. 75, 2337–2340 [DOI] [PubMed] [Google Scholar]
- Walker D. W., Luff A. R. (1995). Functional development of fetal limb muscles: a review of the roles of activity, nerves and hormones. Reprod. Fertil. Dev. 7, 391–398 10.1071/RD9950391 [DOI] [PubMed] [Google Scholar]
- Watchko J. F., Daood M. J., Sieck G. C. (1998). Myosin heavy chain transitions during development. Functional implications for the respiratory musculature. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 119, 459–470 10.1016/S0305-0491(98)00006-6 [DOI] [PubMed] [Google Scholar]
- West J. M., Barclay C. J., Luff A. R., Walker D. W. (1999). Developmental changes in the activation properties and ultrastructure of fast- and slow-twitch muscles from fetal sheep. J. Muscle Res. Cell. Motil. 20, 249–264 10.1023/A:1005433809414 [DOI] [PubMed] [Google Scholar]
- West J. M., Williams N. A., Luff A. R., Walker D. W. (2000). Effect of tibial bone resection on the development of fast- and slow-twitch skeletal muscles in foetal sheep. J. Muscle Res. Cell. Motil. 21, 209–222 10.1023/A:1005676312176 [DOI] [PubMed] [Google Scholar]